Abstract
Klebsiella variicola has been found in various natural niches, alone or in association with other bacteria, and causes diseases in animals and plants with important economic and environmental impacts. K. variicola has the capacity to fix nitrogen in the rhizosphere and soil; produces indole acetic acid, acetoin, and ammonia; and dissolves phosphorus and potassium, which play an important role in plant growth promotion and nutrition. Some members of K. variicola have properties such as halotolerance and alkalotolerance, conferring an evolutionary advantage. In the environmental protection, K. variicola can be used in the wastewater treatment, biodegradation, and bioremediation of polluted soil, either alone or in association with other organisms. In addition, it has the potential to carry out industrial processes in the food and pharmaceutical industries, like the production of maltose and glucose by the catalysis of debranching unmodified oligosaccharides by the pullulanase enzyme. Finally, this bacterium has the ability to transform chemical energy into electrical energy, such as a biocatalyst, which could be useful in the near future. These properties show that K. variicola should be considered an eco-friendly bacterium with hopeful technological promise. In this review, we explore the most significant aspects of K. variicola and highlight its potential applications in environmental and biotechnological processes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00630-z.
Keywords: Nitrogen fixation, Bioremediation, Wastewater treatment
Introduction
The Klebsiella pneumoniae complex is formed by Klebsiella pneumoniae, Klebsiella quasipneumoniae subsp. quasipneumoniae, and Klebsiella quasipneumoniae subsp. similipneumoniae, Klebsiella variicola subsp. variicola, Klebsiella variicola subsp. tropica, Klebsiella quasivariicola, and Klebsiella africana [1–3]. In 2004, based on genetic and biochemical assays, K. variicola was proposed as a novel bacterial species recovered from plants and clinical settings [4]. A misclassification among K. pneumoniae complex has been described, and several K. pneumoniae, K. quasipneumoniae, and K. variicola isolates have been correctly reclassified [5–7]. Several alternatives exist for the differentiation of K. pneumoniae and K. variicola, ranging from PCR, phylogenetic analysis, and whole-genome sequencing approaches [8]. Likewise, matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has been optimized for the routine detection of the related phylogroups of K. pneumoniae, K. quasipneumoniae, and K. variicola, in clinical microbiology laboratories [8].
K. variicola is habitually isolated from a wide range of plant ecosystems, where it plays an important role in nitrogen fixation and plant growth promotion [9–13]. Moreover, it has been identified as part of the microbiota of some insects, which also can be found in several associations with other bacteria in environment like contaminated soil, rivers, and wastewater [12, 14–16].
Recently, the surprising potential of K. variicola for biotechnology processes has been noted. It has also been used for the development of biotechnological processes and environmental protection, such as being a source of renewable energy, fermentation and energy biocatalysts, waste degradation, wastewater treatment, bioremediation of pollutants and biodegradation [12, 14–17]. In this review, we explore the most significant aspects of K. variicola as a promising approach to use in a wide range of environmental applications and industrial processes such as a potential biofertilizer and bioremediator alone or in synergy with other rhizobacteria and mycorrhiza. K. variicola has shown properties that could be exploited for the benefit of improving industrial and agricultural productivity; however, the genes that contribute to some of these beneficial activities are unknown and deserve to be studied in detail.
Although K. variicola causes human and animal infections [8, 18–20] and has found to be a reservoir of important antimicrobial-resistant genes (AMRs) such as extended-spectrum β-lactamases (ESBL), carbapenemases, and colistin resistance [9, 21, 22], its characteristics and biological association needs further investigation in order to identify divergences that led to different host ranges. In addition, the molecular epidemiology based on MultiLocus Sequence Typing (MLST) of K. variicola has previously been addressed using massive sequencing approaches, which contributed considerably to this topic [6, 23, 24]. The specific MLST for K. variicola will be useful as a tool for molecular epidemiology and contributes to highlighting K. variicola as a bacterial species isolated from different environments [25].
The aim of this review is to highlight the wide range of niches and environments in which it is found, its diversity, characteristics, and applications in biological and biotechnological processes.
K. variicola establishes different host associations
K. variicola has been reported in a wide diversity of natural niches (Fig. 1). The mutualistic relationship between leaf-cutter ant colonies and fungi is one example of social insects’ complex behavior (Fig. 1b). The successful colony growth of leaf-cutter ants is supported by the symbiosis of fungal garden and K. variicola, suggesting that colonies obtain a substantial proportion of their nitrogen requirements from this bacterial association [11]. The strain KV321 was isolated from rhizosphere soil of Pisolithus tinctorius-Eucalyptus mycorrhiza. This strain showed high nitrogen-fixation ability and antibiotic resistance, such as to polymyxin, penicillin G, tetracycline, and erythromycin [26]. A similar association has been observed for the misclassified KP5-1 strain, isolated from the microbiota of wild southern green stink bugs (Nezara viridula). The potential of this insect as a vector of cotton disease–causing agents is well known. The opportunistic K. variicola has been documented to cause appreciable boll damage (Fig. 1a) [27].
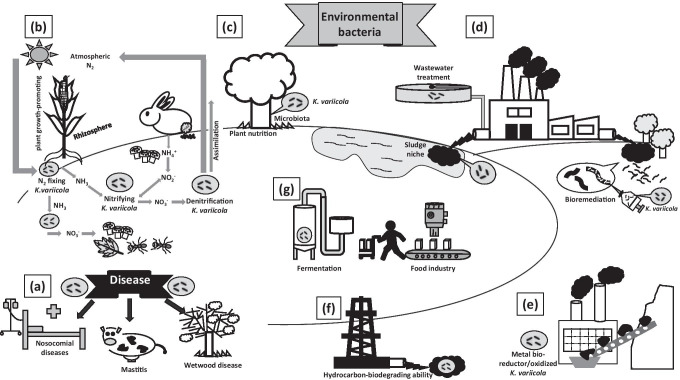
Fig. 1.
Ecology of K. variicola, including properties, niches, and their association with industrial sustainable process. (a) K. variicola as a pathogen in plants, animals, and human. (b) Properties of N2 fixing and denitrification. (c) Plants microbiota in and their contribution in N2 assimilation and plant nutrition. (d) Bioremediation of contaminated soils and water, and participation in wastewater treatment. (e) Metal bioreductor/oxidized properties. (f) Hydrocarbon-biodegrading ability. (g) Participation in fermentation processes and the food and pharma industries
The fruit fly Bactrocera cucurbitae (Melon fly) is an invasive insect that affects the cultivation of fruit and is widely distributed in tropical and sub-tropical regions of the world. The success of this insect depends in part on its relationship with its beneficial intestinal microbiota. These interactions increase the survival and fitness of insects in the environment. The identification of K. variicola as an inhabitant of the melon fly intestinal tract and the presence in the gut microbiota suggests that it produces volatile compounds that contributed to the attraction of both sexes of melon fruit adults [28].
The potential capacity of K. variicola to be involved in or produce diseases is not limited to humans. Bovine mastitis is the condition in which the animal displays physical symptoms in the udder, such as swelling, heat, hardness, redness, and pain, and the milk exhibit a watery appearance and may contain flakes, clots, or pus (Fig. 1a). The diseased cow shows a decrease in milk yield that depends on the specific pathogen causing the infection. K. pneumoniae is one of the primary microbial agents responsible for environment-derived mastitis. In Newfoundland, Canada, 4% of the cases of bovine mastitis from 11 farms were identified as being caused by K. variicola [19]. Moreover, a previous study that investigated the prevalence of Klebsiella species among several animal clinical samples found K. variicola causing infections in dogs, birds, and monkeys [18].
The range of associations of K. variicola extends to very different organism such as the ironwood (Casuarina equisetifolia subsp. equisetifolia), a nitrogen fixation tree of considerable social, economic, and environmental importance in zones of Asia, the Pacific, Africa, and Central America. The decline of ironwood was reported for the first time in Guam in 2002, was associated with wetwood symptoms, and is now affecting thousands of trees and the ecosystem (Fig. 1a). Samples of bacteria obtained from diseased ironwood trees contained the common pathogen Ralstonia solanacearum in association with K. variicola and Klebsiella oxytoca. Causative agents of wetwood in the ironwood trees worldwide have not been completely determined, but scientists attribute the disease to a complex association between R. solanacearum, K. variicola, and K. oxytoca [29].
Bacterial soft rot is a destructive disease that affects many plant species worldwide, including agriculturally important crops, fruits, vegetables, and ornamental plants. Pathogenic bacteria release pectinases that degrade plant cell walls by increasing the turgor pressure within the infected cells, causing the cells to explode and provide organic material to the bacteria. The first report of banana soft rot caused by K. variicola was isolated from an outbreak occurring in the banana-growing regions of Honghe in Yunnan Province, China [30], and another report of bacterial soft rot in carrot was reported in the rural districts of Kolar, Chikkaballapura, and Bengaluru of Karnataka state, India [31].
K. variicola: a plant growth promoter
Plant growth-promoting and free-living soil bacteria play a key role in plant health and nutrition by direct or indirect mechanisms [32, 33]. The direct mechanisms comprise (i) nitrogen fixation, (ii) the production of phytohormones like auxins, especially indole-3-acetic acid (IAA), cytokinins, and gibberellins; (iii) the presence of the enzyme 1-aminocyclopropane-l-carboxylic acid (ACC) deaminase that hydrolyzes ACC and is responsible for reducing the level of ethylene under stress conditions; and (iv) solubilization and conversion of phosphates into orthophosphate, which can be taken up by plant roots [33, 34]. Indirect mechanisms include those that reduce the inhibition of plant growth and development by phytopathogens, and these include the synthesis of siderophores, the scavenging of iron, antibiotic production, and synthesis of fungal cell wall–lysing enzymes [33].
K. variicola is a free-living bacterium since it does not establish a symbiotic relationship with the plant. Different works have shown that K. variicola plays a role as a plant growth–promoting bacterium through direct mechanisms. Nitrogen fixation and production of phytohormones, particularly IAA, have been widely examined [7, 10, 13, 14, 35, 36]. This section discusses what has been found about the plant growth–promoting properties of K. variicola.
The introduction of selected microorganisms that improve the conditions for plant development and yield is one of the major goals in agriculture (Fig. 1b, c). In 2017, Defez et al. performed strain modifications for IAA overproduction in order to assess improvement in nitrogen fixation. The p-iaaM-tms2 construct contained the iaaM gene, which codes for a tryptophan monooxygenase, converting the tryptophan into indole-3-acetamide (IAM), and the tms2 gene which codes for a hydrolase involved in the conversion of IAM into IAA. The introduction of the p-iaaM-tms2 into the endophytic K. variicola RCA26, E. cloacae RCA25, and other model bacteria showed that overproduction of IAA upregulates the nitrogen fixation in these bacterial cultures. Rice plants inoculated with these strains showed upregulation of the nitrogenase activity resulting in a better plant growth and yield (Fig. 1c) [37].
Moreover, IAA is responsible for the division, expansion, and differentiation of plant cells and tissues and stimulates root elongation [34]. Nitrogen-fixing bacteria could be used as bioinoculants to improve the growth and yield of agricultural crops, offering an alternative to the use of chemical nitrogen fertilizers [37]. Nitrogen fixation as a main process in K. variicola is discussed in detail in the “Nitrogen fixation as a trait of K. variicola” section.
The K. variicola D5A isolate was demonstrated to act as a plant growth–promoting rhizobacterium. Analysis of the D5A whole genome sequence identified potential genes involved in plant growth promotion, such as those for IAA biosynthesis, phosphate solubilization, siderophore production, acetoin and 2,3-butanediol synthesis, N2 fixation, chitinase activity, phenazine, 4-hydroxybenzoate, and H2S synthesis, which are present in some other plant growth–promoting rhizobacteria (Liu et al., 2016) [14]. Another report of a K. variicola strain acting as a diazotrophic endophyte on a nonleguminous crop is provided by K. variicola 342 [9]. Wheat plants inoculated with K. variicola 342 had increased dry weight, chlorophyll content, total N, and N concentration [38].
Endophytic K. variicola RBEB3 isolated from the leaves of the red banana cultivar was able to synthesize significant amounts of IAA in cultures. Tomato seeds inoculated with strain RBEB3 had improved germination and seedling growth parameters as well the total biomass and root length compared with the control plants [39]. A similar example is that of K. variicola IARI-PC4-59 isolated from plant samples of two maize genotypes. This strain has the capacity to fix nitrogen, solubilize Zn, and produce IAA and siderophores [40].
Potassium (K) is another essential element for plant growth and development. Soil has rich reserves of K but just 0.1–0.2% can be directly absorbed by plants [41]. Some studies have shown the application of K-solubilizing bacteria (KSB), which decompose silicate minerals and transform the solid into available K that can be directly absorbed by plants. To identify potential KSB strains, Zhang et al. (2014) isolated 17 K. variicola from the tobacco rhizosphere, and at least three of them promoted the growth of tobacco plants and show potential for use as biological K fertilizer that significantly improved seedling height, dry weight, and absorption of N and P [42]. The Jerusalem artichoke (Helianthus tuberosus) is a plant with starchy tubers like potatoes and is native to central North America. The tubers have many health benefits, are suitable for human consumption, and have a high potential in the production of biofuel and high inulin content, and the leaves are used in the pharmaceutical sector [43]. K. variicola UDJA102 × 89–9 was isolated from rhizosphere soil samples in the northeastern regions of Thailand. The isolate had phosphate-solubilizing ability and produced high amounts of several organic acids and IAA in vitro. The dual inoculation of K. variicola and Arbuscular mycorrhizal fungal species in combination with rock phosphate promoted growth and inulin production in Jerusalem artichoke compared to both unfertilized and fertilized controls [36].
K. variicola isolates DX120E and KvMx2 were obtained from the root of a sugarcane plant from the regions of Guangxi, China, and Cuautla, Mexico, respectively. K. variicola DX120E was able to fix nitrogen, produce IAA and siderophores, and solubilize phosphate, thus promoting plant growth [10, 13, 35]. Genome analysis of K. variicola subsp. tropica KvMx2 showed the presence of essential genes for the biosynthesis of IAA, acetoin and 2,3-butanediol, and bglH genes, which are involved in plant growth, development, detoxification of cyanide compounds, and plant colonization. In addition, it contained the nifJ-NifQ nitrogen fixation gene cluster [7]. Other examples of endophyte strains of diverse origins have been reported, such as T29A from sugarcane with tolerance to desiccation [44], F2R9, 6A2, and VI from banana, and 342 isolates from maize [4, 9]. Recently, K. variicola X39 was considered a kingdom-crossing bacterium, and the genomic data and in vitro and in vivo assays showed some genes involved in plant colonization, nitrogen fixation, and defense against oxidative stress [45]. The molecular epidemiology of K. variicola allowed the identification of various isolates from both plants and humans to also be considered kingdom-crossing bacteria which share the same sequence type or phylogenetic relationship [25].
Nitrogen fixation as a trait of K. variicola
One of the most important elements that constitutes the molecules for all forms of life is nitrogen. The fixation of atmospheric nitrogen is a natural process in living organisms and performed by some bacteria and archaea. Natural nitrogen fixation is a clean process that is environmentally friendly and efficient.
N2 fixers, also called diazotrophs, are able to reduce nitrogen gas (triple NΞN bond) to ammonia (NH3). This process changes a biologically inactive form of nitrogen to a compound that can easily be used by organisms. Nitrogen can be used for the synthesis of proteins and nucleic acids, thus playing a critical role in the plant ecosystem (Fig. 1b, c). The nif-operon responsible for nitrogen fixation is chromosomally located in nitrogen fixers like K. variicola but on plasmid in most rhizobial symbionts [4, 46]. Nitrogenase is the central enzyme for nitrogen fixation and it consists of complex of Fe protein encoded by the nifH gene and MoFe protein encoded by nifDK genes. The nif cluster is composed of a set of twenty genes and nifA and nifL genes that are the positive and negative regulatory elements respectively. Full assembly of the nitrogenase complex needs the products of at least twelve nif genes for the processing of catalytic stability and metalloclusters (nifMZ, nifUS, and nifW) and for synthesis of a particular molybdenum cofactor FeMo-co (nifQ, nifB, nifE, nifN, and nifV). The nifH gene has been used to explore the diversity of nitrogen-fixing bacteria [47]. In addition to the nif genes, the rnfABCDEG operon is also important because it encodes a membrane-bound protein complex related to electron transport to nitrogenase [14]. In silico analysis of 328 Klebsiella genomes (including K. pneumoniae, K. quasipneumoniae, and K. variicola) revealed that a nif cluster was present in all K. variicola genomes, supporting its identification as a nitrogen-fixing species [23]. In contrast, it was present in one of K. pneumoniae genomes (a bovine mastitis isolate) and in 50% of K. quasipneumoniae subs. similipneumoniae genomes (human isolates) [23]. However, we do not know if this K. pneumoniae bovine mastitis isolate had the capacity to fix nitrogen. Similar results were reported in 2016, where the authors carried out an analysis of K. variicola, K. pneumoniae, and K. quasipneumoniae genomes in order to identify nitrogen-fixing strains belonging to these bacterial species [5]. They found one K. pneumoniae and five K. quasipneumoniae genomes harbored nitrogen-fixing genes, whereas all K. variicola genomes contained the nif cluster.
The presence of nif cluster in K. pneumoniae genome in these different studies might be related to evolution and speciation events that lead to its loss, probably because K. pneumoniae occupies a niche where nitrogen fixation could be unnecessary and possibly disadvantageous and has thus been selected against. However, this should be studied in detail and confirm that K. pneumoniae has lost this ability to fix nitrogen or perhaps never had it. Both K. quasipneumoniae subsp. quasipneumoniae and K. quasipneumoniae subsp. similipneumoniae can be isolated from human clinical samples and with less frequency in animals (dogs), and there are no reports of this bacterial species in plant samples [23]. The existence of a nif cluster in K. quasipneumoniae subsp. similipneumoniae genomes has been reported; however, not all K. quasipneumoniae subsp. similipneumoniae genomes encode the nif operon genes. It was suggest that the loss of the nif cluster occurred by negative selection, when humans and animals were colonized by K. quasipneumoniae [23]. The role of nif genes in K. quasipneumoniae subs. similipneumoniae isolates remains unclear. Nitrogen fixation has been explored in vertebrates, particularly the role of nitrogen fixation in human gut microbiota attributed to Klebsiella sp. and Clostridium sp. [48].
nif-operon among K. variicola genomes
Nitrogen metabolism of K. variicola may contribute to a better survival in different environmental niches. The phylogenetic diversity of the nifH gene has been widely investigated since it is considered a molecular marker of nitrogen fixation [49]. However, the study of the complete nif operon diversity among K. variicola has been poorly addressed. Blin et al. analyzed the phylogenetic distribution of the nif cluster in Klebsiella isolates in order to understand the origin of their nitrogen fixing ability. They found that the nif cluster was identical in all strains and synteny was observed throughout the nif cluster. Moreover, differences in the utilization of variety of substrates as nitrogen sources were found between K. pneumoniae, K. quasipneumoniae, and K. variicola [49].
To reveal the diversity of nif clusters, we analyzed a set of 199 K. variicola genomes from diverse sources obtained from public GenBank/ENA databases (01/05/2019) and the tree was rooted using two K. quasipneumoniae genomes. The nif-cluster sequences from each K. variicola genome were used to build a Maximum Likelihood phylogeny tree. Figure 2 shows the isolates that were obtained from different sources such as plants, animals, insects, and environments and most frequently in humans. Phylogenetic analysis does not indicate any segregation with respect to the origin of the isolates, as well as for sequence type determined under K. variicola MLST site (https://mlstkv.insp.mx/) (25). The analysis suggests that the rate of substitutions in these genes is restricted (number of mutations per site [0.09]), perhaps to maintain the ability to fix nitrogen. We observe intergenic insertions throughout the nif cluster that do not modify the open reading frames of the genes, which is essential for maintaining the ability to fix nitrogen. However, this should be determined with in vitro assays. Of all the K. variicola isolates described in public databases, only a minor number have been determined to fix nitrogen in vitro. The identification of nitrogen fixing bacteria was determined by acetylene reduction activity in the isolates described in Mexico [4]. The isolates 3, 6A2, and T29A (sugar cane stem) and 801 (pediatric outbreak) were obtained from plants and humans respectively [4, 50]. In the case of the At-22 and DX120E isolates, the nitrogen fixation assay was conducted by enrichment experiments using the natural stable isotope of 15 N [11, 13].
Fig. 2.
Maximum likelihood phylogeny tree of nif-cluster nucleotide sequences was generated using Mega software v7.0.26 from 199 K. variicola genomes obtained from public GenBank/ENA databases. The Tamura-Nei model with discrete Gamma distribution was applied to model evolutionary rate. The tree was rooted using two K. quasipneumoniae nif-cluster operon nif. The circle indicates the origin of the isolates; green plants, red animals, brown insects, blue environmental, and yellow nitrogen fixation detected in vitro
Unlike phylogenetic analysis using DNA sequences, the use of Nif proteins from all K. variicola genomes showed that the sequences were highly conserved (> 98% of identity) (Supplementary File 1). This supposes a selection pressure to the nitrogen fixation system in the bacteria. The case of nifH gene was conserved as the nifDK genes. Among the nifM and nifQ, it showed the most significant variability between their nucleotide sequences and nifT in some genomes. The K. variicola subsp. tropica KvMx2 plant isolate was considered a distant lineage, as its wide variation is observed with respect to the other genomes of K. variicola.
Biotechnological and environmental applications
Bioremediation of pollutants
Free heavy metals used in multiple industrial processes are discharged into the ecosystem and tend to proliferate in agricultural soil and living organisms. Microbial activity plays major roles in the bioremediation of heavy metals by mobilization or immobilization of metals; they can be bioreduced or biooxidized to less toxic forms. K. variicola isolates tolerant to heavy metals were identified from textile effluent wastewater (Fig. 1d), and these isolates possessed significant heavy metal tolerance and bioremediation capacity against nickel (Ni) and cobalt (Co), with a percentage reduction of 49% and 50%, respectively, from polluted samples [15].
K. variicola strain AMIC1 has significant heavy metal tolerance and the potential to be an effective bioremediation tool for Ni and Co. This strain was isolated from textile effluents in Faisalabad, Pakistan. The heavy metal–tolerant K. variicola AMIC1 isolated from industrial wastewater could be used for the development of bioremediation agents to detoxify industry textile effluents in the future [15]. K. variicola strain D5A was isolated from the rhizosphere tall fescue (Festuca arundinacea L.) growing in a soil contaminated with oil. The isolate showed high plant growth–promoting traits, environmental tolerance to a wide range of soil pH, and the ability to adapt to a high saline-alkaline environment. The bacterium also produced the phytohormone IAA, solubilized phosphate, and synthesized siderophores. The inoculation of K. variicola D5A in plants from a petroleum-contaminated saline-alkaline soil promoted the growth of host plants and enhanced phytoremediation efficiency [12, 14] (Fig. 1f).
Water pollution caused by hazardous inorganic and organic pollutants, including textile industries’ dyes and phenols released from various industries affecting the environment, becomes an important concern worldwide. Bio-flocculants are metabolic products of microorganisms produced during their growth and widely used in the treatment of wastewater [51]. In Vietnam, flocculants of K. variicola BF1 were effectively applied in the treatment of cassava starch wastewater from Logom canal, Ho Chi Minh City [52]. Atrazine is the second most widely used herbicide after glyphosate and commonly detected in drinking water. The high solubility of Atrazine in water increases its capacity to contaminate the irrigation water of crops used for human and animal consumption. The characterization of the K. variicola FH-1 showed that it effectively degraded Atrazine. The strain was isolated from agriculture fields exposed to Atrazine for over 10 years and will have ecological promise in the bioremediation of fields polluted by this herbicide [53].
During wastewater treatment, the reduction of hazardous products from human sewage water requires the transformation of ammonium and nitrate to molecular nitrogen (N2) by a nitrifying reaction and aerobic denitrification (Fig. 1b). Nitrous oxide is a product of the oxidation of nitrate only in the absence of oxygen, and is a greenhouse gas more toxic than carbon dioxide. One solution to combat the release of nitrous oxide from wastewater treatment is using aerobically denitrifying organisms with the total capacity to reduce nitrous oxide to nitrogen. K. variicola strains with the ability to heterotrophically nitrify and aerobically denitrify were isolated from different sludge environments. These isolates can remove nitrate, reducing leaching into groundwater, and can be used strategically to treat sewage or animal residues with a high nitrogen content [54] (Fig. 1d).
Agriculture soil quality has been declined mainly by environmental contaminants. Heavy metal soil contaminants can be hazardous to human, animal, and ecosystem health. One of the most common toxic heavy metals acquired by humans from the consumption of contaminated vegetables is lead. Lead accumulates in the bodies of animals and can have carcinogenic effects [55, 56]. The critical situation of pollutants that affect ecological and human health has led to the development of alternative technologies, such as bioremediation using earthworms or microbes for the sanitation of agriculture soils (Fig. 1d). In previous reports, earthworms have been used as indicators of soil quality and health, with the capacity to reduce the concentrations of chromium (Cr), cooper (Cu), lead (Pb), and zinc (Zn) in vermicompost sludge. The utilization of earthworms inoculated with lead-resistant K. variicola for bioremediation was successfully proven in polluted soil [16].
The reactive red 198 Azo dye (RR198) is an organic water-soluble compound that is widely used for cosmetics, leather, paper, and cardboard. In the textile industry, the dyeing and finishing processes consume a significant amount of water leading to the discharge of wastewater contaminated with Azo dyes. An Enterococcus faecalis–Klebsiella variicola bacterial consortium from textile wastewater sludge was determined to remove more than 98% of the RR198 (from an initial concentration of 10–25 mg/L) after 72 h of incubation [57]. Similar report was the bacterial consortium of K. pneumoniae and K. variicola isolated from textile effluent–contaminated soil for decolorizing and degrading different azo dyes [58].
Degradation of waste
Generation of wastes from human activity has a high cost and leads to rapid deterioration of the environment. A major alarming issue is the high rate of fossil fuel consumption and associated emission of toxic gases, and pollution levels are forcing to use alternative energy sources [59, 60]. Among the diverse methods to handle waste, biological mechanisms have the potential to treat wastes and produce green energy as well [61]. Cellulose and lignocellulose are the most abundant materials in nature, and their main uses are in the form of wood for construction, furniture, paper, boards, cloths, foods, and feeds. In developing countries, the cellulose and lignocellulose generated is not utilized and is discarded as waste by the agriculture and food industry, including straw, leaves, cobs, peels, and crop residues. The accumulation of this waste is becoming an environmental problem and the challenge for its reuse by agriculture and food industries by converting it into value-added products, such as sugar, the final product of cellulose degradation by the cellulase [62].
Microbial cellulases are used in the production of pulp and paper, textiles, laundry, biofuel production, food and feed industries, brewing, and agriculture. PRW-1 isolated from soil samples produced efficient cellulase enzymes for the degradation of the cellulose molecule into glucose [63]. The search for coliform bacteria from domestic sewage contaminated with hydrocarbons presents the potential general scheme for the isolation of microorganisms with effective hydrocarbon-biodegrading ability. The report of a K. variicola isolate able to grow on a wide range of aliphatic and aromatic hydrocarbons indicates its high potential for the utilization of hydrocarbons as sole sources of carbon and energy and for the biodegradation of oil spilled in the environment [64] (Fig. 1d, f).
Source material industry
The pullulanase belonging to a family of glycosyl hydrolases is commonly known as α-amylase. This enzyme specifically catalyzes the hydrolysis of α-1,6-glucosidic linkages by debranching unmodified substrates, such as pullulan, β-limit dextrin, amylopectin, and related polymers, to produce linear oligosaccharides. The products of pullulanase, such as maltose, amylose, glucose, maltose syrups, high-purity glucose, and fructose, are used in a wide number of industrial processes, such as the food, fermentation, and pharmaceutical industries. A novel pullulanase enzyme identified and characterized in the isolate K. variicola SHN-1 has the potential for industrial application to lower the cost of starch processing with higher productivity [65] (Fig. 1g).
Fermentation
In food industry, one of the goals is the employment of specific microorganisms for manufacturing refined products. One of the most important biochemical processes in the proteolysis of milk protein is the release of amino acids during fermentation. In the fermentation of milk, a more efficient microorganism could improve the qualities of the derived product by the utilization of consortium K. variicola 35H and Lactobacillus strains in skim milk fermentation. Utilization of this consortium showed the highest production of the essential amino acid leucine (L–Leu) and isoleucine (I–Ile) and the production of volatile compounds important in the aroma and flavor of the fermented dairy products, such as acetoin, ethanol, acetic, hexanoic, and octanoic acids [66] (Fig. 1g).
Potential source of renewable energy
The conversion of biochemical energy to electrical energy using electroactive microorganisms as microbial fuel cells is one of the most effective, environmentally friendly, renewable, and sustainable technologies. Apart from the generation of electricity, microbial fuel cells are an effective solution for different types of wastewater treatment. The generation of electricity from pretreated palm oil mill effluent inoculated with K. variicola obtained from city wastewater is one of the perfect examples of this eco-friendly technology [17, 67].
To overcome the limited availability of fuels and use renewable resources as feed, attempts were made to utilize algal biomass. Bacterial fermentation of algal biomass to glycerol and its further use was explored. K. variicola was genetically modified to obtain strain TB-83D, which could grow well in the presence of 7% (v/v) ethanol and transformed glycerol supplemented with corn steep liquor and yeast extract to produce 34 g/L of ethanol [68].
Industrialized livestock farms generate huge amounts of animal waste slurry. A traditional method for waste management and disposal is to use it as an agricultural fertilizer. A proposed alternative use of the waste slurry is as a substitute for commercial substrates for the fermentation of glycerol to produce ethanol [62]. A methane fermentation digested slurry was used efficiently as a principal carbon source for ethanol production from glycerol by K. variicola strain TB-83D [69] (Fig. 1g). Similar examples come from K. variicola isolate SRP3 and mutant SW3 derived from it. The use of these strains in culture with glycerol as a sole carbon source increases the amount of important products like the 2,3-butanediol (2,3-BD), an important chemical used in diverse industries including chemical, cosmetics, agriculture, and pharmaceutics and the 3-propanediol (PDO), a precursor monomer for the synthesis of important industrial polymers [70, 71].
Considerations
This review compiles an extensive selection of works that describe the niches, properties, and utility of K. variicola from a wide range of environments, highlighting their great industrial potential and usefulness in ecosystem conservation.
We observed that in most published works, the K. variicola isolates were identified by 16S rRNA sequences analysis (Table 1). Furthermore, it has been suggested that identification of the K. pneumoniae complex at species level using 16S rRNA may lead to an incorrect classification [8, 72]. Thus, in order to avoid misclassification, there are different methods available for their proper identification, ranging from the amplification of single genes by PCR, rpoB phylogenetic analysis, genomics, and proteomics approaches. Among the genomics approaches, the ANI and MLST are recommended and widely used for proper identification and typing of bacterial species [8]. The MLST typing scheme for K. variicola (http://mlstkv.insp.mx) integrates the ANI tool in order for proper identification of the bacterial species, allowing the correct differentiation between the members of the K. pneumoniae complex. Considering the MLST as a rigorous system that will improve molecular epidemiology studies, we corroborated the identity of the available K. variicola genomes reported in this review through the ANI and MLST analysis, and all of them were classified as K. variicola (Table 1) [25]. At this point, after the analysis of the identification tools used and reported, we do not have enough criteria to exclude any of the reports included in this review in which K. variicola was identified.
Table 1.
Analysis and data of Klebsiella variicola identification
| Isolated | K. variicola sequences available | Sequences analyzed | NCBI—BLAST analysis ID % 16S rRNA |
ANI analysis ID % Genome |
GenBank accession number | REFERENCE |
|---|---|---|---|---|---|---|
| F2R9 | Whole genome | Whole genome | K. variicola 100 | K. variicola 100 | CP010523.2 | 5 |
| KV321 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | LQMR00000000 | 26 |
| T29A | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | CXPA00000000 | 44 |
| X39 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | CP018307 | 45 |
| 6A2 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | CXPC00000000 | 4 |
| At -22 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | CP001891.1 | 11 |
| D5A | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.2 | LOAR00000000 | 14 |
| DX120E | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.1 | CP009274 | 13 |
| VI | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.1 | In process | 4 |
| KP5-1 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.1 | CP008700.1 | 27 |
| 342 | Whole genome | Whole genome | K. variicola 100 | K. variicola 99.1 | CP000964.1 | 9 |
| KvMx2 | Whole genome | Whole genome | K. variicola 100 | K. variicola 97.1 | FLLH01000001 | 10 |
| F9 49, F9 58 | Analysis of rpoB | rpoB Fragment | K. variicola 100 | NA | NA | 19 |
| sd 1 | 16S rRNA gene | 1363 bases | K. variicola 99.8 | NA | KU353686 | 54 |
| sd -3 | 16S rRNA gene | 1386 bases | K. variicola 99.8 | NA | KU353688 | 54 |
| Kv-1 | 16S rRNA gene | 920 bases | K. variicola 99 | NA | MF179616 | 31 |
| A6123 | 16S rRNA gene | 1408 bases | K. variicola 99 | NA | KM275666.1 | 29 |
| A6126 | 16S rRNA gene | 1408 bases | K. variicola 99 | NA | KM275664.1 | 29 |
| BF1 | 16S rRNA gene | 1498 bases | K. variicola 99 | NA | MH458937.1 | 52 |
| A6127 | 16S rRNA gene | 1408 bases | K. variicola 99 | NA | KM275665.1 | 29 |
| K. variicola | 16S rRNA gene | 1465 bases | K. variicola INA | NA | KT716257 | 67 |
| IARI-PC4-59 | 16S rRNA gene | 1453 bases | K. variicola INA | NA | KT149751.1 | 40 |
| kms0422 | 16S rRNA gene | 1450 bases | K. variicola 99 | NA | KC539418 | 30 |
| AMIC1 | 16S rRNA gene | 1427 bases | K. variicola 99.7 | NA | LT838344 | 15 |
| sd -2 | 16S rRNA gene | 1383 bases | K. variicola 99.7 | NA | KU353687 | 54 |
| XF6 | 16S rRNA gene | 1414 bases | K. variicola 100 | NA | KC853302.1 | 42 |
| BCB5 | 16S rRNA gene | 1394 bases | K. variicola INA | NA | KF224908.1 | 28 |
| BCB2 | 16S rRNA gene | 1385 bases | K. variicola INA | NA | KF224905.1 | 28 |
| UDJA102 × 89–9 | 16S rRNA gene | 1153 bases | K. variicola INA | NA | LC373006.1 | 36 |
| 35H | 16S rRNA gene | 1155 bases | K. variicola INA | NA | KY229768.1 | 66 |
| SB29 | 16S rRNA gene | 900 bases | K. variicola 99 | NA | KU053822.1 | 58 |
| SRP3 | 16S rRNA gene | 740 bases | K. variicola 99 | NA | KR092086 | 70 |
| SW3 | 16S rRNA gene | 738 bases | K. variicola 99 | NA | KY437692 | 71 |
| VITMVCJ1 | 16S rRNA gene | 1471 bases | K. variicola INA | NA | KU359261 | 16 |
| FH-1 | 16S rRNA gene | 1442 bases | K. variicola 99 | NA | MH250202 | 53 |
| RBEB3 | 16S rRNA gene | 1422 bases | K. variicola INA | NA | KF036184.1 | 39 |
| SHN -1 | 16S rRNA gene | 1447 bases | K. variicola 99 | NA | HM037179.2 | 65 |
| SL06/16–2 | 16S rRNA gene | 485 bases | K. variicola 99 | NA | KX279367.1 | 64 |
| PRW 1 | 16S rRNA gene | INA | K. variicola 99.4 | NA | SNA | 63 |
| TB 83D | 16S rRNA gene | INA | K. variicola 99.6 | NA | SNA | 69 |
| RCA26 |
16S rRNA gene nifH gene |
690 bases 390 bases |
K. variicola 99 | NA | KX868071.1 | 37 |
| K. variicola | 16S rRNA gene | INA | K. variicola INA | NA | SNA | 63 |
NA not apply, SNA sequences not available, INA information not available, ANI average nucleotide identity—available at http://mlstkv.insp.mx as K. pneumoniae complex identification tool (Barrios-Camacho etal. 2019)
Concluding remarks
K. variicola is a bacterium of ecological importance, common in natural environments and playing a different role in each of them (Fig. 1). K. variicola could be considered a plant growth promoter, due to its ability to fix nitrogen and produce phytohormones and gibberellins. It has a complex nitrogen fixation system that is widely distributed and conserved in K. variicola genomes. Likewise, this ability has allowed it to inhabit in restricted microenvironments such as in the colonies of leaf-cutting ant and possibly other not yet identified. Further, K. variicola has abilities in wastewater treatment, biodegradation, and bioremediation of polluted soil, either alone or in association with other organisms. In addition, K. variicola has been used for industrial applications in the food and pharmaceutical industries. The high variety of features of K. variicola can be considered a potential tool in the green industry for environmental conservation. There is a high probability that K. variicola isolates obtained from the environment are susceptible to antibiotics, as described above. However, the pathogenicity of K. variicola in humans, animals, and plants needs more research to understand the difference between pathogenic and non-pathogenic strains. It would be important to develop K. variicola isolates lacking virulence factors that are suitable and safe for industrial or environmental applications (Fig. 3).
Fig. 3.
Beneficial impact and contribution of K. variicola in the environment and biotechnological processes
Finally, the identification of closely related species that belong to the genus Klebsiella can clarify their correct differentiation. We encourage the Klebsiella community to implement accurate identification methods not only for their importance at the clinical level, but also for the potential that this bacterium has in biotechnological and industrial applications.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Heatmap of the Nif gene encoding genes. (PPTX 300 KB)
Acknowledgements
Rodríguez-Medina N. was a PhD student of Programa de Doctorado en Ciencias Biomédicas from Universidad Nacional Autónoma de México and a fellow at CONACYT. We thank Dr. Michael Dunn from the Center for Genomic Science-UNAM for reviewing the manuscript
Author contribution
Conceptualization: H. B. C., U. G. R., N. R. M., J. D. B.; writing—original draft preparation: H. B. C., J. D. B., U. G. R., N. R. M.; analyzed the data: A. A. V., N. R. M.; writing—review and editing: H. B. C., J. D. B.; supervision: H. B.
Funding
The Instituto Nacional de Salud Publica supported this work.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rodrigues C, Passet V, Rakotondrasoa A, Brisse S. Identification of Klebsiella pneumoniae, Klebsiella quasipneumoniae, Klebsiella variicola and related phylogroups by MALDI-TOF mass spectrometry. Front Microbiol. 2018;7(9):3000. doi: 10.3389/fmicb.2018.03000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues C, Passet V, Rakotondrasoa A, Diallo TA, Criscuolo A, Brisse S. Description of Klebsiella africana sp. nov., Klebsiella variicola subsp. tropica subsp. nov. and Klebsiella variicola subsp. variicola subsp. nov. Res Microbiol. 2019;170(3):165–170. doi: 10.1016/j.resmic.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol. 2020;18(6):344–359. doi: 10.1038/s41579-019-0315-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblueth M, Martínez L, Silva J, Martínez-Romero E. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst Appl Microbiol. 2004;27(1):27–35. doi: 10.1078/0723-2020-00261. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Li Y, Li S, Tang L, Zheng J, An Q. Genomic identification of nitrogen-fixing Klebsiella variicola, K. pneumoniae and K. quasipneumoniae. J Basic Microbiol. 2015;56(1):78–84. doi: 10.1002/jobm.201500415. [DOI] [PubMed] [Google Scholar]
- 6.Long SW, Linson SE, Ojeda Saavedra M, Cantu C, Davis JJ, Brettin T, Olsen RJ. Whole-genome sequencing of human clinical Klebsiella pneumoniae isolates reveals misidentification and misunderstandings of Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae. mSphere. 2017;2(4):e00290–17. doi: 10.1128/mSphereDirect.00290-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Romero E, Rodríguez-Medina N, Beltrán-Rojel M, Silva-Sánchez J, Barrios-Camacho H, Pérez-Rueda E, Garza-Ramos U. Genome misclassification of Klebsiella variicola and Klebsiella quasipneumoniae isolated from plants, animals and humans. Salud Pública Mex. 2018;60(1):56–62. doi: 10.21149/8149. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Medina N, Barrios-Camacho H, Duran-Bedolla J, Garza-Ramos U. Klebsiella variicola: an emerging pathogen in humans. Emerg Microbes Infect. 2019;8(1):973–988. doi: 10.1080/22221751.2019.1634981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouts DE, Tyler HL, DeBoy RT, Daugherty S, Ren Q, Badger JH, Durkin AS, Huot H, Shrivastava S, Kothari S, Dodson RJ, Mohamoud Y, Khouri H, Roesch LF, Krogfelt KA, Struve C, Triplett EW, Methe BA. Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genet. 2008;4:e1000141. doi: 10.1371/journal.pgen.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyna-Flores F, Barrios-Camacho H, Dantán-González E, Ramírez-Trujillo JA, Lozano Aguirre Beltrán LF, Rodríguez-Medina N, Garza-Ramos U, Suárez-Rodríguez R. Draft genome sequences of endophytic isolates of Klebsiella variicola and Klebsiella pneumoniae obtained from the same sugarcane plant. Genome Announc. 2018;6(12):e00147–18. doi: 10.1128/genomeA.00147-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto-Tomas AA, Anderson MA, Suen G, Stevenson DM, Chu FS, Cleland WW, Weimer PJ, Currie CR. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science. 2009;326:1120–1123. doi: 10.1126/science.1173036. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Hou J, Wang Q, Ding L, Luo Y. Isolation and characterization of plant growth-promoting rhizobacteria and their effects on phytoremediation of petroleum-contaminated saline-alkali soil. Chemosphere. 2014;117:303–308. doi: 10.1016/j.chemosphere.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Wei C, Chen M, Wang H, Li Y, Li Y, Yang L, An Q. Complete genome sequence of endophytic nitrogen-fixing Klebsiella variicola strain DX120E. Stand Genomic Sci. 2015;8(10):22. doi: 10.1186/s40793-015-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Wang Q, Hou J, Tu C, Luo Y, Christie P. Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp. D5A. Sci Rep. 2016;6:26710. doi: 10.1038/srep26710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afzal AM, Rasool MH, Waseem M, Aslam B. Assessment of heavy metal tolerance and biosorptive potential of Klebsiella variicola isolated from industrial effluents. AMB Express. 2017;7:184. doi: 10.1186/s13568-017-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das A, Osborne JW. Enhanced bioremoval of lead by earthworm-Lumbricus terrestris co-cultivated with bacteria-Klebsiella variicola. J Photochem Photobiol B. 2017;175:65–72. doi: 10.1016/j.jphotobiol.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Islam MA, Khan MR, Yousuf A, Wai WC, and Cheng CK (2016) Electricity generation form pretreated palm oil mill effluent using Klebsiella variicola as an inoculum in microbial fuel cell. 4th International Conference on the Development in the in Renewable Energy Technology (ICDRET), Dhaka, 2016, pp. 1–4
- 18.Brisse S, Ev Duijkeren. Identification and antimicrobial susceptibility of 100 Klebsiella animal clinical isolates. Vet Microbiol. 2005;105(3–4):307–312. doi: 10.1016/j.vetmic.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Podder MP, Rogers L, Daley PK, Keefe GP, Whitney HG, Tahlan K. Klebsiella species associated with bovine mastitis in Newfoundland. PLoS One. 2014;9(9):e106518. doi: 10.1371/journal.pone.0106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catalán-Nájera JC, Garza-Ramos U, Barrios-Camacho H. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence. 2017;8(7):1111–1123. doi: 10.1080/21505594.2017.1317412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Feng Y, McNally A, Zong Z. Occurrence of colistin-resistant hypervirulent Klebsiella variicola. J Antimicrob Chemother. 2018;73(11):3001–3004. doi: 10.1093/jac/dky301. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Dong N, Liu C, Zeng Y, Sun Q, Zhou H, Hu Y, Chen S, Shen Z, Zhang R. Prevalence and molecular epidemiology of mcr-1-positive Klebsiella pneumoniae in healthy adults from China. J Antimicrob Chemother. 2020;75(9):2485–2494. doi: 10.1093/jac/dkaa210. [DOI] [PubMed] [Google Scholar]
- 23.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, Brisse S, Cao H, Wilksch J, Gorrie C, Schultz MB, Edwards DJ, Nguyen KV, Nguyen TV, Dao TT, Mensink M, Minh VL, Nhu NT, Schultsz C, Kuntaman K, Newton PN, Moore CE, St rugnell RA, Thomson NR. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA. 2015;112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter RF, Lainhart W, Twentyman J, Wallace MA, Wang B, Burnham CA, Rosen DA, Dantas G. Population structure, antibiotic resistance, and uropathogenicity of Klebsiella variicola. mBio. 2018;9(6):e02481–18. doi: 10.1128/mBio.02481-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrios-Camacho H, Aguilar-Vera A, Beltran-Rojel M, Aguilar-Vera E, Duran-Bedolla J, Rodriguez-Medina N, Lozano-Aguirre L, Perez-Carrascal O, Rojas J, Garza-Ramos U. Molecular epidemiology of Klebsiella variicola obtained from different sources. Sci Rep. 2019;9(1):10610. doi: 10.1038/s41598-019-46998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang SF, Liu Y, Xiao MY, Ruan CJ, Lu ZJ. Draft genome sequence of Klebsiella variicola strain KV321 isolated from rhizosphere soil of Pisolithus tinctorius-Eucalyptus Mycorrhiza. Genome Announc. 2016;4(4):e00676-16. doi: 10.1128/genomeA.00676-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medrano EG, Forray MM, Bell AA. Complete genome sequence of a Klebsiella pneumoniae strain isolated from a known cotton insect boll vector. Genome Announc. 2014;2(4):e00850–14. doi: 10.1128/genomeA.00850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadapad AB, Prabhakar CS, Chandekar SC, Tripathi J, Hire RS. Diversity of bacterial communities in the midgut of Bactrocera cucurbitae (Diptera: Tephritidae) populations and their potential use as attractants. Pest Manag Sci. 2016;72(6):1222–1230. doi: 10.1002/ps.4102. [DOI] [PubMed] [Google Scholar]
- 29.Ayin CM, Schlub RL, Yasuhara-Bell J, Alvarez AM. Identification and characterization of bacteria associated with the decline of ironwood (Casuarina equisetifolia) in Guam. Australas Plant Pathol. 2015;44:225–234. [Google Scholar]
- 30.Fan HC, Zeng L, Yang PW, Guo ZX, Bai TT. First report of banana soft rot caused by Klebsiella variicola in China. Plant Dis. 2015;100:517. doi: 10.1094/PDIS-05-15-0586-PDN. [DOI] [Google Scholar]
- 31.Chandrashekar BS, Prasannakumar MK, Puneeth ME, Kalavati T, Priyanka K, Mahesh HB, Desai RU. First report of bacterial soft rot of carrot caused by Klebsiella variicola in India. N Dis Rep. 2018;37:21. [Google Scholar]
- 32.Lucy M, Reed E, Glick BR. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek. 2004;86(1):1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e. [DOI] [PubMed] [Google Scholar]
- 33.Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil. 2013;366:93–105. [Google Scholar]
- 34.Pindi PK, Sultana T, Vootla PK. Plant growth regulation of Bt-cotton through Bacillus species. 3 Biotech. 2014;4(3):305–315. doi: 10.1007/s13205-013-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei CY, Lin L, Luo LJ, Xing YX, Hu CJ, Yang LT, Li YR, An Q. Endophytic nitrogen-fixing Klebsiella variicola strain DX120E promotes sugarcane growth. Biol Fertil Soils. 2014;50:657–666. [Google Scholar]
- 36.Nacoon S, Jogloy S, Riddech N, Mongkolthanaruk W, Kuyper TW, Boonlue S. Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci Rep. 2020;10(1):4916. doi: 10.1038/s41598-020-61846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Defez R, Andreozzi A, Bianco C. The overproduction of indole-3-acetic acid (IAA) in endophytes upregulates nitrogen fixation in both bacterial cultures and inoculated rice plants. Microb Ecol. 2017;74:441–452. doi: 10.1007/s00248-017-0948-4. [DOI] [PubMed] [Google Scholar]
- 38.Iniguez AL, Dong Y, Triplett EW. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol Plant Microbe Interact. 2004;17:1078–1085. doi: 10.1094/MPMI.2004.17.10.1078. [DOI] [PubMed] [Google Scholar]
- 39.Karthik M, Pushpakanth P, Krishnamoorthy R, Senthilkumar M. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J Hortic Sci Biotechnol. 2017;92(6):1–9. [Google Scholar]
- 40.Marag PS, Suman A. Growth stage and tissue specific colonization of endophytic bacteria having plant growth promoting traits in hybrid and composite maize (Zea mays L.) Microbiol Res. 2018;214:101–113. doi: 10.1016/j.micres.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Meena VS, Maurya BR, Verma JP. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res. 2014;169(5–6):337–347. doi: 10.1016/j.micres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol. 2014;82:18–25. [Google Scholar]
- 43.Bhagia S, Akinosho H, Ferreira J, Ragauskas A. Biofuel production from Jerusalem artichoketuber inulins: a review. Biofuel Res J. 2017;4(2):587–599. [Google Scholar]
- 44.Rodríguez-Andrade O, Corral-Lugo A, Morales-García Y, Quintero-Hernández V, Rivera-Urbalejo A, Molina-Romero D, Martínez-Contreras R, Bernal P, Muñoz-Rojas J. Identification of Klebsiella Variicola T29A genes involved in tolerance to desiccation. Open Microbiol J. 2019;13:256–267. [Google Scholar]
- 45.Guo Y, Zhai Y, Zhang Z, Li D, Wang Z, Li J, He Z, Hu S, Kang Y, Gao Z. Complete genomic analysis of a kingdom-crossing Klebsiella variicola isolate. Front Microbiol. 2018;9:2428. doi: 10.3389/fmicb.2018.02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2:621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 47.Klipp W, Masepohl B, Gallon JR and Newton WE (2005) Genetics and regulation of nitrogen fixation in free-living bacteria. Nitrogen Fixation: Origins, Applications, and Research Progress. 2nd ed. Kluwer Academic Publisher. 10.1007/1-4020-2179-8
- 48.Igai K, Itakura M, Nishijima S, Tsurumaru H, Suda W, Tsutaya T, Tomitsuka E, Tadokoro K, Baba J, Odani S, Natsuhara K, Morita A, Yoneda M, Greenhill AR, Horwood PF, Inoue J, Ohkuma M, Hongoh Y, Yamamoto T, Siba PM, Hattori M, Minamisawa K, Umezaki M. Nitrogen fixation and nifH diversity in human gut microbiota. Sci Rep. 2016;6:31942. doi: 10.1038/srep31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blin C, Passet V, Touchon M, Rocha EPC, Brisse S. Metabolic diversity of the emerging pathogenic lineages of Klebsiella pneumoniae. Environ Microbiol. 2017;19:1881–1898. doi: 10.1111/1462-2920.13689. [DOI] [PubMed] [Google Scholar]
- 50.Garza-Ramos U, Martinez-Romero E, Silva-Sanchez J. SHV-type extended-spectrum beta-lactamase (ESBL) are encoded in related plasmids from enterobacteria clinical isolates from Mexico. Salud Publica Mex. 2007;49:415–421. doi: 10.1590/s0036-36342007000600008. [DOI] [PubMed] [Google Scholar]
- 51.Shahadatab M, Teng TT, Rafatullahc M, Shaikha ZA, Sreekrishnana TR, Ali SW. Bacterial bioflocculants: a review of recent advances and perspectives. Chem Eng J. 2017;328(15):1139–1152. [Google Scholar]
- 52.Nguyen NT, My Phan TH, Tran TN, Velmurugan BK, Kiefer R. Production of novel bio-flocculants from Klebsiella variicola BF1 using cassava starch wastewater and its application. Curr Sci. 2019;117(121):129. [Google Scholar]
- 53.Zhang J, Liang S, Wang X, Lu Z, Sun P, Zhang H, Sun F. Biodegradation of Atrazine by the novel Klebsiella variicola strain FH-1. Biomed Res Int. 2019;2019:4756579. doi: 10.1155/2019/4756579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng Y, Feng J, Shu QL. Isolation and characterization of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae and Klebsiella variicola strains from various environments. J Appl Microbiol. 2018;124(5):1195–1211. doi: 10.1111/jam.13703. [DOI] [PubMed] [Google Scholar]
- 55.Issazadeh K, Savaheli H, Momeni N. Isolation and identification of heavy metal tolerant bacteria from industrial wastewaters in Guilian Province. Int J Adv Biol Biomed Res. 2014;2(6):2066–2071. [Google Scholar]
- 56.Singh R, Gautam N, Mishra A, Gupta R. Heavy metals and living systems: an overview. Indian J Pharmacol. 2011;43(3):246–253. doi: 10.4103/0253-7613.81505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eslami H, Shariatifar A, Rafiee E, Shiranian M, Salehi F, Sadat-Hosseini S, Eslami G, Ghanbari R, Asghar-Ebrahimi A. Decolorization and biodegradation of reactive Red 198 Azo dye by a new Enterococcus faecalis-Klebsiella variicola bacterial consortium isolated from textile wastewater sludge. World J Microbiol Biotechnol. 2019;35(3):38. doi: 10.1007/s11274-019-2608-y. [DOI] [PubMed] [Google Scholar]
- 58.Hassan SED, Fouda A, Azab MS, Saied E. Biological decolorization of different azo dyes using two bacterial strains of Klebsiella spp. and their consortium. Int J Environ Biol. 2015;5(4):104–114. [Google Scholar]
- 59.Bogner J, Pipatti R, Hashimoto S, Diaz C, Mareckova K, Diaz L, Kjeldsen P, Monni S, Faaij A, Gao Q, Zhang T, Abdelrafie-Ahmed M, Sutamihardja RTM, Gregory R, Intergovernmental Panel onClimateChange (IPCC) WorkingGroup III (Mitigation) Mitigation of global greenhouse gas emissions from waste: conclusions and strategies from the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report. Working Group III (Mitigation) Waste Manag Res. 2008;26(1):1–32. doi: 10.1177/0734242X07088433. [DOI] [PubMed] [Google Scholar]
- 60.Schauer JJ. Design criteria for future fuels and related power systems addressing the impacts of non-CO2 pollutants on human health and climate change. Annu Rev Chem Biomol Eng. 2015;6:101–120. doi: 10.1146/annurev-chembioeng-061114-123337. [DOI] [PubMed] [Google Scholar]
- 61.Kalia VC, Prakash J, Koul S. Biorefinery for glycerol rich biodiesel industry waste. Indian J Microbiol. 2016;56:113–125. doi: 10.1007/s12088-016-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liguori R, Ventorino V, Pepe O, Faraco V. Bioreactors for lignocellulose conversion into fermentable sugars for production of high added value products. Appl Microbiol Biotechnol. 2016;100(2):597–611. doi: 10.1007/s00253-015-7125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waghmare PR, Kshirsagar SD, Saratale RG, Govindwar SP, Saratale GD. Production and characterization of cellulolytic enzymes by isolated Klebsiella sp. PRW-1 using agricultural waste biomass. Emir J Food Agric. 2014;26(1):44–59. [Google Scholar]
- 64.Khanafer M, Al-Awadhi H, Radwan S (2017) Coliform bacteria for bioremediation of waste hydrocarbons. Biomed Res Int 2017:1838072. 10.1155/2017/1838072 [DOI] [PMC free article] [PubMed]
- 65.Chen WB, Nie Y, Xu Y. Signal peptide-independent secretory expression and characterization of pullulanase from a newly isolated Klebsiella variicola SHN-1 in Escherichia coli. Appl Biochem Biotechnol. 2013;169(1):41–54. doi: 10.1007/s12010-012-9948-5. [DOI] [PubMed] [Google Scholar]
- 66.Rosales-Bravo H, Morales-Torres HC, Vázquez-Martínez J, Molina-Torres J, Olalde-Portugal V, Partida-Martínez LP. Novel consortium of Klebsiella variicola and Lactobacillus species enhances the functional potential of fermented dairy products by increasing the availability of branched-chain amino acids and the amount of distinctive volatiles. J Appl Microbiol. 2017;123(5):1237–1250. doi: 10.1111/jam.13565. [DOI] [PubMed] [Google Scholar]
- 67.Amirul I, Ahasanul K, Wai WC, Baranitharan E, Cheng CK, Yousuf A, Khan MR. Augmentation of air cathode microbial fuel cell performance using wild type Klebsiella variicola. RSC Adv. 2017;7:4798–4805. [Google Scholar]
- 68.Suzuki T, Seta K, Nishikawa C, Hara E, Shigeno T, Nakajima-Kambe T. Improved ethanol tolerance and ethanol production from glycerol in a streptomycin-resistant Klebsiella variicola mutant obtained by ribosome engineering. Bioresour Technol. 2015;176:156–162. doi: 10.1016/j.biortech.2014.10.153. [DOI] [PubMed] [Google Scholar]
- 69.Seta K, Suzuki T, Kiyoshi K, Shigeno T, Nakajima-Kambe T. Potential use of methane fermentation digested slurry as a low-cost, environmentally-friendly nutrient for bioethanol production from crude glycerol by Klebsiella variicola TB-83D. N Biotechnol. 2018;44:1–5. doi: 10.1016/j.nbt.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Rahman MS, Yuan Z, Ma K, Xu CC, Qin W. Aerobic conversion of glycerol to 2,3-butanediol by a novel Klebsiella variicola SRP3 strain. J Microb Biochem Technol. 2015;7:299–304. [Google Scholar]
- 71.Rahman S, Xu C, Ma K, Guo H, Qin W. Utilization of by-product glycerol from bio-diesel plants as feedstock for 2,3-butanediol accumulation and biosynthesis genes response of Klebsiella variicola SW3. Renew Energy. 2017;114(B):1272–1280. [Google Scholar]
- 72.Martínez J, Martínez L, Rosenblueth M, Silva J, Martínez-Romero E. How are gene sequence analyses modifying bacterial taxonomy? The case of Klebsiella. Int Microbiol. 2004;7(4):261–268. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Heatmap of the Nif gene encoding genes. (PPTX 300 KB)