Abstract
Introduction
The influence of vaccination on composition of the human microbiome at distinct sites has been recognized as an essential component in the development of new vaccine strategies. The HPV vaccine is widely used to prevent cervical cancer; however, the influence of HPV vaccine on the vaginal microbiota has not been previously investigated. In his study, we performed an initial characterization of the microbiome and cytokine composition in the vagina following administration of the bivalent vaccine against HPV 16/18.
Material and methods
In this exploratory study, fifteen women between 18 and 40 years received three doses of the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®). Cervicovaginal samples were collected before the first dose and 30 days after the third dose. HPV genotyping was performed by the XGEN Flow Chip technique. The cytokines IFN-γ, IL-2, IL-12p70, TNF-α, GM-CSF, IL-4, IL-5, IL-10, and IL-13 were quantitated by multiplex immunoassay. The vaginal microbiome was identified by analysis of the V3/V4 region of the bacterial 16S rRNA gene.
Results
The most abundant bacterial species in the vaginal microbiome was Lactobacillus crispatus, followed by L. iners. Bacterial diversity and dominant organisms were unchanged following vaccination. Small decreases in levels of pro and anti-inflammatory cytokines were observed following HPV vaccination, but there was no association between vaginal cytokine levels and microbiome composition.
Conclusion
Vaginal microbiome is not altered following administration of the standard three-dose HPV-16/18 AS04-adjuvanted (Cervarix®) vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00616-x.
Keywords: Microbiome, HPV vaccines, Human papillomavirus 16, Cytokines, Microbiota
Introduction
The vaginal microbiome of reproductive age women has been shown to contain more than 265 species of bacteria, as revealed by culture-independent 16S ribosomal RNA gene amplification, sequencing, and analysis [1]. The predominance of Lactobacillus species in the vagina of the majority of these women has been associated with maintenance of a healthy local environment [2]. Alterations in microbiome composition and relative abundance can be triggered by multiple factors such as age, hormonal changes, sexual behavior, concomitant infections, hygiene, and immune status [3–5]. These changes may adversely affect the magnitude and direction of local immune responses and increase susceptibility to infection [5, 6].
Previous studies have evaluated the influence of the human microbiome composition on the efficacy of oral or parenteral vaccines [7–9]. Therefore, it is reasonable to also assume the possible occurrence of the opposite effect, where the vaccine response alters the resident microbiome. The human papillomavirus (HPV) vaccine targets a sexually transmitted virus that infects the cervix. Although it is parenterally administered, the HPV vaccine results in the appearance of neutralizing IgG antibodies in the vagina as well as the local production of cytokines [10, 11].
The HPV vaccine has been shown to successfully prevent the acquisition of HPV-related diseases, primarily cervical cancer. The vaccine is parenterally administered and induces neutralizing antibodies against the viral capsid antigen L1 (L1-VLP). The vaccine also induces an L1-specific cell-mediated immune response, characterized by the proliferation of CD4+ and CD8+ T lymphocytes and increased cytokine production 11. Anti-HPV antibodies are present in the vagina following immunization and may potentially influence the magnitude of immune responses [11]. This may consequently lead to an alteration in the composition of the microbiome at this site. However, the influence of HPV vaccine on the vaginal microbiota has not been previously investigated.
The aim of the present study was to perform an initial characterization of the vaginal microbiome profile prior to and following administration of three doses of the bivalent vaccine against HPV 16 and 18 (2vHPV, Cervarix®), as well as to evaluate possible changes to the cytokine profile in the vagina.
Material and methods
Enrollment and study design
In this pilot exploratory study, 15 women between 18 and 40 years of age were enrolled. All subjects received medical care at the Women’s Hospital Prof. Dr. José Aristodemo Pinotti (Centro de Atenção Integral à Saúde da Mulher – Hospital da Mulher Prof. Dr. José Aristodemo Pinotti) (CAISM) of the State University of Campinas (Universidade Estadual de Campinas) (UNICAMP), between October, 2017 and August, 2018. This study was approved by the Ethics in Research Committee of the UNICAMP School of Medical Sciences, under registration number CAAE 56933816.0.0000.5404, and all the participants signed informed written consent.
At their initial consultation, the women were evaluated for the presence of the following genital infections: Herpes virus types I and II (Microbial DNA qPCR Arrays, Qiagen®, Roche, Switzerland), Neisseria gonorrhea and Chlamydia trachomatis (Cobas® PCR system, Roche, Switzerland), Candida spp. (Gram staining), bacterial vaginosis (Nugent score), syphilis, and HIV (quick test). Exclusion criteria were positive results in any of the above-mentioned infections, as well as being pregnant, use of an intrauterine device, practicing vaginal douching, having engaged in sexual intercourse within 3 days prior to study enrollment, and/or the use of oral or topical antibiotics within the past 30 days. To eliminate the possible effect of physiological hormonal changes, all women included in the study were using hormonal contraception.
Enrolled women completed a questionnaire on clinical history, socio-demographic profile, and sexual behavior. Subjects then underwent a speculum-based vaginal examination to obtain the following samples: (1) collection of a vaginal sample that was immediately immersed in 1 mL of Amies transport medium (Copan Diagnostic Inc®, USA) for microbiome analysis; (2) collection of a vaginal swab for bacterial vaginosis analysis by Nugent criteria, (3) pH vaginal measurement, (4) collection of an endocervical sample that was then immersed in 10 mL of CellPrev® medium (Kolpast®) for HPV analysis; (5) collection of a cervicovaginal sample that was then immersed in 1 mL of a phosphate-buffered saline (PBS) solution for analysis of vaginal cytokines. Samples in Amies medium and in PBS were immediately stored at – 80 °C until use for extraction of bacterial DNA and measurement of vaginal cytokines. Additional samples for microbiome analysis, HPV detection, and vaginal and serum cytokine levels were again performed 1 month after termination of the vaccination schedule (7 months after the initial collection). So, the assessment of HPV infection, microbiome, and cytokine levels was performed both at the initial visit and at the end of the follow-up (7 months after initical visit).
All subjects received the standard vaccination protocol of three doses of 2vHPV-Cervarix® (bivalent vaccine; GlaxoSmithKline, UK). The first dose was administered at the time of study inclusion, and subsequent doses were administered 1 and 6 months later.
HPV diagnosis
Cervicovaginal samples collected in CellPrev® were analyzed for 36 HPV types (low risk: 6, 11, 40, 42, 43, 44, 54, 55, 61, 62, 67, 69, 70, 71, 72, 81, 84 and 89 and high risk: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82), by XGEN Mult HPV Chip HS12 (Mobius Life Science, PR, Brazil).
Analysis of vaginal microbiome
Genomic DNA was extracted using the QIAamp BiOstic Bacteremia DNA Kit (Qiagen®, USA), according to the manufacturer’s protocol. For the preparation of genomic libraries, we used 12.5 ng of DNA per sample pool, 5 mM of specific primers for the amplification of the highly conserved region (V3 and V4) of the bacterial 16S gene, and 12.5 µL of KAPA HIFI HotStart ReadyMix 2 × (Roche, Switzerland). The 5′-3′ primer sequences for the V3/V4 region of the 16S gene were:
V3-341: (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG) V4-785: (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC)
Negative controls with buffer from the DNA extraction kit were included in the PCR runs. The amplicons were pooled and loaded onto Illumina MiSeq clamshell style cartridge kit v3 at 600 cycles, for paired-end 300 sequencing at a final concentration of 12 pM. The library was clustered to a density of approximately 877 k/mm2, and PhiX 25% spike in control. This procedure was performed by Illumina MiSeq benchtop sequencer at the Molecular Genetics Laboratory (Laboratório de Genética Molecular) at the University of Campinas (UNICAMP). The MiSeq platform was used for image analysis, base calling, and data quality assessment.
The raw data for bioinformatics analysis consisted of 31 paired-end samples, totaling 62 FASTQ files (each sample had forward and reverse reads), from 16 individuals. We used the DADA2 pipeline of QIIME2 (version 2018.11) [12] to perform sequence quality control and feature table construction, including sequence denoising, joining pair ends (where possible), and discarding chimeric artifacts. The analysis proceeded with just the non-chimeric DNA sequences. For the subsequent analyses, we discarded two more samples that yielded too few (< 60) non-chimeric sequences. QIIME2 was also used for OTU identification, diversity analysis, and taxonomic classification with the Greengenes database (version 13_8), available from QIIME2, trained on the region between the specific primers used in this study. Heatmaps were prepared with the Complex Heatmap package (version 1.18.1) of the Bioconductor software (version 3.7), by means of the RStudio interface (version 1.1.463) and the R statistical software (version 3.5.2).
For each library, alpha and beta diversity indexes were calculated, and their analysis refers to the species variety and complexity in a community. For alpha diversity, Chao1 [13] was used to estimate richness, as well as Shannon and Simpson diversity indexes [14, 15]. Then, using Microbiome Analyst [16], a web-based tool, the principal coordinate analyses (PCoA), based on Bray–Curtis dissimilarity distance [17], were constructed to observe the differences in beta diversity between groups.
Analysis of serum and vaginal cytokines
Quantification of pro-inflammatory (IFN-γ, IL-2, IL-12p70, TNF-α, GM-CSF) and anti-inflammatory (IL-4, IL-5, IL-10, IL-13) cytokines was performed in the Bio-Plex 200 Series Plate Reader Systems (Bio-Rad Laboratories Inc., USA), using the MILLIPLEX MAP Human High Sensitivity T Cell Panel-Immunology Multiplex Assay (Merck KGaA, Germany). Processing and analysis of serum and cervicovaginal samples were performed according to the manufacturer’s protocol.
Statistical analysis
Differences in cytokine concentrations between samples were evaluated by the Mann–Whitney test and Student t test using Epi Info™ (version 7.2.2.16) and GraphPad (version 7) software. The Spearman rank correlation test was used to assess correlations between relative abundance of bacterial genera. All analyses were performed using standard software (GraphPad Prism, v7.0 for Windows). All tests were considered significant at p < 0.05.
Results
The women enrolled in the study were between 19 and 40 years old. Most (80%) were white, non-smokers (85%) and had university education (65%). In addition, 95% were nulliparous. Most had only one sexual partner in the previous 6 months and were heterosexual. Additional information on the sociodemographic characteristics of women is shown in Table 1.
Table 1.
Sociodemographic characteristics of study participants
| Variant | Number | Percent | Exact 95% conf. limits | |
|---|---|---|---|---|
| Race | Black/Brown | 4 | 27 | 8–55 |
| White | 11 | 73 | 45–92 | |
| Educational level | High school | 5 | 33 | 12–62 |
| University | 10 | 67 | 33–88 | |
| Smoking | Yes | 2 | 13 | 1.6–40 |
| No | 13 | 87 | 58–89 | |
| Parity | Multiparous | 1 | 7 | 0.2–32 |
| Nulliparous | 14 | 93 | 68–99 | |
| No. of sexual partnersa | One | 9 | 60 | 16–68 |
| Two or more | 6 | 40 | 32–84 | |
| Homosexual relationship | Yes | 2 | 13 | 1.6–40 |
| No | 13 | 87 | 58–89 |
aIn the last 6 months
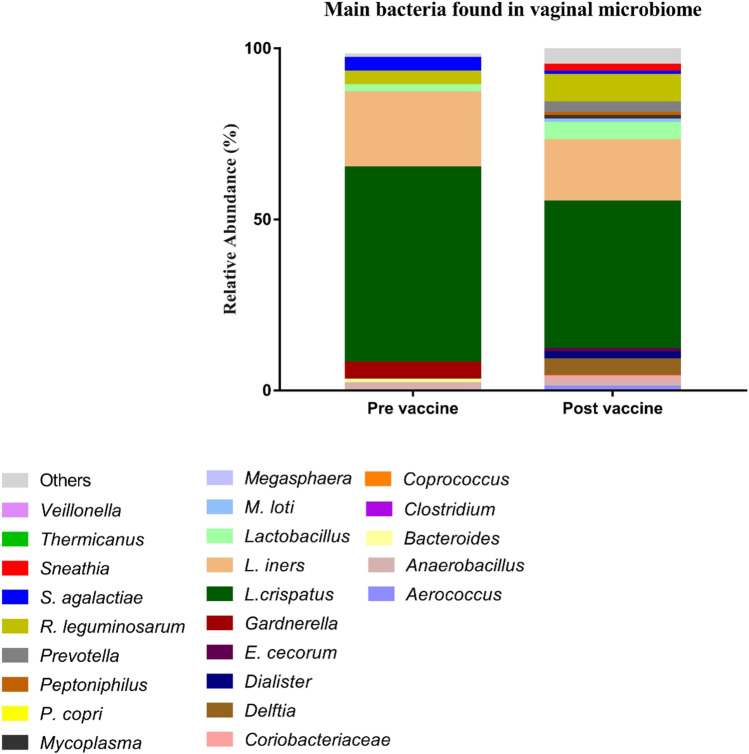
The presence and relative abundance of different bacteria in the vaginal microbiome in the study population as a whole prior to and following the vaccination schedule is shown in Fig. 1. The bacterial composition and abundance of individual bacteria were not significantly altered following vaccine administration. The small reduction in L. crispatus and L. iners abundance following vaccination did not reach statistically significant (Table Supplementary 1).
Fig. 1.
Relative abundance of different bacteria in the vaginal microbiome before (n = 15) and after HPV vaccination (n = 15)
The prevalence of HPV was 50% (04/14) before vaccination and 53% (8/15) 7 months after vaccination. Supplementary Table 2 summarizes the status of HPV infection before and after vaccination. We observed that one woman presenting HPV52 before vaccination had negative results in the post-vaccination period and two women presenting multiple infections before vaccination had single infections in the post-vaccination period. One woman with negative results for HPV before vaccination had positive results in the post-vaccination period. Persistence of the same type of HPV infection was observed in four women, while two women presented infection by a new type of HPV in the post-vaccination period. Altogether, vaginal microbiome data analyzed from both the pre-and post-vaccination periods demonstrated a higher frequency of HPV in vaginal samples with a predominance of L. crispatus (45.5%, 5/11) when compared with samples presenting other types of predominant bacteria (21.05%, 4/19); however, such association was not statistically significant in the evaluated sample size (p = 0.2251).
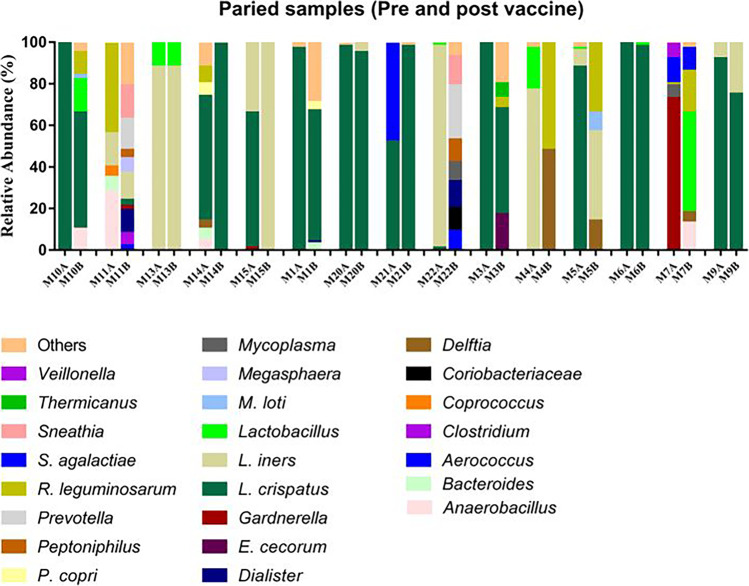
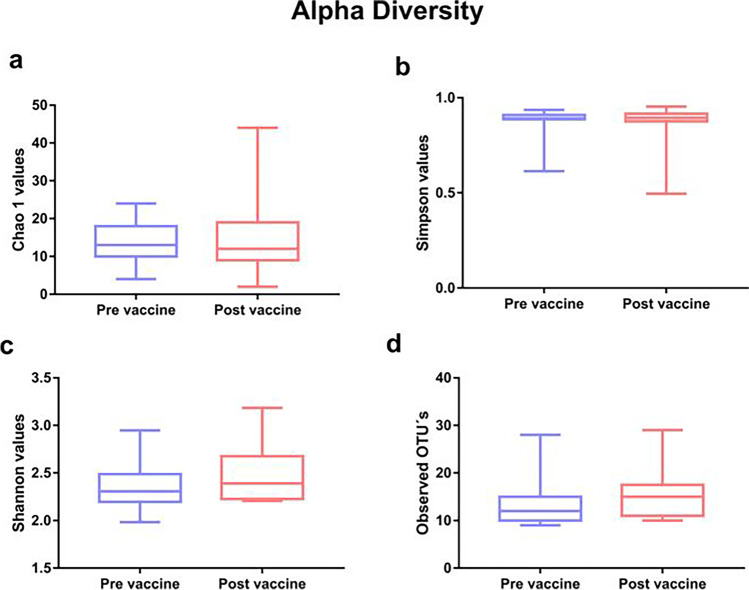
Pre- and post-vaccination microbiome profiles for each of the 15 individual women are shown in Fig. 2. L. iners and L. crispatus were the most abundant bacteria in each of the women. In subjects M5, M14, M15, and M21, there was an apparent change in L. iners and L. crispatus abundance while a post-vaccination increase in bacterial diversity was observed in seven of the women (M1, M3, M7, M10, M11, M14, and M22). Although we have observed in Fig. 2 a greater number of women with diversity in the vaginal microbiome in the post-vaccination period, with a decrease in L.crispatus and L. iners, there were no statistically significant differences in alpha and beta diversity, as shown in Table 2 and Figs. 3 and 4.
Fig. 2.
Relative abundance of individual bacteria in the vaginal microbiome in 15 women before (A) and after (B) HPV vaccination
Table 2.
Alpha diversity indices observed in groups
| Grupos | Diversity | Richness | Observed OTUs | |
|---|---|---|---|---|
| Shannon index | Simpson index | Chao1 | ||
| Pre vaccine |
1.98–2.94 2.35 (0.23) |
0.61–0.93 0.88 (0,07) |
4–24 13.8 (5.23) |
9–28 13.62 (5.009) |
| Post vaccine |
2.20–3.18 2.48 (0.29) |
0.49–0.95 0.85 (0.11) |
2–44 15 (10.48) |
13–29 15.08 (5.29) |
| P | 0.31ª | 0.93ª | 0.91ª | 0.32ª |
*Significant when P < 0.05
ªMann-Whitney test — values shown as minimum and maximum, mean and standard deviation
Fig. 3.
Alpha diversity indices between groups a Chao 1, b Shannon, c Simpson, and Observed OUT (d) between groups
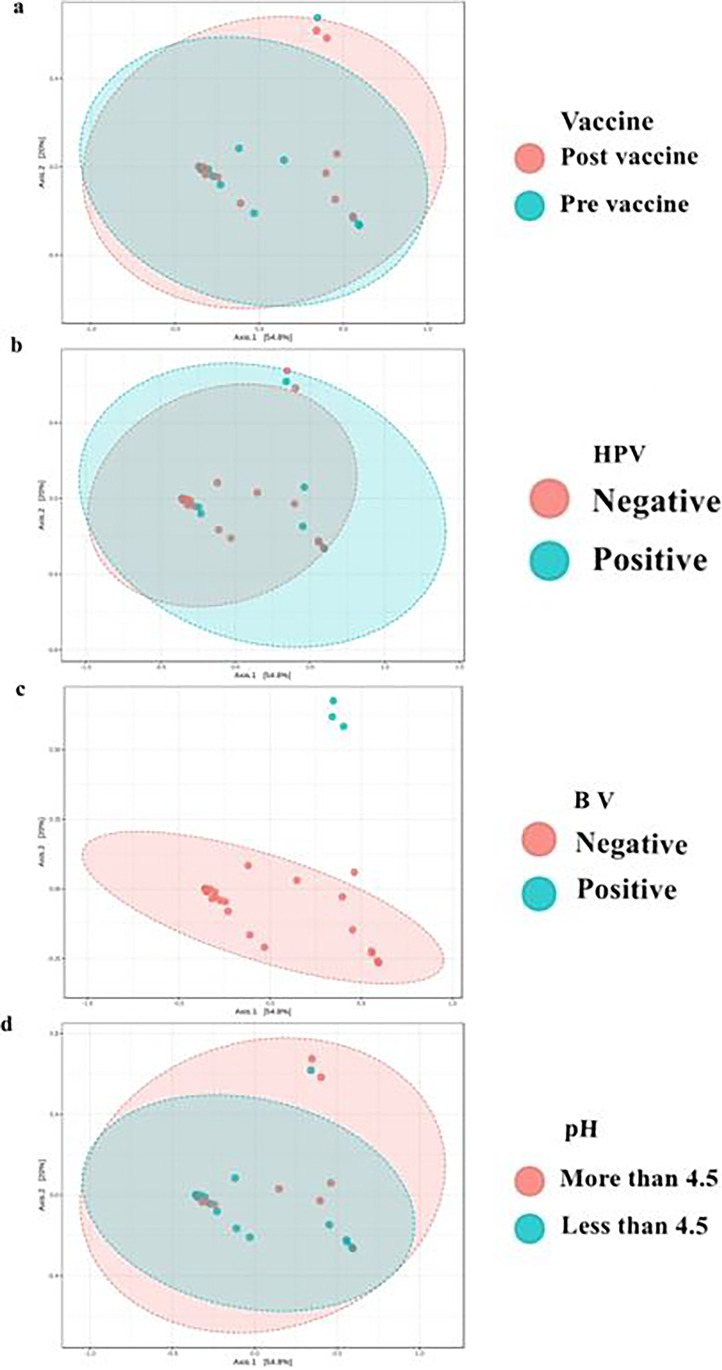
Fig. 4.
Test applied (ANOSIN). Observed metric Brai-Curtis Vaccine R = − 0.045; P = 0.989, HPV R = 0.115; P = 0.085, Bacterial vaginosis R = 0.74; P < 0.001, pH R = − 0.0042; P = 0.432
The evaluation of beta diversity (Fig. 4) also showed no significant differences in the vaginal microbiome before or after vaccination (p = 0.989), in the presence or absence of HPV (p = 0.085) or in relation to vaginal pH (p = 0.432). As expected, there was a statistically significant difference in diversity when comparing women with or without bacterial vaginosis (p < 0.001).
We also evaluated if there were changes in vaginal pro- or anti-inflammatory cytokine levels after vaccine administration. Although concentrations sometimes varied considerably between individuals, small decreases in levels of pro-inflammatory IFN-γ, IL-2, IL-12p70, TNF-α, and anti-inflammatory IL-5, IL-10 and IL-13 mediators following HPV vaccination were statistically significant (Table 3).
Table 3.
Evaluation of vaginal cytokines prior to and following HPV vaccination
| Median (range-pg/mL) | |||
|---|---|---|---|
| Cytokine | Prior HPV vaccination | Following HPV vaccination | P value |
| IFN-γ | 10.5 (7.0–17.50) | 8.50 (6.50–18.50) | 0.048a |
| IL-10 | 16.5 (13.25–162.25) | 13.5 (11.75–30.00) | 0.003a |
| IL-4 | 9.75 (8.00–14.00) | 9.50 (7.50–11.00) | 0.199b |
| TNF-α | 29.75 (19.50–350.00) | 22.00 (18.50–156.3) | 0.033a |
| GM-CSF | 18.75 (12.50–74.25) | 14.5 (9.50–66.00) | 0.133a |
| IL-12p70 | 10.5 (7.25–16.00) | 8.25 (6.50–12.00) | 0.010b |
| IL-13 | 18.00 (13.75–29.00) | 14.50 (12.00–22.25) | 0.013a |
| IL-2 | 23.25 (16.50–47.5) | 25.00 (12.00–48.00) | 0.615a |
| IL-5 | 9.50 (8.25–11.75) | 8.75 (8.00–11.50) | 0.027a |
aMann-Whitney test
bStudent t test
Discussion
The influence of vaccination on composition of the human microbiome at distinct sites has been recognized as an essential component in the development of new vaccine strategies [8, 9, 18]. The HPV vaccine is widely used to prevent cervical cancer [19]. In the present exploratory study, we performed an initial characterization of the vaginal microbiome before and after the standard\2vHPV-Cervarix® vaccine regimen. The major finding is that there was no significant change in the vaginal microbiome in the 15 women analyzed 7 months after initiation of the three-dose immunization regimen.
L. crispatus was the most prevalent vaginal bacterium followed by L. iners, similar to what has been reported in numerous prior investigations of reproductive age women [1, 2, 4]. Small shifts in the microbial profile were observed in several of the women. However, all were statistically insignificant, and alpha and beta diversity analysis revealed that relative abundance of the bacterial population was not significantly altered after vaccination.
The preventive 2vHPV Cervarix® is the L1 virus-like particle vaccine (VLP), which has been shown to have an effect prior to the acquisition of HPV infection. Its action is due to a strong production of neutralizing antibodies against HPV16 and HPV 18 [11, 20]. Activation of the major histocompatibility (MHC)1 and MHC2 presenting pathways improves the efficacy of HPV vaccine. This fact is considered for the development of vaccine adjuvants that increase the activation of CD8 + and CD4 + T cells [21, 22]. Some studies have demonstrated increased production of pro-inflammatory and anti-inflammatory cytokines in peripheral blood or in peripheral blood mononuclear cell cultures in response to VLP [10, 23]. However, in our study, vaginal pro-inflammatory (IFN-γ, IL-12p70, TNF-α) and anti-inflammatory (IL-5, IL-13) cytokine levels decreased in the post-vaccination period. Although statistically significant, the changes were small, and the range of values among individual women was very large in many cases. Thus, analysis of a much larger sample is needed before any conclusions on a possible association between vaccination and vaginal cytokine levels can be validated.
A limitation of the study is the relatively small number of women included and analysis of only a single time point after administration of all three doses of the vaccine. In addition, we did not evaluate changes in vaginal microbiome composition over the same time interval in a comparable population of unvaccinated women. Thus, we cannot rule out any differences may merely represent random variation and are unrelated to vaccine administration. However, since there were no apparent effects of vaccination on microbiome composition, it is likely that this would have paralleled findings in an unvaccinated control group. Nevertheless, our study to evaluate the vaginal microbiome profile in women following vaccination against a sexually transmitted HPV infection is appropriately labeled as exploratory. Therefore, our findings need to be replicated in different populations using a larger number of subjects. It also needs to be determined if changes in microbiome composition may occur at earlier and/or later time periods after cessation of vaccination or if comparable results will be obtained following vaccination of pre-adolescent girls.
The HPV vaccine has been commercially available for over 10 years and has been highly effective in preventing high and low-grade HPV-dependent lesions resulting in cervical cancer or genital warts [19]. Adverse effects have been monitored to corroborate that the introduction of this vaccine in numerous countries was an appropriate measure and was safe in the various populations [19]. Our study, although needing confirmation, highlights that the HPV vaccine may not negatively impact composition of the vaginal microbiome of reproductive age women.
Conclusion
We did not observe any significant changes in the vaginal microbiota profile after the standard\2vHPV-Cervarix® vaccine regimen. Although small decreases in levels of pro- inflammatory IFN-γ, IL-2, IL-12p70, TNF-α and anti-inflammatory IL-5, IL-10, and IL-13 cytokines following HPV vaccination were observed, we found no relationship between the levels of local cytokines and the profile of the vaginal microbiome. This exploratory study suggests that HPV vaccination does not negatively impact the vaginal health of women of reproductive age.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Discacciati MG: project development, data collection, data analysis, manuscript writing. Giraldo PC: project development, data collection, data analysis, manuscript writing. Sanchez JM: project development, data collection. Amaral R: data collection. Migliorini I: data collection. Taddei, CR: data analysis, manuscript writing. Sparvoli LG: data analysis, manuscript writing. Gil CD: data analysis. Witkin SS modified the data analysis, served as English editor and contributed to writing the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by the São Paulo Research Foundation (FAPESP) (Fundação de Apoio à Pesquisa do Estado de São Paulo) (Process No. 2016/083923, Giraldo PC).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not aplicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Tweetable abstract
There was no significant change in the vaginal microbiome in women analyzed 7 months after HPV immunization regimen.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(Supplement_1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG. 2017;124(4):606–611. doi: 10.1111/1471-0528.14390. [DOI] [PubMed] [Google Scholar]
- 3.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2018;25(11):1321–1330. doi: 10.1097/GME.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 4.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis. 2014;209(12):1989–1999. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 5.Torcia MG. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int J Mol Sci. 2019;11(20):266. doi: 10.3390/ijms20020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campisciano G, Zanotta N, Licastro D, De Seta F, Comar M. In vivo microbiome and associated immune markers: new insights into the pathogenesis of vaginal dysbiosis. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-20649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitetta L, Saltzman ET, Thomsen M, Nikov T, Hall S. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes. Vaccines (Basel) 2017;5(4):50. doi: 10.3390/vaccines5040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlasova AN, Takanashi S, Miyazaki A, Rajashekara G, Saif LJ. How the gut microbiome regulates host immune responses to viral vaccines. Curr Opin Virol. 2019;37:16–25. doi: 10.1016/j.coviro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann P, Curtis N. The influence of probiotics on vaccine responses - a systematic review. Vaccine. 2008;36(2):207–213. doi: 10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188(2):327–338. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 11.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines–immune responses. Vaccine. 2012;30(Suppl 5):F83–7. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 12.Bolyen E, Rideout JR, Dillon MR, et al (2018) QIIME 2: Reproducible, interactive, scalable and extensible microbiome data science. PeerJ Prepr [Internet] 852–7. Available from: 10.7287/peerj.preprints.27295v2
- 13.Chao Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11(4):265–270. [Google Scholar]
- 14.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27(4):623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x. [DOI] [Google Scholar]
- 15.Pylro VS, Roesch LFW, Morais DK, Clark IM, Hirsch PR, Tótola MR. Data analysis for 16S microbial profiling from different benchtop sequencing platforms. J Microbiol Methods [Internet] 2014;107:30–37. doi: 10.1016/j.mimet.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45(W1):W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beals EW. Bray-curtis ordination: an effective strategy for analysis of multivariate ecological data. Adv Ecol Res. 1984;14(C):1–55. [Google Scholar]
- 18.Ferreira RB, Antunes LC, Finlay BB. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010;6(11):e1001190. doi: 10.1371/journal.ppat.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human papillomavirus vaccines: WHO position paper (2017). Wkly Epidemiol Rec 92(19):241–68 [PubMed]
- 20.de Oliveira CM, Fregnani JHTG, Villa LL. HPV vaccine: updates and highlights. Acta Cytol. 2019;63:159–168. doi: 10.1159/000497617. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol. 2008;110(3 Suppl 1):S1–10. doi: 10.1016/j.ygyno.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Meyer SI, Fuglsang K, Blaakaer J. Cell-mediated immune response: a clinical review of the therapeutic potential of human papillomavirus vaccination. Acta Obstet Gynecol Scand. 2014;93(12):1209–1218. doi: 10.1111/aogs.12480. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves AK, Giraldo PC, Machado PR, et al. Human papillomavirus vaccine-induced cytokine messenger RNA expression in vaccinated women. Viral Immunol. 2015;28(6):339–342. doi: 10.1089/vim.2015.0008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not aplicable.