Abstract
The multi-step process to obtain an isotretinoin prescription under the iPLEDGE program is challenging for patients, particularly females, hence this retrospective study evaluates the differences in treatment and costs between males and females. While males had a higher total cost of treatment than females, females had a higher treatment cost when medication costs were excluded. Females who missed prescription windows had a longer treatment course and incurred significantly higher treatment costs than females who did not miss a prescription window. The iPLEDGE program places female patients at a disadvantage of incurring higher treatment costs as a consequence of the prescription window requirement.
Keywords: Isotretinoin, iPLEDGE, acne, financial burden, missed prescription window, cost analysis
Isotretinoin prescriptions are regulated by the iPLEDGE program in which female patients, in particular, are held to a strict 7-day window from the date of their pregnancy test during which they must see their provider, complete an iPLEDGE questionnaire, and retrieve isotretinoin from an iPLEDGE-participating pharmacy.1 Failure to complete tasks within the window results in being locked out of iPLEDGE, requiring a repeat pregnancy test to resume eligibility. This study evaluates the costs associated with isotretinoin treatment and the financial consequences of iPLEDGE requirements.
The cost of treatment for patients who received isotretinoin from 2012–2019 at Children’s National Hospital was calculated using costs from Centers for Medicare & Medicaid Services for the appropriate treatment year. Isotretinoin costs were standardized by using data from National Average Drug Acquisition for isotretinoin (Claravis™) 20mg to limit variations in pricing of different brands. Treatment courses were compared by sex and subgroup of female participants with and without missed windows using student t-test continuous variables.
The study included 173 patients (22% White, 22% Black, 38% Hispanic/Latino, mean age 16 years); 36% were female, of which 63% missed at least one window during treatment (Table 1). While there was no significant difference in treatment length between males and females, females who missed windows had a significantly longer treatment than females who did not miss windows (7.67±2.52 vs. 5.63±2.52 months, p=0.001) (Table 2). No significant difference in isotretinoin cumulative dose was observed between males and females or between the female subgroups. The average cost of isotretinoin treatment was $3246.53±1614.49 and $2736.91±1437.01 for males and females, respectively (p=0.029) (Figure 1). Females had a higher treatment cost than males when isotretinoin drug cost was excluded ($671.01±210.69 vs $533.23±281.39, p<0.001). Females who missed windows had a significantly higher treatment cost than females who did not miss windows ($3082.50±1466.70 vs $2138.79±1186.95; p=0.007) and the difference persisted even when isotretinoin drug cost was excluded (p<0.001).
Table 1:
Patient demographics.
| Characteristic | Total | Male | Female |
|---|---|---|---|
| N=173 | N=110 | N = 63 | |
| Age, mean (SD) | 15.97± 1.78 | 15.99± 1.71 | 15.92± 1.91 |
| Race, N (%) | |||
| White | 38 (21.97) | 25 (22.73) | 13 (20.63) |
| African American | 38 (21.97) | 23 (20.91) | 15 (23.81) |
| Other/Unknown | 97 (56.07) | 62 (56.36) | 35 (55.56) |
| Ethnicity, N (%) | |||
| Hispanic/Latino | 65 (37.57) | 40 (36.36) | 25 (39.68) |
| Not Hispanic/Latino | 104 (60.12) | 67 (60.91) | 37 (58.73) |
| Unknown | 4 (2.31) | 3 (2.73) | 1 (1.59) |
| Insurance Type, N (%) | |||
| Medicaid | 126 (72.83) | 80 (72.73) | 46 (73.02) |
| Private | 47 (27.17) | 30 (27.27) | 17 (26.98) |
Table 2:
Comparison of treatment measures stratified by a) Sex and b) Female treatment window designations among iPLEDGE participants, 2012–2019.
| Sex | Female Prescription Window Subgroups | |||||
|---|---|---|---|---|---|---|
| Male | Female | P-Value | No Missed Prescription Window | Missed Prescription Window | P-Value | |
| N=123 | N=71 | N=26 | N=45 | |||
| Mean± SD | Mean± SD | Mean± SD | Mean± SD | |||
| Treatment Length, Months | 6.90± 3.36 | 6.92± 2.69 | 0.963 | 5.63± 2.52 | 7.67± 2.52 | 0.002 |
| Cumulative Dose (mg/kg) | 144.91± 74.97 | 137.33± 74.76 | 0.498 | 122.75± 79.68 | 145.75± 71.31 | 0.214 |
| Mean Number of Pregnancy Tests | N/A | 7.44± 3.18 | N/A | 5.31± 2.45 | 8.67± 2.91 | <.001 |
| Mean Cost of Treatment, US Dollars | 3246.53± 1614.49 | 2736.91± 1437.01 | 0.029 | 2138.79± 1186.95 | 3082.50± 1466.70 | 0.007 |
| Mean Cost of Treatment Excluding Isotretinoin, US Dollars | 533.23± 210.69 | 671.04± 281.39 | 0.005 | 502.53± 268.62 | 768.40±241.87 | <.001 |
Notes: Relapse treatment observations were separated and treated as individual treatments. Treatment observations were excluded if cumulative dose did not exceed 0 mg/kg. Comparisons were made for continuous variables using student t-tests and reported with means and standard deviations. Results with α<.05 were considered statistically significant. Statistical analysis was completed using SAS 9.4.
Figure 1: Breakdown for average cost of treatment stratified by a) sex and b) female treatment window designations among iPLEDGE participants, 2012–2019.
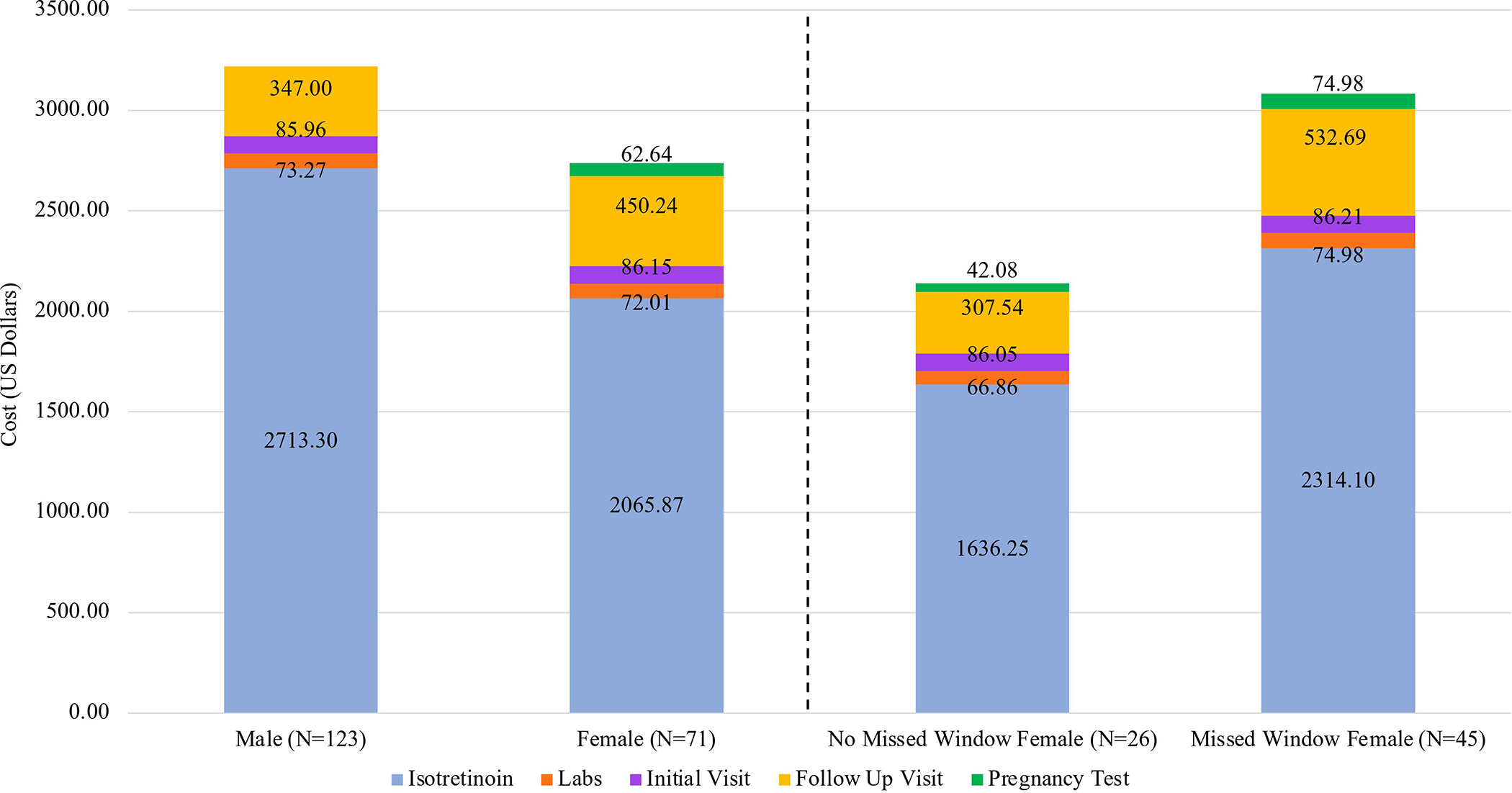
Notes: Cost of isotretinoin was standardized by using applying market cost of Claravis™ 20mg. Initial visit costs were determined using ICD code 99203, and follow-up visit costs were determined using ICD codes 99213 and 99214. Lab costs included AST, ALT, alkaline phosphatase, bilirubin, triglycerides, and cholesterol; laboratory testing was conducted at baseline and at two months of treatment. Pregnancy tests were only attributable to female participants.
Our analysis highlights the financial consequences of the 7-day window and monthly follow-ups mandated by iPLEDGE, as females incurred higher costs than males when isotretinoin, a weight-dependent factor, was excluded and as females who missed windows incurred significantly higher expenses due to additional follow-up visits and repeat pregnancy tests. Importantly, interrupted and prolonged treatment courses for females also increase the duration of exposure to the known teratogen, counter to the aims of iPLEDGE.
Multiple studies have demonstrated that acne may influence the quality of life of females more than males, underscoring the necessity of equal and uninterrupted access to systemic acne treatment.2 Previous research has shown that females and Black patients are undertreated for acne and less likely to be prescribed systemic treatments such as isotretinoin, placing them at a higher risk of long-term dermatologic and psychologic acne-related morbidities.2 Further, when treated with isotretinoin, Black females and females from underserved communities are more likely to miss windows and terminate treatment early; hence, these financial consequences of iPLEDGE requirements, specifically the 7-day window period, may be putting such populations at a disadvantage of treatment completion.3
Individualizing follow-up and pregnancy testing frequency to represent the patient’s reproductive and contraception status, such that patients on long-term acting contraception or patients without childbearing potential can follow-up less frequently, may help to reduce the financial expense.4 The persistence of pregnancies in isotretinoin users, sociodemographic differences in isotretinoin treatment, and the financial consequences of the 7-day window period questions the effectiveness of the iPLEDGE program requirements.3,4 We recommend that future studies evaluate the utility of the 7-day window period in preventing pregnancies in isotretinoin users. The findings in this study underestimate the true expenses because cost of contraception, transportation, and lost wages were excluded from our data. Additionally, approximately three hours of school and $31-$41 in indirect costs were lost every month due to follow-up visits. Utilization of teledermatology may have the potential to decrease both indirect and direct treatment costs by reducing treatment barriers.5 These results are limited by a small sample size and location-specific visit costs. Our study demonstrates the financial consequences of iPLEDGE, providing an opportunity to reconsider the structure of iPLEDGE to reduce the expenses on patients and the healthcare system.
Funding sources:
This project was supported by Award Number UL1TR001876 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflicts of Interest: None declared.
IRB status: Approved by the Children’s National Hospital Institutional Review Board.
References
- 1.Charrow A, Di Xia F, Lu J, Waul M, Joyce C, Mostaghimi A. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PLoS One. 2019;14(3):1–8. doi: 10.1371/journal.pone.0210445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri JS, Shin DB, Wang S, Margolis DJ, Takeshita J. Association of Race/Ethnicity and Sex with Differences in Health Care Use and Treatment for Acne. JAMA Dermatology. 2020;156(3):312–319. doi: 10.1001/jamadermatol.2019.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, Smith E, Kirkorian AY. Evaluating the Barriers to Isotretinoin Treatment for Acne Vulgaris in Pediatric Patients. J Am Acad Dermatol. Published online November 28, 2020. doi: 10.1016/j.jaad.2020.11.055 [DOI] [PubMed] [Google Scholar]
- 4.Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, Patient Safety, and Patient-Centered Care-Time to Reform iPLEDGE. JAMA Dermatol. 2020;156(1):21–22. doi: 10.1001/jamadermatol.2019.3270 [DOI] [PubMed] [Google Scholar]
- 5.Mori WS, Houston N, Moreau JF, et al. Personal Burden of Isotretinoin Therapy and Willingness to Pay for Electronic Follow-up Visits. JAMA Dermatol. 2016;152(3):338. doi: 10.1001/jamadermatol.2015.4763 [DOI] [PubMed] [Google Scholar]


