Abstract
Background:
Prolapse recurrence after transvaginal surgical repair is common; however, its mechanisms are ill-defined. A thorough understanding of how and why prolapse repairs fail is needed to address their high rate of anatomic recurrence and to develop novel therapies to overcome defined deficiencies.
Objective:
To identify mechanisms and contributors of anatomic recurrence after (1) vaginal hysterectomy with uterosacral ligament suspension (native tissue repair [NTR]) vs (2) transvaginal mesh (VM) hysteropexy surgery for uterovaginal prolapse.
Study Design:
This multicenter study was conducted in a subset of participants in a randomized clinical trial by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Ninety-four women with uterovaginal prolapse treated via NTR (N=48) or VM hysteropexy (N=46) underwent pelvic magnetic resonance imaging (MRI) at rest, maximal strain, and post-strain rest (recovery) 30–42 months post-surgery. Participants who desired reoperation prior to 30–42 months were imaged earlier so as to assess the impact of only the index surgery. Using a novel 3D pelvic coordinate system, co-registered mid-sagittal images were obtained to assess study outcomes. MRI-based anatomic recurrence (failure) was defined as prolapse beyond the hymen. The primary outcome was the mechanism of failure (apical descent vs anterior vaginal wall elongation), including the frequency and site of failure. Secondary outcomes included displacement of the vaginal apex and perineal body, and change in length of the anterior wall, posterior wall, vaginal perimeter, and introitus of the vagina from (1) rest to strain and (2) rest to recovery. Group differences in the mechanism, frequency, and site of failure were assessed using Fisher’s exact tests and secondary outcomes were compared using Wilcoxon Rank-Sum tests.
Results:
Of the 88 participants analyzed, 37 (42%) had recurrent prolapse (VM hysteropexy, 29% [13/45]; NTR, 56% [24/43]). The most common site of failure was the anterior compartment (VM hysteropexy, 38%; NTR, 92%). The primary mechanism of recurrence was apical descent (VM hysteropexy, 85%; NTR, 67%). From rest to strain, failures (vs successes) had greater inferior displacement of the vaginal apex (difference = −12 mm; 95%CI = −19 to −6) and perineal body (difference = −7 mm; 95%CI = −11 to −4), and elongation of the anterior vaginal wall (difference = 12 mm; 95%CI = 8 to 16) and vaginal introitus (difference = 11 mm; 95%CI = 7 to 15).
Conclusions:
The primary mechanism of prolapse recurrence following vaginal hysterectomy with uterosacral ligament suspension or VM hysteropexy was apical descent. In addition, greater inferior descent of the vaginal apex and perineal body, lengthening of the anterior vaginal wall, and increased size of the vaginal introitus with strain were associated with anatomic failure. Further studies are needed to provide additional insight into the mechanism by which these factors contribute to anatomic failure.
Keywords: hysteropexy, MRI, pelvic organ prolapse, prolapse surgery, transvaginal mesh, vaginal hysterectomy
CONDENSATION
Anatomic recurrence following vaginal hysterectomy with native tissue suspension and mesh hysteropexy for apical prolapse occurred primarily by descent of the vaginal apex.
INTRODUCTION:
Women’s lifetime risk of surgery for pelvic organ prolapse (POP) is 12.6%, with approximately 300,000 POP surgeries performed annually in the US.1–3 Of those procedures that use patients’ own tissues to correct POP, nearly 25% will fail anatomically at 2 years—increasing up to 46% by 5 years.4,5 These high failure rates of native tissue repairs (NTR) prompted the implementation of transvaginal mesh (VM) to augment POP repair. However, insufficient evidence of the long-term efficacy and safety of VM over NTR led the FDA to halt the sale and distribution of VM products in April 2019.6
The mechanisms of failure following POP surgery are poorly understood. The Pelvic Organ Prolapse Quantification system (POP-Q) is the standard tool used to assess anatomic failure of POP repairs.7 While able to detect anatomic failure, POP-Q is constrained to external vaginal examination; consequently, it is incapable of specifying the pathophysiological mechanisms involved in prolapse recurrence.8
To address this knowledge gap and limits of current practice, the Defining Mechanisms of Anterior Vaginal Wall Descent (DEMAND) study was designed to use magnetic resonance imaging (MRI) to identify mechanisms and contributors to recurrent prolapse following two apical surgeries performed for uterovaginal prolapse: vaginal hysterectomy with native tissue suspension (i.e., NTR) and VM hysteropexy. It was hypothesized that, as the mesh fixes the apex in place, recurrence after VM occurs from anterior vaginal wall elongation. Conversely, since native tissues are predisposed to stretch, it was hypothesized that recurrence after NTR is due to both anterior vaginal wall elongation and descent of the vaginal apex.
MATERIALS AND METHODS:
Study Design:
DEMAND was a multicenter, supplementary study that identified anatomic mechanisms and correlates of prolapse recurrence in a subset of women with symptomatic uterovaginal prolapse enrolled in the Study of Uterine Prolapse Procedures-Randomized (SUPeR) trial9 conducted by the Pelvic Floor Disorders Network. The study protocol received institutional review board approval at each site and all participants provided written informed consent.
Study Population:
All women in SUPeR were offered enrollment. Pelvic MRIs were performed at 30–42 months post-surgery. Participants who desired reoperation prior to 30–42 months were offered earlier imaging so that the impact of only the SUPeR index surgery was analyzed. This cohort consisted of two groups: vaginal hysterectomy with uterosacral ligament suspension and VM hysteropexy. Surgery standardization has been described previously.9 Participants who had an MRI contraindication or whose MRI scans failed to show the full vagina, had incomplete MRI, or were imaged after reoperation were excluded from the analysis (Figure 1).
Figure 1. Participants Flow of the Defining Mechanisms of Anterior Vaginal Wall Descent (DEMAND) Study.
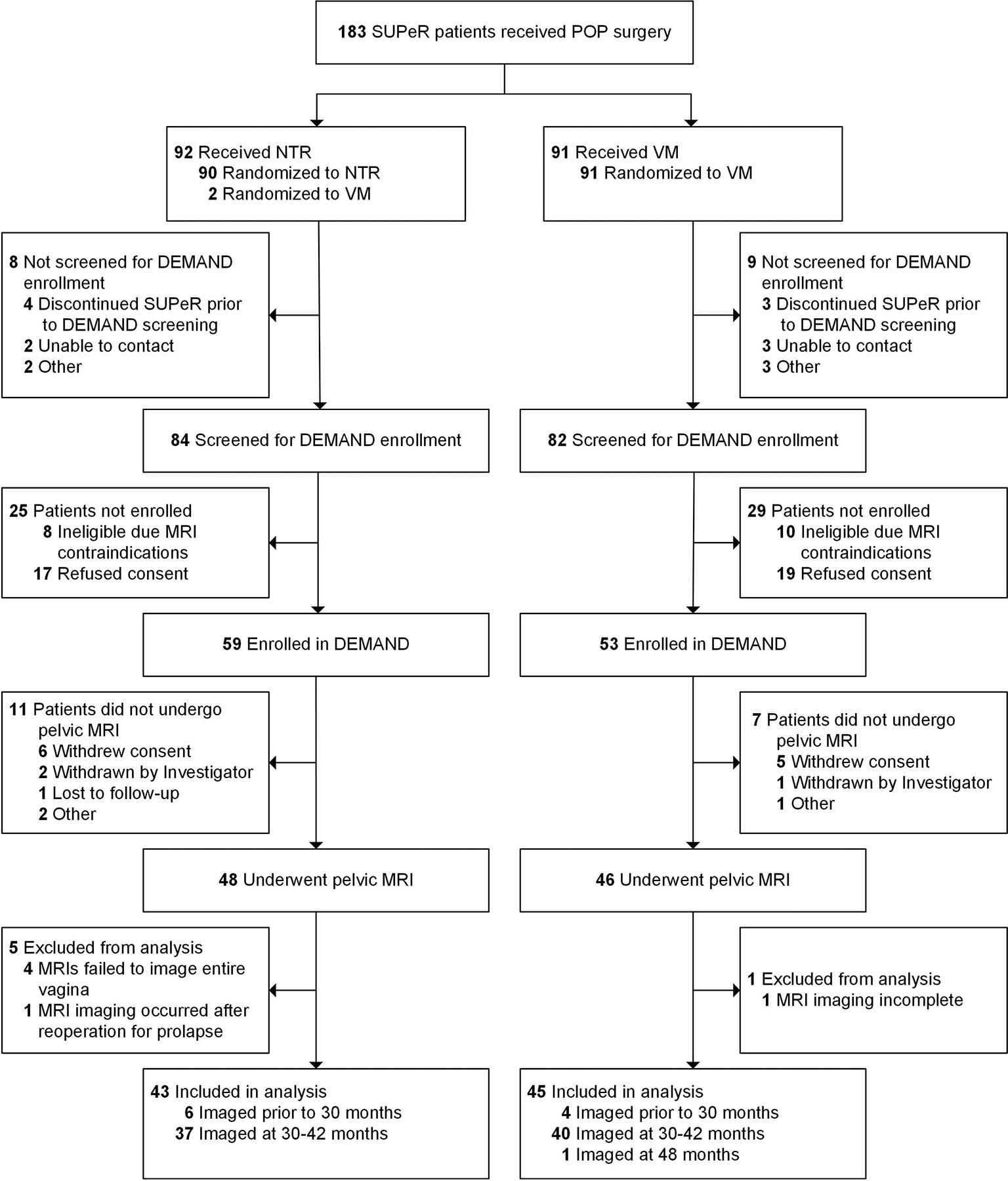
Three patients included in the analysis—2 received native tissue repair (NTR) and 1 received transvaginal mesh (VM) repair—were found ineligible for the intervention in the parent Study of Uterine Prolapse Procedures-Randomized (SUPeR) trial. Among the 43 patients included in the analysis who received NTR, two underwent hysterectomy and sacrospinous ligament suspension.
Abbreviations: DEMAND, Defining Mechanisms of Anterior Vaginal Wall Descent; MRI, magnetic resonance imaging; NTR, native tissue repair; SUPeR, Study of Uterine Prolapse Procedures-Randomized; VM, transvaginal mesh.
MRI Protocol:
The protocol and rationale of the MRI trials have been published previously.10 Prior to imaging, participants were trained by clinicians in person and via a webinar on how to properly maximally strain. Each subject underwent dynamic MRI in the supine position using a 3-T scanner with a pelvic phased array coil. High-resolution, multiplanar T2-weighted pelvic scans were acquired at rest (with prolapse fully reduced), maximal strain (i.e., maximum rest-to-strain vaginal descent out of three trials), and recovery (i.e., post-strain rest with prolapse non-reduced). The recovery scans allowed assessment of how well the vagina returns to its fully-reduced position. At the end of the procedure, patients were asked if they had achieved maximal strain. If they answered “no”, dynamic sequences were repeated until maximal strain had been subjectively achieved. MRIs were imported into 3D Slicer (Version 4.10.0, www.slicer.org) for analysis.11
MRIs were co-registered using a 3D pelvic coordinate system based on the ischial spines and pubic symphysis.10 The anterior-posterior and superior-inferior axes of this system defined the “true midsagittal plane” in the MRI scans.10
MRI Analysis:
A detailed description of the MRI analysis has been previously published.10 In short, the true midsagittal images were used to determine study outcomes. MRI failure was defined as any prolapse beyond the hymen. To assess mechanisms of failure, the vaginal wall was traced and a line was drawn between the posterior margin of the external urethral meatus and anterior margin of the perineal body to delineate the hymen. Protrusion of the vaginal wall beyond the hymenal line at strain indicated failure.
The mechanism of failure was classified as either apical descent or anterior vaginal wall elongation (Figure 2). Anterior vaginal wall elongation was defined as prolapse beyond the hymen associated with >20% lengthening of the anterior wall from rest to strain. Apical descent was characterized as prolapse beyond the hymen associated with descent of the vaginal apex in the absence of anterior vaginal wall elongation from rest to strain. Properties and sensitivity of the failure mechanism definitions were explored (Supplemental Tables 1 and 2). The proportion of failures and the leading edge of prolapse (e.g., anterior, apical, posterior compartment) were also determined.
Figure 2. Mechanisms of Failure by Surgical Group.
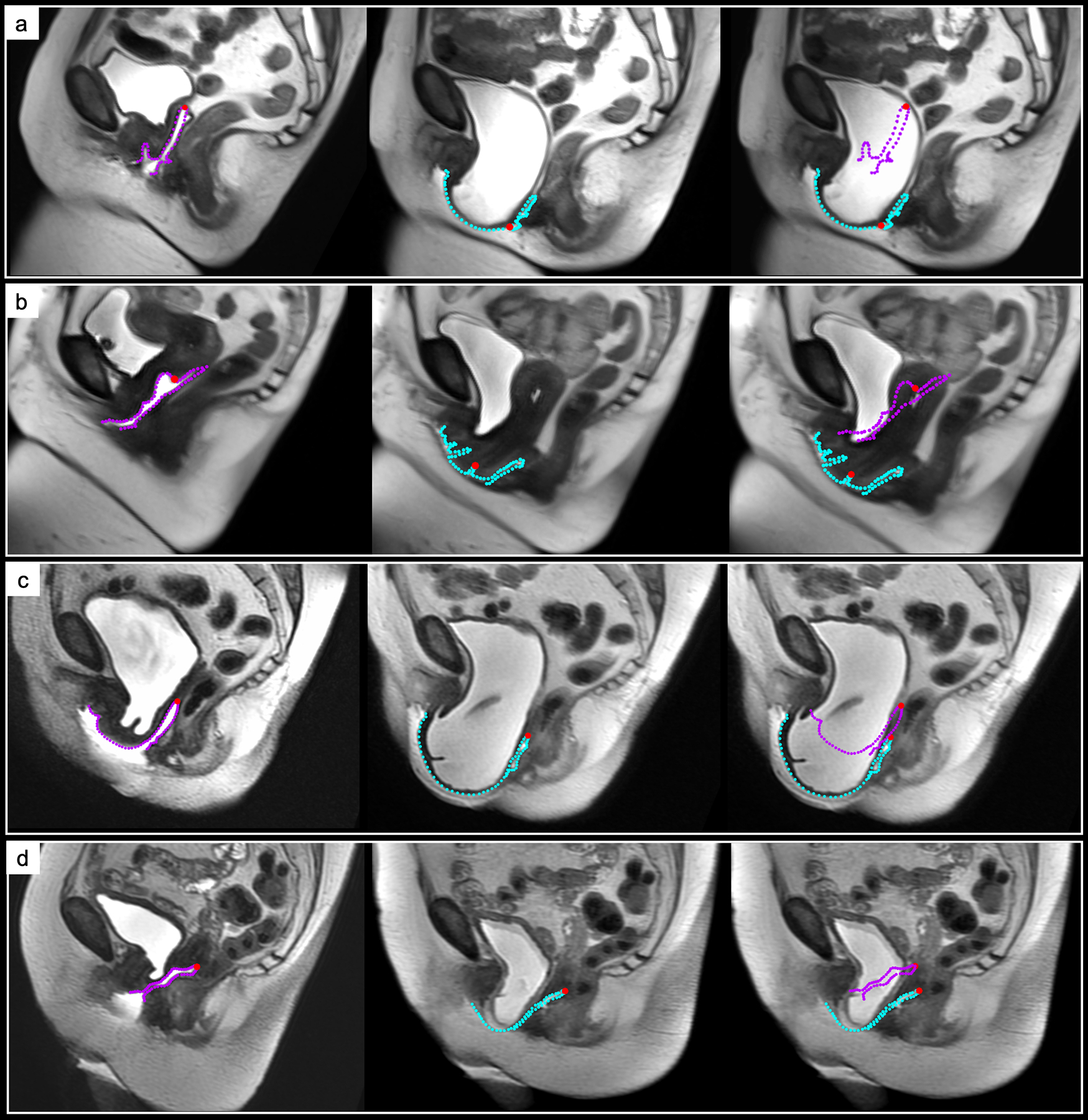
From left to right: Magnetic resonance imaging (MRI) scans and vaginal contours at rest, strain, and strain with the rest vaginal outline overlaid. Apical descent (AD) failure is shown in an (a) native tissue repair (NTR) patient and (b) transvaginal mesh (VM) repair patient. Anterior vaginal wall elongation (AVWE) failure demonstrated in an (c) NTR patient and (d) VM patient. The vaginal perimeter at rest (purple contour) and strain (blue contour) and vaginal apex (red point) are displayed.
Abbreviations: NTR, native tissue repair; VM, transvaginal mesh.
To evaluate vaginal length parameters, the vaginal apex and hymenal line were identified in the MRI to demarcate and outline the anterior and posterior vaginal walls. The contours were then measured to calculate length of the anterior wall, posterior wall, and perimeter of the vagina. Length of the hymenal line approximated the size of the vaginal introitus. The posterior vaginal wall and vaginal introitus length were used to estimate the total vaginal length (TVL) and genital hiatus (GH) POP-Q measures, respectively. The posterior margin of the GH approximated the position of the perineal body. The position of the anterior vaginal wall was defined as the point along the wall corresponding to its half-length to quantify anterior compartment descent.
Using the vaginal landmarks determined previously, the change in position and angulation of the vagina, and displacement of the apex and perineal body from (1) rest to strain and (2) rest to recovery were visualized relative to the 3D pelvic coordinate system.10
Outcomes:
The primary outcome was the mechanism of anatomic failure. Additionally, the frequency and site of failure were determined. Secondary outcomes included displacement of the vaginal apex and perineal body, elongation of the anterior and posterior wall, and change in size of the vaginal introitus from (1) rest to strain and (2) rest to recovery.
Patient demographics and clinical characteristics, as well as secondary outcomes from SUPeR9 collected at baseline and follow-up (i.e., SUPeR study visit date closest to MRI trial date) were compared between (1) NTR vs VM and (2) MRI-based failures vs successes. Agreement between DEMAND and SUPeR outcomes was evaluated as follows: (1) MRI-based anatomic failure (i.e., prolapse beyond the hymen) vs. SUPeR-defined composite failure (i.e., report of bothersome vaginal bulge symptoms, retreatment for prolapse, or any POP-Q measures beyond the hymen)9 and (2) MRI-based vs. clinical-based (POP-Q) measurements of TVL and GH.
Sample Size Calculation:
Because DEMAND was designed primarily as a descriptive study, formal sample size calculations were not conducted. However, based on SUPeR and a preliminary study,9,12 it was estimated that a sample size of 40 for each repair group was needed to achieve 80% power to detect a moderate effect size (0.5 standard deviation) in measures such as displacement of the vaginal apex and elongation of the anterior wall.
Statistical Analysis:
The data were analyzed with SAS Version 9.4 (SAS Institute, Inc., Cary, NC). Group differences in baseline demographics and clinical characteristics were compared using Fisher’s exact tests and Wilcoxon Rank-Sum tests. Fisher’s exact tests were performed to compare the mechanism of failure between the treatment groups. Concordance of failure outcomes between DEMAND and SUPeR was determined using the kappa statistic. Within each repair group, individual and multivariate (canonical) correlations were used to assess whether apical descent or vaginal wall elongation was more closely correlated with vaginal wall descent (i.e., rest-to-strain displacement of the anterior vaginal wall half-length point).
To compare secondary outcomes between groups, Fisher’s exact tests or Wilcoxon Rank-Sum tests were conducted. Correlations in TVL and GH values between MRI and POP-Q were assessed using Pearson correlation.
RESULTS:
Study Population:
Of the 112 women who consented for DEMAND participation, 94 underwent an MRI of which 88 met criteria to be analyzed (45 VM, 43 NTR) (Figure 1). Among these women, 66 (75%) were successes and 22 (25%) were failures (8 VM, 14 NTR) based on SUPeR criteria. Ten women were imaged prior to 30–42 months due to a desire for additional surgical treatment. Baseline demographic and clinical characteristics were similar between the repair groups (Table 1).
Table 1.
Participant Baseline Demographic and Clinical Characteristics by Surgical Repair Performed
| Characteristics | Mesh Repair (N=45) | Native Tissue Repair (N=43) | Risk Difference/ Location Shift (95% CI) | p-valuea |
|---|---|---|---|---|
| Patient Demographics | ||||
| Age (years), Median (P25, P75) | 63.4 (59.7, 67.8) | 65.4 (60.7, 72.3) | −1.5 (−5.0 to 1.6) | 0.39 |
| White, n/N (%) | 36/45 (80) | 36/43 (84) | −4 (−21 to 13) | 0.78 |
| Hispanic/Latina, n/N (%) | 5/43 (12) | 4/41 (10) | 2 (−13 to 17) | >0.99 |
| Married, n/N (%) | 28/45 (62) | 29/43 (67) | −5 (−25 to 15) | 0.66 |
| Associates Degree or Higher, n/N (%) | 27/43 (63) | 30/43 (70) | −7 (−27 to 13) | 0.65 |
| Medicare/Medicaid, n/N (%) | 20/45 (44) | 22/43 (51) | −7 (−28 to 15) | 0.67 |
| Medical History | ||||
| Height (cm), Median (P25, P75) | 160.0 (155.0, 165.0) | 160.0 (157.0, 165.0) | 0.0 (−3.0 to 3.0) | 0.89 |
| Weight (kg), Median (P25, P75) | 73.0 (66.0, 81.0) | 70.0 (64.0, 79.0) | 2.0 (−3.0 to 7.0) | 0.45 |
| BMI (kg/m2), Median (P25, P75) | 28.3 (25.4, 31.6) | 26.4 (24.2, 30.1) | 1.2 (−0.8 to 2.8) | 0.21 |
| Obese (BMI ≥ 30), n/N (%) | 15/45 (33) | 13/43 (30) | 3 (−17 to 23) | 0.82 |
| Gravidity, Median (P25, P75) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0 (0 to 1) | 0.38 |
| Cesarean Delivery, n/N (%) | 3/45 (7) | 4/43 (9) | −3 (−16 to 11) | 0.71 |
| Vaginal Deliveries, Median (P25, P75) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0 (0 to 1) | 0.32 |
| Post-Menopausal, n/N (%) | 43/45 (96) | 42/43 (98) | −2 (−13 to 8) | >0.99 |
| Oral Estrogen, n/N (%) | 2/45 (4) | 3/43 (7) | −3 (−15 to 9) | 0.67 |
| Vaginal Cream/Tablet Estrogen, n/N (%) | 15/45 (33) | 13/43 (30) | 3 (−17 to 23) | 0.82 |
| Skin Patch Estrogen, n/N (%) | 2/45 (4) | 0/43 (0) | 4 (−4 to 15) | 0.49 |
| Smoker, n/N (%) | 9/45 (20) | 10/43 (23) | −3 (−22 to 15) | 0.80 |
| Diabetes, n/N (%) | 7/45 (16) | 4/43 (9) | 6 (−9 to 22) | 0.52 |
| Pulmonary Diseaseb, n/N (%) | 4/45 (9) | 4/43 (9) | 0 (−15 to 13) | >0.99 |
| Cardiovascular Diseasec, n/N (%) | 4/45 (9) | 3/43 (7) | 2 (−11 to 15) | >0.99 |
| Prior Prolapse Surgery, n/N (%) | 0/45 (0) | 3/43 (7) | −7 (−19 to 2) | 0.11 |
| Pelvic Floor Measurements and Symptoms | ||||
| POPQ Measurements (cm), Median (P25, P75) | ||||
| POPQ Ba | 3.0 (2.0, 4.0) | 2.0 (1.0, 4.0) | 0.0 (−1.0 to 1.0) | 0.74 |
| POPQ Bp | 0.0 (−2.0, 2.0) | 0.5 (−1.0, 3.0) | −0.5 (−2.0 to 1.0) | 0.39 |
| POPQ C | 0.0 (−3.0, 2.0) | 0.0 (−2.0, 3.0) | 0.0 (−2.0 to 1.0) | 0.56 |
| POPQ GH Strain | 4.5 (4.0, 5.0) | 4.0 (4.0, 5.0) | 0.0 (−0.5 to 0.5) | 0.70 |
| POPQ PB Strain | 3.0 (2.5, 4.0) | 3.0 (2.5, 4.0) | 0.0 (0.0 to 0.0) | 0.97 |
| POPQ TVL | 9.0 (8.0, 10.0) | 9.0 (8.0, 10.0) | 0.0 (0.0 to 0.5) | 0.86 |
| POPQ Staged, n/N (%) | 0.38 | |||
| Stage 2 | 9/45 (20) | 11/43 (26) | −6 (−24 to 13) | |
| Stage 3 | 33/45 (73) | 26/43 (60) | 13 (−7 to 33) | |
| Stage 4 | 3/45 (7) | 6/43 (14) | −7 (−22 to 6) | |
| Apical Prolapse (POPQ C > 0), n/N (%) | 43/45 (96) | 42/43 (98) | 0 (−21 to 22) | >0.99 |
| Anterior Prolapse (POPQ Aa or Ba > 0), n/N (%) | 20/45 (44) | 22/43 (51) | −2 (−13 to 8) | >0.99 |
| Posterior Prolapse (POPQ Ap or Bp > 0), n/N (%) | 21/45 (47) | 20/43 (47) | −7 (−28 to 15) | 0.67 |
| Any Vaginal Bulge, n/N (%) | 45/45 (100) | 43/43 (100) | ||
| Bothersome Vaginal Bulge, n/N (%) | 43/44 (98) | 43/43 (100) | −2 (−12 to 6) | >0.99 |
| Patient Reported Outcomes | ||||
| PFDI Score, Median (P25, P75) | 127.1 (79.2, 162.5) | 91.7 (70.8, 161.5) | 17.7 (−7.3 to 43.8) | 0.17 |
| UDI Score, Median (P25, P75) | 18.8 (6.3, 40.6) | 18.8 (6.3, 31.3) | 3.1 (−6.3 to 12.5) | 0.45 |
| CRADI Score, Median (P25, P75) | 45.8 (25.0, 66.7) | 33.3 (16.7, 54.2) | 8.3 (−4.2 to 20.8) | 0.16 |
| POPDI Score, Median (P25, P75) | 50.0 (33.3, 66.7) | 45.8 (29.2, 66.7) | 4.2 (−4.2 to 16.7) | 0.33 |
| PFIQ Score, Median (P25, P75) | 47.6 (14.3, 71.4) | 28.6 (9.5, 61.9) | 9.5 (−4.8 to 28.6) | 0.17 |
| UIQ Score, Median (P25, P75) | 23.8 (0.0, 42.9) | 9.5 (0.0, 33.3) | 4.8 (0.0 to 19.0) | 0.19 |
| CRAIQ Score, Median (P25, P75) | 0.0 (0.0, 23.8) | 0.0 (0.0, 14.3) | 0.0 (0.0 to 4.8) | 0.17 |
| POPIQ Score, Median (P25, P75) | 4.8 (0.0, 28.6) | 9.5 (0.0, 28.6) | 0.0 (−4.8 to 4.8) | 0.89 |
| ISI Score, Median (P25, P75) | 4.0 (1.0, 8.0) | 3.0 (1.0, 6.0) | 0.0 (−1.0 to 2.0) | 0.59 |
| FAS Score Median (P25, P75) | 96.2 (84.6, 100.0) | 98.1 (87.5, 100.0) | 0.0 (−3.0 to 0.0) | 0.37 |
| SPS Pain at Rest Score, Median (P25, P75) | 0.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | 0.0 (0.0 to 0.0) | 0.40 |
| SPS Pain during Normal Activities Score, Median (P25, P75) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 0.0 (0.0 to 1.0) | 0.64 |
| SPS Pain during Exercise Score, Median (P25, P75) | 2.0 (0.0, 4.0) | 0.0 (0.0, 2.5) | 0.5 (0.0 to 2.0) | 0.13 |
| SPS Worst Pain Score, Median (P25, P75) | 1.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 0.0 (0.0 to 0.0) | 0.47 |
| BPPS Score, Median (P25, P75) | 0.4 (0.0, 1.1) | 0.1 (0.0, 0.7) | 0.1 (0.0 to 0.4) | 0.11 |
| BIS Score, Median (P25, P75) | 5.0 (2.0, 10.0) | 4.0 (0.0, 8.0) | 1.0 (0.0 to 3.0) | 0.16 |
| PISQ-IR Score among Sexually Active, Median (P25, P75) | 3.5 (3.0, 3.9) | 3.5 (2.9, 3.8) | 0.0 (−0.3 to 0.5) | 0.76 |
| Concomitant Surgical Procedures | ||||
| Anterior Prolapse Repair, n/N (%) | 39/45 (87) | 32/43 (74) | 12 (−5 to 30) | 0.18 |
| Posterior Prolapse Repair, n/N (%) | 26/45 (58) | 26/43 (60) | −3 (−24 to 18) | 0.83 |
| Urinary Incontinence Repair (TVT/TOT), n/N (%) | 24/45 (53) | 24/43 (56) | −2 (−24 to 19) | 0.83 |
BIS, Body Image Scale; BMI, body mass index; BPPS, Body Part Pain Scale; CI, confidence interval; CRADI, Colorectal Anal Distress Inventory; CRAIQ, Colorectal Anal Impact Questionnaire; FAS, Functional Activity Scale; ISI, Incontinence Severity Index; PFDI, Pelvic Floor Distress Inventory; PFIQ, Pelvic Floor Impact Questionnaire; PISQ-IR, Pelvic Organ Prolapse/Incontinence Sexual Function Questionnaire-IUGA Revised; POPDI, Pelvic Organ Prolapse Distress Inventory; POPIQ, Pelvic Organ Prolapse Impact Questionnaire; POPQ, Pelvic Organ Prolapse Quantification; SPS, Surgical Pain Scale; TOT, transobturator tape; TVT, transvaginal tape; UDI, Urogenital Distress Inventory; UIQ, Urinary Impact Questionnaire.
Risk difference, 95% confidence intervals, and p-values were obtained using Fisher’s exact test for categorical measures. Location shift, 95% confidence intervals, and p-values were obtained using Wilcoxon Rank-Sum test for continuous measures with a Hodges-Lehmann estimation of location shift (i.e., estimate of the difference in two distributions, calculated as the median of all paired differences between observations in the two samples). All tests were conducted at a significance level of 0.05.
Pulmonary disease includes any the following: asthma, chronic obstructive pulmonary disease, acute respiratory distress syndrome, and emphysema.
Cardiovascular disease includes any the following: angina, congenital heart failure/heart disease, heart attack, stroke/TIA, peripheral vascular disease.
Pelvic Organ Prolapse Quantification (POPQ) Stages: Stage 2-The vagina is prolapsed between 1 cm above the hymen and 1 cm below the hymen; Stage 3-The vagina is prolapsed more than 1 cm beyond the hymen but is not everted within 2 cm of its length; Stage 4-The vagina is everted to within 2 cm of its length.
Concomitant anterior repair was performed on 39/45 (87%) women in the VM group and 32/43 (74%) in the NTR group (difference=12% [95%CI=−5% to 30%]; P=0.18); concomitant posterior repair was performed on 26/45 (58%) women in the VM group and 26/43 (60%) in the NTR group (difference=−3% [95%CI=−24% to 19%]; P=0.83); concomitant midurethral slings were performed on 24/45 (53%) women in the VM group and 24/43 (56%) in the NTR group (difference=−2% [95%CI=−24% to 19%]; P=0.83).
Comparison of baseline measures in MRI successes and failures demonstrated worse preoperative apical support (POP-Q point C), higher prevalence of pulmonary disease, and more apical prolapse beyond the hymen (POP Q point C > 0) in failures as compared to successes (all P’s <0.05, Supplemental Table 3).
Primary Outcome:
Based on MRI evaluation, 37/88 (42%) women had outcomes of anatomic failure, with 13/45 (29%) in the VM group and 24/43 (56%) in the NTR group. For both repair groups, the primary mechanism of failure was apical descent (11/13 [85%] vs 16/24 [67%]; difference=18% [95%CI=−17% to 44%]; P=.44) while the primary site was the anterior compartment (5/13 [38%] vs 22/24 [92%]; difference=−53% [95%CI=−79% to −19%]; P=.001) (Table 2). There was weak agreement between MRI and SUPeR failure outcomes (Kappa=0.23; 95%CI=0.02 to 0.43; P=.02) (Table 3).
Table 2.
MRI Failure (any point beyond hymen) and Failure Types by Surgical Repair Performed
| MRI Failure Outcomes | Mesh Repair (N=45) | Native Tissue Repair (N=43) | Risk Difference (95% CI) | p-valuea |
|---|---|---|---|---|
| MRI Failure, n/N (%) | 13/45 (29) | 24/43 (56) | 27 (5 to 46) | 0.02 |
| Type of Failure | 0.44 | |||
| Anterior Wall Elongation | 2/13 (15) | 8/24 (33) | −18 (−44 to 17) | |
| Apical Failure | 11/13 (85) | 16/24 (67) | 18 (−17 to 44) | |
| Compartment of Failure | 0.001 | |||
| Anterior | 5/13 (38) | 22/24 (92) | −53 (−79 to −19) | |
| Posterior | 3/13 (23) | 1/24 (4) | 19 (−4 to 49) | |
| Anterior/Posterior | 2/13 (15) | 1/24 (4) | 11 (−10 to 40) | |
| Apex | 3/13 (23) | 0/24 (0) | 23 (3 to 54) |
CI, confidence interval; MRI, magnetic resonance imaging.
Risk difference, 95% confidence intervals, and p-values were obtained using Fisher’s exact test for categorical measures. All tests were conducted at a significance level of 0.05, and p-values <0.05 are bolded.
Table 3.
Agreement between MRI and SUPeR Failure Definitions Overall and within Surgical Repair Performed
| SUPeR Failurea N=22 | SUPeR Successa N=66 | Kappa (95% CI)b | p-valueb | |
|---|---|---|---|---|
| MRI Failure (any point beyond hymen) | ||||
| Overall | 0.23 (0.03 to 0.43) | 0.02 | ||
| MRI Failure, n/N (%) | 14/22 (64) | 23/66 (35) | ||
| MRI Success, n/N (%) | 8/22 (36) | 43/66 (65) | ||
| Mesh Repair | 0.33 (0.01 to 0.62) | 0.03 | ||
| MRI Failure, n/N (%) | 5/8 (63) | 8/37 (22) | ||
| MRI Success, n/N (%) | 3/8 (38) | 29/37 (78) | ||
| Native Tissue Repair | 0.11 (−0.13 to 0.37) | 0.52 | ||
| MRI Failure, n/N (%) | 9/14 (64) | 15/29 (52) | ||
| MRI Success, n/N (%) | 5/14 (36) | 14/29 (48) |
CI, confidence interval; MRI, magnetic resonance imaging; SUPeR, Study of Uterine Prolapse Procedures Randomized.
SUPeR failure is defined as a prolapse beyond the hymen or the presence of bothersome bulge symptoms at the SUPeR visit closest to MRI imaging, or undergoing retreatment (surgery or pessary) for pelvic prolapse through the SUPeR visit closest to MRI imaging. SUPeR success is defined as no prolapse beyond the hymen and the absence of bothersome bulge symptoms at the SUPeR visit closest to MRI imaging as well as no retreatment (surgery or pessary) for pelvic prolapse through the SUPeR visit closest to MRI imaging.
The chance-adjusted measurement of agreement simple Kappa statistic typically ranges from 0 (agreement is totally by chance and expected) to 1 (perfect agreement). Low negative values (0.00 to −0.10) for the Kappa statistic may generally be interpreted as no agreement. 95% confidence intervals were obtained via bootstrapping and p-values were based on exact tests for simple Kappa statistic. All tests were conducted at a 0.05 significance level, and p-values <0.05 are bolded.
In the VM group, vaginal wall descent was highly correlated with apical descent (r=0.83), and less correlated with vaginal wall elongation (r=0.23). Multivariate correlation between vaginal wall descent (on one side) and apical descent and vaginal wall elongation (on the other side) was nearly identical to the correlation with apical descent alone (r=0.84), indicating that the contribution of vaginal wall elongation was minimal. In the NTR group, vaginal wall descent was also more correlated with apical descent (r=0.84) than vaginal wall elongation (r=0.38); however, the multivariate correlation was 0.90, indicating that a greater amount of the variability in vaginal wall descent was accounted for when vaginal wall elongation was also considered.
Secondary Outcomes:
Secondary outcome measures are shown in Supplemental Table 4. Comparisons between MRI-based failures vs successes demonstrated that, from rest to strain, failures had more inferior displacement of the vaginal apex (−33 mm vs −21 mm; difference=−12 mm [95%CI=−19 to −6]; P=.002) and perineal body (−16 mm vs −9 mm; difference=−7 mm [95%CI=−11 to −4]; P<.001) as well as larger change in length of the anterior wall (14 mm vs 2 mm; difference=12 mm [95%CI=8 to 16]; P<.001), posterior wall (−8 mm vs −1 mm; difference= −6 mm [95%CI, −10 to −2]; P=.004), and introitus (15 mm vs 3 mm; difference=11 mm [95%CI=7 to 15]; P<.001). At strain, a larger GH was moderately correlated with more inferior displacement of the vaginal apex (r=−0.51; 95%CI=−0.67 to −0.37; P<0.001). At recovery, the vaginal introitus (or GH) was larger in failures (19 mm vs 14 mm; difference=6 mm [95%CI=2 to 11]; P=.005). Weak-moderate correlation was observed between MRI- and POP-Q-based TVL (r=0.20; 95%CI=−0.01 to 0.39; P=0.07) and GH (r=0.49; 95%CI=0.32 to 0.64; P<.001) measures (Supplemental Table 5). Differences in secondary outcomes by repair group are provided in Supplemental Table 6.
Changes in vaginal position and orientation and the displacement of the vaginal apex and perineal body between MRI failures and successes are shown in Figure 3. From rest to strain, the vagina was more posteriorly-inferiorly deviated in failures, where posterior refers to towards the sacrum and inferior refers to toward the feet. At recovery, the vagina remained more posteriorly-inferiorly displaced in failures.
Figure 3. Vaginal Configuration and Displacement in the Anatomic Space.
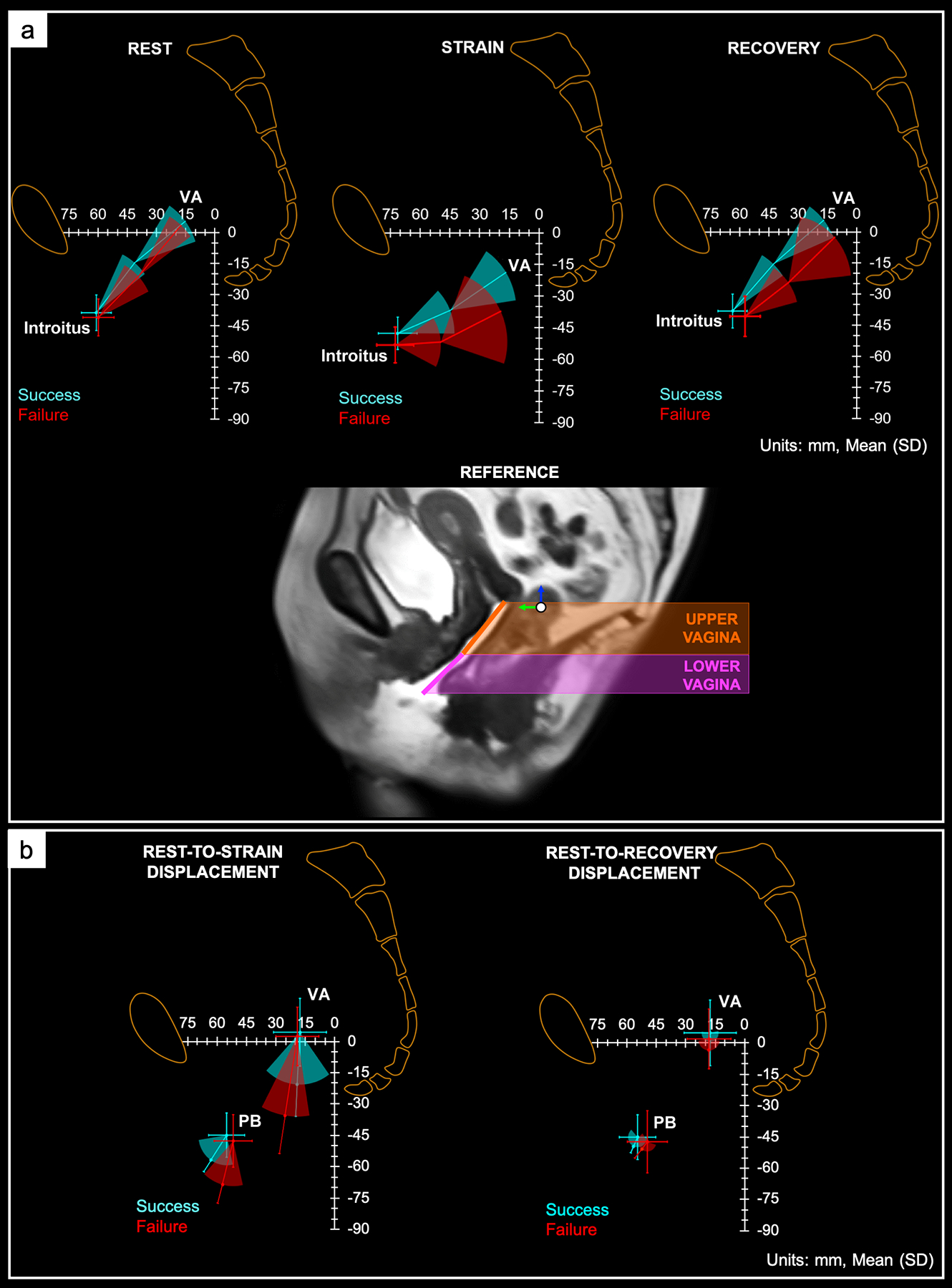
Visual representation of the change in orientation and position of the upper and lower vagina with respect to the pelvic space at rest (left), maximal strain (middle), and recovery (right) in magnetic resonance imaging (MRI) successes and failures. The orientation (i.e., angulation) of the upper and lower vagina and the position of the vaginal apex (VA), and mid-point of the vaginal introitus (introitus) are shown. More posterior-inferior deviation of the upper and lower vagina was observed in MRI failures, signifying poorer apical, mid, and distal vaginal support compared to successes. Data are presented as mean and standard deviation. Units are in mm. (b) Position and displacement vector (mean and standard deviation) of the vaginal apex (VA) and perineal body (PB) from rest to strain (left) and rest to recovery (right). MRI successes (blue) and failures (red) are indicated. The magnitude of the VA and PB displacement from rest to strain was greater in MRI failures vs successes, suggesting less apical and distal vaginal support in failures compared to successes. Rest-to-recovery VA and PB displacement was similar in both outcome groups. Units are in mm.
COMMENT:
Principal Findings:
The primary mechanism of failure in NTR and VM was apical descent; the most frequent site of failure was the anterior compartment. Greater inferior displacement of the apex and perineal body, lengthening of the anterior wall, shortening of the posterior wall, and increased introitus size with strain were observed among failures. MRI and POP-Q measures of TVL and GH were weakly to moderately correlated.
Results:
The anterior compartment was the most common site of anatomic failure following NTR and VM at a rate similar to that previously reported (~73%).13 The primary mechanism of failure was apical descent. Multivariate analyses showed that apical descent was strongly correlated with, and the primary contributor to, anterior vaginal wall descent. In VM, multivariate correlations demonstrated that anterior compartment descent is mainly due to apical descent. Conversely, in NTR, anterior wall elongation and apical descent contributed to anterior compartment descent. In a similar study relating vaginal geometry to anterior vaginal prolapse severity among patients without prior POP surgery, 77% of anterior descent was explained by anterior vaginal wall length and position of the vaginal apex.14 Another study which modeled anterior vaginal wall prolapse showed that a 90% impairment of apical support led to a 530% increase in anterior vaginal prolapse.15
There was weak concordance in failure outcome definitions between DEMAND and SUPeR.9 Poor association between MRI and clinical assessment of POP has been reported in previous literature.16,17
Greater inferior descent of the perineal body and increased vaginal introitus (or GH) size were associated with prolapse recurrence. Also, a larger GH was correlated with greater apical descent. Studies have shown that women with POP have greater inferior displacement of the perineal body and increased introital size from rest to strain compared to women with normal support.18,19 Furthermore, these measures have been correlated with the presence of prolapse and apical support loss.18,19
Increased posterior-inferior deviation of the vagina was observed among failures. Others have reported that posterior-inferior deviation of the vagina, particularly posterior tilting of the lower vagina, increased the risk of anterior compartment prolapse.20–22 This may also apply to the development of recurrent prolapse due to postoperative changes in vaginal position and orientation.20,23
Clinical Implications:
Apical descent and, to a lesser extent, anterior vaginal wall elongation are mechanisms of anatomical failure following POP repair. Apical descent causes the anterior vaginal wall to fall towards the vaginal introitus. Changes in vaginal orientation and position (e.g., posterior-inferior deviation) may leave the anterior compartment more susceptible to intrabdominal forces, facilitating its elongation over time.24,25 Furthermore, an enlarged GH likely allows forces to be distributed along a greater length of the vaginal wall, increasing the likelihood of descent. Together, these factors may raise the risk of prolapse recurrence in the anterior compartment.23, 24, 26–28
In addition, greater perineal body descent and increased vaginal introitus size were associated with anatomical failure. Perineal body position has been correlated with the levator plate angle and displacement, levator hiatus size, and differences in pelvic floor muscle structure and function between women with and without POP.18 This suggests that these structures are interrelated and involved in pelvic floor descent and, potentially, prolapse recurrence.
Research Implications:
This study supports that anatomical failure is common after POP surgery likely due to the fact that current POP surgeries do not address deficiencies in pelvic floor musculature associated with pelvic floor descent.28 The mechanisms and anatomic factors of prolapse recurrence proposed by this study imply that the apical suspension procedures in this study were not sufficient to stabilize the apex in the long-term. The multi-compartmental nature of anatomical failure suggests that compromised mid and distal vaginal support may also play a role in prolapse recurrence.
Future studies are needed that assess surgical outcomes via radiographic and clinical examination to (1) more thoroughly define and compare the efficacy of prolapse procedures and (2) identify anatomic factors and surgical determinants that can optimize surgical outcomes.
Strengths and Limitations:
This study prospectively evaluated and compared long-term anatomic outcomes in women who underwent NTR vs VM using MRI. Past studies have used only POP-Q-based clinical measures to assess anatomic outcomes. Thus, their findings are limited to external measures of prolapse recurrence following surgery.
A major limitation of this study was that the DEMAND population was not a random sample of SUPeR patients (<50% of the original SUPeR population enrolled in DEMAND). Comparisons of groups included in and excluded from (e.g., non-enrolled) the DEMAND analysis are given in Supplemental Tables 7 and 8. Among women included in the DEMAND analysis, a higher proportion were SUPeR failures in the NTR group compared to the VM group (33% vs 18%); similarly, among women not included in the analysis, there was a higher proportion of SUPeR failures in the NTR group than the VM group (25% vs 18%) (Supplemental Table 8). Consequently, the MRI-based failure rates of VM and NTR observed could not be compared.
Additionally, MRIs were obtained in the supine position. Therefore, the extent of prolapse was dependent on patient effort and the degree of prolapse may be underestimated. To overcome this, participants were trained to maximally strain prior to imaging and performed at least three attempts to maximally strain during the MRI trial.10 Further, anatomic measures were taken only in the midsagittal plane. Thus, they were limited to 2-dimensional postoperative morphology, which may not fully capture the 3D nature of prolapse and pelvic anatomy.
Lastly, because the data are based on postoperative MRI alone, it is difficult to distinguish whether anatomical findings are primarily attributed to the surgery performed, the pathophysiological effects of prolapse, or both. Nevertheless, the clinical implication is that future surgeries could consider stabilization of the apex as a priority and novel approaches may be needed to achieve this in transvaginal repair.
CONCLUSIONS:
The primary mechanism of failure following apical prolapse repair with native tissue or mesh was vaginal apex descent. Displacement of the vaginal apex and perineal body, as well as elongation of the anterior wall, posterior wall, and vaginal introitus were significantly associated with anatomical failure. These results suggest that failure of POP repairs not only involves the apical vaginal supports but also the mid and distal supports of the vagina.
Future studies will quantitatively assess 3D postoperative morphology of the vaginal and pelvic floor muscle anatomy with respect to the type and anatomic outcomes of prolapse repair and investigate the integrity of the vaginal supports (i.e., mid, distal, paravaginal). Outcomes will provide insight on factors of apical descent predisposing to anatomical failure.
Supplementary Material
AJOG AT A GLANCE:
A. Why was this study conducted?
To compare mechanisms and contributors of prolapse recurrence using MRI criteria following two transvaginal apical suspension procedures for uterovaginal prolapse: vaginal hysterectomy with uterosacral ligament suspension versus vaginal mesh hysteropexy.
B. What are the key findings?
For both repair groups, the primary mechanism of anatomic failure was apical descent and the most common leading edge of prolapse was the anterior compartment.
Greater inferior displacement of the vaginal apex and perineal body, and elongation of the anterior vaginal wall and vaginal introitus from rest to strain were associated with failure.
C. What does this study add to what is already known?
Our findings identify potential mechanisms and contributors of anatomical failure of apical prolapse repairs.
Failure of prolapse repairs not only involves the apical vaginal supports but also the mid and distal vaginal supports.
Further investigation will provide insight on anatomical predisposing factors of failure.
ACKNOWLEDGMENTS:
In addition to the authors, the following members of the PFDN were involved in the DEMAND study:
UC San Diego Health, San Diego, CA: Michael E. Albo, Marianna Alperin, Joann Columbo, Jodi Curry, Kimberly Ferrante, Kyle Herrala, Sherella Johnson, Anna C. Kirby, Emily S. Lukacz, Charles W. Nager, Erika Ruppert, Erika Wasenda.
Kaiser Permanente, San Diego, CA: Gouri B. Diwadkar, Keisha Y. Dyer, Linda M. Mackinnon, Shawn A. Menefee, Jasmine Tan-Kim, Gisselle Zazueta-Damian.
Duke University Medical Center, Durham, NC: Cindy Amundsen, Yasmeen Bruton, Notorious Coleman-Taylor, Robin Gilliam, Acacia Harris, Akira Hayes, Amie Kawasaki, Nicole Longoria, Shantae McLean, Mary Raynor, Nazema Siddiqui, Anthony G. Visco.
University of Alabama at Birmingham, Dept. OB/GYN, Birmingham, AL: Alicia Ballard, Kathy Carter, David Ellington, Sunita Patel, Nancy Saxon, R. Edward Varner, Velria Willis, Kathy Carter.
Alpert Medical School of Brown University, Providence, RI: Cassandra Carberry, Samantha Douglas, B. Star Hampton, Nicole Korbly, Ann S. Meers, Deborah L. Myers, Vivian W. Sung, Elizabeth-Ann Viscione, Kyle Wohlrab.
University of New Mexico: Karen Box, Gena Dunivan, Peter Jeppson, Julia Middendorf, Rebecca G. Rogers.
University of Pennsylvania, Philadelphia, PA: Lily Arya, Uduak Andy, Norman Butler, Doris Cain, Teresa Carney, Lorraine Flick, Kavita Desai Khanijow, Michelle Kingslee, Daniel Lee, Patricia O’Donnell, Ariana Smith, Donna Thompson.
Magee-Women’s Hospital, Dept. of OB/GYN & Reproductive Sciences, Pittsburgh, PA: Michael Bonidie, Judy Gruss, Jerry Lowder, Jonathan Shepherd, Gary Sutkin, Halina M. Zyczynski.
Cleveland Clinic Foundation, Cleveland, OH: Matthew Barber, Kathleen Dastoli, Maryori Edington, Annette Graham, Geetha Krishnan, Eric Jelovsek, Marie Fidela R. Paraiso, Ly Pung, Cecile Ferrando, Mark Walters.
Northwest Texas Physician Group, Amarillo, TX: Susan Meikle.
Research Triangle International, Research Triangle Park, NC: Andrew Burd, Kate Burdekin, Kendra Glass, Tracey Grant, Scott Grey, Michael Ham, James Pickett, Dennis Wallace, Ryan Whitworth, Amanda Shaffer, Taylor Swankie.
FUNDING/SUPPORT:
This study was conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development-sponsored Pelvic Floor Disorders Network (grants U10 HD054214, U10 HD041267, U10 HD041261, U10 HD069013, U10 HD069025, U10 HD069010, U10 HD069006, U10 HD054215, U01 HD069031) and the National Institutes of Health Office of Research on Women’s Health. Partial support for this study was supplied by Boston Scientific Corporation through a research grant to the Pelvic Floor Disorders Network Data Coordinating Center, RTI International. Research training support was provided by the National Institute of Biomedical Imaging and Bioengineering (5T32EB003392–13) and the National Academies of Sciences, Engineering, and Medicine’s Ford Foundation Predoctoral Fellowship Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Ford Foundation.
ROLE OF THE FUNDER/SPONSOR:
The NICHD project scientist for the PFDN at the time of this study, Dr. Mazloomdoost, had a role in the development of the protocol and management of the study; preparation, review, and approval of the manuscript. The funding of the study was managed by other NIH employees. The NIBIB and Ford Foundation had a role in providing research training support. Boston Scientific had no role in any aspects of this study.
CONFLICT OF INTEREST DISCLOSURES:
All of the authors reported funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Office of Research on Women’s Health and that Boston Scientific Corporation provided partial support through a research grant to the Pelvic Floor Disorders Network (PFDN) Data Coordinating Center, RTI International.
Dr. Moalli reported serving as a consultant to Hologics Medical Inc and receiving research support from the NICHD.
Dr. Abramowitch reported receiving research support from the NICHD.
Dr. Lockhart reported receiving personal fees from the American Institute of Ultrasound in Medicine as deputy editor for the Journal of Ultrasound in Medicine and book royalties from Elsevier and Oxford Publishers.
Dr. Weidner reported receiving personal fees as an assistant editor for the Obstetrical & Gynecological Survey and consultant to Urocure, and research support from the NICHD.
Dr. Ferrando reported receiving personal fees from Coloplast Corp, Boston Scientific Corporation, Medtronic USA, Inc, and Aesculap Inc.
Dr. Nager reported receiving royalties for UpToDate.
Dr. Richter reported receiving personal fees from the International Urogynecological Association and American College of Obstetricians and Gynecologists as an editor for the International Urogynecology Journal and Obstetrics and Gynecology Journal, respectively, and royalties from UpToDate. Dr. Richter also reported receiving research support from Bluewind, Data and Safety Monitoring Board, Renovia, Allergan, NICHD, and National Institute of Aging. Dr. Richter serves as a board member for the Worldwide Fistula Fund and American Urogynecologic Society.
Dr. Rardin reported receiving research support from Solace Therapeutics, Pelvalon, Foundation for Female Health Awareness, and the NICHD.
Dr. Komesu reported receiving funding from Cook-Myosite® and research support from the NICHD.
No other disclosures were reported.
Footnotes
PAPER PRESENTATION INFORMATION:
The work was presented orally and received the Prize Paper Award at the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons, Jacksonville, FL, July 9–12, 2020.
REFERENCES:
- 1.Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–1206. doi: 10.1097/AOG.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith FJ, Holman CDAJ, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–1100. doi: 10.1097/AOG.0b013e3181f73729 [DOI] [PubMed] [Google Scholar]
- 3.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6 [DOI] [PubMed] [Google Scholar]
- 4.Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: The OPTIMAL randomized trial. JAMA - J Am Med Assoc. 2014;311(10):1023–1034. doi: 10.1001/jama.2014.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelovsek JE, Barber MD, Norton P, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA - J Am Med Assoc. 2018;319(15):1554–1565. doi: 10.1001/jama.2018.2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. 2019. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-action-protectwomens-health-orders-manufacturers-surgicalmesh-intended-transvaginal. Accessed January 14, 2020.
- 7.Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q) - a new era in pelvic prolapse staging. J Med Life. 2011;4(1):75–81. /pmc/articles/PMC3056425/?report=abstract. Accessed June 29, 2020. [PMC free article] [PubMed] [Google Scholar]
- 8.Bø K, Freeman R, Chapple CK, Abrams P. Evidence-Based Physical Therapy for the Pelvic Floor : Bridging Science and Clinical Practice. Edinburgh, Scotland:Churchill Livingstone;2015. [Google Scholar]
- 9.Nager CW, Visco AG, Richter HE, et al. Effect of vaginal mesh hysteropexy vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: A randomized clinical trial. JAMA - J Am Med Assoc. 2019;322(11):1054–1065. doi: 10.1001/jama.2019.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moalli PA, Bowen ST, Abramowitch SD, et al. Methods for the defining mechanisms of anterior vaginal wall descent (DEMAND) study. Int Urogynecol J. September 2020:1–10. doi: 10.1007/s00192-020-04511-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easley DC, Menon PG, Abramowitch SD, Moalli PA. Inter-observer variability of vaginal wall segmentation from MRI: A statistical shape analysis approach. In: ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE). Vol 3–2015. American Society of Mechanical Engineers (ASME); 2015. doi: 10.1115/IMECE2015-53499 [DOI] [Google Scholar]
- 13.Morgan DM, Larson K, Lewicky-Gaupp C, Fenner DE, Delancey JOL. Vaginal support as determined by levator ani defect status 6 weeks after primary surgery for pelvic organ prolapse. Int J Gynecol Obstet. 2011;114(2):141–144. doi: 10.1016/j.ijgo.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu Y, Chen L, Summers A, Ashton-Miller JA, Delancey JOL. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. doi: 10.1007/s00192-007-0405-x [DOI] [PMC free article] [PubMed]
- 15.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JOL. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108(2):324–332. doi: 10.1097/01.AOG.0000227786.69257.a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pannu HK, Scatarige JC, Eng J. MRI diagnosis of pelvic organ prolapse compared with clinical examination. Acad Radiol. 2011;18(10):1245–1251. doi: 10.1016/j.acra.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Lakeman MME, Zijta FM, Peringa J, Nederveen AJ, Stoker J, Roovers JPWR. Dynamic magnetic resonance imaging to quantify pelvic organ prolapse: Reliability of assessment and correlation with clinical findings and pelvic floor symptoms. Int Urogynecol J. 2012;23(11):1547–1554. doi: 10.1007/s00192-012-1772-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Y, Summers A, Hussain HK, Guire KE, Delancey JOL. Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am J Obstet Gynecol. 2006;194(5):1427–1433. doi: 10.1016/j.ajog.2006.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowder JL, Oliphant SS, Shepherd JP, Ghetti C, Sutkin G. Genital hiatus size is associated with and predictive of apical vaginal support loss. 2016. doi: 10.1016/j.ajog.2015.12.027 [DOI] [PubMed]
- 20.Lee DD, Siegelman ES, Chua WY, Arya LA, Harvie HS. Comparison of Vaginal Axis in Women Who Have Undergone Hysterectomy Versus Women with an Intact Uterus. Female Pelvic Med Reconstr Surg. 2019;25(4):313–317. doi: 10.1097/SPV.0000000000000557 [DOI] [PubMed] [Google Scholar]
- 21.Morley GW, DeLancey JOL. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158(4):872–881. doi: 10.1016/0002-9378(88)90088-9 [DOI] [PubMed] [Google Scholar]
- 22.Shull BL, Capen CV, Riggs MW, Kuehl TJ. Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166(6 PART 1):1764–1771. doi: 10.1016/0002-9378(92)91567-T [DOI] [PubMed] [Google Scholar]
- 23.Juliato CRT, Santos‐Junior LC, Castro EB, Dertkigil SS, Brito LGO. Vaginal axis after abdominal sacrocolpopexy versus vaginal sacrospinous fixation—a randomized trial. Neurourol Urodyn. 2019;38(4):1142–1151. doi: 10.1002/nau.23970 [DOI] [PubMed] [Google Scholar]
- 24.Senturk MB, Kilicci C, Aydin S, Polat M, Abide Yayla C, Karateke A. Vaginal axis on MRI after unilateral and bilateral sacral hysteropexy: a controlled study. J Obstet Gynaecol (Lahore). 2018;38(1):115–120. doi: 10.1080/01443615.2017.1336754 [DOI] [PubMed] [Google Scholar]
- 25.Balgobin S, Good MM, Dillon SJ, Corton MM. Lowest colpopexy sacral fixation point alters vaginal axis and cul-de-sac depth. Am J Obstet Gynecol. 2013;208(6):488.e1–488.e6. doi: 10.1016/j.ajog.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 26.Summers A, Winkel LA, Hussain HK, DeLancey JOL. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057 [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousuf A, Chen L, Larson K, Ashton-Miller JA, DeLancey JOL. The length of anterior vaginal wall exposed to external pressure on maximal straining MRI: relationship to urogenital hiatus diameter, and apical and bladder location. Int Urogynecol J Pelvic Floor Dysfunct. 2014;25(10):1349–1356. doi: 10.1007/s00192-014-2372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLancey JOL, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 PART 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


