Abstract
Increasing evidences supported that subjective cognitive decline (SCD) might be a potential first symptomatic manifestation of Alzheimer’s disease (AD). The rapidly growing number of SCD individuals who seek medical help and advice also makes it urgent to develop more precise strategy for SCD. Therefore, this study aimed to explore the risk factors for SCD. Logistics and linear regression models were performed to investigate 41 factors for SCD in 1165 participants without objective cognitive impairment. Cochran-Armitage trend test was used to confirm the constant trend toward higher prevalence of SCD with an increasing number of risk factors. A high overall prevalence of SCD was found in total participants (42%). Eight factors were eventually identified as risk factors for SCD, including four stable factors associated with both SCD statues and severity (older age, thyroid diseases, minimal anxiety symptoms, and day time dysfunction; odds ratio (OR) ranging from 1.74 to 2.29) as well as four suggestive factors associated with either SCD statues or severity (female sex, anemia, lack of physical exercises, and living alone; OR ranging from 1.30 to 2.29). The prevalence of SCD gradually increased with the number of risk factors clustering increased in individuals (p for trend <0.001). Five of these eight factors were further proved among individuals with SCD-plus features. These findings revealed several risk factors for SCD, providing some new clues for formulating priority strategies for early prevention of SCD.
Subject terms: Pathogenesis, Predictive markers
Introduction
As the most common form of dementia, Alzheimer’s disease (AD) has become a priority worldwide in terms of both public health and social care [1]. It has been revealed that AD-related pathophysiologies begin a decade or more before the onset of objective cognitive impairment that can be measured with standardized neuropsychological scales [1]. The failure of several previous clinical trials of therapies in the dementia or mild cognitive impairment (MCI) stages further encouraged researchers to shift their focus to the preclinical stage of AD [2, 3]. Subjective cognitive decline (SCD), a cognitive state between objective cognitive impairment and intact cognition, is receiving increasing attention as the potentially first symptomatic manifestation of AD [4]. Longitudinal studies have shown that SCD participants have a higher conversion rate and shorter conversion time to MCI and dementia than cognitively intact individuals [5, 6]. Furthermore, abnormal levels of AD-related biomarkers in cerebrospinal fluid [7], increased amyloid deposition in brain measured by positron-emission tomography (PET) [8, 9] and severer brain atrophy measured by magnetic resonance imaging (MRI) [10] were also found in SCD individuals. All the above evidence confirmed that the exploration of SCD might provide important clues for a preclinical stage closely related to dementia or AD.
It has been widely accepted that genetic and environmental risk factors work together to influence the occurrence and progression of dementia. Our previous meta-analysis showed that one third of the risk factors of AD were modifiable [11], which highlighted the feasibility and importance of early prevention. However, up to now, almost all the previous studies focused on risk factors for objective cognitive impairment [11, 12], and the risk factors for SCD still remained unclear. Since the number of SCD individuals who seek medical help and advice is rapidly growing, it is necessary to detect the risk factors for SCD. In addition, although the outstanding relevance of classical risk factors for dementia was beyond debate, these factors may not be given similar priority in SCD. Therefore, our study was designed to explore risk factors for SCD in a large sample of 1165 cognitively normal (CN) Northern Han Chinese, aiming to provide new clues to early prevention and intervention of SCD.
Methods
Participants
All analyses were performed on the data from the Chinese Alzheimer’s Biomarker and LifestylE (CABLE) study. Initiated in 2017, CABLE study is an ongoing large-scale cohort study majorly focused on AD risk factors and biomarkers in the northern Chinese Han population [13]. The exclusion criteria include: (1) central nervous system infection, head trauma, multiple sclerosis, or other major neurological disorders; (2) major psychological disorders; (3) severe systemic diseases that may affect CSF or blood levels of AD biomarkers including Aβ and tau; and (4) family history of genetic diseases. All participants underwent comprehensive clinical, neuropsychological, psychosocial, and psychiatric evaluations to determine their cognitive diagnoses in compliance with the National Institute on Aging–Alzheimer’s Association (NIA-AA) workgroup diagnostic criteria [14, 15]. The objective cognition was tested by Chinese-modified mini-mental state examination (CM-MMSE: ≤24 for >6 years of education, ≤20 for 1~6 years of education, ≤17 for 0 year of education) and Montreal Cognitive Assessment (MOCA: <24 for >12 years of education, <22 for 7~12 years of education, <19 for <7 years of education). The subjective cognition was tested by a subjective cognitive decline (SCD) scale (detailed below).
Standard protocol approvals, registrations, and patient consents
The CABLE study gained the approval of institutional review board of Qingdao Municipal Hospital. The study procedure was conducted strictly in accordance with the principles of the Declaration of Helsinki and written informed consent was obtained from all participants or their guardians.
Basic information
Basic information of participants were collected including age (<65 years or ≥65 years), sex (male or female), years of education (continuous), and lifestyle factors, including living alone, habit of drinking coffee, habit of drinking tea, lack of physical exercises, living in urban areas, smoking status, and alcohol status were collected through a dichotomy questionnaire (yes or no). Participants’ medical history (yes or no) and current medication information (yes or no) were also collected, including stroke, hypertension, diabetes mellitus, coronary disease, hyperlipoidemia, kidney diseases, cancer, anemia, thyroid diseases, use of anti-hypertension drugs, use of anti-diabetes drugs, and use of vitamins. All the information would be confirmed by available clinical information in the electronic medical record system in Qingdao Municipal Hospital.
Assessment of SCD
The questionnaire of SCD was based on SCD-I recommendations [4, 16]. Two assessment methods, classification and continuous indicators, were used to identify the subjective memory function. People were thought to have SCD status if they answered “yes” for the question “Do you think your memory is declining compared to what it used to be?”, which could not be explained by other diseases or drug abuse. A continuous SCD scale was used to reflect the severity of SCD (see e-Method). Adopting the form of Likert scale and combining with Top nine SCD items [17], it was adapted from subjective memory decline scale [18]. After the adaptation, a subject can score 0–2 points for each question and the greatest total score for 6 questions in the questionnaire is 12 points. Participants would get higher score if they had more serious SCD. At the same time, we also collected the onset time of SCD status, whether the SCD status was confirmed by an observer and whether there were subjective impairments in cognitive domains other than memory (such as difficulty with language or finding words, decreased ability of organization, decreased ability of decision-making and decreased attention).
Despite the growing interest in SCD as the putative first syndrome stage of AD, some evidence also indicated that non-AD medical problems could also underlie SCD. To select SCD individuals who had particularly high risk of objective cognitive decline and an increased likelihood for preclinical AD, a list of SCD-features (SCD-plus) was recommended [19]. Based on this recommendation, 139 participants who met at least three features were classified into a SCD-plus subgroup.
Neuropsychiatric scales and PSQI
Neuropsychiatric symptoms were tested by Hamilton anxiety scale (HAMA) and Hamilton depression scale (HAMD). Participants included in our study did not have significant anxiety (HAMA > 7) and depression (HAMD > 7). Minimal anxiety symptoms (MAS) were defined as 1≤ HAMA score <7, and minimal depression symptoms (MDS) were defined as 1≤ HAMD score <7 [20].
PSQI scale included sleep quality (bad or good), sleep latency (minutes taken from going to bed to falling asleep), sleep duration (hours), bedtime (the usual time to go to bed), sleep efficiency (the ratio of sleep duration-to-time spent in bed), sleep disorders (abnormal behaviors during sleep), sleep assistance (medication from doctors’ prescription or pharmacy to aid sleep), and day time dysfunction (the phenomenon that individuals who are too sleepy to finish daily activities during the day time). All of the above scales were evaluated by professional neurological physicians. In this study, a subset (n = 647, CN = 347, SCD = 300) with complete neuropsychiatric scales and PSQI was used to test these factors.
APOE gene and laboratory indicators of blood
The blood samples were stored in enzyme-free EP tube at –80 °C before DNA was extracted. The APOE ε4 carrier was defined as the carrier of rs7412 or rs429358 with the assistance of restriction fragment length polymorphism (RFLP) technology using QIAamp® DNA Blood Mini Kit (250).
The laboratory blood samples were collected into a blood tube containing silica by vein puncture after participants had been fasting for at least 8 h. Blood samples were tested at Clinical Chemistry Laboratory at Qingdao Municipal Hospital. The samples were centrifuged at 3000g for 10 min to obtain serum. Fasting blood glucose (FBG) levels were measured by glucose hexokinase (HK) method using Glucose Reagent (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Blood urea nitrogen (BUN) levels were measured by urease glutamic acid dehydrogenase (UV liquid) method using Urea Test Kit (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Creatinine (CR) levels were measured by sarcosine oxidase method using Creatinine Test Kit (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Uric acid (UA) levels were determined by uricase method using Uric Acid Test Kit (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Triglyceride (TG) levels were measured by glycerol phosphorus oxidase peroxidase (GPO-PAP) method using Triglycerides Test Kit (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Total cholesterol (TC) levels were measured by cholesterol esterase peroxidase (CHOD-PAP) method using Cholesterol total Test Kit (Ningbo Ruiyuan Biotechnology Co., Ltd, China). Low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were measured by homogeneous method using Creatinine Test Kit (Beckman Coulter Biotechnology (Suzhou) Co., Ltd, China).
Statistical analysis
To describe the variables, we calculated mean ± SD for continuous variables and number (prevalence) for categorical variables. Differences between the two groups were analyzed by Chi-square tests for categorical variables and Wilcoxon tests for numerical variables. False discovery rate (q value) was used to adjust for multiple comparisons.
Risk factors were determined using three models. Firstly, univariate logistic regression models (Model 1) were used to estimate the odds ratio (OR) and 95% confidence interval (CI) for the association of each factor with the risk of SCD. Then all the significant factors in univariate models (p < 0.1) were included in two multivariate models, including the multivariate logistic regression for SCD status (Model 2) and multivariate linear regression for SCD severity (Model 3). In addition, we included age, sex, years of education, and APOE ε4 status in two multivariate models as the basic covariates, no matter whether they were significant or not in Model 1. Furthermore, we conducted subgroup analyses of these risk factors according to age (midlife <65; late life ≥65) and sex (male; female). Then, the Cochran-Armitage trend test was used to confirm the constant trend toward higher prevalence of SCD with an increasing number of risk factors. Finally, we repeated the above three analyses (Model 1–3) in a post hoc analysis to explore the risk factors for SCD-plus.
The multicollinearity was assessed using variance inflation factor (VIF). No multicollinearity existed in each model of the current study. A two-tailed p < 0.05 was considered significant except where specifically noted. Analyses were carried out using R-3.6.1.
Results
Characteristics of participants
A total of 1165 participants were included from the CABLE study consisting of 672 CN controls and 493 SCD participants (Table 1). All participants were cognitively unimpaired (mean CM-MMSE score = 27.95). Female participants accounted for 58.6% and APOE ε4 carriers accounted for 15.45%. Compared with CN individuals, SCD participants were older, more likely to be living alone, having greater percent of hypertension, diabetes mellitus and thyroid diseases, and worse sleep quality (all p values < 0.05).
Table 1.
Characteristics of participants.
| Variables | CN (672) | SCD (493) | Total (1165) | p | q |
|---|---|---|---|---|---|
| Age (≥65 years) | 235 (34.97%) | 248 (50.30%) | 483 (41.46%) | <0.01 | <0.01 |
| Sex (female) | 254 (37.80%) | 227 (46.04%) | 481 (41.29%) | 0.01 | 0.02 |
| Education (years) | 10.07 ± 4.31 | 9.81 ± 4.51 | 9.96 ± 4.40 | 0.88 | 0.88 |
| CM-MMSE score | 28.07 ± 1.99 | 27.78 ± 2.21 | 27.95 ± 2.09 | 0.28 | 0.37 |
| APOE ε4 carrier | 100 (14.88%) | 80 (16.23%) | 180 (15.45%) | 0.59 | 0.66 |
| SCD severity scale | 0.15 ± 0.75 | 2.28 ± 2.14 | 1.30 ± 1.96 | <0.01 | <0.01 |
| Lifestyle | |||||
| Smoking (yes) | 212 (31.55%) | 142 (28.80%) | 354 (30.39%) | 0.31 | 0.39 |
| Alcohol (yes) | 215 (31.99%) | 137 (27.79%) | 352 (30.21%) | 0.14 | 0.23 |
| Living alone (yes) | 22 (3.27%) | 41 (8.32%) | 63 (5.41%) | <0.01 | <0.01 |
| Coffee (yes) | 81 (12.05%) | 56 (11.36%) | 137 (11.76%) | 0.79 | 0.82 |
| Tea (yes) | 421 (62.65%) | 322 (65.31%) | 743 (63.78%) | 0.38 | 0.46 |
| Lack physical exercises (yes) | 341 (50.74%) | 294 (59.63%) | 635 (54.51%) | <0.01 | 0.01 |
| Living in urban (yes) | 492 (73.21%) | 380 (77.08%) | 872 (74.85%) | 0.15 | 0.24 |
| Clinical diseases | |||||
| Stroke (yes) | 14 (2.08%) | 22 (4.46%) | 36 (3.09%) | 0.03 | 0.07 |
| Hypertension (yes) | 227 (33.78%) | 212 (43.00%) | 439 (37.68%) | <0.01 | 0.01 |
| Diabetes mellitus (yes) | 82 (12.20%) | 92 (18.66%) | 174 (14.94%) | <0.01 | 0.01 |
| Coronary disease (yes) | 76 (11.31%) | 82 (16.63%) | 158 (13.56%) | 0.01 | 0.04 |
| Hyperlipoidemia (yes) | 21 (3.13%) | 23 (4.67%) | 44 (3.78%) | 0.23 | 0.33 |
| Kidney diseases (yes) | 19 (2.83%) | 21 (4.26%) | 40 (3.43%) | 0.24 | 0.34 |
| Cancer (yes) | 38 (5.65%) | 31 (6.29%) | 69 (5.92%) | 0.74 | 0.81 |
| Anemia (yes) | 33 (4.91%) | 40 (8.11%) | 73 (6.27%) | 0.04 | 0.07 |
| Thyroid diseases (yes) | 56 (8.33%) | 78 (15.82%) | 134 (11.5%) | <0.01 | 0.00 |
| Anti-hypertension drug (yes) | 156 (23.21%) | 142 (28.80%) | 298 (25.58%) | 0.04 | 0.07 |
| Anti- diabetes drug (yes) | 60 (8.93%) | 67 (13.59%) | 127 (10.90%) | 0.02 | 0.04 |
| Vitamins (yes) | 65 (9.67%) | 72 (14.60%) | 137 (11.76%) | 0.01 | 0.04 |
| Scale* | |||||
| HAMA score (MAS) | 48 (13.79%) | 83 (27.57%) | 131 (20.18%) | <0.01 | <0.01 |
| HAMD score (MDS) | 49 (14.08%) | 74 (24.58%) | 123 (18.95%) | <0.01 | <0.01 |
| PSQI | |||||
| Sleep quality (bad) | 63 (9.38%) | 89 (18.05%) | 152 (13.05%) | <0.01 | <0.01 |
| Sleep latency | 22.55 ± 25.29 | 29.19 ± 31.53 | 25.63 ± 28.53 | <0.01 | 0.01 |
| Sleep duration (hours) | 0.04 | 0.06 | |||
| ≤5 | 57 (16.38%) | 67 (22.26%) | 124 (19.11%) | ||
| 5–6 | 61 (17.53%) | 68 (22.59%) | 129 (19.88%) | ||
| 6−7 | 95 (27.30%) | 81 (26.91%) | 176 (27.12%) | ||
| 7–8 | 100 (28.74%) | 64 (21.26%) | 164 (25.27%) | ||
| >8 | 35 (10.06%) | 21 (6.98%) | 56 (8.63%) | ||
| Bedtime | 0.12 | 0.13 | |||
| Before 8:00 p.m. | 38 (10.92%) | 27 (8.97%) | 65 (10.02%) | ||
| 8:00–9:00 p.m. | 81 (23.28%) | 54 (17.94%) | 135 (20.80%) | ||
| 9:00–10:00 p.m. | 142 (40.80%) | 124 (41.20%) | 266 (40.99%) | ||
| 10:00–11:00 p.m. | 74 (21.26%) | 73 (24.25%) | 147 (22.65%) | ||
| After 11:00 p.m. | 13 (3.74%) | 23 (7.64%) | 36 (5.55%) | ||
| Sleep efficiency (≤70%) | 57 (16.38%) | 61 (20.27%) | 118 (18.18%) | 0.28 | 0.28 |
| Sleep disorders | 248 (71.26%) | 235 (78.07%) | 483 (74.42%) | 0.05 | 0.06 |
| Sleep assistance | 20 (5.75%) | 37 (12.29%) | 57 (8.78%) | 0.01 | 0.01 |
| Day time dysfunction | 16 (4.60%) | 31 (10.30%) | 47 (7.24%) | 0.01 | 0.01 |
| Laboratory indicators | |||||
| FBG (mmol/L) | 5.53 ± 1.14 | 5.60 ± 1.06 | 5.56 ± 1.11 | 0.07 | 0.18 |
| BUN (mmol/L) | 5.76 ± 1.49 | 5.92 ± 1.41 | 5.83 ± 1.46 | 0.10 | 0.21 |
| CR (μmol/L) | 67.88 ± 14.52 | 68.79 ± 14.95 | 68.27 ± 14.70 | 0.39 | 0.62 |
| UA (μmol/L) | 360.34 ± 86.57 | 360.45 ± 83.37 | 360.38 ± 85.17 | 0.88 | 0.88 |
| TG (mmol/L) | 1.53 ± 1.26 | 1.42 ± 0.80 | 1.48 ± 1.09 | 0.59 | 0.67 |
| TC (mmol/L) | 4.83 ± 0.98 | 4.95 ± 1.02 | 4.88 ± 1.00 | 0.04 | 0.15 |
| HDL-C (mmol/L) | 1.20 ± 0.28 | 1.20 ± 0.26 | 1.20 ± 0.27 | 0.58 | 0.67 |
| LDL-C (mmol/L) | 2.83 ± 0.68 | 2.91 ± 0.72 | 2.86 ± 0.70 | 0.04 | 0.15 |
Continuous variables are presented as mean ± SD and categorical variables as number (percentage).
Abbreviations: CN cognitive normal, SCD subjective cognitive decline, MMSE mini-mental state examination, APOE ε4 apolipoprotein E ε4, FBG fasting blood glucose, BUN blood urea nitrogen, CR creatinine, UA uric acid, TG triglyceride, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, HAMA Hamilton anxiety scale, MAS minimal anxiety symptoms, HAMD Hamilton depression scale, MDS minimal depression symptoms, PSQI Pittsburgh sleep quality index.
Differences between two groups were analyzed by Chi-square tests for categorical variables and Wilcoxon tests for numerical variables.
q: Significance after false discovery rate (FDR) correction.
*A subset (n = 647, CN = 347, SCD = 300) with complete neuropsychiatric scales and PSQI.
Factors associated with SCD
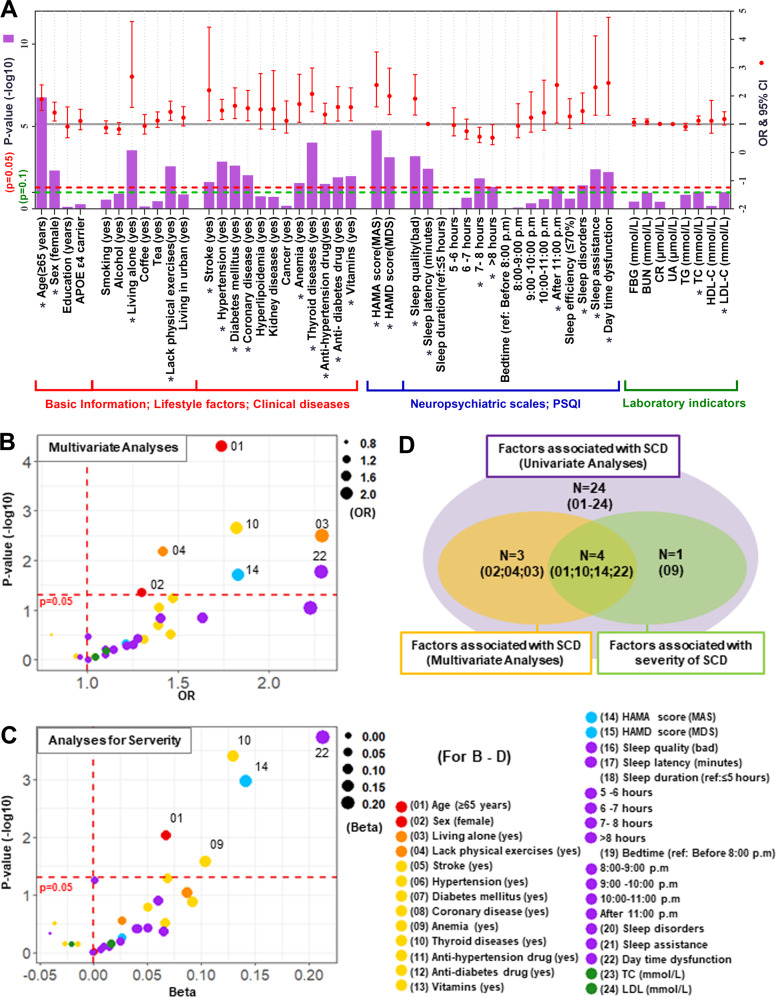
Firstly, 24 factors were screened out in univariate analyses (Model 1), including older age (≥65 years: OR 1.88, 95% CI 1.49–2.39), female sex (OR 1.40, 95% CI 1.11–1.78), living alone (OR 2.68, 95% CI 1.59–4.63), lack of physical exercises (OR 1.43, 95% CI 1.13–1.82), eight disease-related factors (stroke: OR 2.20, 95% CI 1.12–4.43; hypertension: OR 1.48, 95% CI 1.16–1.88; diabetes mellitus: OR 1.65, 95% CI 1.19–2.28; coronary disease: OR 1.56, 95% CI 1.12–2.19; anemia: OR 1.71, 95% CI 1.06–2.77; thyroid disease: OR 2.07, 95% CI 1.44–2.99; anti-hypertension drugs: OR 1.34, 95% CI 1.03–1.74; anti-diabetes drugs: OR 1.60, 95% CI 1.11–2.33; vitamins: OR 1.60, 95% CI 1.12–2.29), MAS (OR 2.38, 95% CI 1.61–3.56), MDS (OR 1.99, 95% CI 1.34–2.98), seven sleep-related factors (bad sleep quality: OR 1.90, 95% CI 1.32–2.76; longer sleep latency: OR 1.01, 95% CI 1.00–1.02; sleep duration (reference: ≤5 h; 7–8 h: OR 0.55, 95% CI 0.34–0.89; >8 h: OR 0.52, 95% CI 0.27–0.98); bed time (reference: before 8:00 p.m.; after 11:00 p.m.: OR 2.38, 95% CI 1.04–5.66); sleep disorders: OR 1.46, 95% CI 1.02–2.10; sleep assistance: OR 2.30, 95% CI 1.32–4.78; day time dysfunction: OR 2.46, 95% CI 1.32–4.78), and two laboratory indicators of peripheral blood (TC: OR 1.13, 95% CI 0.98–1.29; LDL-C: OR 1.18, 95% CI 0.97–1.44) (Fig. 1A and Table S1).
Fig. 1. Risk factors for SCD.
Risk factors for SCD were determined using three models. A Univariate logistic regression models (Model 1) were used to test association of each factor with the risk of SCD. B Then all the significant factors in univariate models (*p < 0.1) were included in the multivariate logistic regression (Model 2) to test their associations with the SCD status. C Similarly, all the significant factors in univariate models (*p < 0.1) were also included in the multivariate linear regression (Model 3) to test their associations with the SCD severity. The age, sex, years of education, and APOE ε4 status were included in two multivariate models as the basic covariates, regardless of their results in Model 1. D The significant results of three models were summarized in a Venn diagram. Abbreviations: SCD: subjective cognitive decline; OR: odds ratio; LCI: lower confidence interval (2.5%); UCI: upper confidence interval (97.5%); APOE ε4: apolipoprotein E ε4; FBG: fasting blood glucose; BUN: blood urea nitrogen; CR: creatinine; UA: uric acid; TG: triglyceride; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; HAMA: Hamilton anxiety scale; MAS: minimal anxiety symptoms; HAMD: Hamilton depression scale; MDS: minimal depression symptoms; PSQI: Pittsburgh sleep quality index.
Then, seven of these 24 factors still had significant associations with SCD risk in multivariate logistic regression models (Model 2), including older age (≥65 years: OR 1.74, 95% CI 1.33–2.27), female sex (OR 1.30, 95% CI 1.01–1.69), living alone (OR 2.29, 95% CI 1.33–4.04), lack of physical exercises (OR 1.42, 95% CI 1.10–1.82), thyroid disease (OR 1.82, 95% CI 1.24–2.69), MAS (OR 1.83, 95% CI 1.10–3.06), and day time dysfunction (OR 2.29, 95% CI 1.17–4.63) (Fig. 1B and Table S2).
Finally, five of these 24 risk factors were further proved associated with SCD severity in multivariate linear regression models (Model 3), including older age (≥65 years: Beta 0.07, 95% CI 0.02–0.12), anemia (Beta 0.10, 95% CI 0.01–0.19), thyroid disease (Beta 0.13, 95% CI 0.06–0.20), MAS (Beta 0.14, 95% CI 0.06–0.23), and day time dysfunction (Beta 0.21, 95% CI 0.10–0.32) (Fig. 1C and Table S3).
Overall, as shown in Fig. 1D, a total of eight risk factors were found associated with SCD in multivariate models, including four stable factors proved by two multivariate models (Model 2 and Model 3: older age, thyroid diseases, MAS, and day time dysfunction) as well as four suggestive factors proved by one of the two multivariate models (Model 2: female sex, lack of physical exercises, and living alone; Model 3: anemia).
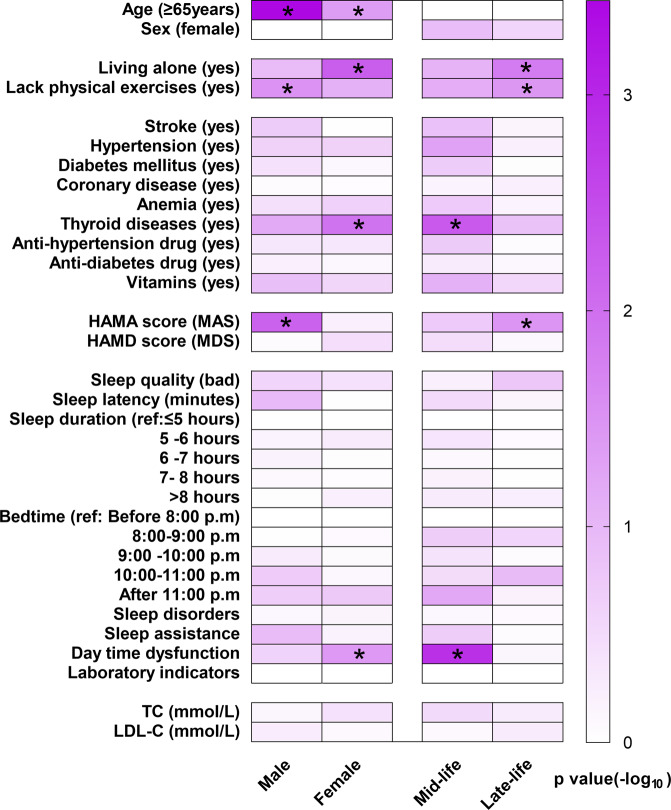
Subgroup analyses by age and sex
Subgroup analyses showed different distribution of risk factors. Living alone, thyroid diseases, and daytime dysfunction were more likely to increase the risk of SCD in females, while lack of physical exercises and MAS increased the risk of SCD in males. Older age was the risk factor of SCD in both female and male subgroups. As for different age subgroups, living alone, lack of physical exercises, and MAS increased the risk of SCD in late life, while thyroid diseases increased the risk of SCD in midlife (Fig. 2 and Tables S4 and S5).
Fig. 2. Subgroup analyses by age and sex.
Multivariate logistic regression was used to test associations between factors and SCD. Abbreviations: TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HAMA: Hamilton anxiety scale; MAS: minimal anxiety symptoms; HAMD: Hamilton depression scale; MDS: minimal depression symptoms.
Trend test and post hoc analyses
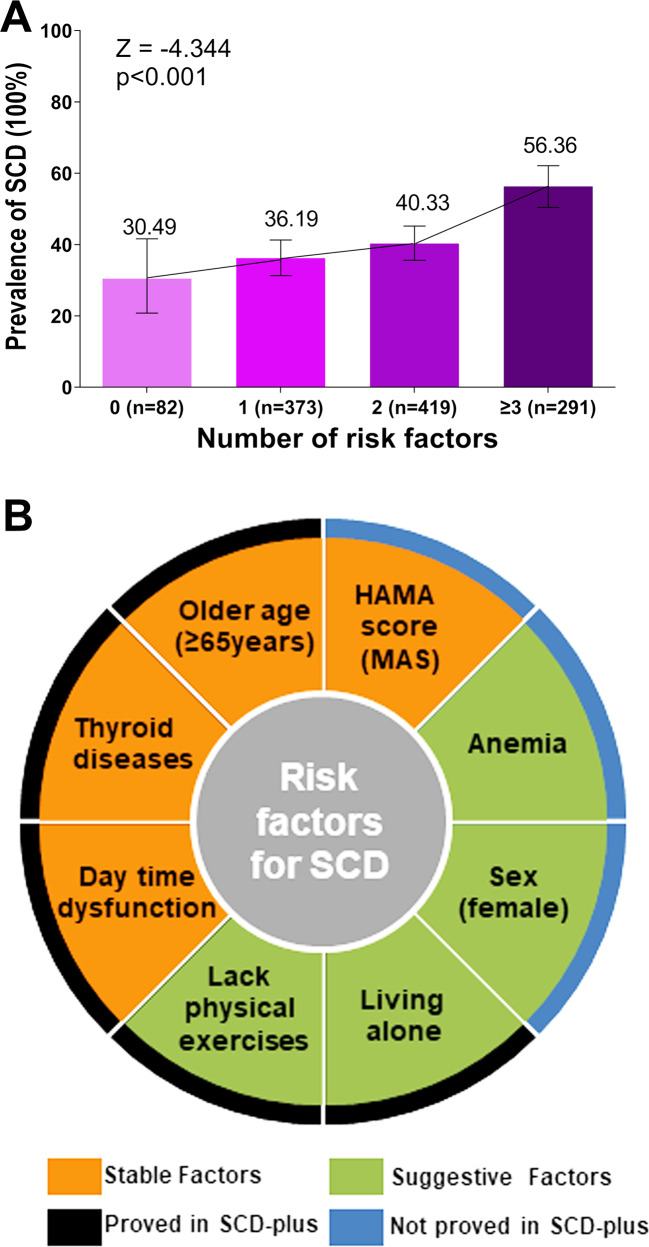
As shown in Fig. 3A, the risk of SCD gradually increased with the number of risk factors clustering in single individuals (p for trend <0.001). Furthermore, five of the eight risk factors for SCD were proved in the post hoc analyses between CN and SCD-plus, including older age, thyroid diseases, day time dysfunction, lack of physical exercises, and living alone (Fig. S1).
Fig. 3. Trend test and the summary of risk factors.
A The Cochran-Armitage trend test was used to confirm the constant trend toward higher prevalence of SCD with an increasing number of risk factors. B A summary chart of risk factors was established. A total of eight factors were found associated with SCD including four stable factors proved by two multivariate models and four suggestive factors proved by one of the two multivariate models. Five of the above eight factors were verified as risk factors for SCD-plus. Abbreviations: SCD: subjective cognitive decline; HAMD: Hamilton depression scale; MDS: minimal depression symptoms.
Discussion
This study explored the risk factors for SCD in a large cohort of participants without objective cognitive impairment. Based on this population, eight factors were eventually identified as risk factors for SCD, including four stable factors (older age, thyroid diseases, MAS, and day time dysfunction) and four suggestive factors (female sex, anemia, lack of physical exercises, and living alone) (Fig. 3B). These findings filled a gap in the field of initial cognitive symptoms and might facilitate a better understanding of the pathophysiological processes involved in the initial stage of cognitive impairment, which might provide new clues to early prevention and intervention.
Notably, we found a high overall prevalence of SCD in total participants (42%). This prevalence in late life reached 51% which was consistent with the previous results varying from 50% to 80% [19, 21, 22]. It was worth noting that though this prevalence in midlife decreased, it also reached 36%. This high prevalence further highlighted the urgency of recognizing initial symptoms of cognitive impairment and their risk factors. Overall, the risk factors for SCD identified in our study were largely supported by previous evidence on AD or dementia. Both older age and female sex are classic risk factors for dementia. Our results on SCD further suggested that the influences of these two factors on cognition already existed as early as the initial stage of symptoms.
Anemia and thyroid diseases were found to increase the risk of SCD in our study. As for anemia, a study based on two independent cohorts showed that lower hemoglobin levels in blood were associated with poor cognitive function and a subsequent Mendelian randomization analysis in the same study further proved that anemia did have a primary causal impact on cognitive impairment in AD [23]. Furthermore, neuroimaging studies also related decreased hemoglobin levels to cortical thinning, white matter hyperintensities, and low cerebral perfusion [24, 25]. As for thyroid diseases, both hyperthyroidism and hypothyroidism were found associated with cognitive impairment or AD [26]. Consistent with our results in midlife, these associations seemed to be more significant in younger adults [26, 27]. Although some other diseases, such as hypertension and diabetes mellitus, were also found associated with dementia or AD [11], our results suggested that anemia and thyroid diseases might be more likely to affect the occurrence of SCD in the early stage of the disease.
An inverse association of physically exercise with the risk of cognitive impairment was widely documented. A meta-analysis that included more than 160,000 participants showed a 45% reduction in the risk of developing AD due to regular physical exercise [28]. It was important to note that in addition to long-term exercises starting from midlife, late-onset exercise interventions in late life also showed obvious effects on delaying brain aging [29]. Furthermore, living alone is a proxy measure of social isolation. A recent meta-analysis proved that living alone was a more important risk factor for dementia than previously identified and 8.9% of the incident dementia in late life (≥65 years) was attributable to living alone [30]. Consistent with this result, our study suggested that living alone might increase the risk of SCD especially in late life. All these findings indicated some important roles of social isolation in cognitive function. However, since previous systematic reviews demonstrated that loneliness was also significantly associated with incident dementia [31], whether the relationship between living alone and SCD was mediated by loneliness still need to be further explored in future studies.
As for neuropsychiatric symptoms, we identified MAS as a stable risk factor for SCD, while MDS was only significant in univariate analyses. Numerous previous studies showed that clinically significant psychiatric symptoms, including anxiety and depression, were associated with brain aging and dementia [32, 33]. There were limited studies focused on minimal psychiatric symptoms. Our recent study showed that even minimal psychiatric symptoms might promote AD-related pathologies and increased the risk of dementia [20]. In addition, recent study focused on SCD individuals also linked psychiatric symptoms to SCD, and showed that individuals with co-occurring SCD and anxiety symptoms had a 25% probability of developing MCI or dementia by 3.1 years [34, 35]. All the above evidence suggested that individuals with psychiatric symptoms, even with minor changes in psychiatric symptoms, should be alert to the risk of cognitive impairment. In addition, accumulating evidence suggested that sleep was closely related to cognitive performance and brain health [36]. In our study, daytime dysfunction was selected from eight sleep indicators as a stable risk factor for SCD. However, the relationships of sleep with cognition and AD-related pathologies seemed to be more complex and heterogeneous across different sleep indicators, and nonlinear relationships have been found by our team and other research groups [13, 36, 37]. Even so, the identification of this risk factor for SCD suggested that sleep might affect cognition at an earlier stage than we expected.
Some strengths enhanced the reliability of our study, including large sample sizes, the use of two SCD measurements, and the adoption of the latest SCD-plus features (five of the above eight factors were verified as risk factors for SCD-plus). There were still some limitations in our study. Firstly, this was a cross-sectional study, which means that the causal relationships between these risk factors and SCD could not be established and still need to be explored in longitudinal studies. Secondly, all participants in our studies were Northern Han Chinese. Our findings should be replicated in other ethnic groups. Thirdly, though this study described a preliminary outline of risk factors for SCD and gave several important suggestions, the more detailed mechanisms of these associations should be further explored in future studies. Fourthly, since SCD may be caused by early pathologies of other types of dementia, combining AD-related biomarkers (such as Aβ or phosphorylated tau in CSF or plasma) to address whether the detected risk factors are specifically related to SCD caused by early AD pathology will be an important direction for future research.
In summary, a high overall prevalence of SCD was found among population without objective cognitive impairments. We identified older age, female sex, anemia, thyroid diseases, lack of physical exercises, living alone, MAS, and day time dysfunction as risk factors for SCD. These findings further deepened the understanding of SCD and provided some new clues for formulating priority strategies for early prevention and intervention of dementia or AD.
Supplementary information
Acknowledgements
The authors thank all participants of the present study as well as all members of staff of the CABLE study for their role in data collection.
Author contributions
C.W., H.H., Y.-N.O., L.T., and J.-T.Y. did the manuscript preparation and drafting. C.W., H.H., Y.-N.O., Y.-L.B., Y.-H.M., L.T., and J.-T.Y. did the clinical assessments and data acquisition. L.T. and J.-T.Y. did the clinical diagnosis. C.W. and H.H. did the data analysis and interpretation. L.T. and J.-T.Y. are responsible for the study conception and design. All authors have contributed to the manuscript revising and editing critically for important intellectual content and given final approval of the version and agreed to be accountable for all aspects of the work presented here.
Funding
This study was supported by grants from the National Natural Science Foundation of China (91849126), the National Key R&D Program of China (2018YFC1314700), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chen Wen, Hao Hu.
Contributor Information
Lan Tan, Email: dr.tanlan@163.com.
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01711-1.
References
- 1.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388:505–17. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378:321–30. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 3.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 4.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–52. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–51. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 6.Buckley RF, Maruff P, Ames D, Bourgeat P, Martins RN, Masters CL, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimers Dement. 2016;12:796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 8.de Rojas I, Romero J, Rodríguez-Gomez O, Pesini P, Sanabria A, Pérez-Cordon A, et al. Correlations between plasma and PET beta-amyloid levels in individuals with subjective cognitive decline: the Fundacio ACE Healthy Brain Initiative (FACEHBI) Alzheimers Res Ther. 2018;10:119. doi: 10.1186/s13195-018-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, et al. Region-specific association of subjective cognitive decline with tauopathy independent of global beta-amyloid burden. JAMA Neurol. 2017;74:1455–63. doi: 10.1001/jamaneurol.2017.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliassen CF, Reinvang I, Selnes P, Grambaite R, Fladby T, Hessen E. Biomarkers in subtypes of mild cognitive impairment and subjective cognitive decline. Brain Behav. 2017;7:e00776. doi: 10.1002/brb3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 12.Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:1201–9. doi: 10.1136/jnnp-2019-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Tan L, Su BJ, Yu H, Bi YL, Yue XF, et al. Sleep characteristics and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: the CABLE study. Alzheimers Dement. 2020;16:1146–52. doi: 10.1002/alz.12117. [DOI] [PubMed] [Google Scholar]
- 14.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13:296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gifford KA, Liu D, Romano R, Jones RN, Jefferson AL. Development of a subjective cognitive decline questionnaire using item response theory: a pilot study. Alzheimers Dement (Amst) 2015;1:429–39. doi: 10.1016/j.dadm.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med. 1997;27:91–8. doi: 10.1017/S0033291796003923. [DOI] [PubMed] [Google Scholar]
- 19.Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19:271–8. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W, Feng W, Shen XN, Bi YL, Ma YH, Li JQ,, et al. Amyloid pathologies modulate the associations of minimal depressive symptoms with cognitive impairments in older adults without dementia. Biol Psychiatry. 2021;89:766–75. doi: 10.1016/j.biopsych.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–22. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 22.van Harten AC, Mielke MM, Swenson-Dravis DM, Hagen CE, Edwards KK, Roberts RO, et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology. 2018;91:e300–e312. doi: 10.1212/WNL.0000000000005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winchester LM, Powell J, Lovestone S, Nevado-Holgado AJ. Red blood cell indices and anaemia as causative factors for cognitive function deficits and for Alzheimer’s disease. Genome Med. 2018;10:51. doi: 10.1186/s13073-018-0556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan B, Venketasubramanian N, Vrooman H, Cheng CY, Wong TY, Chen C, et al. Haemoglobin, magnetic resonance imaging markers and cognition: a subsample of population-based study. Alzheimers Res Ther. 2018;10:114. doi: 10.1186/s13195-018-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolters FJ, Zonneveld HI, Licher S, Cremers L, Heart Brain Connection Collaborative Research Group, Ikram MK, et al. Hemoglobin and anemia in relation to dementia risk and accompanying changes on brain MRI. Neurology. 2019;93:e917–e926. doi: 10.1212/WNL.0000000000008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:4240–8. doi: 10.1210/jc.2015-2046. [DOI] [PubMed] [Google Scholar]
- 27.Chaker L, Wolters FJ, Bos D, Korevaar TI, Hofman A, van der Lugt A, et al. Thyroid function and the risk of dementia: the Rotterdam Study. Neurology. 2016;87:1688–95. doi: 10.1212/WNL.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 28.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda T, Lewis NA, Knight JE, Rush J, Vendittelli R, Kleineidam L, et al. The importance of engaging in physical activity in older adulthood for transitions between cognitive status categories and death: a coordinated analysis of fourteen longitudinal studies. J Gerontol A Biol Sci Med Sci. 2021;76:1661–7. doi: 10.1093/gerona/glaa268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai R, John A, Stott J, Charlesworth G. Living alone and risk of dementia: a systematic review and meta-analysis. Ageing Res Rev. 2020;62:101122. doi: 10.1016/j.arr.2020.101122. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Santabárbara J, Lopez-Anton R, de la Cámara C, Lobo E, Gracia-García P, Villagrasa B, et al. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr Scand. 2019;139:6–14. doi: 10.1111/acps.12966. [DOI] [PubMed] [Google Scholar]
- 33.Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed]
- 34.Liew TM. Subjective cognitive decline, anxiety symptoms, and the risk of mild cognitive impairment and dementia. Alzheimers Res Ther. 2020;12:107. doi: 10.1186/s13195-020-00673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Wang X, Wang Y, Dong H, Lu J, Scheininger T, et al. Anxiety correlates with cortical surface area in subjective cognitive decline: APOE epsilon4 carriers versus APOE epsilon4 non-carriers. Alzheimers Res Ther. 2019;11:50. doi: 10.1186/s13195-019-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91:236–44. doi: 10.1136/jnnp-2019-321896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y, Qu LB, Liu H. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose–response meta-analysis of observational studies. Aging Clin Exp Res. 2019;31:309–20. doi: 10.1007/s40520-018-1005-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.