Abstract
To investigate the potential prognostic value of Serum cystatin C (sCys C) in patients with COVID-19 and determine the association of sCys C with severe COVID-19 illness. We performed a retrospective review of medical records of 162 (61.7 ± 13.5 years) patients with COVID-19. We assessed the predictive accuracy of sCys C for COVID-19 severity by the receiver operating characteristic (ROC) curve analysis. The participants were divided into two groups based on the sCys C cut-off value. We evaluated the association between high sCys C level and the development of severe COVID-19 disease, using a COX proportional hazards regression model. The area under the ROC curve was 0.708 (95% CI 0.594–0.822), the cut-off value was 1.245 (mg/L), and the sensitivity and specificity was 79.1% and 60.7%, respectively. A multivariable Cox analysis showed that a higher level of sCys C (adjusted HR 2.78 95% CI 1.25–6.18, p = 0.012) was significantly associated with an increased risk of developing a severe COVID-19 illness. Patients with a higher sCys C level have an increased risk of severe COVID-19 disease. Our findings suggest that early assessing sCys C could help to identify potential severe COVID-19 patients.
Subject terms: Viral infection, Risk factors
Introduction
In late December 2019, Wuhan, China, a highly infectious respiratory illness due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first reported. This infectious disease was later designated coronavirus disease 2019 (COVID-19)1. As of 8 September 2021, the COVID-19 pandemic has had an unprecedented impact on a global scale with over 220 million cases identified and over four million deaths2. Given the uncontrolled global spread of the COVID-19 pandemic, it is essential to find an easily accessible sensitive biomarker for predicting the severe COVID-19 disease.
Accumulating evidence has supported that COVID-19 comprises a systematic endothelial dysfunction3–5. In addition to the impaired respiratory function and immune system, the kidney might also be one of the main organs affected6. The severity of renal function impairment diverse, ranging from elevated blood urea nitrogen (Bun) or serum creatinine (sCr) levels, to acute kidney injury (AKI) and renal failure6,7. A meta-analysis8 has shown that the incidence of AKI was more prevalent in severe (2.8% [95% CI 1.4–4.2%]) or critical (36.4% [95% CI 14.6–58.3%]) COVID-19 patients than in mild or moderate cases (1.3% [95% CI 0.2–2.4%]), suggesting an association between renal impairment and COVID-19 severity. Cystatin C is a low molecular mass protein (13.3 KD) produced by most nucleated cells. Blood Cystatin C levels are not influenced by ingestion of meat and no tubular secretion of cystatin, and production of Cystatin C is influenced less by age, gender, and muscle mass9. Serum cystatin C (sCys C) is considered a more sensitive biomarker for early renal insufficiency than conventional indicators such as Bun and sCr10. To our knowledge, little is known about the prognostic value of sCys C for COVID-19 severity. We hypothesized that in patients infected with SARS-CoV-2 elevated sCys C increases the risk of developing severe illness. We therefore investigated the association between the sCys C and severe COVID-19 disease in this retrospective observational study.
Methods
Ethics approval and consent to participate
The ethics committee of Fujian Medical University Union Hospital approved the study protocol (NO.2020GFKY005). All clinical investigations were conducted based on the principles expressed in the declaration of Helsinki. Written informed consent was waived by the ethical committee due to the retrospective nature of our study of routine clinical data.
Study design and participants
This is a single-center, retrospective, observational study done at Tumor Center of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), a designated hospital to treat patients with COVID-19. We analyzed consecutive patients admitted between 15 February and 14 March 2020 because of a COVID-19 disease based on World Health Organization interim guidance11. Laboratory confirmation of COVID-19 infection was performed using Reverse Transcription-Polymerase Chain Reaction (RT-PCR) detection by the local health authority as previously described12.
Data collection and outcome measures
We retrospectively reviewed the electronic medical records of 168 consecutive eligible patients with COVID-19 using a digital database. We extracted the epidemiological, demographic, clinical, laboratory data on admission, the chest computed tomography (CT) image, and outcome data using a standardized data collection form. In cases of disagreement, we reached a consensus after team discussion. In case of missing or uncertain data, we obtained and clarified data by direct consulting with attending doctors and other healthcare providers. We constructed a vascular risk factor score based on the following well-documented vascular risk factors in each patient: hypertension, diabetes, dyslipidemia, atrial fibrillation, current smoker, overweight and physical inactivity13–17. Quick Sequential Organ Failure Assessment (qSOFA) was calculated based on data regarding systolic blood pressure, respiratory rate, and mental status within 24 h after admission18 Our primary outcome was severe COVID-19 disease defined as fever or suspected respiratory infection, plus one of: respiratory rate > 30 breaths/min; severe respiratory distress; or SPO2 ≤ 93% on room air based on the interim guidance of the World Health Organization11. Our secondary outcome was death, which was limited by the duration of our observation period. All the authors agreed on the study protocol and reviewed the manuscript.
Measurements of cystatin C, creatinine and Bun
Serum cystatin C was measured by a turbidimetric immunoinhibition assay using the cystatin kit (Tina-quant cystatin C Gen. 2, Roche) with an automatic biochemical analyzer (Cobas c-system, Roche, Switzerland). This method is traceable to a primary reference material with values assigned by the international cystatin C reference material (ERM-DA471/IFCC) as a calibrator19. This procedure had a total coefficient of variation of 2.2% at a cystatin C level of 1.0 mg/L and of 1.4% at a level of 4.0 mg/L. Reference range 0.55–1.09 mg/L. Serum creatinine was determined by a traceable method (enzymatic assay calibrated against the National Institute of Standards and Technology standard reference material [SRM 967]) with an automatic biochemical analyzer (Cobas c-system, Roche, Switzerland)20. The total coefficient of variation is < 4.0%, and the reference range is 44–133 μmol/L. Serum Bun was determined by an enzymatic method with an automatic biochemical analyzer (Cobas c-system, Roche, Switzerland) according to the kit protocol. This method is traceable to a primary reference material (SRM 909b). This procedure had a total coefficient of variation of 1.2% at a level of 7.2 mmol/L and of 0.7% at a level of 35.1 mmol/L. The reference range is 2.9–8.2 mmol/L.
Statistical analysis
We summarized continuous and categorical variables as median (interquartile range, IQR) and numbers (percentages), respectively. We used the t-test or Mann–Whitney test to compare differences in continuous variables, and the chi-square test or Fisher's exact test to compare differences in categorical variables where appropriate. The classification performance of sCys C, Bun and sCr to discriminate between severe and non-severe cases was evaluated by calculating the area under the ROC curve (AUROC) and its 95% confidence intervals (95% CI). We defined the score with the largest Youden Index as the optimal cut-off value for predicting COVID-19 severity. Based on the optimal cutoff value, we calculated the sensitivity and specificity. The differences between AUROC were evaluated by using a method as previously described21. We divided patients into higher and lower group based on sCysC levels above and below the cutoff value. We calculated the absolute event rate per 1000 patient-days for severe COVID-19 illness and death during our observation period. Kaplan–Meier curves depicted the risk for outcome events stratified by sCys C levels (higher vs lower). We implemented univariable and multivariable COX proportional hazards regression models to examine the association between sCys C level and the risk of developing a severe COVID-19. For multivariate analysis, to avoid overfitting in the model, we chose the variables based on previous findings and clinical constraints. Previous studies have shown older age, qSOFA, vascular risk factor burden, and several laboratory abnormal findings to be associated with an increased risk of severe COVID-1913,18. Therefore, we chose qSOFA, vascular risk factor burden, decreased lymphocyte, and increased lactate dehydrogenase (LDH) in addition to age and sex for our multivariable regression model. All analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX) and SPSS for Windows (SPSS 25.0, IBM, Inc., Chicago, IL, USA).
Results
Baseline difference between patients with severe and non-severe COVID-19
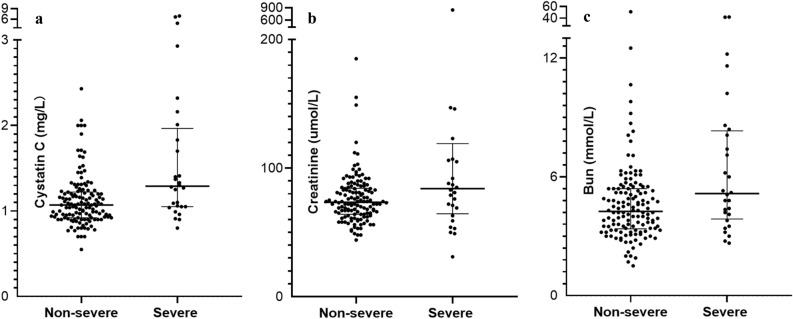
We admitted 168 consecutive patients with COVID-19 from 15 February through 14 March 2020. After excluded four suspected patients and two cases with incomplete data, we included 162 laboratory-confirmed COVID-19 patients (61.7 ± 13.5 years) in the final analysis. Table 1 summarized the demographics, clinical and radiological characteristics in patients with severe COVID-19 illness and non-severe COVID-19 illness. Patients with severe COVID-19 disease were not significantly different from those with non-severe illness regarding their most previous history, exposure to wet seafood market, and onset symptoms. Patients with severe disease were older (70.1 ± 12.7 vs 59.8 ± 13.0, p < 0.001), more likely to be male (20 [71.4%] vs 64 [47.8%], p = 0.023), and more likely to have pre-existent cardio-cerebrovascular disease (8 [28.6%] vs 15 [11.2%], p = 0.017). Regarding routine blood test findings, patients with severe COVID-19 disease were more likely to have decreased lymphocytes (64.3% vs 26.1%, p < 0.001) and increased LDH level (60.7% vs 24.6%, p < 0.001). Supplementary Figs. 1a, 1b, and 1c show the distribution of sCys C, creatinine and Bun levels in severe and non-severe COVID-19 patients. sCys C was significantly increased in patients with severe COVID-19 compared to non-severe patients (1.28 [1.05–1.83] mg/L vs 1.07 [0.92–1.22], p = 0.001, Fig. 1a). Figure 1b,c show the serum levels of creatinine and Bun were higher in severe than in non-severe COVID-19 patients. Regarding chest CT findings, patients with severe COVID-19 illness were more likely to be bilaterally affected (p = 0.024).
Table 1.
Characteristics at baseline among severe and non-severe COVID-19 patients.
| Total (n = 162) | Severe (n = 28) | Non-severe (n = 134) | P-value | |
|---|---|---|---|---|
| Age, (y) mean ± SD | 61.7 ± 13.5 | 70.1 ± 12.7 | 59.8 ± 13.0 | < 0.001 |
| Male, n (%) | 84 (51.9) | 20 (71.4) | 64 (47.8) | 0.023 |
| Current smoker, n (%) | 17 (10.5) | 2 (7.1) | 15 (11.2) | 0.766 |
| Often drinker, n (%) | 3 (1.9) | 0 | 3 (2.2) | > 0.999 |
| Hypertension, n (%) | 50 (30.9) | 12 (42.9) | 38 (28.4) | 0.131 |
| Diabetes, n (%) | 30 (18.5) | 7(25.0) | 23 (17.2) | 0.332 |
| COPD, n (%) | 12 (7.4) | 4 (13.4) | 8 (6) | 0.258 |
| Cardio-cerebrovascular disease, n (%) | 23 (14.2) | 8 (28.6) | 15 (11.2) | 0.017 |
| Digestive disease, n (%) | 15 (9.3) | 1 (3.6) | 14 (10.4) | 0.433 |
| Previous tumor, n (%) | 13 (8.0) | 3 (10.7) | 10 (7.5) | 0.847 |
| Immunosuppresive, n (%) | 3 (1.9) | 1 (1.6) | 2 (1.5) | > 0.999 |
| Wet market exposure, n (%) | 2 (1.2) | 1 (3.6) | 1 (0.7) | 0.772 |
| Clinical manifestion | ||||
| Fever, n (%) | 114 (70.4) | 19 (67.9) | 95 (70.9) | 0.749 |
| Dry cough, n, (%) | 103 (63.6) | 19 (67.9) | 84 (62.7) | 0.605 |
| Productive cough, n (%) | 23 (14.2) | 2 (7.1) | 21 (15.7) | 0.38 |
| Fatigue, n (%) | 56 (34.6) | 9 (32.1) | 47 (35.1) | 0.767 |
| Musle or joint ache, n (%) | 21(13.0) | 2(7.1) | 19 (14.2) | 0.485 |
| Thoracalgia, n (%) | 31(19.1) | 6(21.4) | 25(18.7) | 0.735 |
| Sore throat, n (%) | 23 (14.2) | 4 (14.3) | 19 (14.2) | > 0.999 |
| Diarrhea, n (%) | 12 (7.4) | 3 (10.7) | 9 (6.7) | 0.735 |
| Catarrh, n (%) | 6 (3.7) | 0 | 6 (4.5) | 0.591 |
| Anorexia, n (%) | 47 (29.0) | 28 (28.6) | 39 (29.1) | 0.955 |
| Short of breath, n (%) | 65 (40.1) | 15 (53.6) | 50 (37.3) | 0.11 |
| Headache, n (%) | 19 (11.7) | 3 (10.7) | 16 (11.9) | > 0.999 |
| Total symptoms, (IQR) | 3 [2–4] | 3 [2–4] | 3 [2–4] | 0.815 |
| Regular blood test | ||||
| WBC × 109/L (IQR) | 5.4 [4.4–7.3] | 5.2 [3.9–10.0] | 5.4 [4.4–7.1] | 0.643 |
| Decreased WBC, n (%) | 10 (6.2) | 4 (14.3) | 6 (4.5) | 0.126 |
| Lymphocytes × 109/L (IQR) | 1.4 [0.9–1.7] | 0.8 [0.6–1.4] | 1.4 [1.1–1.7] | < 0.001 |
| Decreased lymphocytes, n (%) | 53 (32.7) | 18 (64.3) | 35 (26.1) | 0.001 |
| sCys C (mg/L) | 1.08 [0.94–1.28] | 1.28 [1.05–1.83] | 1.07 [0.92–1.22] | 0.001 |
| Bun (mmol/L) | 4.4 [3.4–5.5] | 5.1 [3.8–8.4] | 4.3 [3.9–5.4] | 0.014 |
| sCr (umol/L) | 74 [64–87] | 83 [63–107] | 74 [64–84] | 0.042 |
| LDH (U/L) | 197 [164–264] | 271 [203–338] | 187 [160–244] | < 0. 001 |
| Increased LDH, n (%) | 50 [30.9] | 17 [60.7] | 33 [24.6] | < 0. 001 |
| CT findings, n (%) | 0.024 | |||
| Unilateral pneumonia, n (%) | 25 (15.4) | 2 (7.1) | 23 (17.2) | |
| Bilateral pneumonia, n (%) | 85(52.5) | 11 (39.3) | 74 (55.2) | |
| Multiple mottling and Ground-glass opacity, n (%) | 52 (32.1) | 15 (53.6) | 37 (27.6) | |
Legend and abbreviations: COVID-19 = coronavirus disease 2019; SD = Standard deviation; COPD = Chronic obstructive pulmonary disease; IQR = Interquartile range; WBC = white blood cell (109/L; reference range 3.5–9.5); Lymphocytes (× 109/L; reference range 1.1–3.2); sCys C = serum cystatin C (mg/L, reference range 0.55–1.09); Bun = blood urea nitrogen (mmol/L; reference range 2.9–8.2), sCr = serum creatinine (μmol/L; reference range 44–133), LDH = Lactate dehydrogenase (U/L; reference range 109–245); CT = Computed tomography
Figure 1.
Compariosn of serum levels of cystatin C, creatinine, and Bun in COVID-19 patients. (a) Serum level of cystatin C in severe and non-severe COVID-19 patients; (b) Serum level of creatinine in severe and non-severe COVID-19 patients; (c) Serum level of Bun in severe and non-severe COVID-19 patients; Abbreviations: COVID-19 = coronavirus disease 2019, Bun = blood urea nitrogen, sCr = serum creatinine.
Receiver operating characteristic curve analysis
The ROC curves of sCys C value for predicting severe COVID-19 illness was shown in Fig. 2. sCys C resulted numerically in a higher AUROC 0.708 (95% CI 0.594–0.822) than Bun and sCr; 0.622 (95% CI 0.482–0.763) and 0.647 (95% CI 0.523–0.771), respectively (Table 2). The difference was statistically different (p = 0.037). Based on the largest Youden Index (0.398), an optimum cut-off value of 1.245 (mg/L) was used to predict severe COVID-19 illness by using the sCys C, with a sensitivity and specificity of 79.1% and 60.7%, respectively.
Figure 2.
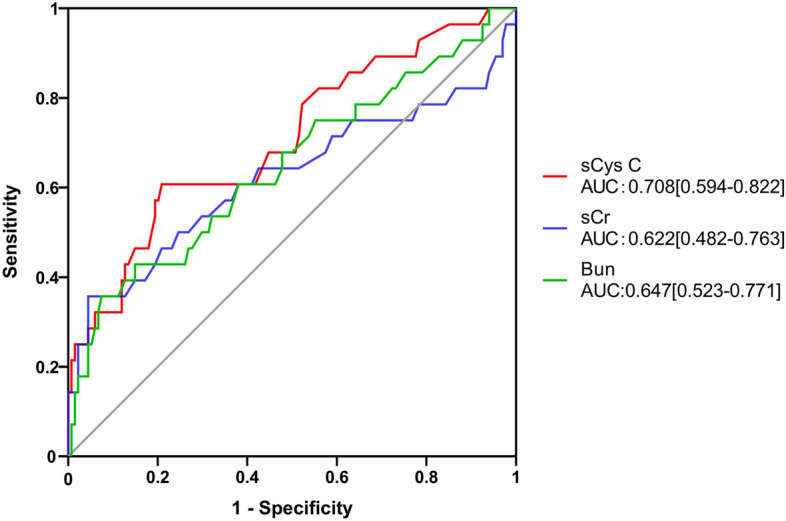
Predictive performances of sCys C, Bun, and sCr using ROC analysis. Comparison of predictive performances of sCys C, Bun, and sCr. Abbreviations: COVID-19 = coronavirus disease 2019, sCys C = serum cystatin C, Bun = blood urea nitrogen, sCr = serum creatinine, ROC = receiver operating characteristic curve.
Table 2.
Predictive performance of sCys C, Bun and sCr for severe COVID-19.
| AUC | 95% CI | P value | |
|---|---|---|---|
| sCys C | 0.708 | 0.594–0.822 | 0.037* |
| Bun | 0.622 | 0.482–0.763 | |
| sCr | 0.647 | 0.523–0.771 |
Abbreviations: sCys C = serum cystatin C; Bun = blood urea nitrogen, sCr = serum creatinine, COVID-19 = coronavirus disease 2019, AUROC = Area under the receiver operating characteristic curve;
*P = 0.037 (Comparison of AUROC of sCys C, Bun and sCr).
Survival analysis and COX proportional hazards regression analysis
The median follow-up time was 42 days [IQR 35–49], providing 6430 patient-days of data. Twenty-eight patients (17.3%) developed severe COVID-19 illness with a median (14 [7–25] days) duration between symptom onset to developing a severe disease, an event rate of 4.35 per 1000-patient days (95% CI 3.01–6.31). Six patients (3.7%) died during hospitalization, an event rate of 0.85 per 1000-patient days (95% CI 0.38–1.90). Kaplan–Meier curves showed an increase in the risk of severe COVID-19 illness (log rank p < 0.001, Fig. 3). In univariable analysis, a higher sCys C level was associated with an increased risk of severe COVID-19 (unadjusted HR 4.95, 95% CI 2.31–10.57). After adjustment for age and sex, a higher sCys C level remained significantly associated with severe COVID-19 (adjusted HR 3.04 95% CI 1.30–7.13). This relationship did not change after additional adjustment for decreased lymphocyte, increased LDH, qSOFA or vascular risk factor burden along with age and sex (Table 3). A sensitivity analysis excluding three patients who reported previous renal disease resulted in similar findings (Table 4).
Figure 3.
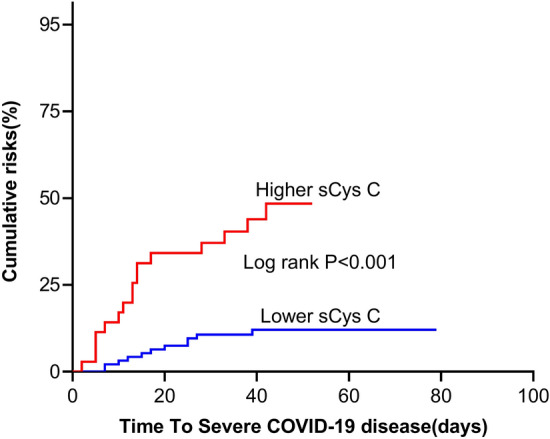
Cumulative probability of severe COVID-19 disease stratified by sCys C level. Higher level means > 1.245 (mg/L); Lower level level means < 1.245 (mg/L). Abbreviations: COVID-19 = coronavirus disease 2019; sCys C = serum cystatin C.
Table 3.
Association between higher sCys C level and severe COVID-19 illness.
| Unadjusted | Adjusted for age and sex | Multivariable-adjusted mode l | Multivariable-adjusted model 2 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| 4.95 [2.31–10.57] | < 0.001 | 3.04 [1.30–7.13] | 0.01 | 2.78 [1.25–6.18] | 0.012 | 2.75 [1.23–6.14] | 0.014 |
Abbreviations: sCys C = serum cystatin C; COVID-19 = coronavirus disease 2019; HR = Hazards ratio;
Multivariable Model 1: Adjusted for age, sex, increased lactate dehydrogenase, decreased lymphocyte, and qSOFA (Quick Sequential Organ Failure Assessment).
Multivariable Model 2: Adjusted for age, sex, increased lactate dehydrogenase, decreased lymphocyte, and vascular risk factor burden.
Table 4.
Association between higher sCys C level and severe COVID-19 illness in a sensitivity analysis.
| Unadjusted | Adjusted for age and sex | Multivariable-adjusted mode l | Multivariable-adjusted model 2 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| 4.80 [2.30–10.38] | < 0.001 | 3.07 [1.30–7.27] | 0.01 | 2.80 [1.24–6.31] | 0.013 | 2.70 [1.20–6.05] | 0.016 |
Abbreviations: sCys C = serum cystatin C; COVID-19 = coronavirus disease 2019; HR = Hazards ratio;
Multivariable Model 1: Adjusted for age, sex, increased lactate dehydrogenase, decreased lymphocyte, and qSOFA (Quick Sequential Organ Failure Assessment).
Multivariable Model 2: Adjusted for age, sex, increased lactate dehydrogenase, decreased lymphocyte, and vascular risk factor burden.
Discussion
The early and fast evaluation of severely ill COVID-19 patients is paramount to ensure early medical monitoring and interventions for these patients due to the medical resource constraints during the COVID-19 pandemic. Our most important finding is that in COVID-19 patients a higher sCys C level is associated with an increased risk of experiencing severe COVID-19 illness. Our results highlighted the considerable predictive performance of sCys C for severe COVID-19 disease on ROC curve analysis. At the time of admission, a sCys C value over 1.245 (mg/L) was highly predictive of developing a severe COVID-19 disease. Our findings were in line with a previous hospital-based study that showed the highest baseline sCys C level was associated with more severe inflammatory status and unfavorable outcomes among COVID-19 patients22. These findings suggest that sCys C could serve as a potential inflammatory target for preventing COVID-19 from the likely progression of critical illness and mortality, in addition to representing early renal insufficiency. Since the sCys C is generally readily available at hospital admission, our findings may contribute to identify COVID-19 patients with poor prognosis at an early stage.
Our ROC analysis showed that sCys C had a better predictive performance for severe COVID-19 than two conventional renal function indicators (Bun and sCr). Accumulating evidence has demonstrated that Bun and sCr levels might not be good indicators for the early detection of renal injury23,24. sCys C is emerging as a novel index with a high sensitivity and specificity for evaluating renal function, independently of age, sex, weight, inflammation, and other factors25,26. The renal clearance of 5–40 kDa molecules like cystatin C (13.3 KDa) is decreased more than that of low molecules like creatinine (0.133 kDa)27. Since cystatin C is mainly excreted via glomerular transport, a reduction in their glomerular filtration rate would result in a simultaneous increase of sCys C levels. Previous studies suggest that a decrease in the pore diameters of the functional pores might account for the increased sCys C levels28,29. Recently, researchers from Lund University (Sweden) proposed the designation ‘Shrunken pore syndrome’ to reflect a reduced pore size of the glomerular membranes, which might help explain the superiority of cystatin C as a predictor of mortality30,31. In line with a previous study32, our findings that sCys C was significantly increased in patients with severe COVID-19 compared to those with non-severe COVID-19 illness (1.28 [1.05–1.83] vs 1.07 [0.92–1.22]) indicate that severe SARS-CoV-2 infection might damage the kidney. Our findings were supported by a previous hospital-based observational study that showed COVID-19 patients with renal involvement had a mortality of 11.2%, compared to 1.2% in those without renal involvement33. These results suggest that renal complications in COVID-19 were associated with higher mortality.
Our findings have important implications for early COVID-19 prevention and treatment. For example, hydroxychloroquine is a safe and highly tolerable approach with minimum side effects. A retrospective longitudinal cohort study showed that hydroxychloroquine treatment (OR 3.891, 95% CI 1.196–12.653) was associated with renal function recovery in patients with lupus nephritis34. A large-sampled multicentered observational study has shown that the exposure of hydroxychloroquine is associated with a decreased risk of hospitalization from COVID-19 (OR 0.53; 95% CI 0.29–0.95)35. Additional, findings from some recent random-controlled studies raise the concern about whether dexamethasone treatment could improve the outcome of COVID-1936,37 Whether patients with higher sCys C levels might benefit form early hospital treatment measures such as hydroxychloroquine or dexamethasone need to be addressed in future large-scale studies.
The underlying mechanisms of the association between sCys C level and COVID-19 severity remain unclear. SARS-CoV-2 infection may directly cause endothelial damage, infects renal tubular epithelium and podocytes, causing mitochondrial dysfunction and acute tubular necrosis. This process is mediated through the angiotensin-converting-enzyme 2 (ACE2) dependent pathway38,39 Moreover, available evidence suopport that kidney impairment in COVID-19 patients may be caused by an interplay of virus-mediated injury, a dysregulated inflammatory response, hypercoagulation, and microangiopathy40. A severe inflammatory process during SARS-CoV-2 infection might also probably induce increased levels of cystatin C similar in size to cytokines like interleukin-6. This hypothesis is supported by a previous study showing that sCys C level was positively correlated with inflammatory indicators such as IL-6, tumor necrosis factor-α, and hypersensitive C-reactive protein41. These aforementioned inflammatory indicators have been reported to be related to the COVID-19 severity42,43. The underlying interaction between circulating cystatin C and viral infection may provide insight into our better understanding of pathophysiological events in COVID-19.
The results of the present study should be interpreted within the constraints of its limitations. First, we conducted this retrospective study at a single-centered hospital with limited sample size. Moreover, we retrospectively collected data from medical records and laboratory data regarding prior renal function in some patients was not accessible. Therefore, we may underestimate the true overall incidence of COVID-19-associated elevated sCys C. However, a sensitivity analysis excluding those patients who reported previous renal disease did not alter the association between a higher sCys C level and a higher COVID-19 risk. Notably, we did not include data regarding urine output, a defining characteristic of acute kidney insufficiency. Therefore, more studies with a broad geographic scope are needed to get a more comprehensive understanding of role of renal impairment in COVID-19. Our strength include the association of sCys C with the COVID-19 severity was verified using previous well-validated cofounders in consecutive laboratory-confirmed participants.
Conclusions
Our findings that COVID-19 patients with a higher sCys C level were at an increased risk suggest that this population needs early prevention and treatment.
Supplementary Information
Acknowledgements
We thank all the patients with their data for this analysis and the medical workers who are on the front line of caring for patients.
Author contributions
Drs H.-W.D. and J.-N.C. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Drs L.-F.L. and X.-L.C. contributed equally to this work and shared first authorship. Study concept and design: Drs L.-F.L. and H.-W.D. Acquisition, analysis, or interpretation of data: Drs L.-F.L., H.-W.D, X.-L. C., J-N. Chen, H.-L. L. and P.-C.X. Drafting of the manuscript: Drs L.-F.L., X.L.-C., and H.-W.D. Critical revision of the manuscript for important intellectual content: Drs. J C, H.-L. L. and P.-C. X.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request (Email: houweidu@fjmu.edu.cn).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luanfeng Lin and Xiaoling Chen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01570-2.
References
- 1.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus resource center. https://coronavirus.jhu.edu/map.html. Accessed 8 Sept 2021.
- 3.Kefale B, Tegegne GT, Degu A, Tadege M, Tesfa D. Prevalence and risk factors of thromboembolism among patients with coronavirus disease-19: A systematic review and meta-analysis. Clin. Appl. Thromb. Hemost. 2020;26:1076029620967083. doi: 10.1177/1076029620967083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amraei R, Rahimi N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells. 2020;9:1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froldi G, Dorigo P. Endothelial dysfunction in Coronavirus disease 2019 (COVID-19): Gender and age influences. Med Hypotheses. 2020;144:110015. doi: 10.1016/j.mehy.2020.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahid U, et al. Acute kidney injury in COVID-19 patients: An inner city hospital experience and policy implications. Am. J. Nephrol. 2020;51:786–796. doi: 10.1159/000511160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020;17:e1003406. doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, et al. Prevalence and impact of acute renal impairment on COVID-19: A systematic review and meta-analysis. Crit Care. 2020;24:356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 2015;68:57–69. doi: 10.1016/bs.acc.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Coll E, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am. J. Kidney Dis. 2020;236:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance. https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (2020). January 28, 2020. Accessed 5 Mar 2020.
- 12.Du HW, et al. Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur. J. Clin. Microbiol. 2021;40:413–417. doi: 10.1007/s10096-020-04024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du HW, et al. The effect of vascular risk factor burden on the severity of COVID-19 illness, a retrospective cohort study. Respir. Res. 2020;21:241. doi: 10.1186/s12931-020-01510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li GJ, et al. Association of prehypertension and cardiovascular risk factor clustering in inner mongolia: A cross-sectional study. BMJ Open. 2017;7:e0153401010. doi: 10.1136/bmjopen-2016-015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emdin CA, et al. Usual blood pressure, atrial fibrillation and vascular risk: Evidence from 4.3 million adults. Int. J. Epidemiol. 2017;46:162–72. doi: 10.1093/ije/dyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, et al. Prevalence, incidence, and mortality of stroke in china: Results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 18.Liu SJ, Yao N, Qiu Y, He C. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am. J. Emerg. Med. 2020;38:2074–2080. doi: 10.1016/j.ajem.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubb A, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem Lab. Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 20.Carobene A, Ceriotti F, Infusino I, Frusciante E, Panteghini M. Evaluation of the impact of standardization process on the quality of serum creatinine determination in Italian laboratories. Clin. Chim. Acta. 2014;427:100–106. doi: 10.1016/j.cca.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Hanley J, McNeil B. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, et al. Serum cystatin C and coronavirus disease 2019: A potential inflammatory biomarker in predicting critical illness and mortality for adult patients. Mediators Inflamm. 2020;2020:3764515. doi: 10.1155/2020/3764515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra R, Siew ED. Biomarkers for the early detection and prognosis of acute kidney injury. Clin. J. Am. Soc. Nephrol. 2017;12:149–173. doi: 10.2215/CJN.01300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017;55:1074–1089. doi: 10.1515/cclm-2016-0973. [DOI] [PubMed] [Google Scholar]
- 25.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann. Clin. Biochem. 2020;37:49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 26.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand. J. Clin. Lab. Invest. 1999;59:587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 27.Grubb A. Shrunken pore syndrome—a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin. Biochem. 2020;83:12–20. doi: 10.1016/j.clinbiochem.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Oberbauer R, et al. Reduction in mean glomerular pore size coincides with the development of large shunt pores in patientswith diabetic nephropathy. Exp. Nephrol. 2001;9:49–53. doi: 10.1159/000020698. [DOI] [PubMed] [Google Scholar]
- 29.Rippe C, et al. Effects of glomerular filtration rate on Ficoll sieving coefficients (theta) in rats. Kidney Int. 2006;69:1326–1332. doi: 10.1038/sj.ki.5000027. [DOI] [PubMed] [Google Scholar]
- 30.Akesson A, et al. Shrunken pore syndrome and mortality: A cohort study of patients with measured GFR and known comorbidities. Scand J. Clin. Lab. Invest. 2020;80:412–422. doi: 10.1080/00365513.2020.1759139. [DOI] [PubMed] [Google Scholar]
- 31.Grubb A, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: 'Shrunken pore syndrome'. Scand. J. Clin. Lab. Invest. 2015;75:333–340. doi: 10.3109/00365513.2015.1025427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang, J.L. et al.. Potential biochemical markers to identify severe cases among COVID-19 patients. https://www.medrxiv.org/content. 10.1101/2020.03.19.20034447v1 (2020).
- 33.Pei G, et al. Renal involvement and early prognosis in patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. 2020;2020(31):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, et al. Recovery of renal function in patients with lupus nephritis and reduced renal function: The beneficial effect of hydroxychloroquine. Lupus. 2020;29:52–57. doi: 10.1177/0961203319890007. [DOI] [PubMed] [Google Scholar]
- 35.Mohana A, et al. Hydroxychloroquine safety outcome within approved therapeutic protocol for COVID-19 outpatients in Saudi Arabia. Int. J. Infect. Dis. 2021;102:110–114. doi: 10.1016/j.ijid.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar J, et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 37.Tomazini BM, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batlle D, et al. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akerfeldt T, Helmersson J, Larsson A. Postsurgical inflammatory response is not associated with increased serum cystatin C values. Clin. Biochem. 2010;43:1138–1140. doi: 10.1016/j.clinbiochem.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElvaney OJ, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request (Email: houweidu@fjmu.edu.cn).