Abstract
The incidence of human papillomavirus (HPV)-associated cancers, especially those from the head and neck region, has increased. The relatively early age of presentation of HPV-positive head and neck cancer (HNC) indicates that viral infection might be acquired early in life. Persistent HPV infection has been recognized as the main risk factor for cancer development, but most studies have focused on evaluating HPV persistence in the genital region. Thus, in this work, we aimed to evaluate the prevalence of HPV in oral cavity and oropharynx in a young population, as well as the possible persistence of the infection after 12 months. Our results indicate that almost half (46.8%) of the analyzed population harbors an HPV infection either in the oral cavity or in the oropharynx. Furthermore, after 1 year of initial identification, half of them eliminated the infection, and only one person (5.26%) exhibited persistence. Interestingly, 50% of the individuals who successfully eliminated the infection acquired a new viral type, indicating that even when the primary infection is effectively eliminated by the immune system, there is a dynamic circulation of HR-HPV types that produce reinfection. This dynamic HPV infection among young individuals could influence the future establishment of cancer in some proportion of the cases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00602-3.
Keywords: HPV infection, HPV persistence, Young population, Oral cavity, Oropharynx, Reinfection
Introduction
Human papillomavirus (HPV) is the etiologic agent of most common sexually transmitted disease in the population worldwide [1]. Although the vast majority of HPV infections are transient and become undetectable within 12–24 months after onset, sometimes the viral infection becomes persistent due to the ability of HPV to avoid the immune response [2]. Consequently, 10–20% of infections become chronic or persistent, representing the primary requirement for the development of cancer [3]. Among the different mechanisms orchestrated by HPV to avoid immune elimination, the negative regulation of interferon expression and the overproduction of IL-10 and transforming growth factor (TGF)-β1 form an immunosuppressive network that inhibits antiviral and antitumor immune responses [4].
HPVs are double-stranded DNA viruses belonging to Papillomaviridae family that infect basal epithelial cells from different mucosal regions including vaginal, penile, oral, and anal zones [5]. HPV causes almost all cervical cancer cases and 25% of head and neck cancer (HNC) cases, particularly those originating in the oropharyngeal region, where the virus has been found in up to 70% of the cases [6]. Currently, more than 200 HPV types have been identified, and have been divided into two groups according to their potential to produce neoplasia: low-risk HPV genotypes, mostly associated with genital warts and recurrent respiratory papillomatosis, which include types 6, 11, 40–44, and 54; and high-risk HPV genotypes including types 16, 18, 26, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 70, 73, and 82, designated as oncogenic and capable of producing cancer [7].
All high-risk genotypes have been detected in the head and neck zone, but most infections are cleared in healthy individuals [8]. However, the number of HPV-positive (HPV +) HNC cases has increased. HPV16 and 18 are the most common in HPV + HNC, followed by the other oncogenic types [9, 10].
Squamous cell carcinoma represents 90% of HNC. These malignancies comprise a heterogeneous group that arises from epithelial cells of the mucous lining of the upper aerodigestive tract which encompass the hypopharynx, nasopharynx, larynx, oropharynx, and the oral cavity [11]. Head and neck squamous cell carcinoma (HNSCC) has an estimated annual incidence of 890,000 cases worldwide with a 50% associated mortality, ranking as the sixth most common cancer worldwide. Oral and oropharynx carcinomas account for about 53.63% of the total HNC cases [12]. These tumors have a strong etiological link with alcohol and tobacco consumption [13]. However, in recent decades, high-risk (HR) HPV infection has been identified as an important and independent risk factor for developing HNSCC. High frequency of oral sex and a high number of sexual partners increase the risk of HPV-related HNSCC [14]. However, HPV infection has been little studied in Mexico in HNC cases, finding a positivity of 43% and HPV16 being the most prevalent type [15, 16].
Regarding the prevalence of HPV in oral and oropharyngeal squamous cell carcinoma (OPSCC), a wide range of data has been reported [6]. However, in recent years, the prevalence of HPV in OPSCC has increased alarmingly, reporting up to 80% positivity for this type of cancer [17]. Although the prevalence of HPV in the genital region is high, infection in the oral cavity region has been less studied, showing a prevalence ranging from 0.1 to 19.3% [5, 18, 19] in different populations, including healthy individuals [20], vaccinated population [21], or population presenting other diseases [22]. Interestingly, 60% HPV positivity was found in the oral mucosa of individuals older than 50 [23]. These studies have focused on evaluating the prevalence at a single time point; however, it is well recognized that HPV oral infection, especially with high-risk genotypes, establishes a predisposition for HNC cancer development. Thus, prospective studies aimed at evaluating viral persistence are needed.
One of the characteristic features of HPV-induced HNSCC is the relatively early age of presentation [24], indicating that this type of cancer might be associated with an HPV infection acquired at an early age. In many countries, the age of debut of sexual activity has diminished in the past decades. In Mexico, more than 50% of the population had the first sexual experience at a mean age of 17.5 and almost 60% did not use a prophylactic method, increasing the risk of acquiring infections [25]. Furthermore, higher incidence of oral sex has been observed as the educational level rises, increasing the risk of presenting an oral HPV-associated lesion [26]. In this sense, the university population represents a group vulnerable to acquiring HPV infection, due to school coexistence and sexual practices. In this study, we determined the prevalence of HPV in oral cavity and oropharynx in a university population cohort and evaluated the persistence after 12 months, in order to understand the dynamics of HPV infection in this particular population.
Methods
Study population and data collection
This prospective study recruited 103 volunteers from a public university in Puebla, Mexico, including students and workers of both sexes aged 18 years and older, to detect the prevalence of HPV infection. Population was categorized according to age into young adults (18–35) and middle-aged adults (36–55). After 12 months of the initial detection, HPV-positive cases were retested to assess viral persistence.
The participants were included in our analysis after answering a questionnaire to collect sociodemographic data and determine any risk factors associated with HNSCC, such as sexual practices and alcohol and tobacco consumption. Sample collection periods were Jan-Jun 2018 for prevalence, and Jan-Jun 2019 for assessing persistence.
Alcohol consumption was determined according to the number, frequency, and type of drinks reported in the questionnaires and divided into low risk, hazardous, and harmful [27]. Additionally, tobacco consumption was calculated based on cigarettes per week and categorized into light, moderate, and heavy smokers [28]. The study was approved by the Institutional Board (reference VIEP 00104) and all participants provided written informed consent before enrolling in the study.
Sampling
Two independent samples were collected using a Cytobrush®, one from the oral cavity and one from the oropharynx, in individuals who avoided oral hygiene one night before collection and with no signs of apparent disease. Cells from the oral cavity were collected by oral brushing, as mucosal scrapings from the internal and external faces of the upper and lower lips, the gingival region, and the anterior and posterior faces of the tongue.
For sampling the oropharynx, the participant was told to tilt the head back with the mouth open and a tongue depressor was introduced to push the tongue down, and the oropharynx was immediately scraped with the brush along the posterior wall of the oropharynx, base of the tongue, tonsils, and soft palate, avoiding the tongue and uvula. Collected cells were placed on slides and fixed for Papanicolaou staining, and the remaining sample was placed in a microtube and centrifuged to obtain a cell pellet.
To evaluate persistence, a second sampling was performed after 12 months in the same manner, only on patients who were HPV positive in the first sampling, either in the oral cavity or in the oropharynx. In addition, a blood sample was taken to assess the blood cell count and biochemistry, as well as IL-10 levels. All cytological swabs were maintained at 4 °C and processed for extraction within the next week. Blood samples were processed the same day of collection.
Papanicolaou staining
Immediately after collection of all oral cavity and oropharynx samples, a smear was performed on a clean slide and fixed with alcohol solutions for 5 min. Slides were stained following the Papanicolaou standard technique, which has been proven effective for evaluation of cytological alterations in this anatomical region, [29–31] and evaluated by two independent pathologists in order to assess pathognomonic signs associated with HPV infection.
HPV detection and genotyping
DNA was extracted and purified using the DNeasy Blood & Tissue Kit (QIAGEN®), following the manufacturer’s instructions, and quantified in a full spectrum Nanodrop® spectrophotometer (Thermo Scientific®). A total of 50 ng of DNA was subjected to β-globin amplification to determine DNA integrity and quality control. After evaluating integrity, HPV detection was performed by amplifying the L1 region using the MY09/11 universal primers through conventional PCR (Supplementary Table 1) [32, 33].
HPV genotype was determined using the commercial kit “MPCR Kit for Human Papilloma Virus Set 2” (Maxim Biotech®) following the manufacturer’s instructions. This kit is based on a multiplex conventional PCR that detects 8 different HPV types (low risk: 6 and 11; high risk: 16, 18, 31, 33, 52, and 58) by amplifying a region of the E6 gene. DNA from HeLa cells was used as a positive control and mixtures without DNA were used as negative controls. PCR conditions are described in Supplementary Table 2. The amplified fragments were analyzed by agarose gel electrophoresis and visualized using a UV light transilluminator (BIO-RAD®). The images were captured with a NIKON® Coolpix P100 camera.
IL-10 quantification
IL-10 levels were analyzed by ELISA using the “Human IL-10 ELISA” kit (ThermoFisher Scientific®) according to the manufacturer’s instructions. A total of 100 μL of serum was used and the level of IL-10 in the samples was obtained by comparison with a standard calibration curve.
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics for Mac, version 25 (IBM Corp., Armonk, NY, USA). Qualitative features are presented as absolute and relative frequencies, and quantitative features are presented as mean ± SD. The association between the presence of HPV and the different risk factors was analyzed using contingency tables to which the chi-square test (χ2) or Fisher’s test was applied. The relative risk was calculated when a result was significant.
Results
Basal features of participants
A total of 103 individuals were initially sampled, and after DNA integrity analyses and exclusion of one participant who was older than 56 years old, we focused on 94 remaining participants. Overall, data analysis included 41 men (43.2%) and 53 women (56.8%) with a mean age of 24.12 ± 8.19 years old. Most of the included individuals were young adults aged 18–35 years old (90.42%) and nine (9.57%) were middle-aged adults between 36 and 55 years old; thus, we categorized the population into two groups according to age. Demographic characteristics of the two groups are indicated in Table 1. Young adults have a mean age of 21.68 ± 3.35 while middle-aged adults were 43.11 ± 5.64 years old.
Table 1.
Sociodemographic characteristics and association between presence of HPV and risk factors of participants at basal sampling and 12 months after HPV detection
| Variable | Young adults | Middle-aged adults | Young and middle-aged adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time at sampling | Time zero | Time zero | 12 months later | ||||||||
| n | 85 | 9 | 19 | ||||||||
| Age range | 18–35 | 36–55 | 20–49 | ||||||||
| Mean age (SD) | 21.68 (3.35) | 43.11 (5.64) | 25.84 (9.88) | ||||||||
| Sociodemographic characteristics | HPV infection | p | Sociodemographic characteristics | HPV infection | p | HPV infection | p | ||||
| Pre | Abs | Pre | Abs | Pre | Abs | ||||||
| Sex n (%) | |||||||||||
|
Male Female |
37 (43.52) 48 (56.47) |
21 (24.70) 20 (23.52) |
16 (18.82) 28 (32.94) |
0.19 |
4 (44.44) 5 (55.55) |
1 (11.11) 2 (22.22) |
3 (33.33) 3 (33.33) |
> 0.99 |
9 (47.36) 1 (5.26) |
7 (36.84) 2 (10.52) |
0.58 |
| Tobacco consumption n (%) | |||||||||||
|
Light Moderate/heavy |
85 (100) 0 |
41 (48.23) 0 |
44 (51.76) 0 |
> 0.99 |
8 (88.88) 1 (11.11) |
2 (22.22) 1 (11.11) |
6 (66.66) 0 |
0.33 |
5 (26.31) 5 (26.31) |
5 (26.31) 3 (3.52) |
0.64 |
| Alcohol consumption n (%) | |||||||||||
|
Low risk Hazardous/harmful |
59 (69.41) 26 (30.58) |
29 (34.11) 12 (14.11) |
30 (35.29) 14 (16.47) |
0.79 |
7 (77.77) 2 (22.22) |
2 (22.22) 1 (11.11) |
5 (55.55) 1 (11.11) |
> 0.99 |
10 (52.63) 0 |
8 (42.10) 1 (5.26) |
> 0.99 |
| Age at sexual debut n (%) | |||||||||||
|
12–15 16–20 21 onwards |
7 (8.23) 73 (85.88) 5 (5.88) |
3 (3.52) 38 (44.70) 0 |
4 (4.70) 35 (41.17) 5 (5.88) |
0.07 |
0 6 (66.66) 3 (33.33) |
0 2 (22.22) 1 (11.11) |
0 4 (44.44) 2 (22.22) |
– |
6 (31.57) 7 (36.84) |
6 (31.57) 0 |
0.043 |
| Lifetime number of sexual partners n (%) | |||||||||||
|
1–2 3 or more |
39 (45.88) 46 (54.11) |
18 (21.17) 23 (27.05) |
21 (24.70) 23 (27.05) |
0.82 |
7 (77.77) 2 (22.22) |
3 (33.33) 0 |
4 (44.44) 2 |
0.50 |
2 (10.52) 8 (42.10) |
5 (26.31) 4 (21.05) |
0.16 |
| Oral sex practice n (%) | |||||||||||
|
Yes No |
67 (78.82) 18 (21.17) |
30 (35.29) 11 (12.94) |
37 (43.52) 7 (8.23) |
0.29 |
5 (55.55) 4 (44.44) |
2 (22.22) 1 (11.11) |
3 (33.33) 3 (33.33) |
> 0.99 |
9 (47.36) 1 (5.26) |
5 (26.31) 4 (21.05) |
0.14 |
| Condom use n (%) | |||||||||||
|
Yes No |
50 (58.82) 35 (41.17) |
18 (21.17) 23 (27.05) |
32 (37.64) 12 (14.11) |
0.007 |
3 (33.33) 6 (66.66) |
1 (11.11) 2 (22.22) |
1 (11.11) 5 (55.55) |
> 0.99 |
5 (26.31) 5 (26.31) |
4 (21.05) 5 (26.31) |
> 0.99 |
| Vaccinated n (%) | |||||||||||
|
Yes No |
8 (9.41) 77 (90.58) |
4 (4.70) 37 (43.52) |
4 (4.70) 40 (47.05) |
> 0.99 |
0 9 (100) |
0 3 (33.33) |
0 6 (66.66) |
> 0.99 |
1 (5.26) 9 (47.36) |
2 (10.52) 7 (36.84) |
0.58 |
Bold numbers indicate a statistically significant value
HPV infection human papillomavirus infection; Pre present; Abs absent
Tobacco consumption was low in both groups, and alcohol consumption results showed that most of the individuals were at low risk, including 69.41% of young and 77.77% of middle-aged adults, with a small proportion of hazardous or harmful drinking habits. Age at sexual debut was significantly different in the tested groups (p = 0.02), where young adults seem to have started at an earlier age compared to middle-aged adults. Condom use was more frequently used by young adults (58.82%) compared to middle-aged adults (33.33%).
Regarding the number of sexual partners, 54.11% of young individuals declared 3 or more, while middle-aged adults indicated a lower number, only one or two partners in life (77.77%). Interestingly, 78.82% of young adults declared oral sex practices while only 55.55% of middle-aged adults did. None of the analyzed characteristics showed statistical difference between groups.
Cytological alterations at initial sampling
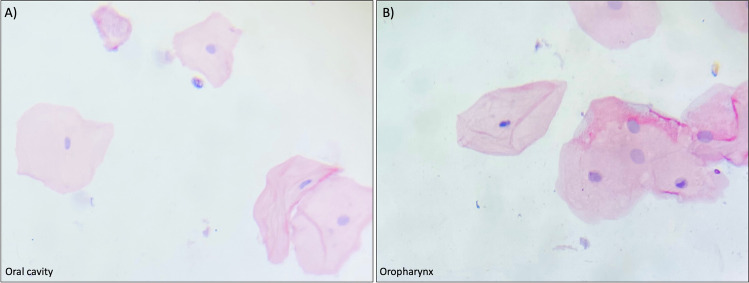
The cytological analysis of all included samples at time zero showed normal morphology of the superficial cells in both the oropharynx and the oral cavity regions of all included subjects. Cells exhibited a polygonal-shaped morphology with eosinophilic staining (Fig. 1).
Fig. 1.
Representative cytologic features of the analyzed population. All samples showed normal characteristics of superficial cells. A Cells from the superficial layer of the oral cavity showing a pyknotic nucleus and eosinophilic staining. B Cells from the superficial layer of the oropharynx showing a pyknotic nucleus and eosinophilic staining. PAP stain. Magnifications were taken under the 20 × objective
HPV prevalence is high in young population
Detection of viral DNA demonstrated a prevalence of HPV in 46.80% of the total subjects (n = 44) with no differences according to age (p = 0.49). Young adults showed 48.23% positivity and middle-aged ones 33.33%. However, there were differences when analyzing HPV presence by anatomical site, since the virus was found exclusively in the oral cavity in 29.78% (n = 28), while it was detected in the oropharynx of only 4.25% (n = 4) of the participants. Moreover, 12.76% of the studied population (n = 12) presented viral infection in both the oral cavity and the oropharynx (Table 2).
Table 2.
HPV prevalence distribution in the oral cavity and the oropharynx
| Prevalence (first sampling) n = 94 |
Probable persistence (12 months later) n = 19* |
|||
|---|---|---|---|---|
| HPV positive n (%) |
HPV negative n (%) |
HPV positive n (%) |
HPV negative n (%) |
|
| Total prevalence n (%) | 44 (46.80) | 50 (53.19) | 10 (52.62) | 9 (47.36) |
| Anatomical site | ||||
| Oral cavity only | 28 (29.78) | 66 (70.21) | 3 (15.78) | 16 (84.21) |
| Oropharynx only | 4 (4.25) | 90 (95.74) | 2 (10.52) | 17 (89.47) |
| Both anatomical regions | 12 (12.76) | 82 (87.23) | 5 (26.31) | 14 (73.68) |
HPV human papillomavirus infection
*Twenty five subjects were lost during follow-up for different reasons: not located, refused to participate, missed appointment
Regarding HPV genotype, it was found that HPV16 was the most frequently detected genotype in the positive samples of both anatomical locations, with a prevalence of 37.5% and 27.5% for oropharynx and oral cavity, respectively. HPV18 was the second most frequent viral type found in the studied population, present in 6.25% of the oropharynx cases and 7.5% of the oral cavity. Additionally, 12.5% of the studied population showed coinfection with two viral types in the oral cavity, from which 10% (n = 4) were HPV16 and HPV18, while 2.5% (n = 1) were infected with HPV18 and HPV11. Interestingly, 51.78% (n = 30) of the samples possessed a different viral type from those included in the detection kit used in this study (Table 3).
Table 3.
HPV genotypes in the oral cavity and oropharynx
| First sampling | Prevalence after 12 months | |||
|---|---|---|---|---|
| Oral cavity n = 40 |
Oropharynx n = 16 |
Oral cavity n = 8 |
Oropharynx n = 7 |
|
| HPV type | n (%) | n (%) | n (%) | n (%) |
| HPV 16 | 11 (27.5) | 6 (37.5) | 0 | 0 |
| HPV 18 | 3 (7.5) | 1 (6.25) | 8 (100) | 6 (85.71) |
| HPV 11 | 0 | 0 | 0 | 1 (14.28) |
| HPV16,18 | 4 (10) | 0 | 0 | 0 |
| HPV18,11 | 1 (2.5) | 0 | 0 | 0 |
| Other HPV types | 21 (52.5) | 9 (56.25) | 0 | 0 |
The existence of different risk factors in the population was evaluated by a using a questionnaire and collected data was associated with the presence of HPV. The majority of the items analyzed showed no association with HPV positivity, indicating that the infection does not depend on the lifestyle of the participants (Table 1), with the exception of condom use in young individuals (p = 0.007), but this item was not associated with HPV infection in middle-aged adults. This indicates that those who use condoms have a lower probability of contracting an HPV infection (RR 0.54, 95% CI 0.34–0.84). Interestingly, alcohol and tobacco consumption were not associated with the presence of HPV in these individuals. Additionally, only 8.51% of the analyzed individuals were vaccinated against HPV.
Young individuals are reinfected by HPV
To assess the likely persistence of HPV infection in the population, the collection of samples and the application of questionnaires were performed in HPV-positive individuals 12 months after the first sample. All individuals initially HPV positive were invited to participate, 19 of them accepted to be included in the second sampling. Two samples were taken from both anatomical sites and analyzed for HPV presence by either Papanicolaou staining or PCR. Additionally, a blood sample was obtained for cell count and IL-10 evaluation. We established a likely persistent infection when the same genotype was found after 12 months.
Most of the population were young adults (84.21%) and the rest middle-aged adults (15.78%) with a mean age of 25.84 ± 9.88 years old. Again, all the participants exhibited high alcohol consumption. Furthermore, 36.84% declared an increase in the number of sexual partners and importantly, the practice of oral sex was again high (73.68%), even after knowing the positive result for HPV infection in the oral cavity and oropharynx region (Table 1).
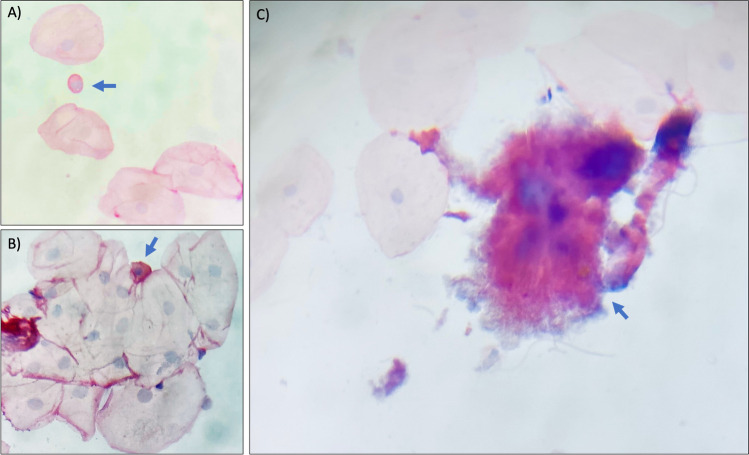
The cytological study performed for the 19 participants to evaluate viral persistence showed normal morphology of the superficial cells in both, the oropharynx and the oral cavity regions, with the exception of one sample from the oral cavity sample where cells with a more prominent nucleus were found, suggestive of cell transformation (Fig. 2A, B). Particularly, this young participant (21 years old) was negative to HPV 12 months later, but instead declared high alcohol intake (37 UU/week) as well as high tobacco consumption (6 cigarettes/day), suggesting that the presence of such factors produced the observed alterations. Additionally, one of the analyzed samples was positive for Actinomyces (Fig. 2C). Interestingly, no evidence of koilocytes was found in any of the samples analyzed (data not shown).
Fig. 2.
Representative cytological alterations found at second sampling. A, B Cells with a prominent nucleus (blue arrow) resembling those from the basal layer. C Actinomyces sp. (blue arrow) in one sample, found in some cases at the second sampling. PAP stain. Magnifications were taken under the 20 × objective
We found that most of the HPV-positive individuals successfully eliminated the infection (94.74%) and only one participant presented persistence (5.26%) for HPV18. Notably, the persistent case was not the one with cellular abnormalities. Interestingly, half of the previously infected subjects who efficiently eliminated the infection acquired a new HPV infection (50%) with a different HPV genotype, indicating a possible reinfection process. Particularly, in the young individuals, 56.25% of them were positive for the infection, while in middle-aged adults, a likely HPV persistence was found in only one case. When analyzing HPV type, we found HPV18 and 11 as the most prevalent viral types in the reinfected population (Table 2). Moreover, when analyzing the associations of the risk factors with HPV reinfection, only hazardous alcohol consumption was associated with getting HPV reinfection (p = 0.043). Thus, low alcohol-consuming individuals have a protecting factor for HPV reinfection (RR 0.5, 95% CI 0.25–0.87).
In order to determine whether alterations in IL-10 levels could be associated with viral persistence or reinfection, IL-10 levels were analyzed by an ELISA assay, finding that IL-10 levels did not show substantial differences among the tested individuals (Supplementary Table 3). This result indicates that the possible reinfection might merely be a random phenomenon. Additionally, all evaluated individuals showed normal levels of HCT, HGB and leukocytes, indicating the absence of anemia, lymphocytosis or any other alteration that could be related to possible viral persistence or reinfection (Supplementary Table 3).
Discussion
The high prevalence of human papillomavirus infection makes this a global public health problem, particularly in young individuals who are continuously exposed to this virus. Additionally, the alarming increase of HPV-associated oral cavity and oropharynx cancers [17, 34] manifests the need to establish epidemiological data in our population, specifically in individuals with age-related risk. The main objective of this study was to determine the presence of HPV in the oral cavity and oropharynx in a university population.
In an initial sampling, we determined HPV prevalence and 12 months later retested the HPV-positive individuals to evaluate the presence of the virus and to look for individuals with a possible persistent infection. The prevalence of HPV, in oral cavity and oropharynx, obtained in the first sampling was 46.31%. Twelve months later, 52.63% of the positive individuals presenting an HPV infection still presented HPV in the analyzed regions (Table 2); both percentages are high compared to similar studies, where virus positivity ranges from 6 to 14.5% in the US [35] and European [20] populations. Moreover, it has been shown that when scraping is used for sample obtention in normal oral mucosa, the prevalence of HPV is about 45%. [23] This is consistent with our results where HPV was detected only by molecular means.
Since the presence of HPV is more frequent in oropharyngeal carcinoma than in oral cancers, [6] we decided to test the obtained samples separately. Our findings showed that the virus was present more frequently in the oral cavity than in the oropharynx at both sampling times. These results contrast with the data found in cancer cases, which show a strong association between HPV and oropharyngeal cancer. [6, 15] Although the association between the virus and OPSCC is well established, it still needs to be investigated whether HPV infection can locate to the tonsil crypts by other means. Additionally, different studies aimed at detecting HPV infection in the head and neck region have based their results on samples taken by rinse or scraping and did not separate the different anatomic areas. [36, 37].
When analyzing the demographic data of the participants, no association was found between the presence of the virus and the items consulted, not even the vaccination status of the participants. Tobacco use was expected to be associated with the presence of the virus because smoking alters a wide range of immune functions in the oral cavity [38]; however, the majority of the study population is within the 18 to 25 age range, which is generally in good health, so the effects of exposure to this agent may not yet be noticeable.
Even when the cytological examination indicated the absence of HPV-induced alterations, the molecular detection of the virus by PCR shows a high sensitivity, which is in agreement with other studies which report a sensitivity of 61.3% for the detection of HPV with a cytological examination and about 90% using molecular identification, specifically PCR. [39, 40] HPV positivity can be due to an incidental infection and viral DNA comes from superficial area peeling and has not been yet integrated to promote detectable changes in the cellular morphology.
Regarding genotyping, HPV16 was found at the highest proportion in both, the oral cavity and the oropharynx at the initial time, followed by HPV18, similar to that found in a study focused on women [36], in a multinational study including 557 healthy Mexican men, [26] and in HNC. [15] In contrast, 12 months later, there were no HPV16-positive cases, being somehow replaced by HPV18 as the most frequent genotype in possible reinfection cases. Therefore, it is relevant to mention the importance of evaluating the presence of HPV at different time points, although a larger study population is required for validating the presence of HPV genotypes.
It is well known that most HPV infections are efficiently eliminated by the immune system within 6–12 months [41], so this study evaluated likely persistent infection cases, considering those presenting the same viral type. We found only one possible persistent case with HPV18. Surprisingly, 47.36% of the participants that eliminated the infection got a new viral type in the second sampling, indicating that the HPV has a dynamic transmission in this population and that a negative result at a certain time does not guarantee the absence of infection in the next months, representing a risk factor for developing lesions and cancer in the future.
Among the factors associated with viral persistence, interleukin 10 (IL-10) is an immunosuppressive cytokine that promotes persistence and progression of lesions related with HPV [4]. The levels of IL-10 obtained in the present work seem not to be associated with HPV presence, since IL-10 levels did not vary significantly between individuals. Additionally, all individuals showed a normal oral mucosa, and the results of the cytological analysis did not show morphological changes suggestive of infection or malignant transformation, with the exception of one case where there was presence of cells with morphological characteristics suggestive of malignancy. It is important to mention that this subject was not the one that showed viral persistence, indicating that there are possibly other factors that are promoting such changes, such as alcohol and tobacco consumption.
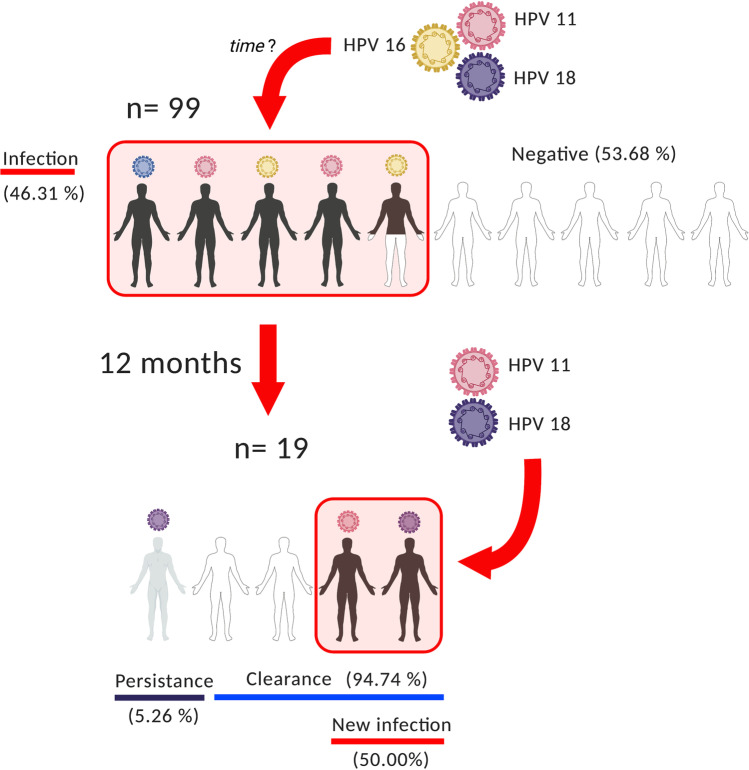
Our results show that in a university population, a reinfection dynamic is occurring since there is a high proportion of individuals in an infection-elimination-infection cycle (Fig. 3). Although the prevalence is not due to the same genotype, the presence of HPV is detected in a population with more sexual opportunities due to scholar coexistence, and consumption of alcohol and tobacco. Furthermore, young people frequently practice oral sex to avoid pregnancy. This trend has negative implications for teenagers’ sexual health, since many seem unaware that sexually transmitted infections (STIs) can be acquired through unprotected oral sex [42]. Certainly, most of the young people are healthy, but this condition could be counterproductive and promote neglectful behaviors, representing a risk factor which could potentiate the HPV infections and cancer development in the future. As a matter of fact, some of the HPV-positive cases refused to participate in the second sampling, indicating a lack of interest for their well-being, and those who were retested indicated a high proportion of oral sex practice, even after a positive result for HPV infection.
Fig. 3.
HPV dynamic infection in young population. Circulating HPV genotypes infect a high proportion of young individuals that clear the infection after 12 months. Although the persistence of oral HPV infection is low, there is an infection-clearance-infection cycle due to the circulating HPV genotypes. This indicates the need for better preventive actions in the young population. Figure was created with BioRender.com
Our results strengthen the need to expand vaccination coverage to men and women in our country since HNC associated with HPV infection is due mainly to high-risk genotypes which are included in the currently available vaccines.
Conclusions
The results obtained indicate that the prevalence of HPV in the oral cavity in a university population is high and could represent a long-term health problem. This work manifests the need to implement strategies aimed at informing about diseases caused by HPV and promoting a culture of safe sex in the young population. Our results can be used to define prevention strategies and timely diagnosis with great impact on the analyzed population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. Elizabeth Langley (Instituto Nacional de Cancerología, Mexico) for her valuable comments on the manuscript.
Author contribution
Conceptualization: JM-M and NGP-S.
Data collection: AM-T, NGP-S, ST-L, DOR-H, JJM-A.
Performing experiments: AM-T, NGP-S, ST-L, DOR-H, JJM-A.
Formal analysis: RJ-L, JM-M, AM-T, DOR-H, ML, NGP-S.
Funding acquisition: JM-M, ML, NGP-S.
Writing and reviewing the manuscript: JM-M, ML, AM-T, DOR-H, ST-L, NGP-S.
Figure design and elaboration: DOR-H, AM-T, JM-M.
Supervision: JM-M.
Funding
This work was partially supported by Benemérita Universidad Autónoma de Puebla Ref: VIEP-BUAP 00104, CONACyT PRONAII-7-VIRUS Y CÁNCER Ref: 303044, and Instituto Nacional de Cancerología, Mexico, Ref: (015/039/IBI) (CEI/998/15) and (017/048/IBI) (CEI/1227/17).
Data availability
All data regarding the present study are included in the manuscript and tables.
Code availability
Not applicable.
Declarations
Ethics approval
The present study was approved by Institutional Board, Ref: VIEP-BUAP 00104.
Consent to participate
Participants provided written informed consent before enrolling in the present study.
Consent for publication
Participants signed informed consent regarding publishing obtained data in this study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Giliane Souza Trindade
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Adriana Morán-Torres and Nidia G. Pazos-Salazar contributed equally to this work.
References
- 1.McQuillan G, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R. Prevalence of HPV in adults aged 18–69: United States, 2011–2014. NCHS Data Brief. 2017;280:1–8. [PubMed] [Google Scholar]
- 2.Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80–85. [PubMed] [Google Scholar]
- 3.Brianti P, De Flammineis E, Mercuri SR, et al. The intersection of HPV epidemiology, genomics and mechanistic studies of HPV-mediated carcinogenesis. Viruses. 2018;18(2):1263–1269. doi: 10.1038/nrc.2018.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres-Poveda K, Bahena-Roman M, Madrid-Gonzalez C, et al. Role of IL-10 and TGF-beta1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol. 2014;5(4):753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candotto V, Lauritano D, Nardone M, et al. HPV infection in the oral cavity: epidemiology, clinical manifestations and relationship with oral cancer. Oral Implantol (Rome) 2017;10(3):209–220. doi: 10.11138/orl/2017.10.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 7.Tumban EA. Current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11(10):922. doi: 10.3390/v11100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood ZC, Bain CJ, Smith DD, Whiteman DC, Antonsson A. Oral human papillomavirus infection incidence and clearance: a systematic review of the literature. J Gen Virol. 2017;98(4):519–526. doi: 10.1099/jgv.0.000727. [DOI] [PubMed] [Google Scholar]
- 9.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 11.Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 12.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed]
- 13.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;6:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibieta-Zarco BR, Carrillo-García A, Ponce-De-León-Rosales S, Flores-Miranda MM, Mohar A, Lizano M. Frequency and genotype distribution of multiple human papillomavirus infections in cancer of the head and neck in a Mexican population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(3):350–357. doi: 10.1016/j.oooo.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Anaya-Saavedra G, Ramírez-Amador V, Irigoyen-Camacho ME, et al. High association of human papillomavirus infection with oral cancer: a case-control study. Arch Med Res. 2008;39(2):189–197. doi: 10.1016/j.arcmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louvanto K, Rintala MA, Syrjänen KJ, Grénman SE, Syrjänen SM. Genotype-specific persistence of genital human papillomavirus (HPV) infections in women followed for 6 years in the Finnish Family HPV Study. J Infect Dis. 2010;202(3):436–444. doi: 10.1086/653826. [DOI] [PubMed] [Google Scholar]
- 19.Bijina BR, Ahmed J, Shenoy N, Ongole R, Shenoy S, Baliga S. Detection of human papilloma virus in potentially malignant and malignant lesions of the oral cavity and a study of associated risk factors. South Asian J Cancer. 2016;4:179–181. doi: 10.4103/2278-330X.195337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonidesová S, Hamšíková E, Klozar J, Tachezy R. The prevalence of oral HPV infection in healthy populations: a systematic review with a focus on European populations. Epidemiol Mikrobiol Imunol Cas Spol pro Epidemiol a Mikrobiol Ces Lek Spol JE Purkyne. 2018;67(4):175–183. [PubMed] [Google Scholar]
- 21.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(3):262–267. doi: 10.1200/JCO.2017.75.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ablanedo-Terrazas Y, Romero-Mora K, Gómez-Palacio M, et al. Prevalence and risk factors for oral human papillomavirus infection in Mexican HIV-infected men. Salud Publica Mex. 2018;60(6):653–657. doi: 10.21149/9834. [DOI] [PubMed] [Google Scholar]
- 23.Lawton G, Thomas S, Schonrock J, Monsour F, Frazer I. Human papillomaviruses in normal oral mucosa: a comparison of methods for sample collection. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 1992;21(6):265–269. doi: 10.1111/j.1600-0714.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 24.Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol. 2017;28(10):2386–2398. doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 25.Gayet C, Gutiérrez JP. Calendario de inicio sexual en México. Comparación entre encuestas nacionales y tendencias en el tiempo. Salud Publica Mex. 2014;56(6):638–647. doi: 10.21149/spm.v56i6.7391. [DOI] [PubMed] [Google Scholar]
- 26.Kreimer AR, Pierce Campbell CM, Lin H-Y, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet (London, England) 2013;382(9895):877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson DA. Defining risk drinking. Alcohol Res Heal J Natl Inst Alcohol Abus Alcohol. 2011;34(2):144–156. [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med (Baltim) 1999;29(3):139–144. doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 29.Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10(2):95–102. [PubMed] [Google Scholar]
- 30.Brunotto M, Zárate AM, Cismondi A, del Fernández M, C, Noher de Halac RI, Valuation of exfoliative cytology as prediction factor in oral mucosa lesions. Med Oral Patol Oral Cir Bucal. 2005;10(Suppl 2):E92–102. [PubMed] [Google Scholar]
- 31.Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, et al. Exfoliative cytology for diagnosing oral cancer. Biotech Histochem Off Publ Biol Stain Comm. 2010;85(3):177–187. doi: 10.3109/10520290903162730. [DOI] [PubMed] [Google Scholar]
- 32.Ayala-Díaz S, Jiménez-Lima R, Ramírez-Alcántara KM, et al. Presence of Papillomavirus DNA sequences in the canine transmissible venereal tumor (CTVT) PeerJ. 2019;7:e7962. doi: 10.7717/peerj.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrillo-García A, Ponce-de-León-Rosales S, Cantú-de-León D, et al. Impact of human papillomavirus coinfections on the risk of high-grade squamous intraepithelial lesion and cervical cancer. Gynecol Oncol. 2014;134(3):534–539. doi: 10.1016/j.ygyno.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Sathish N, Wang X, Yuan Y. Human papillomavirus (HPV)-associated oral cancers and treatment strategies. J Dent Res. 2014;93(7 Suppl):29S–36S. doi: 10.1177/0022034514527969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreimer AR, Bhatia RK, Messeguer AL, González P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010;37(6):386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 36.Louvanto K, Rautava J, Willberg J, et al. Genotype-specific incidence and clearance of human papillomavirus in oral mucosa of women: a six-year follow-up study. PLoS ONE. 2013;8(1):e53413. doi: 10.1371/journal.pone.0053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Losa MDR, Barrera ES, Herrera-Pech V, Conde-Ferráez L, Puerto-Solís M, Ayora-Talavera G. Epidemiology of oral HPV in the oral mucosa in women without signs of oral disease from Yucatan. Mexico Brazilian J Microbiol. 2015;46(1):301–306. doi: 10.1590/S1517-838246120130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha P, Logan HL, Mendenhall WM. Human papillomavirus, smoking, and head and neck cancer. Am J Otolaryngol. 2012;33(1):130–136. doi: 10.1016/j.amjoto.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Poveda K, Piña-Sánchez P, Vallejo-Ruiz V, et al. Molecular markers for the diagnosis of high-risk human papillomavirus infection and triage of human papillomavirus-positive women. Rev Invest Clin. 2020;72(4):198–212. doi: 10.24875/RIC.20000058. [DOI] [PubMed] [Google Scholar]
- 40.De Guglielmo Z, Rodríguez A. Methods used in the identification of human papillomavirus. An Sist Sanit Navar. 2010;33(1):71–77. doi: 10.4321/s1137-66272010000100008. [DOI] [PubMed] [Google Scholar]
- 41.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother Off J Japan Soc Chemother. 2012;18(6):807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 42.Kalmuss D, Davidson A, Cohall A, Laraque D, Cassell C. Preventing sexual risk behaviors and pregnancy among teenagers: linking research and programs. Perspect Sex Reprod Health. 2003;35(2):87–93. doi: 10.1363/3508703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data regarding the present study are included in the manuscript and tables.
Not applicable.