Abstract
Introduction:
Haemophilia A patients require perioperative clotting factor replacement to limit excessive bleeding. Weight-based dosing of Factor VIII (FVIII) does not account for inter-individual pharmacokinetic (PK) variability, and may lead to suboptimal FVIII exposure.
Aim:
To perform an external validation of a previously developed population PK (popPK) model of perioperative FVIII in haemophilia A patients.
Methods:
A retrospective chart review identified perioperative haemophilia A patients at the University of North Carolina (UNC) between April 2014 and November 2019. Patient data was used to externally validate a previously published popPK model proposed by Hazendonk. Based on these validation results, a modified popPK model was developed to characterize FVIII PK in our patients. Dosing simulations were performed using this model to compare FVIII target attainment between intermittent bolus (IB) and continuous infusion (CI) administration methods.
Results:
A total of 521 FVIII concentrations, drawn from 34 patients, were analysed. Validation analyses revealed that the Hazendonk model did not fully capture FVIII PK in the UNC cohort. Therefore, a modified one-compartment model, with weight and age as covariates on clearance (CL), was developed. Dosing simulations revealed that CI resulted in improved target attainment by 16%, with reduced overall FVIII usage by 58 IU/kg, compared to IB.
Conclusion:
External validation revealed a previously published popPK model of FVIII did not adequately characterize UNC patients, likely due to differences in patient populations. Future prospective studies are needed to evaluate our model prior to implementation into clinical practice.
Keywords: dosing simulation, external validation, factor VIII, haemophilia A, perioperative, population pharmacokinetics
1 |. INTRODUCTION
Haemophilia A is a rare X-linked bleeding disorder caused by a qualitative or quantitative Factor VIII (FVIII) deficiency.1 FVIII deficiency prevents adequate thrombin generation and predisposes these patients to chronic recurrent spontaneous bleeds.2 Additionally, haemophilia A patients require exogenous clotting factor administration prior to and after surgical procedures to ensure adequate perioperative haemostasis. In clinical practice, clotting factor replacement is often performed using weight-based FVIII dosing strategies. However, weight-based FVIII dosing does not account for known inter-individual FVIII pharmacokinetic (PK) variability other than weight,3,4 and weight-based dosing can lead to suboptimal (over- or under-exposure) treatment that can result in prolonged bleeding and delayed wound healing.5,6 The source of FVIII PK variability can be attributed to the factor concentrate, age, bleeding intensity (if present), and the level of physical activity.2,7,8 Therefore, in order to facilitate FVIII dosing tailored to a patient’s specific PK parameters, there is a need to develop a novel FVIII dosing strategy that can account for inter-individual variability.
Bayesian adaptive dosing refers to the process of predicting a patient’s optimal dose using both drug concentrations drawn from that patient, as well as established population PK (popPK) modeling.9 If the published popPK model is both robust and has been externally validated, limited PK sampling can performed to allow for individual PK parameters to be estimated, and can then be used to simulate possible dosing schedules.3,10 External validation is crucial because it can determine how robust and reproducible a model is, and is considered the most stringent form of model validation.11 This form of model validation should be performed prior to clinical implementation of a popPK model to ensure model transportability.12
In 2016, Hazendonk et al. published the first popPK model characterizing FVIII PK in the perioperative setting.13 The study was comprehensive, utilized PK data based on the standard one-stage assay (OSA) used in clinical practice, and included a third generation FVIII product. Therefore, this popPK model could be an ideal candidate for clinical implementation of Bayesian adaptive dosing. The objective of this study was to perform an external validation of the Hazendonk popPK model in an independent patient cohort from the University of North Carolina (UNC) Medical Center. If external validation showed that the Hazendonk model did not fully capture FVIII PK in the UNC cohort, a secondary objective was to develop a modified popPK model with data derived from UNC patients.
2 |. MATERIAL AND METHODS
2.1 |. Patient recruitment and sample collection
This study was approved by the UNC Institutional Review Board (UNC 17-3250). A retrospective chart review was conducted to identify eligible patients. Eligibility criteria included adult patients between 18 and 79 years who underwent surgery at UNC Medical Center between April 2014 and November 2019, and were treated with perioperative FVIII concentrates. For this study, standard half-life (SHL) product was used for the replacement therapy. Eligible patients also had quantified FVIII concentrations after the FVIII concentrates were administered on Day 0 as part of standard of care. For eligible patients, baseline clinical and demographic characteristics extracted from the electronic medical record included age, weight, ABO blood type, and FVIII concentration. FVIII concentrations were measured by an OSA.14 Historic FVIII concentrations were reviewed to determine the baseline concentration for each patient. Patients were categorized as mild (initial FVIII concentration >.05 IU/ml), moderate (initial FVIII concentration .01–.05 IU/ml), or severe (initial FVIII concentration < .01 IU/ml) haemophilia.15 Patients were excluded if perioperative FVIII concentrations were not quantified after FVIII concentrates were administered. Descriptive statistics were used to summarize patient baseline characteristics. Surgery was categorized into low, moderate or high risk, according to the International Classification of Disease.16 Major surgeries were defined as those with moderate to high risk.
2.2. External validation of a prior popPK model
The UNC patient dataset was used to assess the predictive performance of the Hazendonk model, which is a two-compartment model with age, blood group O, and major surgery as covariates.13 External validation was performed by fixing all of the fixed and random effect parameters from the Hazendonk model. Predictive performance was evaluated by calculating the bias and precision using mean prediction error (MPE; Equation 1) and mean absolute prediction error (MAPE; Equation 2), respectively, where N is the number of observations, PREDj is the jth population predicted concentration, and observationj is the jth observation. Additionally, predictive performance of the model was also assessed using the goodness of fit plots, prediction-corrected visual predictive checks (pcVPC) and the model was bootstrapped 1000 times to compare the 95% confidence intervals of parameter estimates.
| (1) |
| (2) |
All popPK analyses were performed using NONMEM (version 7.4.3; Icon Development Solutions, Ellicott City, MD, USA). All data manipulation and visualization of diagnostic plots were executed using R (version 3.2, R Foundation for Statistical Computing, Vienna, Austria), and RStudio (version .99, RStudio, Boston, MA, USA), with the packages lattice, latticeExtra, and ggplot2.17–19 Perl-speaks-NONMEM (PsN) (version 4.7.0; Uppsala Pharmacometrics, Uppsala, Sweden) was utilized for the bootstrap analyses.13,17,20,21
2.3 |. UNC model development and validation
Based on previously published models of FVIII, both one- and two-compartment models were evaluated on the UNC dataset. Proportional and mixed (proportional and additive) residual error models were explored. Allometric weight was included a priori with an exponent of .75 for clearance (CL) and 1 for volume of distribution (V).13 Three approaches were employed to account for the endogenous FVIII concentrations at baseline: (1) incorporating an inter-occasion variability to account for different baseline concentrations for each patient (M1); (2) a compartmental reset using the historical lowest FVIII concentrations for each patient (M2)22; and (3) a compartmental reset using the first available FVIII concentrations for each patient (M3). Covariates collected for every patient include sex, ABO blood type, FVIII product, surgery type, age, FVIII baseline concentration, FVIII dose, haemoglobin, and haematocrit. Covariates with sufficient data that showed a clear trend with inter-individual variability (IIV) of CL or V were statistically evaluated. Continuous and categorical covariates were assessed using the Equations 3 and 4, respectively, where
| (3) |
| (4) |
COVi denotes the covariate, which for categorical variables is formatted to be 0 or 1, COVmed denotes the median covariate value across the entire dataset, PARCOV denotes covariate effect on the parameter, and θ denotes the fixed effects of the parameter estimate. Physiologic plausibility, goodness-of-fit plots, reduction in IIV and residual error, and a decrease in the objective function value (OFV) of > 3.8 (p < .05) were used to select the final model. The lower limit of quantification (LLOQ) was defined as <.01 IU/ml. If < 5% of the concentrations were not quantifiable, below the limit of quantification data were excluded from modelling using the method incorporating inter-occasion variability (M1); otherwise, the compartmental reset method using the lowest FVIII value (M2) was used.
2.4 |. Dosing simulations
Based on the population distribution of age, weight, and known baseline FVIII concentration in the UNC patients, 500 virtual patients were simulated. The virtual patients were created based on the demographic distribution from the UNC patient dataset, where the median age for virtual patients was 51.7 years (range 24.3–80.0 years), median weight was 87.1 kg (50.5–135.6 kg), and mean historic low FVIII concentrations for mild, moderate, and severe patients were .27 IU/ml (standard deviation [SD] ± .12 IU/ml), .031 IU/ml; (SD ± .011 IU/ml), and .0045 IU/ml (SD ± .0026 IU/ml), respectively. Simulations were performed to compare FVIII target attainment when FVIII was administered by continuous infusion (CI) versus intermittent bolus (IB). Target attainment was simulated in virtual patients with mild, moderate, and severe haemophilia A. Simulations were only performed for the first 48 h after surgery, as patients typically undergo dose adjustment based on routine plasma FVIII concentration monitoring after 48 h. For the IB strategy, the simulated dose was 50 IU/kg every 8 h; for the simulated CI strategy, the first IV bolus dose was 50 IU/kg, and the CI of 4 IU/kg/h was started simultaneously. For the IB dose, the Cavg was calculated as AUCtau/Tau. The FVIII activity from the first 48 h was simulated, and target attainment was measured as the mean FVIII activity. The proportion of patients who achieved target concentrations was based on an institutional goal of .8–1.2 IU/ml replacement factor activity.
3 |. RESULTS
3.1 |. Patient baseline characteristics
Forty-three adult patients with haemophilia A underwent surgery and received FVIII at UNC between April 2014 and November 2019. Nine patients were excluded due to perioperative FVIII concentrations were not quantified or accessible after FVIII concentrates were administered on Day 0 (n = 6), missing blood type (n = 2), and single FVIII concentration available (n = 1), as shown in Figure 1. Thirty-four patients were included in the final analyses. Among these patients, 97% were male, and most surgeries (91%) were classified as major (Table 1). The one female patient was a symptomatic carrier. The median range of the dosing the patients received was 45.3 IU/kg with a frequency of every 12 h. A total of 521 PK samples were available, with a median of 14 (range 1–34) samples per patient
FIGURE 1.
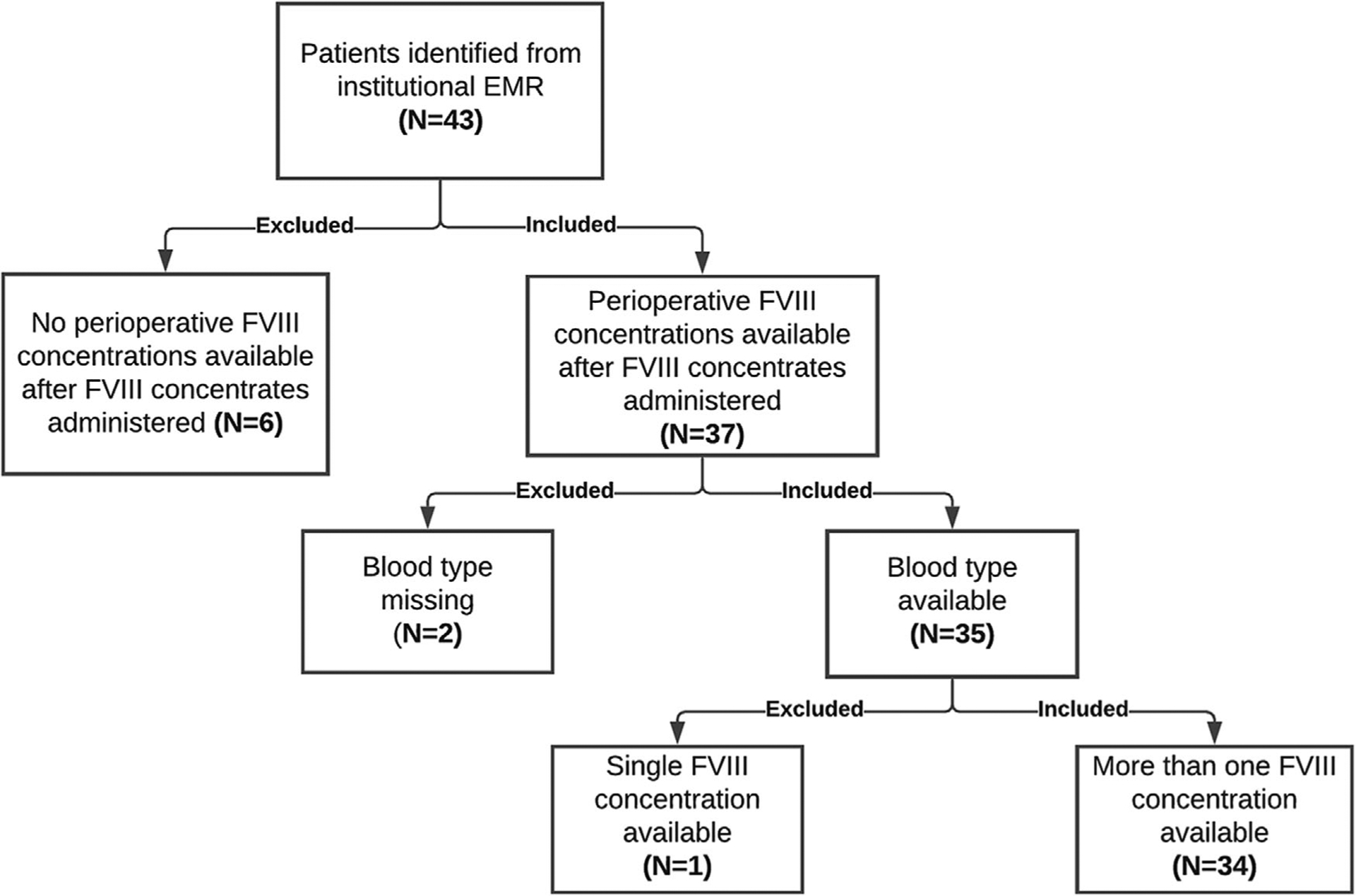
Study schematic. The flow chart below describes the process with which patients were identified from the University of North Carolina Medical Center institutional electronic medical record (n = 43), and why nine patients were excluded. FVIII, factor VIII
TABLE 1.
Patient baseline demographic and clinical characteristics
| Variables | Values |
|---|---|
| Number of patients (N) | 34 |
| Age (years, median [range]) | 51.7 (24.3–80.0) |
| Weight (kg, median [range]) | 87.1 (50.5–135.6) |
| Sex (N, %) | |
| Male | 33 (97.1) |
| Female | 1 (2.9) |
| Blood type (N,%) | |
| Type O | 13 (38.2) |
| Other blood type | 21 (61.8) |
| Haemoglobin (g/dl, median [range]) | 13.1 (5.8–16.7) |
| Haematocrit (%, median [range]) | 38.6 (20.3–48.9) |
| Surgery types (N, %) | |
| Major surgeries | 51 (91.1) |
| Minor surgeries | 5 (8.9) |
| Haemophilia severity | |
| Mild (> .05 IU/ml) | 11 |
| Moderate (.01–.05 IU/ml) | 5 |
| Severe (< .01 IU/ml) | 18 |
| FVIII Dose (IU, median [range]) | 3560 (124.2–7944) |
| Product type | |
| Monoclonal | 135 |
| Recombinant (Xyntha/Refacto) | 270 |
| Recombinant (Obizur) | 13 |
| Infusion duration | |
| ≤5 min | 166 |
| >5min to ≤1h | 22 |
| >1 h | 230 |
3.2 |. External validation of prior population pharmacokinetic model
The MPE and MAPE are shown in Table 2. The predictive performance of the Hazendonk model was evaluated using UNC patient data (n = 34). Less than 80% of the bootstrap runs were minimized successfully. The 95% confidence intervals for each model parameter had wide ranges, with the median values deviating substantially from the original values derived in the Hazendonk model (Table 3). Notably, covariate effects of blood type on CL, surgery type on CL, and age on V in the central compartment, crossed the threshold for non-significance (Table 3). The goodness of fit plot of the external validation showed that the observed versus predicted FVIII concentrations deviated from the line of unity at higher predicted FVIII concentrations, indicating that the Hazendonk model may not adequately characterize FVIII concentrations among the UNC patients (Figure 2).
TABLE 2.
MPE and MAPE for Hazendonk model and the University of North Carolina model predictive performances
| Hazendonk model | UNC model | |
|---|---|---|
| MPE (%) | 24.7 (−96.7, 261) | 14.2 (−54.5, 168) |
| MAPE (%) | 56.7 (1.7, 261) | 41.5 (1.4, 168) |
Abbreviations: MPE, mean prediction error; MAPE, mean absolute prediction error.
The mean, 2.5th and 97.5th percentile of MPE and MAPE are presented in the table.
TABLE 3.
Bootstrap results from fitting UNC dataset to the Hazendonk model
| Parameter | Estimates from the Hazendonk model13 | Estimates from fitting UNC dataset to the Hazendonk model |
|---|---|---|
| Minimization Successful | N/A | 799/1000 |
| CL (ml/h) | 150 | 180 (46, 600) |
| CL-Age | −.172 | −.51 (−1.5, −.016) |
| CL-Blood Type | 1.26 | .92 (.66, 1.3) |
| CL-Surgery Type | .933 | 1.2 (.34, 2.2) |
| V1 (ml) | 2810 | 4100 (2600, 5800) |
| V1-Age | −.0898 | −.65 (−1.5, .47) |
| V2 (ml) | 1900 | 1300 (570, 95000) |
| Q (ml/h) | 160 | 72 (28, 1400) |
| ReFacto Adjustment | .344 | N/A |
| Additive Error (IU/ml) | (Centers 1–3) 15 (Centers 4–5) 5 | .47 (.41, .53) |
| Proportional Error (%) | (Centers 1–3) 18 (Centers 4–5) 23 | 3.3e–03 (3.2e–03, 3.3e–03) |
| IIV-V1a | .0692 | 69 (44, 91) |
| IIV (CL-V1)b | .0437 | .48 (−.14, .88) |
| IIV-CLc | .130 | 33 (21, 82) |
Abbreviations: CL, clearance; IIV, inter-individual variability; Q, intercompartmental clearance between the central and peripheral compartment; UNC, University of North Carolina Medical Center; V1, volume of distribution in the central compartment; V2, volume of distribution in the peripheral compartment. Predictive performance of the Hazendonk model was evaluated using the UNC dataset. Estimates from fitting the UNC dataset are reported as medians with 95% confidence intervals
Variance of the IIV for the central volume of distribution.
Covariance of the IIV between clearance and central volume of distribution.
Variance of the IIV for clearance.
FIGURE 2.
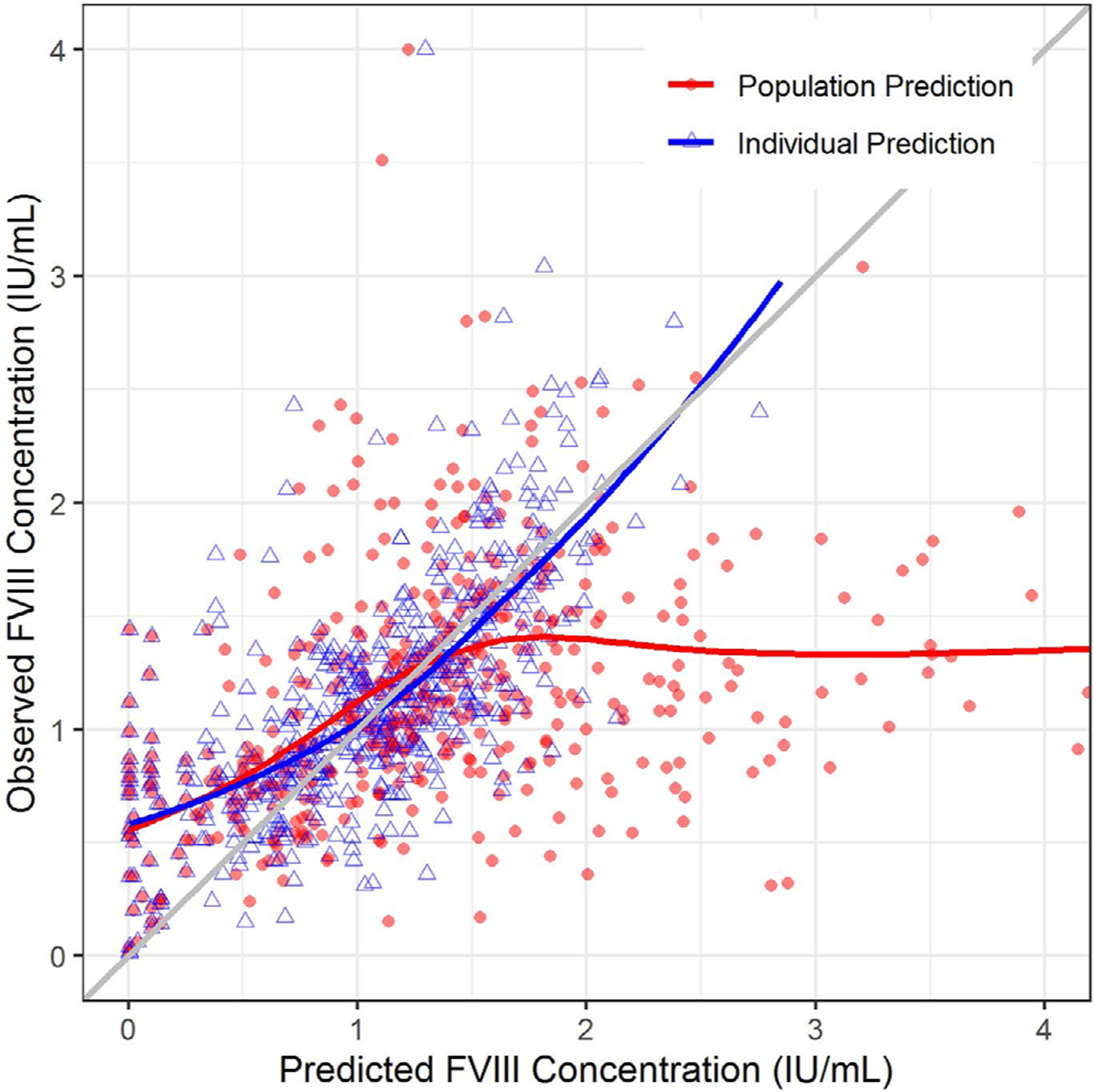
Goodness of fit plot for the external validation. The plot depicts the observed FVIII concentrations (Y-axis) plotted against the model-predicted FVIII concentrations (X-axis). The red solid circles denote population predicted FVIII concentrations, whereas the blue triangles denote the individual predicted FVIII concentrations (which ideally match observed FVIII concentrations in University of North Carolina Medical Center patients). The red and blue lines represent the trend line for the population and individual predictions, respectively. The diagonal grey line represents the line of unity. FVIII, factor VIII
3.3 |. UNC model development and validation
A total of 521 FVIII concentrations were available for the popPK model development, but using observed baseline FVIII concentrations resulted in imprecise parameter estimates. The M1 and M2 strategies attempted to account for the baseline values, but also resulted in imprecise parameter estimates. Therefore, a compartmental initialization method (M3) was used, where the first FVIII concentration measured after the initial bolus marked the baseline. Using the compartmental initialization method resulted in a total of 456 FVIII concentrations available for analyses among the 34 patients. The final modified UNC popPK model was a one-compartment model with age (years) and weight (kg) included as significant covariates:
Theory-based allometry was applied to scale CL and V parameters using a standard weight of 70 kg, and an inverse relationship between age and CL.23 The proportional error model accounted for residual error, and the IIV on CL was less than that on V (Table 4). Based on visual inspection, the UNC data did not support a two-compartment model, unlike the published Hazendonk model. Population predictions from the final modified UNC model (Figure 3) better represented the PK profile of the observed FVIII concentrations than did the Hazendonk model (Figure 2). Last, a pcVPC was performed, and showed that approximately 91% of the prediction-corrected FVIII concentrations fell within the 90% prediction interval based on the final modified UNC popPK model (Figure 4).
TABLE 4.
Final parameter estimates and bootstrap for the UNC model
| Hazendonk et al. | Model | Bootstrap | UNC | Model | Bootstrap |
|---|---|---|---|---|---|
| Minimization successful | N/A | 799/1000 | Minimization successful | N/A | 1000/1000 |
| CL (ml/h)a | 150 | 180 (46, 600) | CL (ml/h)b | 190 | 190 (160, 220) |
| CL: Effect of agec | −.17 | −.51 (−1.5, −.016) | CL: Effect of Aged | −.013 | −.013 (−.024, −.0031) |
| CL: Effect of blood type | 1.26 | .92 (.66, 1.3) | |||
| CL: Effect of severity of surgical procedure | .93 | 1.2 (.34, 2.2) | |||
| V1 (ml) | 2810 | 4100 (2600, 5800) | V1 (ml) | 5800 | 5800 (4300, 7600) |
| V1: Effect of age | −.09 | −.65 (−1.5, .47) | |||
| V2 (ml) | 1900 | 1300 (570, 95000) | |||
| Q (ml/h) | 160 | 72 (28, 1400) | |||
| Additive error (IU/ml) | (Center-based) | .47 (.41, .53) | |||
| Proportional error (%) | (Center-based) | .0033 (.0032, .0033) | Proportional error (%) | 32 | 32 (27, 37) |
| IIV on CL (%) | 26 | 33 (21, 82) | IIV on CL (%) | 47 | 45 (33, 64) |
| Covariance between CL and V1 | .46 | .48 (−.14, .88) | |||
| IIV on V1 (%) | 36 | 69 (44, 91) | IIV on V1 (%) | 74 | 72 (48, 96) |
Abbreviations: CL, clearance; IIV, inter-individual variability; Q, intercompartmental clearance between V1 and V2; UNC, University of North Carolina Medical Center; V1, central compartment volume of distribution; V2, peripheral compartment volume of distribution.
The final estimates for the parameters from the population PK model.
Expected clearance for a 68 kg, 40 year-old, non-O blood type, minor surgery patient.
Expected clearance for a 70 kg, 57 year-old patient.
As an exponent on age centered at 40 year-old
As fractional change per year.
FIGURE 3.
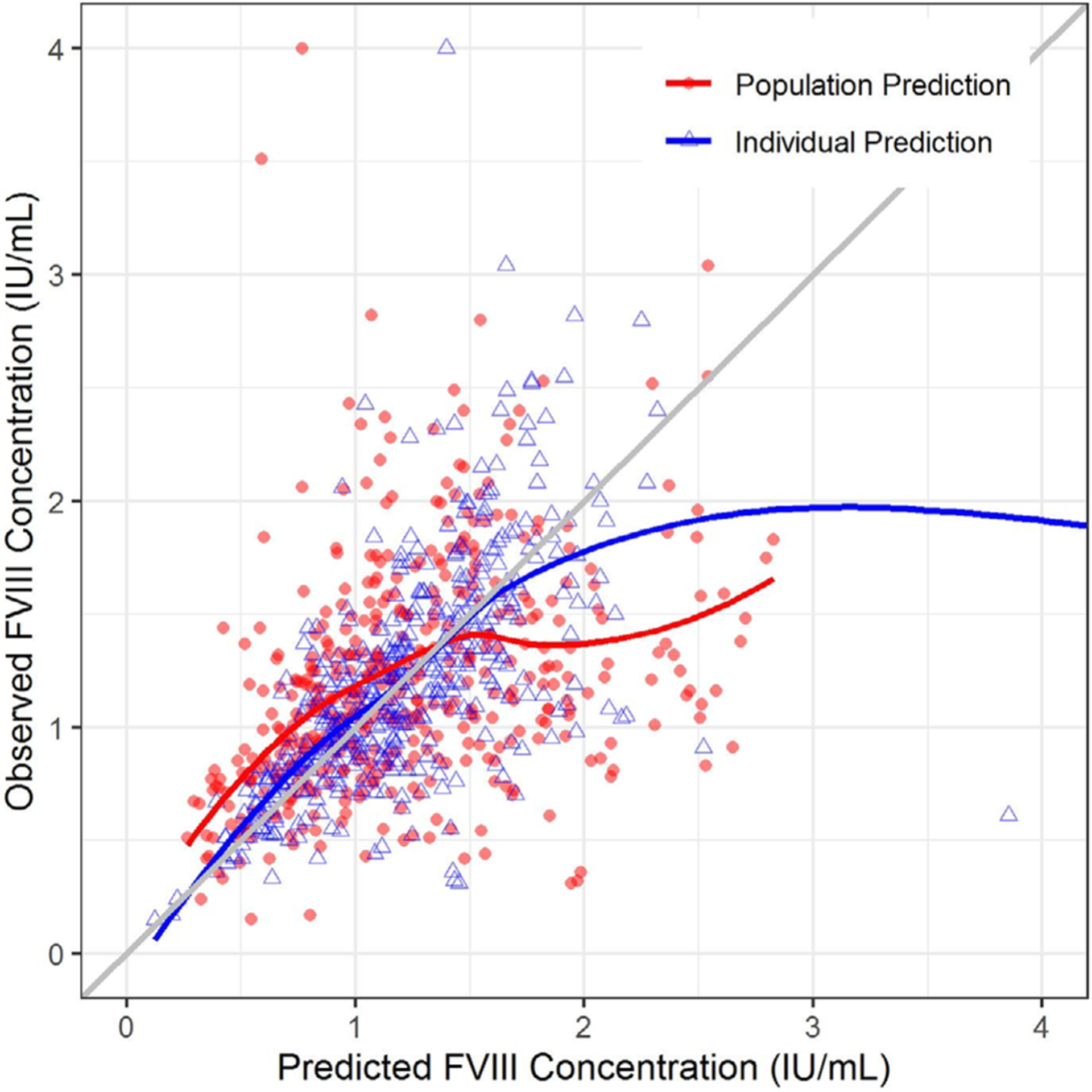
Goodness of fit plot for the University of North Carolina Medical Center model. The plot depicts the observed FVIII concentrations (Y-axis) plotted against the model-predicted FVIII concentrations (X-axis). The red solid circles denote population predicted FVIII concentrations, whereas the blue triangles denote the individual predicted FVIII concentrations. The red and blue lines represent the trend line for the population and individual predictions, respectively. The diagonal grey line represents the line of unity. FVIII, factor VIII
FIGURE 4.
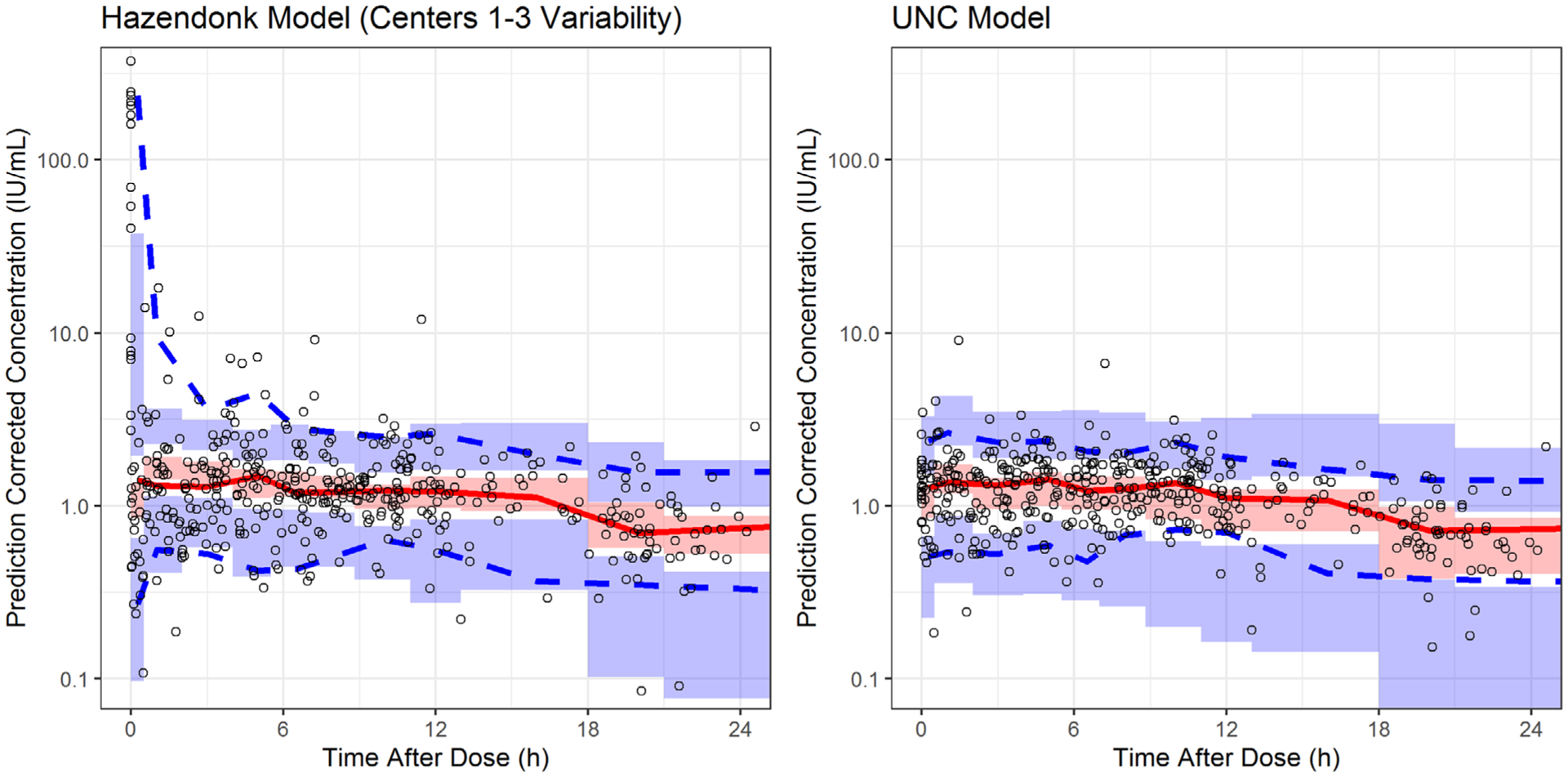
Prediction-corrected visual predictive check. The diagram depicts the visual predictive check of the final UNCMC popPK model as well as the Hazendonk popPK model. The X-axis denotes the time after last dose in hours whereas the Y-axis denotes the prediction corrected FVIII concentrations (IU/ml). The two blue dashed lines represent the 5th and the 95th percentiles of the observed FVIII concentrations while the red solid line represents the median of the observed FVIII concentrations. Shaded area represents the 95% confidence interval predicted by the model for the median, 5th, and the 95th percentile of the FVIII concentrations. Centers 1–3 refer to the three out of the five treatment centers used in the Hazendonk model.13 FVIII, factor VIII; popPK, population PK; UNC, University of North Carolina Medical Center
3.4 |. Dosing simulation
Figure 5 and Table 5 depict the results from the dosing simulations. In simulated severe haemophilia A patients (initial FVIII <.01 IU/ml), the proportion of patients that attained the target FVIII concentrations on Day 2 when treated with IB and CI were 21.0 % and 37.0%, respectively. In simulated moderate patients (initial FVIII .01–.05 IU/ml), the proportion of patients that attained target FVIII concentrations when treated with IB and CI were 20.0% and 20.6%, respectively. In mild haemophilia A patients (initial FVIII > .05 IU/ml), the proportion of patients that attained target FVIII concentrations when treated with IB and CI were 14.8% and 28.2%, respectively. By Day 2, more patients simulated with IB had supratherapeutic FVIII concentrations than with simulated CI (77.0% vs. 50.2%, respectively). Last, the simulation estimated that CI would require less FVIII usage (242 IU/kg) compared to IB (300 IU/kg).
FIGURE 5.
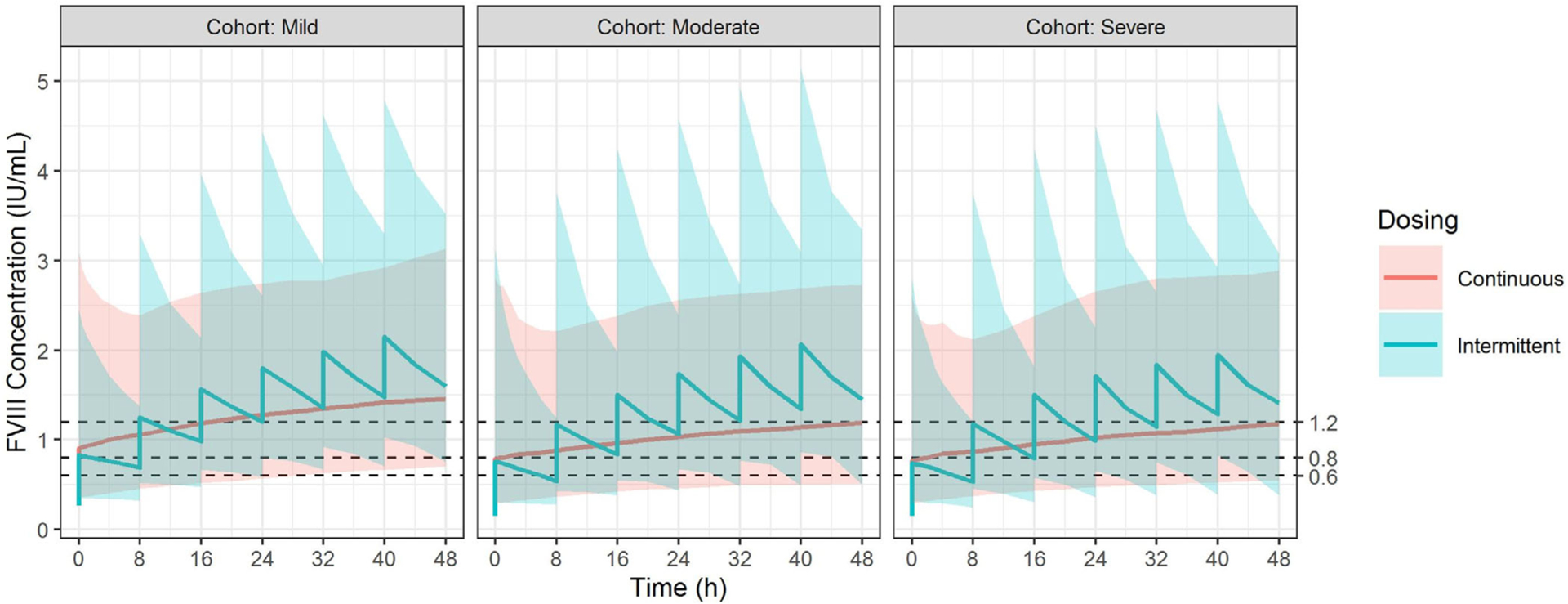
Dosing simulation results for intermittent bolus and continuous infusion. The simulated FVIII concentrations are shown below based on the modified UNC population pharmacokinetic model. The X-axis denotes the time after the patient was admitted while the Y-axis denotes the FVIII concentrations (IU/ml). The three panels from left to right represent patients with mild (initial FVIII concentration > .05 IU/ml), moderate (initial FVIII concentration .01–.05 IU/ml), and severe haemophilia A (initial FVIII concentration < .01 IU/ml). The red line and shaded area represent the median, and the 95% prediction interval of the simulated results for CI while the blue line and shaded area represent those of the IB dose. CI, continuous infusion; FVIII, factor VIII; IB, intermittent bolus
TABLE 5.
Simulation results comparing target attainment between intermittent bolus and continuous infusion
| Mild (initial FVIII >.05 IU/ml) | Moderate (initial FVIII .01–.05 IU/ml) | Severe (initial FVIII < .01 IU/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <.8 IU/ml N (%) | .8–1.2 IU/ml N (%) | >1.2 IU/ml N (%) | <.8 IU/ml N (%) | .8–1.2 IU/ml N (%) | >1.2 IU/ml N (%) | <.8 IU/ml N (%) | .8–1.2 IU/ml N (%) | >1.2 IU/ml N (%) | |
| DAY 1 (Hour 0–24) | |||||||||
| IB | 113 (22.6) | 174 (34.8) | 213 (42.6) | 159 (31.8) | 172 (34.4) | 169 (33.8) | 173 (34.6) | 176 (35.2) | 151 (30.2) |
| CI | 108 (21.6) | 175 (35.0) | 217 (43.4) | 183 (36.6) | 156 (31.2) | 161 (32.2) | 181 (36.2) | 174 (34.8) | 145 (29.0) |
| DAY 2 (Hour 24–48) | |||||||||
| IB | 6 (1.2) | 74 (14.8) | 420 (84.0) | 28 (5.6) | 100 (20.0) | 372 (74.4) | 32 (6.4) | 105 (21) | 363 (72.6) |
| CI | 37 (7.4) | 141 (28.2) | 322 (64.4) | 103 (20.6) | 177 (35.4) | 220 (44.0) | 104 (20.8) | 185 (37.0) | 211 (42.2) |
Abbreviations: IB, intermittent bolus; CI, continuous infusion; N, number of patients.
This table describes the proportion of 500 virtual patients that received either IB or CI FVIII dose.
The number of patients that fall within each category (i.e., subtherapeutic <.8 IU/ml, at goal .8–1.2 IU/ml, and supratherapeutic >1.2 IU/ml) was described in total numbers and percentages.
4. DISCUSSION
The current weight-based dosing algorithm for FVIII in the perioperative period does not account for FVIII PK variability and can lead to suboptimal FVIII exposure, and thus, increased risk of spontaneous bleeding and delayed wound healing in adult haemophilia A patients.5,6 Therefore, precision dosing approaches can improve upon the standard weight-based dosing paradigm to reduce the subtherapeutic FVIII concentrations and spontaneous bleeding and improve stewardship of FVIII usage. However, previous research has demonstrated that popPK models developed outside of the perioperative setting do not reliably predict postoperative FVIII concentrations. Rather, popPK models that specifically characterize perioperative FVIII PK are needed to perform iterative dose adjustments that employ Bayesian methodologies.24,25 However, before these methodologies can be applied to a new target population, external validation of the model is an essential step to ensure model generalizability across patients populations and portability into clinical practice. Therefore, our study aimed to perform an external validation of the previously published Hazendonk model in an independent patient cohort from UNC.
Several differences in the baseline study data utilized for model development may have contributed to the Hazendonk model failing to capture the perioperative FVIII PK among UNC patients. Firstly, many UNC patients (85.3%) received prophylactic FVIII doses prior to hospital admission, resulting in higher predicted than measured FVIII concentrations when fitting the Hazendonk model to these patient data (Figure 2). Adjustment for baseline FVIII concentrations using the M1 and M2 strategies were attempted, but did not resolve the imprecise predictions. Second, 14% of the Hazendonk patients were treated with a B-domain deleted FVIII concentrate. While we attempted to correct for this in the UNC model development, underprediction of FVIII concentrations likely occurred as a result. Third, and likely most important, patient baseline demographics differed significantly between the two cohorts. For instance, there was wider range of total body weight among UNC patients (50–137 vs. 73–90 kg), a higher frequency of major surgery (91.1% vs. 61.4%), and that the UNC cohort was only comprised of adult patients. The Hazendonk model was recently validated to a larger cohort of paediatric population.26 To this point, less than 80% of the bootstrap runs minimized successfully, which could be attributed to the demographic differences between the two cohorts or our relatively small sample size.
Because the Hazendonk model did not adequately characterize FVIII concentrations among UNC patients, a modified popPK model was developed. A one-compartment model adequately characterized the FVIII PK profile in UNC haemophilia A patients undergoing surgery. Age was identified as statistically significant covariate and had an inverse relationship with CL, which suggests FVIII CL decreases with increased age. The UNC dataset was limited to only one or two samples following each dose, which did not support multi-compartmental modelling. Importantly, the resulting final model’s CL and V estimates, scaled to a standard weight of 70 kg, are similar to published reports in adults.27,28 Final parameter estimates from the modified model were comparable to the Hazendonk model estimates, with the exception that we observed a lesser impact of age on CL (Table 4).
Dosing simulations were performed to compare target attainment against FVIII usage and cost, using the two most commonly used FVIII administration methods used in clinical practice: CI and IB dosing. Our findings are similar to those previously published, which demonstrated that CI resulted in a higher percentage of target attainment, and reduced overall FVIII usage.29,30 Notably, simulation results from the modified model revealed that <21% of patients were subtherapeutic at Day 2, which suggests that our model maybe be an effective tool to ensure optimal FVIII exposure, prevent excessive bleeding, and avoid a longer duration of postoperative FVIII use. However, at Day 2 the model also predicted a high percentage of patients with supratherapeutic concentrations. Future application of this model will involve evaluating its use to reduce the percentage of supratherapeutic patients and to reduce unnecessary FVIII usage and costs to both patients and the health-system. Despite these findings, it is important to recognize the increased technical and logistical challenges that are inherent to CI of factor products.
The sample size of our cohort of perioperative UNC haemophilia A patients treated with FVIII (n = 34) is relatively small for an external validation, which could have limited distributions of relevant covariates. This may ultimately explain why covariates identified in the Hazendonk model did not remain significant in our modified UNC popPK model. Despite this limitation, our findings represent an important contribution because they highlight the power of Bayesian-based FVIII adaptive dosing, and should be validated externally at other academic centers. The real-world nature of our study also presented a second limitation because using the recorded FVIII concentrations may not have accurately depicted the true lowest FVIII concentration, which could have affected how baseline FVIII concentrations were defined for each patient. Had patients been followed prospectively prior to surgery, it is possible that quantification of baseline measured FVIII concentrations may have impacted both the Hazendonk model external validation and the UNC popPK model development. Furthermore, our study used OSA to quantify FVIII concentration, which may limit the external validity of these results to studies that use chromogenic assay. Additionally, there was no washout period for patients who received prophylactic FVIII prior to hospital admission, yet our study is innovative because it utilizes real-world data and the final model captures what practicing haematologists will encounter. Similarly, a study conducted by McEneny-King et al. also did not include a washout period as the study utilized real-world data from the Web Accessible Population Pharmacokinetic Service-Haemophilia (WAPPS-Hemo).31 But, McEneny-King did not use a compartmental reset method to account for endogenous FVIII concentrations, which could have limited the ability of their model to capture the full physiologic picture of FVIII in adult haemophilia A patients undergoing surgery. Last, von Willebrand factor (VWF), a carrier protein for FVIII that protects FVIII from protease degradation, could also impact FVIII PK.32,33 However, VWF concentrations are not drawn as routine care at UNC, thereby were not available to PK analysis.
5 |. CONCLUSION
We are the first to perform an external validation of a previously published popPK model of perioperative FVIII for adult haemophilia A patients undergoing surgery.13 The predictive performance of the Hazendonk model did not fully capture FVIII PK in the UNC cohort. Therefore, a modified FVIII popPK model was developed that was more specific to UNC patients. Future prospective studies are needed to evaluate the external validity of the UNC popPK model prior to clinical implementation in this patient population.
ACKNOWLEDGEMENTS
JZ is funded by the National Heart, Lung, and Blood Institute (NHBLI), grant number 5T32HL007149-44. RJB was funded by the National Institute of General Medical Sciences (NIGMS), grant number T32GM086330. WS was funded by a UNC-Nuventra PK/PD Fellowship.
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests.
DATA AVAILABILITY STATEMENT
Deidentified individual data that supports the results will be shared beginning 9 to 36 months following publication provided the investigator who proposes to use the data has approval from an IRB, Independent Ethics Committee (IEC), or Research Ethics Board (REB), as applicable, and executes a data use/sharing agreement with UNC.
REFERENCES
- 1.Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. A United Kingdom Haemophilia Center Doctors’ Organisation (UKHCDO) guideline approved by the British Committee for Standards in Haematology. Haemophilia. 2008;14(4):671–684. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. [DOI] [PubMed] [Google Scholar]
- 3.Björkman S Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia. 2010;16(4):597–605. [DOI] [PubMed] [Google Scholar]
- 4.Brekkan A, Berntorp E, Jensen K, et al. Population pharmacokinetics of plasma-derived factor IX: procedures for dose individualization. J Thromb Haemost. 2016;14(4):724–732. [DOI] [PubMed] [Google Scholar]
- 5.Camire RM. Basic and translational science. Expert Rev Hematol. 2010;3(2):149–151. [DOI] [PubMed] [Google Scholar]
- 6.Collins PW, Fischer K, Morfini M, et al. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17(1):2–10. [DOI] [PubMed] [Google Scholar]
- 7.Carcao M, Iorio A. Individualizing factor replacement therapy in severe hemophilia. Semin Thromb Hemost. 2015;41(8):864–871. [DOI] [PubMed] [Google Scholar]
- 8.Iorio A, Edginton AN, Blanchette V, Spears J. Performing and interpreting individual pharmacokinetic profiles in patients with hemophilia A or B: rationale and general considerations. Res Pract Thromb Haemost. 2018;2(3):535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29(5–6):473–505. [DOI] [PubMed] [Google Scholar]
- 10.Holford NHG, Buclin T. Safe and effective variability-A criterion for dose individualization. Ther Drug Monit. 2012;34(5):565–568. [DOI] [PubMed] [Google Scholar]
- 11.Reuter SE, Evans AM. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet. 2012;51(9):573–590. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. (2019). Population pharma cokinetics Guidance for Industry. https://www.fda.gov/media/128793/download. Accessed 15 March 2021.
- 13.Hazendonk H, Fijnvandraat K, Lock J, et al. A population pharmacokinetic model for perioperative dosing of factor VIII in hemophilia a patients. Haematologica. 2016;101(10):1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan E, Rodgers S. One-stage factor FVIII assays. Methods Mol Biol. 2017;1646:247–263. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26(Suppl. 6):1–158. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization, Executive Board, 143. (2018). International Classification of Diseases: International Statistical Classification of Diseases and Related Health Problems: update on the eleventh revision: report by the Director-General. https://apps.who.int/iris/handle/10665/327092
- 17.Sarkar D Lattice: Multivariate Data Visualization with R. New York: R. Springer; 2008. https://lmdvr.r-forge.r-project.org/figures/figures.html. [Google Scholar]
- 18.Sarkar D, Andrews F. latticeExtra: extra graphical utilities based on lattice. Published. 2016. https://CRAN.R-project.org/package=latticeExtra. Accessed 11 April 2021.
- 19.Ggplot2 WH. Ggplot2: Elegant Graphics for Data Analysis. 2009.
- 20.Sarkar D, Felix A. LatticeExtra: extra graphical utilies based on lattice. CranPackaging. 2017. [Google Scholar]
- 21.Auguie B gridExtra: miscellaneous functions for “Grid” graphics. CranPackaging. 2016. [Google Scholar]
- 22.Wang Y, Liu X. Handling missing dosing history in population pharmacokinetic modeling: an extension to MDM method. CPT Pharmacometrics Syst Pharmacol. 2019;8(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holford NHG, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci. 2017;109:S59–S64. [DOI] [PubMed] [Google Scholar]
- 24.Björkman S, Folkesson A, Jönsson S. Pharmacokinetics and dose requirements of factor VIII over the age range 3–74 years: a population analysis based on 50 patients with long-term prophylactic treatment for haemophilia A. Eur J Clin Pharmacol. 2009;65(10):989–998. [DOI] [PubMed] [Google Scholar]
- 25.Hazendonk H, Moort IV, Fijnvandraat K, et al. A randomised controlled trial on periOperative pharmacokineTIc-guided dosing of CLOTting factor concentrate in haemophilia A. Thromb Haemost. 2015;114(03):639–644. [DOI] [PubMed] [Google Scholar]
- 26.Preijers T, Liesner Ri, Hazendonk HCAM, et al. Validation of a perioperative population factor VIII pharmacokinetic model with a large cohort of pediatric hemophilia A patients. Br J Clin Pharmacol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noe D, Bell W, Ness P, et al. Plasma clearance rates of coagulation factors VIII and IX in factor-deficient individuals. Blood. 1986;67(4):969–972. [PubMed] [Google Scholar]
- 28.Zhang Y, Roberts J, Tortorici M, et al. Population pharmacokinetics of recombinant coagulation factor VIII-SingleChain in patients with severe hemophilia A. J Thromb Haemost. 2017;15(6):1106–1114. [DOI] [PubMed] [Google Scholar]
- 29.Bidlingmaier C, Deml M-M, Kurnik K. Continuous infusion of factor concentrates in children with haemophilia A in comparison with bolus injections. Haemophilia. 2006;12(3):212–217. [DOI] [PubMed] [Google Scholar]
- 30.Batorova A, Martinowitz U. Intermittent injections vs. continuous infusion of factor VIII in haemophilia patients undergoing major surgery. Br J Haematol. 2000;110(3):715–720. [DOI] [PubMed] [Google Scholar]
- 31.Mceneny-King A, Chelle P, Foster G, et al. Development and evaluation of a generic population pharmacokinetic model for standard half-life factor VIII for use in dose individualization. J Pharmacokinet Pharmacodyn. 2019;46(5):411–426. [DOI] [PubMed] [Google Scholar]
- 32.Wise RJ, Dorner AJ, Krane M, et al. The role of von Willebrand factor multimers and propeptide cleavage in binding and stabilization of factor VIII. J Biol Chem. 1991;266(32):21948–21955. [PubMed] [Google Scholar]
- 33.Swystun LL, Ogiwara K, Rawley O, et al. Genetic determinants of VWF clearance and FVIII binding modify FVIII pharmacokinetics in pediatric hemophilia A patients. Blood. 2019;134(11):880–891. [DOI] [PubMed] [Google Scholar]


