Abstract
Aim:
To test the effect of the duration of medication for opioid use disorder (MOUD) use during pregnancy on maternal, perinatal, and neonatal outcomes.
Design:
Retrospective cohort analysis of claims, encounter, and pharmacy data.
Setting:
Pennsylvania, USA.
Participants:
We analyzed 13,320 pregnancies among 10,741 women with opioid use disorder aged 15–44 years enrolled in Pennsylvania Medicaid between 2009 and 2017.
Measurements:
We examined five outcomes during pregnancy and for 12 weeks postpartum: (1) overdose, (2) postpartum MOUD continuation, (3) preterm birth (<37 weeks gestation), (4) term low birthweight (<2,500 grams at ≥ 37 weeks), and (5) neonatal abstinence syndrome (NAS). Our primary exposure was the duration (count of weeks) of any MOUD use, including methadone or buprenorphine, during pregnancy.
Findings:
Among 13,320 pregnancies, 306 (2.3%) were complicated by an overdose, 1,753 (13.2%) resulted in a preterm birth and 6,787 (50.9%) continued MOUD postpartum. Among infants, 874 (7.6%) were low birthweight at term and 7,706 (57.9%) were diagnosed with NAS. As the duration of MOUD use increased, we found a statistically significant decrease in the rate of overdose and preterm birth, a statistically significant increase in the rate of postpartum MOUD continuation and NAS, and a decline in term low birthweight. Specifically, for each additional week of MOUD, the adjusted odds of overdose decreased by 2% (aOR 0.98; 95% CI: 0.97,0.99), preterm birth decreased by 1% (aOR: 0.99; 95% CI: 0.99,1.00), postpartum MOUD continuation increased by 95% (aOR: 1.95; 95% CI: 1.87, 2.04) and NAS increased by 41% (aOR: 1.41; 95% CI 1.35, 1. 47). The odds of term low birthweight did not change (aOR: 1.00; 95% CI: 0.99, 1.00), although the rate declined with a longer duration of MOUD use during pregnancy.
Conclusions:
Longer duration of medication for opioid use disorder use during pregnancy appears to be associated with improved maternal and perinatal outcomes.
INTRODUCTION
Medication for opioid use disorder (MOUD), such as methadone or buprenorphine, markedly reduces the risk of overdose mortality, illicit substance use recurrence and infectious disease acquision1, 2 and increasing MOUD utilization rates has been the primary focus of federal, state and local efforts to combat the opioid epidemic.1, 3 Despite MOUD’s effectiveness, the unique circumstances of pregnancy including the effect of opioid exposure on neonatal outcomes such as neonatal abstinence syndrome (NAS) have contributed to an evolving debate over the risks and benefits of MOUD use during pregnancy.4, 5 Currently, recommendations emphasizing the importance of MOUD use during pregnancy highlight improvements in healthcare utilization, the mitigation of opioid withdrawal symptoms and reductions in illicit substance use which is consistent with benefits found among non-pregnant populations.6, 7 While improvements in these outcomes are critical for all individuals with OUD, research specifically focused on evaluating the effects of MOUD use on outcomes unique to pregnancy and the postpartum period is limited.
In small, single site, retrospective cohort evaluations, a longer duration of MOUD use during pregnancy has been significantly associated with a decreased risk of preterm birth and improved birth weight.8–11 While suggestive of improved outcomes, these analyses primarily focus on within group MOUD duration comparisons, lack a true non-MOUD comparison group and are inadequately powered to evaluate rare but important maternal outcomes, namely, overdose. Conversely, population-level research associated with opioid use during pregnancy has primarily compared perinatal outcomes between opioid-using pregnant women and non-opioid-using pregnant controls.12–15 While this research highlights clear differences in prevalence of adverse birth outcomes among pregnant women who do and do not use opioids, such studies are unable to provide information about the effect of therapeutic interventions, such as MOUD, during pregnancy. Thus, much remains unknown about how MOUD use may modify health outcomes among pregnant women with OUD and their children.
The purpose of the present study is to provide a population-level assessment of health outcomes associated with MOUD use during pregnancy. Specifically, our objective is to examine the association between the duration of MOUD use in pregnancy and maternal (i.e., overdose, postpartum MOUD continuation), perinatal (i.e., preterm birth, term low birthweight) and neonatal (i.e., NAS) health outcomes in a large sample of Medicaid-enrolled pregnant women with OUD.
METHODS
Data sources and study population
In the United States, state Medicaid programs provide healthcare coverage for more than 80% of pregnant women with OUD and their children.16, 17 Thus, for this analysis, we utilized administrative healthcare claims data from the Pennsylvania Department of Health and Human Services Medicaid program. This dataset encompasses a census of all claims, inpatient and outpatient encounters, and pharmacy data for all Medicaid enrollees in Pennsylvania (including Medicaid managed care plans and traditional fee-for-service payments). We identified Medicaid-enrolled females ages 15–44 years who had a live birth from January 1, 2009-September 30, 2017, who had diagnosis of opioid use disorder (OUD) (ICD-9: 304.0X, 305.5X, 304.7X; ICD-10: F11.XXX) during their pregnancy. Live births were identified using the date of delivery in inpatient records. To be included in the cohort, we also required at least 8 weeks of Medicaid enrollment during pregnancy, consistent with prior research.18 Women with a stillbirth were excluded due to the low prevalence in the cohort (N=197, 1.48%) and because our study outcomes necessitated a live birth. If a woman had an interpregnancy interval of less than 24 weeks (<168 days), the later pregnancy was excluded from the analysis. Women with a diagnosis of OUD in remission (ICD-9: 304.03, 304.73, 305.53; ICD-10: F11.11, F11.21) were also excluded. All women were followed from 280 days prior to delivery to 12 weeks after their date of delivery. A 12-week post-delivery follow-up period was selected because Medicaid pregnancy-related eligibility rules require coverage through that period. To identify neonatal outcomes, we linked women with a live birth to their infant record using a family record ID, date of child’s birth, and maternal date of delivery (yielding a 96% match rate). The study cohort included 13,320 maternal-child dyads among whom 1,762 women had more than one pregnancy during the study period.
Demographic information, including age, race and ethnicity, medical and pregnancy-associated co-morbidities, substance use and maternal and perinatal outcomes were obtained from patient enrollment and utilization data files. MOUD utilization data, including buprenorphine prescription fills and methadone treatment services were obtained from pharmacy and professional claims data files, respectively. Neonatal outcomes were obtained from child inpatient and professional data files. Our study design was observational, and our analysis plan was not pre-registered on a publicly available platform. Thus, our results should be considered exploratory. The study was approved by the University of Pittsburgh, Institutional Review Board (IRB).
Patient characteristics
Demographic characteristics included a continuous measure of maternal age at delivery and a categorical measure of patient race and ethnicity (Non-Hispanic white, Non-Hispanic black, Hispanic, and other). Maternal medical co-morbidities including psychiatric disorders, Hepatitis C virus (HCV) and HIV infection, co-occurring non-opioid substance use disorders, chronic hypertension, diabetes mellitus, gestational diabetes mellitus and gestational hypertensive disorders were identified by ICD-9 and ICD-10 diagnostic codes that were associated with the pregnancy. Psychiatric disorders included depressive disorders, anxiety disorders, bipolar disorder, and schizophrenia. Co-occurring non-opioid substance use disorders were categorized as: tobacco use disorders, alcohol use disorders, and non-opioid substance use disorders (including cocaine, marijuana, amphetamine, hallucinogen, sedatives and other substance abuse or dependence during pregnancy). Psychotropic medications were measured in patient pharmacy records and were defined as any prescription fill for a selective-serotonin-reuptake inhibitor (SSRI), gabapentinoid (i.e., gabapentin, pregabalin), or benzodiazepine during the pregnancy (full list of National Drug Codes used is available upon request).
Duration of MOUD use in pregnancy
Our exposure was defined as the count of weeks in pregnancy with any MOUD use and included the use of either methadone only, buprenorphine only, or both methadone and buprenorphine during pregnancy. Methadone use was identified using procedure codes, and buprenorphine use was identified using National Drug Codes (NDC) in outpatient pharmacy records (available from the authors upon request). We did not include naltrexone because it is not routinely used during pregnancy. To construct the exposure measure, we used the Proportion of Days Covered (PDC) algorithm,19 which calculates a proportion from 0 to 1 of all days with MOUD use during pregnancy, and then we converted days to weeks. Because we sought a flexible exposure measure that allowed for gaps in treatment that occur in the real world, we considered the total count of weeks of MOUD use during pregnancy, which did not have to be consecutive. The weeks of MOUD use during pregnancy ranged from 0 weeks (no treatment) to 40 weeks (continuous use with initiation before pregnancy). Because most women who newly enroll in Medicaid during pregnancy are not insured prior to enrollment,20 MOUD use was defined as “none” during the weeks gestation when participants were not observed in Medicaid enrollment files.
The purpose of our analysis was to evaluate differences in health outcomes when MOUD was and was not used during pregnancy and not to compare drug-specific differences in outcomes following the use of methadone versus buprenorphine. However, we conducted a sensitivity analyses to compare the effect of buprenorphine only use compared to no MOUD use and methadone only use compared to no MOUD use in pregnancy on our outcomes of interest (Appendix Table B). Pregnant women who received both methadone and buprenorphine during pregnancy (n=410, 3.08%) were excluded from the sensitivity analysis. Results from these analyses were highly consistent with our combined MOUD measure for the postpartum MOUD use and NAS diagnoses. For maternal overdose, preterm birth, and term low birthweight, we lacked statistical power to detect precise estimates in buprenorphine and methadone subpopulations but note that these results are not inconsistent with our primary analysis.
Maternal, perinatal, and neonatal outcomes
We examined 5 health outcomes associated with MOUD use during pregnancy. First, we examined maternal health outcomes which included overdose and postpartum MOUD continuation. Postpartum MOUD continuation was defined as at least 1 claim for any MOUD (buprenorphine or methadone) prescribed during the first 12 weeks postpartum. Overdose was defined as any overdose event observed during pregnancy or within the first 12 weeks postpartum using ICD-9 and ICD-10 codes for opioid overdose, consistent with prior research (T400X1A, T400X2A, T400X3A, T400X4A, T401X1A, T401X2A, T401X3A, T401X4A, T403X1A, T403X2A, T403X3A, T403X4A, T402X1A, T402X2A, T402X3A, T402X4A, T404X1A, T404X2A, T404X3A, T404X4A, T40601A, T40602A, T40603A, T40604A, T40691A, T40692A, T40693A, T40694A, E8500, E8501, E8502, E9350, E9351, E9352, 96500, 96501, 96502, 96509).21 Second, we examined perinatal health outcomes which included whether infants were born preterm (<37 weeks’ gestation) and among infants who were born at term gestations (≥ 37 weeks), whether they were low birthweight (<2,500 grams). Term low birthweight and preterm birth outcomes were identified using ICD-9 and ICD-10 diagnostic codes on infants’ claims related to their birth hospitalization. Finally, we examined whether infants were identified as having NAS which was defined based on ICD-9 and ICD-10 diagnostic codes (779.5, P96.1) during the birth hospitalization or during the first 7 days of life. In prior research, hospital administrative data has a high positive predictive value (PPV) in identifying cases of clinically diagnosed NAS both with and without pharmacotherapy.22 Iatrogenic cases of NAS were excluded, consistent with prior research.23 A comprehensive list of the ICD-9 and ICD-10 codes used to identify our exposure, covariate and outcome measures are described in Appendix Table A.
Statistical analysis
First, descriptive statistics were calculated to examine the characteristics of the study population which were stratified by duration of MOUD use during pregnancy (no MOUD, 1–10 weeks, 11–20 weeks, > 20 weeks) and assessed for unadjusted differences between each group (Table 1). We used multivariable regression models to determine the adjusted association between duration of MOUD use in pregnancy and maternal, perinatal, and neonatal outcomes. Based on exploratory data analysis, we modeled weeks’ MOUD in pregnancy as a continuous variable in the models with maternal overdose, term low birthweight, and preterm birth as outcomes. We modeled weeks’ MOUD in pregnancy using a restricted cubic spline term with knots at 1, 5, 10, and 20 weeks’ MOUD duration in the models with postpartum MOUD continuation and NAS as outcomes. Because approximately 10% of women in our cohort had more than one pregnancy during the study time period, we used a robust standard error estimate to account for non-independence of observations within a woman.24 We used log-linear regression to model the association between the duration of MOUD in pregnancy and overdose; for all other outcomes, we used logistic regression. All regression analyses controlled for a set of covariates that are expected to confound the association between the duration of MOUD use in pregnancy and health outcomes.25, 26 Covariates in the analysis for postpartum MOUD continuation, maternal overdose and NAS included a continuous measure of maternal age at delivery, maternal race and ethnicity, an indicator of any psychiatric co-morbidity, an indicator of HCV or HIV infection, an indicator of tobacco use disorder, an indicator of any other non-opioid substance use disorder, and three individual indicators for any use of psychotropic medications: SSRIs, gabapentinoids, and benzodiazepines. Covariates in the analysis for term low birthweight and preterm birth also included an indicator for chronic hypertension, diabetes mellitus, gestational diabetes mellitus and gestational hypertensive disorder. We then derived average predicted probabilities and associated 95% confidence intervals (CIs) of outcomes for each week of MOUD use in pregnancy, using marginal standardization.27
Table 1.
Characteristics of pregnancies with opioid use disorder in Pennsylvania Medicaid, overall and stratified by duration of use of medications for opioid use disorder (MOUD), 2009–2017
| Overalla,b n=13,320 | No MOUD observeda,b n=5,566 | 1–10 weeks MOUDa,b N=2,718 | 11–20 weeks MOUDa,b N=1,416 | >20 weeks MOUD N=3,620a,b | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age [years; mean ±SD] | 28.3 (±4.7) | 28.1 (±4.9) | 27.9 (±4.5) | 28.2 (±4.6) | 29.0 (±4.5) |
| Race and ethnicity | |||||
| Non-Hispanic white | 11635 (87.4) | 4497 (80.8) | 2,466 (90.7) | 1,300 (91.8) | 3,372 (93.2) |
| Non-Hispanic black | 1032 (7.8) | 688 (12.4) | 146 (5.4) | 68 (4.8) | 130 (3.6) |
| Hispanic | 434 (3.3) | 280 (5.0) | 58 (2.1) | 33 (2.3) | 63 (1.7) |
| Other | 219 (1.6) | 101 (1.8) | 48 (1.8) | 15 (1.1) | 55 (1.5) |
| Medical comorbidities | |||||
| Psychiatric disorderc | 7501 (56.3) | 3015 (54.2) | 1,435 (52.8) | 848 (59.9) | 2,203 (60.9) |
| Hepatitis C virus (HCV) infection | 4030 (30.3) | 1316 (23.6) | 975 (35.9) | 506 (35.7) | 1,233 (34.1) |
| HIV infection | 73 (0.6) | 32 (0.57) | 12 (0.4) | <11 (<1.0) | 19 (0.52) |
| Co-occurring substance use disorders | |||||
| Tobacco use disorder | 10194 (76.5) | 4083 (73.4) | 2,108 (77.6) | 1,126 (79.5) | 2,877 (79.5) |
| Non-opioid substance use disordersd | 7217 (54.2) | 2819 (50.7) | 1,542 (56.7) | 842 (59.5) | 2,014 (55.6) |
| Alcohol use disorder | 942 (7.1) | 489 (8.8) | 155 (5.7) | 110 (7.8) | 188 (5.2) |
| Psychotropic medicationse | |||||
| SSRIs | 2718 (20.4) | 1094 (19.7) | 509 (18.7) | 296 (20.9) | 819 (22.6) |
| Gabapentins | 1303 (9.8) | 477 (8.6) | 265 (9.8) | 164 (11.6) | 397 (10.9) |
| Benzodiazepines | 2006 (15.1) | 714 (12.8) | 395 (14.5) | 221 (15.6) | 676 (18.7) |
| Medications for opioid use disorder (MOUD) | |||||
| Methadone | 3855 (28.9) | -- | 1931 (71.0) | 563 (39.8) | 1361 (37.6) |
| Buprenorphine | 4309 (32.3) | -- | 1198 (44.1) | 899 (63.5) | 2212 (61.1) |
| Timing of MOUD initiation | |||||
| Pre-pregnancy | -- | -- | 541 (19.9) | 364 (25.7) | 2410 (66.6) |
| First trimester | -- | -- | 852 (31.4) | 450 (31.8) | 3,191 (88.2) |
| Second trimester | -- | -- | 619 (22.8) | 787 (55.6) | 429 (11.9) |
| Third trimester | -- | -- | 1,247 (45.9) | 179 (12.6) | <11 (<1.0) |
| Outcomes | |||||
| Overdose | 306 (2.3) | 134 (2.4) | 81 (2.9) | 31 (2.2) | 60 (1.7) |
| Postpartum MOUD utilization | 6787 (50.9) | 570 (10.2) | 1572 (57.8) | 1201 (84.8) | 3444 (95.1) |
| Term Low Birthweight (<2500 grams at ≥ 37 weeks) | 874 (7.6) | 320 (6.6) | 223 (9.7) | 113 (9.4) | 218 (6.8) |
| Preterm Birth (<37 weeks) | 1753 (13.2) | 730 (13.1) | 409 (15.1) | 207 (14.6) | 407 (11.2) |
| Neonatal Abstinence Syndrome (NAS) | 7706 (57.9) | 2115 (38.0) | 1963 (72.2) | 974 (68.8) | 2654 (73.3) |
n (%) unless otherwise specified
duration of MOUD is defined as the total number of weeks with any buprenorphine or methadone treatment in pregnancy; categories of MOUD duration are shown here
defined as a diagnosis of a depressive, anxiety or bipolar disorder, or schizophrenia
defined as a diagnosis of cocaine, marijuana, amphetamine, hallucinogen and/or sedatives use during pregnancy
defined as outpatient prescription fills for psychotropic medications in pregnancy
Results are reported in two ways. First, we report the average predicted probabilities of outcomes for each week of MOUD use in pregnancy to allow for interpretation of the association between MOUD and outcomes across the entire range of MOUD duration in pregnancy. Second, we present the adjusted odds ratios (aORs) and associated 95% CIs from the regression models to allow for the interpretation of each marginal week of MOUD use in pregnancy. We also calculated the aORs for each marginal 5 weeks, 10 weeks, or 20 weeks’ duration of MOUD use in pregnancy by exponentiating the multiplied coefficient (while accounting for spline effects in terms of postpartum MOUD continuation and NAS). For all analyses, statistical significance of duration of MOUD is assessed in terms of confidence intervals. All programming and statistical analyses were performed using the SAS software version 9.4.
RESULTS
From January 1, 2009-September 30, 2017, we identified 354,891 Medicaid enrolled pregnancies with a live birth. Within this sample, we identified a cohort of 13,320 pregnancies (3.8%) among 10,741 women who had a diagnosis of OUD. The characteristics of our cohort, stratified by duration of MOUD use during pregnancy (no MOUD, 1–10 weeks, 11–20 weeks, >20 weeks), are described in Table 1. Among 13,320 pregnancies, 5,566 (41.8%) did not have an observed pharmacy fill or claim for MOUD during pregnancy, 2,718 (20.4%) had duration of MOUD from 1–10 weeks, 1,416 (10.6%) had duration of MOUD from 11–20 weeks, and 3,620 (27.2%) had duration of MOUD >20 weeks. Overall, 306 (2.3%) pregnancies were complicated by an overdose, 1,753 (13.2%) resulted in a preterm birth and 6,787 (50.9%) continued MOUD use during 12 weeks postpartum. Among pregnancies complicated by at least one an overdose event, 238 (77.8%) had an overdose that occurred during pregnancy, 57 (18.63%) had an overdose that occurred postpartum, and 11 (3.59%) had an overdose that occurred during both pregnancy and postpartum. Among pregnancies with MOUD use, 3,855 (28.9%) used methadone and 4,309 (32.3%) used buprenorphine. Among infants in our cohort, 874 (7.6%) were low birthweight at term and 7,706 (57.9%) were diagnosed with NAS.
Co-occurring medical disorders were common. More than half of pregnancies with OUD had a diagnosis of a psychiatric disorder (56.3%), which did not vary meaningfully by duration of MOUD in pregnancy. In the overall sample, nearly one-third had a diagnosis of HCV infection (30.3%), although the prevalence was lower (23.6%) among those with no MOUD. Most used tobacco (76.5%), more than half (54.2%) had co-occurring non-opioid substance use disorder, and 7.1% had an alcohol use disorder. While the prevalence of co-occurring non-opioid use disorders did not differ meaningfully by MOUD duration, the prevalence was lower among those with no MOUD. The use of prescribed psychotropic medications was not uncommon; approximately 20% of pregnant women used a SSRI, 10% used a gabapentinoid, and 15% used a benzodiazepine during pregnancy. Psychotropic medication use did not differ meaningfully by MOUD duration.
Figure 1 demonstrates the trend in maternal outcomes associated with an increasing duration of MOUD use during pregnancy. After adjusting for covariates, we found a statistically significant decrease in rate of overdose (Figure 1A) and a statistically significant increase in the rate of postpartum MOUD continuation (Figure 1B) as the duration of MOUD use increased during pregnancy. Figure 2 demonstrates the trend in perinatal and neonatal outcomes associated with an increasing duration of MOUD use during pregnancy. After adjusting for covariates, we found a statistically significant decline in the rate of preterm birth (Figure 2A) and a decline in the rate of term low birthweight (Figure 2B) as the duration of MOUD use during pregnancy increased. Conversely, we found a statistically significant increase in the rate of NAS as the duration of MOUD use during pregnancy increased (Figure 2C).
Figure 1.
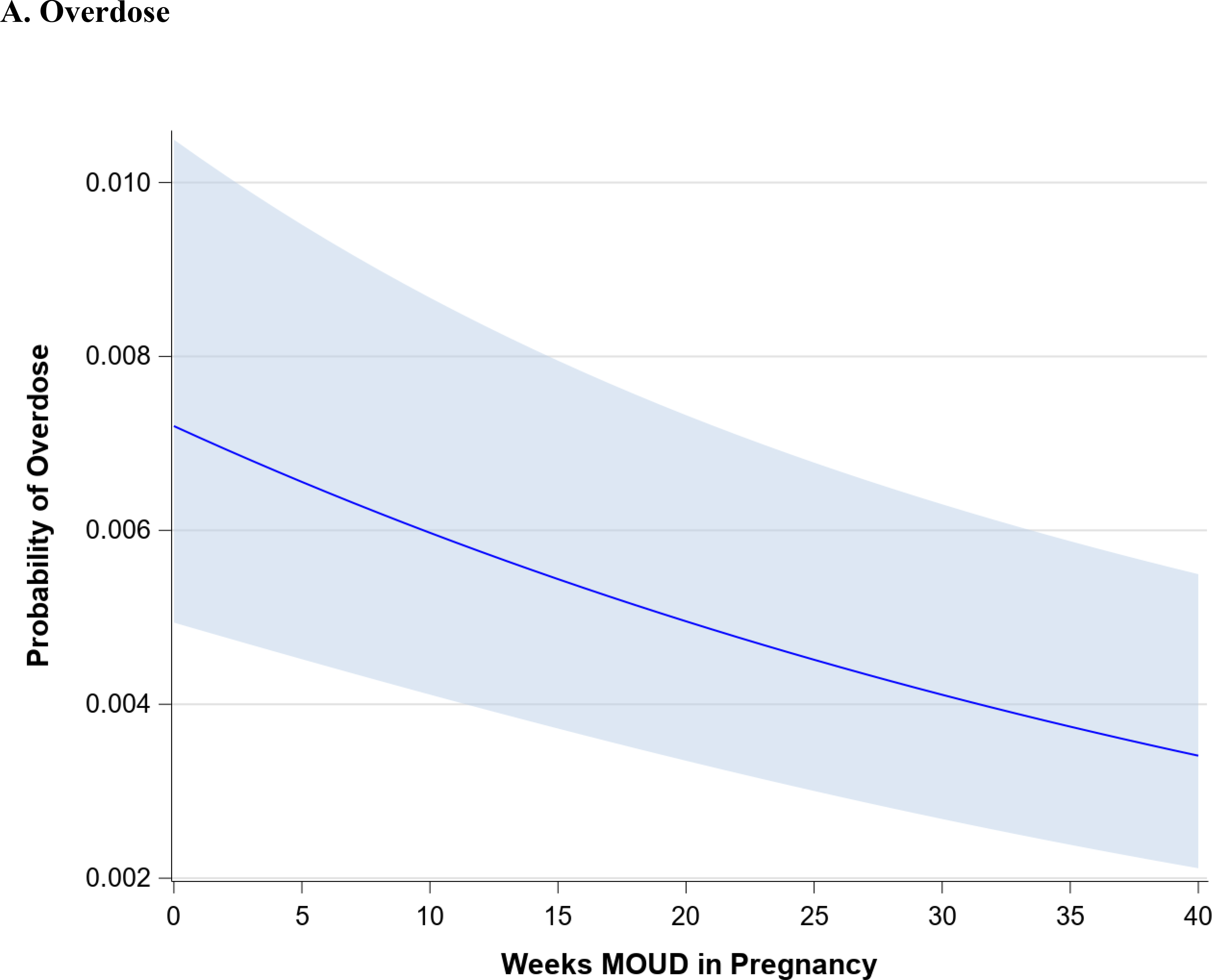
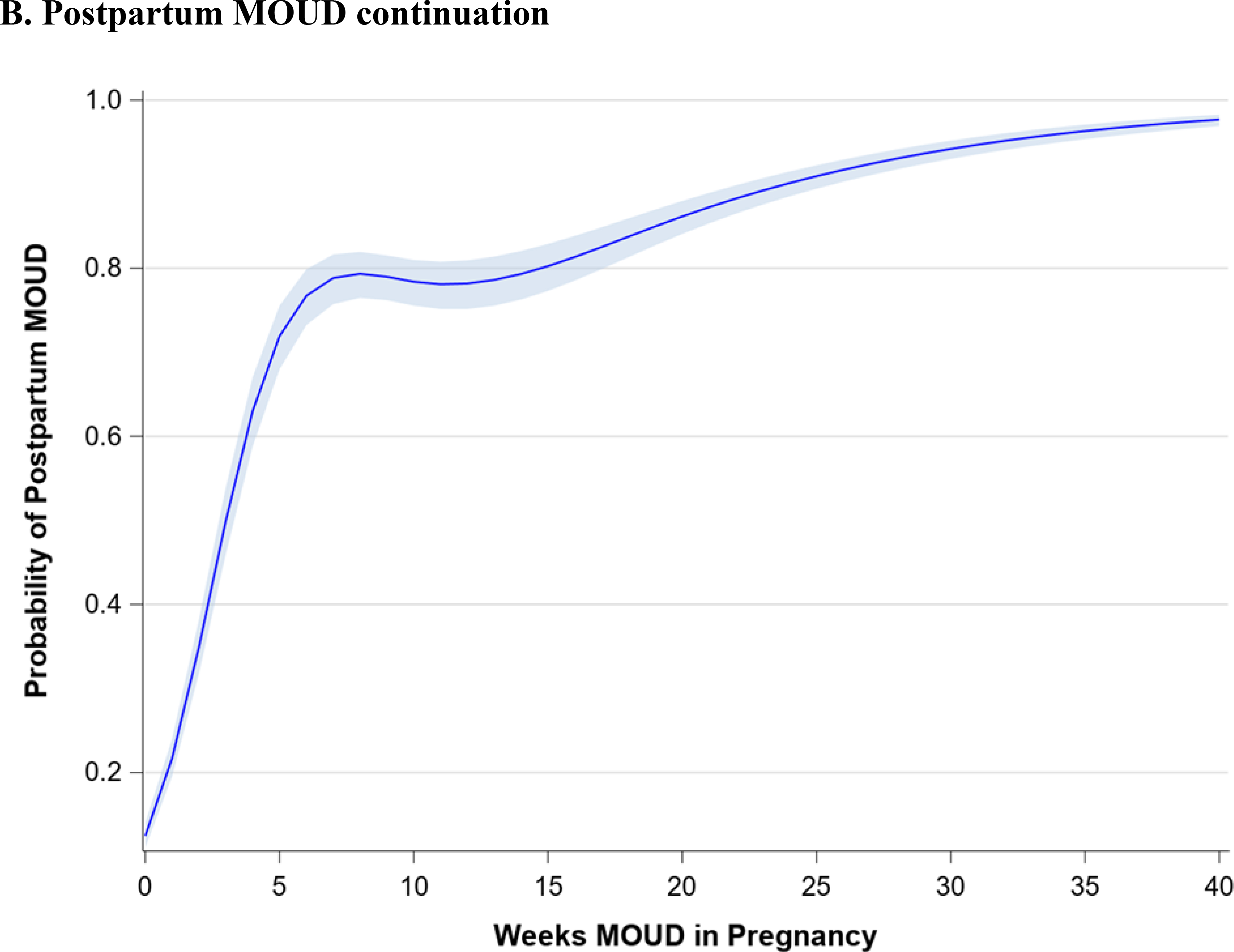
(a,b), Maternal outcomes associated with medications for opioid use disorder (MOUD) use during pregnancy. Association between duration of MOUD in pregnancy and (a), overdose and (b) postpartum MOUD continuation. Average predicted probabilities and 95% confidence intervals (CIs) were derived using marginal standardization from multivariable logistic regression models (log-binomial model for overdose) adjusted for age, race/ethnicity, psychiatric disorders, non-OUD substance use disorders, tobacco use disorder, alcohol use disorder, maternal HIV or hepatitis C virus (HCV) infection, and any prescription use of either selective serotonin re-uptake inhibitors (SSRIs), gabapentins or benzodiazepines. Robust sandwich estimator was used to account for non-independence of observations within each person. Weeks’ duration of MOUD in pregnancy is modeled as a continuous exposure for overdose and as a restricted cubic spline term for postpartum MOUD continuation. Overdose defined as any overdose event in pregnancy or within 12 weeks after delivery; postpartum MOUD utilization is any utilization in first 12 weeks after delivery.
Figure 2.
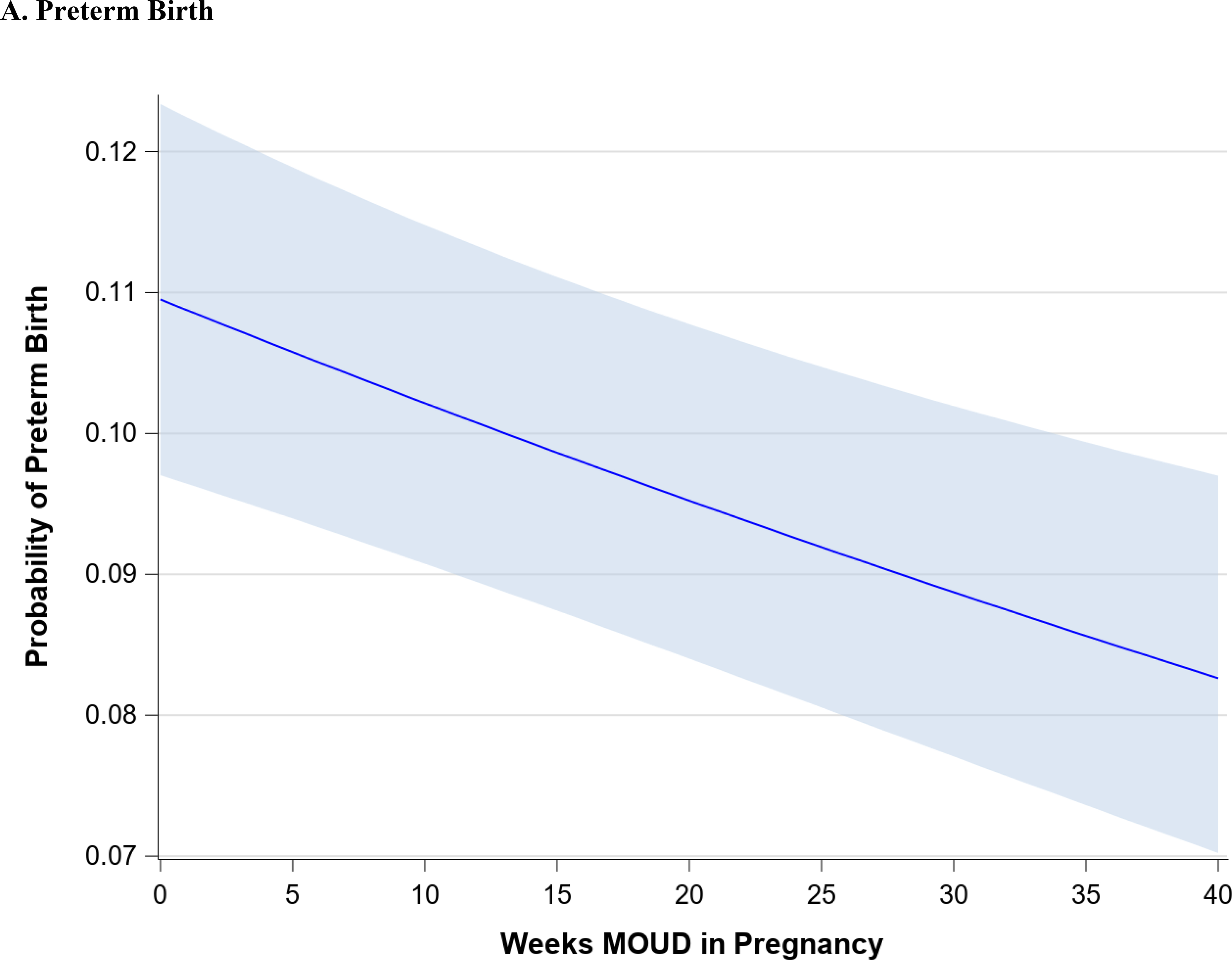
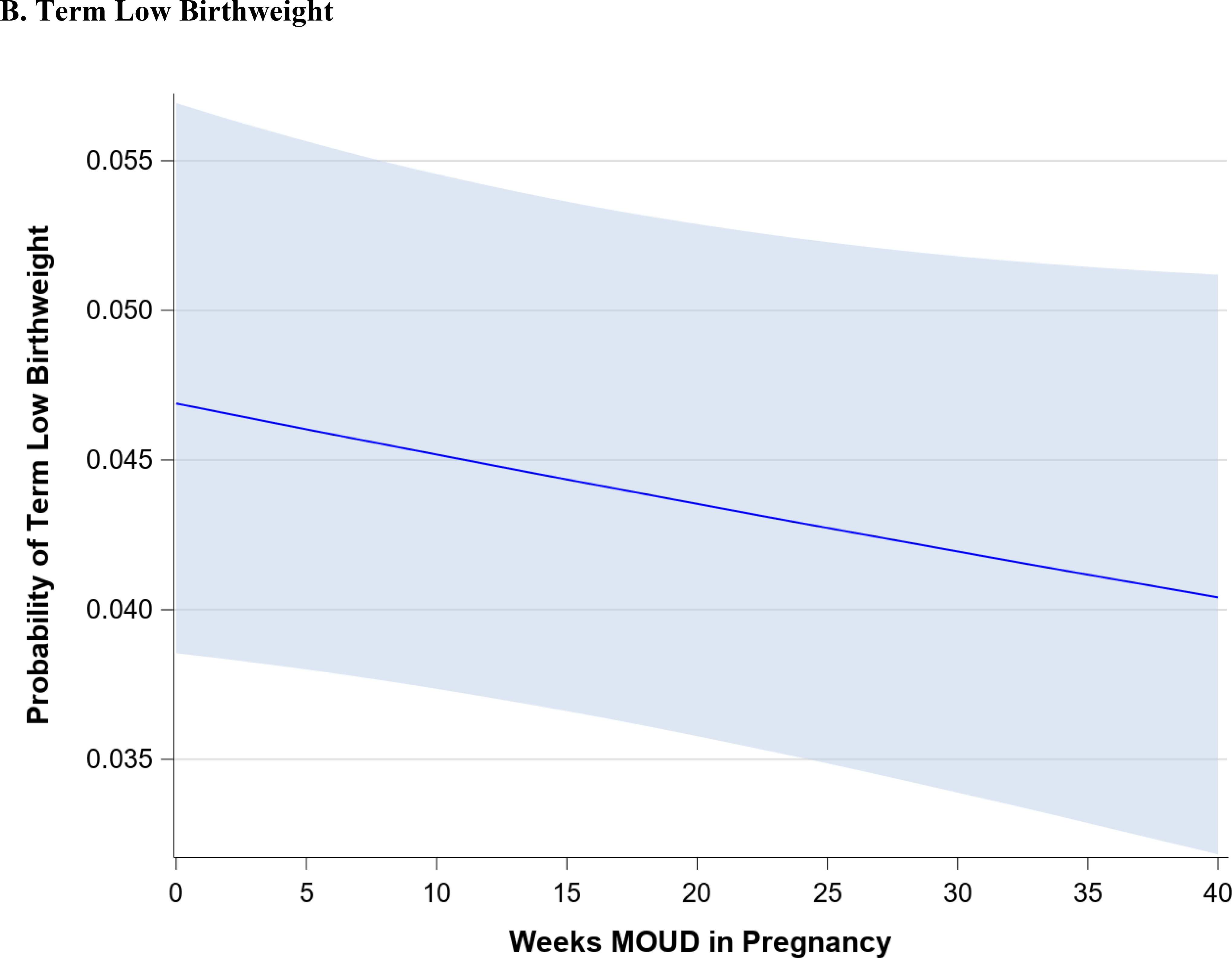
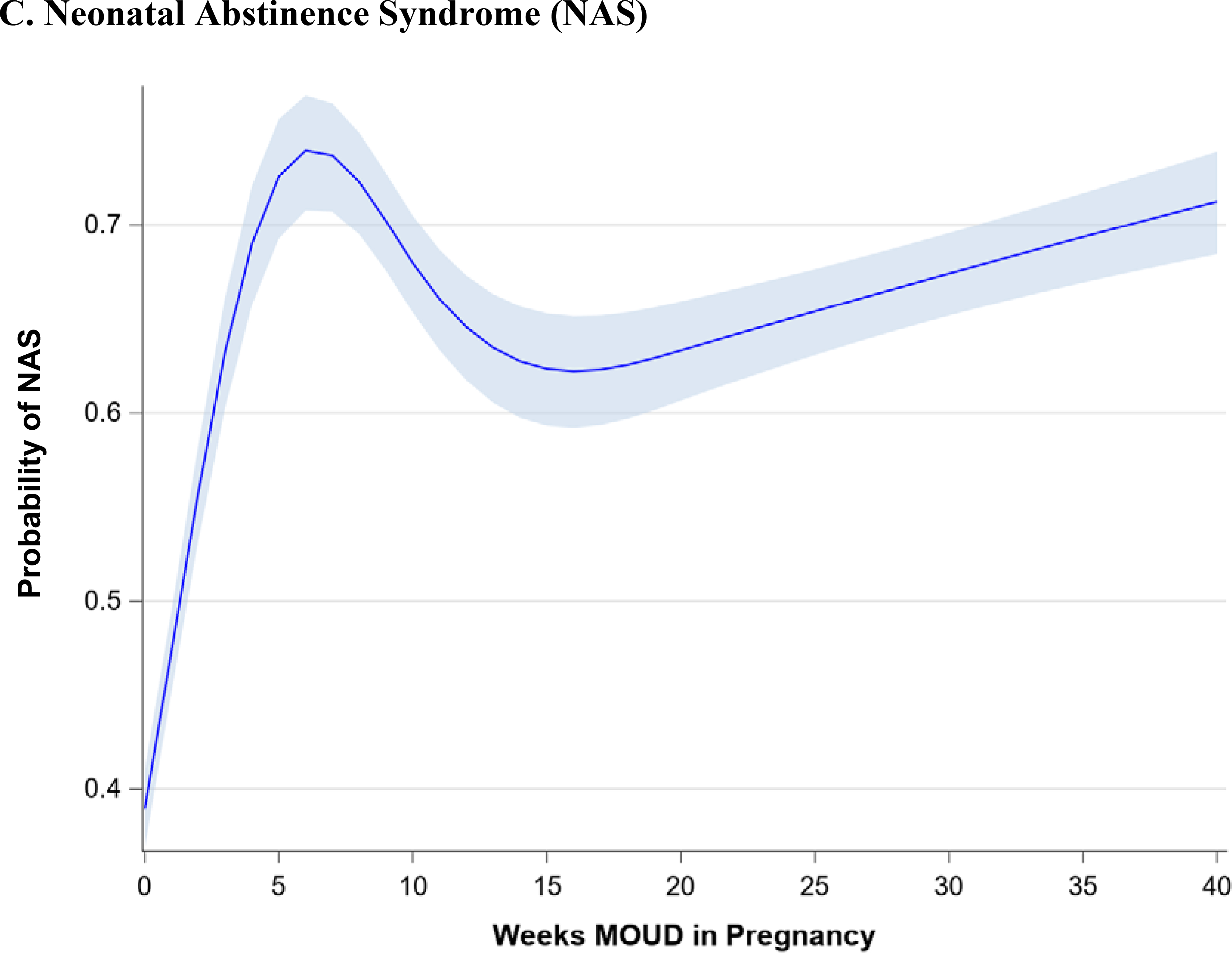
(a-c) Perinatal and neonatal outcomes associated with medications for opioid use disorder (MOUD) use during pregnancy. Association between duration of MOUD in pregnancy and (a) preterm birth, (b), term low birth weight and (c), neonatal abstinence syndrome (NAS). Average predicted probabilities and 95% confidence intervals (CIs) were derived using marginal standardization from multivariable logistic regression models (log-binomial model for overdose) adjusted for age, race/ethnicity, psychiatric disorders, non-OUD substance use disorders, tobacco use disorder, alcohol use disorder, maternal HIV or hepatitis C virus (HCV) infection, chronic hypertension, diabetes mellitus, gestational diabetes mellitus, gestational hypertensive disorder and any prescription use of either SSRIs, gabapentinoids or benzodiazepines. Robust sandwich estimator was used to account for non-independence of observations within each person. Weeks’ duration of MOUD in pregnancy is modeled as a continuous exposure for term low birth weight and preterm birth and as a restricted cubic spline term for NAS. Term low birth weight is < 2500 g among infants born at ≥ 37 weeks’ gestation; preterm birth is < 37 weeks’ gestation; NAS is based on diagnoses in the first 90 days of life, excluding codes indicating iatrogenic withdrawal.
Table 2 demonstrates the change in the average predicted probabilities of maternal, perinatal, and neonatal outcomes associated with 5-week increment increases in the duration of MOUD utilization. The average predicted probabilities of maternal overdose steadily decline with increasing duration of MOUD in pregnancy. Among those with no MOUD in pregnancy, the average predicted probability of overdose is 0.7% (95% CI: 0.5%, 1.0%), among those with 20 weeks of MOUD in pregnancy, the average predicted probability of overdose is 0.5 % (95% CI: 0.3%, 0.7%), and among those with continual MOUD use in pregnancy the average predicted probability of overdose is 0.3% (95% CI: 0.2%,0.5%). Similarly, average predicted probabilities of term low birthweight and preterm birth steadily decline with increasing duration of MOUD in pregnancy.
Table 2.
Average predicted probabilities (95% CIs) of maternal, perinatal, and neonatal outcomes by duration of MOUD use in pregnancy
| Maternal | Perinatal | Neonatal | |||
|---|---|---|---|---|---|
| MOUD duration, weeks | Overdosea | Postpartum MOUD continuationb | Term Low Birthweightc | Preterm Birthd | NASe |
| 0 | 0.7 (0.5,1.0) | 12.6 (11.1,14.0) | 4.7 (3.9,5.7) | 11.0 (9.7,12.3) | 39.0 (36.9,41.1) |
| 5 | 0.7 (0.5,1.0) | 71.9 (68.0,75.5) | 4.6 (3.8,5.6) | 10.6 (9.4,11.9) | 72.6 (69.3,75.6) |
| 10 | 0.6 (0.4,0.9) | 78.4 (75.5,81.0) | 4.5 (3.7,5.5) | 10.2 (9.1,11.5) | 68.0 (65.3,70.5) |
| 15 | 0.5 (0.4,0.8) | 80.2 (77.3,82.9) | 4.4 (3.7,5.4) | 9.9 (8.7,11.1) | 62.4 (59.3,65.3) |
| 20 | 0.5 (0.3,0.7) | 86.2 (84.1,88.0) | 4.4 (3.6,5.3) | 9.5 (8.4,10.8) | 63.3 (60.7,65.9) |
| 25 | 0.5 (0.3,0.7) | 90.9 (89.5,92.2) | 4.3 (3.5,5.2) | 9.2 (8.1,10.5) | 65.4 (63.1,67.6) |
| 30 | 0.4 (0.3,0.6) | 94.2 (93.0,95.2) | 4.2 (3.4,5.2) | 8.9 (7.7,10.2) | 67.4 (65.2,69.6) |
| 35 | 0.4 (0.2,0.6) | 96.3 (95.3,97.1) | 4.1 (3.3,5.1) | 8.6 (7.4,9.9) | 69.4 (66.9,71.7) |
| 40 | 0.3 (0.2,0.5) | 97.7 (96.9,98.3) | 4.0 (3.2,5.1) | 8.3 (7.0,9.7) | 71.2 (68.4,73.9) |
Derived using marginal standardization from multivariable logistic regression models (log-binomial model for overdose) adjusted for age, race/ethnicity, psychiatric disorders, non-OUD substance use disorders, tobacco use disorder, alcohol use disorder, maternal HIV or HCV infection, and any prescription use of either SSRIs, gabapentinoids, or benzodiazepines. Models for term low birthweight and preterm birth were also adjusted for chronic hypertension, diabetes mellitus, gestational diabetes mellitus, gestational hypertensive disorders. Weeks duration of MOUD in pregnancy was modeled as a continuous exposure for overdose, term low birthweight, and preterm birth; and as a restricted cubic spline term for postpartum MOUD use and NAS. Robust sandwich estimator was used to account for non-independence of observations within each person.
Overdose defined as any overdose event in pregnancy or within 12 weeks after delivery
Postpartum MOUD utilization is any utilization in first 12 weeks after delivery
Term low birthweight is <2500 g among infants born at >=37 weeks gestation
Preterm birth is <37 weeks gestation
NAS is based on diagnoses in the first 90 days of life, excluding codes indicating iatrogenic withdrawal.
There are non-linear associations between duration of MOUD in pregnancy and average predicted probabilities of postpartum MOUD continuation and NAS. Generally, any use of MOUD in pregnancy is associated with a very high probability of MOUD use postpartum. At 5 weeks’ duration of MOUD in pregnancy, the average predicted probability of postpartum MOUD use is 71.9% (95% CI: 68.0%, 75.5%); at 20 weeks’ duration of MOUD in pregnancy, the average predicted probability of postpartum MOUD continuation increases to 86.2% (95% CI: 84.1%, 88.0%), and at >=25 weeks’ duration of MOUD in pregnancy, the average predicted probability of postpartum MOUD continuation exceeds 90%. There is a much higher average predicted probability of NAS among those with any MOUD in pregnancy, relative to those with no MOUD, although there is not an increased probability of NAS diagnosis with increasing duration of MOUD in pregnancy. For example, the average predicted probability of NAS with no MOUD in pregnancy is 39.0% (95% CI: 36.9%, 41.1%), compared to 72.6% (95% CI: 69.3%, 75.6%) at 5 weeks’ duration of MOUD and 71.2% (95% CI: 68.4%, 73.9%) with continual MOUD use in pregnancy.
Table 3 shows the adjusted association between each additional week of MOUD use during pregnancy and maternal, perinatal, and neonatal outcomes (bivariate associations are shown in Appendix Table C). For each additional week of MOUD use during pregnancy, the odds of maternal overdose decreased by 2% (aOR 0.98; 95% CI: 0.97,0.99), and the odds of preterm birth decreased by 1% (aOR: 0.99; 95% CI: 0.99,1.00). For each additional week of MOUD, the odds of term low birthweight did not change (aOR: 1.00; 95% CI: 0.99, 1.00), although there was a downward trend in odds of term low birthweight with longer duration of MOUD in pregnancy. Specifically, for an additional 10 weeks’ MOUD duration in pregnancy, the odds of term low birthweight decreased by 4% (aOR: 0.96; 95% CI: 0.92, 1.01), although the confidence interval for the aOR spans 1.0. For each additional week of MOUD use during pregnancy, the odds of postpartum MOUD continuation nearly doubled (aOR: 1.95; 95% CI: 1.87, 2.04). For one additional week of MOUD in pregnancy, the odds of NAS diagnosis increased by 41% (aOR: 1.41; 95% CI 1.35, 1.47); however, this association was nonlinear, as longer duration of MOUD in pregnancy did not monotonically increase.
Table 3.
Adjusted association between each marginal week of MOUD use in pregnancy and maternal, perinatal, and neonatal outcomes
| Outcome | Adjusted Odds Ratio (95% CI) | |||
|---|---|---|---|---|
| 1 week | 5 weeks | 10 weeks | 20 weeks | |
| Postpartum MOUD continuationa | 1.95 (1.87,2.04) | 18.00 (15.18,21.35) | 25.49 (22.29,29.16) | 43.74 (38.48,49.72) |
| Overdoseb | 0.98 (0.97,0.99) | 0.91 (0.87,0.95) | 0.83 (0.76,0.91) | 0.67 (0.56,0.82) |
| Term Low Birthweightc | 1.00 (0.99,1.00) | 0.98 (0.96,1.01) | 0.96 (0.92,1.01) | 0.93 (0.84,1.02) |
| Preterm Birthd | 0.99 (0.99,1.00) | 0.96 (0.94,0.98) | 0.93 (0.89,0.96) | 0.86 (0.79,0.92) |
| NASe | 1.41 (1.35,1.47) | 4.14 (3.54,4.86) | 3.33 (2.98,3.71) | 2.71 (2.46, 2.98) |
From multivariable logistic regression models (log-binomial model for overdose) adjusted for age, race/ethnicity, psychiatric disorders, non-OUD substance use disorders, tobacco use disorder, alcohol use disorder, maternal HIV or HCV infection ncy, and any prescription use of either SSRIs, gabapentins, or benzodiazepines. Models for term low birthweight and preterm birth were also adjusted for chronic hypertension, diabetes mellitus, gestational diabetes mellitus, gestational hypertensive disorders. Weeks duration of MOUD in pregnancy is modeled as a continuous exposure for overdose, term low birthweight, and preterm birth; and as a restricted cubic spline term for postpartum MOUD use and NASS. Robust sandwich estimator was used to account for non-independence of observations within each person.
Postpartum MOUD utilization is any utilization in first 12 weeks after delivery
Overdose defined as any overdose event in pregnancy or within 12 weeks after delivery
Term low birthweight is <2500 g among infants born at >=37 weeks gestation
Preterm birth is <37 weeks gestation
NOWS is based on diagnoses in the first 90 days of life, excluding codes indicating iatrogenic withdrawal.
DISCUSSION
MOUD is a highly effective intervention that has been consistently shown to decrease the risk of overdose morbidity and mortality, improve social functioning and reduce the risk of infectious disease acquisition in individuals with OUD.1 Our findings provide evidence that in pregnancy, a longer duration of MOUD use may also result in improvements in pregnancy-associated outcomes including a reduced risk of preterm birth. Up to 20% of pregnancies complicated by OUD result in preterm birth, a rate that is twice the national average, and infants born to women with OUD are significantly more likely to be small for gestational age compared to infants born to women without OUD.12–14 Thus, our findings indicate that efforts to improve MOUD utilization during pregnancy should focus on ensuring that pregnant women with OUD receive treatment interventions such as MOUD as early as possible during the pregnancy (i.e. first trimester for those who do not initiate MOUD prior to conception) and that continuing MOUD use throughout pregnancy should be prioritized, as a longer duration of use during pregnancy was associated with a steady improvement in perinatal health outcomes.
Our findings also indicate that duration of MOUD use is associated with a reduced risk of overdose during pregnancy and postpartum which is consistent with prior research in non-pregnant populations. In a comparative effectiveness study of different OUD treatment pathways among over 40,000 individuals with OUD, only MOUD treatment with methadone or buprenorphine was associated with a decreased risk of overdose and opioid-related morbidity compared to treatment pathways that did not include MOUD.28 Overdose is a leading cause of pregnancy-associated mortality in the US.29–32 In 2016, 10% of all pregnancy-associated deaths in the United States were due to opioids and the majority of these deaths (70%) occurred during pregnancy or after a pregnancy termination.32 While previous evaluations have largely focused on the contribution of opioid overdose to pregnancy-associated mortality, the effect of interventions such as MOUD use on overdose trends has received considerably less attention. In an evaluation of 242 overdose events among a cohort of pregnant and postpartum women with OUD in Massachusetts, overdose rates were lower among women who received MOUD, but did not achieve statistical significance.30 This analysis had a significantly smaller sample size and MOUD use duration was evaluated categorically (vs. continuously) which may have diminished the statistical power necessary to identify a significant association between MOUD and overdose, a relatively rare outcome.
The postpartum period is a time of unique vulnerability for women with substance use disorders and continued engagement in postpartum treatment is critical to improve maternal and neonatal health outcomes.33 Despite this, the postpartum period is associated with MOUD discontinuation which places parenting women at increased risk for illicit substance use recurrence and overdose. Among women engaged in methadone treatment during pregnancy, over half of women discontinued treatment before 6 months postpartum.34 In this analysis, we found a significant association between the duration of MOUD during pregnancy and postpartum MOUD continuation indicating that earlier engagement in OUD treatment during pregnancy may allow for additional time to provide necessary psychosocial services (i.e. counseling, transportation, housing) and to develop therapeutic patient-provider relationships which may improve treatment engagement. Because the prescribing provider may change between pregnancy and the postpartum period, efforts should also be made to minimize gaps in MOUD utilization after delivery which is necessary to sustain long-term recovery.
Newborns chronically exposed to opioids in utero are at increased risk for NAS which has increased dramatically due to the opioid epidemic.35, 36 While NAS is an expected and treatable group of symptoms that typically occur after birth, not all newborns exposed to opioids during pregnancy will develop significant signs of withdrawal. Variability in NAS symptoms and severity depends on a variety of factors including the type, quantity and duration of drug use, maternal metabolism and co-occurring substance use as well as genetic and epigenetic factors.37–40 Prior research indicates that approximately half of NAS cases are associated with MOUD use during pregnancy.41 However, by examining MOUD duration, we found that NAS diagnoses sharply increased with any MOUD initiation, but did not increase linearly with duration of MOUD. Notably, results were highly consistent in sensitivity analyses examining buprenorphine and methadone separately. These findings point to identification bias in screening for and identification of NAS. Specifically, the newborns of women who use MOUD during pregnancy may be more likely to be identified as opioid-exposed and subsequently evaluated for signs and symptoms of NAS compared to the newborns of women who did not receive MOUD during pregnancy.
Importantly, the association between MOUD use and NAS has prompted an increased interest in medically-assisted withdrawal or “detoxification” during pregnancy to prevent NAS and mitigate in-utero opioid exposure.4, 5 While our findings indicate an association between initiation, but not duration, of MOUD use in pregnancy and the diagnosis of NAS, existing evidence fails to consistently demonstrate a decrease in NAS in the absence of MOUD, largely due to high rates of illicit opioid use recurrence among pregnant women who do not receive MOUD. In a systematic review of medically assisted opioid withdrawal during pregnancy, only 2 out of 15 studies reported a lack of NAS following maternal detoxification during pregnancy.42 Even when NAS occurs, the diagnosis may have a protective effect on infant mortality rates among opioid-exposed infants by initiating a cascade of healthcare supports and interventions.43 In an evaluation of 7,207 maternal-infant dyads with prenatal opioid exposure, the odds of mortality in opioid-exposed infants without NAS was 72% greater than infants with NAS.43
While our findings provide new evidence regarding the effect of the duration of MOUD use on outcomes during pregnancy, our results must be interpreted with certain limitations. Our sample represents a cohort of pregnant women with OUD enrolled in the Pennsylvania Medicaid program which may limit the generalizability of our results to women who are uninsured or who are privately insured and to pregnant women in other regions of the United States. However, Pennsylvania’s Medicaid program is the 4th largest in the US, with expenditures of $27.6 billion in 2016,44 and has demographic and socioeconomic profiles that closely track national averages of Medicaid program enrollees.45 In the United States, state Medicaid programs provide healthcare coverage for more than 80% of pregnant women with OUD and their children which further increases the generalizability of our findings.16, 17 Social determinants of health such as limited access to prenatal care, environmental stress and unstable housing can also adversely affect perinatal health outcomes, but we are unable to account for these factors in administrative data. Further, women classified as having no MOUD use during pregnancy in our dataset may have received MOUD from a non-Medicaid-billing provider or a provider that only accepted cash payments for services.46 We were also unable to account for the type and frequency of illicit or non-prescribed opioid use among women who did not have MOUD use observed in our dataset. However, due to the significant increase in the overdose rates found among women who did not use MOUD during pregnancy, our findings indicate that illicit opioid use was common. Finally, population-level, observational retrospective cohort analyses are vulnerable to bias and the possibility for unmeasured confounding exists in our evaluation despite efforts to account for these factors in multivariable analyses. Specifically, we found differences in the rates of tobacco use, non-opioid substance use and alcohol use disorders among pregnant women with different durations of MOUD use during pregnancy which may have confounded the effect of MOUD duration on outcomes. We were also unable to account for differences in healthcare settings and quality which could have influenced MOUD access and utilization patterns during pregnancy.
Our results indicate that MOUD use is associated with meaningful reductions in maternal and perinatal morbidity. For example, our estimates suggest that if all women with OUD continuously used MOUD in pregnancy, it would result in a 57% decline in overdose and a 25% decline in preterm birth, compared to if women with OUD did not use MOUD during pregnancy. These results support current recommendations to initiate and continue MOUD use during pregnancy and postpartum as part of a comprehensive approach to substance use treatment that includes access to obstetric and behavioral healthcare, social services, and resource support (e.g., housing, transportation).7 The effect of maternal opioid use on neonatal outcomes should not be minimized as NAS has had a significant impact on women, children and the healthcare community. Efforts to mitigate the effects of in-utero opioid exposure on outcomes for both women and their children should continue to be aggressively pursued as non-pharmacologic interventions that increase mother-infant bonding such as rooming-in and breastfeeding have been shown to profoundly decrease the rate of NAS.47 Further, the effect of any exposure and intervention on outcomes during pregnancy must take into account the balance of these effects on both mothers and their children because ensuring the health and well-being of mothers provides the best opportunities to improve outcomes for their children.48
Supplementary Material
Acknowledgements:
This research was supported by an inter-governmental agreement between the University of Pittsburgh and the Pennsylvania Department of Human Services. We also greatly acknowledge support for this analysis from Jenny Lo-Ciganic, PhD, MS, MSPharm.
Funding disclosure:
Research reported in this publication was supported by the National Institute on Drug Abuse under Award Number R01DA045675 (Krans and Jarlenski).
Conflicts of interest: Dr. Krans is an investigator on grants to Magee-Womens Research Institute from the National Institutes of Health, Gilead, and Merck outside of the submitted work. The other authors report no conflicts of interest.
Contributor Information
Elizabeth E. Krans, Department of Obstetrics, Gynecology & Reproductive Sciences, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, Pennsylvania.
Joo Yeon Kim, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
Qingwen Chen, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
Scott D. Rothenberger, Center for Research on Health Care Data Center, Division of General Internal Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Alton Everette James, III, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
David Kelley, Pennsylvania Department of Human Services, Harrisburg, Pennsylvania.
Marian P. Jarlenski, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. May 29 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- 2.Amerian Society of Addiction Medicine (ASAM). (2015). National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Available at https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. [DOI] [PMC free article] [PubMed]
- 3.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. April 27 2019;393(10182):1760–1772. doi: 10.1016/S0140-6736(18)33078-2 [DOI] [PubMed] [Google Scholar]
- 4.Campbell WA. Opioid detoxification during pregnancy: the door continues to open. Am J Obstet Gynecol. September 2016;215(3):258–60. doi: 10.1016/j.ajog.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 5.Caritis SN, Panigrahy A. Opioids affect the fetal brain: reframing the detoxification debate. Am J Obstet Gynecol. December 2019;221(6):602–608. doi: 10.1016/j.ajog.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. HHS Publication No. (SMA) 18–5054. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. [Google Scholar]
- 7.Committee on Obstetric Practice. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol. August 2017;130(2):e81–e94. doi: 10.1097/AOG.0000000000002235 [DOI] [PubMed] [Google Scholar]
- 8.Almario CV, Seligman NS, Dysart KC, Berghella V, Baxter JK. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. Am J Obstet Gynecol. September 2009;201(3):326 e1–6. doi: 10.1016/j.ajog.2009.05.052 [DOI] [PubMed] [Google Scholar]
- 9.Meyer M, Benvenuto A, Howard D, et al. Development of a substance abuse program for opioid-dependent nonurban pregnant women improves outcome. J Addict Med. June 2012;6(2):124–30. doi: 10.1097/ADM.0b013e3182541933 [DOI] [PubMed] [Google Scholar]
- 10.Peles E, Schreiber S, Bloch M, Dollberg S, Adelson M. Duration of methadone maintenance treatment during pregnancy and pregnancy outcome parameters in women with opiate addiction. J Addict Med. March 2012;6(1):18–23. doi: 10.1097/ADM.0b013e318229bb25 [DOI] [PubMed] [Google Scholar]
- 11.Burns L, Mattick RP, Lim K, Wallace C. Methadone in pregnancy: treatment retention and neonatal outcomes. Addiction. February 2007;102(2):264–70. doi: 10.1111/j.1360-0443.2006.01651.x [DOI] [PubMed] [Google Scholar]
- 12.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. December 2014;121(6):1158–65. doi: 10.1097/ALN.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 13.Cleary BJ, Donnelly JM, Strawbridge JD, et al. Methadone and perinatal outcomes: a retrospective cohort study. Am J Obstet Gynecol. February 2011;204(2):139 e1–9. doi: 10.1016/j.ajog.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Azuine RE, Ji Y, Chang HY, et al. Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated With Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw Open. June 5 2019;2(6):e196405. doi: 10.1001/jamanetworkopen.2019.6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, Salihu HM. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:906723. doi: 10.1155/2014/906723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Admon LK, Bart G, Kozhimannil KB, Richardson CR, Dalton VK, Winkelman TNA. Amphetamine- and Opioid-Affected Births: Incidence, Outcomes, and Costs, United States, 2004–2015. Am J Public Health. January 2019;109(1):148–154. doi: 10.2105/AJPH.2018.304771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and Costs of Neonatal Abstinence Syndrome Among Infants With Medicaid: 2004–2014. Pediatrics. April 2018;141(4)doi: 10.1542/peds.2017-3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemans-Cope L, Lynch V, Howell E, et al. Pregnant women with opioid use disorder and their infants in three state Medicaid programs in 2013–2016. Drug Alcohol Depend. February 1 2019;195:156–163. doi: 10.1016/j.drugalcdep.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 19.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. Jul-Aug 2006;40(7–8):1280–88. doi: 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 20.Daw JR HL, Swartz K, Sommers BD. Women in the United States experience high rates of coverage ‘churn’ in months before and after childbirth. Health Aff. 2017;36(4)doi: 10.1377/hlthaff.2016.1241 [DOI] [PubMed] [Google Scholar]
- 21.Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of Machine-Learning Algorithms for Predicting Opioid Overdose Risk Among Medicare Beneficiaries With Opioid Prescriptions. JAMA Netw Open. March 1 2019;2(3):e190968. doi: 10.1001/jamanetworkopen.2019.0968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maalouf FI, Cooper WO, Stratton SM, et al. Positive Predictive Value of Administrative Data for Neonatal Abstinence Syndrome. Pediatrics. January 2019;143(1)doi: 10.1542/peds.2017-4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strahan AE, Guy GP Jr., Bohm M, Frey M, Ko JY. Neonatal Abstinence Syndrome Incidence and Health Care Costs in the United States, 2016. JAMA Pediatr. December 16 2019;doi: 10.1001/jamapediatrics.2019.4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White HA. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. May 1980;48(4):817–838. doi:doi: 10.2307/1912934 [DOI] [Google Scholar]
- 25.Jarlenski M, Kim JY, Ahrens KA, et al. Healthcare Patterns of Pregnant Women and Children Affected by OUD in 9 State Medicaid Populations. J Addict Med. February 5 2021;doi: 10.1097/ADM.0000000000000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaman SL, Isaacs K, Leopold A, et al. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. J Addict Med. May/Jun 2017;11(3):178–190. doi: 10.1097/ADM.0000000000000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Intern J Epidemiology. June 2014;43(3):962–70. doi: 10.1093/ije/dyu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open. February 5 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smid MC, Stone NM, Baksh L, et al. Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths. Obstet Gynecol. June 2019;133(6):1131–1140. doi: 10.1097/AOG.0000000000003279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff DM, Nielsen T, Terplan M, et al. Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts. Obstet Gynecol. August 2018;132(2):466–474. doi: 10.1097/AOG.0000000000002734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal Deaths From Suicide and Overdose in Colorado, 2004–2012. Obstet Gynecol. December 2016;128(6):1233–1240. doi: 10.1097/AOG.0000000000001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gemmill A, Kiang MV, Alexander MJ. Trends in pregnancy-associated mortality involving opioids in the United States, 2007–2016. Am J Obstet Gynecol. January 2019;220(1):115–116. doi: 10.1016/j.ajog.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 33.Krans EE, Campopiano M, Cleveland LM, et al. National Partnership for Maternal Safety: Consensus Bundle on Obstetric Care for Women With Opioid Use Disorder. Obstet Gynecol. August 2019;134(2):365–375. doi: 10.1097/AOG.0000000000003381 [DOI] [PubMed] [Google Scholar]
- 34.Wilder C, Lewis D, Winhusen T. Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder. Drug Alcohol Depend. April 1 2015;149:225–31. doi: 10.1016/j.drugalcdep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 35.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. May 28 2015;372(22):2118–26. doi: 10.1056/NEJMsa1500439 [DOI] [PubMed] [Google Scholar]
- 36.Leech AA, Cooper WO, McNeer E, Scott TA, Patrick SW. Neonatal Abstinence Syndrome In The United States, 2004–16. Health Aff. May 2020;39(5):764–767. doi: 10.1377/hlthaff.2019.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wachman EM, Hayes MJ, Lester BM, et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J Pediatrics. September 2014;165(3):472–8. doi: 10.1016/j.jpeds.2014.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wachman EM, Hayes MJ, Sherva R, et al. Variations in opioid receptor genes in neonatal abstinence syndrome. Drug Alcohol Depend. October 1 2015;155:253–9. doi: 10.1016/j.drugalcdep.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy UM, Davis JM, Ren Z, Greene MF, Opioid Use in Pregnancy NAS, Childhood Outcomes Workshop Invited S. Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. July 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrick SW, Barfield WD, Poindexter BB, Committee On F, Newborn COSU, Prevention. Neonatal Opioid Withdrawal Syndrome. Pediatrics. November 2020;146(5)doi: 10.1542/peds.2020-029074 [DOI] [PubMed] [Google Scholar]
- 41.Jones HE, Terplan M, Meyer M. Medically Assisted Withdrawal (Detoxification): Considering the Mother-Infant Dyad. J Addict Med. Mar/Apr 2017;11(2):90–92. doi: 10.1097/ADM.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 42.Terplan M, Laird HJ, Hand DJ, et al. Opioid Detoxification During Pregnancy: A Systematic Review. Obstet Gynecol. May 2018;131(5):803–814. doi: 10.1097/AOG.0000000000002562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leyenaar JK, Schaefer AP, Wasserman JR, Moen EL, O’Malley AJ, Goodman DC. Infant Mortality Associated With Prenatal Opioid Exposure. JAMA Pediatr. April 12 2021;doi: 10.1001/jamapediatrics.2020.6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser Family Foundation. State Health Facts: Total Medicaid Spending in FY 2015. Accessed April 12, 2017. http://kff.org/medicaid/state-indicator/total-medicaid-spending
- 45.Medicaid.gov. Pennsvylania 2021. Accessed March 17th, 2021. https://www.medicaid.gov/state-overviews/stateprofile.html?state=pennsylvania
- 46.Patrick SW, Buntin MB, Martin PR, et al. Barriers to Accessing Treatment for Pregnant Women with Opioid Use Disorder in Appalachian States. Substance Abuse. June 27 2018:1–18. doi: 10.1080/08897077.2018.1488336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacMillan KDL, Rendon CP, Verma K, Riblet N, Washer DB, Volpe Holmes A. Association of Rooming-in With Outcomes for Neonatal Abstinence Syndrome: A Systematic Review and Meta-analysis. JAMA Pediatr. April 1 2018;172(4):345–351. doi: 10.1001/jamapediatrics.2017.5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris LH. Rethinking maternal-fetal conflict: gender and equality in perinatal ethics. Obstet Gynecol. November 2000;96(5 Pt 1):786–91. doi: 10.1016/s0029-7844(00)01021-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


