Abstract
Vitamin D has been linked to various physiological functions in pregnant women and their fetuses. Previous studies have suggested that some per- and polyfluoroalkyl substances (PFAS) may alter serum vitamin D concentrations. However, no study has investigated the relationship between PFAS and vitamin D in pregnant women. This study aims to evaluate the associations of serum PFAS with serum total and free 25-hydroxyvitamin D (25(OH)D) during pregnancy in a cohort of African American women in Atlanta, GA. Blood samples from 442 participants were collected in early pregnancy (8–14 weeks of gestation) for PFAS and 25(OH)D measurements, and additional samples were collected in late pregnancy (24–30 weeks) for the second 25(OH)D measurements. We fit multivariable linear regressions and weighted quantile sum (WQS) regressions to estimate the associations of individual PFAS and their mixtures with 25(OH)D concentrations. We found mostly positive associations of total 25(OH)D with PFHxS (perfluorohexane sulfonic acid), PFOS (perfluorooctane sulfonic acid), PFDA (perfluorodecanoic acid), and NMeFOSAA (N-methyl perfluorooctane sulfonamido acetic acid), and negative associations with PFPeA (perfluoropentanoic acid). For free 25(OH)D, positive associations were observed with PFHxS, PFOS, PFOA (perfluorooctanoic acid), and PFDA, and a negative association with PFPeA among the women with male fetuses in the models using 25(OH)D measured in late pregnancy. In mixture models, a quartile increase in WQS index was associated with 2.88 ng/mL (95%CI 1.14–4.59) and 5.68 ng/mL (95%CI 3.31–8.04) increases in total 25(OH)D measured in the early and late pregnancy, respectively. NMeFOSAA, PFDA, and PFOS contributed the most to the overall effects among the eight PFAS. No association was found between free 25(OH)D and the PFAS mixture. These results suggest that PFAS may affect vitamin D biomarker concentrations in pregnant African American women, and some of the associations were modified by fetal sex.
Keywords: Vitamin D, Per- and Polyfluoroalkyl Substance (PFAS), Chemical mixtures, Weighted quantile sum (WQS) regression, Endocrine disruptors
Introduction
Per- and polyfluoroalkyl substances (PFAS) have been manufactured and used from the 1940s. Due to their unique hydrophobic and lipophobic properties, PFAS have been applied in numerous consumer products such as stain- and water-resistant fabrics and textiles, nonstick coatings on food wrappers and cookware, personal care products, and firefighting foams (Herzke et al., 2012; Sunderland et al., 2018). Because of their ubiquity as well as the persistence, previous studies have commonly detected PFAS in the environment and biological samples, leading to critical concerns to the public (Harris et al., 2017; Kim et al., 2020; Lau et al., 2007; Paul et al., 2009). Thus, major manufacturers have voluntarily phased out the production of PFAS since 2002. However, over 98% the participant in the 2015–2016 National Health and Nutrition Examination Survey (NHANES) have detectable serum perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) concentrations (US Department of Health and Human Services, 2019). Additionally, exposure to PFAS has been associated with endocrine disruption (Abbott et al., 2007; Li et al., 2020; White et al., 2011), metabolic syndrome (Frisbee et al., 2010), reduced immune function (Grandjean et al., 2012; Granum et al., 2013; Stein et al., 2016), developmental issues (Abbott et al., 2007; Lam et al., 2014), and adverse skeletal health (Koskela et al., 2016, 2017) in experimental and observational studies (Ballesteros et al., 2017; Cluett et al., 2019; Frisbee et al., 2010; Hu et al., 2019; Johnson et al., 2014; Khalil et al., 2016; Kim et al., 2018; Lau et al., 2006; Di Nisio, et al., 2020a; Rappazzo et al., 2017; Steenland et al., 2010).
Vitamin D significantly contributes to development and progression of chronic diseases, such cancers, autoimmune diseases, metabolic diseases, and cardiovascular diseases in addition to maintaining skeletal health (Bikle, 2014; Mousavi et al., 2019; Norman & Powell, 2014). During pregnancy, vitamin D homeostasis is essential for placentation and maintaining maternal and fetal health (Luk et al., 2012; Ponsonby et al., 2010; Wagner & Hollis, 2018; Zehnder et al., 2002). For example, vitamin D controls the secretion of some placental hormones, reduces infection, limits the production of pro-inflammatory cytokines, and supports intrauterine growth by providing calcium and phosphorous, and enhancing skeletal ossification (Barrera et al., 2007, 2008; Shin et al., 2010). In two meta-analyses of epidemiological studies, levels of serum 25-hydroxyvitamin D (25(OH)D), a metabolite of vitamin D, during pregnancy were inversely associated with risks of adverse pregnancy and birth outcomes including pre-eclampsia, gestational diabetes, preterm birth, and small-for-gestational age (Aghajafari et al., 2013; Wei et al., 2013). The effect of PFAS exposure on vitamin D hemostasis during pregnancy is of interest because perturbation of maternal hormone levels in this susceptible window can result in profound health risks in both pregnant women and their fetuses (Wagner & Hollis, 2018).
Environmental endocrine-disrupting chemicals (EDCs) have been proven to affect steroid and thyroid hormone metabolisms via different actions, such as interaction with hormone receptors and serum protein transporters, and influences on steroidogenesis and clearance (Ghassabian & Trasande, 2018; Sanderson, 2006; Yang et al., 2015). Vitamin D metabolism may also be altered by EDCs through similar pathways because its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D), is akin to the molecular structure of classic steroid hormones, and vitamin D receptor belongs to the same superfamily of steroid and thyroid receptors (Norman, 2008; Pike & Meyer, 2010; Schug et al., 2011). Previous epidemiological studies have shown that vitamin D metabolism was disturbed by exposures to EDCs including polychlorinated biphenyls (Morales et al., 2013), organochlorine pesticides (Yang et al., 2012), bisphenol A, and phthalates (Erden et al., 2014; Johns et al., 2016, 2017). Additionally, studies suggest that PFAS can affect bone mineral density in both adults and children (Khalil et al., 2016, 2018; Cluett et al., 2019; Hu et al., 2019), and the disturbance of vitamin D by PFAS exposure could be a potential explanation (Di Nisio et al., 2020b). Therefore, we hypothesized that PFAS, which act as EDCs to disrupt sex steroids and thyroid hormones (Benninghoff et al., 2011; Li et al., 2020; Weiss et al., 2009), may also affect vitamin D metabolism.
Only few epidemiologic studies have investigated the association between PFAS and the vitamin D system. These studies have shown inconsistent results and some of them have suffered from limited statistical power due to small sample sizes (Di Nisio et al. 2020b; Etzel et al., 2019; Khalil et al., 2018). Additionally, we are not aware of any study investigating the association in pregnant women. In the present study, we aimed to investigate the association of individual and combined serum PFAS levels with circulating serum total and free 25(OH)D concentrations in a population-based cohort of pregnant women.
2. Materials and Methods
2.1. Study population
This study utilized samples and data from the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Study, a prospective birth cohort study in Atlanta, Georgia. The details of this cohort were described in a previous report (Corwin et al., 2017). The participants were enrolled from two hospitals, Emory University Hospital (privately owned) and Grady Memorial hospital (publicly run), to enhance a wider coverage of socioeconomic status. The inclusion criteria were U.S.-born African Americans women by self-report, between 8–14 weeks of gestation, between age 18–40 years, able to communicate in English, experiencing no chronic medical condition nor taking prescribed medications. In the present study, we analyzed data from 442 women enrolled between March 2014 and May 2018. These subjects represent the first group of the participants in the cohort, whose pregnancy ended with a live birth with blood samples collected for PFAS and 25(OH)D measurements. Written informed consent was obtained from the participants at enrollment. Our study was reviewed and approved by Emory’s Institutional Review Board (approval reference number 68441).
2.2. Data collection
Data were collected at two routine clinical visits (Enrollment/Visit 1, at 8–14 weeks of gestation; Visit 2, at 24–30 weeks of gestation) through questionnaire administration and medical record abstraction. Sociodemographic information such as education, marital and cohabiting status, insurance status, income-to-poverty ratio, and tobacco, marijuana, and alcohol use was gathered by self-report and prenatal clinical records. Clinical data including maternal age, parity, fetal sex, and gestational age at time of sampling were ascertained from prenatal clinical records, and body mass index (BMI) was derived from height and weight measured at Visit 1.
A Food Frequency Questionnaire (FFQ) was administrated on a subset of women (n=292) at Visit 1 and Visit 2 to collect the information of fish and vitamin D supplement intake over the previous three months. Vitamin D supplement intake information was extracted by the question “how often did you take vitamin D supplements”, and fish intake information was obtained from the question “how often did you eat fish”. The FFQ used in this study was modified to collect intake information from pregnant women and validated in various low-income pregnant women population (Baer et al., 2005).
2.3. Biological specimens and assays
For blood sample collections, the laboratory technicians extracted additional blood from the routine blood draws at two prenatal clinical visits for research purposes. The blood samples were transported to the laboratory and centrifuged for serum separation. The serum samples were then stored at −80°C for future analyses. PFAS were measured in the serum samples from Visit 1, and total and free 25(OH)D were measured in the serum samples from both prenatal visits.
2.3.1. Quantification of PFAS
Aliquots of maternal serum were measured at two laboratories from the Children’s Health Exposure Analysis Resource (CHEAR) -- Wadsworth Center/New York University Laboratory Hub (Wadsworth/NYU) and the Laboratory of Exposure Assessment and Development for Environmental Research (LEADER) at Emory University. CHEAR laboratories, supported by the U.S. National Institute of Environmental Health Sciences for the purpose of environmental exposure assessments, have followed the same quality control procedures to provide harmonized and quality data (Balshaw et al., 2017). All 442 samples were analyzed for PFHxS, PFOS, PFOA, and PFNA by these two laboratories, among which 351 samples were measured for 10 additional PFAS, including perfluorobutane sulfonic acid (PFBS), perfluorooctane sulfonamide (PFOSA), N-methyl perfluorooctane sulfonamido acetic acid (NMeFOSAA), N-ethyl perfluorooctane sulfonamido acetic acid (NEtFOSAA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), and perfluorododecanoic acid (PFDoA) by Wadsworth/NYU.
Further details of the analytical methods were described previously (Chang et al., 2020; Honda et al., 2018). Briefly, each sample was spiked with internal standards, extracted by solid phase extraction, and analyzed by liquid chromatography interfaced with tandem mass spectrometry (LC-MS/MS). Quantification of PFAS was performed using isotope dilution calibration. Wadsworth/NYU and LEADER have been certified by the German External Quality Assessment Scheme (http://g-equas.de/) twice each year for PFAS measurements. The results from these two laboratories have good agreements on 11 overlapped samples with Pearson correlation coefficients ranging from 0.88 to 0.93 and the relative percent differences (RPD) ranging from 0.12% to 20.2% (median 4.8%) (Table S1).
2.3.1. Quantification of vitamin D biomarkers
Serum total and free 25(OH)D were analyzed in the Vitamin D Research Laboratory at Emory University School of Medicine. The automated competitive binding chemiluminescence 25(OH)D assay (Immunodiagnostic Systems Ltd, Fountain Hills, AZ) was utilized to measure total 25(OH)D, with a detection range of 7–120 ng/mL. A competitive enzyme-linked immunosorbent assay (DIAsource ImmunoAssays, Louvain-la-Neuve, Belgium), calibrated against a symmetrical dialysis method, was used to measured free 25(OH)D. This method allows a direct measurement of the free fraction of 25(OH)D with a detection range of 2.4 –17.1 pg/mL and the limit of blank of 1.5 pg/mL. The laboratory participates in Vitamin D Metabolites Quality Assurance Program at the National Institute of Standards and Technology (Lippa et al., 2020) and the Vitamin D External Quality Assessment Scheme (http://www.deqas.org/), providing interlaboratory comparisons to warrant the reliability of 25(OH)D measurements. The definition of vitamin D deficiency was based on the reference range for the general population prescribed by the Endocrine Society: total 25(OH)D ≤ 20 ng/mL (Holick et al., 2011).
2.5. Statistical analysis
All the analyses were performed in R (version 3.6.1). Arithmetic means and standard deviations of total and free 25(OH)D at two prenatal visits were tabulated by selected population characteristics, and the measurements of serum 25(OH)D and PFAS below the limits of detection (LODs) were imputed as in the descriptive analysis (Hornung & Reed, 1990). No data transformation was performed for total and free 25(OH)D concentrations because the empirical histograms approximated normal distributions in this population. Due to right-skewed distribution, PFAS concentrations were natural-log transformed to reduce the impact of outliers before further analyses. PFBS, PFOSA, PFHxSA, PFHpA, NEtFOSAA, and PFDoA, which were less frequently detected (<15%), were excluded from this analysis, resulting in a total of eight PFAS were analyzing in this study. We used paired t-test to compare the means of total and free 25(OH)D concentrations from Visit 1 and Visit 2. Pearson correlation coefficients were calculated to investigate the correlations between the serum concentrations of total and free 25(OH)D at two visits and serum PFAS.
Next, we fit multivariable linear regressions to estimate the associations between PFAS exposure and 25(OH)D concentrations measured at two prenatal visits individually. For the Visit 1 models, both the exposure and the outcome were collected at the same prenatal visit (Visit 1), which was considered as a cross-sectional study design; whereas for the Visit 2 models, the exposure and outcome were collected at Visit 1 and Visit 2, respectively, which was considered as a prospective cohort study design. To explore the dose-response relationships, we modeled difference in total and free 25(OH)D concentrations with successive exposure categories (the PFAS with >90% detection frequencies grouped into quartiles; the PFAS with 40–50% detection frequencies were categorized into three groups: <LODs, and low and high exposure groups divided by median values of detectable levels) using multivariable linear regressions. Moreover, test for trend was performed by modeling the exposure categories as ordinal variables and used p-values for trend <0.05 as the criteria for monotonic effects. The odds of vitamin D deficiency were also modeled using multivariable logistic regressions.
We chose covariates guided by a directed acyclic graph (DAG) to identify potential confounding variables in the causal association between PFAS exposure and 25(OH)D concentrations (Figure S1) (Greenland et al., 1999). The covariates include maternal age (continuous, years), education (less than high school, high school, some college, college and above), BMI (<18.5, 18.5–25, 25–30, ≥30 kg/m2), parity (0, 1, ≥2), fetal sex (male, female), marijuana use (during pregnancy, not during pregnancy), tobacco use (during pregnancy, not during pregnancy), and season of sample collection for 25(OH)D (spring: March to May, summer: June to August, fall: September to November, winter: December to February). We assessed potential effect modification of PFAS by fetal sex because vitamin D metabolism through the placenta could be modulated by sex steroid hormones, leading to differential sensitivities to PFAS exposure by fetal sex (Liu et al., 2018; Olmos-Ortiz et al., 2016). We included interaction terms in the models and used p-value <0.10 as the cut-off for significance. Moreover, the effect estimates by fetal sex were derived from the model with the interaction term.
Additionally, a weighted quantile sum (WQS) regression was performed to evaluate the joint associations of the highly correlated eight PFAS levels with serum 25(OH)D concentrations using the R gwqs and miWQS packages. WQS can address the collinearity issues of highly correlated exposures and identify the major contributors of pollutant mixture. Details of the WQS methods can be found elsewhere (Carrico et al., 2015). Specifically, we divided the dataset into a training set (40%) and a validation set (60%). Each exposure is empirically assigned a weight based on their associations with the outcomes by using bootstrap samples in a training set. We categorized the eight PFAS into quartiles and the bootstrapped weights are multiplied by these PFAS quantiles. The products of quartiles and weights were then summed to create an index for the PFAS mixtures. Next, the index is used to estimate the overall effect of the PFAS mixture on 25(OH)D using a validation set. The basic WQS regression model is:
where β0 is the intercept, β1 is the regression coefficient for the WQS index and can be interpreted as the effect of the PFAS mixture on the outcomes, zi′ represents the values of other covariates from the ith subject, and φ denotes the corresponding regression coefficients. The term represents the WQS index for each participant, ωj is the weight for the jth PFAS (0 ≤ ωj ≤ 1, ) and can be used to identify the important chemicals in the PFAS mixture (a priori cut-point = 1/number of chemicals = 1/8 =0.125); PFASqji is the quantile for the jth PFAS from the ith subject. g(μ) is a monotonic link function linking the predictor to the mean of the continuous outcome variables. The effects of the PFAS mixture on 25(OH)D were estimated for both directions separately, using the analysis constrained in either positive or negative directions. Effect modification by fetal sex was evaluated by including an interaction term with the WQS index in the model, and the interaction term was removed if the p-value was higher than 0.10 (Brunst et al., 2017; Preston et al., 2020).
Single imputation with was conducted for the biomarkers with higher detection frequencies (87–99%), i.e., PFHxS, PFOS, PFOA, PFNA, and total and free 25(OH)D. A multiple imputation framework was adopted for the biomarkers with lower detection frequencies (40–50%), i.e., PFPeA, PFDA, PFUnDA, and NMeFOSAA, because a high percentage of the measurements below the LODs could introduce biases into the analyses. We imputed the values below the LOD by randomly sampling from a lognormal distribution with the estimated parameters from maximum likelihood estimates (Gilbert, 1987). To incorporate the uncertainty, ten datasets were created based on the different estimated parameters from bootstrapping. These datasets were independently analyzed in the statistical models and combined to reflect variabilities of the imputation process (Hargarten & Wheeler, 2020; Lubin et al., 2004). Analyses investigating the association between PFAS and 25(OH)D which produced p-values less than 0.05 were considered statistically significant.
Several sensitivity analyses were performed to ensure the robustness of our results. First, to assess the impact of the potential diet confounders, which were only available for some participants in the study, we performed sensitivity analyses on a subset of participants (n=292) to adjust for fish (yes/no) and vitamin D supplement intake (yes/no) in addition to the other covariates in the main models. Additionally, to remove the effect of vitamin D supplement intake completely, we further excluded the participants who took vitamin D supplement during pregnancy in the analyses (n=160). Second, because both PFAS and 25(OH)D can bind to albumin and circulate in the blood (Bikle & Schwartz, 2019; Forsthuber et al., 2020), we used data from NHANES 2011–2014 to examine the influence of albumin on the association between PFAS and 25(OH)D. The detailed statistical analyses for NHANES data were described in the supplement. Third, the results of single imputation with and multiple imputation for the biomarkers with lower detection frequencies, i.e., PFPeA, PFDA, PFUnDA, and NMeFOSAA, were compared to determine the impact of different imputation methods.
3. Results
3.1. Distribution of study variables
The demographic characteristics of the cohort are described in Table 1. The majority of the participating women were between 18 and 25 years of age, and predominantly at a lower socioeconomic status – about 55% with a high school or below education, 57% below 150% income-to-poverty ratio, 78% with Medicaid as medical insurance, and 60% enrolled at a public hospital. A total of 52% of the participants had given birth one or more times; 21% were overweight, and 36% were obese.
Table 1.
Serum 25(OH)D concentrations by selected population characteristics in pregnant African American women in the Atlanta area, 2014–2018 (n=442).
| Visit 1 | Visit 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n (%)a | Total 25(OH)Db (ng/mL) Mean ± SD | n (%)a | Free 25(OH)Db (pg/mL) Mean ± SD | n (%)a | Total 25(OH)Db (ng/mL) Mean ± SD | n (%)a | Free 25(OH)Db (pg/mL) Mean ± SD |
| All participants (N=442) | 436 (98.6) | 19.5 ± 8.7 | 439 (99.3) | 3.7 ± 1.4 | 337 (76.2) | 23.3 ± 11.1 | 341 (77.1) | 4.0 ± 1.7 |
| Age (year) | ||||||||
| 18–25 | 241 (54.5) | 18.9 ± 8.5 | 241 (54.5) | 3.6 ± 1.4 | 184 (41.6) | 22.7 ± 10.6 | 186 (42.1) | 3.9 ± 1.7 |
| 25–30 | 111 (25.1) | 20.1 ± 8.8 | 114 (25.8) | 3.7 ± 1.3 | 81 (18.3) | 23.3 ± 10.9 | 84 (19.0) | 3.9 ± 1.5 |
| 30–35 | 65 (14.7) | 20.7 ± 8.8 | 65 (14.7) | 3.9 ± 1.5 | 57 (12.9) | 24.1 ± 10.9 | 57 (12.9) | 4.2 ± 1.7 |
| >35 | 19 (4.3) | 20.8 ± 11.8 | 19 (4.3) | 4.4 ± 2.3 | 15 (3.4) | 27.5 ± 17.1 | 14 (3.2) | 4.6 ± 2.1 |
| Education | ||||||||
| Less than high school | 71 (16.1) | 19.6 ± 8.1 | 71 (16.1) | 3.6 ± 1.2 | 50 (11.3) | 23.5 ± 9.8 | 50 (11.3) | 4.0 ± 1.5 |
| High school | 166 (37.6) | 17.6 ± 8.3 | 167 (37.8) | 3.4 ± 1.4 | 126 (28.5) | 21.9 ± 11.2 | 129 (29.2) | 3.7 ± 1.5 |
| Some college | 130 (29.4) | 20.2 ± 8.6 | 130 (29.4) | 3.7 ± 1.3 | 102 (23.1) | 22.4 ± 10.2 | 103 (23.3) | 4.1 ± 1.8 |
| College and above | 69 (15.6) | 22.9 ± 9.6 | 71 (16.1) | 4.2 ± 1.7 | 59 (13.3) | 27.5 ± 12.4 | 59 (13.3) | 4.5 ± 1.7 |
| Income-to-poverty ratio (%) | ||||||||
| <100 | 184 (41.6) | 19.2 ± 9.2 | 186 (42.1) | 3.6 ± 1.3 | 144 (32.6) | 22.0 ± 10.8 | 147 (33.3) | 3.8 ± 1.5 |
| 100–150 | 67 (15.2) | 16.9 ± 7.1 | 68 (15.4) | 3.3 ± 1.0 | 50 (11.3) | 21.6 ± 9.6 | 51 (11.5) | 3.6 ± 1.2 |
| 150–300 | 93 (21.0) | 18.6 ± 7.8 | 94 (21.3) | 3.7 ± 1.4 | 75 (17.0) | 21.1 ± 9.3 | 75 (17.0) | 4.0 ± 1.4 |
| >300 | 31 (7.0) | 24.6 ± 9.9 | 31 (7.0) | 4.7 ± 2.1 | 28 (6.3) | 30.5 ± 14.7 | 28 (6.3) | 4.7 ± 2.2 |
| Married or cohabitating | ||||||||
| No | 207 (46.8) | 20.5 ± 8.8 | 209 (47.3) | 3.8 ± 1.5 | 163 (36.9) | 24.2 ± 11.7 | 165 (37.3) | 4.1 ± 1.7 |
| Yes | 229 (51.8) | 18.7 ± 8.7 | 230 (52.0) | 3.6 ± 1.3 | 174 (39.4) | 22.5 ± 10.4 | 176 (39.8) | 3.9 ± 1.6 |
| Insurance | ||||||||
| Private | 95 (21.5) | 22.5 ± 9.1 | 96 (21.7) | 4.1 ± 1.7 | 81 (18.3) | 25.5 ± 12.1 | 80 (18.1) | 4.3 ± 1.8 |
| Public (Medicaid) | 341 (77.1) | 18.7 ± 8.5 | 343 (77.6) | 3.6 ± 1.3 | 256 (57.9) | 22.6 ± 10.7 | 261 (59.0) | 3.9 ± 1.6 |
| Hospital | ||||||||
| Private (Emory) | 175 (39.6) | 21.3 ± 9.1 | 175 (39.6) | 3.9 ± 1.5 | 140 (31.7) | 24.3 ± 11.1 | 140 (31.7) | 4.1 ± 1.6 |
| Public (Grady) | 261 (59.0) | 18.4 ± 8.3 | 264 (59.7) | 3.5 ± 1.4 | 197 (44.6) | 22.6 ± 11.0 | 201 (45.5) | 3.9 ± 1.7 |
| Parity | ||||||||
| 0 | 210 (47.5) | 19.7 ± 8.3 | 210 (47.5) | 3.7 ± 1.5 | 148 (33.5) | 25.2 ± 11.8 | 148 (33.5) | 4.3 ± 1.8 |
| 1 | 119 (26.9) | 20.4 ± 9.5 | 121 (27.4) | 3.8 ± 1.5 | 101 (22.9) | 23.3 ± 10.6 | 103 (23.3) | 3.9 ± 1.6 |
| >2 | 107 (24.2) | 18.3 ± 8.7 | 108 (24.4) | 3.5 ± 1.2 | 88 (19.9) | 20.1 ± 9.7 | 90 (20.4) | 3.5 ± 1. |
| BMI (kg/m2) | ||||||||
| < 18.5 | 15 (3.4) | 18.0 ± 10.1 | 15 (3.4) | 3.8 ± 1.8 | 13 (2.9) | 23.2 ± 12.5 | 13 (2.9) | 4.3 ± 1.9 |
| 18.5–25 | 169 (38.2) | 20.3 ± 8.1 | 171 (38.7) | 3.7 ± 1.4 | 129 (29.2) | 25.4 ± 10.2 | 132 (29.9) | 4.1 ± 1.9 |
| 25–30 | 94 (21.3) | 20.4 ± 9.8 | 94 (21.3) | 3.8 ± 1.6 | 68 (15.4) | 24.7 ± 12.8 | 70 (15.8) | 3.9 ± 1.7 |
| ≥ 30 | 158 (35.7) | 18.4 ± 8.5 | 159 (36.0) | 3.6 ± 1.3 | 127 (28.7) | 20.4 ± 10.3 | 126 (28.5) | 3.9 ± 1.3 |
| Fetal Sex | ||||||||
| Male | 208 (47.1) | 20.5 ± 9.4 | 208 (47.1) | 3.9 ± 1.6 | 163 (36.9) | 24.4 ± 11.5 | 165 (37.3) | 4.1 ± 1.8 |
| Female | 225 (50.9) | 18.6 ± 8.1 | 228 (51.6) | 3.5 ± 1.2 | 174 (39.4) | 22.3 ± 10.6 | 176 (39.8) | 3.9 ± 1.5 |
| Marijuana Use | ||||||||
| Not during pregnancy | 343 (77.6) | 20.1 ± 8.6 | 344 (77.8) | 3.8 ± 1.5 | 261 (59.0) | 24.4 ± 11.1 | 264 (59.7) | 4.1 ± 1.7 |
| During pregnancy | 93 (21.0) | 17.5 ± 9.1 | 95 (21.5) | 3.3 ± 1.1 | 76 (17.2) | 19.6 ± 10.3 | 77 (17.4) | 3.5 ± 1.4 |
| Tobacco Use | ||||||||
| Not during pregnancy | 377 (85.3) | 19.9 ± 8.6 | 378 (85.5) | 3.7 ± 1.4 | 289 (65.4) | 23.6 ± 11.2 | 291 (65.8) | 4.1 ± 1.7 |
| During pregnancy | 59 (13.3) | 17.5 ± 9.3 | 61 (13.8) | 3.5 ± 1.3 | 48 (10.9) | 21.4 ± 9.8 | 50 (11.3) | 3.7 ± 1.4 |
| Alcohol Use | ||||||||
| Not during pregnancy | 400 (90.5) | 19.5 ± 8.7 | 403 (91.2) | 3.7 ± 1.4 | 307 (69.5) | 23.3 ± 11.1 | 311 (70.4) | 4.0 ± 1.6 |
| During pregnancy | 36 (8.1) | 19.5 ± 8.8 | 36 (8.1) | 3.6 ± 1.6 | 30 (6.8) | 23.4 ± 11.0 | 30 (6.8) | 4.0 ± 1.9 |
| Sampling season c | ||||||||
| Winter (Dec-Feb) | 79 (17.9) | 15.9 ± 7.5 | 81 (18.3) | 3.4 ± 1.3 | 115 (26.0) | 21.4 ± 11.1 | 119 (26.9) | 3.7 ± 1.5 |
| Spring (Mar-May) | 96 (21.7) | 17.7 ± 7.0 | 96 (21.7) | 3.5 ± 1.2 | 70 (15.8) | 23.0 ± 12.6 | 71 (16.1) | 3.8 ± 1.6 |
| Summer (Jun-Aug) | 123 (27.8) | 24.6 ± 8.0 | 121 (27.4) | 4.2 ± 1.4 | 70 (15.8) | 24.0 ± 10.1 | 70 (15.8) | 4.3 ± 1.8 |
| Fall (Sep-Nov) | 138 (31.2) | 18.4 ± 9.3 | 141 (31.9) | 3.5 ± 1.5 | 81 (18.3) | 25.7 ± 10.1 | 81 (18.3) | 4.3 ± 1.7 |
| Vitamin D deficiency (Total 25(OH)D ≤ 20 ng/mL) | ||||||||
| No | 198 (44.8) | 27.1 ± 6.2 | 196 (44.3) | 4.3 ± 1.5 | 197 (44.6) | 30.5 ± 8.2 | 196 (44.3) | 4.7 ± 1.8 |
| Yes | 238 (53.8) | 13.3 ± 4.6 | 238 (53.8) | 3.2 ± 1.1 | 140 (31.7) | 13.1 ± 4.7 | 140 (31.7) | 3.1 ± 0.9 |
| The participants with Food frequency questionnaires (n=292) | 287 (98.3) | 19.6 ± 8.9 | 291 (99.7) | 3.8 ± 1.5 | 238 (81.5) | 23.3 ± 11.5 | 243 (83.2) | 4.0 ± 1.7 |
| Vitamin D supplement in the past three months c | ||||||||
| No | 158 (55.1) | 18.4 ± 8.2 | 161 (55.3) | 3.6 ± 1.3 | 129 (54.2) | 21.7 ± 11.7 | 131 (53.9) | 3.8 ± 1.5 |
| Yes | 129 (44.9) | 21.1 ± 9.5 | 130 (44.7) | 4.0 ± 1.6 | 109 (45.8) | 25.2 ± 11.0 | 112 (46.1) | 4.3 ± 1.9 |
| Fish consumption in the past three months c | ||||||||
| No | 56 (19.5) | 21.2 ± 8.3 | 55 (18.9) | 4.0 ± 1.5 | 89 (37.4) | 22.6 ± 11.2 | 91 (37.4) | 3.9 ± 1.5 |
| Yes | 231 (80.5) | 19.3 ± 9.0 | 236 (81.1) | 3.7 ± 1.5 | 149 (62.6) | 23.7 ± 11.6 | 152 (62.6) | 4.1 ± 1.8 |
Note: Visit 1 = 25(OH)D collected at 8–14 weeks of gestation; Visit 2 = 25(OH)D collected at 24–30 weeks of gestation; n = sample number; SD = standard deviation.
The sample numbers might not be summed up to the total sample number due to missingness.
The values below limits of detection (LODs) were replaced by .
Longitudinal variables: data were collected at two visits.
In the paired t-test analyses, the mean concentrations of total and free 25(OH)D significantly increased from Visit 1 to Visit 2 (p <0.01 for both total and free). On average, the total 25(OH)D levels were 19.5 ng/mL (standard deviation (SD) = 8.7) and 23.3 ng/mL (SD = 11.1), and the free 25(OH)D concentrations were 3.7 pg/mL (SD = 1.4) and 4.0 pg/mL (SD = 1.7) at Visit 1 and Visit 2, respectively. Similar concentrations were found in the subset of the participants with FFQ data. Total and free 25(OH)D concentrations were generally higher among people in high socioeconomic groups, including the women with college and above education, ≥300% income-to-poverty ratio, private insurance, and being enrolled in the private hospital. In addition, higher 25(OH)D concentrations were mostly observed among the women with no partner, less parity, and with male fetuses. The other variables shown different 25(OH)D concentrations include the consumption of marijuana, tobacco, and vitamin D supplement, and the season of sample collection. Among two prenatal visits, detection frequencies for total and free 25(OH)D were in a range of 87–94% in this study population (Table S2).
The exposure distributions of PFAS of this cohort have been described in detail previously (Chang et al., 2020). Briefly, PFHxS, PFOS, PFOA, and PFNA were detected in >95% samples with PFOS having the highest geometric mean (2.03 ng/mL, geometric SD = 2.08). NMeFOSAA, PFPeA, PFDA, and PFUnDA were detected in approximately 40–50% of the participants (Table S3). Total 25(OH)D was positively correlated with most PFAS (r = 0.10–0.34), and negatively correlated with PFPeA (r = −0.23 and −0.21). Free 25(OH)D was weakly correlated with PFDA (r = 0.13 and 0.15) and PFOS (r = 0.10) but showed no correlation with the other PFAS. Moderate and strong correlations were found between total and free 25(OH)D concentrations with the coefficients ranging from 0.43 to 0.77 (Schober et al., 2018) (Table S4).
3.2. Association between individual PFAS and vitamin D biomarkers
The associations between serum PFAS and 25(OH)D are presented in Table 2 by fetal sex. Each natural-log unit increase in PFHxS, PFOS, PFDA, and NMeFOSAA was associated with a significant increase in total 25(OH)D concentrations among the women with either male or female fetuses, except for NMeFOSAA in the Visit 1 model. PFHxS in the Visit 2 models showed the largest effects (βmale = 4.71, 95%CI 2.28–7.14; βfemale = 3.53, 95%CI 1.28–5.77). Negative associations between total 25(OH)D and serum PFPeA were observed among the women with male fetuses in both the Visit 1 and 2 models (Visit 1: βmale = −2.23, 95%CI −3.50, −0.95, pint = 0.14; Visit 2: βmale = −3.53, 95%CI −5.68, −1.38, pint = 0.08), and null associations among those with female fetuses (Visit 1: βfemale = −0.88, 95%CI –2.21, 0.45; Visit 2: βfemale = −0.77, 95%CI −2.70, 1.16). Additionally, some significant but inconsistent associations of total 25(OH)D across the Visit 1 and 2 models were found in PFOA, PFNA, and PFUnDA. For free 25(OH)D, positive associations were found in PFHxS, PFOS, PFOA, and PFDA, and negative association was found in PFPeA only among the women with male fetuses in the Visit 2 models. PFHxS also showed the largest effects for free 25(OH)D (βmale = 0.41, 95%CI 0.03–0.79, pint = 0.05). The associations between PFAS and 25(OH)D among all participants were presented in Table S5.
Table 2.
Adjusted differences in serum 25(OH)D concentrations with per natural-log unit increase of serum PFAS concentrations (ng/mL) by fetal sex in pregnant African American women in the Atlanta area, 2014–2018.
| 25(OH)D | PFHxSa | PFOSa | PFOAa | PFNAa | PFPeAb | PFDAb | PFUnDAb | NMeFOSAAb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | β (95% CI) |
p | ||
| Totala Visit 1 (ng/mL) | M c | 2.58 (0.86, 4.30) | <0.01 | 2.79 (1.28, 4.31) | <0.01 | 0.39 (−0.86, 1.64) | 0.54 | 0.26 (−0.92, 1.45) | 0.66 | -2.23 (−3.50, −0.95) | <0.01 | 1.59 (0.62, 2.57) | <0.01 | -1.00 (−1.92, −0.08) | 0.03 | 1.21 (0.34, 2.08) | 0.01 |
| F c | 4.15 (2.58, 5.72) | <0.01 | 3.56 (2.01, 5.12) | <0.01 | 1.20 (−0.17, 2.56) | 0.09 | 1.49 (0.12, 2.87) | 0.03 | −0.88 (−2.21, 0.45) | 0.20 | 1.88 (0.95, 2.81) | <0.01 | 0.25 (−0.65, 1.16) | 0.58 | 0.77 (−0.09, 1.62) | 0.08 | |
| p int | 0.18 | 0.48 | 0.39 | 0.18 | 0.14 | 0.67 | 0.05 | 0.49 | |||||||||
| n | 433 | 433 | 433 | 433 | 346 | 346 | 346 | 346 | |||||||||
| Totala Visit 2 (ng/mL) | M c | 4.71 (2.28, 7.14) | <0.01 | 4.64 (2.43, 6.84) | <0.01 | 2.91 (1.15, 4.67) | <0.01 | 1.42 (−0.36, 3.19) | 0.12 | -3.53 (−5.68, −1.38) | <0.01 | 2.59 (1.12, 4.06) | <0.01 | −1.17 (−2.56, 0.23) | 0.10 | 1.90 (0.60, 3.20) | 0.01 |
| F c | 3.53 (1.28, 5.77) | <0.01 | 2.65 (0.69, 4.61) | 0.01 | 0.76 (−1.13, 2.66) | 0.43 | 1.11 (−0.84, 3.06) | 0.26 | −0.77 (−2.70, 1.16) | 0.44 | 2.52 (1.06, 3.99) | <0.01 | 0.02 (−1.36, 1.40) | 0.98 | 1.31 (0.03, 2.60) | 0.05 | |
| p int | 0.48 | 0.18 | 0.10 | 0.82 | 0.08 | 0.95 | 0.22 | 0.54 | |||||||||
| n | 336 | 336 | 336 | 336 | 261 | 261 | 261 | 261 | |||||||||
| Freea Visit 1 (pg/mL) | M c | 0.13 (−0.18, 0.43) | 0.42 | 0.18 (−0.09, 0.45) | 0.19 | 0.07 (−0.14, 0.28) | 0.52 | 0.00 (−0.21, 0.20) | 0.97 | −0.21 (−0.47, 0.06) | 0.13 | 0.14 (−0.05, 0.34) | 0.14 | −0.14 (−0.31, 0.04) | 0.12 | 0.08 (−0.07, 0.23) | 0.29 |
| F c | −0.17 (−0.44, 0.10) | 0.21 | −0.19 (−0.44, 0.05) | 0.12 | −0.09 (−0.32, 0.13) | 0.43 | −0.17 (−0.40, 0.06) | 0.14 | 0.06 (−0.16, 0.28) | 0.60 | 0.05 (−0.12, 0.22) | 0.57 | −0.03 (−0.18, 0.12) | 0.70 | 0.04 (−0.11, 0.19) | 0.59 | |
| p int | 0.14 | 0.04 | 0.30 | 0.28 | 0.14 | 0.48 | 0.36 | 0.70 | |||||||||
| n | 436 | 436 | 436 | 436 | 348 | 348 | 348 | 348 | |||||||||
| Freea Visit 2 (pg/mL) | M c | 0.41 (0.03, 0.79) | 0.03 | 0.40 (0.06, 0.74) | 0.02 | 0.29 (0.03, 0.56) | 0.03 | 0.20 (−0.07, 0.47) | 0.14 | -0.37 (−0.72, −0.02) | 0.04 | 0.28 (0.05, 0.52) | 0.02 | −0.12 (−0.36, 0.13) | 0.36 | 0.05 (−0.15, 0.25) | 0.62 |
| F c | −0.10 (−0.45, 0.25) | 0.57 | −0.07 (−0.38, 0.23) | 0.63 | −0.01 (−0.30, 0.28) | 0.94 | −0.23 (−0.52, 0.07) | 0.13 | 0.23 (−0.05, 0.52) | 0.11 | 0.04 (−0.18, 0.26) | 0.72 | 0.01 (−0.20, 0.22) | 0.93 | 0.04 (−0.15, 0.22) | 0.71 | |
| p int | 0.05 | 0.04 | 0.13 | 0.04 | 0.01 | 0.15 | 0.45 | 0.91 | |||||||||
| n | 341 | 341 | 341 | 341 | 264 | 264 | 264 | 264 |
Note: CI = confidence interval; p = p-value; Visit 1 = 25(OH)D collected at 8–14 weeks of gestation; Visit 2 = 25(OH)D collected at 24–30 weeks of gestation; M = male fetus; F = female fetus; pint = p-value for interaction term; n = sample number.
The values below limits of detection (LODs) were replaced by .
The values below LODs were multiply imputed by a lognormal distribution and maximum likelihood estimation due to their lower detection frequencies (43–49%).
The models were adjusted for maternal age, education, BMI, parity, fetal sex, tobacco use, marijuana use, and season of sample collection for 25(OH)D, and PFAS × fetal sex interaction term. The effect estimates (β) by fetal sex were derived from the model with the interaction term.
Table S6 and Figure S2 show the dose-response relationships between PFAS and 25(OH)D by fetal sex. Monotonic responses of total 25(OH)D were generally found in PFHxS, PFOS, PFDA, and NMeFOSAA among the women with both fetal sexes, and PFPeA and PFUnDA among the women with male fetuses. For free 25(OH)D, significant p-values for trend were observed in PFOS, PFPeA, and PFDA among the women with male fetuses.
There were 238 (54%) and 140 (32%) participants at Visit 1 and Visit 2 who were vitamin D deficient. As shown in Table S7, increases in serum PFHxS, PFOS, PFDA, and NMeFOSAA concentrations were generally associated with decreased odds of vitamin D deficiency among the women with both fetal sexes, with PFHxS among the women with female fetuses in the Visit 1 model showing the largest effects (ORfemale = 0.32, 95%CI 0.19–0.54). However, an increase in PFPeA concentrations was associated with increased odds of vitamin D deficiency among the women with male fetuses (ORmale = 1.58, 95%CI 1.00–2.48 for Visit 1; ORmale = 2.06, 95%CI 1.24–3.44 for Visit 2). Some significant but inconsistent findings were shown in PFOA, PFNA, and PFUnDA across the Visit 1 and 2 models.
3.3. Association between the PFAS mixture and vitamin D biomarkers
Figure 1 summarizes the results of WQS regression analyses (see also Table S8). Because there was no significant effect modification by fetal sex in the WQS analyses, we presented the effects of the PFAS mixtures on 25(OH)D for all the participants collectively. The WQS index was positively associated with total 25(OH)D. More specifically, a quartile increase in the WQS index was associated with increases of 2.88 ng/mL (95%CI 1.14–4.59) and 5.68 ng/mL (95%CI 3.31–8.04) total 25(OH)D in the Visit 1 and Visit 2 models, respectively. Within the PFAS mixture, NMeFOSAA (weight = 0.36 for Visit 1; 0.38 for Visit 2), PFDA (weight = 0.41 for Visit 1; 0.17 for Visit 2), and PFOS (weight = 0.24 for Visit 2) had weights exceeding the cut-point of 0.125, suggesting major contributions of these PFAS to the overall effect of the mixture. No negative regression coefficients in the bootstrapped models for total 25(OH)D were found; thus, we were unable to present the results of negative direction models. Additionally, no association was found between free 25(OH)D concentrations and the PFAS mixture in both directionalities.
Figure 1. Associations of the PFAS mixture with (a) total 25(OH)D concentrations, and with (b) free 25(OH)D concentrations based on weighted quantile sum regression (WQS) analyses in pregnant African American women in the Atlanta area, 2014–2018.
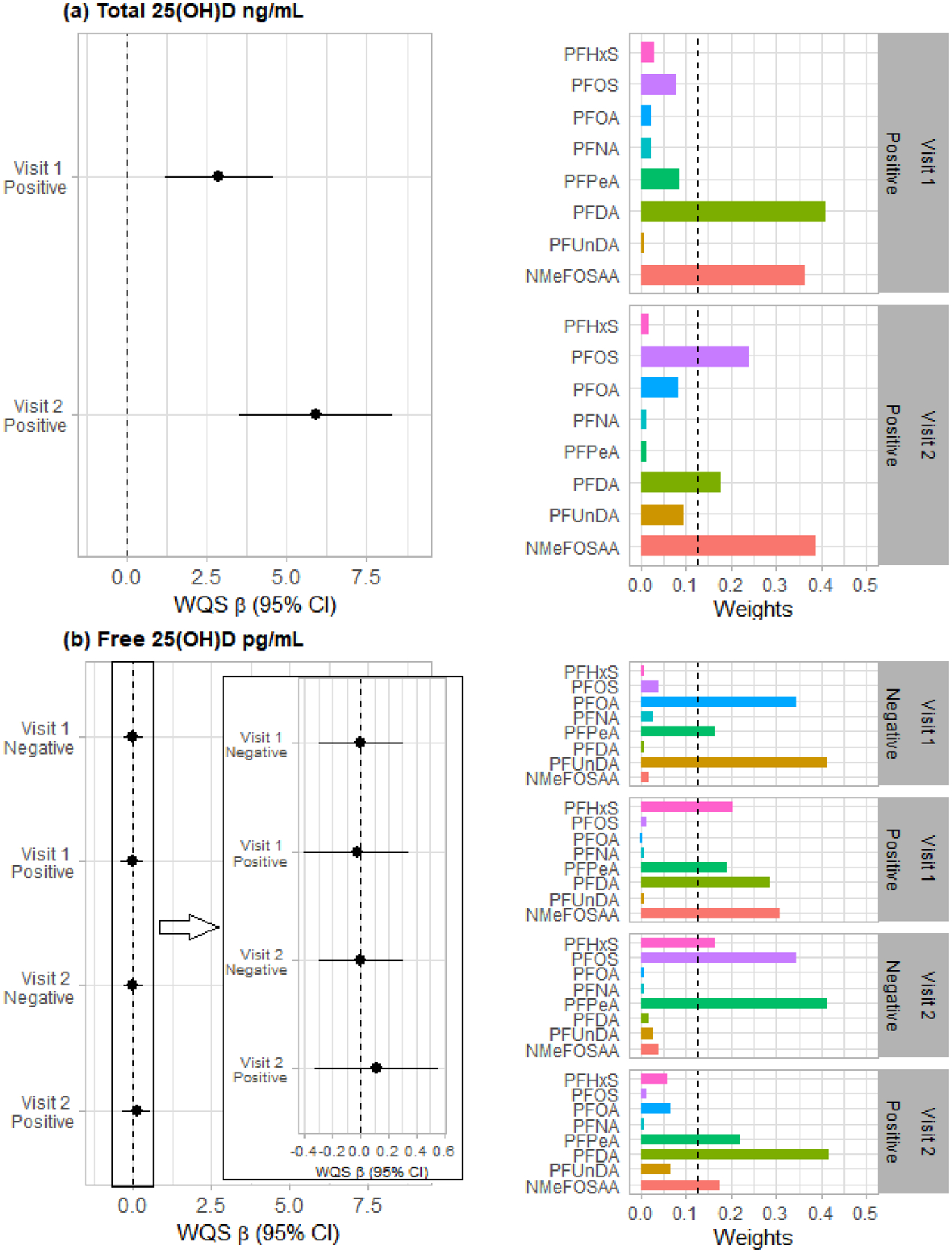
The models were adjusted for maternal age, education, BMI, parity, fetal sex, tobacco use, marijuana use, and season of sample collection for 25(OH)D. We ran each model twice, one in positive and one in negative direction of effects. Sample numbers are 346, 261, 348, and 264 for the models of total 25(OH)D at Visit 1, total 25(OH)D at Visit 2, free 25(OH)D at Visit 1, and free 25(OH)D at Visit 2, respectively. Note: Visit 1 = 25(OH)D collected at 8–14 weeks of gestation; Visit 2 = 25(OH)D collected at 24–30 weeks of gestation; the dashed line on the right bar chart represent the a priori cut-point for identification of important agents: 1/numer of chemicals = 1/8 = 0.125.
3.4. Sensitivity analysis
In the sensitivity analyses, additionally adjusting for fish and vitamin D supplement intake or excluding the participants taking vitamin D supplement did not substantially change the results among a subset of the participants (Table S9). Similarly, additional adjustment for albumin had little impact on the estimates in the NHANES 2011–2014 participants, even when stratifying by age, race/ethnicity, and sex (Table S10). Table S8 and S11 shows the difference between single imputation with and multiple imputation for the values below the LODs. Changes in estimates were calculated between the two imputation methods, and the range of percentage change [(βmultiple-βsingle)/βsingle] was between −133% and 33% with a median of −35%. We observed overall larger effect sizes using single rather than multiple imputation.
Discussion
In this cohort of 442 healthy pregnant African American women, we report general findings of positive associations of circulating total 25(OH)D with PFHxS, PFOS, PFDA, and NMeFOSAA concentrations. We noted positive associations of total 25(OH)D with PFOA and PFNA, and negative associations of total 25(OH)D with PFPeA and PFUnDA among certain fetal sex. Although the statistical significance levels of these findings were inconsistent between the Visit 1 and the Visit 2 models for PFOA, PFNA, PFUnDA, and NMeFOSAA, the direction of associations remains consistent for the same PFAS. For free 25(OH)D, we observed positive associations with PFHxS, PFOS, PFOA, and PFDA, and an inverse association with PFPeA among the women with male fetuses in the Visit 2 models. A joint effect of the eight PFAS was also positively associated with total 25(OH)D concentrations, with NMeFOSAA, PFDA, and PFOS as the most important contributors, explaining 79–85% of the total weight. No significant association between free 25(OH)D and the PFAS mixture was found.
To date, limited human research have evaluated the associations between PFAS exposure and vitamin D biomarkers. Altered vitamin D levels associated with serum PFAS concentrations were observed in the general U.S. population using the data from NHANES 2003–2010 participants (n=7040), where a positive association of total 25(OH)D with PFHxS and an inverse association with PFOS concentrations were found predominantly in non-Hispanic whites than the other races/ethnicities (Etzel et al., 2019). No association between total 25(OH)D and PFAS was reported by Khalil et al. (2018) or Di Nisio et al. (2020b) with their smaller cohorts of obese children aged 8–12 years (n=47) and healthy males aged 18–21 years (n=100), respectively. The inconsistent findings across these studies suggest that more epidemiological studies with larger samples size are needed.
It is somewhat unexpected to observe that most PFAS exposures are associated with elevated 25(OH)D concentrations given the majority of environmental pollutants showing inverse associations with total 25(OH)D (Johns et al., 2016, 2017; Morales et al., 2013; Yang et al., 2012). Although the positive associations could be due to residual confounding, including behaviors and socioeconomic status, it is also possible that our results only partially captured non-monotonic dose-response relationships, which have been observed in numerous EDCs, especially given the relatively narrow range of serum PFAS concentrations in an environmental exposure cohort or lower 25(OH)D concentrations in African Americans (Ginde et al., 2010; Li et al., 2007; Vandenberg et al., 2012). The positive associations also suggest that PFAS may have different actions in the vitamin D system from the other environmental pollutants (Etzel et al., 2019).
The observed positive associations could also be explained by a compensatory mechanism due to inefficient binding of vitamin D to its receptor. PFOA was shown to compete for vitamin D receptors with 1,25(OH)2D, the active metabolite of vitamin D (Di Nisio et al., 2020b) (Figure S3). The competition may reduce the activation of vitamin D receptor on the responsive gene expression and cause a functional hypovitaminosis D. For example, CYP24A1, a major 25(OH)D-inactivating cytochrome P450 enzyme in the liver, can be transcriptionally upregulated by activated vitamin D receptors (Jones et al., 2012; Ohyama et al., 1993). The antagonistic activity of PFOA via receptor competition may result in downregulation of CYP24A1, thus elevation of circulating 25(OH)D. Similarly, PFOA may dysregulate CYP27B1 in the kidney, and lead to altered levels of 1,25(OH)2D and of 25(OH)D (Bikle, 2014; Johnson et al., 2014). It is worth noting that besides acting as a passive antagonist through competing for receptor binding, an EDC ligand bound to the hormone receptor may also function as agonist or active antagonist to induce or repress gene expression through recruiting coactivators or corepressors in a tissue context-dependent manner (Li et al., 2007; Smith et al., 1997; Smith & O’Malley, 2004). It is therefore possible that the biological effects of PFAS through vitamin D receptors can be bidirectional. Moreover, the homeostasis of vitamin D is also tightly regulated through feedbacks involving parathyroid hormone, calcium, phosphorus, and fibroblast growth factor; thus, it is possible for PFAS to influence vitamin D levels through interacting with the concentrations of these metabolites (Christakos et al., 2010; Johns et al., 2017). However, future studies are necessary to establish the actions of PFAS on the vitamin D system and elucidate the clinical and public health relevance of these findings.
The effects of PFAS on free 25(OH)D were not as predominant and consistent as those on total 25(OH)D across the Visit 1 and the Visit 2 models. Free 25(OH)D is present at very low concentrations (i.e., parts per-trillion, 10−12) with low variance, which makes their measurements very challenging; the current immunoassay method has not been rigorously validated in a broad human population with various physiological conditions (Feldman et al., 2017; Jukic et al., 2018). Thus, the potential measurement errors coupled with relatively small variance could bias the associations between PFAS and free 25(OH)D to the null. Additionally, the associations between PFAS and total 25(OH)D may be driven by vitamin D binding proteins (DBP) or the affinity of DBP for 25(OH)D, which, in turn, may largely impact the levels of total 25(OH)D but not free 25(OH)D since approximately 85% of total 25(OH)D is bound to DBP and <1% is in its free form. Previous studies have shown that DBP production increases with elevated estrogen, glucocorticoids, and certain cytokine such as IL-6, and the affinity of DBP for 25(OH)D was also affected by estrogen concentrations (Best et al., 2019; Bikle & Schwartz, 2019; Pop et al., 2015). Since these physiological factors were also associated with PFAS exposure (Benninghoff et al., 2011; Li et al., 2020; Liu et al., 2020; Pereiro, 2014; Son et al., 2009), it is possible that PFAS only indirectly influence DBP and total 25(OH)D through affecting endocrine systems or immune responses.
Some evidence of effect modification by fetal sex was observed in this study. Generally, we found larger effects on both total and free 25(OH)D among the women with male fetuses in the Visit 2 models. The heterogeneous effect by fetal sex may be due to the differences in vitamin D systems in the placenta. Previous studies have shown the levels of vitamin D receptors and CYP24A1 gene expression were higher in the placentas of women with male than female fetuses (Liu et al., 2018). Moreover, testosterone, which is higher on average in male fetuses, stimulates CYP24A1 and inhibits CYP27B1 gene expressions in the placenta (Olmos-Ortiz et al., 2016). It is thus likely that the clearance of 25(OH)D through CYP24A1 may be higher in the women with male fetuses than female fetuses, rendering it more sensitive to perturbations by PFAS as discussed above. Additionally, we observed larger effects of PFAS on both total and free 25(OH)D concentrations in the Visit 2 than the Visit 1 models. These findings could be explained by the higher means of total and free 25(OH)D concentrations measured at Visit 2 than at Visit 1. Although a higher mean of total 25(OH)D at Visit 2 is expected due to the increase in DBP during pregnancy, it is unclear why free 25(OH)D is also higher since its concentrations often remain the same or decrease during gestation (Bikle & Schwartz, 2019; Tsuprykov et al., 2019).
In addition to the single-PFAS models, we also investigated the associations between the PFAS mixtures and 25(OH)D concentrations. We identified that PFHxS has the strongest positive association with total 25(OH)D in the single-PFAS models but found PFHxS contributed little weight to the overall effects in the WQS regression models. Accordingly, PFPeA was inversely associated with total 25(OH)D in the single-chemical models, but no overall negative association between the PFAS mixture and total 25(OH)D was found. Although free 25(OH)D was significantly associated with some individual PFAS in women with male fetuses in the Visit 2 models, no significant effects nor significant effect modifications were observed in the mixture models. The inconsistent results between the mixture and single chemical models indicate the possibility of confounding effects among PFAS in the single-chemical models and also highlight the importance of incorporating mixture analysis when there are high correlations and similar biological functions among the exposures of interest (Carrico et al., 2015).
Although we found that serum PFAS were associated with decreased odds of vitamin D deficiency, it is unlikely that PFAS would be “protective” to the vitamin D system. Because of its longer half-life, 25(OH)D is considered the best indicator to monitor vitamin D status compared with the other metabolites in the vitamin D system such as 1,25(OH)2D. Accordingly, vitamin D deficiency, which is associated with many adverse health outcomes, was often diagnosed by low serum total 25(OH)D concentrations (e.g., ≤ 20 ng/mL) (Holick et al., 2011). However, the reference level of vitamin D deficiency remains controversial, especially among African Americans due to genetic polymorphisms (Powe et al., 2018). Thus, the clinical implication of this finding remains unknown and needs further investigation. However, our findings in the models with the continuous 25(OH)D indicate that elevated PFAS concentrations were associated with changes in 25(OH)D concentrations and may cause perturbation on the vitamin D system.
Although the use of serum biomarkers to assess PFAS exposure is advantageous because of their ability to provide an integrated internal dose, many physiological conditions that influence serum biomarker concentrations may also affect or be affected by the health outcomes of interest, suggesting a possibility of introducing confounding effects. For example, it is possible that the observed associations were partly confounded by a third unknown factor which transports, metabolizes, or excretes both serum PFAS and 25(OH)D in the same fashion. This confounding issue is especially concerning in a cross-sectional study design (Fitz-Simon et al., 2013; Savitz & Wellenius, 2018; Steenland et al., 2009). A strength of our study was the repeated 25(OH)D measurements, which provide an opportunity to examine the associations in not only a cross-sectional (the Visit 1 models) but a prospective cohort (the Visit 2 models) study design. Confounding is less problematic in a cohort study design because the third unknown confounding factor may not simultaneously affect the exposure and outcome measured at two different time points. Additionally, the mixture models, which mutually adjusted for the other PFAS, can remove the confounding effects if the physiological parameters (e.g., transportation, metabolism, and excretion) regulating the eight PFAS are correlated (Fletcher & Webster, 2020).
Our study was limited in several ways. First, several potential confounders that were either not measured or only measured in a subset of the participants, such as vitamin D supplement intake, fish intake, and albumin concentrations, were not included in the main analyses. However, we performed sensitivity analyses on either a subset of our cohort or different sub-populations in the NHANES to evaluate the impact of these covariates. The results show little impact of these variables on the associations between PFAS and 25(OH)D. Second, the low detection frequencies of PFPeA, PFDA, PFUnDA, and NMeFOSAA could bias the results. We found single imputation with biased the effect estimates away from the null hypothesis in this study; thus, we presented the results using multiple instead of single imputation to mitigate the impact of the measurements below their respective LODs. Third, the possible compensatory mechanism due to vitamin D receptor competition might be unmasked by evaluating parathyroid hormone. Parathyroid hormone as well as the other vitamin D related metabolites and proteins such as calcium, phosphorous, and DBP, which we did not measure, may improve our understanding of how PFAS may disturb vitamin D metabolism. Finally, our results from pregnant African American women limit the generalizability to other populations.
Conclusions
Our study provides suggestive evidence that exposure to PFAS might disturb vitamin D metabolism among pregnant African American women and that some of these effects might be modified by fetal sex. These results show potential explanations of the relationships between PFAS exposure and some adverse health effects reported by the previous studies, such as adverse skeletal health, and pregnancy and birth outcomes. Future experimental and observational studies are warranted to understand the underlying biological mechanisms, to confirm the findings in different populations, and to determine the implications of these findings to clinical practice and public health.
Supplementary Material
Acknowledgements
This study was funded by the National Institute of Health (NIH) research grants [R01NR014800, R01MD009064, R24ES029490, R01MD009746], NIH Center Grants [P50ES02607, P30ES019776, UH3OD023318, U2CES026560, U2CES026542], and Environmental Protection Agency (USEPA) center grant [83615301]. Furthermore, we thank our colleagues -Nathan Mutic, Cierra Johnson, Erin Williams, Priya D’Souza, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, Cassandra Hall, and the clinical health care workers and staff at the recruiting sites for helping with data and sample collection and sample chemical analyses in the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbott AG, Jones KC, & Sweetman AJ (2009). A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environmental Science & Technology, 43(2), 386–392. 10.1021/es802216n [DOI] [PubMed] [Google Scholar]
- Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, & Lau C (2007). Perfluorooctanoic Acid–Induced Developmental Toxicity in the Mouse is Dependent on Expression of Peroxisome Proliferator–Activated Receptor-alpha. Toxicological Sciences, 98(2), 571–581. 10.1093/toxsci/kfm110 [DOI] [PubMed] [Google Scholar]
- Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, & Rabi DM (2013). Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ, 346(mar26 4), f1169–f1169. 10.1136/bmj.f1169 [DOI] [PubMed] [Google Scholar]
- Baer HJ, Blum RE, Rockett HR, Leppert J, Gardner JD, Suitor CW, & Colditz GA (2005). Use of a food frequency questionnaire in American Indian and Caucasian pregnant women: A validation study. BMC Public Health, 5(1), 135. 10.1186/1471-2458-5-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, & Lopez-Espinosa M-J (2017). Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environment International, 99, 15–28. 10.1016/j.envint.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Balshaw DM, Collman GW, Gray KA, & Thompson CL (2017). The Children’s Health Exposure Analysis Resource (CHEAR): Enabling Research into the Environmental Influences on Children’s Health Outcomes. Current Opinion in Pediatrics, 29(3), 385–389. 10.1097/MOP.0000000000000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera D, Avila E, Hernández G, Halhali A, Biruete B, Larrea F, & Díaz L (2007). Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. The Journal of Steroid Biochemistry and Molecular Biology, 103(3), 529–532. 10.1016/j.jsbmb.2006.12.097 [DOI] [PubMed] [Google Scholar]
- Barrera D, Avila E, Hernández G, Méndez I, González L, Halhali A, Larrea F, Morales A, & Díaz L (2008). Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reproductive Biology and Endocrinology, 6(1), 3. 10.1186/1477-7827-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, & Williams DE (2011). Estrogen-Like Activity of Perfluoroalkyl Acids In Vivo and Interaction with Human and Rainbow Trout Estrogen Receptors In Vitro. Toxicological Sciences, 120(1), 42–58. 10.1093/toxsci/kfq379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best CM, Pressman EK, Queenan RA, Cooper E, & O’Brien KO (2019). Longitudinal changes in serum vitamin D binding protein and free 25-hydroxyvitamin D in a multiracial cohort of pregnant adolescents. The Journal of Steroid Biochemistry and Molecular Biology, 186, 79–88. 10.1016/j.jsbmb.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD (2014). Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chemistry & Biology, 21(3), 319–329. 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, & Schwartz J (2019). Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Frontiers in Endocrinology, 10. 10.3389/fendo.2019.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, Wright RO, Baccarelli A, & Wright RJ (2017). Maternal Lifetime Stress and Prenatal Psychological Functioning and Decreased Placental Mitochondrial DNA Copy Number in the PRISM Study. American Journal of Epidemiology, 186(11), 1227–1236. 10.1093/aje/kwx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, & Needham LL (2007). Serum Concentrations of 11 Polyfluoroalkyl Compounds in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Science & Technology, 41(7), 2237–2242. 10.1021/es062686m [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, & Needham LL (2007). Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environmental Health Perspectives, 115(11), 1596–1602. 10.1289/ehp.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, & Factor-Litvak P (2015). Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological, and Environmental Statistics, 20(1), 100–120. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-J, Ryan PB, Smarr M, Kannan K, Panuwet P, Dunlop AL, Corwin EJ, & Barr DB (2020). Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations and Predictors of Exposure among Pregnant African American Women in the Atlanta Area, Georgia. Environmental Research, 110445. 10.1016/j.envres.2020.110445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, Ajibade DV, Dhawan P, Fechner AJ, & Mady LJ (2010). Vitamin D: Metabolism. Endocrinology and Metabolism Clinics of North America, 39(2), 243–253. 10.1016/j.ecl.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, Gold DR, Coull B, Gordon CM, Rosen CJ, Oken E, Sagiv SK, & Fleisch AF (2019). Per- and Polyfluoroalkyl Substance Plasma Concentrations and Bone Mineral Density in Midchildhood: A Cross-Sectional Study (Project Viva, United States). Environmental Health Perspectives, 127(8), 087006. 10.1289/EHP4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, & Dunlop AL (2017). Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC Pregnancy and Childbirth, 17(1), 161. 10.1186/s12884-017-1357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, De Rocco Ponce M, Giadone A, Rocca MS, Guidolin D, & Foresta C (2020a). Perfluoroalkyl substances and bone health in young men: A pilot study. Endocrine, 67(3), 678–684. 10.1007/s12020-019-02096-4 [DOI] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, De Toni L, Sabovic I, Guidolin D, Dall’Acqua S, Acquasaliente L, De Filippis V, Plebani M, & Foresta C (2020b). Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Scientific Reports, 10(1), 16789. 10.1038/s41598-020-74026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erden ES, Genc S, Motor S, Ustun I, Ulutas KT, Bilgic HK, Oktar S, Sungur S, Erem C, & Gokce C (2014). Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine, 45(2), 311–318. 10.1007/s12020-013-0022-z [DOI] [PubMed] [Google Scholar]
- Etzel TM, Braun JM, & Buckley JP (2019). Associations of serum perfluoroalkyl substance and vitamin D biomarker concentrations in NHANES, 2003–2010. International Journal of Hygiene and Environmental Health, 222(2), 262–269. 10.1016/j.ijheh.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, & Hewison M (2017). Vitamin D: Volume 1: Biochemistry, Physiology and Diagnostics (4th ed.). Academic Press. https://books.google.com/books?hl=en&lr=&id=CutGDgAAQBAJ&oi=fnd&pg=PP1&dq=Vitamin+D:+Volume+1:+Biochemistry,+Physiology+and+Diagnostics&ots=QJXjAcu1Bp&sig=VPlG6ibVCZCVh2F3ZPBBK7-KNXk#v=onepage&q=Vitamin%20D%3A%20Volume%201%3A%20Biochemistry%2C%20Physiology%20and%20Diagnostics&f=false [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, & Armstrong B (2013). Reductions in Serum Lipids with a 4-year Decline in Serum Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid. Epidemiology (Cambridge, Mass.), 24(4), 569–576. 10.1097/EDE.0b013e31829443ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, & Webster TF (2020). PFAS and cholesterol in epidemiological studies: causality, biomarkers, mixtures and relative potency. 32nd Annual Conference of the International Society for Environmental Epidemiology (virtual meeting). [Google Scholar]
- Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H, & Gundacker C (2020). Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environment International, 137, 105324. 10.1016/j.envint.2019.105324 [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, & Ducatman AM (2010). Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Archives of Pediatrics & Adolescent Medicine, 164(9), 860–869. 10.1001/archpediatrics.2010.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, & Trasande L (2018). Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Frontiers in Endocrinology, 9. 10.3389/fendo.2018.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RO (1987). Statistical methods for environmental pollution monitoring. John Wiley & Sons. [Google Scholar]
- Ginde AA, Sullivan AF, Mansbach JM, & Camargo CA Jr (2010). Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. American journal of obstetrics and gynecology, 202(5), 436–e1. 10.1016/j.ajog.2009.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, & Heilmann C (2012). Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA, 307(4), 391–397. 10.1001/jama.2011.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, Loveren H van, Løvik M, & Nygaard UC (2013). Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. Journal of Immunotoxicology, 10(4), 373–379. 10.3109/1547691X.2012.755580 [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, & Robins JM (1999). Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass.), 10(1), 37–48 [PubMed] [Google Scholar]
- Hargarten PM, & Wheeler DC (2020). Accounting for the uncertainty due to chemicals below the detection limit in mixture analysis. Environmental Research, 186, 109466. 10.1016/j.envres.2020.109466 [DOI] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, Oken E, & Sagiv SK (2017). Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environmental Science & Technology, 51(9), 5193–5204. 10.1021/acs.est.6b05811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzke D, Olsson E, & Posner S (2012). Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway – A pilot study. Chemosphere, 88(8), 980–987. 10.1016/j.chemosphere.2012.03.035 [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, & Weaver CM (2011). Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 96(7), 1911–1930. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- Honda M, Robinson M, & Kannan K (2018). A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environmental Chemistry, 15(2), 92–99. 10.1071/EN17192 [DOI] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, Coull B, Furtado JD, Qi L, Grandjean P, & Sun Q (2019). Perfluoroalkyl substances and changes in bone mineral density: A prospective analysis in the POUNDS-LOST study. Environmental Research, 179, 108775. 10.1016/j.envres.2019.108775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB (2014). Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. International Journal of Hygiene and Environmental Health, 217(1), 52–61. 10.1016/j.ijheh.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, & Meeker JD (2017). Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis. Environmental Health Perspectives, 125(8). 10.1289/EHP1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, & Meeker JD (2016). Relationships Between Urinary Phthalate Metabolite and Bisphenol A Concentrations and Vitamin D Levels in U.S. Adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. The Journal of Clinical Endocrinology and Metabolism, 101(11), 4062–4069. 10.1210/jc.2016-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, & Woodruff TJ (2014). The Navigation Guide—Evidence-Based Medicine Meets Environmental Health: Systematic Review of Human Evidence for PFOA Effects on Fetal Growth. Environmental Health Perspectives, 122(10), 1028–1039. 10.1289/ehp.1307893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AMZ, Hoofnagle AN, & Lutsey PL (2018). Measurement of Vitamin D for Epidemiologic and Clinical Research: Shining Light on a Complex Decision. American Journal of Epidemiology, 187(4), 879–890. 10.1093/aje/kwx297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, & Kannan K (2016). Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environmental Health Perspectives, 124(1), 81–87. 10.1289/ehp.1307909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, Hangartner T, & Kannan K (2018). Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environmental Research, 160, 314–321. 10.1016/j.envres.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Kim K, Bennett DH, Calafat AM, Hertz-Picciotto I, & Shin H-M (2020). Temporal trends and determinants of serum concentrations of per- and polyfluoroalkyl substances among Northern California mothers with a young child, 2009–2016. Environmental Research, 186, 109491. 10.1016/j.envres.2020.109491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Moon S, Oh B-C, Jung D, Ji K, Choi K, & Park YJ (2018). Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS ONE, 13(5). 10.1371/journal.pone.0197244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela A, Finnilä MA, Korkalainen M, Spulber S, Koponen J, Håkansson H, Tuukkanen J, & Viluksela M (2016). Effects of developmental exposure to perfluorooctanoic acid (PFOA) on long bone morphology and bone cell differentiation. Toxicology and Applied Pharmacology, 301, 14–21. 10.1016/j.taap.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, & Tuukkanen J (2017). Perfluoroalkyl substances in human bone: Concentrations in bones and effects on bone cell differentiation. Scientific Reports, 7(1), 6841. 10.1038/s41598-017-07359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, & Woodruff TJ (2014). The Navigation Guide—Evidence-based medicine meets environmental health: Integration of animal and human evidence for PFOA effects on fetal growth. Environmental Health Perspectives, 122(10), 1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, & Seed J (2007). Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicological Sciences, 99(2), 366–394. 10.1093/toxsci/kfm128 [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, & Strynar MJ (2006). Effects of Perfluorooctanoic Acid Exposure during Pregnancy in the Mouse. Toxicological Sciences, 90(2), 510–518. 10.1093/toxsci/kfj105 [DOI] [PubMed] [Google Scholar]
- Li L, Andersen ME, Heber S, & Zhang Q (2007). Non-monotonic dose–response relationship in steroid hormone receptor-mediated gene expression. Journal of Molecular Endocrinology, 38(5), 569–585. 10.1677/JME-07-0003 [DOI] [PubMed] [Google Scholar]
- Li J, Cao H, Feng H, Xue Q, Zhang A, & Fu J (2020). Evaluation of the Estrogenic/Antiestrogenic Activities of Perfluoroalkyl Substances and Their Interactions with the Human Estrogen Receptor by Combining In Vitro Assays and In Silico Modeling. Environmental Science & Technology, 54(22), 14514–14524. 10.1021/acs.est.0c03468 [DOI] [PubMed] [Google Scholar]
- Lippa KA, Bedner M, Tai S, & Burdette CQ (2020). NIST/NIH Vitamin D Metabolites Quality Assurance Program (VitDQAP): Final Report. https://www.nist.gov/publications/nistnih-vitamin-d-metabolites-quality-assurance-program-vitdqap-final-report
- Liu H, Pan Y, Jin S, Li Y, Zhao L, Sun X, Cui Q, Zhang B, Zheng T, Xia W, Zhou A, Campana AM, Dai J, & Xu S (2020). Associations of per-/polyfluoroalkyl substances with glucocorticoids and progestogens in newborns. Environment International, 140, 105636. 10.1016/j.envint.2020.105636 [DOI] [PubMed] [Google Scholar]
- Liu NQ, Larner DP, Yao Q, Chun RF, Ouyang Y, Zhou R, Tamblyn JA, Wagner CL, & Hewison M (2018). Vitamin D-deficiency and sex-specific dysregulation of placental inflammation. The Journal of Steroid Biochemistry and Molecular Biology, 177, 223–230. 10.1016/j.jsbmb.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, & Hartge P (2004). Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives, 112(17), 1691–1696. 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk J, Torrealday S, Neal Perry G, & Pal L (2012). Relevance of vitamin D in reproduction. Human Reproduction (Oxford, England), 27(10), 3015–3027. 10.1093/humrep/des248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales E, Gascon M, Martinez D, Casas M, Ballester F, Rodríguez-Bernal CL, Ibarluzea J, Marina LS, Espada M, Goñi F, Vizcaino E, Grimalt JO, & Sunyer J (2013). Associations between blood persistent organic pollutants and 25-hydroxyvitamin D3 in pregnancy. Environment International, 57–58, 34–41. 10.1016/j.envint.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Mousavi SE, Amini H, Heydarpour P, Amini Chermahini F, & Godderis L (2019). Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environment International, 122, 67–90. 10.1016/j.envint.2018.11.052 [DOI] [PubMed] [Google Scholar]
- Nelson JW, Scammell MK, Hatch EE, & Webster TF (2012). Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environmental Health, 11(1), 10. 10.1186/1476-069X-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW (2008). From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. The American Journal of Clinical Nutrition, 88(2), 491S–499S. 10.1093/ajcn/88.2.491S [DOI] [PubMed] [Google Scholar]
- Norman PE, & Powell JT (2014). Vitamin D and cardiovascular disease. Circulation Research, 114(2), 379–393. 10.1161/CIRCRESAHA.113.301241 [DOI] [PubMed] [Google Scholar]
- Ohyama Y, Noshiro M, Eggertsen G, Gotoh O, Kato Y, Björkhem I, & Okuda K (1993). Structural characterization of the gene encoding rat 25-hydroxyvitamin D3 24-hydroxylase. Biochemistry, 32(1), 76–82. 10.1021/bi00052a011 [DOI] [PubMed] [Google Scholar]
- Olmos-Ortiz A, García-Quiroz J, López-Marure R, González-Curiel I, Rivas-Santiago B, Olivares A, Avila E, Barrera D, Halhali A, Caldiño F, Larrea F, & Díaz L (2016). Evidence of sexual dimorphism in placental vitamin D metabolism: Testosterone inhibits calcitriol-dependent cathelicidin expression. The Journal of Steroid Biochemistry and Molecular Biology, 163, 173–182. 10.1016/j.jsbmb.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B, & Harlow SD (2019). Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environmental Research, 175, 186–199. 10.1016/j.envres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereiro N (2014). Regulation of corticosterone secretion is modified by PFOS exposure at different levels of the hypothalamic–pituitary–adrenal axis in adult male rats. Toxicology Letters, 11. [DOI] [PubMed] [Google Scholar]
- Pike JW, & Meyer MB (2010). The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-Dihydroxyvitamin D3. Endocrinology and Metabolism Clinics of North America, 39(2), 255–269. 10.1016/j.ecl.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsonby A-L, Lucas RM, Lewis S, & Halliday J (2010). Vitamin D status during Pregnancy and Aspects of Offspring Health. Nutrients, 2(3), 389–407. 10.3390/nu2030389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop LC, Shapses SA, Chang B, Sun W, & Wang X (2015). VITAMIN D–BINDING PROTEIN IN HEALTHY PRE- AND POSTMENOPAUSAL WOMEN: RELATIONSHIP WITH ESTRADIOL CONCENTRATIONS. Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 21(8), 936–942. 10.4158/EP15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OR Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, & Thadhani R (2013). Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. New England Journal of Medicine, 369(21), 1991–2000. 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, Rifas-Shiman SL, Pearce EN, Calafat AM, Fleisch AF, & Sagiv SK (2020). Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: A mixtures approach. Environment International, 139, 105728. 10.1016/j.envint.2020.105728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, & Hines EP (2017). Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. International Journal of Environmental Research and Public Health, 14(7), 691. 10.3390/ijerph14070691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT (2006). The Steroid Hormone Biosynthesis Pathway as a Target for Endocrine-Disrupting Chemicals. Toxicological Sciences, 94(1), 3–21. 10.1093/toxsci/kfl051 [DOI] [PubMed] [Google Scholar]
- Savitz DA, & Wellenius GA (2018). Invited Commentary: Exposure Biomarkers Indicate More Than Just Exposure. American Journal of Epidemiology, 187(4), 803–805. 10.1093/aje/kwx333 [DOI] [PubMed] [Google Scholar]
- Schober P, Boer C, & Schwarte LA (2018). Correlation coefficients: appropriate use and interpretation. Anesthesia & Analgesia, 126(5), 1763–1768. [DOI] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, & Heindel JJ (2011). Endocrine disrupting chemicals and disease susceptibility. The Journal of Steroid Biochemistry and Molecular Biology, 127(3–5), 204–215. 10.1016/j.jsbmb.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Choi MY, Longtine MS, & Nelson DM (2010). Vitamin D Effects on Pregnancy and the Placenta. Placenta, 31(12), 1027–1034. 10.1016/j.placenta.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, & O’Malley BW (1997). Coactivator and Corepressor Regulation of the Agonist/Antagonist Activity of the Mixed Antiestrogen, 4-Hydroxytamoxifen. Molecular Endocrinology, 11(6), 657–666. 10.1210/mend.11.6.0009 [DOI] [PubMed] [Google Scholar]
- Smith CL, & O’Malley BW (2004). Coregulator Function: A Key to Understanding Tissue Specificity of Selective Receptor Modulators. Endocrine Reviews, 25(1), 45–71. 10.1210/er.2003-0023 [DOI] [PubMed] [Google Scholar]
- Son H-Y, Lee S, Tak E-N, Cho H-S, Shin H-I, Kim S-H, & Yang J-H (2009). Perfluorooctanoic acid alters T lymphocyte phenotypes and cytokine expression in mice. Environmental Toxicology, 24(6), 580–588. 10.1002/tox.20459 [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, & Savitz DA (2010). Epidemiologic Evidence on the Health Effects of Perfluorooctanoic Acid (PFOA). Environmental Health Perspectives, 118(8), 1100–1108. 10.1289/ehp.0901827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, & Vaccarino V (2009). Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate With Serum Lipids Among Adults Living Near a Chemical Plant. American Journal of Epidemiology, 170(10), 1268–1278. 10.1093/aje/kwp279 [DOI] [PubMed] [Google Scholar]
- Stein CR, McGovern KJ, Pajak AM, Maglione PJ, & Wolff MS (2016). Perfluoroalkyl and Polyfluoroalkyl Substances and Indicators of Immune Function in Children Aged 12 – 19 years: NHANES. Pediatric Research, 79(2), 348–357. 10.1038/pr.2015.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, & Allen JG (2019). A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of exposure science & environmental epidemiology, 29(2), 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuprykov O, Buse C, Skoblo R, & Hocher B (2019). Comparison of free and total 25-hydroxyvitamin D in normal human pregnancy. The Journal of Steroid Biochemistry and Molecular Biology, 190, 29–36. 10.1016/j.jsbmb.2019.03.008 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2019). National Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019508.pdf
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, & Myers JP (2012). Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocrine Reviews, 33(3), 378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, & Hollis BW (2018). The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Frontiers in Endocrinology, 9. 10.3389/fendo.2018.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-Q, Qi H-P, Luo Z-C, & Fraser WD (2013). Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine, 26(9), 889–899. 10.3109/14767058.2013.765849 [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PEG, van Leeuwen SPJ, & Hamers T (2009). Competitive Binding of Poly- and Perfluorinated Compounds to the Thyroid Hormone Transport Protein Transthyretin. Toxicological Sciences, 109(2), 206–216. 10.1093/toxsci/kfp055 [DOI] [PubMed] [Google Scholar]
- White SS, Fenton SE, & Hines EP (2011). Endocrine disrupting properties of perfluorooctanoic acid. The Journal of Steroid Biochemistry and Molecular Biology, 127(1–2), 16–26. 10.1016/j.jsbmb.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-H, Lee Y-M, Bae S-G, Jacobs DR, & Lee D-H (2012). Associations between Organochlorine Pesticides and Vitamin D Deficiency in the U.S. Population. PLoS ONE, 7(1). 10.1371/journal.pone.0030093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang O, Kim HL, Weon J-I, & Seo YR (2015). Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. Journal of Cancer Prevention, 20(1), 12–24. 10.15430/JCP.2015.20.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, & Hewison M (2002). The Ontogeny of 25-Hydroxyvitamin D3 1α-Hydroxylase Expression in Human Placenta and Decidua. The American Journal of Pathology, 161(1), 105–114. 10.1016/S0002-9440(10)64162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


