Abstract
In this study, different dairy products such as ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk were manufactured by using either Lactobacillus acidophilus DSM 20,079 or Lactobacillus acidophilus NCFM. The counts of L. acidophilus in the samples on days 1, 15, and 30 of the storage were determined. Additionally, the samples contained L. acidophilus were passed through a dynamic gastrointestinal model designed in laboratory conditions to compare the protective effect of different dairy products on viability of L. acidophilus against stress factors of the gastrointestinal model. The counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the samples decreased by between 0.04 and 0.37 log units and by between 0.11 and 0.27 log units, respectively, within 30 days of storage. During the passage through the gastrointestinal model, the highest percentage reduction in the counts of L. acidophilus was determined in yoghurt followed by fermented acidophilus milk, white pickled cheese, and ice cream, respectively. The reduction in the counts of L. acidophilus in the samples during the passage through the model increased with extension of storage time. The results of this study showed that the reduction in the counts L. acidophilus in the samples during the passage through the model was influenced significantly by the matrix of the dairy product and storage period.
Keywords: Ice cream, Yoghurt, White pickled cheese, Fermented acidophilus, Lactobacillus acidophilus, Dynamic gastrointestinal model
Introduction
An increased demand on the probiotic microorganisms occurred due to increase of the resistance of pathogens against antibiotics and tendency of consumers for functional foods instead of pharmaceuticals [1]. Fermented dairy products with probiotic microorganisms are recognized as functional foods due to their health-promoting properties on the consumer’s body [2]. The uptake of many of probiotic microorganisms occurs via fermented dairy products, which are considered among the most important probiotic carrier foods [3].
The viability of probiotic microorganism in the fermented dairy products is influenced by various factors such as chemical composition of the fermentation environment (source of carbohydrate), pH value after fermentation, total solid content of milk, the usage of nutrients, growth supporters or preventers, type of bacterial strain, interaction between the strains, sugar concentration (osmotic pressure), dissolved oxygen, level of inoculation, temperature of incubation, time of fermentation, and storage conditions [4]. Probiotic microorganisms have to survive during the processing as well as during the passage through the gastrointestinal system [5, 6]. The resistances to stomach acid and bile salts are two essential properties for the survival of probiotic microorganisms in the human gastrointestinal tract. Various in vivo and in vitro studies have been carried out to determine the viability of probiotic microorganisms in the gastrointestinal conditions [7–9]. Determination of probiotic properties of microorganisms used in the production of foods and assessment of the factors affecting the viability of probiotic microorganisms during transit through gastrointestinal system via in vivo studies are very complex [10, 11]. Compared with in vivo studies, in vitro studies are easy, fast, and reliable, and can be used without ethical constraints. There are two types of in vitro gastrointestinal models simulating gastrointestinal digestive system, namely dynamic and static types. In dynamic models, physical and mechanical processes and temporal changes occurring in vivo conditions can be simulated. In static models, physical processes that occur in vivo conditions such as mixing, shear, and hydration are not accounted and digestion products remain mostly immobile [12, 13].
Several strategies have been suggested to protect probiotic microorganisms against gastrointestinal conditions, such as stress adaptation, use of prebiotics, microencapsulation, two-step fermentation application, and use of oxygen impermeable containers as well as selection of appropriate carrier food matrix [5, 6]. The food matrix has an important role for the gastrointestinal resistance of probiotic microorganisms [14]. Sanders and Marco [15] reported that probiotic microorganisms survive better in dairy products such as drinking milk, cheese, and yoghurt compared with either saline or buffer solutions during exposure to gastrointestinal conditions.
Although various studies on the use of probiotic bacteria in the manufacture of dairy products have been carried out, as far as we know, no study has been conducted that compares the viability of the same probiotic bacteria used in the production of different dairy products during passage through the dynamic in vitro gastrointestinal model. The aim of the present study was to determine the viability of two strains of L. acidophilus used in the production of different dairy products, such as ice cream, yoghurt, and white pickled cheese as well as fermented acidophilus milk and to assess the viability of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in the yoghurt samples and the viability of total mesophilic aerobic bacteria and total Lactococcus spp. in the white pickled cheese samples during storage for 30 days and after passage through the dynamic in vitro gastrointestinal model. Furthermore, the effect of strain type of L. acidophilus used in the production of the different dairy products on the survival of L. acidophilus was investigated.
Materials and methods
Bacterial strains
In this work, two well-documented probiotic strains, L. acidophilus DSM 20,079 and L. acidophilus NCFM [16, 17], were used. L. acidophilus DSM 20,079 (Leibniz Institute DSMZ, Braunschweig, Germany) and L. acidophilus NCFM (Howaru® Dophilus) were kindly provided by the Technical University of Munich (Germany) and obtained from Danisco A/S (Copenhagen, Denmark), respectively. The cheese starter culture (R-704-DVS) containing mainly Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis, and yoghurt starter culture (CH-1 Yo-Flex) containing L. delbrueckii subsp. bulgaricus and S. thermophilus were purchased from Chr. Hansen A/S (Horsholm, Denmark).
Manufacture of the dairy products
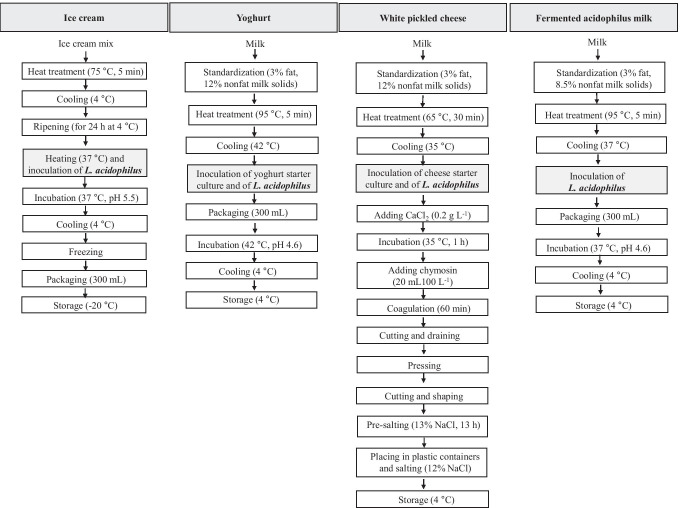
Raw cow’s milk (pH value of 6.7 ± 0.1, titratable acidity value of 0.1 ± 0.0%, total solid content of 11.8 ± 0.1%, protein content of 3.2 ± 0.2%, fat content of 2.8 ± 0.3%, and ash content of 1.1 ± 0.1%) used in this study was purchased from the Dairy Processing Unit of the Faculty of Agriculture at Akdeniz University. Figure 1 illustrates the production lines for the manufacture of the dairy products as ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk containing L. acidophilus DSM 20,079 or L. acidophilus NCFM, indicating the inoculation steps of L. acidophilus. Ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk were produced in laboratory scale. All productions were made in duplicate.
Fig. 1.
Production lines for the manufacture of ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk containing L. acidophilus, indicating the inoculation steps of L. acidophilus
Ice cream
The ice cream was produced according to the method of Ergin et al. [18]. In 2.5 kg of ice cream formulation, 269 g skim milk powder, 450 g sucrose, 86 g butter, 14 g stabilizer, and 1681 g water were used. Ice cream mix composition was 3% (w/w) fat, 10% (w/w) non-fat milk solids, 18% (w/w) sugar, 0.5% (w/w) stabilizer, and 68.5% (w/w) water. The mixture was heated at 75 °C for 5 min and cooled to 4 °C. The mixture was homogenized using a mechanical mixer (Bosch, Mixxo Quattro MSM 7700, Jesenice, Slovenia) during the heat treatment. The cooled mix was ripened overnight at 4 °C. After the ripening period, the temperature of the mix was adjusted to 37 °C and the mix was inoculated with L. acidophilus DSM 20,079 or L. acidophilus NCFM so as to obtain a final concentration of at least 108 cfu (colony-forming unit) L. acidophilus per gram of the ice cream. The mix was fermented at 37 °C until a pH of 5.5 was reached. The fermentation was ended by cooling the mix to 4 °C, followed by freezing. The ice cream mixes were frozen in a batch type freezer (M10C, Mehen Food Machine Manufacture Co. Ltd., Nanjing, China) with a 10 kg capacity and without air incorporation. The ice cream samples were packaged in 300 mL glass cups with lids and stored at − 20 °C for 30 days.
Yoghurt
Yoghurt production was performed as described by [19]. The milk was standardized to have 3% (w/w) fat and 12% (w/w) non-fat milk solids. After heating at 95 °C for 5 min, it was cooled to 42 °C. The milk was inoculated with the yoghurt starter culture at the ratio of 0.03 g L−1, and then L. acidophilus DSM 20,079 or L. acidophilus NCFM inoculated into the milk to achieve a concentration of at least 108 cfu L. acidophilus per gram of the yoghurt. The inoculated milk was filled into 300 mL glass cups with lids and incubated at 42 °C until a pH of 4.6 was achieved. After cooling, the yoghurt samples were stored at 4 °C for 30 days.
White pickled cheese
White pickled cheese samples were manufactured according to the method of Kasımoğlu, Göncüoğlu, and Akgün [20] with some modifications. After standardization of milk to 3% (w/w) fat and 12% (w/w) non-fat milk solids, heat treatment was applied to milk at 65 °C for 30 min, and then it was cooled to 35 °C. The milk was inoculated with the cheese starter culture at 0.04 g L−1, and then L. acidophilus DSM 20,079 or L. acidophilus NCFM inoculated into the milk so as to obtain a final concentration of at least 108 cfu L. acidophilus per gram of the white pickled cheese. After inoculation, CaCl2 (0.2 g L−1) was added to the milk and the milk was incubated at 35 °C for 1 h. Then, chymosin was added to the milk at a level sufficient to coagulate the milk in 60 min (20 mL 100 L−1; Mayasan A.S., Istanbul, Turkey). The coagulum was cut into cubes (1 cm3) and held for 15 min for whey separation. The curds were transferred into perforated molds lined with cheesecloth for further drainage of whey.
and pressed (7 kg weight for 10 L−1 milk) for about 15 h at 20 °C. Then, the curd was cut into cubic pieces (7 × 7 × 7 cm3) to shape and these shaped curds were placed into brine (13%, w/v, NaCl) at 20 °C for about 13 h. After brine-salting, cheeses were placed in plastic containers. The plastic containers were filled with brine (12%, w/v, NaCl), closed, and then stored at 4 °C for 30 days.
Fermented acidophilus milk
Fermented acidophilus milk production was performed according to method of Božanić et al. [21], with minor modifications. After standardization of milk to 3% (w/w) fat and 8.5% (w/w) non-fat milk solids, it was heated at 95 °C for 5 min and then cooled to 37 °C. The milk was inoculated with L. acidophilus DSM 20,079 or L. acidophilus NCFM to achieve a concentration of at least 108 cfu L. acidophilus per milliliter of the fermented acidophilus milk. The inoculated milk was filled into 300 mL glass cups with lids and incubated at 37 °C until a pH of 4.6 reached. The samples were stored at 4 °C for 30 days.
Design of dynamic gastrointestinal model
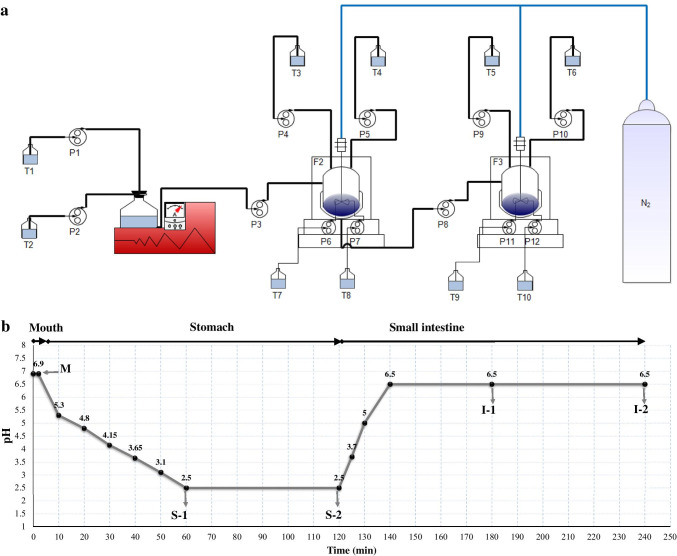
A dynamic gastrointestinal model to simulate the physiological condition characteristics of human upper gastrointestinal tract consists of three main parts in order to simulate the mouth, stomach, and small intestine (Fig. 2a). For the part representing the mouth, a 750 mL bottle placed into the temperature-controlled water bath (JS Research Inc., JSRC-22(C)(CL), Chungcheongnam-do, Korea) and for the parts representing stomach and small intestine two temperature-controlled glass bioreactors (New Brunswick Scientific Co., BF-115, New Jersey, USA, and Electrolab Biotech Ltd., FerMac 320 Bioreactor, Tewkesbury, UK, respectively) have been used. The working volume of each bioreactor was 3.0 L. The bottle and bioreactors were autoclaved at 121 °C for 15 min before use. The temperatures of the water bath and bioreactors were kept constant at 37.0 ± 0.1 °C. In the bioreactors, pH value and mixing speed were controlled, and anaerobic conditions were maintained by purging with nitrogen during experiments. Mucin from porcine stomach (M2378, Sigma-Aldrich Co., St. Louis, USA) and α-amylase from porcine pancreas (A3176, Sigma-Aldrich Co., St. Louis, USA) to simulate saliva fluid, mucin and pepsin from porcine gastric mucosa (P700, Sigma-Aldrich Co., St. Louis, USA) to simulate stomach solution, and pancreatin from porcine pancreas (P7545, Sigma-Aldrich Co., St. Louis, USA) and bile salt mixture (B3426, Sigma-Aldrich Co., St. Louis, USA) to simulate small intestine solution were used in the model. All solutions of enzymes were freshly prepared. Furthermore, the time spent in the gastrointestinal model was controlled and arranged as follows: 2 min in the mouth part, 2 h in the stomach part, and 2 h in the small intestine part (Fig. 2b). In the model, total twelve peristaltic pumps were used in order to pump the simulated digestion solutions, control pH values, and as well as transfer the sample, which was exposed to the processes in the model, from mouth to stomach and from stomach to small intestine. The flow rates of these pumps were determined in preliminary experiments. Four of these pumps served for the flow of NaHCO3 (1 M) and HCl (1 M) to arrange the pH values in the stomach and small intestine parts of the model. Approximately 300 g of yoghurt, ice cream, and fermented acidophilus milk samples containing 108 cfu g−1 or mL−1 of L. acidophilus and 100 g of white pickled cheese samples containing 108 cfu g−1 of L. acidophilus were used in the experiments in the dynamic gastrointestinal model. The yoghurt, ice cream, or fermented acidophilus milk samples were mixed with sterile water at room temperature at a ratio of 1:1 (w/v or v/v), while the white pickled cheese sample was mixed with sterile water at room temperature at a ratio of 1:5 (w/v). The sample-water mixture was homogenized using a stomacher (Laboratory blender stomacher 80, Seward Medical, London, UK) for 2 min. Digestion conditions were modified from gastrointestinal models previously developed by Madureira et al. [22], Marteau et al. [23], and Sumeri et al. [10] (Table 1). The homogenized mixture was added into 750 mL of sterile bottle placed into the water bath to perform the study of the mouth part of the model. After introducing the mixture into the mouth part, the pH of the mixture in the bottle was adjusted to 6.9 with 40% NaOH solution. Then, the saliva solution was added to the bottle containing the mixture at a flow rate of 5 mL min−1. One hundred milliliters of 0.01 M HCl was added into the first bioreactor called stomach part of the model to imitate the empty stomach. Then, the contents of the bottle representing the mouth part of the model were pumped at 100 mL min−1 into the stomach part containing HCl. Then, the stomach solution was added to the bioreactor at a flow rate of 0.25 mL min−1. In the process of adding the stomach solution completed about 60 min, the pH value of the contents of the stomach part was gradually reduced to 2.5. After completion of the addition of the stomach solution, the pH value of the contents of the stomach part was kept at 2.5 for 60 min. Thereafter, the contents of the bioreactor representing the stomach part of the model were pumped at 100 mL min−1 into the second bioreactor called small intestine part of the model. Then, the small intestine solution was added to the bioreactor at a flow rate of 3 mL min−1. In the process of adding the small intestine solution completed about 20 min, the pH value of the contents of the small intestine part was gradually increased to 6.5. After completion of the addition of the small intestine solution, the pH value of the contents of the small intestine part was kept at 6.5 for 100 min. Time-dependent pH changes in the contents of the parts of the model are shown in Fig. 2b. At three different times of storage, after the passage of the dairy products containing L. acidophilus through the dynamic gastrointestinal model, the counts of L. acidophilus in all dairy products, the counts of L. delbrueckii subsp. bulgaricus and S. thermophilus in yoghurt samples, and the counts of total mesophilic aerobic bacteria and total Lactococcus spp. in white pickled cheese samples were determined. The experiments performed with the dynamic in vitro gastrointestinal model were replicated in duplicate.
Fig. 2.
Dynamic in vitro gastrointestinal model to simulate the physiological conditions of human upper gastrointestinal tract. T1: 40% NaOH solution (to adjust pH to 6.9); T2: saliva solution; T3: 1 M HCl solution (to reduce pH from 6.9 to 2.5); T4: stomach solution; T5: 1 M NaHCO3 solution (to increase pH from 2.5 to 6.5); T6: small intestine solution; T7 and T9: 1 M HCl solution (to keep pH values constant at 2.5); T8 and T10: 1 M NaHCO3 solution (to keep pH values constant at 6.5); F1: mouth part of the model; F2: stomach part of the model; F3: small intestine part of the model; N2: nitrogen; P (1–12): peristaltic pumps (a). Sampling positions in the dynamic gastrointestinal model. M: end of mouth; S-1: stomach after 1 h digestion; S-2: stomach after 2 h digestion; I-1: small intestine after 1 h; I-2: small intestine after 2 h (b)
Table 1.
Description and preparation of the dynamic in vitro model system
| Intestinal segment | Description | Preparation | pH | Retention time (min) | Volume (mL) |
|---|---|---|---|---|---|
| Mouth | Chewing | 300 g of yoghurt, ice cream and fermented acidophilus milk samples were mixed with 300 g of sterile water at 37 °C, 100 g of white pickled cheese samples were mixed with 500 g of sterile water at 37 °C, and the samples were homogenized | - | - | |
| Simulated saliva solution | 2 g L−1 α-amylase and 1 g L−1 mucin were dissolved in sterile water. The simulated saliva solution (0.05 mL g−1 sample) was added to the mouth part (5 mL min−1) | 6.9 | 2 | 10 | |
| Stomach | Simulated stomach solution | 25 g L−1 pepsin and 23 g L−1 mucin were dissolved in sterile stomach buffer solution*. The sample exposed to digestion in the mouth part was fed to the reactor simulating the stomach (100 mL min−1). The simulated stomach solution was fed to the stomach part (0.25 mL min−1) | 6.9→2.5 | 120 | 15 |
| Small intestine | Simulated small intestine solution | 1 g L−1 pancreatin and 12 g L−1 mucin were dissolved in sterile small intestine buffer solution**. The sample exposed to digestion in the stomach part was fed to the reactor simulating the small intestine (100 mL min−1). The simulated intestine solution was fed to the intestine part (3 mL min −1) | 2.5→6.5 | 120 | 60 |
*Stomach buffer solution: 2.2 g L−1 KCl, 6.2 g L−1 NaCl, 1.2 g L−1 NaHCO3, 0.22 g L−1 CaCl2
**Small intestine buffer solution: 0.6 g L−1 KCl, 5.0 g L−1 NaCl, 0.25 g L−1 CaCl2
Analysis of the dairy products
Physicochemical analysis
Total solid content (%), fat content (%), protein content (%), and ash content (%) of the samples and the raw milk used for the manufacture of the dairy products were determined using gravimetric, Gerber, Kjeldahl, and gravimetric methods, respectively [24]. Percentage of titratable acidity for the samples was measured by the method of Bradley et al. [25]. The pH values of the milk and the samples were measured using a pH meter (Orion 2-Star, Thermo Scientific, Bremen, Germany).
Microbiological analysis
The microbiological analysis of the ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples taken from the dynamic gastrointestinal model was conducted on days 1, 15, and 30 of the storage of the samples. The pour plate technique was used.
Microbiological analysis of ice cream samples
L. acidophilus counts were determined after frozen storage at − 20 °C. Serial dilutions of the ice cream samples (1 g) were made in 9 mL ringer solution (1/4). One milliliter aliquot dilutions were poured onto plates of the MRS agar (Merck KGaA, Darmstadt, Germany). All plates were incubated anaerobically at 37 °C for 72 h and the results were expressed as colony-forming units per gram of the sample [26].
Microbiological analysis of yoghurt samples
L. acidophilus counts in the yoghurt samples were determined using MRS agar with bromocresol green and clindamycin (MRS-BC agar). The plates were incubated anaerobically at 37 °C for 72 h [2]. For the determination of the counts of L. delbrueckii subsp. bulgaricus, MRS agar was used and the plates were incubated at 45 °C for 72 h [25]. The counts of S. thermophilus were conducted using M17 agar (Merck KGaA, Darmstadt, Germany) containing 1% (w/v) lactose after incubation at 45 °C for 24 h [27].
Microbiological analysis of white pickled cheese samples
The counts of total mesophilic aerobic bacteria were performed using Plate Count Agar (Merck KGaA, Darmstadt, Germany) and total Lactococcus spp. were enumerated on M17 agar according to the methods of Evrendilek et al. [28] and IDF [29], respectively. MRS-sorbitol agar was used for the selective enumeration of L. acidophilus according to the method described by Ong et al. [30]. The agar was prepared by adding 10 mL of sterile solutions (10% (w/v), membrane filtered) of sorbitol (Sigma-Aldrich Co., St. Louis, USA) to 90 mL of molten MRS agar just before pouring. The plates were incubated anaerobically at 37 °C for 72 h.
Microbiological analysis of fermented acidophilus milk samples
L. acidophilus counts were performed using MRS agar with anaerobic incubation at 37 °C for 72 h [31].
The percentage of reduction in the counts of L. acidophilus in the dynamic in vitro gastrointestinal model .
The percentage reduction in the counts of L. acidophilus in the 1-, 15-, and 30-day stored samples during the passage through each part of the dynamic in vitro gastrointestinal model was calculated according to the slightly modified method of Kos et al. [32] using the equation given below:
where N0 is the count of L. acidophilus in the 1-, 15-, and 30-day stored samples before exposure to each part of the gastrointestinal model and N is the count of L. acidophilus in the 1-, 15-, and 30-day stored samples exposed to each part of the gastrointestinal model.
Statistical analyses
In this study, all measurements were carried out in duplicate. All statistical analyses were performed using SAS Statistical Software (release for Windows, SAS Institute Inc., Cary, NC, USA). A two-factor ANOVA was conducted to determine the effects of gastrointestinal model conditions and storage period on the count of L. acidophilus DSM 20,079 and L. acidophilus NCFM in all samples. The Duncan’s multiple range test was used to detect differences among the means.
Results and discussion
Physicochemical properties
The mean total solids, protein, fat and ash contents, and titratable acidity and pH values of the ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples manufactured by using L. acidophilus DSM 20,079 or L. acidophilus NCFM are presented in Table 2. The chemical composition of the samples in the present study was generally similar to that found by Ranadheera et al. [33] for the ice cream, by Ribeiro et al. [34] for the yoghurt, by Kasımoğlu et al. [20] for the white pickled cheese, and by Akpınar [35] for the fermented acidophilus milk. The titratable acidity values of the samples investigated in this study increased with 30-day storage period, while the pH values of the samples decreased during storage. These agree with the results reported by Afzaal et al. [36] for ice cream, by Moschopoulou et al. [37] for yoghurt, by Kılıç et al. [38] for cheese, and by Junaid et al. [39] for fermented acidophilus milk. During 30-day storage, the decreases in the pH values of yoghurt, cheese, and fermented asidophilus milk produced by both strains of L. acidophilus and the increases in the titratable acidity values of yoghurt and fermented asidophilus milk produced by both strains of L. acidophilus and of ice cream produced by L. acidophilus DSM 20,079 were significant. The increase in titratable acidity and decrease in pH during storage are most probably due to formation of lactic acid from lactose by lactic acid bacteria [40].
Table 2.
Physicochemical properties of the ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples produced by using L. acidophilus DSM 20,079 or L. acidophilus NCFM after storage
| Analysis | L. acidophilus | Storage period | Ice cream | Yoghurt | Cheese | Fermented acidophilus milk |
|---|---|---|---|---|---|---|
| Total solids (%) | DSM 20,079 | 1-day | 29.1 ± 0.3 | 15.2 ± 0.2 | 41.4 ± 0.1 | 11.5 ± 0.1 |
| NCFM | 1-day | 29.2 ± 0.4 | 15.1 ± 0.1 | 41.2 ± 0.1 | 11.5 ± 0.1 | |
| Fat (%) | DSM 20,079 | 1-day | 2.9 ± 0.2 | 3.0 ± 0.1 | 16.7 ± 0.1 | 3.0 ± 0.1 |
| NCFM | 1-day | 2.9 ± 0.1 | 3.0 ± 0.1 | 16.8 ± 0.1 | 3.0 ± 0.1 | |
| Protein (%) | DSM 20,079 | 1-day | 3.4 ± 0.1 | 4.2 ± 0.1 | 17.9 ± 0.1 | 3.5 ± 0.1 |
| NCFM | 1-day | 3.5 ± 0.1 | 4.1 ± 0.1 | 17.7 ± 0.6 | 3.4 ± 0.1 | |
| Ash (%) | DSM 20,079 | 1-day | 0.8 ± 0.1 | 1.0 ± 0.1 | 2.7 ± 0.1 | 1.0 ± 0.1 |
| NCFM | 1-day | 0.8 ± 0.1 | 1.0 ± 0.1 | 2.7 ± 0.1 | 1.0 ± 0.1 | |
| Titratable acidity (%) | DSM 20,079 | 1-day | 0.33 ± 0.01b* | 1.05 ± 0.07c | 0.26 ± 0.05a | 0.91 ± 0.01b |
| DSM 20,079 | 15-day | 0.35 ± 0.01ab | 1.25 ± 0.15b | 0.29 ± 0.01a | 0.98 ± 0.01ab | |
| DSM 20,079 | 30-day | 0.38 ± 0.02a | 1.44 ± 0.04a | 0.31 ± 0.04a | 1.03 ± 0.03a | |
| NCFM | 1-day | 0.34 ± 0.01a | 1.06 ± 0.09c | 0.25 ± 0.01a | 0.93 ± 0.01b | |
| NCFM | 15-day | 0.35 ± 0.03a | 1.38 ± 0.11b | 0.26 ± 0.03a | 1.07 ± 0.02a | |
| NCFM | 30-day | 0.36 ± 0.01a | 1.53 ± 0.06a | 0.30 ± 0.01a | 1.10 ± 0.01a | |
| pH | DSM 20,079 | 1-day | 5.52 ± 0.01a | 4.45 ± 0.03a | 5.86 ± 0.01a | 4.58 ± 0.01a |
| DSM 20,079 | 15-day | 5.50 ± 0.01a | 4.20 ± 0.02b | 5.81 ± 0.01ab | 4.49 ± 0.02b | |
| DSM 20,079 | 30-day | 5.48 ± 0.01a | 4.01 ± 0.03c | 5.78 ± 0.01b | 4.43 ± 0.01b | |
| NCFM | 1-day | 5.51 ± 0.01a | 4.47 ± 0.01a | 5.90 ± 0.01a | 4.55 ± 0.01a | |
| NCFM | 15-day | 5.50 ± 0.01a | 4.28 ± 0.05b | 5.87 ± 0.01ab | 4.46 ± 0.01b | |
| NCFM | 30-day | 5.47 ± 0.01a | 4.06 ± 0.01c | 5.84 ± 0.01b | 4.37 ± 0.03c |
Values (mean ± standard deviation) of total solids, fat, protein and ash contents, and titratable acidity and pH values of the samples. *For the titratable acidity and pH values of the each sample manufactured by using same strain of L. acidophilus, means with different letters show the difference in the same column (P < 0.05)
Microbiological properties of the dairy products during storage
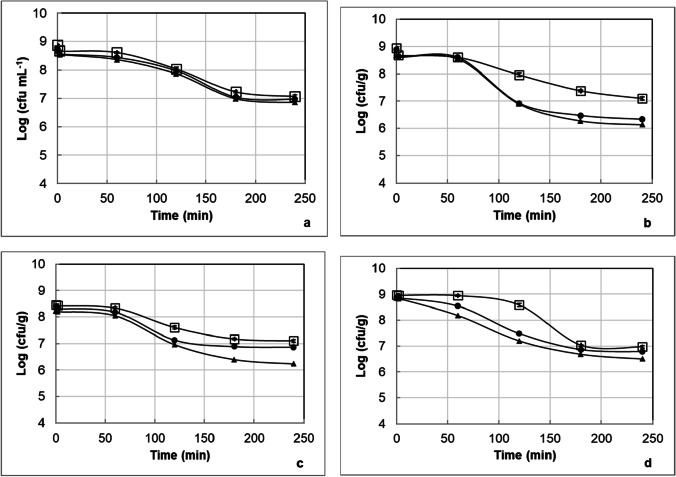
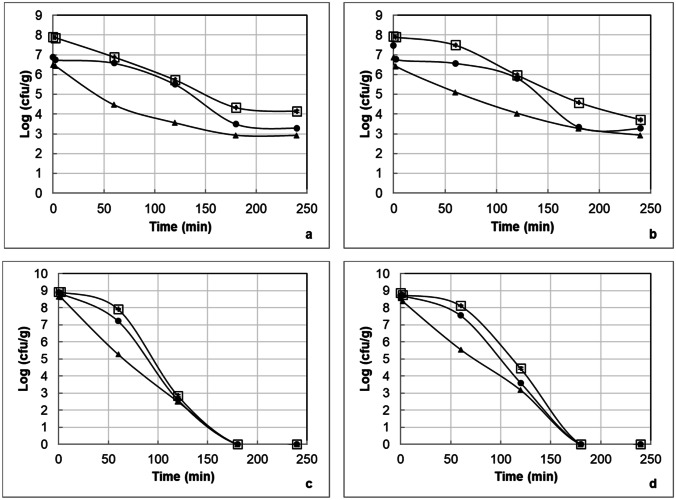
The survival of L. acidophilus NCFM and L. acidophilus DSM 20,079 in ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples on the 1st, 15th, and 30th days of the storage is illustrated in Fig. 3 and Fig. 4, respectively.
Fig. 3.
The counts of L. acidophilus in the ice cream (a), yoghurt (b), white pickled cheese (c), and fermented acidophilus milk (d) samples produced with L. acidophilus DSM 20,079 on the 1st (white square), 15th (black circle), and 30th (black triangle) days of the storage during the passage through the dynamic in vitro gastrointestinal model
Fig. 4.
The counts of L. acidophilus in the ice cream (a), yoghurt (b), white pickled cheese (c), and fermented acidophilus milk (d) samples produced with L. acidophilus NCFM on the 1st (white square), 15th (black circle), and 30th (black triangle) days of the storage during the passage through the dynamic in vitro gastrointestinal model
The counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the ice cream samples stored 1 day at − 20 °C were approximately 8.9 log cfu g−1. At the end of the 30-day storage period, the counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the ice cream samples decreased about 0.4 and 0.2 log units, respectively. The incorporation of oxygen into the ice cream mix may reduce the count of probiotic bacteria in the ice cream. The freezing process may have influenced the number of probiotic bacteria in the ice cream, because batch freezing, used in the present study, is less efficient for incorporating air as compared to continuous freezing [41]. Another explanation for this finding may be the stress adaptation of the strains of L. acidophilus used in this study. The fermentation of ice cream mix may provide protection to the probiotic bacteria in the ice cream against cold stress, since the resistance of probiotic bacteria in the ice cream to cold stress may be improved by applying acid stress caused by the fermentation of ice cream mix [18].
In the present study, the viable counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the yoghurt samples stored 1 day at 4 °C were 8.66 and 8.94 log cfu g−1, respectively. The viable counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the yoghurt samples decreased about 0.04 and 0.27 log units, respectively, within 30 days of the storage. Geraldi et al. [42] and Ribeiro et al. [34] reported that the number of L. acidophilus in probiotic yoghurt decreased from 8.18 to 7.28 log cfu g−1 and from 8.74 to 7.64 log cfu mL−1, respectively, during 30 days storage at about 4 °C. Nighswonger et al. [43] reported that some strains of L. acidophilus in yoghurt lost viability during storage at 7 °C for 28 days, while others maintained at near constant levels in yoghurt during the storage. However, Ng et al. [44] found that the count of L. acidophilus NCFM in yoghurt reduced by 4.6 logs during 28-day storage at 4 °C. They reported that L. acidophilus NCFM could be adversely affected from the elevated level of hydrogen peroxide produced by L. delbrueckii ssp. bulgaricus when grown in co-culture with the yoghurt starter culture. The contrasting findings regarding the viability of L. acidophilus NCFM in yoghurt during storage between the present study and the study of Ng et al. [44] may be due to the difference in strains of yoghurt starter bacteria used in the production of yoghurt. The survival of the probiotic bacteria may sometimes be threatened by the metabolic activities, e.g., productions of lactic acid and hydrogen peroxide, of yoghurt starter bacteria during incubation of milk and storage of yoghurt [6].
The counts of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the white pickled cheese samples remained at a constant level of 8 log cfu g−1 during the 30 days of the storage at 4 °C. Kılıç et al. [38] reported that the viability of probiotic bacteria was satisfactory in white pickled cheese even at the end of storage periods. Kasımoğlu et al. [20] manufactured probiotic white pickled cheese samples with L. acidophilus and found the numbers of L. acidophilus in the 30-day stored samples were greater than 7.0 log cfu g−1.
The numbers of L. acidophilus NCFM and L. acidophilus DSM 20,079 in the fermented acidophilus milk samples stored 1 day at 4 °C were 8.92 and 8.96 log cfu mL−1, respectively, and these counts remained stable up until the end of the storage period (30 days). Božanić et al. [21] reported fermented acidophilus cow’s milk contained, after 30 days of storage, over 7.5 log cfu mL−1 of viable L. acidophilus. pH value is a critical factor for the survival of L. acidophilus in acidophilus milk, with a decrease in pH value of acidophilus milk less than 4.5 reported to affect the viability of L. acidophilus [45]. Since the pH value of the fermented acidophilus milk samples containing Lb. acidophilus was around 4.5 during the 30-day storage period in this study, the viability of Lb. acidophilus in the fermented acidophilus milk was not negatively affected by the pH value.
The differences in strains of L. acidophilus and L. acidophilus-carrying dairy products, such as ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk, did not affect the viability of L. acidophilus in the product during the storage. A longer storage time of the dairy products samples resulted in slight decrease in the survival of the strains of L. acidophilus. In order to achieve the health benefits of probiotic bacteria for humans, the minimum viable count of probiotic bacteria should be ≥ 6 log cfu g−1 or mL−1 in the product up to the expiry date [18]. However, the numbers of viable L. acidophilus in all dairy products investigated in this study were equal or above 8 log cfu g−1 or mL−1 at the end of 30-day storage period.
The effects of the storage period on the counts of L. acidophilus in the samples during passage through the dynamic in vitro gastrointestinal model are given in Table 3. The statistical analysis showed that the effects of gastrointestinal model conditions and storage period on the counts of L. acidophilus DSM 20,079 and L. acidophilus NCFM in the samples were significant (P < 0.001). The counts of L. acidophilus NCFM in the ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples decreased 1.40, 2.22, 2.16, and 2.41 log units at the end of the small intestine part of the model, respectively, according to the number of L. acidophilus NCFM in the samples at the end of the mouth part of the model, while the counts of L. acidophilus DSM 20,079 in the ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples decreased 1.63, 2.11, 1.57, and 2.12 log units, respectively. A decrease in the counts of the strains of L. acidophilus was recorded in the samples during the passage through the model (Figs. 3 and 4). The decrease in the viable counts of L. acidophilus in the samples during the passage through the model increased significantly for all products with extension of storage time, as shown in Table 3.
Table 3.
Effects of the storage period on the counts of L. acidophilus in the samples during passage through the dynamic in vitro gastrointestinal model
| Count of L. acidophilus NCFM (log cfu g−1 or mL−1) | Count of L. acidophilus DSM 20,079 (log cfu g−1 or mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ice cream | Yoghurt | White pickled cheese | Fermented acidophilus milk | Ice cream | Yoghurt | White pickled cheese | Fermented acidophilus milk | |
| Gastrointestinal model conditions | *** | *** | *** | *** | *** | *** | *** | *** |
| End of mouth | 8.67 ± 0.07 a* | 8.66 ± 0.03 a | 8.74 ± 0.14 a | 8.81 ± 0.03 a | 8.60 ± 0.02 a* | 8.64 ± 0.01 a | 8.31 ± 0.04 a | 8.88 ± 0.02 a |
| Stomach after 1 h | 8.34 ± 0.17 b | 8.60 ± 0.02 a | 8.28 ± 0.16 b | 8.73 ± 0.03 b | 8.48 ± 0.05 b | 8.59 ± 0.02 a | 8.18 ± 0.06 b | 8.56 ± 0.14 b |
| Stomach after 2 h | 8.09 ± 0.16 c | 7.17 ± 0.19 b | 8.00 ± 0.13 c | 7.82 ± 0.36 c | 7.96 ± 0.03 c | 7.26 ± 0.22 b | 7.23 ± 0.12 c | 7.76 ± 0.27 c |
| Small intestine after 1 h | 7.46 ± 0.18 d | 6.61 ± 0.15 c | 6.71 ± 0.17 d | 6.67 ± 0.28 d | 7.09 ± 0.05 d | 6.71 ± 0.21 c | 6.82 ± 0.14 d | 6.86 ± 0.06 d |
| Small intestine after 2 h | 7.27 ± 0.15 e | 6.44 ± 0.16 d | 6.58 ± 0.22 d | 6.40 ± 0.27 e | 6.97 ± 0.04 e | 6.53 ± 0.19 d | 6.74 ± 0.16 e | 6.76 ± 0.09 e |
| Storage period (days) | *** | *** | *** | *** | *** | *** | *** | *** |
| 1 | 8.27 ± 0.17 a | 7.88 ± 0.25 a | 8.03 ± 0.24 a | 8.16 ± 0.25 a | 7.93 ± 0.02 a | 7.95 ± 0.21 a | 7.73 ± 0.19 a | 8.09 ± 0.30 a |
| 15 | 8.10 ± 0.16 b | 7.44 ± 0.33 b | 7.69 ± 0.31 b | 7.75 ± 0.34 b | 7.80 ± 0.23 b | 7.39 ± 0.34 b | 7.47 ± 0.21 b | 7.71 ± 0.28 b |
| 30 | 7.53 ± 0.21 c | 7.25 ± 0.38 c | 7.26 ± 0.33 c | 7.14 ± 0.44 c | 7.73 ± 0.23 c | 7.30 ± 0.36 c | 7.17 ± 0.27 c | 7.48 ± 0.30 c |
Values are expressed mean ± standard deviation
*Values with different letters show the difference in the same column (P < 0.05), ***P < 0.001
The effects of dairy products, gastrointestinal model conditions, and storage period on the percent reduction of L. acidophilus counts in the samples are given in Table 4. The results showed that reduction of viable L. acidophilus counts was influenced significantly (P < 0.05) by the type of dairy products, gastrointestinal model conditions, and storage period. The highest decrease in the counts of L. acidophilus was determined in yoghurt sample followed by fermented acidophilus milk, white pickled cheese, and ice cream, respectively. However, the differences in the percentage reduction in counts of L. acidophilus NCFM between yoghurt and fermented acidophilus milk and in the percentage reduction in counts of L. acidophilus DSM 20,079 between white pickled cheese and ice cream were not significant (P > 0.05). When the viabilities of the two strains of L. acidophilus in the samples were taken in consideration, L. acidophilus in the ice cream samples was more resistant to the harsh conditions of the dynamic in vitro gastrointestinal model, while L. acidophilus in the yoghurt samples was less resistant to the same conditions of the model. Furthermore, during the passage of the dairy products containing L. acidophilus through the parts of the model and prolonged storage, the reduction of viable counts of L. acidophilus in the samples increased (Table 4).
Table 4.
Effects of dairy products, gastrointestinal model conditions, and storage period on the reduction of L. acidophilus counts in the samples
| Reduction of L. acidophilus NCFM counts (%) | Reduction of L. acidophilus DSM 20,079 counts (%) | |
|---|---|---|
| Dairy products | ||
| Ice cream | 8.47 ± 1.25c* | 10.79 ± 1.54c * |
| Yoghurt | 13.38 ± 2.13a | 14.56 ± 2.02a |
| White pickled cheese | 12.62 ± 1.94b | 10.88 ± 1.45c |
| Fermented acidophilus milk | 13.00 ± 2.36ab | 12.74 ± 1.90b |
| Gastrointestinal model conditions | ||
| End of mouth | 0.25 ± 0.15e | 1.23 ± 0.23e |
| Stomach after 1 h | 2.87 ± 0.62d | 2.97 ± 0.37d |
| Stomach after 2 h | 11.08 ± 1.35c | 13.33 ± 1.07c |
| Small intestine after 1 h | 21.47 ± 1.27b | 21.14 ± 0.77b |
| Small intestine after 2 h | 23.66 ± 1.30a | 22.54 ± 0.81a |
| Storage period (days) | ||
| 1 | 8.95 ± 1.27c | 10.01 ± 1.29c |
| 15 | 11.21 ± 1.67b | 12.93 ± 1.53b |
| 30 | 15.44 ± 1.98a | 13.78 ± 1.67a |
*Different superscript letters after values indicate significant differences using Duncan’s multiple range test (P < 0.05). Values are expressed mean ± standard deviation
Kailasapathy [46] reported that yoghurt was not suitable for use as probiotics carrier food, due to post acidification that occurred in yoghurt storage resulting in major cell death of probiotic bacteria. Sharp et al. [47] showed that Lactobacillus casei 334e in yoghurt had lower resistance than that in cheese to acid stress (pH 2). A possible reason for this was explained by the authors that lower pH of yoghurt (pH 4.3) might be the reason for the sublethal damage to L. casei 334e in yoghurt during storage compared with low-fat cheese (pH 5.1). Ranadheera et al. [48] evaluated effect of carrier food type (goat’s milk ice cream, plain yoghurt, and fruit yoghurt) on in vitro gastrointestinal survival of Lactobacillus acidophilus LA-5, and ice cream was found to improve the bile tolerance of the L. acidophilus LA-5 compared to plain yoghurt and fruit yoghurt.
Among food products, ice cream is known to be an advantageous vehicle to deliver probiotic bacteria to human body since it has relatively high pH values (> 5.5) and high total solid contents providing protection for probiotic cells [49, 50]. Moreover, metabolizable sugars can protect the probiotic bacteria. Corcoran et al. [51] reported that the presence of 19.4 mM glucose resulted in up to 6 log enhanced survival of Lactobacillus rhamnosus GG in simulated gastric juice at pH 2.0 following 90 min of exposure as compared to the control without glucose. The authors indicated that in acid conditions, glucose provides ATP to F0F1-ATPase via glycolysis and allows protons to be removed from the cell thereby enhancing probiotic survival. In our study, the lowest decrease in the viability of L. acidophilus in ice cream samples after exposure to the dynamic in vitro gastrointestinal model could depend mainly on milk proteins, fat and lactose contents and relatively high pH values of ice cream samples [52]. Cheese has also a relatively high pH values, solid consistency (high fat and protein contents) compared to other dairy products and good vehicles for probiotics like ice cream [53, 54]. However, the high salt concentration in cheese could be a potential problem for viability of probiotics during long shelf life of cheese and after passage through gastrointestinal tract [55]. Moreover, the results of this study showed that although a decrease in the numbers of L. acidophilus in the dairy products was observed, L. acidophilus still survived (≥ 106 cfu g−1) in the dynamic in vitro gastrointestinal model, except for the white pickled cheese and fermented acidophilus milk samples produced by using L. acidophilus NCFM only on the 30th days of storage (Figs. 3 and 4).
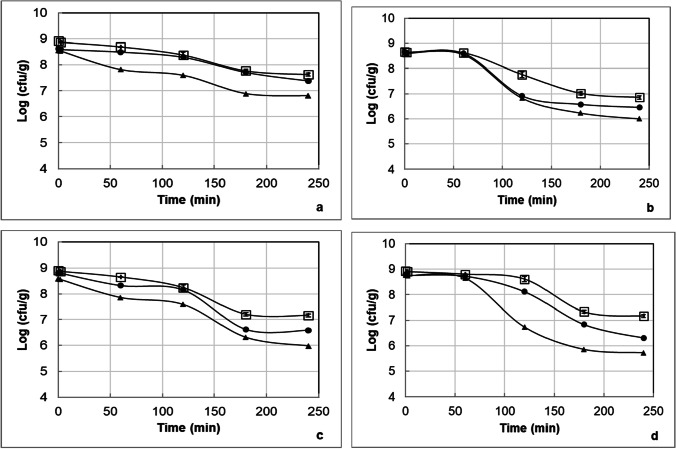
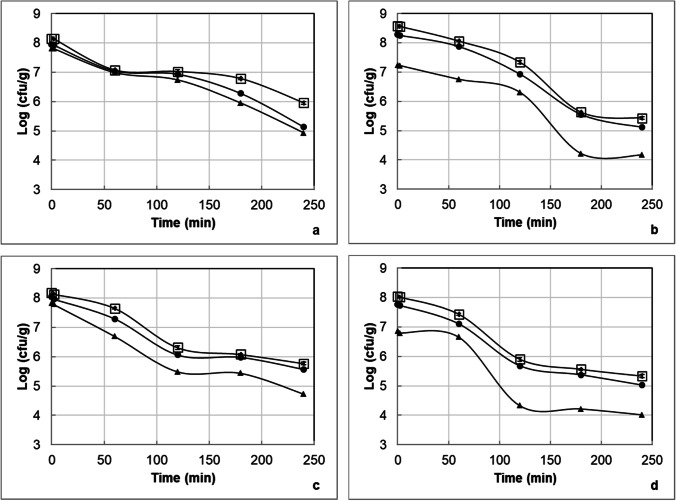
In Fig. 5a–d , the changes in the counts of L. delbrueckii subsp. bulgaricus and S. thermophilus are illustrated for the yoghurt samples containing L. acidophilus DSM 20,079 or L. acidophilus NCFM within 30 days of storage during the passage through the dynamic in vitro gastrointestinal model. In the yoghurt sample, a decrease for L. delbrueckii subsp. bulgaricus was observed; however, the decrease in the count of S. thermophilus was much pronounced and after 180 min of the passage through the dynamic in vitro gastrointestinal model no viable counts of S. thermophilus could be recorded. During 30-day storage, the viable counts of L. delbrueckii subsp. bulgaricus in the yoghurt samples containing L. acidophilus DSM 20,079 and L. acidophilus NCFM decreased in the range of 3.6–3.8 log units and 4.0 and 4.2 log units, respectively, after the passage through the dynamic in vitro gastrointestinal model. According to these findings, it is possible to say that the strain of L. delbrueckii subsp. bulgaricus used in the production of yoghurt is more resistant than the strain of S. thermophilus used in the production of yoghurt to the harsh conditions of the dynamic in vitro gastrointestinal model. Hernández-Galán et al. [56] did not observe any protective effect of the dairy matrices (skimmed milk, whole milk, rennet gel from skimmed milk, and rennet gel from whole milk) on survival of Streptococcus thermophilus TIL 257 during dynamic in vitro digestion. Furthermore, García‐Hernández et al. [57] found that Lactobacillus delbrueckii subsp. bulgaricus CECT 4005 T and Streptococcus thermophilus CECT 801 in yoghurt were more sensitive to gastric juice than intestinal juice because of their high ability to resist intestinal conditions. Pacheco et al. [58] reported that Lactobacillus delbrueckii subsp. bulgaricus NRRL-734 could survive in a high number under simulated gastrointestinal conditions when it was consumed together with food with a viscous consistency because of slowed diffusion processes and less interaction between gastrointestinal conditions and Lactobacillus delbrueckii subsp. bulgaricus cells.
Fig. 5.
The counts of L. delbrueckii subsp. bulgaricus in the yoghurt samples produced with L. acidophilus DSM 20,079 (a) or L. acidophilus NCFM (b) and in the counts of S. thermophilus in the yoghurt samples produced with L. acidophilus DSM 20,079 (c) or L. acidophilus NCFM (d) on the 1st (white square), 15th (black circle), and 30th (black triangle) days of the storage during the passage through the dynamic in vitro gastrointestinal model
In Fig. 6a–d , the changes in the counts of total mesophilic aerobic bacteria and total Lactococcus spp. are shown for the white pickled cheese samples containing L. acidophilus DSM 20,079 or L. acidophilus NCFM within 30 days of storage during the passage through the dynamic in vitro gastrointestinal model. About three-log reduction in the counts of total mesophilic aerobic bacteria and total Lactococcus spp. in the white pickled cheese samples was detected after exposure of the samples to the gastrointestinal model for approximately 4 h. The decrease in the counts of total mesophilic aerobic bacteria and total Lactococcus spp. in the white pickled cheese samples during the passage through the model increased significantly with extension of storage time. The counts of total mesophilic aerobic bacteria and total Lactococcus spp. in the cheese samples stored 30 day at 4 °C ranged between 4.2–4.9 log cfu g−1 and 4.0–4.7 log cfu g−1, respectively. Our study was generally in agreement with the studies by Sumeri et al. [59] and Adouard et al. [60] who reported that the resistance of cheese bacteria against gastrointestinal stresses varied depending on their species, genotypes, and physiological states.
Fig. 6.
The counts of total mesophilic aerobic bacteria in the white pickled cheese samples produced with L. acidophilus DSM 20,079 (a) or L. acidophilus NCFM (b) and in the counts of total Lactococcus spp. in the white pickled cheese samples produced with L. acidophilus DSM 20,079 (c) or L. acidophilus NCFM (d) on the 1st (white square), 15th (black circle), and 30th (black triangle) days of the storage during the passage through the dynamic in vitro gastrointestinal model
Conclusion
The dynamic gastrointestinal model designed in this study can effectively be used for comparative survival researches of probiotic bacteria in dairy products. The results obtained in the present study showed that there was no difference among ice cream, yoghurt, white pickled cheese, and fermented acidophilus milk samples in terms of carrying food of L. acidophilus if the gastrointestinal model part of the study was not considered. The matrix of the dairy product and storage period has significant effects on the survival of L. acidophilus during passage through the dynamic in vitro gastrointestinal model. This study demonstrates that among the examined dairy products, ice cream is the most protective product under in vitro gastrointestinal digestion conditions concerning the survival of L. acidophilus. The importance of the food matrix containing probiotic bacteria with regard to their survival during the passage through the gastrointestinal tract should be studied more extensively.
Acknowledgements
The authors thank Prof. Dr. Jörg Hinrichs and Dr. Zeynep Atamer of the University of Hohenheim for their critical comments and suggestions in the preparation of the manuscript.
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Akdeniz University (Project number: 2014.03.0121.009) and the Alexander von Humboldt Foundation (Project number: 3.4-/1116798-TUR-IP).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong A, Saint Ngu DY, Dan LA, Ooi A, Lim RLH. Detection of antibiotic resistance in probiotics of dietary supplements. Nutr J. 2015;14:95. doi: 10.1186/s12937-015-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips M, Kailasapathy K, Tran L. Viability of commercial probiotic cultures (L. acidophilus, Bifidobacterium sp., L. casei, L. paracasei and L. rhamnosus) in cheddar cheese. Int J Food Microbiol. 2006;108:276–280. doi: 10.1016/j.ijfoodmicro.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Zoumpopoulou G, Pot B, Tsakalidou E, Papadimitriou K. Dairy probiotics: beyond the role of promoting gut and immune health. Int Dairy J. 2017;67:46–60. doi: 10.1016/j.idairyj.2016.09.010. [DOI] [Google Scholar]
- 4.Homayouni A, Alizadeh M, Alikhah H, Zijah V. Functional dairy probiotic food development: trends, concepts, and products. In: Rigobelo E, editor. Probiotics. Rijeka: IntechOpen; 2012. pp. 772–797. [Google Scholar]
- 5.Casarotti SN, Penna ALB. Acidification profile, probiotic in vitro gastrointestinal tolerance and viability in fermented milk with fruit flours. Int Dairy J. 2015;41:1–6. doi: 10.1016/j.idairyj.2014.08.021. [DOI] [Google Scholar]
- 6.Meybodi NM, Mortazavian AM, Arab M, Nematollahi A. Probiotic viability in yoghurt: a review of influential factors. Int Dairy J. 2020 doi: 10.1016/j.idairyj.2020.104793. [DOI] [Google Scholar]
- 7.Valente GLC, Acurcio LB, Freitas LPV, Nicoli JR, Silva AM, Souza MR, Penna CFAM. Short communication: In vitro and in vivo probiotic potential of Lactobacillus plantarum B7 and Lactobacillus rhamnosus D1 isolated from Minas artisanal cheese. J Dairy Sci. 2019;102:5957–5961. doi: 10.3168/jds.2018-15938. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan D, Mallappa RH, Grover S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control. 2020;108:106872. doi: 10.1016/j.foodcont.2019.106872. [DOI] [Google Scholar]
- 9.Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L. Progress in microencapsulation of probiotics: a review. Compr Rev Food Sci Food Saf. 2020;19:857–874. doi: 10.1111/1541-4337.12532. [DOI] [PubMed] [Google Scholar]
- 10.Sumeri I, Arike L, Adamberg K, Paalme T. Single bioreactor gastrointestinal tract simulator for study of survival of probiotic bacteria. Appl Microbiol Biotechnol. 2008;80:317–324. doi: 10.1007/s00253-008-1553-8. [DOI] [PubMed] [Google Scholar]
- 11.Mainville I, Arcand Y, Farnworth E. A dynamic model that simulates the human upper gastrointestinal tract for the study of probiotics. Int J Food Microbiol. 2005;99:287–296. doi: 10.1016/j.ijfoodmicro.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Gaikwad V, Holmes M, Murray B, Povey M, Wang Y, Zhang Y. Development of a simple model device for in vitro gastric digestion investigation. Food Funct. 2011;2:174–182. doi: 10.1039/C0FO00159G. [DOI] [PubMed] [Google Scholar]
- 13.Parada J, Aguilera JM. Effect of food microstructure on nutrition bioavailability and health. In: Ciesarova Z, Plasencia FP, editors. Chemical Food Safety and Health. London: Nova Science Publishers; 2013. pp. 218–223. [Google Scholar]
- 14.Gomand F, Borges F, Burgain J, Guerin J, Revol-Junelles AM, Gaiani C. Food matrix design for effective lactic acid bacteria delivery. Annu Rev Food Sci T. 2019;10:285–310. doi: 10.1146/annurev-food-032818-121140. [DOI] [PubMed] [Google Scholar]
- 15.Sanders ME, Marco ML. Food formats for effective delivery of probiotics. Annu Rev Food Sci T. 2010;1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- 16.Goderska K, Czarnecki Z. Characterization of selected strains from Lactobacillus acidophilus and Bifidobacterium bifidum. Af J Microbiol Res. 2007;1(6):65–78. [Google Scholar]
- 17.Sanders ME, Klaenhammer TR. Invited review: The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2011;84(2):319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 18.Ergin F, Atamer Z, Arslan AA, Gocer EMC, Demir M, Samtlebe M, Hinrichs J, Kücükcetin A. Application of cold-and heat-adapted Lactobacillus acidophilus in the manufacture of ice cream. Int Dairy J. 2016;59:72–79. doi: 10.1016/j.idairyj.2016.03.004. [DOI] [Google Scholar]
- 19.Kücükcetin A. Effect of heat treatment and casein to whey protein ratio of skim milk on graininess and roughness of stirred yoghurt. Food Res Int. 2008;41:165–171. doi: 10.1016/j.foodres.2007.11.003. [DOI] [Google Scholar]
- 20.Kasımoğlu A, Göncüoğlu M, Akgün S. Probiotic white cheese with Lactobacillus acidophilus. Int Dairy J. 2004;14:1067–1073. doi: 10.1016/j.idairyj.2004.04.006. [DOI] [Google Scholar]
- 21.Božanić R, Tratnik L, Herceg Z, Marić O. The influence of milk powder, whey protein concentrate and inulin on the on the quality of cow and goat acidophilus milk. Acta Aliment. 2004;33:337–346. doi: 10.1556/AAlim.33.2004.4.4. [DOI] [Google Scholar]
- 22.Madureira AR, Amorim M, Gomes AM, Pintado ME, Malcata FX. Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res Int. 2011;44:465–470. doi: 10.1016/j.foodres.2010.09.010. [DOI] [Google Scholar]
- 23.Marteau P, Minekus M, Havenaar R, Huis J. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci. 1997;80:1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- 24.VDLUFA (2003) Handbuch der landwirtschaftlichen Versuchs-und Untersuchungsmethodik, Methodenbuch Band VI-Chemische, physikalische und mikrobiologische Untersuchungsverfahren für Milch, Milchprodukte und Molkereihilfsstoffe. Verlag Darmstadt, Germany: Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten
- 25.Bradley R, Arnold E, Barbano D, Semerad R, Smith D, Vines B (1992) Chemical and physical methods. In: Marshall RT (ed) Standard methods for the examination of dairy products. American Public Health Association, Washington, DC, pp 433–531
- 26.IDF . Dairy starter cultures of lactic acid bacteria (LAB). Standard of identity. Standard 149A. Brussels: International Dairy Federation; 1997. [Google Scholar]
- 27.Tabasco R, Paarup T, Janer C, Peláez C, Requena T. Selective enumeration and identification of mixed cultures of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. paracasei subsp. paracasei and Bifidobacterium lactis in fermented milk. Int Dairy J. 2007;17:1107–1114. doi: 10.1016/j.idairyj.2007.01.010. [DOI] [Google Scholar]
- 28.Evrendilek GA, Koca N, Harper J, Balasubramaniam V. High-pressure processing of Turkish white cheese for microbial inactivation. J Food Protect. 2008;71:102–108. doi: 10.4315/0362-028X-71.1.102. [DOI] [PubMed] [Google Scholar]
- 29.IDF . Milk and mik products enumeration of microorganisms-colony count at 30 °C. Standard 100A. Brussels: International Dairy Federation; 1987. [Google Scholar]
- 30.Ong L, Henriksson A, Shah N. Development of probiotic Cheddar cheese containing Lactobacillus acidophilus, Lact. casei, Lact. paracasei and Bifidobacterium spp. and the influence of these bacteria on proteolytic patterns and production of organic acid. Int Dairy J. 2006;16:446–456. doi: 10.1016/j.idairyj.2005.05.008. [DOI] [Google Scholar]
- 31.Moayednia A. The shifts of acidophilus milk at the refrigerator. JFBT. 2012;2:65–70. [Google Scholar]
- 32.Kos B, Suskovic J, Goreta J, Matosic S. Effect of protectors on the viability of Lactobacillus acidophilus M92 in simulated gastrointestinal conditions. Food Technol Biotech. 2000;38:121–128. [Google Scholar]
- 33.Ranadheera CS, Evans C, Adams M, Baines S. Production of probiotic ice cream from goat's milk and effect of packaging materials on product quality. Small Rumin Res. 2013;112:174–180. doi: 10.1016/j.smallrumres.2012.12.020. [DOI] [Google Scholar]
- 34.Ribeiro MCE, Chaves KS, Gebara C, Infante FN, Grosso CR, Gigante ML. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res Int. 2014;66:424–431. doi: 10.1016/j.foodres.2014.10.019. [DOI] [Google Scholar]
- 35.Akpınar A (2008) Acidophlus milk properties with to produce added different flavour components. PhD thesis, Ege University. İzmir. 01.02.2016. https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp. 2 Jan 2016
- 36.Afzaal M, Khan AU, Saeed F, Arshad MS, Khan MA, Saeed M, Maan AA, Khan MK, Ismail Z, Ahmed A, Tufail T, Ateeq H, Anjum FM. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in ice cream. Food Sci Nutr. 2020;8:1649–1656. doi: 10.1002/fsn3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Moschopoulou E, Sakkas L, Zoidou E, Theodorou G, Sgouridou E, Kalathaki C, Liarakou A, Chatzigeorgiou A, Politis I, Moatsou G. Effect of milk kind and storage on the biochemical, textural and biofunctional characteristics of set-type yoghurt. Int Dairy J. 2018;77:47–55. doi: 10.1016/j.idairyj.2017.09.008. [DOI] [Google Scholar]
- 38.Kılıç GB, Kuleaşan H, Eralp İ, Karahan AG. Manufacture of Turkish Beyaz cheese added with probiotic strains. LWT-Food Sci T. 2009;42:1003–1008. doi: 10.1016/j.lwt.2008.12.015. [DOI] [Google Scholar]
- 39.Junaid M, Javed I, Abdullah M, Gulzar M, Younas U, Nasir J, Ahmad N. Development and quality assessment of flavored probiotic acidophilus milk. J Anim Plant Sci. 2013;23:1342–1346. [Google Scholar]
- 40.Mousavi M, Heshmati A, Garmakhany AD, Vahidinia A, Taheri M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT-Food Sci T. 2019;102:80–88. doi: 10.1016/j.lwt.2018.12.023. [DOI] [Google Scholar]
- 41.Parussolo G, Busatto RT, Schmitt J, Pauletto R, Schons PF, Ries EF. Synbiotic ice cream containing yacon flour and Lactobacillus acidophylus NCFM. LWT-Food Sci T. 2017;82:192–198. doi: 10.1016/j.lwt.2017.04.049. [DOI] [Google Scholar]
- 42.Geraldi MV, Tulini FL, Souza VM, De Martinis EC. Development of yoghurt with juçara pulp (Euterpe edulis M.) and the probiotic Lactobacillus acidophilus La5. Probiotics Antimicro. 2018;10:71–76. doi: 10.1007/s12602-017-9280-z. [DOI] [PubMed] [Google Scholar]
- 43.Nighswonger BD, Brashears MM, Gilliland SE. Viability of Lactobacillus acidophilus and Lactobacillus casei in fermented milk products during fefrigerated storage. J Dairy Sci. 1996;79:212–219. doi: 10.3168/jds.S0022-0302(96)76353-1. [DOI] [PubMed] [Google Scholar]
- 44.Ng EW, Yeung M, Tong PS. Effects of yogurt starter cultures on the survival of Lactobacillus acidophilus. Int J Food Microbio. 2011;145:169–175. doi: 10.1016/j.ijfoodmicro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Farag MA, El Hawary EA, Elmassry MM. Rediscovering acidophilus milk, its quality characteristics, manufacturing methods, flavor chemistry and nutritional value. Crit Rev Food Sci Nutr. 2019;60(18):3024–3041. doi: 10.1080/10408398.2019.1675584. [DOI] [PubMed] [Google Scholar]
- 46.Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT-Food Sci T. 2006;39:1221–1227. doi: 10.1016/j.lwt.2005.07.013. [DOI] [Google Scholar]
- 47.Sharp M, McMahon DJ, Broadbent JR. Comparative evaluation of yogurt and low-fat cheddar cheese as delivery media for probiotic Lactobacillus casei. J Food Sci. 2008;73:375–377. doi: 10.1111/j.1750-3841.2008.00882.x. [DOI] [PubMed] [Google Scholar]
- 48.Ranadheera CS, Evans C, Adams M, Baines S. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat's milk ice cream and yogurt. Food Res Int. 2012;49:619–625. doi: 10.1016/j.foodres.2012.09.007. [DOI] [Google Scholar]
- 49.Homayouni A, Ehsani M, Azizi A, Razavi S, Yarmand M. Growth and survival of some probiotic strains in simulated ice cream conditions. J Appl Sci. 2008;8:379–382. doi: 10.3923/jas.2008.379.382. [DOI] [Google Scholar]
- 50.Homayouni A, Azizi A, Ehsani M, Yarmand M, Razavi S. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. doi: 10.1016/j.foodchem.2008.03.036. [DOI] [Google Scholar]
- 51.Corcoran B, Stanton C, Fitzgerald G, Ross R. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol. 2005;71:3060–3067. doi: 10.1128/AEM.71.6.3060-3067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cruz AG, Antunes AEC, Sousa ALOP, Faria JAF, Saad SMI. Ice-cream as a probiotic food carrier. Food Res Int. 2009;42:1233–1239. doi: 10.1016/j.foodres.2009.03.020. [DOI] [Google Scholar]
- 53.Flach J, van der Waal MB, van den Nieuwboer M, Claassen E, Larsen OF. The underexposed role of food matrices in probiotic products: reviewing the relationship between carrier matrices and product parameters. Crit Rev Food Sci Nutr. 2018;58:2570–2584. doi: 10.1080/10408398.2017.1334624. [DOI] [PubMed] [Google Scholar]
- 54.Hayes M, Coakley M, O’sullivan L, Stanton C. Cheese as a delivery vehicle for probiotics and biogenic substances. Aust J Dairy Technol. 2006;61:132–141. [Google Scholar]
- 55.Neffe-Skocińska K, Rzepkowska A, Szydłowska A, Kołożyn-Krajewska D. Trends and possibilities of the use of probiotics in food production. In: Holban AM, Grumezescu AM, editors. Alternative and Replacement Foods. Cambridgen: Elsevier Academic Press; 2018. pp. 65–94. [Google Scholar]
- 56.Hernández-Galán L, Cattenoz T, Le Feunteun S, Canette A, Briandet R, Le-Guin S, Guedon E, Castellote J, Delettre J, Bony ED. Effect of dairy matrices on the survival of Streptococcus thermophilus, Brevibacterium aurantiacum and Hafnia alvei during digestion. Food Res Int. 2017;100:477–488. doi: 10.1016/j.foodres.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 57.García-Hernández J, Moreno Y, Chuan C, Hernández M. In vivo study of the survival of Lactobacillus delbruecki subsp. bulgaricus CECT 4005T and Streptococcus thermophilus CECT 801 by DVC-FISH after consumption of fermented milk. J Food Sci. 2012;77:593–597. doi: 10.1111/j.1750-3841.2012.02918.x. [DOI] [PubMed] [Google Scholar]
- 58.Pacheco KC, del Toro GV, Martínez FR, Durán-Páramo E. Viability of Lactobacillus delbrueckii under human gastrointestinal conditions simulated in vitro. Am J Agric Biol Sci. 2010;5:37–42. doi: 10.3844/ajabssp.2010.37.42. [DOI] [Google Scholar]
- 59.Sumeri I, Adamberg S, Uusna R, Sarand I, Paalme T. Survival of cheese bacteria in a gastrointestinal tract simulator. Int Dairy J. 2012;25:36–41. doi: 10.1016/j.idairyj.2011.12.016. [DOI] [Google Scholar]
- 60.Adouard N, Magne L, Cattenoz T, Guillemin H, Foligné B, Picque D, Bonnarme P. Survival of cheese-ripening microorganisms in a dynamic simulator of the gastrointestinal tract. Food Microbiol. 2016;53:30–40. doi: 10.1016/j.fm.2015.03.002. [DOI] [PubMed] [Google Scholar]