Abstract
People report substituting cannabis for pain medications, but whether cannabidiol (CBD) is used similarly remains unknown. CBD products can be CBD alone (isolate), hemp extract (containing <0.3% Δ-9-tetrahydrocannabinol [THC], other cannabinoids, and terpenes), or CBD-cannabis (containing >0.3% THC). In a secondary analysis from a cross-sectional survey, we examined substitution patterns among n = 878 individuals with fibromyalgia who currently used CBD. We sub-grouped participants by most commonly used CBD product (CBD isolate, hemp, CBD-cannabis, no preference) and whether they substituted CBD for medications. We investigated rationale for substituting, substitution-driven medication changes, CBD use patterns, and changes in pain-related symptoms (e.g., sleep, anxiety). The study population was 93.6% female and 91.5% Caucasian, with an average age of 55.5 years. The majority (n = 632, 72.0%) reported substituting CBD products for medications, most commonly NSAIDs (59.0%), opioids (53.3%), gabapentanoids (35.0%), and benzodiazepines (23.1%). Most substituting participants reported decreasing or stopping use of these pain medications. The most common reasons for substitution were fewer side effects and better symptom management. Age, hemp products, past-year use of marijuana, and higher somatic burden were all associated with substituting (p’s ≤ 0.05). Those who substituted reported larger improvements in health and pain than those who did not. Participants using CBD-cannabis reported significantly more substitutions than any other group (p’s ≤ 0.001) and larger improvements in health, pain, memory, and sleep than other subgroups. This widespread naturalistic substitution for pain medications suggests the need for more rigorous study designs to examine this effect.
Keywords: cannabidiol, substitution, fibromyalgia, hemp, opioids
Introduction
Fibromyalgia (FM) is a common chronic pain condition that affects 2-4% of the population.15 People with FM typically experience widespread pain and co-occurring symptoms, including fatigue, sleep disturbances, and cognitive dysfunction.15 FM symptom management is difficult: non-pharmacological FM interventions (such as acupuncture, massage, and nutrition) are typically not covered by insurance and it can be difficult to find qualified practitioners for these non-pharmacological modalities.3, 7 Further, while there are three approved medications for FM (duloxetine, pregabalin, and milnacipran), these medications typically only result in modest pain relief carry a significant side effect burden.25 Indeed, consumer surveys of people with FM typically do not report these medications as being especially helpful, instead rating them as among the more harmful therapies.24 As such, many individuals with FM investigate alternative therapies for symptom relief, including Cannabis sativa (i.e., cannabis) and active compounds derived from cannabis (cannabinoids).
Studies show that people use cannabis to manage chronic pain (including FM) and other conditions,9, 39 with many individuals reporting successful substitution cannabis for opioids and other pain medications.10, 11, 30 However, little is understood about how this substitution is affected by the two most common cannabinoids: Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC causes analgesic, sleep-inducing, and appetite stimulating effects in humans, but at higher doses also carrying abuse potential and causing side effects including intoxication, mood alterations, and memory problems.34 While preclinical studies and human studies in healthy controls show analgesic synergism between THC and opioids,17, 35 whether these effects translate to opioid-sparing outcomes among clinical pain populations has not been verified. In contrast to THC, CBD is non-intoxicating, has little or no abuse potential, and is far less studied in humans, with its sole verified therapeutic effect as an anticonvulsant for orphan epileptic conditions.41 CBD products may also be found independently of the medical or adult-use cannabis markets due to the removal of hemp-derived products (containing <0.3% THC) from the Controlled Substances Act in 2018.18 As such, people may use CBD products independently of or concurrently with medical cannabis.19 In small clinical trials, CBD has also been shown to improve subjective anxiety33 as well as cue-induced anxiety and craving among individuals in recovery from heroin addiction,27 and preclinical research suggests that CBD causes anti-inflammatory and analgesic effects.32, 37 Similarly to THC, however, whether these findings translate to opioid-sparing outcomes among clinical pain populations remains unknown, although many individuals report using CBD products for chronic pain, arthritis, and related symptoms.13
We recently showed that CBD product use is common among a large national sample of individuals with FM.8 We performed a secondary analysis of individuals from that study who reported current CBD product use to investigate whether CBD products were used as a substitute for pain medications. We hypothesized that many individuals would report successfully substituting CBD products for their pain medications, citing fewer side effects and better symptom management as their rationale for doing so. We hypothesized that those who reported substituting CBD for other medications would report greater symptom improvements than those who did not, as substitution is likely indicative of CBD products causing symptom relief. Finally, we explored relationships between type of CBD product (CBD isolate, hemp-derived products, and products with >0.3% THC), reported symptom relief, and CBD product use characteristics.
Methods
As described previously, we collaboratively designed the survey, drawing on commonly asked questions about CBD products in the FM community (LM) as well as our previous work on cannabis substitution in the context of chronic pain (KFB, DW).10, 11 We recruited participants who identified as having chronic pain in April and May of 2020 by sending out an anonymized survey link (Qualtrics, Provo, UT) to a National Fibromyalgia Association listserv and through press releases and social media platform.
We collected demographic information including sex, age, race/ethnicity, household income, education level, employment status, and location. We classified locations as having legal cannabis (i.e., through adult and/or medical cannabis laws) or no legal cannabis. We also inquired about past-year use of marijuana, defined as containing little or no CBD. CBD was defined as “a component of cannabis that does not get you high”, while THC was defined as “the component of cannabis that does get you high”. The parent study included N=2,701 complete surveys of individuals with FM who had never used CBD products, used CBD products in the past, or currently used CBD products. In the current analysis, we only included participants from the parent study who were >18 years or older and currently used CBD products (n = 878). As described previously, study procedures were approved by the Institutional Review Board at the University of Michigan Medical School (HUM00170424). All respondents freely consented to participate, could terminate their participation at any time, and were not compensated for participation.
FM and other chronic pain symptoms
As measures of clinical pain symptoms, participants completed the 2011 FM Survey Criteria and the Complex Medical Symptom Inventory (CMSI). The 2011 FM Survey criteria consists of questions that measure widespreadness of pain (0-19 tender points) and symptom severity (e.g., sleep problems, trouble thinking).50 The 2011 FM survey criteria are scored continuously, with values ranging from 0-31. The CMSI measures functional somatic burden and screens for chronic overlapping pain conditions, which share many features with fibromyalgia and present similar treatment challenges.45–47, 49 The CMSI is scored from 0-41, with higher scores indicating more symptoms of COPCs.
Type of CBD product
Participants indicated their most commonly used CBD product from a list of: don’t know, CBD isolate (solely CBD), full spectrum CBD with <0.3% THC, or CBD with >0.3% THC. We then subgrouped participants based on their most commonly used CBD product: CBD isolate (n=235, hereafter referred to as “isolate”), CBD products with <0.3% THC (n=367, hereafter referred to as “hemp”), CBD products with >0.3% THC (n=180, hereafter referred to as “CBD-cannabis”). Participants who did not answer this question and thus had no known preference were also included as a comparison group (n=96, hereafter referred to as “no preference”).
Frequency of CBD product use
Participants indicated how frequently they used CBD products, both in days per week (1 to 7) and times per day (1 to 10).
Administration routes
Participants selected all the ways in which they administered CBD, including smoking CBD-dominant flower, vaporizing CBD-flower, vaporizing concentrates, eating, topical applications, tinctures, and other. Examples of each administration route were provided in the survey (e.g., edibles such as gummies, cookies, and candies). Data are reported as categories and as the number of administration routes used.
Substitution for other medications
We asked participants whether they had substituted CBD products for pain medications, providing them with a list that included opioids, benzodiazepines, gabapentanoids, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), non-steroidal anti-inflammatory drugs (NSAIDs), disease modifying rheumatic drugs (DMARDs), and other. We provided examples of common drug names for each class listed. Participants indicated for which classes they had substituted CBD, as well as changes in medication use following substitution (stopped using through increased a lot). Data are reported by category and by the number of medication classes.
We also assessed substitution rationale by asking participants to rank their most important reasons for substituting cannabis for medication as described previously.11, 30 Participants could rank all that apply from: fewer adverse effects from CBD products, fewer withdrawal effects from CBD products, the ability to obtain CBD products versus (drug), greater social acceptance of CBD products, better symptom management with CBD products, and other. If “other” was selected, participants could describe their substitution rationale in a free text entry.
CBD product effectiveness of symptoms
Participants selected symptoms for which they used CBD products from a list of FM symptoms that included pain, insomnia or sleep problems, anxiety, fatigue, depression, memory or clarity of thought, and other.48 For each symptom selected, participants rated how their symptom had changed since using CBD products using a 7-point Likert scale slider based on the Patient Global Impression of Change ranging from “very much worse” to “very much improved”, resulting in continuous values from 1.0 to 7.0.23 Participants rated their change in overall health using the same 7-point Likert scale.
Statistical analysis
We first characterized the study population using descriptive statistics. We sub-grouped participants by their preferred CBD product and by whether the substituted CBD products for medications. We assessed differences in proportions for categorical variables (e.g., income level, relationship status) using Pearson’s Chi-square (X2) test, and reported results as frequency (percent, %). We assessed between-group differences in continuous variables (e.g., age, differences in symptom changes) using t-tests for two groups (those substituting vs. those who did not) and analysis of variance (ANOVA) for three or more groups (i.e., by CBD product preference). We used the Benjamini-Hochberg procedure to control for a false discovery rate (FDR) of 0.05 due to multiple testing for reported changes in overall health and symptoms.6 We conducted post-hoc testing for ANOVA results that were statistically significant after FDR adjustment using Tukey’s honestly significant difference test. We used multivariable logistic regression to investigate potential explanatory variables related to substitution, using relevant demographic (sex, age), clinical (CMSI, FM-score), and CBD product use variables (e.g., CBD product preference group, past year marijuana use). We report continuous variables as mean ± standard deviation (SD) if normally distributed, otherwise as median ± interquartile range (IQR). All analyses were conducted in STATA/SE 14.2 (StataCorp, College Station, TX).
Results
Demographics and clinical characteristics
Study participants lived in 48 states (no representation from Wyoming and Vermont), Canada (4.8%) and other countries (1.7%, most commonly United Kingdom and all from countries where English is spoken). The states with the greatest representation among the study population were California (12.9%), Michigan (6.0%), Florida (4.5%), Pennsylvania (4.2%), and Texas (4.0%). Participants overwhelmingly identified as white/Caucasian, were on average 55.5 ± 12.2 years old, and 45.7% were college-educated or had obtained a higher degree (Table 1). The most commonly reported co-morbid chronic pain diagnoses with FM were chronic low back pain (52.5%), irritable bowel syndrome (47.7%), osteoarthritis (44.9%), and migraine (42.5%).
Table 1.
Sociodemographic characteristics for sample: overall and by preferred CBD product
| Overall (n=878) |
Isolate (n=235) |
Hemp (n=367) |
CBD-Cannabis (n=180) |
No preference (n=96) |
X2 | F | p | |
|---|---|---|---|---|---|---|---|---|
| Sex | 16.7 | 0.053 | ||||||
| Female | 95.0% | 93.6% | 95.6% | 95.6% | 94.8% | |||
| Male | 4.1% | 5.1% | 4.1% | 3.3% | 3.1% | |||
| Gender non-conforming | 0.9% | 1.3% | 0.3% | 1.1% | 2.1% | |||
| Age | 4.2 | 0.006 | ||||||
| Mean (SD) | 55.5 (12.4) | 55.4 (12.4) | 55.7 (12.1) | 53.3 (12.2) | 58.7 (11.3) | |||
| Annual Household Income (US$) | 11.7 | 0.07 | ||||||
| Less than $50,000 | 39.4% | 37.9% | 37.9% | 36.7% | 54.2% | |||
| $50,001-$99,999 | 31.8% | 31.1% | 31.9% | 36.1% | 25.0% | |||
| $100,000+ | 22.0% | 25.1% | 21.8% | 22.2% | 14.6% | |||
| Missing | 6.8% | 6.0% | 8.4% | 5.0% | 6.3% | |||
| Education | 20.8 | 0.01 | ||||||
| High school degree, GED, or less | 10.7% | 7.7% | 11.7% | 8.3% | 18.8% | |||
| Associates degree or some college | 43.6% | 39.1% | 44.4% | 47.8% | 43.8% | |||
| Bachelor’s degree (BA, BS, AB, BBA) | 24.6% | 31.1% | 24.3% | 18.3% | 21.9% | |||
| Masters, Professional or Doctoral degree | 20.6% | 22.1% | 19.3% | 23.9% | 15.6% | |||
| Missing | 0.5% | 0.0% | 0.3% | 1.7% | 0.0% | |||
| Past-year marijuana use | 152.4 | <0.001 | ||||||
| None | 46.7% | 66.4% | 49.9% | 10.6% | 54.2% | |||
| Recreational only | 4.2% | 3.8% | 4.1% | 2.8% | 8.3% | |||
| Medical only | 33.1% | 20.9% | 32.4% | 55.0% | 25.0% | |||
| Medical and recreational | 16.0% | 8.9% | 13.6% | 31.7% | 12.5% | |||
| Employment Status | 23.2 | 0.33 | ||||||
| Unemployed (looking for work) | 2.1% | 2.1% | 1.6% | 2.8% | 2.1% | |||
| Student | 0.7% | 0.9% | 0.5% | 1.1% | 0.0% | |||
| Full time (40+ hours week) | 19.7% | 22.6% | 21.0% | 17.2% | 12.5% | |||
| Part time (<40 hours per week) | 7.7% | 6.0% | 7.9% | 10.0% | 7.3% | |||
| Unemployed (not looking for work) | 3.8% | 3.0% | 4.4% | 2.8% | 5.2% | |||
| Retired | 30.0% | 29.8% | 28.3% | 26.1% | 43.8% | |||
| Self-employed | 5.2% | 6.8% | 6.0% | 4.4% | 0.0% | |||
| Unable to work | 30.8% | 28.9% | 30.0% | 35.6% | 29.2% | |||
| Missing | 0.1% | 0.0% | 0.3% | 0.0% | 0.0% | |||
| Relationship Status | 21.3 | 0.046 | ||||||
| Single (never married) | 8.7% | 6.4% | 8.4% | 12.8% | 7.3% | |||
| Married | 62.4% | 62.6% | 64.9% | 59.4% | 58.3% | |||
| In a domestic partnership | 6.3% | 3.8% | 6.8% | 8.9% | 5.2% | |||
| Divorced | 17.2% | 19.1% | 14.7% | 16.1% | 24.0% | |||
| Widowed | 5.1% | 8.1% | 4.6% | 2.8% | 4.2% | |||
| Missing | 0.3% | 0.0% | 0.5% | 0.0% | 1.0% | |||
| Race/Ethnicity (could select ≥1) | ||||||||
| American Indian/Alaska Native | 2.3% | 1.7% | 2.2% | 3.9% | 1.0% | |||
| Asian | 1.4% | 0.9% | 1.4% | 1.7% | 2.1% | |||
| Black or African American | 2.8% | 2.1% | 3.3% | 2.2% | 4.2% | |||
| Hispanic or Latino | 5.6% | 6.4% | 4.6% | 6.7% | 5.2% | |||
| Native Hawaiian/Other Pacific Islander | 0.3% | 0.0% | 0.5% | 0.6% | 0.0% | |||
| White/Caucasian | 89.9% | 91.5% | 90.2% | 88.9% | 86.5% | |||
| Other | 1.8% | 1.7% | 1.9% | 1.1% | 3.1% | |||
| Missing | 0.5% | 0.0% | 0.5% | 0.6% | 1.0% | |||
| Legal Cannabis | 42.0 | <0.001 | ||||||
| Yes | 75.0% | 72.3% | 69.2% | 93.9% | 68.8% | |||
| No | 24.4% | 27.7% | 29.7% | 6.1% | 30.2% | |||
| Missing | 0.6% | 0% | 1.1% | 0% | 1.0% | |||
| CMSI | 2.2 | 0.08 | ||||||
| Mean (SD) | 21.1 (7.8) | 20.5 (7.5) | 21.0 (7.8) | 22.4 (7.6) | 20.8 (8.9) | |||
| FM Score | 1.6 | 0.18 | ||||||
| Mean (SD) | 18.7 (5.7) | 18.1 (5.6) | 18.8 (5.6) | 19.3 (5.6) | 18.7 (6.2) |
Legal cannabis designates living in a place with legal recreational or medical cannabis. Marijuana was defined as cannabis with little or no CBD. Access to legal cannabis and past-year cannabis use were strongly associated with CBD product preferences. CMSI: Complex multi-symptom inventory. FM Score: 2011 FM survey criteria score. SD: Standard deviation.
There were significant age differences between CBD product groups, (F = 4.2, p = 0.006), driven by differences between the CBD-cannabis group and no preference group (mean difference = 5.4, 95% CI [1.46 - 9.4], p = 0.003). There were also significant differences in the distribution of past-year marijuana use (p<0.001), with the most use among the CBD-cannabis group and the least among the CBD isolate group. Compared to other subgroups, a higher proportion of individuals in the CBD-cannabis subgroup lived in places with legal cannabis. There were no differences in CMSI and FM score among participants in CBD product subgroups.
Those who substituted CBD products for other medications were significantly younger than those who did not (mean difference = 3.3: 95% CI [1.5 - 5.1], p < 0.001). (Supplementary Table 1) There were also significant differences in CBD product preference between those who substituted and those who did not (X2 = 14.8, p = 0.002): a greater proportion of those who substituted used CBD-cannabis and hemp, while a greater proportion of those who did not substitute used isolate. There were no differences in FM score between groups, although those in the substitution group had a significantly higher CMSI score than those who did not (mean difference = 1.6: 95% CI [0.5 – 2.8], p = 0.003).
Logistic regression modeling: associations of variables with substitution
In univariable analyses, lower age, CMSI, CBD product subgroup (hemp and CBD-cannabis), and past year marijuana use were all associated with higher odds of substitution. (Table 2) These associations held in the full model excepting the relationship between CBD-cannabis and substitution which was no longer statistically significant.
Table 2.
Associations of demographic, clinical, and CBD use characteristics with substitution
| Predictor | Univariable associations OR (95% CI) | p-value | Full model OR (95% CI) | p-value |
|---|---|---|---|---|
| Age | 0.98 (0.96 – 0.99) | <0.001 | 0.98 (0.97 – 0.99) | 0.005 |
| Sex | 1.02 (0.55 – 1.87) | 0.95 | 0.89 (0.47 – 1.70) | 0.73 |
| CMSI | 1.03 (1.01 – 1.05) | 0.008 | 1.03 (1.0 – 1.05) | 0.029 |
| FM-score | 1.02 (0.99 – 1.04 | 0.20 | 0.98 (0.95 – 1.01) | 0.24 |
| Past-year marijuana use | 1.39 (1.22 – 1.59) | <0.001 | 1.32 (1.14 – 1.53) | <0.001 |
| CBD-preference (Isolate as reference) | ||||
| Hemp | 1.67 (1.16 – 2.40) | 0.005 | 1.55 (1.06 – 2.25) | 0.022 |
| CBD-cannabis | 2.13 (1.35 – 3.36) | 0.001 | 1.37 (0.93 – 2.26) | 0.22 |
| No preference | 1.07 (0.65–1.77) | 0.78 | 1.02 (0.61 – 1.72) | 0.94 |
All results were produced using logistic regression modeling. OR = odds ratio; CI = confidence interval.
Substituting CBD products for other medications
Overall, 72.0% (n = 632) of participants indicated that they had substituted CBD products for pain medications (Table 3). Among these participants, the medications for which CBD products were most commonly substituted were NSAIDs (59.0%), opioids (53.3%), gabapentanoids (35.0%), and benzodiazepines (23.1%). The highest proportion of participants reported substituting CBD products for medications in the CBD-cannabis subgroup (79.3%), followed by the hemp subgroup (74.7%). There were more substitutions in the CBD-cannabis subgroup than any other subgroup: CBD-cannabis vs. isolate (0.74, 95% CI: 0.35 - 1.12, p<0.001), CBD-cannabis vs. hemp (0.6, 95% CI: 0.26 - 0.94, p < 0.001), CBD-cannabis vs. no preference (0.75, 95% CI: 0.26 - 1.25, p < 0.001). Over half of participants reported that they stopped using each respective medication classes other than NSAIDs as a result of this substitution, and less than 3% indicated that they had increased use of any medication class following substitution (Figure 1). The most common reasons for substituting CBD products for pain medications across medication classes were fewer adverse side effects (43.2% - 63.0%) and better symptom management (17.9% - 38.3%).
Table 3.
Substituting CBD products for pain medications
| Total (n=878) | Isolate (n=235) |
Hemp (n=367) |
CBD-Cannabis (n=180) |
No preference (n=96) |
X2 | F | P | |
|---|---|---|---|---|---|---|---|---|
| Substitutions | 14.8 | 0.002 | ||||||
| Substituted | 632 (72.0%) | 151 (64.3%) | 274 (74.7%) | 143 (79.4%) | 64 (66.7%) | |||
| No substitutions | 237 (27.0%) | 81 (34.5%) | 88 (24.0%) | 36 (20.0%) | 32 (33.3%) | |||
| Missing | 9 (1.0%) | 3 (1.3%) | 5 (1.4%) | 1 (0.6%) | 0 (0.0%) | |||
| # substitutions | 10.8 | <0.001 | ||||||
| Mean (SD) | 2.2 (1.3) | 1.9 (1.2) | 2.0 (1.2) | 2.7 (1.5) | 1.9 (1.2) | |||
| Substitutions: Medication Class | ||||||||
| Opioids | 337 (53.3%) | 69 (45.7%) | 148 (54.0%) | 88 (61.5%) | 32 (50.0%) | 7.7 | 0.052 | |
| NSAIDs | 373 (59.0%) | 85 (56.3%) | 165 (60.2%) | 87 (60.8%) | 36 (56.3%) | 1.03 | 0.79 | |
| DMARDs | 31 (4.9%) | 7 (4.6%) | 11 (4.0%) | 11 (7.7%) | 2 (3.1%) | 3.3 | 0.35 | |
| SNRIs | 102 (16.1%) | 16 (10.6%) | 42 (15.3%) | 36 (25.2%) | 8 (12.5%) | 7.8 | 0.005 | |
| SSRIs | 82 (13.0%) | 18 (11.9%) | 29 (10.6%) | 25 (17.5%) | 10 (15.6%) | 4.5 | 0.21 | |
| Gabapentanoids | 221 (35.0%) | 46 (30.5%) | 90 (32.8%) | 65 (45.5%) | 20 (31.3%) | 9.2 | 0.03 | |
| Benzodiazepines | 146 (23.1%) | 32 (21.2%) | 57 (20.8%) | 51 (35.7%) | 6 (9.4%) | 20.6 | <0.001 | |
| Other | 67 (10.6%) | 18 (11.9%) | 23 (8.4%) | 18 (12.6%) | 8 (12.5%) | 2.5 | 0.47 |
The majority of the study population substituted CBD for pain medications, and those using CBD with >0.3% THC (i.e., CBD-cannabis) substituted significantly more than those who did not. Younger participants substituted more than those who did not.
Figure 1. Substitution of CBD for pain medications among study population.
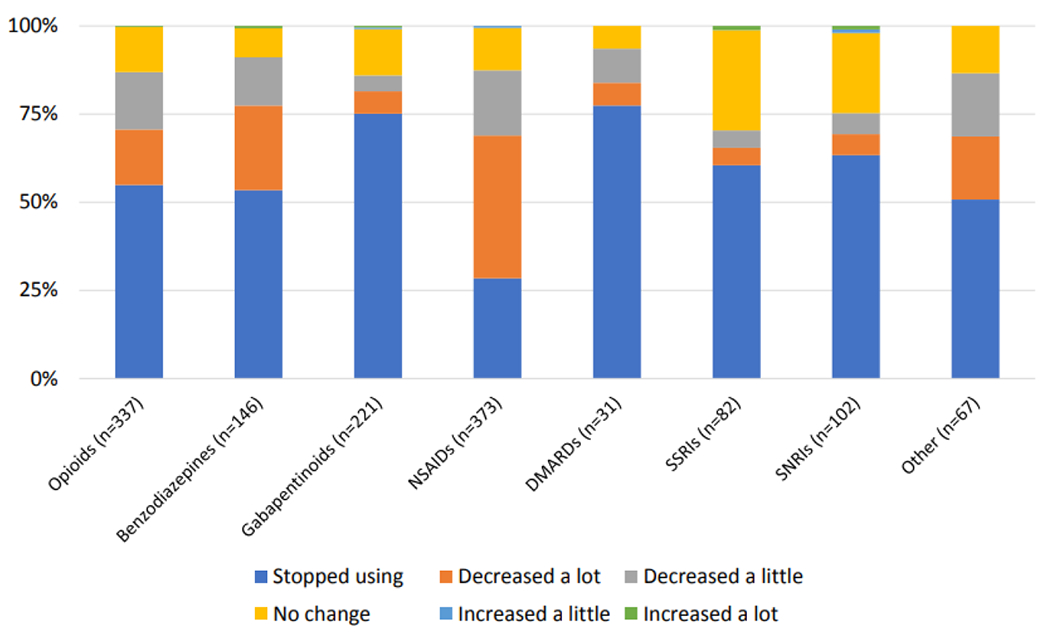
Participants reported large decreases in pain medication use across numerous medication classes.
CBD use characteristics by CBD product subgroup
Overall, the most common routes of ingestion were tincture (65%), topicals (49%), and eating (33.6%) (Table 3). While these top three routes held among all subgroups, those in the “CBD-cannabis” subgroup used inhalation administration routes (smoking, vaporizing flower or concentrates) far more than participants in other CBD product preference subgroups (p’s < 0.001). Similarly, those in the substitution subgroup used inhalation routes more than those who did not substitute (p’s < 0.001) (Table 4). Both the CBD-cannabis subgroup and substitution subgroup also reported using the greatest number of administration routes on average. Overall, participants used CBD products 5.4 ± 2.0 times per week and 2.0 ± 1.3 times per day. Those in the CBD-cannabis and hemp subgroup used CBD products more days per week than those in the isolate and “no preference” group (p’s < 0.001). Those in the CBD-cannabis subgroup used CBD products more times per day than any other subgroup. Similarly, there was more frequent CBD product use in the substitution subgroup (both in days per week and times per day) than in the no substitution subgroup.
Table 4.
CBD use characteristics by CBD product subgroup and substitution
| Overall (n=878) | Isolate (n=235) | Hemp (n=367) | CBD-Cannabis (n=180) | No preference (n=96) | F | X2 | p | Subs | No subs | t | X2 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARs | |||||||||||||
| Smoking | 13.0% | 3.4% | 9.5% | 30.6% | 16.8% | 73.1 | <0.001 | 16.3% | 4.7% | 20.4 | <0.001 | ||
| Vaporizing (flower) | 6.5% | 2.1% | 5.4% | 15.6% | 4.2% | 33.1 | <0.001 | 7.9% | 3.0% | 6.9 | 0.009 | ||
| Vaporizing concentrates | 12.8% | 8.5% | 10.6% | 24.4% | 9.5% | 28.2 | <0.001 | 15.2% | 5.9% | 13.4 | <0.001 | ||
| Eating | 33.6% | 30.8% | 28.1% | 50.6% | 29.5% | 29.8 | <0.001 | 36.3% | 26.3% | 7.7 | 0.005 | ||
| Topicals | 49.0% | 50.9% | 46.3% | 53.9% | 45.3% | 3.6 | 0.30 | 51.5% | 41.9% | 6.3 | 0.012 | ||
| Tinctures | 65.0% | 58.1% | 74.1% | 67.2% | 42.1% | 40.5 | <0.001 | 67.5% | 58.5% | 6.2 | 0.013 | ||
| Other | 9.8% | 9.8% | 12.3% | 4.4% | 10.5% | 8.4 | 0.04 | 9.4% | 11.0% | 0.5 | 0.46 | ||
| # ARs | |||||||||||||
| Mean (SD) | 1.9 (1.1) | 1.6 (0.8) | 1.9 (1.1) | 2.5 (1.2) | 1.6 (0.9) | 27.1 | <0.001 | 2.0 (1.1) | 1.5 (0.8) | 6.7 | <0.001 | ||
| Use frequency | |||||||||||||
| Days/week (Mean, SD) | 5.4 (2.0) | 5.0 (2.1) | 5.7 (1.9) | 5.6 (1.9) | 4.7 (2.2) | 10.4 | <0.001 | 5.5 (2.0) | 5.2 (2.2) | 2.1 | 0.02 | ||
| Times/day* (Mean, SD) | 2.0 (1.1) | 1.9 (1.1) | 1.9 (1.1) | 2.3 (1.4) | 1.8 (1.0) | 7.0 | <0.001 | 2.0 (1.2) | 1.8 (0.9) | 2.8 | 0.002 |
Those in the CBD-cannabis and substitution group used inhalation routes at a higher rate than any other group. Those in the hemp group and CBD-cannabis group used cannabis more days per week than other CBD subgroups.
: 6 participants indicated using CBD products > 10 times/day and were excluded from analyses as unknowns. AR: Administration Route. Sub: Substitutions. No Subs: No substitutions.
Changes in symptoms by CBD product subgroup and by substitution
Self-reported changes in symptoms and overall health by CBD product subgroup and by whether participants substituted are displayed in Figures 2a and 2b, respectively. After adjustment for FDR using the Benjamini-Hochberg procedure, there were statistically significant differences in health (p < 0.001), pain (p = 0.004), sleep (p = 0.02), and memory (p = 0.024). Generally, the greatest symptom improvements were reported in the CBD-cannabis subgroup. Across all symptoms and overall health, those who substituted reported greater symptom improvements than those who did not, although these differences were only statistically significant after adjusting for FDR for overall health and pain (both p-values < 0.001).
Figure 2. a. Changes in symptoms based on CBD products use. b. Changes in symptoms by substitutions.
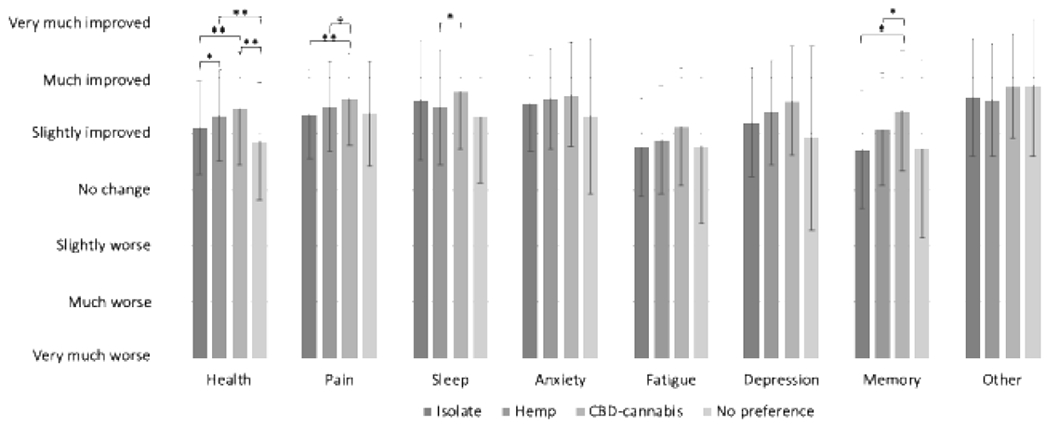
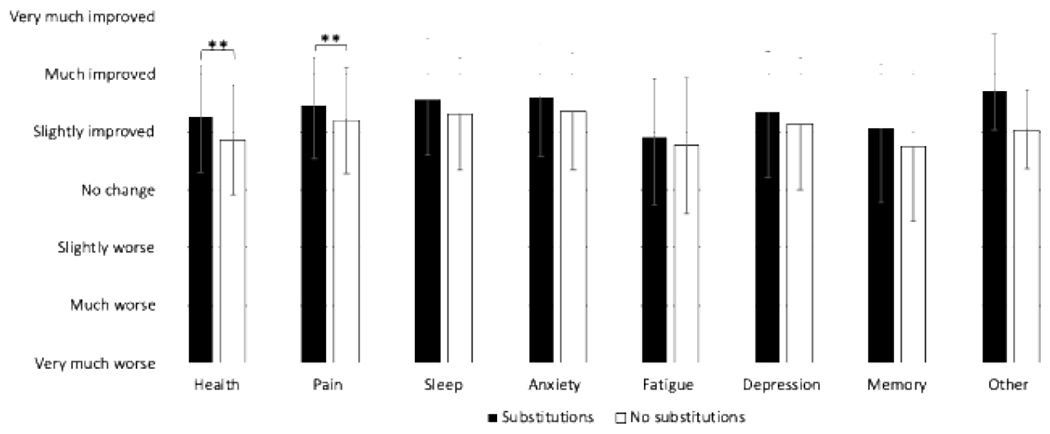
Symptom changes ranged from very much worse (1) through very much improved (7). Error bars indicate standard deviation. Brackets indicate pairwise differences with Tukey’s test, with comparisons only conducted for symptom differences that were statistically significant via ANOVA after adjustment using the Benjamini-Hochberg procedure. *: Indicates p < 0.05 **: Indicates p < 0.005
Discussion
To our knowledge, this study is among the first reports showing deliberate substitution of CBD products for pain medications among individuals with FM, predominantly for opioids and NSAIDs. Strikingly, the majority of participants (70.4% - 93.6% depending on medication class) reported that this substitution was successful – i.e., that they stopped using or reduced their use of that other medication. We also show differential effects on substitution and symptom management based on the type of CBD product used. Those who substituted CBD products for other pain medications reported greater improvements across all symptoms (significantly so for overall health and pain) than those who did not. We expected this, reasoning that those who find CBD products to be effective enough to use them in place of other medications would report greater benefit. Taken together, these findings are consistent with a recent prospective cohort study of people with chronic pain currently on opioids (n = 131), which showed that 8 weeks of 15-30mg per day of hemp-derived CBD extract improved pain and sleep, and allowed 53% to decrease or discontinue opioid medication.14 Our results also align with numerous observational studies that report substitution of medical cannabis products for opioids and other pain medications,1, 10, 11, 30 with younger age associated with substitution and the top reasons for substituting focusing on harm reduction (fewer side effects) and effectiveness (better symptom management).11
In contrast to these medical cannabis studies in which THC-dominant products and smoking/vaporizing were predominantly used, however, many participants in our study who substituted used CBD isolate or hemp products alone and predominantly tinctures and topical administration routes. This is an important distinction as hemp products may have fewer risks than high-THC medical cannabis products, which include abuse potential and cognitive impairment.34 It also shows that while there is some overlap between participants in our study and those using medical cannabis (e.g., the CBD-cannabis group), many people using CBD products do not fit the classic image of a medical cannabis patient. While many participants in the isolate group still substituted CBD products for pain medications, our findings also point to THC’s role as an enhancer of substitution effects. Indeed, both past-year marijuana use and use of hemp products were significantly associated with higher odds of substitution, which may signify that the presence of even small quantities of THC could enhance therapeutic benefits of cannabinoid products, either on pain or related symptoms.
The reported effects may due to the proposed analgesic synergism between THC and other pain medications (e.g., opioids17, 35 and gabapentanoids2) or with CBD31 , which could theoretically allow individuals to lower their doses of these medications while obtaining similar symptomatic relief. CBD also has potential interactions with other drugs often prescribed to people with fibromyalgia, such as selective norepinephrine reuptake inhibitors (e.g., duloxetine), benzodiazepines (e.g., clobazam), and anti-convulsants (e.g., pregabalin) which may change the level or effect of these drugs and potentially contribute to substitution.5 THC and THC analogs such as nabilone have also been shown to improve sleep among individuals with fibromyalgia and other chronic conditions,44 and the known bidirectional relationship between poor sleep and pain sensitivity21 could mean that the improvements in sleep associated with THC feed back into further improving pain and related symptoms. Lastly, it is possible that THC’s intoxicating and euphoric effects (especially in higher doses) make cannabis products more pleasurable to use and thus inflate reported improvements in symptoms. Regardless of whether these or other reasons contribute to this finding, more research is warranted to examine CBD’s therapeutic and medication-sparing effects – both alone and in tandem with THC – in the context of the ongoing opioid epidemic.
Our substitution findings also complement preliminary clinical trials that have been conducted using various CBD formulations. For example, in a recent clinical trial performed among individuals in recovery from heroin addiction, CBD isolate significantly decreased cue-related anxiety and craving compared with placebo, as well as reducing physiological symptoms of stress (e.g., heart rate, cortisol levels), leading the authors to suggest that CBD should be further investigated for potential use in treating opioid use disorder.27 Similarly, 300mg/day of CBD (administered in olive oil) for four weeks significantly improved social anxiety and fear of negative evaluation among teenagers in a small clinical trial conducted in Japan,33 and administration of 300mg of pure CBD in corn oil decreased anxiety in public speaking tasks.29 While such high doses are cost-prohibitive, 25mg of hemp-derived CBD extract did reduce significantly anxiety among psychiatric patients in a large, longitudinal case series.40 While these combined findings are preliminary and reflect effects on both trait and state anxiety, they suggest that it is possible that CBD’s anxiolytic effects aided with the reported substitution in our study since co-morbid anxiety is known to be associated with worse pain and related symptoms.4 Preliminary clinical trials conducted with CBD (alone or hemp-derived) in chronic pain contexts typically show modest improvements in pain (neuropathic, temporomandibular joint disorder, knee osteoarthritis),26, 36, 51 although inhaled CBD-dominant cannabis was shown to be unhelpful at reducing pain in FM.42 The corroboration of these findings in our naturalistic CBD product use environment highlights the need for further rigorous study of CBD to better understand when and how these therapeutic benefits occur.
Clinical implications
Fourteen percent of Americans reported current use of CBD products in 2019,13 making it vital for clinicians to understand the risks and benefits of CBD products. CBD products derived from hemp (Cannabis sativa with <0.3% THC) are no longer designated Schedule I and are thus available in most states. In contrast, products that contain >0.3% THC are still illegal under federal law, but are available in the thirty-five states with legal medical cannabis.43 While CBD does appear to have a favorable side effect profile compared to other medications and very little abuse potential,28 little is known about long-term use of CBD products in adults with chronic pain.43 Further, CBD products are poorly regulated and inaccurately labeled,12 with some containing THC or contaminants (e.g., synthetic cannabinoids).38 Some CBD product manufacturers also make unverified health claims – many of which relate to chronic pain and related symptoms – despite a dearth of rigorous clinical data.22 As such, patients with FM who are using CBD products may benefit from practical tips on products (i.e., avoiding those without independent safety/potency testing), potential drug-drug interactions (e.g., with valproate), 20 and harm-reduction practices such as: 1) starting at low doses and titrating up slowly; 2) use of tinctures and topical products rather than smoking; and 3) monitoring symptoms and coming up with a treatment plan in concert with their physician. 31 As our findings and the literature show, many people with FM express dissatisfaction with their current medication regimen24 – so much so that they substitute CBD products for these medications. By providing evidence-based support for patients and rigorously collecting clinical outcomes and related information on doses, substitutions, and adverse effects, physicians can aid with clarifying the scientific and legal quagmire in which CBD products now reside.
Limitations
Our study was limited in several ways. First, our cross-sectional design makes our results subject to recall bias and prevent us from investigating the reported substitutions or symptom changes over time. We also stress that while many participants in this sub-analysis reported substituting CBD products for pain medications, this behavior may not be as common as it appears among people who try CBD products. Indeed, as our primary article with this cohort shows, a similar number of people with FM tried CBD products and discontinued it – mainly because CBD was not effective.8 Second, we do not have any objective measures of substitution (e.g., pre- and post-CBD urine drug screens), relying instead on self-report. We also do not have any data on which side effects prompted substitution. Third, CBD products are incredibly popular, which may result in strong expectancy effects that could cause a powerful placebo effect. Fourth, our study population was >90% women, so may not generalize to men, who often report different cannabis use behaviors.16 Fifth, we did not investigate how CBD product dose affected substitution behaviors. Sixth, our survey was conducted during the nationwide US lockdown due to the COVTD-19 pandemic, which may have affected our results. However, as previously reported >93% of participants in our survey were using CBD products prior to lockdown,8 suggesting that initiation of use was likely not due to this pandemic.
Conclusions
Among people with FM using CBD products, the majority report substituting CBD products for opioids and other pain medications. Those using CBD products with >0.3% THC reported the greatest number of substitutions, and participants who substituted reported more improvement in symptoms than those who did not. Differentiating between the substitution potential of hemp vs. medical cannabis CBD products could inform more judicious clinical use of cannabinoid products. These findings ought to be followed up by more rigorous studies (e.g., prospective cohorts and clinical trials) as well as mechanistic studies to more thoroughly examine CBD’s opioid-sparing and analgesic potential.
Supplementary Material
Highlights.
72% of participants with fibromyalgia substituted CBD for pain medications.
Participants often substituted CBD for opioids (53.3%) and benzodiazepines (23.1%).
70-94% of reported substitutions resulted in stopped or reduced use of medications.
Participants substituted for harm reduction reasons (e.g., fewer side effects).
Perspective:
This article shows that people with fibromyalgia are deliberately substituting CBD products for conventional pain medications despite the dearth of evidence suggesting CBD products may be helpful for fibromyalgia. CBD’s medication-sparing and therapeutic potential should be examined in more rigorous study designs.
Acknowledgements
We are very grateful to the many participants who generously took the time to complete this survey.
Disclosures:
Dr. Boehnke sits on a data safety and monitoring board for an ongoing clinical trial with Vireo Health (unpaid). Dr. Gagnier consults for Bartimus Frickleton Robertson Rader P.C., and for the Law Office of Robert J. Krakow, P.C., on topics unrelated to the content of this manuscript. Dr. Williams is a consultant to Community Health Focus Inc. Ms. Matallana founded the National Fibromyalgia Association and is the CEO of Community Health Focus Inc. The National Fibromyalgia Association provided funding support for recruitment efforts. Dr. Boehnke’s effort on this publication was partially supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number K01DA049219 (KFB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abuhasira R, Schleider LB, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 49:44–50, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Atwal N, Casey SL, Mitchell VA, Vaughan CW. THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology. 144:115–121, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Bair MJ, Matthias MS, Nyland KA, Huffman MA, Stubbs DL, Kroenke K, Damush TM. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med. 10:1280–1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 70:890–897, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran P, Elsohly M, Hill KP. Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: a Comprehensive Review. J Gen Intern Med. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 57:289–300, 1995 [Google Scholar]
- 7.Boehnke KF. Pain Management: Assembling a Tool Kit, Building a Life. JAMA. 320:2201–2202, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Boehnke KF, Gagnier JJ, Matallana L, Williams DA. Cannabidiol Use for Fibromyalgia: Prevalence of Use and Perceptions of Effectiveness in a Large Online Survey. J Pain. 2021 [DOI] [PubMed] [Google Scholar]
- 9.Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL. Qualifying Conditions Of Medical Cannabis License Holders In The United States. Health Aff (Millwood). 38:295–302, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehnke KF, Litinas E, Clauw DJ. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J Pain. 17:739–744, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Boehnke KF, Scott JR, Litinas E, Sisley S, Williams DA, Clauw DJ. Pills to Pot: Observational Analyses of Cannabis Substitution Among Medical Cannabis Users With Chronic Pain. J Pain. 20:830–841, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA. 318:1708–1709, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenan M: 14% of Americans Say They Use CBD Products. Available at: https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx Accessed 4-21-2020, 2020
- 14.Capano A, Weaver R, Burkman E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med.1–6, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 311:1547–1555, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. The Lancet Psychiatry. 3:954–964, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 43:2046–2055, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corroon J, MacKay D, Dolphin W. Labeling of Cannabidiol Products: A Public Health Perspective. Cannabis and Cannabinoid Research. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corroon J, Phillips JA. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 3:152–161, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Santos RG, Guimaraes FS, Crippa JAS, Hallak JEC, Rossi GN, Rocha JM, Zuardi AW. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 1–10, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 14:1539–1552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher E, Moore RA, Fogarty AE, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice ASC, Rowbotham M, Wallace M, Eccleston C. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain. 2020 [DOI] [PubMed] [Google Scholar]
- 23.Guy W: ECDEU assessment manual for psychopharmacology, U.S. Department of Health, Education, and Welfare, Rockville, MD, 1976, pp. 76–338. [Google Scholar]
- 24.Hauser W, Jung E, Erbsloh-Moller B, Gesmann M, Kuhn-Becker H, Petermann F, Langhorst J, Thoma R, Weiss T, Wolfe F, Winkelmann A. The German fibromyalgia consumer reports - a cross-sectional survey. BMC Musculoskelet Disord. 13:74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 11:505–521, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hunter D, Oldfield G, Tich N, Messenheimer J, Sebree T. Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. Osteoarthritis and Cartilage. 26:S26–S26, 2018 [Google Scholar]
- 27.Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, Salsitz E. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am J Psychiatry. 176:911–922, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Iffland K, Grotenhermen F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2:139–154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimaraes FS, Crippa JA. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 41:9–14, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: A survey of authorized medical cannabis patients. Int J Drug Policy. 42:30–35, 2017 [DOI] [PubMed] [Google Scholar]
- 31.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 49:12–19, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 97:9561–9566, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masataka N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders. Front Psychol. 10:2466, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Academies of Sciences, Engineering, and Medicine: The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, Washington (DC), 2017. [PubMed] [Google Scholar]
- 35.Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, Lintzeris N, Khor KE, Farrell M, Smith A, Le Foll B. Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology. 42:1752–1765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nitecka-Buchta A, Nowak-Wachol A, Wachol K, Walczynska-Dragon K, Olczyk P, Batoryna O, Kempa W, Baron S. Myorelaxant Effect of Transdermal Cannabidiol Application in Patients with TMD: A Randomized, Double-Blind Trial. J Clin Med. 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 158:2442–2451, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poklis JL, Mulder HA, Peace MR. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci Int. 294:e25–e27, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagy I, Bar-Lev Schleider L, Abu-Shakra M, Novack V. Safety and Efficacy of Medical Cannabis in Fibromyalgia. J Clin Med. 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm J. 23:18–041, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K, Group GS. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 391:1085–1096, 2018 [DOI] [PubMed] [Google Scholar]
- 42.van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 160:860–869, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin Proc. 94:1840–1851, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 110:604–610, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Warren JW, Clauw DJ. Functional somatic syndromes: sensitivities and specificities of self-reports of physician diagnosis. Psychosom Med. 74:891–895, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Warren JW, Clauw DJ, Langenberg P. Prognostic factors for recent-onset interstitial cystitis/painful bladder syndrome. BJU Int. 111:E92–97, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Warren JW, Howard FM, Cross RK, Good JL, Weissman MM, Wesselmann U, Langenberg P, Greenberg P, Clauw DJ. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology. 73:52–57, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Williams DA. Phenotypic Features of Central Sensitization. J Appl Biobehav Res 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 35:339–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 38:1113–1122, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Xu DH, Cullen BD, Tang M, Fang Y. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Current Pharmaceutical Biotechnology. 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


