Abstract
Asthma is a complex disease with heterogenous phenotypes and endotypes that are incompletely understood. Obesity, obstructive sleep apnea, and gastroesophageal reflux disease co-occur in asthmatics at higher rates than non-asthmatics. While these diseases share risk factors, there are some data suggesting these comorbidities have shared inflammatory pathways, drive the development of asthma or worsen asthma control. This review discusses the epidemiology, pathophysiology, management recommendations and key knowledge gaps of these common comorbidities.
Keywords: Asthma, obesity, obstructive sleep apnea, gastroesophageal reflux disease
Asthma is a heterogeneous disease that is complicated by multiple comorbidities and known risk factors. Comorbidities like obesity, gastroesophageal reflux disorder (GERD), and obstructive sleep apnea (OSA), contribute to disease severity in subjects with asthma. There are a number of shared risk factors among these comorbidities however, emerging evidence demonstrates that there is shared pathophysiology that drive the development and impact control of these conditions. A better understanding of the mechanisms by which these comorbidities contribute to asthma severity may provide insight towards targeted therapeutic approaches for asthmatics with these conditions.
This review discusses the current epidemiologic and pathophysiologic evidence tying each of these comorbidities to asthma and reviews key areas for future research.
OBESITY AND ASTHMA:
Analysis of literature
Asthma and obesity are associated with asthma risk increased by 50% in overweight and obese individuals(1). Obesity appears to have a causal role in asthma, as prospective studies indicate that obesity precedes asthma onset(2–7), and weight gain is associated with the development of asthma in susceptible individuals(3, 4). Obesity rates worldwide continue to rise(8), so a corresponding increase in obese asthma prevalence can be predicted.
Obese asthma has been described as two predominant phenotypes, with markedly different inflammatory profiles(9). Obese individuals with early onset allergic asthma (EOAA) have asthma which developed in childhood independent of obesity and is driven by type 2 inflammation. In EOAA, obesity worsens asthma symptoms, but asthma does not completely resolve with weight loss(9). Late onset nonatopic asthma (LONA) is a different clinical phenotype, in which asthma develops after childhood as a consequence of obesity and is more likely to resolve with weight loss(9). Cluster analyses have consistently identified LONA in older obese women, who have non-T2 inflammation, that is refractory to management with corticosteroids(10–12). The complexity of clinical expression makes obese asthma difficult to manage(13, 14).
Treatments:
Weight loss is the obvious solution to treat obese asthma, and there are multiple strategies that can be used. In uncontrolled studies, bariatric surgery improves asthma outcomes including symptoms, medication use and exacerbation rates(15–17). A systematic review found 6 trials and concluded that weight loss was generally associated with improvements in asthma-related quality of life, asthma control and lung function, regardless of the approach used to achieve weight loss(18). Of particular interest is one three-arm trial which randomized overweight/obese adults with asthma to either dietary intervention, exercise intervention, or a combination and found that 5–10% weight loss resulted in improvement asthma control in 58% and asthma quality of life in 83% of subjects, irrespective of the type of intervention(19). The majority of evidence demonstrates that weight loss improves asthma outcomes, though notably Forno and colleagues demonstrated improvement in asthma control after bariatric surgery only in those who had metabolic syndrome(20).
Improving diet quality is beneficial for obese asthma, independent of weight loss. Obesogenic diets are typically of poor quality, characterized by the consumption of energy-dense foods, high in fat and low in fiber(21), promoting inflammation(22). Clinical intervention studies show that fatty acids induce airway inflammation(23), soluble fiber reduces airway inflammation and improves asthma control (24, 25) and increased intake of fruits and vegetables reduces exacerbation risk(26, 27).
Increasing physical activity is a key element of weight loss interventions for asthma. In addition to weight control, physical activity induces a wide range of benefits, including reduction in all-cause mortality, cardiovascular disease, type 2 diabetes, cancer and depression(28). Physical activity consistently decreases asthma exacerbation rates and healthcare utilization and improves asthma quality of life among all asthma subjects(29, 30). Freitas and colleagues demonstrate that independent of weight loss, asthma control can be improved in obese asthmatics via an exercise intervention, where a corresponding improvement in aerobic capacity is achieved(31).
Pharmacological treatments targeted at specific mechanistic pathways are needed. For example, nitric oxide (NO) bioavailability in the airways of obese asthmatics is reduced, and this contributes to airway dysfunction and substandard response to inhaled corticosteroids(32). In a proof-of-concept, open-label pilot study, 41 obese asthmatics were treated with 15 g/d L-citrulline for 2 weeks. Short-term L-citrulline treatment improved asthma control and exhaled NO(32). A 2010 study found treatment with glucagon-like peptide-1 receptor antagonists in diabetics with asthma reduced asthma exacerbations compared to other diabetes medications(33), and now is a potential treatment for asthmatics with metabolic syndrome.
Knowledge gaps
Effective weight loss strategies are urgently needed and remain a public health priority, relevant to the current global epidemic of chronic lifestyle diseases. Considering the scale of the problem, it is critical that alternative treatment strategies also are developed to improve the management of individuals for whom weight loss is not achieved.
Metabolic abnormalities induced by obesity may contribute directly to asthma pathology, which is an important area for future research. The relationship between asthma and features of metabolic syndrome, including insulin resistance and dyslipidemia, persists after accounting for BMI in many studies(34). While these metabolic abnormalities often co-exist with obesity, there are non-obese asthmatics with other features of metabolic syndrome who may benefit from targeted interventions. High serum triglycerides and low HDL-cholesterol are associated with asthma risk(35). Rastogi and colleagues reported an inverse association between HDL-cholesterol levels and monocyte activation and an inverse association between monocyte activation and pulmonary function that was mediated by HDL-cholesterol in a study of obese children with asthma(36). This suggests the potential for lifestyle or pharmacological interventions aimed at increasing HDL-cholesterol levels as a therapeutic approach for obese asthma.
Gut microbiome manipulation also may have the potential to improve the management of obese asthma. Dietary changes recommended for obese asthma are associated with improvements in the gut microbiome profile which may have immunomodulatory effects in the airways(37, 38). The role of gut microbiota in modulating asthmatic immune responses warrants further investigation.
The role of adipokines, cytokines produced by adipose tissue, as a therapy for obese asthma is another area that requires further research. Evidence is strong for an immunomodulatory role of adiponectin, leptin and resistin in murine models(39). However, human data are less convincing, with majority of evidence coming from epidemiologic observational studies. The therapeutic potential of adipokines in asthma remains unknown.
OBSTRUCTIVE SLEEP APNEA AND ASTHMA
Analysis of literature
Though OSA and asthma are both common medical conditions, subjects with asthma versus those without have a higher prevalence of OSA(40, 41). The prevalence of OSA in asthmatics was found to be 49.5% (95% confidence interval 36.4%, 62.6%), and the pooled odds ratio (OR) for prevalent OSA was 2.64 (1.76, 3.52) among asthmatics compared to non-asthmatics in a meta-analysis(41). Similarly, a large cross-section study found an OR of 2.7 (1.6, 4.6) after adjusting for obesity and other confounders(42). A number of longitudinal studies demonstrate an increased risk of developing incident OSA among subjects with asthma after adjustment for BMI or obesity(43–45). The relationship between OSA and asthma severity is less well understood. Studies show an increased risk of OSA among subjects with severe asthma compared to moderate asthma(46–49), though others find no difference(50, 51).
OSA is linked to worse asthma outcomes including increased emergency department visits, increased number of asthma exacerbations, decreased quality of life and asthma control(45, 52–59). Wang and colleagues were able to demonstrate increased odds of severe exacerbations with an increased apnea hypopnea index (AHI), adjusted OR 1.33 (1.15, 1.52)(59). Despite these findings, others have not found a significant relationship between OSA and asthma outcomes(47, 60).
Pathophysiology
While there are strong epidemiologic ties between OSA and asthma, the mechanism of this association is complex and not well understood, and there is likely a bidirectional association.
Impact of asthma on OSA
Animal studies and a small study in asthmatics show a depressed ventilatory response to hypoxia that appears to be mediated by the carotid sinus and increased Th1 inflammation(61–63). Inhaled corticosteroids (ICS) inhalers are a mainstay of asthma management, though ICS use led to increases in upper airway collapsibility measured by changes in passive critical closing pressure, particularly among older, male subjects with worse baseline asthma control, in a small study of ICS-naïve subjects with mild asthma(64). ICS use is associated with habitual snoring and OSA with frequency of snoring showing a dose-dependent relationship(48).
Impact of OSA on asthma
Features of OSA, particularly chronic intermittent hypoxia (CIH), may lead to inflammation and/or remodeling of the lower airways that may increase asthma morbidity. CIH increases airflow obstruction(65), markers of airway remodeling and fibrosis(66, 67), and Th1 inflammation and peri-bronchial neutrophils in mouse models of asthma(65, 67). Studies indicate that asthmatics with high risk for OSA versus those without were more likely to have sputum neutrophilia (50, 68) and higher matrix metalloprotease 9 and IL-8(68). IL-8 levels were found to correlate with increasing AHI (rho=0.72, p=0.006)(68). There is a well-described neutrophil predominant endotype of asthma(12), suggesting a possible role of shared pathophysiology.
Shared inflammatory pathways
L-arginine and NO metabolism are dysregulated in asthma(69–71). In obesity associated asthma, there is an imbalance in L-arginine and asymmetric dimethyl arginine, leading to inhibition and uncoupling of NO synthase, which is the enzyme that converts L-arginine into L-citrulline and NO(70, 72, 73). There is also evidence that L-arginine metabolism is dysregulated in OSA. OSA subjects have decreased L-arginine bioavailability, decreased NO and increased arginase in cross sectional studies(74–76). This dysregulation seen in OSA subjects share some features with obese asthmatics(70, 72, 73). The role of L-arginine metabolism in subjects with both asthma and OSA has not been investigated to date.
The impact of treating OSA on asthma outcomes
There are limited data on the impact of treating OSA on asthma outcomes. Continuous positive airway pressure (CPAP) use is associated in observational studies with decreased daytime symptoms after adjustment for obesity(57), improved quality of life(77) and improved asthma control(78) and decreased decline in FEV1(46). Quasi-experimental studies show improvement in asthma control and quality of life, though changes in bronchial responsiveness were variable between studies(79, 80). The only randomized control trial to date, found no difference in asthma control, however those randomized to CPAP had improved quality of life(81). In one observational study, the use of an oral appliance for OSA treatment demonstrated improvement in asthma control(82). Overall, studies to date have consistently demonstrated an improvement in asthma quality of life associated with CPAP, though the effects on asthma control and objective measures of lung function remain less clearly understood.
Knowledge gaps
There is much to be learned about the relationship between asthma and OSA. Asthma is a complex and heterogenous disease with poorly defined clinical phenotypes. Some studies suggest that subjects with OSA and asthma have increased Th1 inflammation and neutrophil predominance(50, 68), though which phenotypes of asthma are at highest risk of developing OSA is not well defined. This is particularly important to understand as Th1 inflammation does not respond to ICS treatment as well as Th2-driven asthma, and data suggest that ICS may increase risk of OSA. Given that OSA risk increases with the duration of asthma, it is possible that age of asthma onset and asthma morbidity may impact the development of OSA. There are not yet life-course or birth cohort studies to examine the natural history and risk factors that may lead to incident OSA. These studies, and others also are needed to better understand mechanisms of incident OSA among asthmatics, especially since there is a lack of understanding of how inflammation, upper airway changes and treatment lead to OSA in some asthmatics. A better understanding of asthma phenotypes also would aid in identifying asthmatics who would benefit from screening in the clinical setting.
GASTROESOPHAGEAL REFLUX AND ASTHMA
Analysis of literature
Asthma and GERD are both common clinical conditions, but their frequent presence as comorbid conditions raises the possibility of shared biological mechanisms underlying these two diseases. Epidemiologic data suggest that GERD occurs in 30–80% of asthmatics subjects(83). Esophageal manometry and 24-hour esophageal pH testing demonstrates frequent GERD in the absence of overt symptoms in stable asthmatics(84), though notably, the majority of subjects reported cough due to laryngopharyngeal reflux independent of other reflux symptoms(85).
Previous studies demonstrate variable associations of GERD with asthma severity and exacerbations. GERD was identified as a significant risk factor, OR 4.9 (1.4, 17.8), for recurrent exacerbations after covariate adjustment in a cross-sectional study of 152 subjects(52). Furthermore, GERD has been associated with exacerbation frequency in large asthma cohort studies(86, 87). Using data from the Severe Asthma Research Project-3, Denlinger and colleagues found an increased rate of asthma exacerbations among those with GERD, rate ratio 1.6 (1.3, 2.0)(86).
Pathophysiology:
There are two proposed mechanisms by which GERD impacts asthma severity and exacerbations: 1) micro-aspiration of gastric contents causing airway inflammation, respiratory symptoms, and lung injury(88, 89), and 2) vagal nerve stimulation that occurs from reflux of acidic gastric contents into the lower esophagus leading to bronchoconstriction and respiratory symptoms(90, 91). Furthermore, bronchoconstriction and hyperinflation in asthma may induce acid reflux due to changes in the pressure gradient between the abdomen and the chest changing lower esophageal sphincter (LES) tone(92).
Impact of GERD treatment on asthma outcomes:
Results of clinical trials of acid suppression on asthma outcomes are inconsistent. Littner and colleagues found that twice daily lansoprazole improved overall quality of life and reduced asthma exacerbations in subjects with symptomatic reflux, with the most pronounced effect occurring in more severe asthmatics(93). These findings have not been replicated in other trials among those with mild to moderate asthma(94, 95), including asthmatics without symptoms of GERD(95). Others have found improvement in asthma symptoms without improvement in lung function(96, 97).
A meta-analysis in 2002 of existing trials by the Cochrane Systematic Review group did not recommend the treatment of GERD in subjects with uncontrolled asthma due to lack of evidence to support their routine use(98). Since that time, trials investigating the impact of GERD treatment on asthma outcomes is limited. However, the common occurrence of these two comorbid conditions and the conflicting data regarding GERD treatment would suggest that additional larger scale randomized clinical trials in this area are warranted.
Knowledge gaps
The routine treatment of GERD in poorly controlled asthmatics without GERD symptoms remains controversial due to the inconsistency of the existing data. Given the common occurrence of these two comorbid conditions, additional investigation of the impact of GERD and GERD treatment on asthma outcomes is essential to address some ongoing knowledge gaps. Additionally, future research should aim to address several unanswered questions with respect to the relationship between GERD and asthma outcomes: 1) efficacy of GERD treatment in manometry or pH probe confirmed subjects with and without GERD symptoms, 2) the impact of proximal versus distal esophageal reflux, and 3) a better understanding of the impact of medication and surgical options for GERD. Well-designed randomized controlled trials are needed to address each of these key issues.
THREE COLINEAR DISEASES: OBESITY, OSA & GERD
There are strong associations and suspected causal pathways between obesity, OSA, GERD and asthma. The estimated prevalence and co-occurrence of each of these diseases is described in Table 1. Among those with asthma, the prevalence of GERD and OSA is higher than that of the general population. The same is true for the prevalence of asthma among those with obesity and OSA. There are certainly shared risk factors that partially explain the high rates of comorbidity, however there are proposed pathophysiological mechanisms that each disease drives the development of or worsens control of another comorbid condition. Table 2 summarizes evidence for treatment of these conditions among asthmatics and key knowledge gaps.
Table 1:
Estimated prevalence of asthma, obesity, OSA and GERD
| Among those with: | ||||
|---|---|---|---|---|
| Prevalence of: | Asthma | Obesity | OSA | GERD |
| Asthma | 8.0%(108) | 16.6%(109) | 35.1%(110) | 4.6%(111) |
| Obesity | 38.8%(112) | 42.4%(113) | 13.5–67.9% women 44.6–82.8% men(114) |
27.9%(115) |
| OSA | 49.5%(41) | 21.8–34.4% women 22.2–62.6% men(116) |
4.5%(117) | 65%(118) |
| GERD | 50.9%(111) | 39.0%(119) | 73%(120) | 30.9%(121) |
The prevalence of each condition (row) among those with asthma, obesity, OSA and GERD (columns)
Table 2:
Evidence for treatment and knowledge gaps in treating obesity, GERD, and OSA in asthma
| Evidence for treatment | Knowledge gaps | |
|---|---|---|
| Obesity | Weight loss • Increased asthma quality of life, asthma control, improved lung function Physical activity • Increased asthma control Improved diet quality • Increased fruits and vegetables associated with decreased exacerbations • Increased soluble fiber associated with increased asthma control |
• Effective weight loss strategies • Targeted pharmacologic therapies for metabolic abnormalities including increased HDL, L-citrulline supplementation, decreased triglycerides • Gut microbiome manipulation • Better understanding of immunomodulatory effects and possible treatment targeting adipokines |
| OSA | CPAP for sleep apnea • Improves asthma quality of life • Improved asthma control and decreased daytime symptoms in observational studies |
• Impact of CPAP treatment in preventing incident asthma • Utility of routine screening for OSA among asthmatics |
| GERD | Treating symptomatic GERD may improve • Asthma quality of life • Asthma symptoms |
• Benefit of screening for and treating asymptomatic GERD • Treatment duration • Impact of medical vs surgical treatment for GERD |
Obesity and OSA:
There are many hypothesized mechanisms between OSA and obesity. Increased neck circumference and upper airway tissue in obesity may directly lead to intermittent obstruction that causes sleep apnea(99). Additionally, obese subjects have lower lung volumes, more pronounced when supine. Intermittent hypoxia from OSA leads to increased insulin resistance and leptin levels, both of which are associated with obesity and the metabolic syndrome(100). Weight loss decreases AHI and OSA(100). Treating OSA with CPAP, along with calorie restrictions, leads to more weight loss than calorie restriction alone among obese subjects with OSA(101).
Obesity and GERD:
Obese subjects have increased prevalence of esophageal dysmotility, transient relaxation of LES tone and hiatal hernia, all of which increase the incidence of GERD(102). Obesity also leads to increased intraabdominal pressure, increasing the likelihood of gastric contents being pushed through the LES. Increased leptin, which occurs in obesity, is associated with the development of dysplasia and Barrett’s esophagus(102).
GERD and OSA:
There are a number of theories to causally link GERD and OSA. OSA is thought to lead to increased intrathoracic pressure during apneic episodes, causing the LES to open, with concomitant increase in abdominal pressure. This leads to an increase in gastric reflux. Conversely, nocturnal GERD symptoms may lead to increased sleep arousals, decreased sleep and laryngeal edema leading to increased upper airway collapse. Conflicting data exist for these two theories(103). CPAP decreases GERD symptoms, implying that this treatment may be efficacious for both diseases(104).
Asthma, obesity, OSA and GERD:
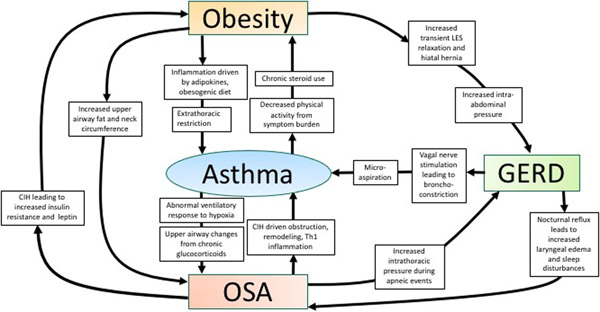
While these four conditions are separately linked, to date, no investigation has examined the prevalence of these disorders together. Figure 1 shows a framework for how these disorders relate to each other with proposed mechanisms. Obesity, OSA, GERD and some endotypes of asthma share similar features of Th1-driven inflammation. This may represent a common underlying pathway, though no investigator has identified any common markers of inflammation in subjects with asthma and all three comorbidities.
Figure 1:
Relationships and proposed mechanisms between asthma, obesity, obstructive sleep apnea and gastroesophageal reflux disease
Treatment of OSA can improve asthma symptoms and decrease GERD, inconsistently associated with weight loss(40, 99, 102, 105, 106). Weight loss improves and/or resolves OSA, GERD and asthma in individual studies(40, 99, 102). Finally, treatment of GERD improves asthma control(96, 97) and sleep disturbances(107), though does not lead to weight loss. In summary, though not studied collectively, the identification and treatment of these comorbidities has a positive impact on asthma control.
CONCLUSIONS:
Asthma is associated with comorbid obesity, OSA and GERD and the interplay among these diseases is not fully understood. In studies on the effects of obesity, OSA and GERD individually, there appears to be shared and bidirectional mechanisms that drive the development of asthma and/or worsen asthma control. Data from studies of individual comorbidities and asthma demonstrate improvement in many domains of asthma control and/or lung function with treatment. Although there are many gaps in the understanding of these relationships, it is important to identify and treat these comorbidities in subjects with poorly controlled asthma, even if they may not impact asthma outcomes.
Abbreviations:
- GERD
gastroesophageal reflux disease
- OSA
obstructive sleep apnea
- EOAA
early onset allergic asthma
- LONA
late onset atopic asthma
- NO
nitric oxide
- OR
odds ratio
- AHI
apnea hypopnea index
- ICS
inhaled corticosteroids
- CIH
chronic intermittent hypoxia
- CPAP
continuous positive airway pressure
- LES
lower esophageal sphincter
Footnotes
Disclosure: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. American journal of respiratory and critical care medicine. 2007;175(7):661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54(5):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camargo CA Jr., Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Archives of internal medicine. 1999;159(21):2582–8. [DOI] [PubMed] [Google Scholar]
- 4.Beckett WS, Jacobs DR Jr., Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. American journal of respiratory and critical care medicine. 2001;164(11):2045–50. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. American journal of epidemiology. 2002;155(3):191–7. [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122(4):1256–63. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. The European respiratory journal. 2004;24(5):740–4. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Archives of internal medicine. 2002;162(13):1477–81. [DOI] [PubMed] [Google Scholar]
- 9.Bates JHT, Poynter ME, Frodella CM, Peters U, Dixon AE, Suratt BT. Pathophysiology to Phenotype in the Asthma of Obesity. Annals of the American Thoracic Society. 2017;14(Supplement_5):S395–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. American journal of respiratory and critical care medicine. 2008;178(3):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefaudeux D, De Meulder B, Loza MJ, Peffer N, Rowe A, Baribaud F, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. The Journal of allergy and clinical immunology. 2017;139(6):1797–807. [DOI] [PubMed] [Google Scholar]
- 12.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2010;181(4):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respiratory medicine. 2007;101(11):2240–7. [DOI] [PubMed] [Google Scholar]
- 14.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, et al. Effect of obesity on clinical presentation and response to treatment in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2006;43(7):553–8. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, Chapman L, O’Brien P. Marked improvement in asthma after Lap-Band surgery for morbid obesity. Obesity surgery. 1999;9(4):385–9. [DOI] [PubMed] [Google Scholar]
- 16.Dhabuwala A, Cannan RJ, Stubbs RS. Improvement in co-morbidities following weight loss from gastric bypass surgery. Obesity surgery. 2000;10(5):428–35. [DOI] [PubMed] [Google Scholar]
- 17.Macgregor AM, Greenberg RA. Effect of Surgically Induced Weight Loss on Asthma in the Morbidly Obese. Obesity surgery. 1993;3(1):15–21. [DOI] [PubMed] [Google Scholar]
- 18.Okoniewski W, Lu KD, Forno E. Weight Loss for Children and Adults with Obesity and Asthma. A Systematic Review of Randomized Controlled Trials. Annals of the American Thoracic Society. 2019;16(5):613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43(1):36–49. [DOI] [PubMed] [Google Scholar]
- 20.Forno E, Zhang P, Nouraie M, Courcoulas A, Mitchell JE, Wolfe BM, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PloS one. 2019;14(4):e0214730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jessri M, Wolfinger RD, Lou WY, L’Abbé MR. Identification of dietary patterns associated with obesity in a nationally representative survey of Canadian adults: application of a priori, hybrid, and simplified dietary pattern techniques. The American journal of clinical nutrition. 2017;105(3):669–84. [DOI] [PubMed] [Google Scholar]
- 22.Wood LG. Diet, Obesity, and Asthma. Annals of the American Thoracic Society. 2017;14(Supplement_5):S332–s8. [DOI] [PubMed] [Google Scholar]
- 23.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. The Journal of allergy and clinical immunology. 2011;127(5):1133–40. [DOI] [PubMed] [Google Scholar]
- 24.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLoughlin R, Berthon BS, Rogers GB, Baines KJ, Leong LEX, Gibson PG, et al. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine. 2019;46:473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free radical research. 2008;42(1):94–102. [DOI] [PubMed] [Google Scholar]
- 27.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. The American journal of clinical nutrition. 2012;96(3):534–43. [DOI] [PubMed] [Google Scholar]
- 28.Committee PAGA. Physical activity guidelines advisory committee report, 2008. Washington, DC: US Department of Health and Human Services. 2008;2008:A1–H14. [Google Scholar]
- 29.Nyenhuis SM, Dixon AE, Ma J. Impact of Lifestyle Interventions Targeting Healthy Diet, Physical Activity, and Weight Loss on Asthma in Adults: What Is the Evidence? The journal of allergy and clinical immunology In practice. 2018;6(3):751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panagiotou M, Koulouris NG, Rovina N. Physical Activity: A Missing Link in Asthma Care. Journal of clinical medicine. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. American journal of respiratory and critical care medicine. 2017;195(1):32–42. [DOI] [PubMed] [Google Scholar]
- 32.Holguin F, Grasemann H, Sharma S, Winnica D, Wasil K, Smith V, et al. L-Citrulline increases nitric oxide and improves control in obese asthmatics. JCI insight. 2019;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. American journal of respiratory and critical care medicine. 2021;203(7):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serafino-Agrusa L, Spatafora M, Scichilone N. Asthma and metabolic syndrome: Current knowledge and future perspectives. World journal of clinical cases. 2015;3(3):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenger RV, Gonzalez-Quintela A, Linneberg A, Husemoen LL, Thuesen BH, Aadahl M, et al. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respiratory medicine. 2013;107(6):816–24. [DOI] [PubMed] [Google Scholar]
- 36.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. American journal of respiratory and critical care medicine. 2015;191(2):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunological reviews. 2017;278(1):277–95. [DOI] [PubMed] [Google Scholar]
- 38.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature communications. 2015;6:7320. [DOI] [PubMed] [Google Scholar]
- 39.Sood A, Shore SA. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. Journal of allergy. 2013;2013:785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad B, Nyenhuis SM, Imayama I, Siddiqi A, Teodorescu M. Asthma and Obstructive Sleep Apnea Overlap: What Has the Evidence Taught Us? American journal of respiratory and critical care medicine. 2020;201(11):1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong DL, Qin Z, Shen H, Jin HY, Wang W, Wang ZF. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Scientific reports. 2017;7(1):4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya N, Kepnes LJ. Ambulatory office visits and medical comorbidities associated with obstructive sleep apnea. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;147(6):1154–7. [DOI] [PubMed] [Google Scholar]
- 43.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130(6):1779–83. [DOI] [PubMed] [Google Scholar]
- 44.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. Jama. 2015;313(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen TC, Lin CL, Wei CC, Chen CH, Tu CY, Hsia TC, et al. Risk of Obstructive Sleep Apnea in Adult Patients with Asthma: A Population-Based Cohort Study in Taiwan. PloS one. 2015;10(6):e0128461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang TY, Lo YL, Lin SM, Huang CD, Chung FT, Lin HC, et al. Obstructive sleep apnoea accelerates FEV(1) decline in asthmatic patients. BMC pulmonary medicine. 2017;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. The Journal of allergy and clinical immunology. 2009;124(2):371–6. [DOI] [PubMed] [Google Scholar]
- 48.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135(5):1125–32. [DOI] [PubMed] [Google Scholar]
- 49.Teodorescu M, Polomis DA, Gangnon RE, Fedie JE, Consens FB, Chervin RD, et al. Asthma Control and Its Relationship with Obstructive Sleep Apnea (OSA) in Older Adults. Sleep disorders. 2013;2013:251567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teodorescu M, Broytman O, Curran-Everett D, Sorkness RL, Crisafi G, Bleecker ER, et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. The journal of allergy and clinical immunology In practice. 2015;3(4):566–75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep medicine. 2008;9(5):494–9. [DOI] [PubMed] [Google Scholar]
- 52.ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. The European respiratory journal. 2005;26(5):812–8. [DOI] [PubMed] [Google Scholar]
- 53.Yii ACA, Tan JHY, Lapperre TS, Chan AKW, Low SY, Ong TH, et al. Long-term future risk of severe exacerbations: Distinct 5-year trajectories of problematic asthma. Allergy. 2017;72(9):1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jordan HT, Stellman SD, Reibman J, Farfel MR, Brackbill RM, Friedman SM, et al. Factors associated with poor control of 9/11-related asthma 10–11 years after the 2001 World Trade Center terrorist attacks. The Journal of asthma : official journal of the Association for the Care of Asthma. 2015;52(6):630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon AE, Clerisme-Beaty EM, Sugar EA, Cohen RI, Lang JE, Brown ED, et al. Effects of obstructive sleep apnea and gastroesophageal reflux disease on asthma control in obesity. The Journal of asthma : official journal of the Association for the Care of Asthma. 2011;48(7):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teodorescu M, Polomis DA, Hall SV, Teodorescu MC, Gangnon RE, Peterson AG, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138(3):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teodorescu M, Polomis DA, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. The Journal of asthma : official journal of the Association for the Care of Asthma. 2012;49(6):620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim MY, Jo EJ, Kang SY, Chang YS, Yoon IY, Cho SH, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;110(4):253–7, 7.e1. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Liu K, Hu K, Yang J, Li Z, Nie M, et al. Impact of obstructive sleep apnea on severe asthma exacerbations. Sleep medicine. 2016;26:1–5. [DOI] [PubMed] [Google Scholar]
- 60.Tay TR, Radhakrishna N, Hore-Lacy F, Smith C, Hoy R, Dabscheck E, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology (Carlton, Vic). 2016;21(8):1384–90. [DOI] [PubMed] [Google Scholar]
- 61.Xu F, Zhuang J, Zhou T, Lee LY. Ovalbumin sensitization alters the ventilatory responses to chemical challenges in guinea pigs. Journal of applied physiology (Bethesda, Md : 1985). 2005;99(5):1782–8. [DOI] [PubMed] [Google Scholar]
- 62.Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, et al. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. Journal of applied physiology (Bethesda, Md : 1985). 2006;101(6):1795–802. [DOI] [PubMed] [Google Scholar]
- 63.Eckert DJ, Catcheside PG, Smith JH, Frith PA, McEvoy RD. Hypoxia suppresses symptom perception in asthma. American journal of respiratory and critical care medicine. 2004;169(11):1224–30. [DOI] [PubMed] [Google Scholar]
- 64.Teodorescu M, Xie A, Sorkness CA, Robbins J, Reeder S, Gong Y, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(2):183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, et al. Effects of chronic intermittent hypoxia on allergen-induced airway inflammation in rats. American journal of respiratory cell and molecular biology. 2015;52(2):162–70. [DOI] [PubMed] [Google Scholar]
- 66.Zhou JP, Lin YN, Li N, Sun XW, Ding YJ, Yan YR, et al. Angiotensin-(1–7) Rescues Chronic Intermittent Hypoxia-Aggravated Transforming Growth Factor-β-Mediated Airway Remodeling in Murine and Cellular Models of Asthma. The Journal of pharmacology and experimental therapeutics. 2020;375(2):268–75. [DOI] [PubMed] [Google Scholar]
- 67.Baek KJ, Cho JY, Rosenthal P, Alexander LEC, Nizet V, Broide DH. Hypoxia potentiates allergen induction of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway remodeling in a mouse model. Clinical immunology (Orlando, Fla). 2013;147(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taillé C, Rouvel-Tallec A, Stoica M, Danel C, Dehoux M, Marin-Esteban V, et al. Obstructive Sleep Apnoea Modulates Airway Inflammation and Remodelling in Severe Asthma. PloS one. 2016;11(3):e0150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asosingh K, Lauruschkat CD, Alemagno M, Frimel M, Wanner N, Weiss K, et al. Arginine metabolic control of airway inflammation. JCI insight. 2020;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott JA, North ML, Rafii M, Huang H, Pencharz P, Subbarao P, et al. Asymmetric dimethylarginine is increased in asthma. American journal of respiratory and critical care medicine. 2011;184(7):779–85. [DOI] [PubMed] [Google Scholar]
- 71.Xu W, Comhair SAA, Janocha AJ, Lara A, Mavrakis LA, Bennett CD, et al. Arginine metabolic endotypes related to asthma severity. PloS one. 2017;12(8):e0183066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holguin F. Arginine and nitric oxide pathways in obesity-associated asthma. Journal of allergy. 2013;2013:714595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. American journal of respiratory and critical care medicine. 2013;187(2):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yüksel M, Okur HK, Pelin Z, Öğünç AV, Öztürk L. Arginase activity and nitric oxide levels in patients with obstructive sleep apnea syndrome. Clinics (Sao Paulo, Brazil). 2014;69(4):247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.İn E, Özdemir C, Kaman D, Sökücü SN. Heat Shock Proteins, L-Arginine, and Asymmetric Dimethylarginine Levels in Patients With Obstructive Sleep Apnea Syndrome. Archivos de bronconeumologia. 2015;51(11):544–50. [DOI] [PubMed] [Google Scholar]
- 76.Ozkan Y, Firat H, Simşek B, Torun M, Yardim-Akaydin S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep & breathing = Schlaf & Atmung. 2008;12(2):149–54. [DOI] [PubMed] [Google Scholar]
- 77.Davies SE, Bishopp A, Wharton S, Turner AM, Mansur AH. Does Continuous Positive Airway Pressure (CPAP) treatment of obstructive sleep apnoea (OSA) improve asthma-related clinical outcomes in patients with co-existing conditions?-A systematic review. Respiratory medicine. 2018;143:18–30. [DOI] [PubMed] [Google Scholar]
- 78.Kauppi P, Bachour P, Maasilta P, Bachour A. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep & breathing = Schlaf & Atmung. 2016;20(4):1217–24. [DOI] [PubMed] [Google Scholar]
- 79.Lafond C, Sériès F, Lemière C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. The European respiratory journal. 2007;29(2):307–11. [DOI] [PubMed] [Google Scholar]
- 80.Serrano-Pariente J, Plaza V, Soriano JB, Mayos M, López-Viña A, Picado C, et al. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy. 2017;72(5):802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng SSS, Chan TO, To KW, Chan KKP, Ngai J, Yip WH, et al. Continuous positive airway pressure for obstructive sleep apnoea does not improve asthma control. Respirology (Carlton, Vic). 2018;23(11):1055–62. [DOI] [PubMed] [Google Scholar]
- 82.Bachour P, Bachour A, Kauppi P, Maasilta P, Mäkitie A, Palotie T. Oral appliance in sleep apnea treatment: respiratory and clinical effects and long-term adherence. Sleep & breathing = Schlaf & Atmung. 2016;20(2):805–12. [DOI] [PubMed] [Google Scholar]
- 83.Sandur V, Murugesh M, Banait V, Rathi PM, Bhatia SJ, Joshi JM, et al. Prevalence of gastro-esophageal reflux disease in patients with difficult to control asthma and effect of proton pump inhibitor therapy on asthma symptoms, reflux symptoms, pulmonary function and requirement for asthma medications. Journal of postgraduate medicine. 2014;60(3):282–6. [DOI] [PubMed] [Google Scholar]
- 84.Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. American journal of respiratory and critical care medicine. 2000;162(1):34–9. [DOI] [PubMed] [Google Scholar]
- 85.Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest. 1999;115(3):654–9. [DOI] [PubMed] [Google Scholar]
- 86.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. American journal of respiratory and critical care medicine. 2017;195(3):302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blakey JD, Price DB, Pizzichini E, Popov TA, Dimitrov BD, Postma DS, et al. Identifying Risk of Future Asthma Attacks Using UK Medical Record Data: A Respiratory Effectiveness Group Initiative. The journal of allergy and clinical immunology In practice. 2017;5(4):1015–24.e8. [DOI] [PubMed] [Google Scholar]
- 88.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Critical care medicine. 2011;39(4):818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tuchman DN, Boyle JT, Pack AI, Scwartz J, Kokonos M, Spitzer AR, et al. Comparison of airway responses following tracheal or esophageal acidification in the cat. Gastroenterology. 1984;87(4):872–81. [PubMed] [Google Scholar]
- 90.Wright RA, Miller SA, Corsello BF. Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology. 1990;99(1):71–3. [DOI] [PubMed] [Google Scholar]
- 91.Wu DN, Tanifuji Y, Kobayashi H, Yamauchi K, Kato C, Suzuki K, et al. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest. 2000;118(6):1553–6. [DOI] [PubMed] [Google Scholar]
- 92.Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. American journal of respiratory and critical care medicine. 2002;166(9):1206–11. [DOI] [PubMed] [Google Scholar]
- 93.Littner MR, Leung FW, Ballard ED 2nd, Huang B, Samra NK. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest. 2005;128(3):1128–35. [DOI] [PubMed] [Google Scholar]
- 94.Kiljander TO, Harding SM, Field SK, Stein MR, Nelson HS, Ekelund J, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. American journal of respiratory and critical care medicine. 2006;173(10):1091–7. [DOI] [PubMed] [Google Scholar]
- 95.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. The New England journal of medicine. 2009;360(15):1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: A double-blind, placebo-controlled crossover study with omeprazole. Chest. 1999;116(5):1257–64. [DOI] [PubMed] [Google Scholar]
- 97.Broers C, Tack J, Pauwels A. Review article: gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Alimentary pharmacology & therapeutics. 2018;47(2):176–91. [DOI] [PubMed] [Google Scholar]
- 98.Gibson PG, Henry RL, Coughlan JL. Gastro-oesophageal reflux treatment for asthma in adults and children. The Cochrane database of systematic reviews. 2003(2):Cd001496. [DOI] [PubMed] [Google Scholar]
- 99.Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Current opinion in pulmonary medicine. 2019;25(6):602–8. [DOI] [PubMed] [Google Scholar]
- 100.Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, et al. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep medicine and disorders : international journal. 2017;1(4). [PMC free article] [PubMed] [Google Scholar]
- 101.Mao Y, Ambrogini E, Lendel I, Goulden P. Treating Obstructive Sleep Apnea with Continuous Positive Airway Pressure May Aid Weight Loss in Patients with Obesity on a Calorie Restriction Diet. J Obes Chronic Dis. 2020;3(2):42–8. [Google Scholar]
- 102.Chang P, Friedenberg F. Obesity and GERD. Gastroenterology clinics of North America. 2014;43(1):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim KG, Morgenthaler TI, Katzka DA. Sleep and Nocturnal Gastroesophageal Reflux: An Update. Chest. 2018;154(4):963–71. [DOI] [PubMed] [Google Scholar]
- 104.Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130(4):1003–8. [DOI] [PubMed] [Google Scholar]
- 105.Quan SF, Budhiraja R, Clarke DP, Goodwin JL, Gottlieb DJ, Nichols DA, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013;9(10):989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pleava R, Mihaicuta S, Serban CL, Ardelean C, Marincu I, Gaita D, et al. Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension-An Observational Study. Journal of clinical medicine. 2020;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh JH. Gastroesophageal reflux disease: recent advances and its association with sleep. Annals of the New York Academy of Sciences. 2016;1380(1):195–203. [DOI] [PubMed] [Google Scholar]
- 108.Centers for Disease Control and Prevention. Most Recent National Asthma Data 2021. [Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
- 109.Wang L, Wang K, Gao X, Paul TK, Cai J, Wang Y. Sex difference in the association between obesity and asthma in U.S. adults: Findings from a national study. Respiratory medicine. 2015;109(8):955–62. [DOI] [PubMed] [Google Scholar]
- 110.Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Primary care respiratory journal : journal of the General Practice Airways Group. 2009;18(4):328–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56(12):1654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Centers for Disease Control and Prevention. Asthma and Obesity [Available from: https://www.cdc.gov/asthma/asthma_stats/asthma_obesity.htm.
- 113.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS data brief. 2020(360):1–8. [PubMed] [Google Scholar]
- 114.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. The New England journal of medicine. 2006;354(22):2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep medicine. 2010;11(5):441–6. [DOI] [PubMed] [Google Scholar]
- 117.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Preventive medicine. 2010;51(1):18–23. [DOI] [PubMed] [Google Scholar]
- 118.Vela MF, Kramer JR, Richardson PA, Dodge R, El-Serag HB. Poor sleep quality and obstructive sleep apnea in patients with GERD and Barrett’s esophagus. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(3):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. The American journal of gastroenterology. 2005;100(6):1243–50. [DOI] [PubMed] [Google Scholar]
- 120.Valipour A, Makker HK, Hardy R, Emegbo S, Toma T, Spiro SG. Symptomatic gastroesophageal reflux in subjects with a breathing sleep disorder. Chest. 2002;121(6):1748–53. [DOI] [PubMed] [Google Scholar]
- 121.Delshad SD, Almario CV, Chey WD, Spiegel BMR. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology. 2020;158(5):1250–61.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]