Abstract
Neonicotinoids are replacement insecticides increasingly used for organophosphates, methylcarbamates, and pyrethroids. Experimental evidence suggests neonicotinoids may affect glucose metabolism and insulin secretion through pancreatic β cell dysfunction, oxidative stress, and inflammation. However, no epidemiologic study has investigated neonicotinoids as potential diabetogens. We examined associations between neonicotinoids with insulin and glucose homeostasis parameters among 1381 non-diabetic adults in the National Health and Nutrition Examination Survey (2015–2016). Urinary concentrations of acetamiprid, clothianidin, imidacloprid, N-desmethyl-acetamiprid, and 5-hydroxy-imidacloprid were quantified. Fasting plasma glucose, insulin, and hemoglobin A1c (HbA1c) were assessed. Insulin resistance was defined as a homeostatic model assessment of insulin resistance ≥2.5. We used weighted linear and logistic regression to estimate associations between detectable neonicotinoids with insulin and glucose homeostasis parameters. Weighted detection frequencies for imidacloprid, 5-hydroxy-imidacloprid, and N-desmethyl-acetamiprid were 4.4%, 21.5%, and 32.8%, respectively. Detectable imidacloprid (β=−4.7 μIU/mL, 95% CI −8.5, −0.8) and 5-hydroxy-imidacloprid (β=−2.4 μIU/mL, 95% CI −4.6, −0.2) were associated with lower fasting plasma insulin levels. Individuals with detectable 5-hydroxy-imidacloprid had lower odds of insulin resistance (OR=0.3, 95% CI 0.2, 0.7). We observed evidence of sexually dimorphic associations between N-desmethyl-acetamiprid with glucose (pint=0.079) and 5-hydroxy-imidacloprid with HbA1c (pint=0.038), with patterns suggesting positive associations in males and negative associations in females. Associations between 5-hydroxy-imidacloprid and insulin were modified by BMI (pint=0.013). We additionally observed age modified associations between 5-hydyroxy-imidacloprid and glucose (pint=0.048). Results suggest neonicotinoids may be associated with insulin and glucose homeostasis indices and call for prospective studies to examine the metabolic impact of these replacement insecticides in humans.
Keywords: Neonicotinoids, glucose, insulin, HbA1c, HOMA-IR, insulin resistance
1. Introduction
Neonicotinoids were introduced in the 1990s as insecticides and have since served as a replacement for organophosphates, methylcarbamates, and pyrethroids. By 2014, neonicotinoids accounted for more than 25% of the insecticide market worldwide (Bass et al., 2015). Environmental studies have detected neonicotinoids in soil, water, sewage, dust, and fauna (Abdel-Ghany et al., 2016; Anderson et al., 2015; Berheim et al., 2019; Bradley et al., 2017; Goulson, 2013; MacDonald et al., 2018; Montiel-Leon et al., 2018; Sadaria et al., 2017; Salis et al., 2017; Sultana et al., 2018; Yamamoto et al., 2012; Zhou et al., 2018). The main route of human exposure is through intake of contaminated water and food. Unlike other pesticides, washing food products cannot easily remove neonicotinoids since they exist in the plant flesh (Chen et al., 2014; Craddock et al., 2019). Neonicotinoids have also been detected in fine particulate matter (PM2.5), with inhalation as another route of exposure among both occupational and non-occupational populations (Zhou et al., 2020). A study of indoor dust samples in Wuhan, China observed significant increases in neonicotinoid residues from 2016 to 2018 (Wang et al., 2019).
Biomonitoring studies have revealed urinary concentrations of neonicotinoids in populations from China, Greece, Japan, Spain, and Sri Lanka (Kabata et al., 2016; Lopez-Garcia et al., 2017; Osaka et al., 2016; Ueyama et al., 2014; Wang et al., 2015; Zhang et al., 2018; Zhang et al., 2019). Concentrations of urinary neonicotinoids in women have steadily increased from 1994 to 2011 in Japan (Ueyama et al., 2015). , Approximately half of the participants from the 2015–2016 National Health and Nutrition Examination Survey (NHANES) had detectable concentrations of at least one of six neonicotinoid biomarkers (acetamiprid, clothianidin, imidacloprid, thiacloprid, N-desmethyl-acetamiprid, and 5-hydroxy-imidacloprid) (Ospina et al., 2019).
Concerns regarding the toxicity of neonicotinoids have emerged following several in vitro and in vivo studies reporting genotoxic, hepatic, neurological, and reproductive effects in mammals (Cimino et al., 2017; Gu et al., 2013; Han et al., 2018; Hirano et al., 2015; Toor et al., 2013). Experimental evidence highlights neonicotinoid’s role in glucose metabolism and insulin secretion. A reduction in insulin stimulated glucose uptake was observed in an in vitro study of three cell culture models, including adipocyte (3T3-L1), hepatocyte (HepG2), and myotubule cells (C2C12), that were treated with imidacloprid for 4–6 days after treatment with insulin (Kim et al., 2013). Findings suggest that imidacloprid may contribute to type 2 diabetes mellitus (T2DM) development by impairing downstream targets of glucose transporter 4 (GLUT4) translocation in myotubes and adipocytes (Kim et al., 2013). Neonicotinoids may disrupt insulin receptor substrate-1 (IRS-1) activation via calcium-dependent mechanisms (Kim et al., 2013). However, no human study has examined the relationship between neonicotinoid exposure and insulin or glucose homeostasis parameters.
The objective of the study was to assess the associations between detectability of five neonicotinoid biomarkers in urine with insulin and glucose homeostasis parameters in adults. In the absence of longitudinal data, a cross-sectional analysis of data from NHANES 2015–2016 was used. We also examined whether sex modifies the relationship between neonicotinoids with insulin and glucose homeostasis parameters since neonicotinoid metabolism differs by sex, with females more readily metabolizing neonicotinoids than males (Song et al., 2020). We additionally explored whether BMI status and age modifies these associations (Mesnage et al., 2018; Park et al., 2013; Sun et al., 2016).
2. Methods
2.1. Data source and study participants
Our study sample included individuals who participated in the 2015–2016 NHANES, a cross-sectional, population-based, multistage probability sample of the civilian, non-institutionalized population that allows the estimates to represent the US population. Briefly, NHANES participants completed questionnaires and received physical examinations and donated blood and urine samples. The National Center for Health Statistics Ethic Review Board approved all procedures and content, and all participants provided written informed consent. A total of 9,971 participants were included in the NHANES from 2015–2016. Of these participants, 2,014 had information on urinary concentrations of neonicotinoids, which were measured among a random one-third subsample of participants, and at least one measure of either fasting plasma glucose, insulin, or hemoglobin A1c (HbA1c). We further excluded individuals: 1) aged <20 years (n=366); 2) who had confirmed pregnancies (n=17) or who had a pregnancy status that could not be confirmed (n=5); and 3) who were ever diagnosed with diabetes, taking diabetic drugs, or taking insulin (n=245). In total, 1,381 participants were included in this study.
2.2. Assessment of neonicotinoids
Four parent neonicotinoid compounds (acetamiprid, clothianidin, imidacloprid, and thiacloprid) and two metabolites (N-desmethyl-acetamiprid and 5-hydroxy-imidacloprid) were quantified in urine samples that were collected and stored at −7°C until analysis. Details regarding the analytical method, including quality control materials, calibration standards, reagent blanks, and method accuracy, are described by Baker et al. (2019). Briefly, the analytical method entailed using 0.2 mL of urine for enzymatic hydrolysis of urinary conjugates of the target analytes, online solid phase extraction, reversed phase high-performance liquid chromatography separation, and isotope dilution-electrospray ionization tandem mass spectrometry detection. Limits of detection (LOD) for the neonicotinoid compounds were: 0.3 μg/L for acetamiprid, 0.2 μg/L for clothianidin and N-desmethyl-acetamiprid, 0.4 μg/L for imidacloprid and 5-hydroxy-imidacloprid, and 0.03 μg/L for thiacloprid.
2.2. Insulin and glucose homeostasis parameters
Blood samples were collected from participants during the same study visit as urine samples. The average time between blood and urine collection can be up to 4 hours. Participants were instructed to fast for at least 8 hours. All samples were frozen and shipped to the University of Missouri’s Diabetes Diagnostic Laboratory for quantification of fasting plasma glucose, serum insulin, and HbA1c. Fasting plasma glucose was measured with a UV test to detect glucose in plasma using the Roche Cobas 311 instrument. Insulin was measured in serum with a two-site immunoenzymometric assay using the Tosoh AIA System analyzer. HbA1c was measured in whole blood samples with a Tosoh Automated Glycohemoglobin Analyzer HLC-723G8. Coefficients of variation for fasting plasma glucose, insulin, and HbA1c were 0.8–1.8%, 2.84–4%, and 1.1–1.4%, respectively. Insulin resistance was assessed using homeostatic model assessment (HOMA-IR) with the formula: HOMA-IR = fasting serum insulin (uIU/mL) × fasting plasma glucose (mmol/L) / 22.5 (Matthews et al., 1985). Higher HOMA-IR values are indicative of increased sensitivity of insulin resistance, with HOMA-IR values ≥2.5 indicating insulin resistance (Muniyappa et al., 2008). Individuals were considered as having a prediabetic status if they had either a HbA1c value between 5.7–6.4% or a fasting glucose measure between 100–125 mg/dL based on criteria from the American Diabetes Association. Individuals having both a HbA1c value <5.7% and a glucose <100 mg/dL were considered as non-prediabetic.
2.3. Statistical methods
We calculated descriptive statistics for neonicotinoid concentrations detected among adults, including weighted percent detections and percentiles. Neonicotinoids are not widely detected in urine samples, with weighted detection frequencies of 0.3%, 7.6%, 4.4%, 0.04%, 21.5%, and 32.8% for acetamiprid, clothianidin, imidacloprid, thiacloprid, 5-hydroxyimidacloprid, and N-desmethyl-acetamiprid, respectively (Supplemental Table S1). Given the low detection frequencies of quantified neonicotinoid parent compounds and metabolites, we examined neonicotinoids as a binary variable, specifically as detect versus non-detect. Our analysis focused on three parent compounds (acetamiprid, clothianidin, imidacloprid) and two metabolites (5-hydroxy-imidacloprid, N-desmethyl-acetamiprid). We performed analysis of variance (ANOVA) to assess whether the distribution of insulin and glucose homeostasis parameters significantly differed by population characteristics, such as sex, race/ethnicity, poverty income ratio, body mass index (BMI), daily caloric intake, and levels of physical activity.
To estimate associations between detectable concentrations of neonicotinoids and insulin and glucose homeostasis parameters, including glucose, insulin, HbA1c, and HOMA-IR, we conducted linear regression models. For dichotomized outcomes of insulin resistance and prediabetes, we used logistic regression models to estimate the odds of having insulin resistance or prediabetes with detectable concentrations of neonicotinoids compared to non-detectable concentrations. All models considered strata, primary sampling units, and survey weights to ensure generalizability to the US population since NHANES uses a complex multistage design. The survey weight for neonicotinoids was used for the present analyses. We considered variables based on a priori knowledge of their potential associations with neonicotinoids and/or insulin and glucose homeostasis parameters. These included age, sex, race/ethnicity, poverty income ratio, BMI, daily caloric intake (assessed using a 24-hour dietary recall), physical activity (assessed using a self-reported questionnaire based on the Global Physical Activity Questionnaire (WHO, 2005)), serum cotinine, hypertension, and family history of T2DM. Inclusion in the final model were based on bivariate analyses (p<0.20), and included the following: age (continuous), sex (male/female), race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, Other/multiple races), poverty income ratio (low income, middle income, high income), BMI (underweight/normal, overweight, obese), serum cotinine (<1, 1–9, 10+ ng/mL), hypertension status (yes/no), and family history of T2DM (yes/no).
We evaluated effect measure modification by sex by including cross product terms between sex and detectability of neonicotinoid metabolites (5-hydroxy-imidacloprid, N-desmethyl-acetamiprid) in the models, with p<0.10 considered statistically significant. We also examined whether BMI (underweight/normal, overweight/obese) and age (20–45 years, 46+ years) modified the relationship between detectability of neonicotinoid metabolites and insulin and glucose homeostasis parameters. In separate sensitivity analyses, we additionally adjusted for total daily caloric intake and physical activity levels. We assessed for multicollinearity using the variance inflation factor (VIF) and observed no evidence of multicollinearity.
3. Results
3.1. Study participants
Participants included in the present study were an average of 46.7±16.8 years of age, with a slightly higher percentage of males (51.3%) compared to females (48.7%) (Table 1). Almost two-thirds of the participants self-identified as non-Hispanic White. A majority had a poverty income ratio that was considered as either middle or high income. Approximately two-thirds of the participants had a BMI ≥25 kg/m2, indicating an overweight or obese status. Of participants with available physical activity information (56.8%), approximately half met the requirements of physical activity based on guidelines recommended for US adults (either 150 minutes of moderate intensity aerobic activity or 75 minutes of vigorous aerobic activity per week) (Services, 2018). Almost three-quarters had serum cotinine measures <1 ng/mL, indicative of being non-smokers. Approximately 46% and 23% reported having hypertension and a family history of diabetes, respectively.
Table 1.
Characteristics of adults with insulin and glucose homeostasis parameters in NHANES 2015–2016a
| Characteristics | n (%) | Insulin (μIU/mL) Mean (SD) | HOMA-IR Mean (SD) | Glucose (ng/mL) Mean (SD) | HbA1c Mean (SD) | Detectable Imidacloprid n (%) | Detectable 5-hydroxy-imidacloprid n (%) |
|---|---|---|---|---|---|---|---|
| Total | 1381 | 12.4 (12.7) | 3.4 (4.2) | 103.6 (18.2) | 5.5 (0.5) | 60 (4.4) | 269 (21.5) |
| Age (years) | |||||||
| 20–45 | 671 (49.3) | 12.5 (15.3) | 3.4 (5.3) | 106.4 (15.6)* | 5.3 (0.5)* | 32 (54.3) | 136 (51.0) |
| 46+ | 710 (50.7) | 12.3 (10.3) | 3.3 (3.0) | 100.9 (20.0)* | 5.6 (0.5)* | 28 (45.7) | 133 (49.0) |
| Sex | |||||||
| Male | 654 (48.7) | 14.4 (15.3)* | 4.0 (5.2)* | 106.4 (15.6)* | 5.5 (0.5) | 31 (60.5) | 131 (52.1) |
| Female | 727 (51.3) | 10.5 (9.1)* | 2.7 (2.7)* | 100.9 (20.0)* | 5.5 (0.5) | 29 (39.5) | 138 (47.9) |
| Race/ethnicity | |||||||
| Non-Hispanic White | 465 (65.6) | 12.1 (13.4) | 3.3 (4.5) | 103.3 (15.4) | 5.4 (0.5)* | 22 (68.5) | 84 (66.3) |
| Non-Hispanic Black | 308 (10.3) | 11.8 (10.2) | 3.1 (3.1) | 103.0 (31.5) | 5.6 (0.8)* | 11 (8.4) | 63 (10.7) |
| Non-Hispanic Asian | 155 (5.5) | 11.1 (10.4) | 3.0 (3.1) | 104.6 (16.3) | 5.5 (0.4)* | 13 (10.6) | 40 (7.1) |
| Hispanic | 396 (14.5) | 14.5 (11.7) | 3.8 (3.4) | 104.6 (15.5) | 5.5 (0.5)* | 12 (11.1) | 69 (11.8) |
| Other/multiracial | 57 (4.1) | 12.6 (9.2) | 3.5 (3.3) | 105.7 (24.9) | 5.6 (0.6)* | 2 (1.3) | 13 (4.1) |
| Poverty Income Ratio | |||||||
| <1.3 (low income) | 370 (18.5) | 12.2 (9.1) | 3.3 (2.9) | 106.3 (23.1) | 5.5 (0.7) | 15 (18.7) | 72 (17.1)* |
| 1.3–3.4 (middle income) | 473 (34.4) | 13.3 (16.1) | 3.8 (5.8) | 104.2 (22.4) | 5.5 (0.5) | 14 (27.5) | 72 (20.8)* |
| ≥3.5 (high income) | 394 (47.1) | 11.7 (10.7) | 3.0 (3.0) | 101.7 (12.0) | 5.4 (0.4) | 25 (53.8) | 102 (62.1)* |
| BMI | |||||||
| Underweight/Normal | 396 (32.2) | 6.5 (4.2)* | 1.6 (1.3)* | 98.9 (12.7)* | 5.4 (0.4)* | 21 (29.1) | 71 (25.0) |
| Overweight | 441 (31.0) | 9.6 (8.4)* | 2.4 (2.4)* | 102.0 (16.1)* | 5.4 (0.5)* | 17 (37.2) | 91 (34.1) |
| Obese | 538 (36.8) | 19.5 (16.4)* | 5.5 (5.7)* | 108.8 (22.0)* | 5.6 (0.6)* | 22 (33.8) | 103 (40.9) |
| Daily caloric intake (kcal) | |||||||
| <1485 | 305 (20.5) | 13.2 (15.9) | 3.8 (6.1) | 105.4 (25.3) | 5.5 (0.6) | 14 (19.7) | 49 (16.8) |
| 1485–2012 | 313 (23.2) | 12.1 (11.9) | 3.3 (3.8) | 102.6 (16.1) | 5.5 (0.5) | 7 (7.8) | 53 (15.2) |
| 2013–2603 | 337 (29.1) | 13.3 (13.0) | 3.5 (3.6) | 104.7 (17.8) | 5.5 (0.5) | 15 (33.8) | 77 (33.1) |
| 2603+ | 332 (27.2) | 11.6 (10.2) | 3.1 (2.9) | 102.1 (11.9) | 5.4 (0.5) | 16 (38.8) | 72 (35.0) |
| Levels of physical activity | |||||||
| Standard | 335 (49.1) | 12.1 (14.4) | 3.1 (4.1) | 100.2 (12.2) | 5.4 (0.4) | 15 (33.0) | 74 (44.1) |
| Substandard | 367 (50.9) | 10.0 (7.5) | 2.6 (2.3) | 100.1 (12.2) | 5.4 (0.4) | 27 (67.0) | 89 (55.9) |
| Serum cotinine (ng/mL) | |||||||
| <1 (Non-smoker) | 969 (71.2) | 11.8 (11.2) | 3.2 (4.0) | 103.2 (16.7) | 5.5 (0.5) | 48 (81.3) | 207 (77.4) |
| 1–9 (ETS) | 53 (3.3) | 15.1 (19.6) | 3.9 (5.5) | 100.7 (10.0) | 5.3 (0.6) | 1 (0.8) | 13 (4.5) |
| 10+ (Smoker) | 354 (25.6) | 13.6 (14.9) | 3.6 (4.3) | 105.0 (21.9) | 5.5 (0.6) | 11 (17.8) | 47 (18.1) |
| Hypertension | |||||||
| No | 675 (54.1) | 10.9 (12.3)* | 2.9 (4.4)* | 100.0 (14.7)* | 5.3 (0.4)* | 35 (72.4)* | 127 (55.6) |
| Yes | 706 (45.9) | 14.4 (12.9)* | 3.9 (3.7)* | 108.3 (21.0)* | 5.6 (0.6)* | 25 (27.6)* | 142 (44.4) |
| Family history of diabetes | |||||||
| No | 1057 (77.0) | 10.6 (8.5)* | 2.8 (2.6)* | 101.9 (14.2)* | 5.4 (0.4)* | 43 (74.1) | 200 (77.6) |
| Yes | 324 (23.0) | 18.3 (20.1)* | 5.2 (6.9)* | 109.2 (26.5)* | 5.6 (0.7)* | 17 (25.9) | 69 (22.4) |
Percent, mean, and SD values presented are weighted to account for the NHANES complex survey design
Statistically different at p<0.05
Mean values of glucose, insulin, HbA1c, and HOMA-IR were 103.6±18.2 ng/mL, 12.4±12.7 μIU/mL, 5.5±0.5%, and 3.4±4.2, respectively. Participants who were 20–45 years had higher glucose levels compared to those who were 46+ years. In contrast, HbA1c values were significantly higher among older individuals. We observed significantly higher glucose, insulin, and HOMA-IR levels among males. Individuals who self-identified as either non-Hispanic Black or as other/multiple races had higher HbA1c values. Significantly higher values of all insulin and glucose homeostasis parameters were observed among participants who were obese, hypertensive, and those with a family history of diabetes.
3.2. Insulin and glucose homoeostasis parameters
Of the participants included in the study, 296 (20.9%) and 624 (42.2%) had insulin resistance and prediabetes, respectively. No association was noted between detectability of neonicotinoids and glucose or HbA1c levels (Table 2). However, a statistically significant inverse association was observed between imidacloprid and its metabolite 5-hydroxy-imidacloprid with insulin levels. Specifically, participants with detectable imidacloprid and 5-hydroxy-imidacloprid had decreases of 4.7 μIU/mL (95% CI −8.5, −0.8) and 2.4 μIU/mL (95% CI −4.6, −0.2) of insulin, respectively. Detectability of imidacloprid was also significantly associated with lower HOMA-IR (β=−1.5, 95% CI −2.9, −0.1). There was suggestive evidence of a negative association between 5-hydroxy-imidacloprid and HOMA-IR, though the finding was only borderline significant (β=−0.7, 95% CI −1.5, 0.05). However, individuals with detectable 5-hydroxy-imidacloprid had lower odds of having insulin resistance (OR=0.3, 95% CI 0.2, 0.7) (Table 3). Results do not support a relationship between detectability of neonicotinoids and increased odds of being prediabetic.
Table 2.
Associations between detectable urinary concentrations of neonicotinoids with insulin and glucose homeostasis parameters in adults, NHANES 2015–2016a
| Insulin | HOMA-IR | Glucose | HbA1c | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD)b | β (95% CI) | Mean (SD)b | β (95% CI) | Mean (SD)b | β (95% CI) | Mean (SD)b | β (95% CI) | |
| Acetamiprid | 10.3 (8.9) | −2.4 (−8.7, 3.9) | 2.7 (2.3) | −0.6 (−2.4, 1.1) | 102.8 (13.0) | 0.9 (−11.8, 13.6) | 5.4 (0.4) | −0.1 (−0.4, 0.2) |
| Clothianidin | 10.5 (7.8) | 0.2 (−3.7, 4.2) | 2.8 (2.4) | 0.01 (−1.2, 1.2) | 104.0 (17.4) | 0.2 (−7.1, 7.4) | 5.4 (0.5) | −0.02 (−0.1, 0.1) |
| Imidacloprid | 8.8 (6.7) | −4.7 (−8.5, −0.8) | 2.3 (1.8) | −1.5 (−2.9, −0.1) | 101.3 (11.7) | −2.1 (−8.2, 4.0) | 5.4 (0.4) | −0.1 (−0.3, 0.1) |
| N-desmethyl-acetamiprid | 12.5 (12.6) | 0.5 (−1.8, 2.7) | 3.4 (4.9) | 0.2 (−0.6, 1.1) | 102.5 (14.4) | −1.0 (−3.5, 1.6) | 5.5 (0.5) | 0.003 (−0.1, 0.1) |
| 5-hydroxy-imidacloprid | 11.6 (9.0) | −2.4 (−4.6, −0.2) | 3.1 (2.7) | −0.7 (−1.5, 0.05) | 103.0 (12.9) | −1.0 (−4.5, 2.5) | 5.5 (0.5) | 0.0003 (−0.1, 0.1) |
Units: glucose, mg/dL; insulin, μIU/mL
Adjusted by age, sex, race/ethnicity, poverty income ratio, BMI, serum cotinine, hypertension status, and family history of type 2 diabetes mellitus.
Mean (SD) for individuals with detectable urinary concentrations of neonicotinoids
Table 3.
Associations between detectable urinary concentrations of neonicotinoids and odds of insulin resistance and prediabetes in adults, NHANES 2015–2016a
| Insulin Resistance | Prediabetes | |||
|---|---|---|---|---|
| n (%)b | OR (95% CI) | n (%)b | OR (95% CI) | |
| Acetamiprid | 2 (0.3) | 0.6 (0.1, 3.3) | 4 (0.3) | 0.8 (0.1, 7.5) |
| Clothianidin | 20 (6.7) | 1.4 (0.4, 8.6) | 46 (7.6) | 1.2 (0.6, 2.3) |
| Imidacloprid | 7 (2.8) | 0.5 (0.2, 1.1) | 18 (3.0) | 0.4 (0.1, 1.4) |
| N-desmethyl -acetamiprid | 106 (34.5) | 0.8 (0.5, 1.3) | 213 (34.9) | 1.2 (0.8, 1.7) |
| 5-hydroxy-imidacloprid | 52 (19.6) | 0.3 (0.2, 0.7) | 117 (21.9) | 1.0 (0.7, 1.4) |
Units: glucose, mg/dL; insulin, μIU/mL
Adjusted by age, sex, race/ethnicity, poverty income ratio, BMI, serum cotinine, hypertension status, and family history of type 2 diabetes mellitus.
Number and weighted percentage of individuals with IR or prediabetes by detectable urinary concentrations of neonicotinoids
3.3. Effect measure modification by sex, BMI, and age
Sex modified the association between N-desmethyl-acetamiprid and glucose (pint=0.079) (Figure 1A). While sex-stratified findings did not reach statistical significance, males with detectable N-desmethyl-acetamiprid had higher levels of glucose (β=2.3 ng/mL, 95% CI −0.6, 5.2) compared to the lower glucose levels that were observed in females (β=−3.7 ng/mL, 95% CI −9.1, 1.6). Sex also modified the relationship between 5-hydroxy-imidacloprid (pint=0.038) with HbA1c, with evidence suggesting a positive association among males and a negative association among females.
Figure 1.
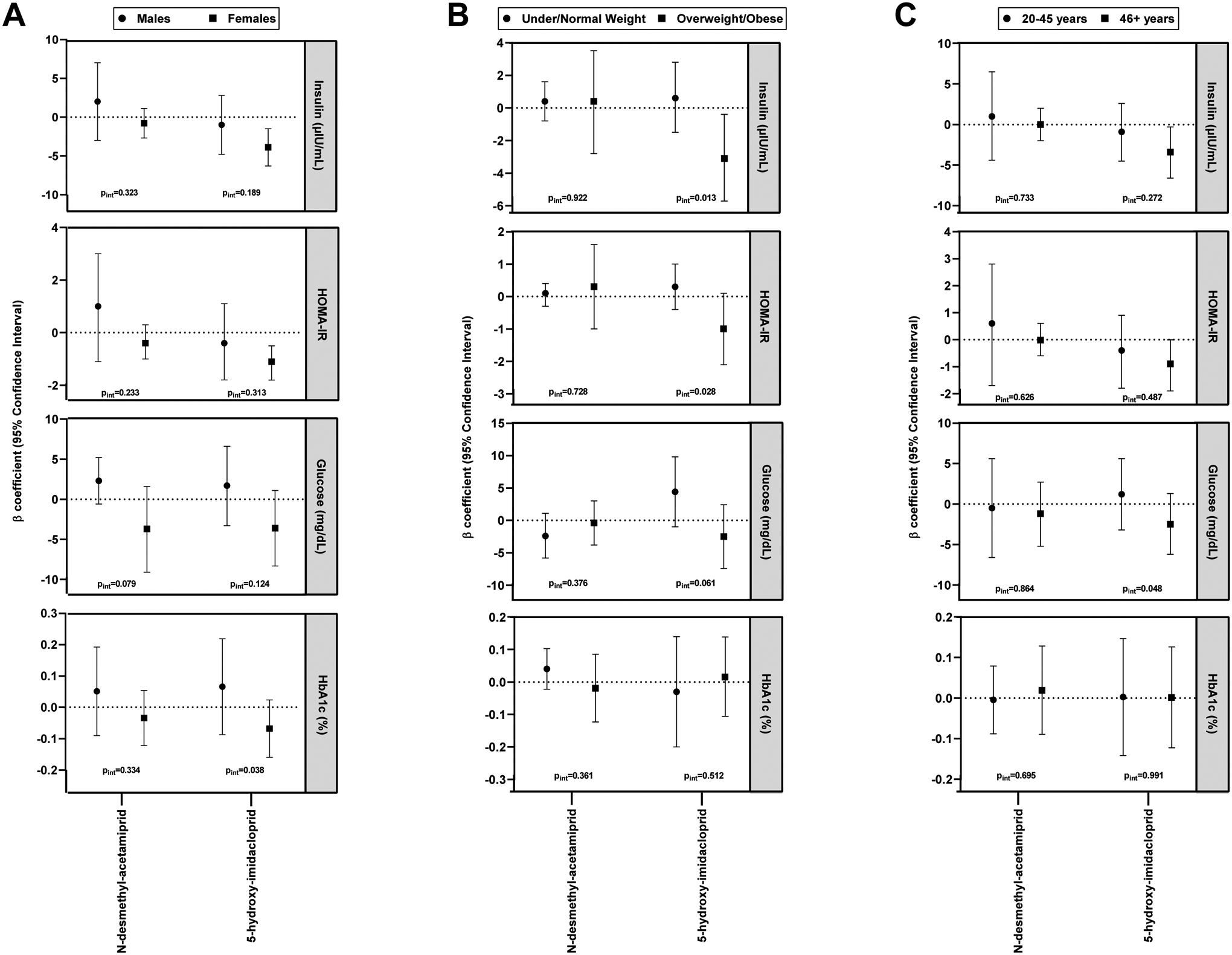
Associations between detectability of urinary neonicotinoids with insulin and glucose homeostasis parameters in adults by sex (A), BMI status (B), and age (C), NHANES 2015–2016. Adjusted by age, sex, race/ethnicity, poverty income ratio, BMI, serum cotinine, hypertension status, and family history of type 2 diabetes mellitus.
With regard to BMI, we observed evidence that detectability of 5-hydroxy-imidacloprid was significantly associated with decreased insulin levels among individuals who were overweight/obese (β=−3.1 μIU/mL, 95% CI −5.7, −0.4), while there was no relationship among those who were under/normal weight (β=0.6 μIU/mL, 95% CI −1.5, 2.8) (pint=0.013) (Figure 1B). A similar pattern was observed for 5-hydroxy-imidacloprid with glucose (pint=0.061) and HOMA-IR (pint=0.028) by BMI status, albeit stratified associations were not statistically significant. Lastly, the association between 5-hydroxy-imidacloprid and glucose was modified by age (pint=0.048) (Figure 1C). We observed suggestive evidence that younger individuals (20–45 years) with detectable 5-hydroxy-imidacloprid had increased glucose levels (β=1.2 ng/mL, 95% CI −3.2, 5.6), while older individuals (46+ years) had lower glucose levels (β=−2.5 ng/mL, 95% CI −6.2, 1.3).
3.4. Sensitivity analyses
Our overall findings did not differ after adjusting for daily caloric intake, though the relationship between detectable 5-hydroxy-imidacloprid with HOMA-IR was now only borderline significant (Supplemental Table S2). Adjustment for physical activity resulted in a borderline significant association between detectable 5-hydroxy-imidacloprid and insulin (β=−3.3 μIU/mL, 95% CI −7.1, 0.5) (Supplemental Table S3). However, we continued to observe negative associations between imidacloprid with insulin and HOMA-IR. Further, individuals with detectable 5-hydroxy-imidacloprid had lower odds of insulin resistance in both sensitivity analyses (data not shown). We additionally found lower odds of prediabetes among individuals with detectable imidacloprid compared to those with non-detectable concentrations after taking into account physical activity (OR=0.3, 95% CI 0.1, 0.7).
4. Discussion
Our study provides the first epidemiological evidence on the association between neonicotinioids with insulin and glucose homeostasis parameters in a cross-sectional national survey. We found that detectability of imidacloprid and 5-hydroxy-imidacloprid were associated with lower insulin and HOMA-IR levels in adults. Individuals with detectable 5-hydroxyimidacloprid had lower odds of having insulin resistance compared to those with non-detectable concentrations. However, no relationship was noted between any of the neonicotinoid compounds or metabolites with glucose or HbA1c levels. Effect measure modification by sex was noted between N-desmethyl-acetamiprid with glucose and between 5-hydroxy-imidacloprid with HbA1c, with suggestive positive associations in males. We noted that the association between 5-hydroxy-imidacloprid with insulin was modified by BMI status, with significant negative associations among individuals who were overweight/obese compared to null associations in those who were under/normal weight. Lastly, higher glucose levels were observed among younger individuals with detectable 5-hydroxy-imidacloprid, while lower levels were noted among older adults.
Although we did not find a relationship between neonicotinoids and glucose levels, elevated glucose has been reported after imidacloprid exposure in male and female rats, suggesting a hyperglycemic effect (Bhardwaj et al., 2010; Khalil et al., 2017). Similar to findings from the present study, Khalil et al. (2017) also reported reductions in insulin following imidacloprid exposure in rats (Khalil et al., 2017). Reductions in insulin levels may be due to a number of mechanisms. Increased inflammation and reactive oxygen species may have contributed to the observed effect as studies have found neonicotinoids increase oxidative stress (El-Gendy et al., 2010; Kapoor et al., 2010). While oxidative stress is necessary for pancreatic β cell neogenesis and proliferation, levels that are beyond the optimal range are not conducive for proper function. Oxidative stress induced by imidacloprid could contribute to our findings as oxidative stress stimulates serine kinase of p38 mitogen-activated protein kinase, which reduces insulin signaling (Henriksen et al., 2011). Oxidative stress may also impair insulin signaling through disturbances to the cellular redistribution of signaling components and altering mitochondrial activity (Bloch-Damti and Bashan, 2005; Morino et al., 2006). Further, Khalil et al. (2017) observed atrophy of pancreatic β cells in the histopathological examination, impaired pancreatic islets, and subsequent insulin signaling dysfunction following imidacloprid exposure. Lastly, down-regulation of the muscarinic receptors of pancreatic β cells can contribute to the reduced production of insulin (van Koppen and Kaiser, 2003).
However, our findings of an inverse association between detectability of imidacloprid and its metabolite with insulin levels were not consistent with other toxicological studies (Ndonwi et al., 2020; Sun et al., 2017; Sun et al., 2016). In an experimental study of pregnant nulliparous Wistar rats, imidacloprid exposure significantly increased fasting glucose, insulin, and insulin resistance (HOMA-IR) in both mothers and offspring, with insulin resistance persisting until adulthood for offspring even after cessation of exposure (Ndonwi et al., 2020). Male mice dosed with imidacloprid, specifically 6 mg/kg bw/day, had significantly higher blood insulin levels compared to the control group (Sun et al., 2016). Similar conclusions were noted in female mice fed a diet containing imidacloprid for 12 weeks (Sun et al., 2017). Findings from these toxicological studies could be due to imidacloprid’s impairment of GLUT4 activation, a downstream target of protein kinase B (AKT) that is considered to be a major insulin-regulated glucose transporter (Kim et al., 2013). Depletion of GLUT4 expression is a key contributor of insulin resistance (Garvey et al., 1998). Imidacloprid may play a role in the development of insulin resistance by impairing IRS-1 through calcium-dependent mechanisms (Kim et al., 2013). In addition, chronic exposure to imidacloprid in Wister rats increased pro-inflammatory cytokines, including interleukin-1β, interleukin-6, interferon gamma, and tumor necrosis factor-α (TNF- α) in the liver and brain (Duzguner and Erdogan, 2012). TNF-α may contribute to the pathology of insulin resistance by inhibiting insulin signaling through the activation of protein tyrosine phosphatase and promoting phosphorylation of IRS-1 at serine 307, which negatively regulates insulin signaling. The contrasting findings between our study and other toxicological studies regarding the relationship between imidacloprid and its metabolite with insulin may be due to the differing exposure doses, varying timeframes from exposure to the measurement of insulin and glucose homeostasis parameters, and in the endocrine milieu that make it difficult to fully extrapolate the results from animal models to humans.
We found that the relationship between detectability of neonicotinoids with glucose and HbA1c varied by sex. Specifically, N-desmethyl-acetamiprid and 5-hydroxy-imidacloprid were associated with increased glucose and HbA1c in males and lower levels in females, albeit stratified associations were not statistically significant. The source and implications for this finding of sex differences remains elusive. The varying directionality of the association between neonicotinoid metabolites with glucose and HbA1c by sex may be due to the differences in the metabolism of these contaminants in males and females. Males metabolize imidacloprid more efficiently than females (EMEA, 2009), which has been suggested to increase reactive oxygen species (ROS) production and contribute to oxidative stress (Yardimci et al., 2014). In a study investigating the oxidative potential of imidacloprid in rats, Yardimici et al. (2014) observed higher oxidative toxicity in the kidney of male rats compared to female rats. Oxidative stress is hypothesized as a pathogenic factor that ultimately results in the development of T2DM via β-cell dysfunction, insulin resistance, and impaired glucose tolerance (Ceriello and Motz, 2004). The inverse associations between detectable neonicotinoid metabolites with glucose and HbA1c among females could also be attributed to differences in sex hormones. There is some indication that females may be more resistant to diabetogenic actions due to estrogens serving as a protective factor (Gupte et al., 2015; Louet et al., 2004). Imidacloprid has been found to inhibit the gene expression of cyp19 in male lizards (Yang et al., 2020), which is involved in estrogen biosynthesis (Simpson et al., 1994). Mitochondrial function may also play a role in the observed sex differences in metabolic regulation and diabetes susceptibility (Tramunt et al., 2020). Emerging evidence indicates mitochondrial dysfunction is associated with T2DM, though the exact pathogenic mechanism is uncertain (Patti and Corvera, 2010). Neonicotinoids have been shown to disrupt mitochondrial function in bumblebees and cotton bollworms (Moffat et al., 2015; Nareshkumar et al., 2018).
We noted significant multiplicative interaction between BMI status and detectability of 5-hydroxy-imidacloprid in the association with insulin and HOMA-IR. In particular, there were decreases in insulin and HOMA-IR among those who were overweight/obese with detectable 5-hydroxy-imidacloprid, while no relationship was observed among those who were under/normal weight. Neonicotinoids may play a role in obesity development by altering thyroid hormone homeostasis, increasing oxidative stress, and inducing lipid accumulation (Mesnage et al., 2018; Park et al., 2013; Wang et al., 2018; Wang et al., 2020; Yan et al., 2020). However, toxicological studies investigating neonicotinoids as potential obesogens have reported mixed results. While a number of animal models have concluded neonicotinoid exposure results in substantial weight gain, (Lukowicz et al., 2018; Sun et al., 2017; Sun et al., 2016; Tanaka, 2012), others have observed decreased weight gain (Badgujar et al., 2013; Bhardwaj et al., 2010; Burke et al., 2018; Mosbah et al., 2018; Terayama et al., 2016; Terayama et al., 2018). Our findings are suggestive that individuals who may have a higher adiposity are more susceptible to perturbations by neonicotinoids with regard to insulin homeostasis parameters. However, it is unclear whether the perturbations are unidirectionally inverse. Given that we were only able to examine neonicotinoids as binary, we could not explore if there were non-monotonic dose response relationships between neonicotinoid concentrations and any of the glucose and insulin homeostasis parameters. Lastly, our results indicate that age modifies the association between detectable 5-hydroxy-imidacloprid and glucose levels. There were positive associations among those ages 20–45 years and negative associations among those ≥46 years, albeit age stratified associations were not statistically significant. It is unclear why younger individuals may experience increased glucose levels and worse glycemic control with detectable 5-hydroxyimidacloprid. Several sensitive developmental windows have been identified for perturbations by endocrine disrupting chemicals to the metabolic milieu, including preconception, gestation, and puberty (Sargis and Simmons, 2019). It remains to be determined whether there are additional periods of susceptibility during adulthood in which environmental exposures may have a greater impact on disease risk. Our findings support the theory that early to mid-adulthood could potentially be a time period that insults from environmental chemicals may play a bigger role in the pathogenesis of diabetes.
Our study has several strengths. We used a large, nationally representative sample of the general population in the US, increasing the generalizability of our findings. Second, we took into account a number of important confounders, including sociodemographics, dietary intake, physical activity, and family history of T2DM. We further explored whether effect modification by sex, BMI status, and age was present in the associations between neonicotinoids and insulin and glucose homeostasis parameters.
Despite these strengths, our findings should be interpreted with caution given the study limitations. First, the NHANES is cross-sectional in nature, which precludes us from establishing temporality. Second, exposure misclassification is also a concern as we relied on a single urine sample to assess neonicotinoid concentrations. While the half-life of neonicotinoid compounds is not well established in humans, findings from animal models suggest that neonicotinoids have short half-lives (Ford and Casida, 2006a; Ford and Casida, 2006b). Pharmacokinetic models of neonicotinoids in urine samples of healthy Japanese adults reported half-lives of approximately 14 hours, 40 hours, and 35 hours for clothianidin, desmethyl-acetamiprid, and imidacloprid, respectively (Harada et al., 2016). It is estimated that 2.6%, 63.7%, and 12.7% of acetamiprid, clothianidin, and imidacloprid, respectively, were excreted unchanged within 96 hours (Harada et al., 2016). In addition, a study examining the temporal variability of urinary neonicotinoids and their metabolites for 44 consecutive days observed poor to moderate reproducibility for spot urine samples of clothiandin, imidacloprid, and N-desmethyl-acetamiprid, with intraclass correlation coefficients [ICCs] of 0.08, 0.36, and 0.42, respectively (Li et al., 2020). Therefore, it is possible that exposure misclassification may be present since the timing of exposure quantification may not be representative of true exposure concentrations. However, any exposure misclassification is likely be nondifferential and would likely bias our estimates toward the null. Third, we defined exposure as binary based on detectability due to the low percent detection frequencies of the quantified neonicotinoids and their metabolites. This prohibited us from exploring any type of dose response relationship between neonicotinoids with insulin and glucose homeostasis parameters. Fourth, reverse causality must be considered as individuals with metabolic disease risk may alter dietary intake, which could contribute to neonicotinoid exposure if their consumption of fruits and vegetables increased. Our analyses did not adjust for consumption of fruits and vegetables. Lastly, a number of pesticides, including chlorpyrifos, organophosphates, organochlorines, and pyrethroids, have been identified as chemicals associated with prediabetes as well as diabetes (Hansen et al., 2014; Juntarawijit and Juntarawijit, 2018; Montgomery et al., 2008; Starling et al., 2014; Tang et al., 2014). We did not take into consideration potential synergistic or antagonistic effects of these compounds on the relationship between neonicotinoids with insulin and glucose homeostasis parameters.
5. Conclusion
The present study is the first to investigate whether neonicotinoids are associated with insulin and glucose homeostasis parameters in humans. Results suggest a potential relationship between neonicotinoids and alteration of insulin levels. Although there were significant negative associations reported in the current study, it is possible that the observed decreased in insulin resistance may be due to the method in which we examined neonicotinoids, which did not allow us to further examine for potential non-monotonic dose response relationships. We were also limited to one urinary measure of neonicotinoids, which may not accurately reflect the general body burden of these contaminants. We encourage future epidemiological studies to investigate the relationship between neonicotinoids with insulin and glucose homeostasis parameters using a prospective study design with repeated measurements of neonicotinoids across various populations, particularly in geographical areas where detectability of neonicotinoids is higher, such as China and Japan, where detectability has been reported to be over 80% for several neonicotinoid parent and metabolite compounds (Song et al., 2020; Ueyama et al., 2015; Ueyama et al., 2014; Zhang et al., 2019). We also recommend epidemiological studies examine potentially susceptible populations with high exposure to neonicotinoids, particularly individuals involved with farming and agriculture (Lopez-Galvez et al., 2020; Suwannarin et al., 2020; Tao et al., 2019).
Supplementary Material
Highlights.
We examined detectable neonicotinoids with insulin and glucose homeostasis parameters
Detectable imidacloprid and 5-hydroxy-imidacloprid were associated with lower insulin
Sex modified the relationship between N-desmethyl-acetamiprid with glucose and 5-hydroxy-imidacloprid with HbA1c
5-hydroxy-imidacloprid was inversely associated with insulin among overweight/obese individuals
Funding:
This work was supported by the National Institute on Minority Health and Health Disparities (L32 MD015437).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: Disclaimer:
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The authors declare no competing financial interest.
References
- Abdel-Ghany MF, Hussein LA, El Azab NF, El-Khatib AH, Linscheid MW. Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography-tandem mass spectrometry coupled with QuEChERS. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1031: 15–28. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Dubetz C, Palace VP. Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 2015; 505: 409–22. [DOI] [PubMed] [Google Scholar]
- Badgujar PC, Jain SK, Singh A, Punia JS, Gupta RP, Chandratre GA. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ Toxicol Pharmacol 2013; 35: 408–18. [DOI] [PubMed] [Google Scholar]
- Baker SE, Serafim AB, Morales-Agudelo P, Vidal M, Calafat AM, Ospina M. Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 2019; 411: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Denholm I, Williamson MS, Nauen R. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 2015; 121: 78–87. [DOI] [PubMed] [Google Scholar]
- Berheim EH, Jenks JA, Lundgren JG, Michel ES, Grove D, Jensen WF. Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer. Sci Rep 2019; 9: 4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S, Srivastava MK, Kapoor U, Srivastava LP. A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem Toxicol 2010; 48: 1185–90. [DOI] [PubMed] [Google Scholar]
- Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 2005; 7: 1553–67. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Journey CA, Romanok KM, Barber LB, Buxton HT, Foreman WT, et al. Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams. Environ Sci Technol 2017; 51: 4792–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Niibori Y, Terayama H, Ito M, Pidgeon C, Arsenault J, et al. Mammalian Susceptibility to a Neonicotinoid Insecticide after Fetal and Early Postnatal Exposure. Sci Rep 2018; 8: 16639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004; 24: 816–23. [DOI] [PubMed] [Google Scholar]
- Chen M, Tao L, McLean J, Lu CS. Quantitative Analysis of Neonicotinoid Insecticide Residues in Foods: Implication for Dietary Exposures. Journal of Agricultural and Food Chemistry 2014; 62: 6082–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino AM, Boyles AL, Thayer KA, Perry MJ. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ Health Perspect 2017; 125: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock HA, Huang D, Turner PC, Quiros-Alcala L, Payne-Sturges DC. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ Health 2019; 18: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzguner V, Erdogan S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pestic Biochem Physiol 2012; 104: 58–64. [Google Scholar]
- El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 2010; 48: 215–21. [DOI] [PubMed] [Google Scholar]
- EMEA. European Medicines Agency. Scientific Discussion, CVMP/0297/03, 2009.
- Ford KA, Casida JE. Chloropyridinyl neonicotinoid insecticides: diverse molecular substituents contribute to facile metabolism in mice. Chem Res Toxicol 2006a; 19: 944–51. [DOI] [PubMed] [Google Scholar]
- Ford KA, Casida JE. Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. Chem Res Toxicol 2006b; 19: 1549–56. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest 1998; 101: 2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 2013; 50: 977–987. [Google Scholar]
- Gu YH, Li Y, Huang XF, Zheng JF, Yang J, Diao H, et al. Reproductive effects of two neonicotinoid insecticides on mouse sperm function and early embryonic development in vitro. PLoS One 2013; 8: e70112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res 2015; 2015: 916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tian Y, Shen X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018; 192: 59–65. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Jors E, Lander F, Condarco G, Schlunssen V. Is cumulated pyrethroid exposure associated with prediabetes? A cross-sectional study. J Agromedicine 2014; 19: 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada KH, Tanaka K, Sakamoto H, Imanaka M, Niisoe T, Hitomi T, et al. Biological Monitoring of Human Exposure to Neonicotinoids Using Urine Samples, and Neonicotinoid Excretion Kinetics. PLoS One 2016; 11: e0146335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 2011; 51: 993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Yanai S, Omotehara T, Hashimoto R, Umemura Y, Kubota N, et al. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J Vet Med Sci 2015; 77: 1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ Health Prev Med 2018; 23: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata R, Nanayakkara S, Senevirathna S, Harada KH, Chandrajith R, Hitomi T, et al. Neonicotinoid concentrations in urine from chronic kidney disease patients in the North Central Region of Sri Lanka. J Occup Health 2016; 58: 128–33. [DOI] [PubMed] [Google Scholar]
- Kapoor U, Srivastava MK, Bhardwaj S, Srivastava LP. Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL). J Toxicol Sci 2010; 35: 577–81. [DOI] [PubMed] [Google Scholar]
- Khalil SR, Awad A, Mohammed HH, Nassan MA. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ Toxicol Pharmacol 2017; 55: 165–174. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Yoon KS, Clark JM, Park Y. Imidacloprid, a neonicotinoid insecticide, induces insulin resistance. J Toxicol Sci 2013; 38: 655–60. [DOI] [PubMed] [Google Scholar]
- Li AJ, Martinez-Moral MP, Kannan K. Variability in urinary neonicotinoid concentrations in single-spot and first-morning void and its association with oxidative stress markers. Environ Int 2020; 135: 105415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Galvez N, Wagoner R, Canales RA, de Zapien J, Calafat AM, Ospina M, et al. Evaluating imidacloprid exposure among grape field male workers using biological and environmental assessment tools: An exploratory study. Int J Hyg Environ Health 2020; 230: 113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia M, Romero-Gonzalez R, Lacasana M, Garrido Frenich A. Semiautomated determination of neonicotinoids and characteristic metabolite in urine samples using TurboFlow coupled to ultra high performance liquid chromatography coupled to Orbitrap analyzer. J Pharm Biomed Anal 2017; 146: 378–386. [DOI] [PubMed] [Google Scholar]
- Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 2004; 6: 180–5. [DOI] [PubMed] [Google Scholar]
- Lukowicz C, Ellero-Simatos S, Regnier M, Polizzi A, Lasserre F, Montagner A, et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ Health Perspect 2018; 126: 067007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AM, Jardine CM, Thomas PJ, Nemeth NM. Neonicotinoid detection in wild turkeys (Meleagris gallopavo silvestris) in Ontario, Canada. Environ Sci Pollut Res Int 2018; 25: 16254–16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9. [DOI] [PubMed] [Google Scholar]
- Mesnage R, Biserni M, Genkova D, Wesolowski L, Antoniou MN. Evaluation of neonicotinoid insecticides for oestrogenic, thyroidogenic and adipogenic activity reveals imidacloprid causes lipid accumulation. J Appl Toxicol 2018; 38: 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat C, Pacheco JG, Sharp S, Samson AJ, Bollan KA, Huang J, et al. Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). FASEB J 2015; 29: 2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. Am J Epidemiol 2008; 167: 1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Leon JM, Duy SV, Munoz G, Amyot M, Sauve S. Evaluation of on-line concentration coupled to liquid chromatography tandem mass spectrometry for the quantification of neonicotinoids and fipronil in surface water and tap water. Anal Bioanal Chem 2018; 410: 2765–2779. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006; 55 Suppl 2: S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbah R, Djerrou Z, Mantovani A. Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug Chem Toxicol 2018; 41: 206–212. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008; 294: E15–26. [DOI] [PubMed] [Google Scholar]
- Nareshkumar B, Akbar SM, Sharma HC, Jayalakshmi SK, Sreeramulu K. Imidacloprid impedes mitochondrial function and induces oxidative stress in cotton bollworm, Helicoverpa armigera larvae (Hubner: Noctuidae). J Bioenerg Biomembr 2018; 50: 21–32. [DOI] [PubMed] [Google Scholar]
- Ndonwi EN, Atogho-Tiedeu B, Lontchi-Yimagou E, Shinkafi TS, Nanfa D, Balti EV, et al. Metabolic effects of exposure to pesticides during gestation in female Wistar rats and their offspring: a risk factor for diabetes? Toxicol Res 2020; 36: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, et al. Exposure characterization of three major insecticide lines in urine of young children in Japan-neonicotinoids, organophosphates, and pyrethroids. Environ Res 2016; 147: 89–96. [DOI] [PubMed] [Google Scholar]
- Ospina M, Wong LY, Baker SE, Serafim AB, Morales-Agudelo P, Calafat AM. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ Res 2019; 176: 108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim Y, Kim J, Yoon KS, Clark J, Lee J, et al. Imidacloprid, a neonicotinoid insecticide, potentiates adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem 2013; 61: 255–9. [DOI] [PubMed] [Google Scholar]
- Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 2010; 31: 364–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaria AM, Sutton R, Moran KD, Teerlink J, Brown JV, Halden RU. Passage of fiproles and imidacloprid from urban pest control uses through wastewater treatment plants in northern California, USA. Environ Toxicol Chem 2017; 36: 1473–1482. [DOI] [PubMed] [Google Scholar]
- Salis S, Testa C, Roncada P, Armorini S, Rubattu N, Ferrari A, et al. Occurrence of imidacloprid, carbendazim, and other biocides in Italian house dust: Potential relevance for intakes in children and pets. J Environ Sci Health B 2017; 52: 699–709. [DOI] [PubMed] [Google Scholar]
- Sargis RM, Simmons RA. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 2019; 62: 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services UDoHaH. Physical activity guidelines for Americans. US Department of Health and Human Services, Washington, DC, 2018. [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 1994; 15: 342–55. [DOI] [PubMed] [Google Scholar]
- Song S, Zhang T, Huang Y, Zhang B, Guo Y, He Y, et al. Urinary Metabolites of Neonicotinoid Insecticides: Levels and Recommendations for Future Biomonitoring Studies in China. Environ Sci Technol 2020; 54: 8210–8220. [DOI] [PubMed] [Google Scholar]
- Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med 2014; 71: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana T, Murray C, Kleywegt S, Metcalfe CD. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018; 202: 506–513. [DOI] [PubMed] [Google Scholar]
- Sun Q, Qi W, Xiao X, Yang SH, Kim D, Yoon KS, et al. Imidacloprid Promotes High Fat DietInduced Adiposity in Female C57BL/6J Mice and Enhances Adipogenesis in 3T3-L1 Adipocytes via the AMPKalpha-Mediated Pathway. J Agric Food Chem 2017; 65: 65726581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, et al. Imidacloprid Promotes High Fat DietInduced Adiposity and Insulin Resistance in Male C57BL/6J Mice. J Agric Food Chem 2016; 64: 9293–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannarin N, Prapamontol T, Isobe T, Nishihama Y, Nakayama SF. Characteristics of Exposure of Reproductive-Age Farmworkers in Chiang Mai Province, Thailand, to Organophosphate and Neonicotinoid Insecticides: A Pilot Study. Int J Environ Res Public Health 2020; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Res B Dev Reprod Toxicol 2012; 95: 151–9. [DOI] [PubMed] [Google Scholar]
- Tang M, Chen K, Yang F, Liu W. Exposure to organochlorine pollutants and type 2 diabetes: a systematic review and meta-analysis. PLoS One 2014; 9: e85556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Dong F, Xu J, Phung D, Liu Q, Li R, et al. Characteristics of neonicotinoid imidacloprid in urine following exposure of humans to orchards in China. Environ Int 2019; 132: 105079. [DOI] [PubMed] [Google Scholar]
- Terayama H, Endo H, Tsukamoto H, Matsumoto K, Umezu M, Kanazawa T, et al. Acetamiprid Accumulates in Different Amounts in Murine Brain Regions. Int J Environ Res Public Health 2016; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama H, Qu N, Endo H, Ito M, Tsukamoto H, Umemoto K, et al. Effect of acetamiprid on the immature murine testes. Int J Environ Health Res 2018; 28: 683–696. [DOI] [PubMed] [Google Scholar]
- Toor HK, Sangha GK, Khera KS. Imidacloprid induced histological and biochemical alterations in liver of female albino rats. Pestic Biochem Physiol 2013; 105: 1–4. [DOI] [PubMed] [Google Scholar]
- Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020; 63: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama J, Harada KH, Koizumi A, Sugiura Y, Kondo T, Saito I, et al. Temporal Levels of Urinary Neonicotinoid and Dialkylphosphate Concentrations in Japanese Women Between 1994 and 2011. Environ Sci Technol 2015; 49: 14522–8. [DOI] [PubMed] [Google Scholar]
- Ueyama J, Nomura H, Kondo T, Saito I, Ito Y, Osaka A, et al. Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults. J Occup Health 2014; 56: 461–8. [DOI] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther 2003; 98: 197–220. [DOI] [PubMed] [Google Scholar]
- Wang A, Mahai G, Wan Y, Jiang Y, Meng Q, Xia W, et al. Neonicotinoids and carbendazim in indoor dust from three cities in China: Spatial and temporal variations. Sci Total Environ 2019; 695: 133790. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu T, Liu F, Zhang J, Wu Y, Sun H. Occurrence and Profile Characteristics of the Pesticide Imidacloprid, Preservative Parabens, and Their Metabolites in Human Urine from Rural and Urban China. Environ Sci Technol 2015; 49: 14633–40. [DOI] [PubMed] [Google Scholar]
- Wang X, Anadon A, Wu Q, Qiao F, Ares I, Martinez-Larranaga MR, et al. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu Rev Pharmacol Toxicol 2018; 58: 471–507. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu P, Chang J, Li W, Yang L, Tian H. Unraveling the toxic effects of neonicotinoid insecticides on the thyroid endocrine system of lizards. Environ Pollut 2020; 258: 113731. [DOI] [PubMed] [Google Scholar]
- WHO. WHO STEPS Surveillance Manual: the WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance. World Health Organization, Geneva, Switzerland, 2005. [Google Scholar]
- Yamamoto A, Terao T, Hisatomi H, Kawasaki H, Arakawa R. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionisation. J Environ Monit 2012; 14: 2189–94. [DOI] [PubMed] [Google Scholar]
- Yan S, Wang D, Teng M, Meng Z, Yan J, Li R, et al. Perinatal exposure to 2-Ethylhexyl Diphenyl Phosphate (EHDPHP) affected the metabolic homeostasis of male mouse offspring: Unexpected findings help to explain dose- and diet- specific phenomena. J Hazard Mater 2020; 388: 122034. [DOI] [PubMed] [Google Scholar]
- Yang L, Shen Q, Zeng T, Li J, Li W, Wang Y. Enrichment of imidacloprid and its metabolites in lizards and its toxic effects on gonads. Environ Pollut 2020; 258: 113748. [DOI] [PubMed] [Google Scholar]
- Yardimci M, Sevgiler Y, Rencuzogullari E, Arslan M, Buyukleyla M, Yilmaz M. Sex-, tissue-, and exposure duration-dependent effects of imidacloprid modulated by piperonyl butoxide and menadione in rats. Part I: oxidative and neurotoxic potentials. Arh Hig Rada Toksikol 2014; 65: 387–98. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang X, Li Z, Jin H, Lu Z, Yu C, et al. Simultaneous determination of nine neonicotinoids in human urine using isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Environ Pollut 2018; 240: 647–652. [DOI] [PubMed] [Google Scholar]
- Zhang T, Song S, Bai X, He Y, Zhang B, Gui M, et al. A nationwide survey of urinary concentrations of neonicotinoid insecticides in China. Environ Int 2019; 132: 105114. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Guo J, Wang Z, Zhang B, Sun Z, Yun X, et al. Levels and inhalation health risk of neonicotinoid insecticides in fine particulate matter (PM2.5) in urban and rural areas of China. Environ Int 2020; 142: 105822. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu X, Fu X, Yu B, Wang D, Zhao C, et al. Development of a fast and sensitive method for measuring multiple neonicotinoid insecticide residues in soil and the application in parks and residential areas. Anal Chim Acta 2018; 1016: 19–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


