Abstract
CARD14-associated papulosquamous eruption (CAPE) is a proposed term that encompasses features ranging from psoriasis to pityriasis rubra pilaris (PRP) in association with CARD14 mutations. The early onset of the disease, prominent facial involvement, family history of an autosomal dominant trait, and poor response to conventional treatment are characteristics of CAPE that distinguish it from classical psoriasis and PRP. We describe the clinical features, family history, and response to therapy in three unrelated children with CAPE and compare these characteristics with those of previously described pediatric patients. Testing for CARD14 mutations in children with early onset of features of psoriasis or pityriasis rubra pilaris and resistance to conventional therapy should be considered.
Keywords: CARD14, child, pityriasis rubra pilaris, psoriasis, ustekinumab
1 |. INTRODUCTION
Activating mutations in CARD14, encoding caspase recruitment domain family, member 14, manifest clinically in a variety of presentations, most often pityriasis rubra pilaris (PRP) or plaque psoriasis, but less commonly pustular psoriasis, erythrodermic psoriasis, or acute generalized exanthematous pustulosis. Patients with CARD14 mutations can be distinguished from more common forms of psoriasis and acquired PRP by the early age of onset (primarily before 1 year of age), prominent facial involvement (cheeks, chin and ears), frequent family history of psoriasis or PRP, and limited response to conventional topical and systemic therapies for psoriasis or PRP. Increases in CARD14 expression have been shown to activate the IL-23/Th17 pathway, and best responses have been described with ustekinumab.1
2 |. CASE REPORTS
2.1 |. Patient 1
A 12-month-old Caucasian girl developed an erythematous, desquamative diaper dermatitis at 10 months of age that slowly progressed to involve the entire body. Physical examination showed confluent erythema and scale in the diaper area, trunk, buttocks, axillae, and neck, with scattered scaly, erythematous plaques on the upper and lower limbs, scalp, and face (Figure 1). No palmoplantar involvement, islands of sparing, or follicular papules were present. She did not have ectropion or nail changes. Family history revealed a maternal aunt who suffered from mild-to-moderate plaque psoriasis, but her mother was unaffected. Skin biopsy revealed psoriasiform dermatitis with regular acanthosis, elongation of rete ridges, and a diminished granular layer.
FIGURE 1.
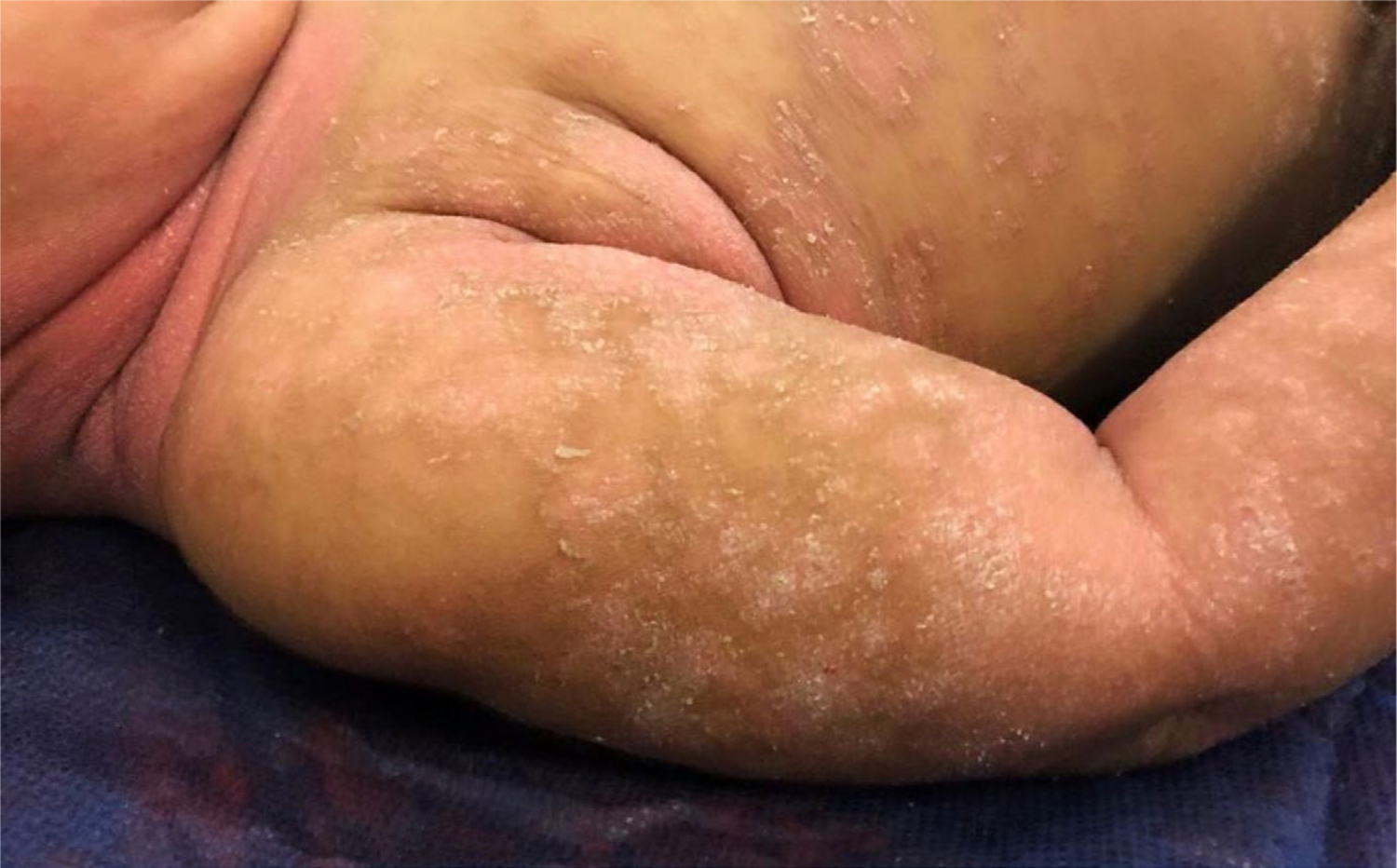
Patient 1: Erythematous plaques with fine scaling on the upper limbs and neck
Twice daily application of hydrocortisone 1% cream for one week to affected area led to a partial reduction in scales and erythema. However, she continued with several flares during three months with minimal response to more potent topical corticosteroids. Genomic DNA was extracted from blood lymphocytes and revealed heterozygous mutation in CARD14 (c.1604A>G, p. Gln535Arg).
2.2 |. Patient 2
A 7-year-old Caucasian boy had a history of a recurrent pruritus and desquamative dermatitis that started on his cheeks at 3 months of age. It was initially diagnosed as atopic dermatitis. Physical examination revealed well-demarcated orange-red scaling plaques and follicular papules on the trunk and limbs with distinct islands of sparing. He also had thick scaly plaques on the scalp, forehead, malar region, philtrum, and chin with sparing of the infralabial region (Figure 2). Bilateral ectropion, palmoplantar keratoderma, and scleroderma-like changes in the hands were also noted. Nail changes were not observed. He had a family history of a maternal grandfather with psoriasis, but his mother was unaffected. Histopathological evaluation of biopsy sections revealed epidermal acanthosis, alternating orthokeratosis and parakeratosis, both vertically and horizontally, as well as follicular dilation. The dermis displayed superficial perivascular lymphocytic infiltrate.
FIGURE 2.
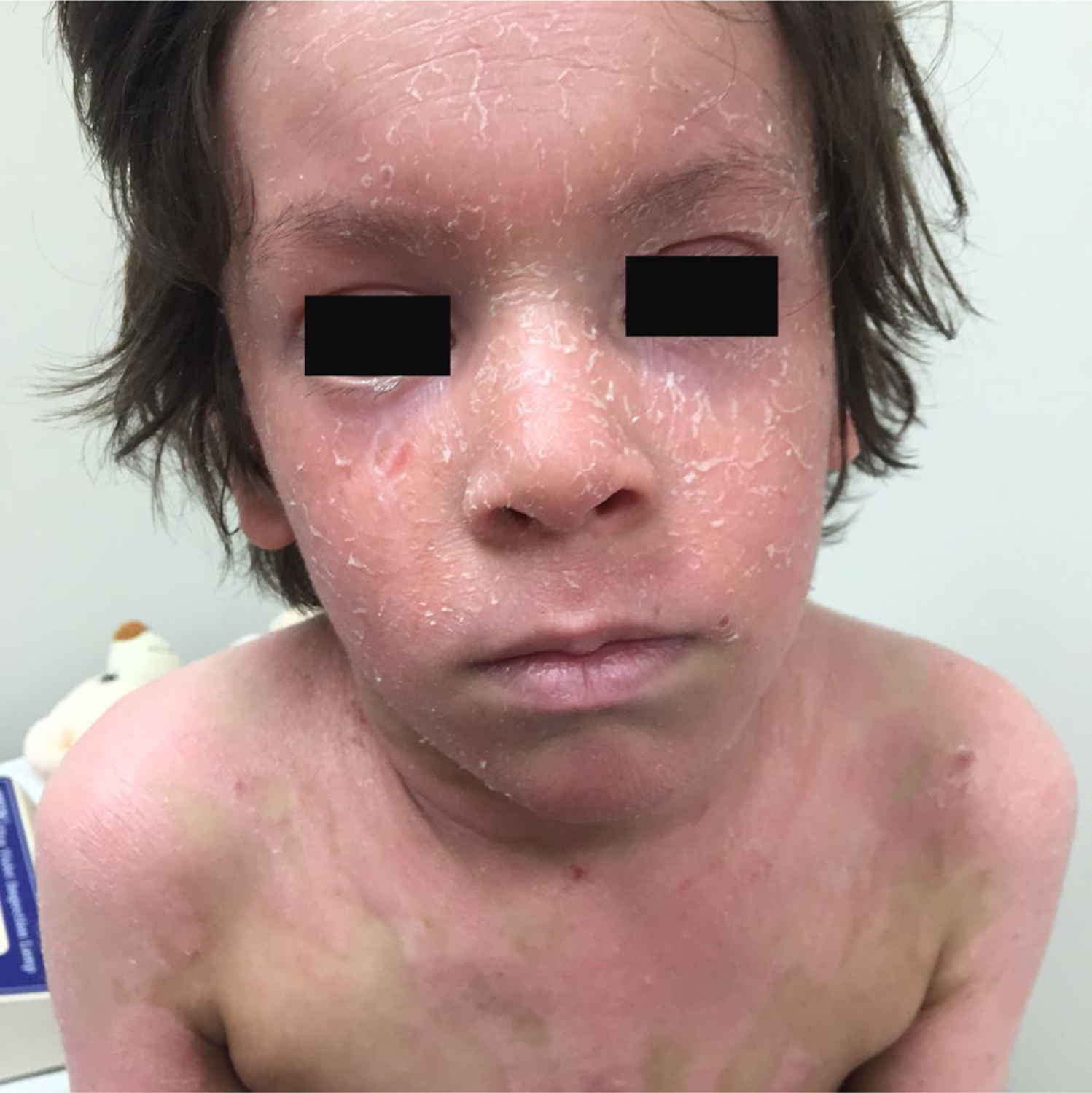
Patient 2: Thick scaly plaques on the face and trunk with distinct islands of sparing
Pityriasis rubra pilaris type V was diagnosed, and isotretinoin was initiated at a dosage of 0.5–1 mg/kg/d. Mometasone 0.1% cream was applied twice daily to affected areas for the first month and then daily for the more erythematous plaques. However, only partial clinical improvement was noted after three months and the isotretinoin was stopped.
He showed a partial response after eight months to a trial of methotrexate (0.4 mg/kg/wk), but subsequently progressed to erythroderma, with 80% body surface area (BSA) affected during a viral infection, despite the continued methotrexate. Given the inadequate response to conventional treatments, CARD14 analysis was performed on DNA extracted from blood lymphocytes and showed a heterozygous CARD14 variant c.365T>C (p. Met119Thr).
Ustekinumab, 2 mg/kg (45 mg), was injected subcutaneously at weeks 0, 4, and 12. After the first month, there was rapid improvement noted, with a reduction in total BSA from 80% to 20%. Methotrexate was discontinued without worsening. At the third month of treatment, he had a flare, so the frequency of ustekinumab was increased to every eight weeks, resulting in further reduction of total BSA to 11%. He continued another four months of treatment without adverse events, despite the higher and more frequent dosing.
2.3 |. Patient 3
A 5-year-old Caucasian boy developed a desquamative dermatitis at 6 months of age that progressed to well-demarcated erythematous scaly plaques on the cheeks, chin, ears, buttocks, arms, lateral aspects of the thighs, and legs. Follicular prominence was noted in plaques on the legs (Figures 3 and 4). Palmoplantar involvement, ectropion, and nail changes were absent. His mother had psoriasis from 8 years of age; his maternal uncle and maternal grandfather also had plaque psoriasis. Lesional erythema, scale, and associated pruritus were improved satisfactorily with a regimen of once daily alclometasone 0.05% ointment for facial lesions and mometasone 0.1% cream for non-facial sites, as well as daily calcipotriene 0.005% ointment. However, minimal improvement was noted with persistent plaques at all sites of involvement. The patient, as well as all affected family members, was tested for CARD14 mutations using DNA extracted from blood lymphocytes, and all family members affected by psoriasis had a heterozygous CARD14 deletion (c.437_439del (p.146_147del) in exon 4). This deletion of AAG from the codons of two contiguous amino acids retains the first amino acid (Lys at position 146) but effectively cuts out the Glu at 147. The resultant CARD14 protein has an altered conformation, promoting formation of the CARD14-containing complex that activates NF-κB.
FIGURE 3.
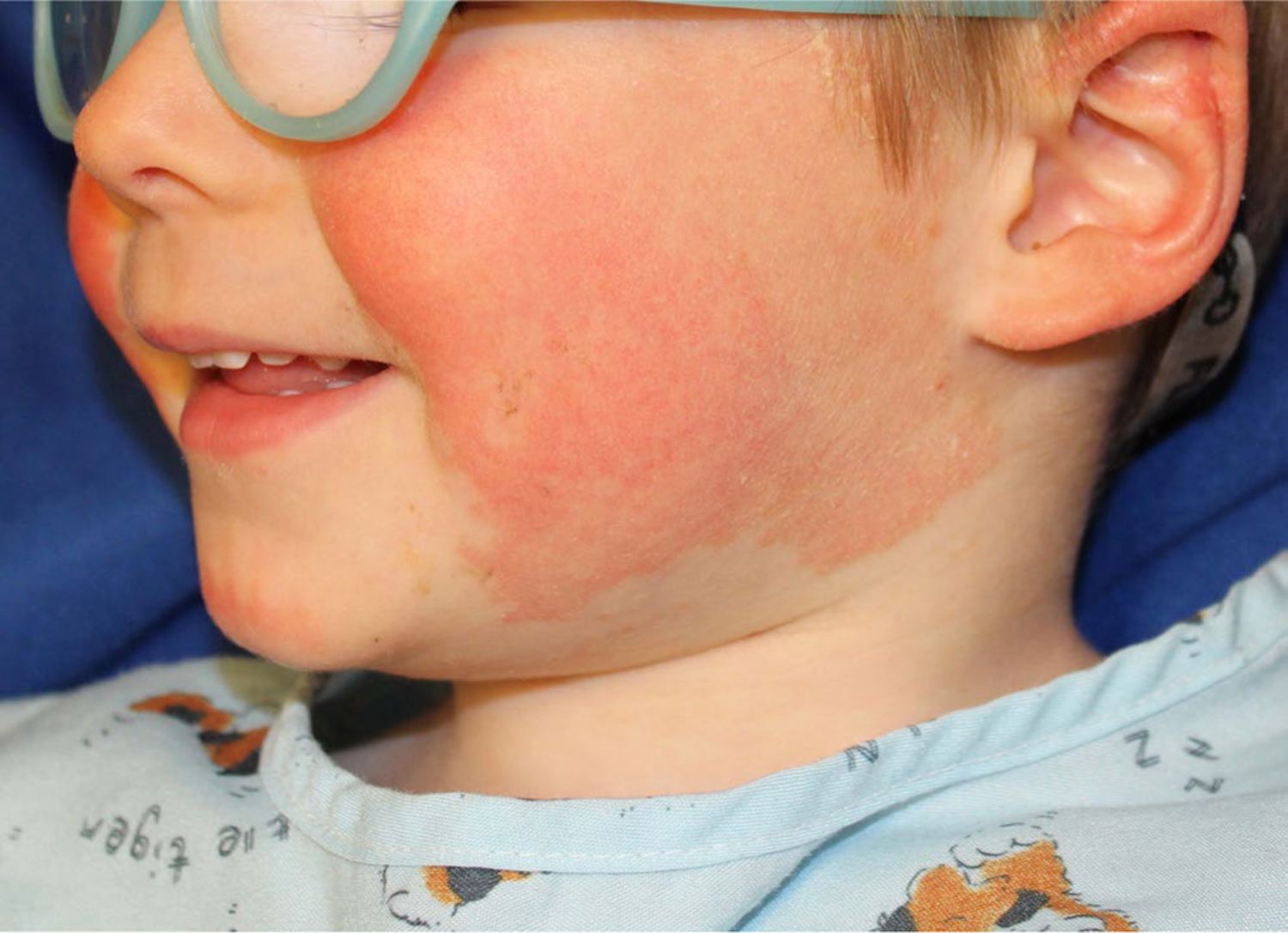
Patient 3: Well-demarcated erythematous scaly plaques on the cheeks, chin, and ears
FIGURE 4.
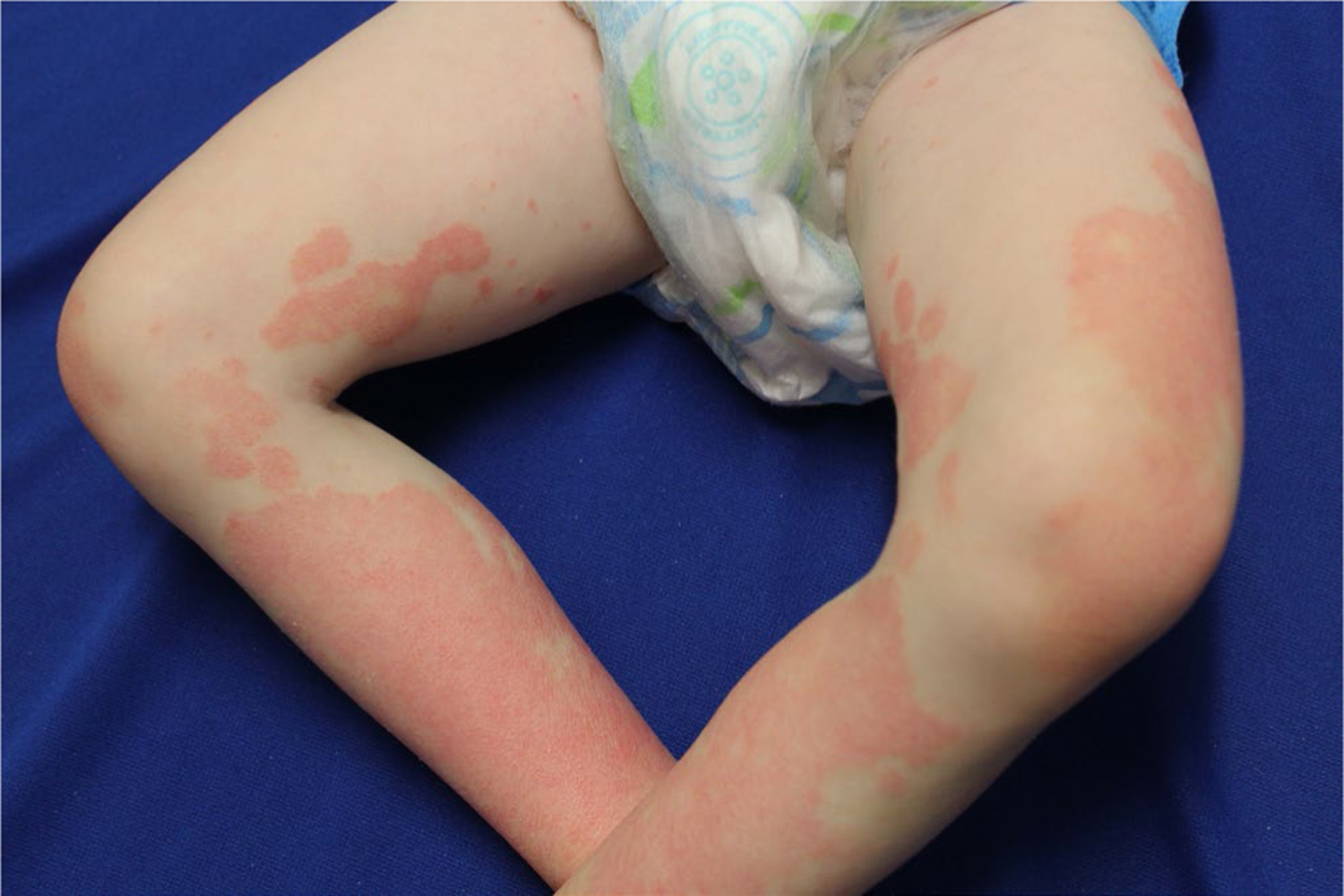
Patient 3: Symmetric patches on lateral thighs and legs
3 |. DISCUSSION
We have described the clinical characteristics of three children with autosomal dominant-activating mutations in CARD14 and reviewed the 31 previously reported pediatric patients (Table 1).1–7 The mean age of disease onset overall was 12.4 months versus 6 months of age for our cases. All three of our patients had a positive family history of psoriasis in extended family members. However, only one had an affected first-degree family member (Patient #3), and in this family with several more distant affected members, perfect genotype-phenotype concordance was found (the CARD14 variant found only in those affected by psoriasis and wild-type CARD14 in unaffected family members). The other family members of Patients #1 and #2 were not tested for a CARD14 mutation, making it unclear whether the family exhibited incomplete penetrance in a parent or the affected child had a de novo mutation.
TABLE 1.
Pediatric patients with CARD14-associated papulosquamous eruption (CAPE)
| Characteristics | Craiglow BG et al1 | Signa S et al2 | Chiramel MJ et al3 | Takeichi T, Terawaki S, et al4 | Takeichi T, Sugiura K, et al5 | Fuchs-Telem D et al6 | Wu T, et al7 | Frare C et al |
|---|---|---|---|---|---|---|---|---|
| Patients (N) | 15 | 2 (twins) | 2 (siblings) | 1 | 2 | 8 | 1 | 3 |
| Mean Age of Onset | 1.1 y (13 had onset at ≤1 y; a 2 y/o and an 8 y/o) | 9 mo | 17 mo | Infancy | 1 m | 14 mo | 24 mo | 6 mo |
| Clinical Features (N; % of patients) | ||||||||
| Facial involvement | 14 | 2 | 2 | 1 | 2 | 8 | 0 | 3 |
| Trunk involvement | 13 | 2 | 2 | 1 | 2 | 0 | 0 | 2 |
| Follicular papules | 6 | 0 | 0 | 0 | 0 | 8 | 0 | 2 |
| Island of sparing | 5 | 0 | 0 | 0 | 0 | 8 | 0 | 1 |
| PPK | 12 | 0 | 0 | 0 | 0 | 8 | 1 | 1 |
| Sclerodermatous changes of the hands | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Nail changes | 0 | 2 | 2 | 0 | 0 | 3 | 0 | 0 |
| Ectropion | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Histopathology | NA | Parakeratotic cornified layer and epidermis with marked elongation of rete ridges and hypogranulosis, Inflammatory cells and dilatation of blood vessels in the dermis (N = 2) | Chronic psoriasiform dermatitis with suprapapillary thinning and hypogranulosis (N = 1) | Hyperkeratosis, parakeratosis, acanthosis and slight spongiosis; neutrophilic infiltration in the stratum corneum, lymphocytic infiltration in the dermis | Hyperkeratosis, parakeratosis and acanthosis and lymphocytic infiltration in the dermis (N = 1) Hyperkeratosis, parakeratosis, follicular dilation filled with keratin and lymphocytic infiltration in the upper dermis (N = 1) |
Alternating orthokeratosis and parakeratosis, follicular plugging, mild lymphocytic infiltration in the upper dermis (N = 8) | Hyperkeratosis, acanthosis and lymphocytic infiltration in the dermis | Regular acanthosis, elongation of rete ridges and hypogranulosis (N = 1) Alternating orthokeratosis and parakeratosis, follicular dilation, perivascular lymphocytic infiltration in the dermis (N = 1) |
| Treatment and Response | Ustekinumab (N = 6) (5)-NC; (1)-MP Ixekizumab (N = 1)-MP Etanercept (N = 9) (1)-NC; (7)-MP; (1)-W Adalimumab (N = 1)-MP Methotrexate (N = 10) (1)-NC; (9)-MP Cyclosporine (N = 2)-MP Acitretin (N = 5) (4)-MP; (1)-W Isotretinoin (N = 4) (1)-NC; (3)-MP |
Ustekinumab (N = 2)-C Etanercept (N = 2)-MP Cyclosporine (N = 2)-MP Retinoids (N = 2)-MP Local and systemic steroids (N = 2)-MP |
Cyclosporine (N = 1)-MP Acitretin(N = 1)-MP Local steroids, coal tar, calcipotriene (N = 1)-MP |
Systemic steroids (N = 1)-NC | NA | NA | NA | Ustekinumab (N = 1)-NC Methotrexate (N = 1)-MP Isotretinoin (N = 1)-MP Local steroids and calcipotriene (N = 2)-MP |
| Family Historya of PRP, PsO or PsA | Positive (N = 10) None (N = 5) | Positive | None | NA | None (N = 1) NA (N = 1) |
Positive (N = 6) None (N = 2) |
Positive | Positive (N = 3) |
Abbreviations: C, complete; MP, minimal/partial; N, number of patients; NA, not available; NC, near complete; PRP, pityriasis rubra pilaris; PsA, psoriatic arthritis; PsO, psoriasis; W, worse.
Family history: Parents, siblings, or extended family member affected (grandparents, aunts, uncles, cousins); None: no affected family member.
In the 31 previously reported cases, clinical features typical of PRP such as follicular papules were observed in 45%, islands of sparing in 42%, palmoplantar keratoderma in 68%, and sclerodermatous changes of the hands in 6%.1–7 One of our three patients presented these manifestations, and two of them only had follicular papules. Biopsy samples of previously reported cases have shown alternating orthokeratosis and parakeratosis with follicular plugging in patients with clinical manifestations of PRP and regular acanthosis with elongated rete ridges in patients with features of psoriasis. One of our biopsies was typical of PRP, and the other showed a psoriasiform dermatitis, reflecting the clinical features of the patients, as previously described for CAPE.8 As described in 93% of the 31 previously described patients, all of our patients had facial involvement that occurred early as symmetric, well-demarcated pink-red plaques involving the cheeks, chin, and ears with sparing of the infralabial region.1 Ectropion developed in more severe cases (6% of previous reports and one of ours). Truncal involvement was present in 64% of previous cases and two of our cases.
At least partial recalcitrance to conventional treatment (local and oral steroids, oral retinoids, methotrexate, and cyclosporine) is a key finding. Patient 1 had a partial response with topical steroid application, which the family deemed satisfactory. Minimal to partial responses have been described with antagonists of tumor necrosis factor-α (TNF-α).1,2 Notably, 7 of 8 patients treated with ustekinumab had near complete to complete response, four of them at a dosage of 0.75 mg/kg-1.1 mg/kg every 12 weeks, one at a dosage of 1.2 mg/kg every 8 weeks,1 and two at a dosage of 2 mg/kg every 8 weeks.2 Our patient had a near-complete response at a dosage of 2 mg/kg every 8 weeks. Patients with CAPE may require higher dose and more frequent dosing of ustekinumab to achieve clinical remission than children taking ustekinumab for psoriasis without a CARD14 variant.
Better understanding of the pathogenesis of monogenic skin disorders has led to repurposing of medications used for common diseases, such as psoriasis. CARD14 activates NF-kB and MAPK signaling pathways, leading to recruitment and differentiation of inflammatory cells with increased production of IL-23 by dendritic cells and of IL-17 and IL-22 by T cells.9 Given the activation of this IL-23/Th17 pathway with CARD14 mutations, a better therapeutic response to ustekinumab versus TNF-α inhibition or broad immunosuppressants is not surprising. Furthermore, recognition that lesional skin in PRP shows activation of the IL-23/Th17 pathway, even without CARD14 mutation,10–14 has led to successful intervention with ustekinumab15,16 and the IL-17 pathway inhibitors secukinumab,17,18 ixekizumab,19 and brodalumab.20 Despite the positive experience with targeted biologics in children with CAPE, long-term potential efficacy and risks are unknown.
Herein, we describe three pediatric patients with CAPE, and each has overlapping features of psoriasis and PRP. We suggest performing genetic testing on patients with onset during infancy, a positive family history, typical facial compromise, or poor response to traditional therapy. Considering the often refractory response to many conventional therapies, detection of a CARD14 mutation should prompt providers to choose inhibitors of IL-12/23, IL-23, or IL-17, rather than inhibitors of the TNF-α pathway.
Funding information
This work was supported by the Foundation for Ichthyosis and Related Skin Types and the National Institutes of Health (R01 AR068392 to KAC and R01 AR050266 to AMB)
Footnotes
CONFLICT OF INTEREST
KAC and ASP have received honoraria from Janssen.
REFERENCES
- 1.Craiglow BG, Boyden LM, Hu R, et al. CARD14-associated papulosquamous eruption: a spectrum including features of psoriasis and pityriasis rubra pilaris. J Am Acad Dermatol. 2018;79(3):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Signa S, Campione E, Rusmini M, et al. Whole exome sequencing approach to childhood onset familial erythrodermic psoriasis unravels a novel mutation of CARD14 requiring unusual high doses of ustekinumab. Pediatr Rheumatol Online J. 2019;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiramel MJ, Sathishkumar D, Edison ES, George R. Two cases of CARD14-associated papulosquamous eruption from India. Pediatr Dermatol. 2020;37(4):692–694. [DOI] [PubMed] [Google Scholar]
- 4.Takeichi T, Terawaki S, Kubota Y, et al. A patient with CARD14-associated papulosquamous eruptions showing atopic dermatitis-like features. J Eur Acad Dermatol Venereol. 2021;35(1):e58–e59. 10.1111/jdv.16799. [published online ahead of print, Jul 3, 2020]. [DOI] [PubMed] [Google Scholar]
- 5.Takeichi T, Sugiura K, Nomura T, et al. Pityriasis rubra pilaris type V as an autoinflammatory disease by CARD14 mutations. JAMA Dermatol. 2017;153(1):66–70. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs-Telem D, Sarig O, van Steensel MA, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91(1):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Banerjee S, Deng J, et al. Familial pityriasis rubra pilaris in a Chinese family caused by a novel mutation in CARD14 gene. Indian J Dermatol Venereol Leprol. 2020;86(1):81–84. [DOI] [PubMed] [Google Scholar]
- 8.Ring NG, Craiglow BG, Panse G, et al. Histopathologic findings characteristic of CARD14-associated papulosquamous eruption. J Cutan Pathol. 2020;47(5):425–430. [DOI] [PubMed] [Google Scholar]
- 9.Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90(5):784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eytan O, Sarig O, Sprecher E, van Steensel MA. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171(2):420–422. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano M, Lembo L, Fania L, Abeni D, Didona D, Didona B. Ustekinumab treatment of pityriasis rubra pilaris: a report of five cases. J Dermatol. 2018;45(2):202–206. [DOI] [PubMed] [Google Scholar]
- 12.Lwin SM, Hsu CK, Liu L, Huang HY, Levell NJ, McGrath JA. Beneficial effect of ustekinumab in familial pityriasis rubra pilaris with a new missense mutation in CARD14. Br J Dermatol. 2018;178(4):969–972. [DOI] [PubMed] [Google Scholar]
- 13.Nieto-Benito LM, Baniandrés-Rodríguez O, Moreno-Torres A, Hernández-Martín A, Torrelo-Fernández A, Campos-Domínguez M. Clinical response to ustekinumab in CARD14-associated papulosquamous eruption (CAPE) with a new missense mutation in CARD14: a case report and systematic review. J Eur Acad Dermatol Venereol. 2020;34(11):e728–e730. [DOI] [PubMed] [Google Scholar]
- 14.Feldmeyer L, Mylonas A, Demaria O, et al. Interleukin 23-helper T cell 17 axis as a treatment target for pityriasis rubra pilaris. JAMA Dermatol. 2017;153(4):304–308. [DOI] [PubMed] [Google Scholar]
- 15.Min MS, Shroff A, Rose S, Lebwohl M, Guttman-Yassky E. Ustekinumab as therapy for psoriasis in a 2-year-old girl. J Eur Acad Dermatol Venereol. 2016;30(11):109–110. [DOI] [PubMed] [Google Scholar]
- 16.Bonomo L, Raja A, Tan K, Guttman-Yassky E. Successful treatment of juvenile pityriasis rubra pilaris with ustekinumab in a 7-year-old girl. JAAD Case Rep. 2018;4(2):206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang JY, Ye RX, Tian X, Zhang SQ, Zhang XB. Secukinumab Monotherapy successfully treated severe refractory type V (atypical juvenile) pityriasis rubra pilaris: a case report and literature review. Dermatol Ther. 2020;33(6):e14097. [DOI] [PubMed] [Google Scholar]
- 18.Cole D, Mohammad T, Lim H. Rapid response of pityriasis rubra pilaris with psoriasis overlap to secukinumab. Br J Dermatol. 2019;181(6):1331–1332. [DOI] [PubMed] [Google Scholar]
- 19.Hanfstingl K, Pekar-Lukacs A, Motz R, Guenova E, Hoetzenecker W. Successful treatment of pityriasis rubra pilaris with ixekizumab. Case Rep Dermatol. 2018;10(2):97–100. 10.1159/000488902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Felice C, Graceffa D, Morrone A, Bonifati C. Familial pityriasis rubra pilaris successfully treated with brodalumab. Int J Dermatol. 2020;59(7):885–887. [DOI] [PubMed] [Google Scholar]


