Abstract
Background:
Social and financial hardships explain approximately 50% of the observed disparity in asthma-related readmissions between Black and White children.
Objectives:
To determine whether asthma-related readmissions differed by degree of African ancestry and the extent to which such an association would also be explained by socio-environmental risk factors.
Methods:
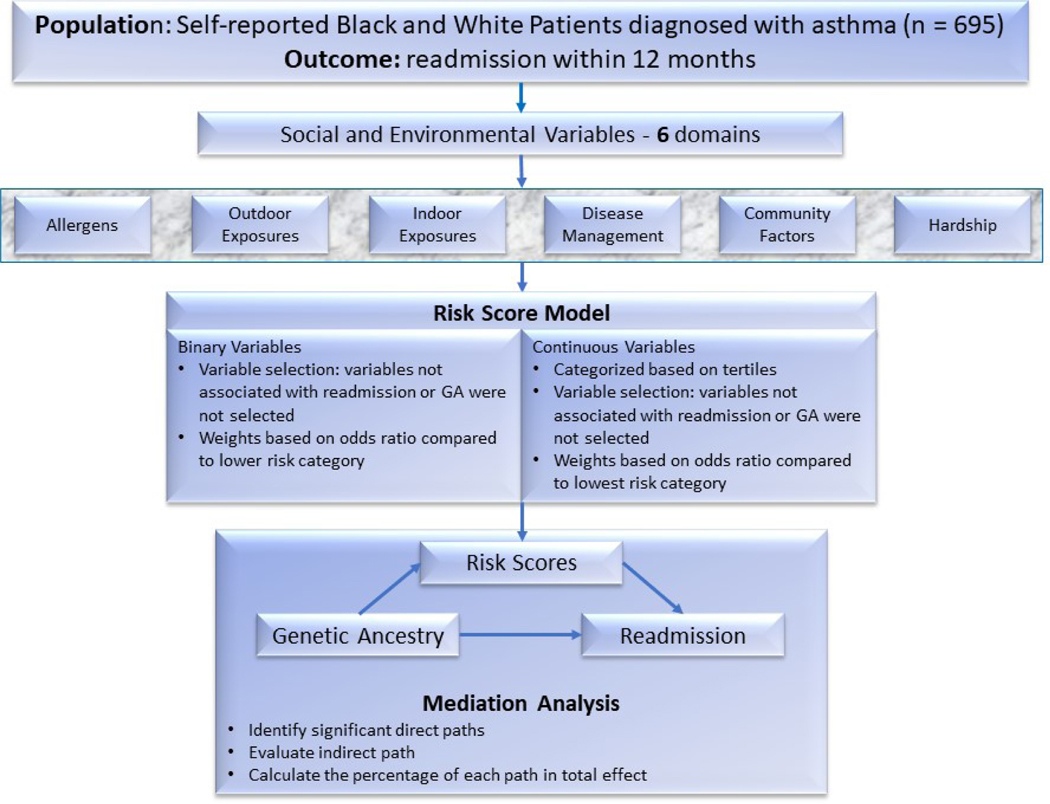
This study used data from a prospective cohort study of 695 Black and White children aged 1–16 years with an asthma-related admission. The primary outcome was a similar readmission within 12 months. Each subject’s African ancestry was determined by single nucleotide polymorphisms, on a continuous scale ranging from 0–1 (0=no African ancestry; 1=100% African ancestry). We also assessed 37 social, environmental, and clinical variables that we clustered into six domains (e.g., hardship, disease management). Survival and mediation analyses were conducted.
Results:
A total of 134 (19.3%) children were readmitted within 12 months. Higher African ancestry was associated with asthma readmission (OR 1.11, 95% CI: 1.05–1.18 for every 10% increase in African ancestry) with adjustment for age and sex. The association between African ancestry and readmission was mediated by hardship (sβ=3.42, p<0.001) and disease management (sβ=0.046, p=0.001), accounting for >50% of African ancestry’s effect on readmission. African ancestry was no longer significantly associated with readmission (sβ=0.035, p=0.388) after accounting for these mediators.
Conclusions:
African ancestry was strongly associated with readmission and the association was mediated by family hardships and disease management. These results are consistent with the notion that asthma-related racial disparities are driven by factors like structural racism and social adversity.
Keywords: Asthma, readmission, social factors, environmental exposure risk, genetic ancestry, mediation analysis
Capsule Summary
While prior work has predominately focused on self-reported race, genetic heterogeneity within racial groups is substantial. Incorporating genetic ancestry variation into an analysis could help dissect the relationship of ancestry, socio-environmental context, and asthma readmission.
Graphical Abstract
Introduction
Asthma is a common, complex, and costly chronic condition of childhood. In the U.S., asthma results in nearly $82 billion in overall costs each year [1]. In 2016, the prevalence of asthma was 15.7% in self-reported Black children, twice that of White population [2]. Asthma morbidity, hospitalizations, emergency visits, and deaths are also higher among self-reported Black children compared with White children [3, 4]. In addition, children of low-income families in urban communities disproportionately experience asthma morbidity and mortality [5].
Previous studies suggest that racial disparities in asthma outcomes may be due, at least in part, to differential exposures to social and environmental determinants [6, 7]. We recently found that 50% of the Black-White disparity in hospital readmissions due to asthma could be explained by financial and social hardships [8]. Adding environmental, disease management, and access variables to the model explained an additional 30% of the observed disparity [8].
While prior work has predominately focused on self-reported race, the degree of genetic heterogeneity within self-reported racial groups is substantial. Studies show that self-identified race misclassifies the genetics of many people who do not map exactly to ancestral patterns of their assigned origin [9]. Thus, grouping human populations by self-reported race oversimplifies human genetic diversity. Genetic ancestry describes the architecture between populations due to genetic heritage. The distribution of African and European ancestry within admixed African American populations can be described using high density DNA markers[10]. Genetic ancestry analyses have been successfully used to identify genomic regions that harbor susceptibility loci for cardiovascular disease, multiple sclerosis, prostate cancer, obesity, and asthma [11–15]. Such analyses have been used to influence implementation of so-called precision medicine. However, without looking at ancestry markers in concert with known influential socio-environmental factors, they could lead to incorrect inferences about genetic causes of health disparities.
In this context, it is important to recognize that ancestry is a statistical construct based on genetic polymorphisms whereas race is a social construct. Both genetically determined ancestry and self-reported race and ethnicity are known to be associated with socio-environmental exposures. Here, we sought to use patients’ genetic ancestry, as determined by genome wide single nucleotide polymorphism (SNP) markers, and socio-environmental exposures to understand the mediating pathways that might explain disparities in hospital readmissions. Hospital readmission is a relatively objective and measurable indicator of poorly controlled disease at the population level and has been used for prior research on disparities [16, 17]. Moreover, mediation analyses are widely used in molecular biology to identify underlying causal mechanisms [18]. With this analytic approach, we sought to specifically determine the relative contributions of genetic and non-genetic factors as they relate to child asthma readmission among a cohort with an index admission.
METHODS
The Methods section in this article’s Online Repository at www.jacionline. org provides additional details on the methods used in this study.
Study design and population
The Greater Cincinnati Asthma Risk Study (GCARS) was a population-based, prospective observational cohort that enrolled 774 children, aged 1 to 16 years, admitted between August 2010 and October 2011 to Cincinnati Children’s Hospital Medical Center (CCHMC), an urban tertiary care hospital. Details related to GCARS inclusion and exclusion criteria have been previously described [8, 19, 20]. Briefly, patients were identified by use of the evidence-based clinical pathway for acute asthma or bronchodilator-responsive wheezing. Children were excluded if they had significant respiratory or cardiovascular comorbidity, if they lived outside the CCHMC primary service area, or if they had a non–English speaking caregiver. The analyses pursued here include only those children identified by their caregivers as non-Hispanic and either Black/African American or White/Caucasian through a face-to-face survey at admission. The number of subjects eligible for inclusion was 695 after applying those exclusion criteria. A complete flow chart describing the population accrual for this study can be found in Figure 1.
Figure 1.
The flowchart of this study. The cohort has a population of 774. We then selected non-Hispanic White patients and Black patients which reduced the total sample size to 695. A total of 37 binary variables or continuous variables were grouped into 6 domains for score calculation. The scores were used as covariates to fit logistic regression models in mediation analysis.
Asthma readmission outcome
Readmission to the hospital within 12 months of enrollment (initial admission) was captured using hospital billing data. Accuracy was verified by review of the electronic medical record to ensure that each readmission event met the same inclusion and exclusion criteria as the index admission [21].
Variables and Domains
We selected for inclusion those variables known to be associated with asthma readmission and/or shown to affect the association between race and readmission based on previous studies (Table 1) [8, 22]. Since we had multiple correlated mediating variables, we built a standardized and scaled risk factor scoring scheme, similar to those developed in previous genetic risk score studies [23–26]. We grouped variables into six domains discussed below and used the scoring scheme to assign weights to the risk factors in each domain. The detailed description of variables selected in each domain can be found in the Methods section in this article’s Online Repository.
Table 1.
Description of variables in the study. The Domains used in the risk score model is marked in bold.
| Overall (n=695) | Readmitted within 12 months (n=134) | Not readmitted within 12 months (n=561) | p-value | |
|---|---|---|---|---|
| Self-reported race | ||||
| Black | 448 (64.5%) | 106 (79.1%) | 342 (61.0%) | <0.001 |
| White | 247 (35.5%) | 28 (20.9%) | 219 (39.0%) | |
| Age, Mean (SD) | 6.28 (4.03) | 6.11 (3.64) | 6.33 (4.12) | 0.561 |
| Gender, male | 456 (65.6%) | 89 (66.4%) | 367 (65.4%) | 0.906 |
| Global Ancestry, Mean (SD) | 0.523 (0.378) | 0.630 (0.326) | 0.497 (0.385) | < 0.001 |
| Allergens, IgE, above 0.35 kU/L | ||||
| Alternaria alternata/A tenuis | 262 (43.0%) | 57 (49.1%) | 205 (41.7%) | 0.175 |
| Aspergillus fumigatus | 246 (41.1%) | 55 (47.8%) | 191 (39.5%) | 0.129 |
| Dermatophagoides pteronyssinus | 256 (42.2%) | 52 (45.2%) | 204 (41.5%) | 0.529 |
| Dermatophagoides farina | 267 (43.9%) | 53 (45.3%) | 214 (43.6%) | 0.816 |
| Cat Dander | 256 (42.2%) | 49 (42.6%) | 207 (42.1%) | >0.999 |
| Dog Dander | 318 (52.1%) | 62 (53.0%) | 256 (51.9%) | 0.917 |
| Mouse epithelium | 86 (14.3%) | 17 (15.0%) | 69 (14.1%) | 0.909 |
| American cockroach | 125 (20.6%) | 24 (20.5%) | 101 (20.7%) | > 0.999 |
| Ragweed | 192 (32.4%) | 35 (31.0%) | 157 (32.7%) | 0.808 |
| White Oak | 208 (34.7%) | 43 (37.4%) | 165 (34.0%) | 0.566 |
| Outdoor exposures, mean (SD) | ||||
| Distance to nearest highway (km) | 2.90 (3.43) | 2.28 (2.33) | 3.05 (3.63) | 0.019 |
| PM 2.5 (μg/m3) | 13.6 (0.265) | 13.6 (0.244) | 13.6 (0.270) | 0.840 |
| Greenspace index (0–1) | 0.642 (0.096) | 0.634 (0.097) | 0.643 (0.096) | 0.338 |
| Elemental carbon (μg/m3) | 0.431 (0.135) | 0.448 (0.139) | 0.427 (0.133) | 0.105 |
| Indoor exposure | ||||
| Furry pets | 367 (53.1%) | 77 (57.9%) | 290 (52.0%) | 0.257 |
| Cockroaches | 83 (12.1%) | 16 (12.0%) | 67 (12.1%) | >0.999 |
| Rodents | 56 (8.15%) | 11 (8.27%) | 45 (8.12%) | >0.999 |
| Carpets | 192 (27.9%) | 36 (27.1%) | 156 (28.1%) | 0.895 |
| Mold/mildew | 108 (15.8%) | 21 (15.9%) | 87 (15.8%) | >0.999 |
| Water leaks | 167 (24.3%) | 35 (26.3%) | 132 (23.8%) | 0.625 |
| Cracks/holes in walls or ceilings | 160 (23.5%) | 41 (31.3%) | 119 (21.6%) | 0.026 |
| Cotinine in serum greater than 100 pg/mL | 356 (58.9%) | 73 (68.2%) | 283 (56.9%) | 0.041 |
| Disease management | ||||
| Patients sleep outside routinely | 235 (34.2%) | 62 (46.3%) | 173 (31.2%) | 0.001 |
| Patients regularly missed medicine | 165 (27.3%) | 38 (30.9%) | 127 (26.3%) | 0.370 |
| Patients run out of medicine | 201 (35.7%) | 45 (36.6%) | 156 (35.5%) | 0.901 |
| Community factors, mean (SD) | ||||
| Deprivation index (0–1) | 0.468 (0.162) | 0.497 (0.159) | 0.461 (0.162) | 0.018 |
| Property crime rate (per 1000 residents) | 35.7 (15.6) | 37.0 (20.7) | 35.4 (19.3) | 0.371 |
| Violent crime rate (per 1000 residents) | 8.70 (11.2) | 9.43 (9.86) | 8.53 (11.5) | 0.403 |
| Family Hardships | ||||
| Annual income < $15,000 per household | 570 (83.7%) | 121 (92.4%) | 449 (81.6%) | 0.004 |
| Caregiver education high school or less | 296 (44.4%) | 66 (52.8%) | 230 (42.5%) | 0.047 |
| Caregiver single, never married | 452 (65.4%) | 102 (76.1%) | 350 (62.8%) | 0.005 |
| Family not owned home | 545 (78.9%) | 120 (89.6%) | 425 (76.0%) | 0.001 |
| Family had any other financial hardship | 497 (71.5%) | 105 (78.4%) | 392 (69.9%) | 0.064 |
| Family had any other social hardship | 500 (71.9%) | 106 (79.1%) | 394 (70.2%) | 0.051 |
| Family not owned vehicle | 200 (29.0%) | 53 (39.6%) | 147 (26.5%) | 0.004 |
| Insurance type public/self-pay | 549 (79.0%) | 125 (93.3%) | 424 (75.6%) | <0.001 |
| Primary access score | ||||
| <75 | 258 (37.1%) | 54 (40.3%) | 204 (36.4%) | 0.621 |
| 75–99 | 305 (43.9%) | 54 (40.3%) | 251 (44.7%) | |
| 100 | 132 (19.0%) | 26 (19.4%) | 106 (18.9%) |
Chi square for categorical variable and ANOVA for continuous variable
Outdoor environmental exposures
Outdoor environmental variables referred to exposures based on the geolocation of subjects. Variables include the distance from residential address to nearest major highway and exposures to greenspace, elemental carbon attributable to traffic (ECAT), and particulate matter 2.5 (PM 2.5). Distances to highway in kilometers were calculated using the “rgeos” package in R Software (version 3.6.2, R Core Team). Exposures to greenspace were estimated using satellite-derived normalized difference vegetation index (NDVI) images. The averages of NDVI within 400m of the residential addresses were calculated and normalized to a range of −1 to 1, with higher values representing more surrounding greenspace [27]. Exposures to ECAT and PM 2.5 were estimated using the R packages “ecat” and “AiR” by averaging the daily data from 2010 to 2011 at 1 × 1 km grid resolution [28, 29].
Indoor environmental exposures
Indoor exposure variables included caregiver report of the following: furry pets, cockroaches, rodents, carpets, mold/mildew, water leaks, and cracks/holes in the walls or ceilings. Exposure to tobacco smoke was measured using serum cotinine concentrations, defined as positive if the concentration was above the detection level of 100 pg/ml [20].
Disease management
Disease management variables were obtained from survey responses by caregivers. Survey questions included assessments of how many nights per week the patients slept away from home, whether the patients missed medicine doses, and whether the family ran out of medicines. Access to healthcare services was measured using the access subscale of Parent’s Perception of Primary Care (P3C) instrument. Those with subscale scores of less than 75, 75 to 99, and 100 were considered to have low access, medium access, and excellent access, respectively [30].
Community factors
Community factors included a socioeconomic deprivation index (DPVI) and a measure of violent/property crimes per 1,000 people based on the census tract of the patient’s residence. The DPVI was estimated using a principal components analysis of six different 2015 American Community Survey (ACS) variables measuring aspects of community socioeconomic deprivation within a given US census tract [31]. Crime data from 2010 were obtained from the Cincinnati Police Data Initiative (https://data.cincinnati-oh.gov/Safety/PDI-Police-Data-Initiative-Crime-Incidents/k59e-2pvf) and the US FBI Uniform Crime Reporting (UCR) Program (https://www.fbi.gov/services/cjis/ucr/). For rural places with no available crime data and places that overlapped two or more census tracts, data from the nearest neighboring tract or the one with the largest overlap were used.
Family hardships
Family-level measures of socioeconomic status and financial and social hardships by survey. Variables included income, caregiver education attainment, marital status, home and vehicle ownership, and several other hardships defined in our previous studies, [8, 22] such as recent history of borrowing money, inability to borrow money or receive help from family/friends, and inability to acquire a loan when needed [8]. Insurance status was dichotomized as either public/self-pay or private.
Allergens
Allergen-specific IgE testing assessed sensitization to the following allergens: outdoor allergens (rag-weed and white oak), animal dander (cat, dog, and mouse epithelium), American cockroach, Alternaria alternata/A tenuis, Aspergillus fumigatus, Dermatophagoides pteronyssinus, and Dermatophagoides farina using the Quantitative ImmunoCAP Fluorescent Enzyme Immunoassay (ARUP Laboratories, Salt Lake City, Utah). Test results were considered positive when IgE was greater than or equal to 0.35 kU/L per convention [20].
Self-reported race
Race was defined according to caregiver report. Respondents could choose ≥1 of the following US Census categories: White/Caucasian, Black/African American, Asian/Oriental or Pacific Islander, American Indian or Alaskan Native, or other. Those children that were identified by their caregiver as White/Caucasian and/or Black/African American were included in this study.
Genotyping and genetic ancestry
Genotyping was performed using Multi-Ethnic Global Array (MEGA) that contains SNP sets tailored toward diverse ancestry populations (Illumina) [32]. Genotypes were determined using the Genetrain2 algorithm in Illumina Genome Studio software. Genotype data were available over 1.43 million variants. We performed quality control on the genotyped data. Variants were filtered for a call rate <0.95. We filtered SNPs with significant deviation from HWE in control at p<1E-5. The quality control step retained all the self-identified 695 Black and White samples in the cohort and their 1.37 million autosomal variants.
Statistical analysis
Global ancestry estimates
African ancestry proportion was estimated along with the African and European (YRI and CEU) sample data from the 1000 Genomes Project as the reference population [33]. We estimated the global ancestry (GA) of each subject using the ADMIXTURE software program [34]. GA is the average proportion of each contributing population across the genome of an admixed population. GA was measured on a continuous scale, ranging from 0 to 1 where 0 indicated 0% and 1 indicated 100% African ancestry. Logistic regression models assessed the association between GA and readmission using this continuous scale. An additional survival analysis with time to asthma readmission using Cox proportional hazards model within one year was performed. In the survival analysis, GA was categorized into three ancestral groups using 0.2 and 0.8 as cutoffs (<20%, 20–80%, and >80% African ancestry).
Constructing risk score model for social and environmental variables
The risk score for each domain was calculated as the weighted sum of the variables within that domain where variables and weights in each domain were calculated through a risk score model constructed using the variables associated with readmission and/or with those that changed the association between GA and readmission. We performed a variable selection and weight assignment step which has been used in previous studies [23, 35], to remove bias generated by including unrelated variables before calculating weights. Instead of the step-wise backward variable selection method [26], which only includes the variables associated with the outcome, our selection method considered the variables that could affect the association between GA and readmission [23] (see in this article’s Online Repository at www.jacionline.org for details on risk scoring and survival analysis methods).
Mediation analysis
Mediation analyses add a putative mediator, M, to an established association model, X → Y. In this way, X is thought to cause M, and M causes Y (X → M → Y representing the indirect effect of X on Y). Such an approach is used to determine the pathway between an independent variable X and dependent variable Y [36]. Here, we were interested in the GA → readmission pathway.
We estimated direct effects of GA on readmission, adjusted for age and gender. Once the weights assignment and variable selection in the scoring scheme were done, a mediation analysis with structural equation modelling (SEM) was performed for each domain with calculated risk scores using the R Package “lavaan” [37]. We formulated the direct and indirect path based on previous reports [8], and calculated the p-value of each connection for the two domains along paths. Nonsignificant associations among domains (p>0.10) were not shown in SEM output (Figure 2), except for the effects directly connected with readmission.
Figure 2.
Final Structural Equation Model for all the domains selected. The values shown are standardized coefficients (sβ), with significant sβ (p<0.10) marked in bold.
In our analysis of total effects, each series of arrows connecting GA to readmission through included domains is considered one “path” to represent how GA affects readmission through those potentially mediating construct(s). The total effect (Ω) from GA to readmission, is the sum of direct effect (θ) and all indirect effects (δ).
The indirect effect for each path is calculated as the product of all the standardized coefficients (sβ) within this path [38].
The percentage of each indirect effect over total effect was assessed to show the relative contribution for each path. Analyses were performed using R software (version 3.6.2, R Core Team).
Results
Among the 695 included patients, 134 (19.3%) were readmitted within 12 months of their initial admission (Table 1). There were 456 male patients and 239 female patients; readmission was not associated with gender (p=0.91). The mean age was 6.3 years. Readmission was not associated with patient age. A total of 11.4% of patients (n=26) with GA less than 0.2 were readmitted within 12 months, while the percentages of readmission within 12 months for those with GA between 0.2 and 0.8, and GA greater than 0.8 were 22.4% (n=43) and 23.6% (n=65), respectively. GA was strongly correlated with self-reported race (Spearman’s correlation, r=0.93).
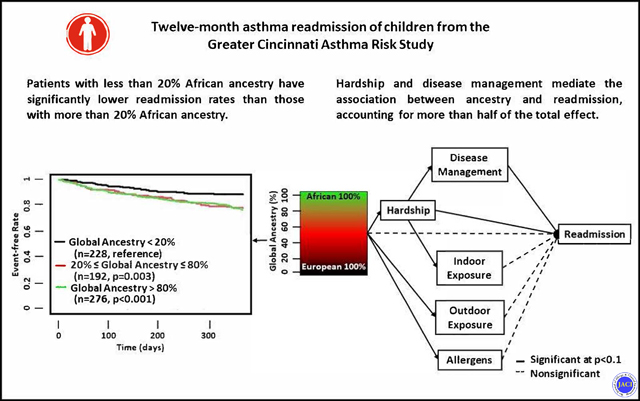
There was a significant association between increased African ancestry and increased readmission after adjustment for age and gender (OR 1.11 for every 10% increase in African ancestry, 95% CI: 1.05–1.18). Survival analysis revealed that those patients with GA less than 20% African ancestry had significantly lower readmission rates than those with higher than 20% African ancestry (Figure 3).
Figure 3.
Kaplan-Meier (K-M) plot showing the survival status within 365 days after first admission, illustrative of the time to readmission based on the proportion of African ancestry. Global ancestry (GA) of <0.2 indicates those with <20%, 0.2–0.8 (20%−80%) and >0.8 (>80%) of African ancestry. The K-M plot for each group was shown. The p-value shown was the coefficient compared to base group (GA<0.2).
We identified 13 variables from 5 of the 6 domains, as well as race that were associated with readmission and/or affected the associations between GA and readmission (Table 2 and Supplementary Table 1). No variables in the community domain, which included the deprivation index and crime rates, were associated with readmission or GA, so that domain was not retained. The AUC in the model with the 13 selected variables examining readmission was 0.67 (95% CI 0.61–0.74), while the AUC in the model with the 13 variables, but no weight assignment was 0.63 (95% CI 0.56–0.70). The AUC in the model including all the variables was 0.61 (95% CI 0.53–0.68).
Table 2.
Variables selected in the final risk score model. The weights were used in calculating the score of each assigned domain.
| Categories/Variables | Weights Assigned |
|---|---|
|
| |
| Allergens (IgE > 0.35 kU/L) | |
| Alternaria alternata/A tenuis | 1 |
| Aspergillus fumigatus | 1 |
| White oak | 1 |
|
| |
| Outdoor Exposures | |
| Distance to nearest highway (km) not in the highest tercile | 1 |
|
| |
| Indoor Exposure | |
| Cracks/holes in walls or ceilings | 2 |
| Cotinine in serum greater than 100 pg/mL | 2 |
|
| |
| Disease Management | |
| Patients sleep outside routinely | 2 |
|
| |
| Family Socioeconomic Hardship | |
| Annual income < $15000 per household | 2 |
| Caregiver single, never married | 1 |
| Caregiver education high school or less | 1 |
| Family does not own home | 2 |
| Family does not own vehicle | 1 |
| Insurance type: public/self-pay | 4 |
In mediation analyses, we investigated the associations among GA, socio-environmental domains, and readmission (Figure 2). Variables related to hardship (sβ=0.013, p=0.02) and disease management (sβ=0.035, p=0.042) were significantly associated with readmission. The association between GA and readmission was not significant (sβ=0.035, p=0.388) in the model adjusting for these domains. The association between GA and readmission was mainly mediated by hardship (sβ=3.42, p<0.001), and by disease management (sβ=0.046, p=0.001). Hardship accounted for 40% of the association between GA and readmission (Table 3). Hardship in turn was mediated by two additional variables (disease management and indoor exposures) which accounted for 4.8% and 9.0% of the total effect, respectively. In addition, GA had significant associations with allergens (sβ=0.59, p<0.001), and outdoor exposures (sβ=0.26, p<0.001) but not indoor exposures (sβ=−0.07, p=0.11) or disease management (sβ=0.13, p=0.20). These last two domains were, however, significantly associated with hardship. In a final sensitivity analysis that only included significant paths, we found no direct effect of GA on readmission.
Table 3.
Summary of direct and indirect effects on readmission. Each effect is the sum of standardized coefficients along each path. For each effect, the relative effect compared to the total effect is shown in percentage.
| Effects with 95% CI | Percentage | |
|---|---|---|
| Total Effects | 0.115 | |
| Direct Effects | ||
| GA – Readmission | 0.035 (−0.045 – 0.115) | 30.7 |
| Indirect effects*, mediated by | ||
| Disease Management | 0.004 | 3.81 |
| Hardship (GA – Hardship – Readmission) | 0.046 | 40.1 |
| Via Management | 0.006 | 4.84 |
| Via Indoor Exposure | 0.010 | 9.00 |
| Outdoor Exposure | 0.007 | 5.82 |
| Allergen | 0.009 | 7.86 |
| Indoor Exposure | −0.001 | −0.75 |
indirect effects are the product of several coefficients and was not possible to calculate the CI.
Discussion
The study is the first, to our knowledge, to demonstrate that increased African ancestry is positively associated with increased likelihood of pediatric asthma readmission. Yet, our findings suggest that this association is mediated by hardship and the association of hardship with other exposures. In other words, children with higher proportions of African ancestry experienced increased asthma readmission because of adverse social and environmental exposures and not direct biologic consequences of their genetics. Those with increased African ancestry were more likely to be poor, have a single parent, sleep away from home more frequently, be sensitized to common fungi, and be exposed to pollution. Ancestry is a genetic construct but here its health ramifications stem from the social consequences of racism. This study suggests future research examining a genetic basis for asthma disparities should include social and environmental variables and affirms the need for comprehensive social and environmental strategies to reduce asthma disparities.
This study is also novel in systematically investigating GA along with social, environmental, and clinical factors affecting asthma-related readmission using multivariable mediation analysis. While prior authors have examined the effects of individual variables or groups of variables that can affect asthma readmission [39–48], this research combines a range of variables to evaluate how GA is linked to child asthma readmission through non-genetic pathways.
Disease management had the highest direct effect on readmission. The only significant variable present in this domain was whether patients sleep away from home routinely, a marker for disrupted disease management routines. Children sleeping away from home can result from factors including caregiver marital status, income, and work shifts [49]. Having children sleep under the supervision of other caregivers may disrupt their routine care, the division of caregiver responsibility may be unclear, and there may be a lack of adequate adult supervision of child medication administration [49, 50]. However, from Figure 2, we see that GA is not directly related to children sleeping away from home. GA does, however, indirectly affect disease management through caregiver marital status which, in turn, significantly alters associations between readmission and GA (Table S1). Where patients sleep, the availability and use of their medicine there, and education for all the child’s main caregivers should be a high priority for clinicians seeking to reduce the likelihood their patients experience a readmission.
Measures of hardship also directly affected asthma readmission. Variables in this domain included income, home and car ownership, caregiver education, caregiver marital status, and insurance type (Table 2). Insurance type had the strongest association with readmission amongst all variables after adjustment by GA, age, and gender. It was previously documented that children with private insurance may have lower rates of asthma readmission due to better access to health care [30]. It is also possible that children with public insurance (Medicaid) are more likely to go to an emergency department (ED) during an exacerbation and children seen in the ED are more likely to be admitted than if they are seen in a primary care office for their exacerbation [30]. Medicaid is also likely a marker for other unmeasured social risk factors.
Caregiver marital status and family income in the hardship domain were significant covariates affecting the associations between sleeping away and readmission [49]. The results confirmed this relationship, and suggested disease management is a mediator for hardship. It was found that caregiver marital status and income had significant associations with children who routinely slept away from home [49]. We also found indoor exposures significantly mediate the effect of hardship on readmission. However indoor exposures themselves were not directly related to asthma readmission (Figure 2).
Outdoor exposures and allergic sensitization were both significantly related to GA but not with asthma readmission. Sensitization to Alternaria alternata/A tenuis, Aspergillus fumigatus, and white oak affected the associations between GA and readmission (Table S1, Table 2). This is in agreement with previous studies that showed African American patients have higher total serum IgE levels and more sensitization to fungus [40, 51]. Other allergen sensitizations that were shown previously to be associated with race did not significantly affect associations between readmission and GA (Table S1). Allergen sensitization affects asthma morbidity [52, 53]; however, the effects on asthma readmission were not significant. Instead, distance to highway was the only variable in the outdoor exposure domain that mediated the causal relationship between GA and readmission. Distance to highway determines patients’ exposures to traffic-related pollutants, such as PM 2.5, PM 10, and elemental carbon [54] which have been previously associated with altered pulmonary function in children [55].
Our results are subject to several limitations. First, this study was not designed to explore the frequency with which individual respiratory infections may have exacerbated a child’s asthma. That is, we did not have a domain to assess potential differences in viral triggers of wheeze across ancestry groups. We also adjusted our analyses for age which is likely linked to viral-induced wheeze in this population. Second, environmental exposure measurements are based on home address and medication use is based on self-report. Direct measurement of these variables would more accurately capture exposures but was not possible in the GCARS data. Third, while we comprehensively assessed social, environmental, and clinical variables, unmeasured variables, including the effects of racial discrimination in a child or family’s day-to-day experiences, likely affect the results of mediation analysis [56] and complicate our ability to fully illuminate the forces that perpetuates the existing disparity. Future work assessing how we might better identify, measure, and account for such additional variables, and prioritize relevant interventions, would be a major step forward. Fourth, our sample was children identified by their parent or caregiver as Black and/or White and who had already been hospitalized at CCHMC. Thus, our results do not reflect the contribution of genetic and non-genetic factors in other populations (e.g., Hispanic/Latinx, an admixed population with African, European, and Native American genetic ancestry contributions). Future work should test our model in other populations. Fifth, our scoring method to create domain was calculated based on our sample distribution. It may not represent interactions with other variables. In future research with larger study samples, other weights or methods could be considered and further examination of individual variables, instead of weighted domains, and may improve the model. Sixth, although children of different ancestries may have predispositions to asthma risk, differences in how genes turned on (or off), as a result of one’s environment or experience may play a critical part in perpetuation of disparities. Future work could consider epigenetic mechanisms in racial disparities. Despite these limitations, our study has key strengths that help unravel socio-environmental risk for asthma readmission.
In conclusion, increased African ancestry is associated with increased asthma readmission. The mediation analysis suggests family hardships and poor disease management resulting from hardships are the main reasons why children with high African ancestry experience higher rates of readmissions. Importantly, the absence of a direct GA effect on readmission after accounting for these mediating pathways is consistent with the notion that asthma-related racial disparities are likely driven by factors linked to structural racism and social adversities. These results suggest future studies of reported race, genetic ancestry, and health outcomes should include measurement of relevant social and environmental variables. Further, precision medicine studies based on genetic ancestry should strongly consider inclusion of social and environmental variables to assess genetic contributions most accurately. Moreover, precision medicine interventions may often need to target non-genetic social and environmental pathways.
Supplementary Material
Key Messages:
Survival analyses revealed that patients with higher African ancestry had a higher readmission rate following an asthma admission than patients with lower African ancestry.
Mediation analyses revealed that the association between African ancestry and readmission was no longer significant after adjusting for social, environmental, and clinical variables.
Family hardship and disease management mediated or accounted for over 50% of the association between African ancestry and asthma readmission.
Abbreviations:
- ACS
American Community Survey
- AUC
area under curve
- CCHMC
Cincinnati Children’s Hospital Medical Center
- DPVI
deprivation index
- ECAT
elemental carbon attributable to traffic
- ED
emergency department
- GA
Global ancestry
- GCARS
Greater Cincinnati Asthma Risk Study
- K-M
Kaplan-Meier
- MEGA
Multi-Ethnic Global Array
- NDVI
normalized difference vegetation index
- OR
odds ratio
- P3C
Parent’s Perception of Primary Care
- PM
particulate matter
- ROC
receiver operating characteristics
- Sβ
standardized coefficients
- SEM
structural equation modelling
- SNPS
single nucleotide polymorphisms
Footnotes
Declaration of Interests: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nurmagambetov T, Kuwahara R, and Garbe P, The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc, 2018. 15(3): p. 348–356. [DOI] [PubMed] [Google Scholar]
- 2.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs : Asthma in Children — United States, 2001–2016, in MMWR Morb Mortal Wkly Rep. 2018. p. 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Simon AE, and Rossen LM, Changing Trends in Asthma Prevalence Among Children. Pediatrics, 2016. 137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adejare AA, Gautam Y, Madzia J, Mersha TB Unraveling racial disparities in asthma emergency department visits using electronic healthcare records and machine learning. J Asthma, 2020: p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacheco CM, Ciaccio CE, Nazir N, Daley CM, DiDonna A, Choi WS, et al. , Homes of low-income minority families with asthmatic children have increased condition issues. Allergy and Asthma Proceedings, 2014. 35(6): p. 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsen S. and Nazroo JY, Relation between racial discrimination, social class, and health among ethnic minority groups. American journal of public health, 2002. 92(4): p. 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mersha TB and Abebe T, Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human genomics, 2015. 9(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AF, Huang B, Auger KA, Ryan PH, Chen C, Kahn RS Explaining racial disparities in child asthma readmission using a causal inference approach. JAMA pediatrics, 2016. 170(7): p. 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mersha TB, Mapping asthma-associated variants in admixed populations. Front Genet, 2015. 6: p. 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautam Y, Ghandikota S, Chen S, Mersha TB PAMAM: Power analysis in multiancestry admixture mapping. Genet Epidemiol, 2019. 43(7): p. 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. , Discovery and refinement of loci associated with lipid levels. Nature Genetics, 2013. 45(11): p. 1274-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. , Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nature Genetics, 2013. 45(11): p. 1353-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, et al. , Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nature Genetics, 2007. 39(5): p. 645–649. [DOI] [PubMed] [Google Scholar]
- 14.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. , Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nature Genetics, 2011. 43(9): p. 887–U103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Seibold MA, Aldrich MC, Williams LK, Reiner AP, Colangelo L, et al. , Genetic Ancestry in Lung-Function Predictions. New England Journal of Medicine, 2010. 363(4): p. 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auger KA, Teufel RJ, Harris JM, Gay JC, Del Beccaro MA, Neuman MI, et al. , Children’s Hospital Characteristics and Readmission Metrics. Pediatrics, 2017. 139(2). [DOI] [PubMed] [Google Scholar]
- 17.Sills MR, Hall M, Colvin JD, Macy ML, Cutler GJ, Bettenhausen JL, et al. , Association of Social Determinants With Children’s Hospitals’ Preventable Readmissions Performance. JAMA Pediatr, 2016. 170(4): p. 350–8. [DOI] [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health, 2016. 37: p. 17–32. [DOI] [PubMed] [Google Scholar]
- 19.Beck AF, Moncrief T, Huang B, Simmons JM, Sauers H, Chen C. et al. , Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. The Journal of pediatrics, 2013. 163(2): p. 574–580. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howrylak JA, Spanier AJ, Huang B, Peake RW, Kellogg MD, Sauers H, et al. , Cotinine in children admitted for asthma and readmission. Pediatrics, 2014: p. peds. 2013–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanier AJ, Beck AF, Huang B, McGrady ME, Drotar DD, Peake RW, et al. , Family hardships and serum cotinine in children with asthma. Pediatrics, 2015. 135(2): p. e416–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. , Role of financial and social hardships in asthma racial disparities. Pediatrics, 2014. 133(3): p. 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo RS, Subramanian SV, Pearce N, Florez JC and McKinlay JB Relative Contributions of Socioeconomic, Local Environmental, Psychosocial, Lifestyle/Behavioral, Biophysiological, and Ancestral Factors to Racial/Ethnic Disparities in Type 2 Diabetes. Diabetes Care, 2016. 39(7): p. 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balavarca Y, Weigl K, Thomsen H. and Brenner H. Performance of individual and joint risk stratification by an environmental risk score and a genetic risk score in a colorectal cancer screening setting. International Journal of Cancer, 2020. 146(3): p. 627–634. [DOI] [PubMed] [Google Scholar]
- 25.Park SK, Tao Y, Meeker JD, Harlow SD and Mukherjee B. Environmental Risk Score as a New Tool to Examine Multi-Pollutants in Epidemiologic Research: An Example from the NHANES Study Using Serum Lipid Levels. Plos One, 2014. 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biagini Myers JM, et al. , A Pediatric Asthma Risk Score to better predict asthma development in young children. Journal of Allergy and Clinical Immunology, 2019. 143(5): p. 1803-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brokamp C, LeMasters GK, and Ryan PH, Residential mobility impacts exposure assessment and community socioeconomic characteristics in longitudinal epidemiology studies. Journal of Exposure Science and Environmental Epidemiology, 2016. 26(4): p. 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brokamp C, Jandarov R, Hossain M. and Ryan P. Predicting Daily Urban Fine Particulate Matter Concentrations Using a Random Forest Model. Environmental Science & Technology, 2018. 52(7): p. 4173–4179. [DOI] [PubMed] [Google Scholar]
- 29.Ryan PH, LeMasters GK, Biswas P, Levin L, Hu S, Lindsey M, Bernstein DI, et al. , A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environmental Health Perspectives, 2007. 115(2): p. 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auger KA, Kahn RS, Davis MM, Beck AF and Simmons JM Medical Home Quality and Readmission Risk for Children Hospitalized With Asthma Exacerbations. Pediatrics, 2013. 131(1): p. 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM and Hall ES Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Annals of Epidemiology, 2019. 30: p. 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeWan AT, Egan KB, Hellenbrand K, Sorrentino K, Pizzoferrato N, Walsh KM, et al. , Whole-exome sequencing of a pedigree segregating asthma. BMC Med Genet, 2012. 13: p. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.1000 Genomes Project Consortium. A global reference for human genetic variation. Nature, 2015. 526(7571): p. 68-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander DH, Novembre J, and Lange K, Fast model-based estimation of ancestry in unrelated individuals. Genome Res, 2009. 19(9): p. 1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang H, Edwards AM, Bomback AS, Ballantyne CM, Brillon D, Callahan MA, et al. , Development and Validation of a Patient Self-assessment Score for Diabetes Risk. Annals of Internal Medicine, 2009. 151(11): p. 775–W255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Introduction to statistical mediation analysis. Introduction to statistical mediation analysis. 2008, New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates. x, 477-x, 477. [Google Scholar]
- 37.Rosseel Y, lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software, 2012. 48(2): p. 1–36. [Google Scholar]
- 38.Kline RB, Principles and practice of structural equation modeling. 2011: Third edition. New York: : Guilford Press, [2011] ©2011. [Google Scholar]
- 39.Weiss S, Environmental risk factors in childhood asthma. Clinical and Experimental Allergy, 1998. 28: p. 29–34. [DOI] [PubMed] [Google Scholar]
- 40.Beck AF, Huang B, Kercsmar CM, Guilbert TW, McLinden DJ, Lierl MB, et al. , Allergen sensitization profiles in a population-based cohort of children hospitalized for asthma. Annals of the American Thoracic Society, 2015. 12(3): p. 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS and Kahn RS Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. The Journal of pediatrics, 2014. 164(6): p. 1396–1402. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neophytou AM, White MJ, Oh SS, Thakur N, Galanter JM, Nishimura KK, et al. , Air pollution and lung function in minority youth with asthma in the GALA II (Genes–Environments and Admixture in Latino Americans) and SAGE II (Study of African Americans, Asthma, Genes, and Environments) studies. American journal of respiratory and critical care medicine, 2016. 193(11): p. 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhry S, Burchard EG, Borrell LN, Tang H, Gomez I, Naqvi M, et al. , Ancestry–environment interactions and asthma risk among Puerto Ricans. American journal of respiratory and critical care medicine, 2006. 174(10): p. 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baye TM, Kovacic MB, Myers JMB, Martin LJ, Lindsey M, Patterson TL, et al. , Differences in candidate gene association between European ancestry and African American asthmatic children. PloS one, 2011. 6(2): p. e16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forno E. and Celedón JC, Asthma and ethnic minorities: socioeconomic status and beyond. Current opinion in allergy and clinical immunology, 2009. 9(2): p. 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litonjua AA, Carey VJ, Weiss ST and Gold DR Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatric pulmonology, 1999. 28(6): p. 394–401. [DOI] [PubMed] [Google Scholar]
- 47.Thakur N, Oh SS, Nguyen EA, Martin M, Roth LA, Galanter J, et al. , Socioeconomic Status and Childhood Asthma in Urban Minority Youths. The GALA II and SAGE II Studies. American Journal of Respiratory and Critical Care Medicine, 2013. 188(10): p. 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur N, Martin M, Castellanos E, Oh SS, Roth LA, Eng C, et al. , Socioeconomic Status and Asthma Control in African American Youth in SAGE II. The Journal of asthma : official journal of the Association for the Care of Asthma, 2014. 51(7): p. 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moncrief T, Beck AF, Olano K, Huang B. and Kahn RS Routinely Sleeping Away from Home and the Association with Child Asthma Readmission. Journal of Community Health, 2014. 39(6): p. 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klinnert MD, Kaugars AS, Strand M. and Silveira L. Family psychological factors in relation to children’s asthma status and behavioral adjustment at age 4. Family Process, 2008. 47(1): p. 41–61. [DOI] [PubMed] [Google Scholar]
- 51.Nyenhuis SM, Krishnan JA, Berry A, Calhoun WJ, Chinchilli VM, Engle L, et al. , Race is associated with differences in airway inflammation in patients with asthma. Journal of Allergy and Clinical Immunology, 2017. 140(1): p. 257-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE and Sampson HA Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clinical and Experimental Allergy, 2009. 39(9): p. 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gruchalla RS, Pongracic J, Plaut M, Evans III R, Visness CM, Walter M, et al. , Inner City Asthma Study: Relationships among sensitivity, allergen exposure, and asthma morbidity. Journal of Allergy and Clinical Immunology, 2005. 115(3): p. 478–485. [DOI] [PubMed] [Google Scholar]
- 54.Spira-Cohen A, Chen LC, Kendall M, Sheesley R. and Thurston GD Personal exposures to traffic-related particle pollution among children with asthma in the South Bronx, NY. Journal of Exposure Science and Environmental Epidemiology, 2010. 20(5): p. 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK, et al. , Altered pulmonary function in children with asthma associated with highway traffic near residence. International Journal of Environmental Health Research, 2009. 19(2): p. 139–155. [DOI] [PubMed] [Google Scholar]
- 56.Luria CJ, Sitarik AR, Havstad S, Zoratti EM, Kim H, Wegienka GR, et al. , Association between asthma symptom scores and perceived stress and trait anxiety in adolescents with asthma. Allergy and Asthma Proceedings, 2020. 41(3): p. 210–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.