Graphical abstract
This study investigated the anti-inflammatory activity of Lactiplantibacillus plantarum IDCC 3501 isolated from kimchi (Korean fermented food) and its safety. When lipopolysaccharide (LPS)-induced RAW 264.7 macrophages were treated with cell-free supernatant from L. plantarum IDCC 3501, the mRNA expression level of inflammatory markers (i.e., TNF-α, IL-1β, and IL-6) was significantly reduced. In addition, the decreased cell viability by LPS was recovered and NO production in LPS-induced cell was also decreased. For the safety assessment, the genes responsible for antibiotic resistance and virulence were not detected from the genome analysis of this strain. Consistent with this, minimal inhibitory concentrations against various antibiotics, biogenic amines, and d-lactate production, as well as enzymatic and hemolysis activities, indicated that L. plantarum IDCC 3501 did not produce any harmful compounds during fermentation. Furthermore, no acute toxicity and mortality were observed in a murine mouse model. Based on our findings, L. plantarum IDCC 3501 is safe and beneficial for human consumption.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00603-2.
Keywords: Anti-inflammatory, Lactic acid bacteria, Lactiplantibacillus plantarum, Probiotics, Safety evaluation
Introduction
Lactiplantibacillus plantarum, formally known as Lactobacillus plantarum, is a major widespread species among lactobacilli due to its ecological niches, existing in dairy products, fermented foods, and host’s mouth and intestinal tract [1]. As a facultatively heterofermentative species, L. plantarum has various beneficial health effects, such as antimicrobial activity [2], antiobesity [3], immune-boosting [4], and anti-inflammatory effects [5]. For this reason, L. plantarum has been used in various industrial food fermenters as a starter. As a probiotic, this species typically possesses acid/bile tolerance and intestinal adhesion activity [6, 7], and alleviating acute and chronic inflammation.
Inflammation is an immunity-mediated response to viral or pathogenic infection, toxic compounds, or irradiation [8]. This response is typically regulated by pro-inflammatory mediators (e.g., iNOS and COX-2) and cytokines (e.g., TNF-α, IL-1β, and IL-6) [9], resulting in the recruitment of immune cells (e.g., macrophages) and systemic responses. Mainly, gut inflammation often causes acute symptoms, such as diarrhea, gastric bleeding, and abdominal pain and chronic symptoms, such as inflammatory bowel disease and colorectal cancer [10]. Therefore, the anti-inflammatory effect of probiotics has been intensively studied in the last decades to improve gut health. For example, L. plantarum has significant positive effects on Crohn’s disease and ulcerative colitis by modulating the intestinal microbiota, suppressing pathogens, and boosting the immune system [11, 12].
Previously, L. plantarum IDCC 3501 was reported to have the following characteristics as probiotics: coaggregation with pathogens, 25.0–66.1%; hydrophobicity, 39.2%; acid tolerance, 84.9%; and antibacterial effects against nine pathogens including Escherichia coli O157:H7 and Salmonella Typhimurium [13]. However, the safety of L. plantarum IDCC 3501 needs to be carefully examined for human consumption on the strain basis.
In this study, the anti-inflammatory potential of L. plantarum IDCC 3501 and its safety were investigated. Here, cell viability, nitric oxide (NO) production, and the expression of inflammatory markers were investigated in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. In vitro and in vivo studies, including hemolysis, minimal inhibitory concentration (MIC) tests, whole-genome analysis, and single-dose acute oral toxicity test in rats, were performed.
Materials and methods
Bacterial strain, culture conditions, and preparation of cell-free supernatants
Lactiplantibacillus plantarum IDCC 3501 (ATCC BAA-2838), isolated from kimchi (Korean fermented food), has been manufactured in Ildong Bioscience (Pyeongtaek-si, Korea) since 2015. L. plantarum IDCC 3501 and Lacticaseibacillus rhamnosus GG were anaerobically cultured in De Man, Rogosa, and Sharpe (MRS; BD Difco, Franklin Lakes, NJ, USA) medium at 37 °C in a static incubator. Staphylococcus aureus ATCC 25,923 was used as a positive strain for hemolysis assay, and it was cultured in brain heart infusion (BHI; BD Difco) medium at 37 °C with shaking at 200 rpm. Supernatants of 16 h cultured L. plantarum IDCC 3501 and L. rhamnosus GG (positive control) were prepared by centrifugation at 8,000 rpm and filtered using a 0.22-μm syringe filter (Merck Millipore, Burlington, MA, USA).
Cell culture
RAW 264.7 cells, one of the most representative macrophage-like cell lines, were purchased from Korean Cell Line Bank (Seoul, Korea) and were cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, MA, USA), and supplemented with 10% (v/v) fetal bovine serum and 1% (w/v) penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
Macrophage viability assay
The viability of RAW 264.7 cells was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, the cells (1 × 105 cells·well−1) were seeded in a 96-well plate for 24 h and treated with L. plantarum IDCC 3501 cell-free supernatants for 2 h. Then, MTT was added to each well and incubated for 4 h at 37 °C. After removal of media and MTT, dimethyl sulfoxide (DMSO) was added to the well to dissolve the formazan. Finally, the developed color was measured at 540 nm using a microplate reader (BioTek, Winooski, VT, USA).
Measurement of nitric oxide production
Nitric oxide contents were determined by the Griess reaction (Promega, Madison, WI, USA). Briefly, 50 µL of cell supernatant was collected and mixed with 50 µL of Griess A (1% sulfanilamide) and 50 µL of Griess B (0.1% N-1-naphthylethylenediamine dihydrochloride). The mixture was then incubated at room temperature for 10 min, and nitric oxide contents were determined at 540 nm according to the calibration curve.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA from RAW 264.7 cells treated with LPS and supernatant of either L. plantarum IDCC 3501 or L. rhamnosus GG was isolated using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription (Superscript IV First-Strand Synthesis System; Thermo Fisher Scientific) was then performed to synthesize cDNA with 2 μg of total RNA and random hexamers. Finally, PCR was performed using the primers listed in Table S2, and the bands were analyzed and quantified using Image Lab (Bio-Rad, Hercules, CA, USA).
Gene search for antibiotic resistance and virulence
The complete genome sequence of L. plantarum IDCC 3501 was previously reported, consisting of a circular 3,242,587-bp chromosome with a GC content of 44.52% (Table S1) [14] (GenBank accession no. CP031702). The assembled sequence was compared with the reference sequences in the ResFinder database, using ResFinder v.3.2 (https://cge.cbs.dtu.dk/servies/ResFinder). The search parameters were sequence identity greater than 80% and a 60% coverage. Virulence genes were searched using the BLASTn algorithm with VFDB database [15]. The identification thresholds were identity greater than 70%, coverage greater than 70%, and E-value less than 1E−5.
Determination of MIC
The susceptibility of L. plantarum IDCC 3501 to ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, and chloramphenicol was evaluated as followed by CLSI (Clinical Laboratory Standards Institute) protocol [16]. A single colony was inoculated into MRS broth and cultured for 16–18 h. The cultured cells and antibiotic solution were mixed in a 96-well plate to obtain an initial cell density of 5 × 105 CFU mL−1 and antibiotic concentration of 0.125–1024 µg mL−1. The plate was then anaerobically incubated at 37 °C for 20 h. Finally, optical density was measured using a microplate reader (BioTek), and MICs for each antibiotic were determined as the lowest concentration that completely inhibited cell growth.
Biogenic amine and lactate concentration determination
The supernatants from L. plantarum IDCC 3501 were collected by centrifugation at 15,928 × g for 5 min at 4 °C and were filtered using a 0.2-μm pore-size membrane. Then, 1 mL of supernatant was mixed with 200 µL of saturated NaHCO3, 20 µL of 2 M NaOH, and 0.5 mL of dansyl chloride (10 mg mL−1 acetone), and the mixture was incubated at 70 °C for 10 min for derivatization. The derivatized sample was added with 200 µL of proline (100 mg mL−1 H2O) and incubated in a dark room for 15 min. The sample was made up to 5 mL with acetonitrile and filtered with a 0.45-µm membrane. Finally, biogenic amines were analyzed using high-performance liquid chromatography (HPLC; LC-NETI/ADC, Jasco, Macclesfield, UK) equipped with an Athena C18 column (4.6 mm × 250 mm, ANPEL Laboratory analysis, Shanghai, China). Aqueous acetonitrile solution (67:33 of H2O) was used as the mobile phase at 0.8 mL min−1. Peaks were detected at 254 nm using a UV detector (UV-2075 Plus, Jasco, Macclesfield, UK) and quantified according to the calibration curves of each biogenic amine.
The quantities of l-lactate and d-lactate in L. plantarum IDCC 3501 supernatant were measured using an assay kit (Megazyme, Bray, Ireland) according to the manufacturer’s protocol.
Hemolytic and extracellular enzyme activities
L. plantarum IDCC 3501 and Staphylococcus aureus ATCC 25923 were streaked on sheep blood agar plates (BBL Microbiology Systems, Cockeysville, MD, USA) and incubated at 37 °C overnight. Then, β-hemolytic activity was determined by observing clear zones around colonies.
Enzyme activities of L. plantarum IDCC 3501 were assessed using the API-ZYM kit (BIOMÉRIUX, Marcy-l’Étoile, France), which can evaluate 19 hydrolytic enzyme activities according to the manufacturer’s protocol.
Acute oral toxicity test
An acute oral toxicity test was performed by Korea Testing and Research Institute (Hwasun-gun, Jeollanam-do, Korea) according to the OECD guidelines (Test No. 425) [17]. The procedure is shown in Fig. S3. Briefly, 12 Crl:CD(SD) female rats aged 9–10 weeks were divided into 4 groups of 3 rats each. Each group’s rats were orally administered at 300 mg kg−1 or 2000 mg kg−1, corresponding to 3.4 × 1011–3.6 × 1011 CFU and 2.3 × 1012–3.4 × 1012 CFU, respectively. Then, toxicity, morality, and body changes were observed for 14 days. Finally, 100mL isoflurane was treated to euthanize the rats, and an autopsy for the examination of organs was performed.
Results and discussion
There are many reports about whole cells (e.g., alive or heat-killed cells) or cell wall components of lactic acid bacteria. Recently, much attention has been paid to cell-free supernatants containing biologically active metabolites which are secreted by live bacteria [18]. According to Aguilar-Toalá [19], the term “postbiotics” refers to a preparation of inanimate microorganisms and/or their components (i.e., exopolysaccharides, supernatant, cell wall fragments, bacterial lysates, enzymes, and various metabolites) that confers a health benefit on the host. Thus, in this study, we were focusing on the use of the cell-free supernatants, a type of postbiotics, as an effective anti-inflammatory agent.
Effects of L. plantarum IDCC 3501 on cell viability
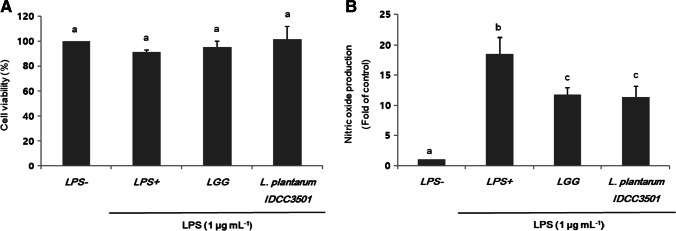
The cytotoxicity of the supernatants of L. plantarum IDCC 3501 on RAW 264.7 cells, which inflammation was induced by 1 μg per mL of LPS, was determined using MTT assay (Fig. 1A). The supernatants of L. rhamnosus GG were also applied as a control. After treatment using the supernatants of L. plantarum IDCC 3501, the decreased cell viability by LPS was recovered, showing no negative effect on cell viability. Like cell viability results, LPS addition showed a remarkable increase in NO production, which shows early stimulation exerted on activated macrophages (Fig. 1B). However, the supernatants of L. plantarum IDCC 3501 considerably inhibited NO production in a level of more than 60%. Thus, both cell viability and NO production results suggested that L. plantarum IDCC 3501 supernatants positively affected the relief of oxidative stress in cells.
Fig. 1.
Effects of L. plantarum IDCC 3501 and L. rhamnosus GG on (A) cell viability and (B) NO production in LPS-induced RAW 264.7 cells. The results were expressed as mean ± standard deviation from three independent experiments. Different letters indicate significant differences at p-value < 0.05 by Duncan’s studentized range test
The effect of L. plantarum IDCC 3501 on pro-inflammatory cytokines
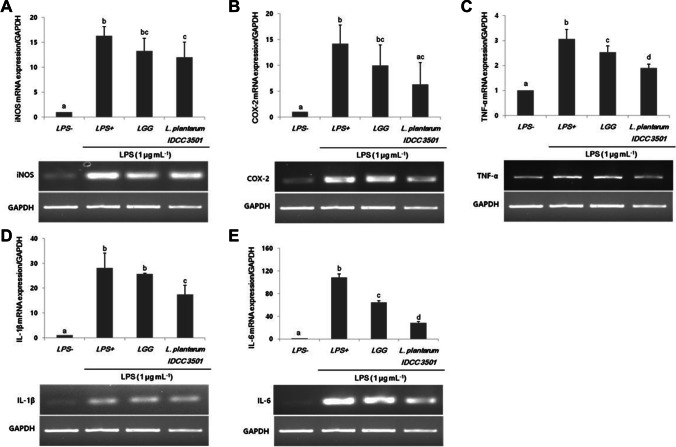
Activated macrophages which were regulated by LPS-induced pro-inflammatory cytokine mediators could release various cytokines to enhance immune defense mechanisms, such as TNF-α, IL-1β, and IL-6 in RAW 264.7 cells. To demonstrate that L. plantarum IDCC 3501 inhibits LPS-induced nitrite and PEG2 production, iNOS and COX-2 expression were investigated using semi-RT-PCR (Fig. 2A and B). The expression levels of both iNOS and COX-2 were significantly increased in LPS-induced cells. L. plantarum IDCC 3501 decreased iNOS and COX-2 expression by 26.5% and 55.5% than the values of LPS-induced cells, respectively. Although TNF-α, IL-1β, and IL-6 were inhibited by 38.2%, 37.9%, and 73.8%, respectively (Fig. 2C–E), IL-6 was the mostly affected among these cytokines.
Fig. 2.
Effects of L. plantarum IDCC 3501 and L. rhamnosus GG on mRNA expression of (A) iNOS, (B) COX-2, (C) TNF-α, (D) IL-1β, and (E) IL-6 in LPS-induced RAW 264.7 cells. The results were expressed as mean ± standard deviation from three independent experiments. Different letters indicate significant differences at p-value < 0.05 by Duncan’s studentized range test
Previous studies have shown anti-inflammatory response on intestinal epithelial cells and macrophages by reducing pro-inflammatory mediators or cytokines [19]. Among them, the deregulation of IL-6 has been implicated in the pathogenesis of many diseases, especially Crohn’s disease and colon cancer [20]. According to our findings, the supernatants of L. plantarum IDCC 3501 exert anti-inflammatory effects because it leads to iNOS, COX-2, TNF-α, IL-1β, and IL-6 inhibition under viable conditions (Fig. 2). Thus, cell-free bacterial supernatants are promising anti-inflammatory agents for treating gut inflammation. The administration of our strains to infected mice can clarify the effects for further study. In previous study, the effect of the ID-JPL 936 (mixtures of L. plantarum IDCC 3501, L. johnsonii IDCC 9203, and Bifidobacterium lactis IDCC 4301) on inhibition of pro-inflammatory cytokines expression was investigated in a mouse model [21]. The administration of the mixture significantly reduced disease activity index (DAI), improved contraction of colon length, and decreased the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Thus, the anti-inflammatory effects of L. plantarum IDCC 3501 in this study could be further explained [21].
Whole-genome analysis and antibiotic susceptibility of L. plantarum IDCC 3501
In lactic acid bacteria, antibiotic resistance and virulence factors are easily acquired from an exogenous source. Gene transfer commonly involves bacterial conjugative plasmids, transposable elements, and integron systems [22, 23]. Thus, whole-genome analysis is becoming an essential procedure for safety assessment in the probiotic industry. Notably, L. plantarum IDCC 3501 has no antibiotic resistance genes and virulence factors [15]. Meanwhile, L. plantarum IDCC 3501 showed susceptibility to all antibiotics, except kanamycin (Table 1). Lactobacillus strains are susceptible to antibiotics related to protein synthesis (e.g., chloramphenicol, erythromycin, clindamycin, and tetracycline) but are aminoglycosides-resistant (neomycin, kanamycin, streptomycin, and gentamicin) [24]. For example, approximately 79% of isolated probiotic strains showed kanamycin resistance [25]. Thus, L. plantarum IDCC 3501 resistance to kanamycin might be considered aminoglycoside intrinsic because of membrane impermeability by potential efflux mechanisms [26].
Table 1.
Minimal inhibitory concentration and antibiotic resistance gene of L. plantarum IDCC 3501
| Class | β-Lactam | Glycopeptide | Aminoglycoside | Macrolide | Lincomycin | Tetracycline | Amphenicol | ||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | AMP | VAN | GEN | KAN | STR | ERY | CLI | TET | CHL |
|
Breakpoint (µg mL−1)a MIC Antibiotic resistance gene |
2 0.25–0.5 no |
n.r. > 512 no |
16 8–16 no |
64 256 no |
n.r. 32 no |
1 < 0.125 no |
4 0.5 no |
32 4 no |
8 2–8 no |
Abbreviations: AMP ampicillin; VAN vancomycin; GEN gentamicin; KAN kanamycin; STR streptomycin; ERY erythromycin; CLI clindamycin; TET tetracycline; CHL chloramphenicol; MIC minimal inhibitory concentration; n.r. not required
aEFSA (2018)
According to hemolytic activity results identified as a virulence factor, L. plantarum IDCC 3501 showed no hemolysis zones on the sheep blood agar plates, unlike S. aureus ATCC 25,923 which was used as a positive control showing a distinct hemolytic zone (Fig. S1). In conclusion, L. plantarum IDCC 3501 was safe regarding antibiotic resistance and virulence factors.
In vitro safety evaluation of L. plantarum IDCC 3501
In this study, observations of biogenic amines (BAs), d-lactate, and potential toxin-producing enzymes in L. plantarum IDCC 3501 were evaluated. BAs are low molecular weight compounds derived by the decarboxylation of amino acids. BAs are present in various foods and beverages, such as meat, fish, cheese, and vegetables [27]. However, BAs are precursors for forming N-nitroso compounds known as cancer-causing agents [28]. Also, high BA consumption may cause symptoms, such as headache, heart palpitations, vomiting, diarrhea, and hypertensive crises in humans and animals [29]. In this study, BAs’ production including tyramine, histamine, putrescine, 2-phenethyamine, and cadaverine was not observed during L. plantarum IDCC 3501 fermentation (data not shown).
Lactate, a key molecule produced during the fermentation of lactic acid bacteria, exists in two forms, d-lactate and l-lactate. In humans, more than 99% of lactate found in the blood is l-lactate. Even though d-lactate appears in human tissue, it possesses D-2-hydroxy acid dehydrogenase that converts d-lactate to pyruvate, resulting in a decrease in acidosis risk [30]. In this study, L. plantarum IDCC 3501 produced 22.64 g L−1 of l-lactate (61.70%) and 14.05 g L−1 of d-lactate (38.30%) (Table 2). d-Lactate production in L. plantarum IDCC 3501 was much lower than in other lactic acid bacteria. Next, an essential criterion in safety assessment is the absence of harmful enzymatic activity. For example, α-chymotrypsin has been associated with virulence and local tissue injury during infective endocarditis [31]. β-Glucuronidase can cleave glucuronic acid–conjugated carcinogens related to toxic compounds [32]. Also, β-glucosidase may have damaging effects on the colon [33]. In this study, L. plantarum IDCC 3501 had no α-chymotrypsin and β-glucuronidase (Table 3 and Fig. S2). Although a low level of β-glucosidase activity was shown, it was relatively low compared with other lactic acid bacteria. In contrast, β-galactosidase expressed in probiotics has contributed to the relief of lactose maldigestion symptoms [34]. Also, aminopeptidases, such as leucine arylamidase, valine arylamidase, and cystine arylamidase, are involved in cheese ripening by supplying free amino acids that further affect metabolism of cheese flavor [35]. Conclusively, L. plantarum IDCC 3501 retained these beneficial enzymes (Table 3 and Fig. S2).
Table 2.
The ratio of d-lactate to l-lactate
| Strain | d-Lactate | l-Lactate |
|---|---|---|
| L. plantarum IDCC 3501 |
14.05 ± 0.48 g L−1 (38.30%) |
22.64 ± 0.47 g L−1 (61.70%) |
Table 3.
Enzymatic activities of L. plantarum IDCC 3501
| Enzyme | IDCC 3501 | KCC-10a | 0147b | 0612b | JCM 1057c |
|---|---|---|---|---|---|
| Alkaline phosphate | − | − | + | − | − |
| Esterase (C4) | − | + | + | − | + |
| Esterase lipase (C8) | − | + | + | − | − |
| Lipase (C14) | − | − | − | − | − |
| Leucine arylamidase | + | + | + | + | + |
| Valine arylamidase | + | + | + | + | + |
| Cystine arylamidase | − | + | + | − | − |
| Trypsin | − | + | − | − | − |
| α-Chymotrypsin | − | − | − | − | − |
| Acid phosphatase | + | − | + | + | + |
| Naphthol-AS-BI-phosphohydrolase | + | + | + | + | + |
| α-Galactosidase | + | + | − | − | − |
| β-Galactosidase | + | − | + | + | + |
| β-Glucuronidase | − | + | − | − | − |
| α-Glucosidase | + | − | + | − | + |
| β-Glucosidase | + | + | + | + | + |
| N-Acetyl-β-glucosaminidase | + | + | + | − | + |
| α-Mannosidase | − | − | − | − | − |
| α-Fucosidase | − | − | − | − | − |
In vivo safety evaluation of L. plantarum IDCC 3501 by oral administration
A 14-day oral acute toxicity study in female rats aged 9–10 weeks was performed to investigate the oral consumption safety of L. plantarum IDCC 3501 (Table 4). L. plantarum IDCC 3501 did not cause any toxic symptoms or mortality, and the rats lived up to 14 days after administering 300 mg or 2,000 mg single dose per kg body weight. Also, there were no significant behavior changes, skin effects, impairment in feed intake, and body weight. Thus, these results show that L. plantarum IDCC 3501 is safe, both in vitro and in vivo.
Table 4.
Body weight evolution of the tested rats by L. plantarum IDCC 3501 and its dosage
| Group | Dose (g kg−1 BW1) |
Day after administration | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | ||
| 9 week aged | 300 | 214.6 ± 5.1 | 234.5 ± 4.0 | 245.6 ± 4.6 | 249.8 ± 3.3 | 258.3 ± 8.3 |
| 2,000 | 223.4 ± 11.4 | 241.5 ± 15.5 | 249.1 ± 12.0 | 263.6 ± 15.0 | 267.9 ± 23.0 | |
| 10 week aged | 300 | 234.4 ± 11.4 | 257.6 ± 10.1 | 261.4 ± 10.2 | 269.0 ± 7.8 | 276.2 ± 8.9 |
| 2,000 | 238.8 ± 7.1 | 254.8 ± 7.8 | 264.8 ± 5.1 | 267.5 ± 4.5 | 277.2 ± 5.1 | |
Based on the Student’s t-tests using STATISTICA 7.0, there was no significant difference among the rats
1BW body weight
Conclusion
In this study, the anti-inflammatory potential was verified by downregulation of genes associated with pro-inflammatory mediators and cytokines in RAW 264.7 macrophage cells. Also, the safety of L. plantarum IDCC 3501 was demonstrated with various in vitro and in vivo tests. Thus, L. plantarum IDCC 3501 is considered to be safe and beneficial for consumption.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
S-Y Yang, WY Bang, and SA Chae carried out the in vitro experiments and S-Y Yang drafted the manuscript. SA Chae and M Lee carried out the cell experiments. O–H Ban carried out the genetic studies, S-J Kim participated in the design of the study, and YH Jung and J Yang conceived the study and participated in its design and coordination, and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Ildong Bioscience and the National Research Foundation of Korea (NRF) funded by Korea government (Ministry of Science and ICT; Grant No. 2020R1C1C1005251).
Data availability
All data generated or analyzed during this study are included in this published article and its additional information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young Hoon Jung, Email: younghoonjung@knu.ac.kr.
Jungwoo Yang, Email: yjw@ildong.com.
References
- 1.Siezen RJ, Tzeneva VA, Castioni A, Wels M, Phan HTK, Rademaker JLW, Starrenburg MJC, Kleerebezem M, Molenaar D, Van Hylckama Vlieg JET. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ Microbiol. 2010;12:758–773. doi: 10.1111/j.1462-2920.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 2.Dinev T, Beev G, Tzanova M, Denev S, Dermendzhieva D, Stoyanova A. Antimicrobial activity of Lactobacillus plantarum against pathogenic and food spoilage microorganisms: a review. Bulg J Vet Med. 2018;21:1–16. doi: 10.15547/bjvm.1050. [DOI] [Google Scholar]
- 3.Tsai Y-T, Cheng P-C, Pan T-M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl Microbiol Biotechnol. 2014;98:1–10. doi: 10.1007/s00253-013-5346-3. [DOI] [PubMed] [Google Scholar]
- 4.Duary RK, Bhausaheb MA, Batish VK, Grover S. Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Mol Biol Rep. 2012;39:4765–4775. doi: 10.1007/s11033-011-1269-1. [DOI] [PubMed] [Google Scholar]
- 5.Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9:555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins. 2017;9:111–122. doi: 10.1007/s12602-017-9264-z. [DOI] [PubMed] [Google Scholar]
- 7.Jeong M, Kim JH, Yang H, Kang SD, Song S, Lee D, Lee JS, Yoon Park JH, Byun S, Lee KW. Heat-killed Lactobacillus plantarum KCTC 13314BP enhances phagocytic activity and immunomodulatory effects via activation of MAPK and STAT3 pathways. J Microbiol Biotechnol. 2019;29:1248–1254. doi: 10.4014/jmb.1905.05066. [DOI] [PubMed] [Google Scholar]
- 8.Shih R-H, Wang C-Y, Yang C-M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le B, Yang SH. Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol Rep. 2018;5:314–317. doi: 10.1016/j.toxrep.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchinaka A, Azuma N, Mizumoto H, Shiho N, Moeko M, Mamoru Y, Kiyoshi A, Yuki K, Yuichiro Y, Toyoaki M, Kohzo N. Anti-inflammatory effects of heat-killed Lactobacillus plantarum L-137 on cardiac and adipose tissue in rats with metabolic syndrome. Sci Rep. 2018;8:1–20. doi: 10.1038/s41598-018-26588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayalakshmi S, Adeyemi DE, Choi IY, Sultan G, Madar IH, Park M-K. Comprehensive in silico analysis of lactic acid bacteria for the selection of desirable probiotics. LWT-Food Sci Technol. 2020;130:109617. doi: 10.1016/j.lwt.2020.109617. [DOI] [Google Scholar]
- 14.Kim T-Y, Kim Y-H, Moon JS, Kwon H-S, Choi SK. Complete genome sequence of probiotic Lactobacillus plantarum IDCC3501 isolated from kimchi. Korean J Microbiol. 2019;55:438–440. [Google Scholar]
- 15.Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, Jin Q. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100 (ISBN 978-1-68440-066-9)
- 17.OECD (2008) Test No. 425: Acute oral toxicity: up-and-down procedure. OECD Guidelines for the Testing of Chemicals, Section 4, Part 425, Paris: OECD Publishing
- 18.De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evi Based Complementy Altern Med. 2018;2018:1756308. doi: 10.1155/2018/1756308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- 20.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res Ther. 2002;4:1–10. doi: 10.1186/ar400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Je I-G, Lee D-G, Jeong D-G, Hong DH, Yoon J-M, Moon JS, Park SB. The probiotic, ID-JPL934, attenuates dextran sulfate sodium-induced colitis in mice through inhibition of proinflammatory cytokines expression. J Med Food. 2018;21:858–865. doi: 10.1089/jmf.2017.4152. [DOI] [PubMed] [Google Scholar]
- 22.Chang L, Zhang Z-Y, Dong K, Yuan J-P, Guo X-K. Antibiotic resistance of probiotic strains of lactic acid bacteria isolated from marketed foods and drugs. Biome Environ Sci. 2009;22:401–412. doi: 10.1016/S0895-3988(10)60018-9. [DOI] [PubMed] [Google Scholar]
- 23.Preena PG, Swaminathan TR, Kumar VJR, Singh ISB. Antimicrobial resistance in aquaculture: a crisis for concern. Biologia. 2020;75:1497–1517. doi: 10.2478/s11756-020-00456-4. [DOI] [Google Scholar]
- 24.Zhou J, Pillidge C, Gopal P, Gill HS. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol. 2005;98:211–217. doi: 10.1016/j.ijfoodmicro.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Temmerman R, Pot B, Huys G, Swings J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol. 2003;81:1–10. doi: 10.1016/S0168-1605(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 26.Todorov SD, Perin LM, Carneiro BM, Rahal P, Holzapfel W, Nero LA. Safety of Lactobacillus plantarum ST8Sh and its bacteriocin. Probiotics Antimicrob Proteins. 2017;9:334–344. doi: 10.1007/s12602-017-9260-3. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Capillas C, Herrero AM. Impact of biogenic amines on food quality and safety. Foods. 2019;8:62. doi: 10.3390/foods8020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karovičová J, Kohajdová Z. Biogenic amines in food. Chemical Papers. 2005;59:70–79. [Google Scholar]
- 29.Barbieri F, Montanari C, Gardini F, Tabanelli G. Biogenic amine production by lactic acid bacteria: a review. Foods. 2019;8:17. doi: 10.3390/foods8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueimonde M, Frias R, Ouwehand A. Assuring the continued safety of lactic acid bacteria used as probiotics. Biologia. 2006;61:755–760. doi: 10.2478/s11756-006-0153-2. [DOI] [Google Scholar]
- 31.Delgado S, O’sullivan E, Fitzgerald G, Mayo B. Subtractive screening for probiotic properties of Lactobacillus species from the human gastrointestinal tract in the search for new probiotics. J Food Sci. 2007;72:M310–5. doi: 10.1111/j.1750-3841.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 32.Awolade P, Cele N, Kerru N, Gummidi L, Oluwakemi E, Singh P. Therapeutic significance of β-glucuronidase activity and its inhibitors: a review. Eur J Med Chem. 2020;187:111921. doi: 10.1016/j.ejmech.2019.111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son S-H, Jeon H-L, Yang S-J, Sim M-H, Kim Y-J, Lww N-K, Paik H-D. Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Sci Biotechnol. 2018;27:123–129. doi: 10.1007/s10068-017-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bujnakova D, Strakova E, Kmet V. In vitro evaluation of the safety and probiotic properties of lactobacilli isolated from chicken and calves. Anaerobe. 2014;29:118–127. doi: 10.1016/j.anaerobe.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Valan Arasu M, Jung M-W, Ilavenil S, Jane M, Kim D-H, Kee K-D, Park H-S, Hur T-Y, Choi G-J, Lim Y-C, Al-Dhabi NA, Choi K-C. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J App Microbiol. 2013;115:1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- 36.Ng SY, Koon SS, Padam BS, Chye FY. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang) CYTA-J FOOD. 2015;13:563–572. [Google Scholar]
- 37.Nishida S, Ishii M, Nishiyama Y, Abe S, Ono Y, Sekimizu K. Lactobacillus paraplantarum 11–1 isolated from rice bran pickles activated innate immunity and improved survival in a silkworm bacterial infection model. Front Microbiol. 2017;8:436. doi: 10.3389/fmicb.2017.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional information files.