Abstract
Introduction
The COVID-19 pandemic has had a global impact on cancer care but the extent to which this has affected the management of colorectal cancer (CRC) in different countries is unknown. CRC management in Denmark was thought to have been relatively less impacted than in other nations during the first wave of the pandemic. The aim of this study was to determine the pandemic’s impact on CRC in Denmark.
Methods
The Danish national cancer registry identified patients with newly diagnosed with CRC from 1 March 2020 to 1 August 2020 (pandemic interval) and corresponding dates in 2019 (prepandemic interval). Data regarding clinicopathological demographics and perioperative outcomes were retrieved and compared between the two cohorts.
Results
Total CRC diagnoses (201 versus 359 per month, P = 0.008) and screening diagnoses (38 versus 80 per month, P = 0.016) were both lower in the pandemic interval. The proportions of patients presenting acutely and the stage at presentation were, however, unaffected. For those patients having surgery, both colonic and rectal cancer operations fell to about half the prepandemic levels: colon (187 (i.q.r. 183–188) to 96 (i.q.r. 94–112) per month, P = 0.032) and rectal cancers (63 (i.q.r. 59–75) to 32 (i.q.r. 28–42) per month, P = 0.008). No difference was seen in surgical practice or postoperative 30-day mortality rate (colon 2.2 versus 2.2 per cent, P = 0.983; rectal 1.0 versus 2.9 per cent, P = 0.118) between the cohorts. Treatment during the pandemic interval was not independently associated with death at 30 or 90 days.
Conclusion
The initial wave of the COVID-19 pandemic reduced the number of new diagnoses made and number of operations but had limited impact on technique or outcomes of CRC care in Denmark.
This study investigated the impact of the initial wave of the COVID-19 pandemic on colorectal cancer care in Denmark. In contrast with other nations, surgical treatment and outcomes were largely unchanged. However, significant reductions in new diagnoses were noted.
Introduction
The initial wave of the COVID-19 pandemic had a global impact on the provision of cancer care, manifest in postponement of and alterations to standard therapy1. The need to divert resources to the pandemic response forced many countries to cancel elective operations, with an estimated 2.3 million cancer operations cancelled worldwide at the height of the pandemic2,3. In some nations, cessation of screening programmes was also necessary4. As the severity of the consequences of infection with SARS-CoV-2 became apparent, including markedly increased rates of severe postoperative respiratory complications, questions arose as to whether standard surgical practices could be continued safely5–8. Despite its recognized benefits, surgeons were advised against the use of minimally invasive surgery, due to theoretical concerns of infection of personnel through the generation of aerosols containing SARS-CoV-29. More specifically to colorectal cancer, other guidelines advocating an increased use of diverting or end stomas were published, with the rationale of reducing the incidence of complications at a time when intensive care resources were already stretched10,11.
Response to the pandemic and its impact on healthcare systems in different countries has not been uniform. Denmark was one of the first European nations to introduce national lockdown measures and was able to achieve comparatively good control of the initial wave12. Although the cancellation of benign elective operations was still necessary, cancer care continued to be provided largely unchanged. No alterations to national guidelines were made, in terms of the use of minimally invasive surgery or anastomotic techniques. There remained concerns, however, that cancer care may have been adversely affected due to various patient factors, including reluctance to seek medical help due to fear of infection or of adding to the burden of healthcare during the pandemic. Delays of this nature might have led to an increase in acute presentations as well as diagnosis at a later stage of disease with detrimental effects on outcomes13. It has also been unclear if the continuation of standard surgical care had adverse effects on perioperative morbidity and mortality rates.
The differing outcomes of the response to the initial wave of the COVID-19 pandemic provide the opportunity to learn vital lessons for the future. Such lessons may not only be of importance for the management of subsequent waves of the current pandemic, particularly given the continued emergence of novel Sars-CoV-2 variants, but also for pandemics of the future14. The aim of the present study was to determine if any alterations in the diagnosis, management or short-term outcomes of patients with colorectal cancer occurred following the initial wave of the pandemic in Denmark, through an analysis of the national cancer registry.
Methods
This was a nationwide cohort study based on data from the Danish Colorectal Cancer Group (DCCG) database and performed in accordance with STROBE guidelines15. This database includes at least 95 per cent of all patients diagnosed with colorectal cancer in Denmark and has recently been validated, with an overall data accuracy of over 95 per cent16. The first documented case of Sars-CoV-2 infection in Denmark occurred on 27 February 2020. For this study the start of the pandemic interval was therefore defined as 1 March 2020. The end of the initial pandemic interval was defined as 1 August 2020, as data regarding clinicopathological demographics and perioperative outcomes are not yet available for patients diagnosed after this date. A prepandemic interval was defined as the corresponding dates from the previous calendar year (1 March to 1 August 2019) to account for seasonal variations in diagnosis and treatment patterns. Patients diagnosed with a new colorectal cancer during the study interval were eligible for inclusion. This study was approved by the Danish Data Protection Agency (P-2020–517).
The following variables were retrieved from the DCCG database for each patient: age, sex, ASA grade, performance status, Charlson Co-morbidity Index score, BMI, Union for International Cancer Control (UICC) stage, mode of diagnosis (screening versus non-screening), mode of presentation (acute versus non-acute), tumour location and presence of synchronous tumours. Additional variables retrieved in patients undergoing surgery included time from diagnosis to surgery, operative approach, conversion rates, type and site of anastomosis formation, stoma formation, neoadjuvant therapy, time to and type of adjuvant therapy, death at 30 days, morbidity at 30 days and death at 90 days.
The primary endpoint was the number of new cancer diagnoses made in each interval. Secondary endpoints included the number of screening diagnoses made in each interval, the proportion of patients proceeding to surgery and the 30- and 90-day mortality rates of patients undergoing surgery for UICC stage I–III colorectal cancer. Univariable analyses were performed using the χ2 test for categorical data and the Mann–Whitney–Wilcoxon test for continuous data. All analyses were two-sided and considered statistically significant with P < 0.050. The following potential prognostic factors for death at 30 and 90 days were investigated using univariable analyses: patient group (pandemic versus prepandemic), age, sex, cancer location (colon versus rectum), ASA grade, performance status, Charlson Co-morbidity Index score, BMI, acute presentation and mode of surgery (minimally invasive versus open). These factors were chosen a priori. Factors found to have P < 0.100 on univariable analysis were then combined in multivariable logistic regression analyses. All statistical analyses were performed using SPSS®, version 27.0 (IBM, Armonk, New York, USA).
Results
A total of 2794 patients were diagnosed with colorectal cancer during the study interval, with a 45.4 per cent reduction in the number of diagnoses made during the pandemic interval (prepandemic 1807 diagnoses versus pandemic 987). The median number of new diagnoses fell from 359 (i.q.r. 343–379) per month in the prepandemic interval to 201 (i.q.r. 197–213) per month in the pandemic interval (P = 0.008) (Fig. 1a). A corresponding reduction in the number of screening diagnoses was observed during the pandemic interval (prepandemic 388 screening diagnoses versus pandemic 201) with a 48.2 per cent reduction, from a median of 80 (i.q.r. 76–87) diagnoses per month in the prepandemic interval to 38 (i.q.r. 34–42) diagnoses per month (P = 0.016) (Fig. 1b). A significant reduction was noted in the number of operations performed in the pandemic interval for both colon (187 (i.q.r. 183–188) to 96 (i.q.r. 94–112) per month, P = 0.032) and rectal cancers (63 (i.q.r. 59–75) to 32 (i.q.r. 28–42) per month, P = 0.008) (Fig. 2). The clinicopathological characteristics of these patients are summarized in Table 1. No significant differences in tumour stage were observed between the two time intervals. A greater proportion of patients in the pandemic group had an ASA grade of 3 or above, although this was not accompanied by significant differences in performance status or Charlson Co-morbidity Index score.
Fig. 1.

The impact of the COVID-19 pandemic on colorectal cancer diagnoses in Denmark
a A comparison of all new colorectal cancer diagnoses between the prepandemic and pandemic intervals. b A comparison of screening colorectal cancer diagnoses between the prepandemic and pandemic intervals. c Number of new COVID-19 infections from January to October 2020, adapted from the WHO COVID-19 dashboard17.
Fig. 2.
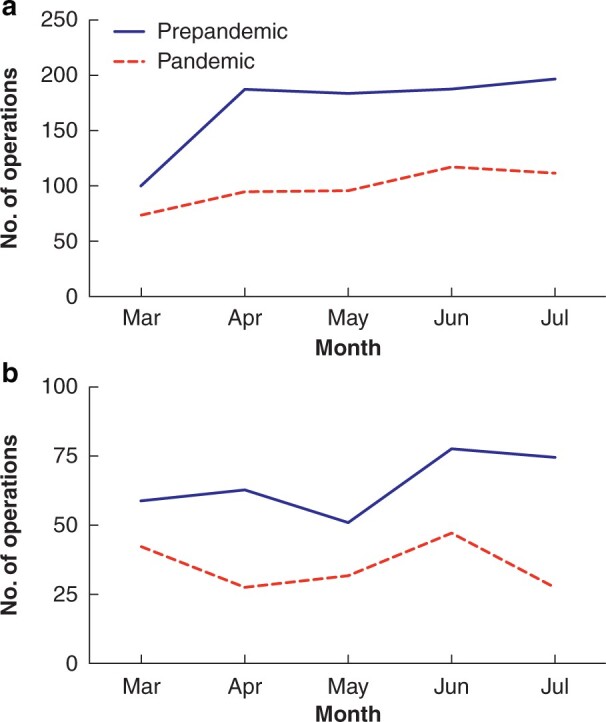
The impact of the COVID-19 pandemic on operations in Denmark
Operations for a colon and b rectal cancers.
Table 1.
The clinicopathological characteristics of patients diagnosed with colorectal cancer
| Characteristics | Prepandemic | Pandemic | P |
|---|---|---|---|
| (n = 1807) | (n = 987) | ||
| Male : female ratio | 941 : 866 | 524 : 463 | 0.608 |
| Age at diagnosis (years)* | 72 (65–80) | 73 (65–80) | 0.472 |
| Screening diagnosis | 388 (21.5) | 201 (20.4) | 0.740 |
| ASA grade | 0.035 | ||
| 1 | 314 (17.4) | 129 (13.1) | |
| 2 | 856 (47.4) | 481 (48.7) | |
| 3 | 527 (29.2) | 317 (32.1) | |
| 4 | 46 (2.5) | 30 (3.0) | |
| 5 | 3 (0.2) | 0 (0) | |
| Missing data | 61 (3.4) | 30 (3.0) | |
| Performance status | 0.059 | ||
| 0 | 988 (54.7) | 529 (53.6) | |
| 1 | 445 (24.6) | 260 (26.3) | |
| 2 | 181 (10.0) | 119 (12.1) | |
| 3 | 91 (5.0) | 46 (4.7) | |
| 4 | 34 (1.9) | 10 (1.0) | |
| Missing data | 68 (3.8) | 23 (2.3) | |
| Median CCI score | 1 (0–2) | 1 (0–2) | 0.301 |
| BMI | 0.035 | ||
| <18.5 | 77 (4.3) | 29 (2.9) | |
| 18.5–24.9 | 737 (40.8) | 378 (38.3) | |
| 25.0–29.9 | 561 (31.0) | 328 (33.2) | |
| 30.0–34.9 | 256 (14.2) | 159 (16.1) | |
| >35 | 85 (4.7) | 65 (6.6) | |
| Missing data | 91 (5.0) | 28 (2.8) | |
| UICC stage | 0.090 | ||
| 0 | 9 (0.5) | 1 (0.1) | |
| 1 | 534 (29.6) | 312 (31.6) | |
| 2 | 368 (20.4) | 193 (19.6) | |
| 3 | 522 (28.9) | 307 (31.1) | |
| 4 | 374 (20.7) | 174 (17.6) | |
| Tumour location | 0.197 | ||
| Colon | 1299 (71.9) | 732 (74.2) | |
| Rectum | 508 (28.1) | 255 (25.8) | |
| Synchronous tumours | 65 (3.6) | 27 (2.7) | 0.223 |
Values in parentheses are percentages unless otherwise stated; χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results. *values are median (i.q.r.). CCI, Charlson Co-morbidity Index; UICC, Union for International Cancer Control.
Outcomes of patients with stage I–III colon cancer
A total of 1599 patients were diagnosed with stage I–III colon cancer during the study interval. No differences in tumour stage, acute presentations or in the proportion of patients proceeding to surgery were identified. Formal colectomy was performed in a total of 1381 patients (Table 2). There was no difference in the time between diagnosis and surgery between the prepandemic and pandemic groups (median 13 versus 13 days, P = 0.776). No differences in the use of minimally invasive surgery or in the number of patients receiving primary anastomoses or stomas were noted.
Table 2.
The treatment of patients undergoing formal colectomy for stage I–III colon cancer
| Treatment | Prepandemic | Pandemic | P |
|---|---|---|---|
| (n = 872) | (n = 509) | ||
| Time from diagnosis to surgery (days)* | 13 (7–21) | 13 (8–20) | 0.776 |
| Acute presentation | 70 (8.2) | 33 (6.5) | 0.292 |
| Operative approach | 0.212 | ||
| Laparotomy | 138 (15.8) | 64 (12.6) | |
| Laparoscopic | 641 (73.5) | 394 (77.4) | |
| Robotic | 93 (10.7) | 51 (10.0) | |
| Number of conversions | 63 (8.6) | 42 (9.4) | 0.607 |
| Anastomosis formation | 785 (90.0) | 461 (90.6) | 0.741 |
| Type of anastomosis | 0.759 | ||
| Handsewn | 436 (55.5) | 261 (56.6) | |
| Stapled | 331 (42.7) | 191 (41.4) | |
| Not specified | 18 (2.3) | 9 (2.0) | |
| Site of anastomosis formation | 0.214 | ||
| Intracorporeal | 115 (14.6) | 62 (13.4) | |
| Extracorporeal | 561 (71.5) | 374 (81.1) | |
| Not specified | 109 (13.9) | 25 (5.4) | |
| Stoma formation | 30 (3.4) | 19 (3.7) | 0.777 |
| Neoadjuvant therapy | 20 (2.3) | 8 (1.6) | 0.359 |
| Referred for adjuvant therapy | 306 (35.1) | 152 (29.9) | 0.047 |
| Received adjuvant therapy | 187 (61.1) | 99 (65.1) | 0.403 |
| Adjuvant therapy not indicated | 46 (15.0) | 23 (15.1) | 0.978 |
| Time to adjuvant therapy (days)* | 33 (27–41) | 30 (24–39) | 0.007 |
| Type of adjuvant therapy† | 0.154 | ||
| FOLFOX | 9 (4.8) | 5 (5.1) | |
| CAPOX | 92 (49.2) | 39 (39.4) | |
| Capecitabine | 68 (36.4) | 44 (44.4) | |
| 5-FU | 10 (5.3) | 2 (2.0) | |
| Not specified | 8 (4.3) | 9 (9.1) |
Values in parentheses are percentages unless stated otherwise; χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results.
*values are median (i.q.r.).
†Percentages presented are those of all patients receiving adjuvant therapy. FOLFOX, leucovorin, fluorouracil and oxaloplatin; CAPOX, capecitabine and oxaliplatin; 5-FU, 5-fluorouracil.
Neoadjuvant therapy was rarely given to these patients with colon cancer, with no significant difference in its use identified between cohorts (Table 2). However, a significant reduction in the number of patients referred for adjuvant therapy following surgery was seen in the pandemic cohort (29.9 versus 35.1 per cent, P = 0.047). Despite this, no differences in the proportion of referred patients who went on to receive adjuvant therapy or in the types of therapeutic agents used were noted between the two cohorts. Patients in the pandemic group had a slightly shorter interval between surgery and the start of adjuvant therapy (median 30 versus 33 days, P = 0.007). No alterations in the type of adjuvant therapy used in each time interval were noted.
There were no differences in 30- or 90-day postoperative mortality rates between the two cohorts (Table 3). Rates of surgical and medical complications were similar between cohorts, with no difference in the incidence of respiratory complications (44 of 872 patients (5.0 per cent) in the prepandemic cohort versus 19 of 509 (3.7 per cent), P = 0.141). Although the rates of anastomotic leak were similar between cohorts (24 of 785 patients (3.1 per cent) in the prepandemic cohort versus 13 of 461 patients (2.8 per cent), P = 0.812), less severe leaks were slightly more common in the pandemic cohort (grade A or B, 0 of 24 versus 3 of 13, P = 0.049) and a greater proportion of these anastomoses were preserved (3 of 24 versus 4 of 13, P = 0.176).
Table 3.
Postoperative morbidity and death in patients undergoing formal colectomy for stage I–III colon cancer
| Morbidity and death details | Prepandemic | Pandemic | P |
|---|---|---|---|
| Death at 30 days | 19 (2.2) | 11 (2.2) | 0.983 |
| Death at 90 days | 27 (3.1) | 14 (2.8) | 0.741 |
| 30-day morbidity | 151 (17.3) | 99 (19.5) | 0.321 |
| Surgical complication | 119 (13.7) | 65 (12.8) | 0.644 |
| Specific surgical complications | 0.862 | ||
| Bleeding | 25 (2.9) | 11 (2.2) | |
| Dehiscence | 11 (1.2) | 8 (1.6) | |
| Obstruction | 27 (1.3) | 19 (3.7) | |
| SSI—intra-abdominal | 13 (1.5) | 9 (1.8) | |
| SSI—superficial | 6 (0.7) | 3 (0.6) | |
| Stoma-related | 6 (0.7) | 2 (0.4) | |
| Anastomotic leak (rate) | 24 (3.1) | 13 (2.8) | 0.812 |
| Grade of anastomotic leak | 0.049 | ||
| A | 0 (0) | 1 (7.7) | |
| B | 0 (0) | 2 (15.4) | |
| C | 24 (100) | 10 (76.9) | |
| Consequence of anastomotic leak | 0.176 | ||
| Anastomosis preserved | 3 (12.5) | 4 (30.8) | |
| Anastomosis resected | 21 (87.5) | 9 (69.2) | |
| Medical complications | 90 (10.3) | 53 (10.4) | 0.957 |
| Type of medical complication | 0.141 | ||
| Respiratory | 44 (5.0) | 19 (3.7) | |
| Cardiac | 16 (1.8) | 5 (1.0) | |
| Sepsis | 24 (2.8) | 11 (2.2) | |
| Renal failure | 6 (0.7) | 7 (1.4) | |
| Thromboembolic | 7 (0.8) | 3 (0.6) | |
| Other | 22 (2.5) | 22 (4.3) |
Values in parentheses are percentages. χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results. SSI, surgical-site infection.
Outcomes of patients with stage I–III rectal cancer
A total of 637 patients were diagnosed with stage I–III rectal cancer during the study interval, with no differences in tumour stage, tumour height or the proportion of patients proceeding to surgery between the cohorts. Formal proctectomy was performed in 476 patients, whose treatment is summarized in Table 4. Patients in the pandemic group had a shorter median time to surgery (19 versus 21 days, P = 0.029). No differences in the use of minimally invasive surgery or the types of operations performed were noted. In those patients undergoing anterior resection, there was no difference in the rates of formation of a defunctioning stoma.
Table 4.
The treatment of patients undergoing formal proctectomy for stage I–III rectal cancer
| Treatment | Prepandemic | Pandemic | P |
|---|---|---|---|
| (n = 304) | (n = 172) | ||
| Time from diagnosis to surgery (days)* | 21 (13–80) | 19 (12–38) | 0.029 |
| Operative approach | |||
| Laparotomy | 21 (6.9) | 7 (4.1) | 0.128 |
| Laparoscopic | 154 (50.7) | 86 (50.0) | |
| Robotic | 123 (40.5) | 79 (45.9) | |
| TaTME | 6 (2.0) | 0 (0) | |
| Number of conversions | 11 (3.9) | 8 (4.9) | 0.626 |
| Operation performed | |||
| Anterior resection | 172 (56.6) | 84 (48.8) | 0.424 |
| Hartmann’s | 26 (8.6) | 16 (9.3) | |
| APE | 104 (34.2) | 71 (41.3) | |
| Proctocolectomy | 2 (0.7) | 1 (0.6) | |
| Defunctioning stoma formation† | 66 (38.4) | 30 (35.7) | 0.680 |
| Neoadjuvant therapy‡ | |||
| Total | 74 (24.3) | 50 (29.1) | 0.259 |
| Chemotherapy | 6 (8.1) | 5 (10.0) | 0.290 |
| Radiotherapy | 19 (25.7) | 17 (34.0) | |
| Chemoradiotherapy | 49 (66.2) | 27 (54.0) | |
| Immunotherapy | 0 (0) | 1 (2.0) | |
| Referred for adjuvant therapy | 112 (36.8) | 41 (23.8) | 0.004 |
| Received adjuvant therapy | 55 (49.1) | 29 (70.7) | 0.017 |
| Adjuvant therapy not indicated | 37 (12.2) | 6 (3.5) | 0.002 |
| Time to adjuvant therapy (days)* | 35 (29–45) | 30 (25–40) | 0.013 |
| Type of adjuvant therapy§ | |||
| FOLFOX | 5 (9.1) | 3 (10.3) | 0.255 |
| CAPOX | 17 (30.9) | 6 (20.7) | |
| Capecitabine | 30 (54.5) | 14 (48.3) | |
| 5-FU | 2 (3.6) | 5 (17.2) | |
| Other | 1 (1.8) | 1 (3.4) |
Values in parentheses are percentages unless stated otherwise; χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results. *values are median (i.q.r.). †Percentages presented are those of all patients undergoing anterior resection. ‡Percentages presented are those of all patients receiving neoadjuvant therapy. §Percentages presented are those of all patients receiving adjuvant therapy. TaTME, transanal total mesorectal excision; APE, abdominoperineal excision; FOLFOX, leucovorin, fluorouracil and oxaloplatin; CAPOX, capecitabine and oxaliplatin; 5-FU, 5-fluorouracil.
No difference in the proportion of patients undergoing neoadjuvant therapy was seen, with similar rates of in the use of chemotherapy, radiotherapy and chemoradiotherapy (Table 4). However, a significant reduction was again seen in the proportion of patients referred for adjuvant therapy in the pandemic group (23.8 versus 36.8 per cent, P = 0.004). Despite this, after referral, a greater proportion of patients in the pandemic group went on to receive adjuvant therapy (70.7 versus 49.1 per cent, P = 0.017), with a corresponding reduction in the proportion of patients that were referred in whom adjuvant therapy was not indicated (3.5 versus 12.2 per cent, P = 0.002). Patients in the pandemic group also had a shorter interval between surgery and the start of adjuvant therapy (median 30 versus 35 days, P = 0.013).
No significant differences were noted in perioperative mortality or morbidity rates between the two cohorts (Table 5). No difference in the rate of respiratory complications (2.6 per cent in the prepandemic cohort versus 3.9 per cent, P = 0.702) or in the incidence (8.1 per cent in the prepandemic cohort versus 6.0 per cent, P = 0.531) or management of anastomotic leaks was noted.
Table 5.
Postoperative morbidity and death in patients undergoing surgery for stage I–III rectal cancer.
| Morbidity and death details | Prepandemic | Pandemic | P |
|---|---|---|---|
| Death at 30 days | 3 (1.0) | 5 (2.9) | 0.118 |
| Death at 90 days | 5 (1.6) | 5 (2.9) | 0.356 |
| 30-day morbidity | 89 (29.3) | 43 (25.0) | 0.317 |
| Surgical complications | 71 (23.4) | 31 (18.0) | 0.168 |
| Specific surgical complications | 0.650 | ||
| Bleeding | 4 (1.3) | 3 (1.7) | |
| Dehiscence | 1 (0.3) | 0 (0) | |
| Obstruction | 12 (3.9) | 11 (6.4) | |
| SSI—intra-abdominal | 11 (3.6) | 5 (2.9) | |
| SSI—superficial | 7 (2.3) | 2 (1.2) | |
| Stoma-related | 14 (4.6) | 6 (3.5) | |
| Anastomotic leak (rate) | 14 (8.1) | 5 (6.0) | 0.531 |
| Grade of anastomotic leak | |||
| A | 1 (7.1) | 0 (0) | 0.771 |
| B | 4 (28.6) | 2 (40.0) | |
| C | 9 (64.3) | 3 (60.0) | |
| Consequence of anastomotic leak | |||
| Anastomosis preserved | 9 (64.3) | 3 (60.0) | 0.845 |
| Anastomosis resected | 5 (35.7) | 2 (40.0) | |
| Medical complications | 30 (9.9) | 17 (9.9) | 0.995 |
| Type of medical complication | |||
| Respiratory | 8 (2.6) | 5 (2.9) | 0.702 |
| Cardiac | 5 (1.6) | 3 (1.7) | |
| Sepsis | 1 (0.3) | 3 (1.7) | |
| Renal failure | 5 (1.6) | 2 (1.2) | |
| Thromboembolic | 3 (1.0) | 1 (0.6) | |
| Other | 15 (4.9) | 8 (4.7) |
Values in parentheses are percentages. χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results. SSI, surgical-site infection.
Prognostic factors for perioperative death
To investigate further the potential impact of the pandemic on perioperative death, potential prognostic factors for death at 30 and 90 days were investigated in uni- (data not shown) and multivariable analyses (Table 6). Undergoing operation in the pandemic interval was not significantly associated with perioperative death. In contrast, age, ASA grade, performance status and open surgery were all found to be significant prognostic factors for death at both 30 and 90 days. Subgroup analyses of patients with ASA grade 3 and above did not demonstrate an independent association between the pandemic interval and perioperative death.
Table 6.
Multivariable analyses of prognostic factors for death at 30 and 90 days in patients undergoing colectomy or proctectomy for stage I–III colorectal cancer
| Patient details | Death at 30 days |
Death at 90 days |
||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Patient group | ||||
| Prepandemic | Reference | – | Reference | – |
| Pandemic | 1.55 (0.89, 2.68) | 0.120 | 1.39 (0.87, 2.23) | 0.167 |
| Age | 1.07 (1.03, 1.11) | <0.001 | 1.07 (1.03, 1.10) | <0.001 |
| ASA grade | ||||
| 1 | Reference | – | Reference | – |
| 2 3 |
1.13 (0.24, 5.26) | 0.875 | 1.66 (0.37, 7.34) | 0.506 |
| 3.76 (0.80, 17.64) | 0.094 | 3.62 (0.80, 16.37) | 0.094 | |
| ≥4 | 25.38 (4.50, 143.2) | <0.001 | 18.42 (3.46, 97.93) | <0.001 |
| Performance status | ||||
| 0 | Reference | – | Reference | – |
| 1 | 1.38 (0.62, 3.05) | 0.430 | 1.85 (0.93, 3.66) | 0.079 |
| 2 | 2.99 (1.25, 7.17) | 0.014 | 3.95 (1.85, 8.46) | <0.001 |
| ≥3 | 4.48 (1.64, 12.28) | 0.004 | 6.23 (2.57, 15.13) | <0.001 |
| Charlson Co-morbidity Index | 0.86 (0.72, 1.02) | 0.079 | 1.02 (0.89, 1.17) | 0.782 |
| Acute presentation | 0.60 (0.24, 1.48) | 0.263 | 0.63 (0.28, 1.42) | 0.265 |
| Cancer type | ||||
| Colon | * | * | Reference | – |
| Rectum | * | * | 0.86 (0.45, 1.66) | 0.651 |
| Mode of surgery | ||||
| MIS | Reference | – | Reference | – |
| Open | 3.15 (1.61, 6.14) | <0.001 | 2.43 (1.52, 3.98) | <0.001 |
Values in parentheses are 95 per cent confidence intervals. χ2 test for categorical data and the Mann–Whitney–Wilcoxon test were used to obtain P values. Bold characters represent statistically significant results. * Not significant on univariable analysis. MIS, minimally invasive surgery (laparoscopic, robotic or transanal total mesorectal excision).
Treatment of stage IV colorectal cancer
A total of 548 patients were diagnosed with stage IV colorectal cancer during the study interval. No differences in the proportion of patients undergoing surgery or the proportion of patients treated with curative intent was noted. No difference in the proportion of patients treated with neoadjuvant therapy was noted between cohorts, although slight alterations in the patterns of intraoperative treatment were identified, with no patients undergoing hyperthermic intraperitoneal chemotherapy in the pandemic cohort.
Discussion
While modelling studies and surveys from several countries indicate significant alterations to cancer care because of the COVID-19 pandemic, there has been limited direct evidence of the consequences of such alterations to date13,17–20. The UK has reported national-level data, describing substantial alterations to standard colorectal care during the pandemic interval21,22. The present study provides notable contrasts to these data, demonstrating that standard surgical practices were maintained during the pandemic in Denmark, albeit with a reduction in the number of diagnoses made and cancer operations performed. While caution should be applied when comparing the pandemic’s impact in different healthcare systems, the current study provides insight into the impact of the relative success or failure of initial containment of the pandemic on subsequent cancer care. While the UK and Denmark have similar healthcare systems, they differed markedly in their initial response to the pandemic. National lockdown measures were introduced 2 weeks after the first confirmed case in Denmark, whereas such measures were not introduced in the UK until 2 months after the first confirmed case. Despite following initially similar trajectories in terms of new cases, the initial wave of the COVID-19 pandemic had dramatically more severe consequences in the UK, particularly in terms of COVID-19-related deaths (Fig. 3). As such, the present study provides data on colorectal cancer care in a country with a more aggressive initial containment strategy.
Fig. 3.
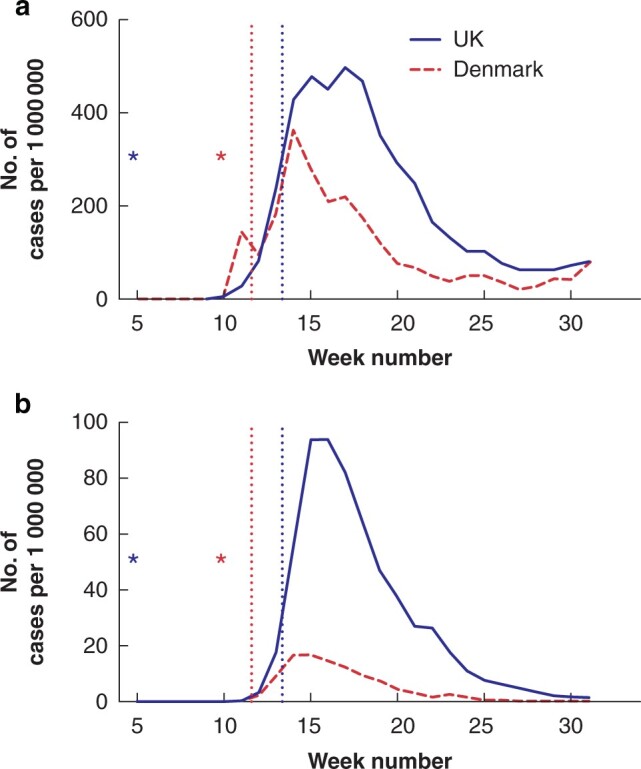
A comparison of the initial wave of the COVID-19 pandemic in the UK and Denmark—adapted from the WHO COVID-19 dashboard 17
a New cases of COVID-19 each week per 1 000 000 inhabitants. b COVID-19-related deaths each week per 1 000 000 inhabitants. The stars indicate the first confirmed case in each nation. The dotted lines indicate the introduction of national lockdown measures.
The need to alter standard surgical care was not unique to the UK, with both national and international studies demonstrating increases in open surgery, stoma formation and the use of short-course radiotherapy as an alternative to surgery in patients with rectal cancer21–23. No such alterations in surgical techniques were identified in the present study and there was no evidence of an increase in the use of radiotherapy as a potential alternative to surgery. The debate on the safety of the continued use of minimally invasive surgery during the pandemic continues, despite a lack of scientific evidence to support the theoretical concerns of transmission of the SARS-CoV-2 virus in surgically generated aerosols9 and the documented benefits of minimally invasive techniques compared with open surgery for colorectal cancer24,25. Similarly, while stomas reduce the clinical consequences of anastomotic leaks, diverting stomas are associated with significant risks themselves26,27. Decisions to alter standard surgical practice in other nations may have been unavoidable due to the demands of the pandemic response, particularly in countries where hospital admissions with COVID-19 overwhelmed the healthcare system.
Despite the limited impact on surgical practices, the present study found similar substantial reductions in both total diagnoses, screening diagnoses and numbers of operations performed during the pandemic interval. The number of new diagnoses the number of screenings diagnoses both fell by almost half. The reasons are unclear, as the national bowel cancer screening programme and cancer referrals pathways continued largely unaltered during the pandemic. It is possible that the reduction in diagnoses represents a reduction in patient engagement with primary care, either due to a reluctance to seek medical help on behalf of the patient or to increased inaccessibility of these services, with many general practitioners having to perform a greater number of remote consultations in the pandemic interval, with stricter triage criteria for in-person consultations. National-level data from the UK demonstrated a similar reduction in the number of new colorectal cancer diagnoses, accompanied by reductions in both cancer referrals and colonoscopies21. Diagnostic delays and acute presentations have well recognised adverse effects on survival outcomes28,29. Despite no difference in the proportion of patients presenting acutely or with more advanced disease in the present study, the detrimental effects of delays in diagnosis are likely to become evident in time. Modelling studies suggest that even modest disruptions to colorectal cancer screening programmes will lead to significant increases in future disease-related deaths30.
There was a reduction in the proportion of patients referred for adjuvant therapy following surgery for colorectal cancer in the pandemic interval. This occurred in the absence of a difference in staging between the two cohorts. In those with rectal cancers, the accompanying increase in the proportion of referred patients receiving adjuvant therapy and decrease in the proportion of patients in whom adjuvant therapy was not indicated suggests that more appropriate referrals were made during the pandemic. However, these patterns were not replicated in patients with colon cancers, raising the possibility that these alterations in referral practices may have led to the undertreatment of some patients. Despite the recommended changes to other aspects of colorectal cancer care during the pandemic, updated European guidelines advised against delays in adjuvant therapy, with such delays recognized to affect survival adversely31–33. Despite the reductions outlined above, the time interval between surgery and the start of adjuvant therapy was shortened during the pandemic study interval.
One of the major global concerns with the continuation of surgery during the pandemic was the marked increase in postoperative mortality rate following SARS-CoV-2 infection8, with the dilemma of balancing the risks of surgery against delaying and compromising cancer care. Although international collaborative studies have provided crucial and timely data to guide clinicians, these studies demonstrate a selection bias towards countries with a high incidence of SARS-CoV-2 infection, limiting the generalizability of these results to countries with a lower incidence8,23. The incidence of SARS-CoV-2 infection in Denmark has been relatively low and therefore the present study may be more relevant to countries with similar levels of infection34. No clinically significant alterations in postoperative morbidity or mortality rates were noted between the two cohorts in this study, and whilst the rates of 30-day mortality were slightly higher following surgery for rectal cancer in the pandemic interval, this mortality rate is in keeping with previously reported rates, in both Denmark and internationally35–37. Subsequent multivariable analyses did not find any evidence of an independent association between the pandemic interval and increased risks of perioperative death. These national level data suggest that colorectal surgery can be continued safely without increases in postoperative complications in countries with a relatively low incidence of SARS-CoV-2 infection.
While the comprehensive nature and fidelity of the data set are major strengths in the present study, there are limitations. Caution must be applied to comparisons of colorectal cancer care in other nations, not only due to differences in the structure of healthcare systems, including the resilience of such systems to catastrophic events, but also in the timing and severity of the initial wave of the pandemic. Many nations have now been affected by second and third waves of the COVID-19 pandemic. As in other nations, the severity of the second wave was far worse than the first in Denmark, and, while cancer-care pathways remained unaltered, it would be of interest to investigate whether the increased severity of the second wave affected management and outcomes. Given the relatively indolent nature of colorectal cancer, it may be years until the real effects of delayed diagnoses during the pandemic interval become evident.
Disclosure. The authors declare no conflicts of interest.
References
- 1. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E.. The impact of the COVID-19 pandemic on cancer care. Nat Cancer 2020;1:565–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVIDSurg Collaborative. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg 2020;107:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020;107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan KK, Lau J.. Cessation of cancer screening: an unseen cost of the COVID-19 pandemic? Eur J Surg Oncol 2020;46:2154–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A. et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital system. Cancer Discov 2020;10:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang W, Guan W, Chen R, Wang W, Li J, Xu K. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z. et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020;396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Boghdady M, Ewalds-Kvist BM.. Laparoscopic surgery and the debate on its safety during COVID-19 pandemic: a systematic review of recommendations. Surgeon 2021;19:e29–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. England RCoSo. Updated Intercollegiate General Surgery Guidance on COVID-192020. https://www.rcseng.ac.uk/coronavirus/joint-guidance-for-surgeons-v2/ (accessed on 18 February 2021).
- 11. Beamish AJ, Brown C, Abdelrahman T, Harper ER, Harries RI, Egan RJ; Welsh Surgical Research Initiative (WSRI) Collaborative. International surgical guidance for COVID-19: validation using an international Delphi process – cross-sectional study. Int J Surg 2020;79:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown F. Denmark second European country to impose lockdown against coronavirus. Metro. 2020. (accessed 11 March 2020).
- 13. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R. et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE.. SARS-CoV-2 variants of concern are emerging in India. Nat Med 2021;27:1131–1133. [DOI] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 16. Klein MF, Gogenur I, Ingeholm P, Njor SH, Iversen LH, Emmertsen KJ; DCCG Validation Group. Validation of the Danish Colorectal Cancer Group (DCCG.dk) database – on behalf of the Danish Colorectal Cancer Group. Colorectal Dis 2020;22:2057–2067. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/?gclid=CjwKCAjwnK36BRBVEiwAsMT8WJ3y00_BUzvrLsvbl3uthuoTH_Occ45gyEUbpYRyEqAzll3aZB6TYxoCcM0QAvD_BwE (accessed on 18 February 2021).
- 18. Santoro GA, Grossi U, Murad-Regadas S, Nunoo-Mensah JW, Mellgren A, Di Tanna GL. et al. ; DECOR-19 Collaborative Group. DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): global perspective from an international survey. Surgery 2021;169:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. CRC COVID Research Collaborative. The impact of the COVID-19 pandemic on colorectal cancer service provision. Br J Surg 2020;107:e521–e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caricato M, Baiocchi GL, Crafa F, Scabini S, Brisinda G, Clementi M. et al. ; Italian Colorectal Anastomotic Leakage (iCral) study group. Colorectal surgery in Italy during the Covid19 outbreak: a survey from the iCral study group. Updates Surg 2020;72:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunner M, Krautz C, Kersting S, Weber GF, Stinner B, Benz SR. et al. Oncological colorectal surgery during the COVID-19 pandemic – a national survey. Int J Colorectal Dis 2020;35:2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J. et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol 2021;6:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuryba A, Boyle JM, Blake HA, Aggarwal A, van der Meulen J, Braun M. et al. Surgical treatment and outcomes of colorectal cancer patients during the COVID-19 pandemic: a national population-based study in England. Ann Surg Open 2021;2:e071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. COVIDSurg Collaborative. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ. et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477–484. [DOI] [PubMed] [Google Scholar]
- 26. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM. et al. ; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 27. Schlesinger NH, Smith H.. The effect of a diverting stoma on morbidity and risk of permanent stoma following anastomotic leakage after low anterior resection for rectal cancer: a nationwide cohort study. Int J Colorectal Dis 2020;35:1903–1910. [DOI] [PubMed] [Google Scholar]
- 28. Cottam J, Richards K, Hasted A, Blackman A.. Results of a nationwide prospective audit of stoma complications within 3 weeks of surgery. Colorectal Dis 2007;9:834–838. [DOI] [PubMed] [Google Scholar]
- 29. McPhail S, Elliss-Brookes L, Shelton J, Ives A, Greenslade M, Vernon S. et al. Emergency presentation of cancer and short-term mortality. Br J Cancer 2013;109:2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E. et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Jonge L, Worthington J, van Wifferen F, Iragorri N, Peterse EFP, Lew JB. et al. ; COVID-19 and Cancer Global Modelling Consortium working group 2. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol 2021;6:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vecchione L, Stintzing S, Pentheroudakis G, Douillard JY, Lordick F.. ESMO management and treatment adapted recommendations in the COVID-19 era: colorectal cancer. ESMO Open 2020;5:e000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turner MC, Farrow NE, Rhodin KE, Sun Z, Adam MA, Mantyh CR. et al. Delay in adjuvant chemotherapy and survival advantage in stage III colon cancer. J Am Coll Surg 2018;226:670–678. [DOI] [PubMed] [Google Scholar]
- 34. Becerra AZ, Aquina CT, Mohile SG, Tejani MA, Schymura MJ, Boscoe FP. et al. Variation in delayed time to adjuvant chemotherapy and disease-specific survival in stage III colon cancer patients. Ann Surg Oncol 2017;24:1610–1617. [DOI] [PubMed] [Google Scholar]
- 35. Murray AC, Mauro C, Rein J, Kiran RP.. 30-day mortality after elective colorectal surgery can reasonably be predicted. Tech Coloproctol 2016;20:567–576. [DOI] [PubMed] [Google Scholar]
- 36. Iversen LH, Ingeholm P, Gogenur I, Laurberg S.. Major reduction in 30-day mortality after elective colorectal cancer surgery: a nationwide population-based study in Denmark 2001–2011. Ann Surg Oncol 2014;21:2267–2273. [DOI] [PubMed] [Google Scholar]
- 37. Gietelink L, Wouters MW, Bemelman WA, Dekker JW, Tollenaar RA, Tanis PJ; Dutch Surgical Colorectal Cancer Audit Group. Reduced 30-day mortality after laparoscopic colorectal cancer surgery: a population based study from the Dutch Surgical Colorectal Audit (DSCA). Ann Surg 2016;264:135–140. [DOI] [PubMed] [Google Scholar]


