Abstract
Sox9 is a high-mobility-group domain-containing transcription factor required for chondrocyte differentiation and cartilage formation. We used a yeast two-hybrid method based on Son of Sevenless (SOS) recruitment to screen a chondrocyte cDNA library and found that the catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase A (PKA-Cα) interacted specifically with SOX9. Next we found that two consensus PKA phosphorylation sites within SOX9 could be phosphorylated by PKA in vitro and that SOX9 could be phosphorylated by PKA-Cα in vivo. In COS-7 cells cotransfected with PKA-Cα and SOX9 expression plasmids, PKA enhanced the phosphorylation of wild-type SOX9 but did not affect phosphorylation of a SOX9 protein in which the two PKA phosphorylation sites (S64 and S211) were mutated. Using a phosphospecific antibody that specifically recognized SOX9 phosphorylated at serine 211, one of the two PKA phosphorylation sites, we demonstrated that addition of cAMP to chondrocytes strongly increased the phosphorylation of endogenous Sox9. In addition, immunohistochemistry of mouse embryo hind legs showed that Sox9 phosphorylated at serine 211 was principally localized in the prehypertrophic zone of the growth plate, corresponding to the major site of expression of the parathyroid hormone-related peptide (PTHrP) receptor. Since cAMP has previously been shown to effectively increase the mRNA levels of Col2a1 and other specific markers of chondrocyte differentiation in culture, we then asked whether PKA phosphorylation could modulate the activity of SOX9. Addition of 8-bromo-cAMP to chondrocytes in culture increased the activity of a transiently transfected SOX9-dependent 48-bp Col2a1 chondrocyte-specific enhancer; similarly, cotransfection of PKA-Cα increased the activity of this enhancer. Mutations of the two PKA phosphorylation consensus sites of SOX9 markedly decreased the PKA-Cα activation of this enhancer by SOX9. PKA phosphorylation and the mutations in the consensus PKA phosphorylation sites of SOX9 did not alter its nuclear localization. In vitro phosphorylation of SOX9 by PKA resulted in more efficient DNA binding. We conclude that SOX9 is a target of cAMP signaling and that phosphorylation of SOX9 by PKA enhances its transcriptional and DNA-binding activity. Because PTHrP signaling is mediated by cAMP, our results support the hypothesis that Sox9 is a target of PTHrP signaling in the growth plate and that the increased activity of Sox9 might mediate the effect of PTHrP in maintaining the cells as nonhypertrophic chondrocytes.
The transcription factor SOX9 contains a high-mobility-group (HMG)-type DNA-binding domain that shows 50% identity to that of the mammalian testis-determining factor SRY (35) and a transcription activation domain located at the carboxyl terminus of the molecule (27, 31). During embryonic development, expression of Sox9 parallels that of the gene for type II collagen (Col2a1) in all chondrocyte progenitors and chondrocytes (27, 36, 37). In addition, Sox9 is also expressed in gonadal ridges in male and female embryos, and later, whereas its expression is strongly downregulated in female gonads, it is found at high levels in the Sertoli cells of male gonads (13, 26). Sox9 is also expressed in otic vesicles and in discrete areas of the heart, kidney, and nervous system of mouse embryos (27, 37). Our recent experiments using mouse embryo chimeras derived from Sox9−/− embryonic stem cells demonstrated that Sox9 is required for chondrocyte differentiation and cartilage formation (3). In contrast to wild-type chondrocytes, the mutant cells had the aspect of undifferentiated mesenchymal cells and could not express chondrocyte-specific markers such as Col2a1 and the genes for the α2 chain of type IX collagen (Col9a2), the α2 chain of type XI collagen (Col11a2), and aggrecan. In addition, neither cartilage nor type II collagen formed in teratomas derived from Sox9−/− embryo stem cells. Thus, Sox9 has an essential role in determining the fate of chondrocytes. In humans, heterozygous mutations in and around the SOX9 gene cause campomelic dysplasia (CD), a lethal disorder involving abnormalities in skeletal structures derived from cartilage (8, 10, 15, 25, 33). In many cases the disease is believed to be due to SOX9 haploinsufficiency (19, 25). The skeletal anomalies in CD patients include bowing and angulation of the long bones, micrognathia, hypoplasia of the pelvis and scapulae, cleft palate, and a missing pair of ribs. Sex reversal is found in 75% of XY CD patients, which implies that SOX9 also functions in sex determination in humans (24, 34). Sox9 binds to essential sequences in chondrocyte-specific enhancers of the Col2a1 (19) and the Col11a2 (5) genes, and forced expression of SOX9 activates these enhancers in nonchondrocytic cells. Ectopic expression of SOX9 also activates the Col2a1 gene in transgenic mice (2). These experiments provided evidence that these genes are direct targets for Sox9.
Two other members of the Sox family of transcription factors, L-Sox5 and Sox6, also bind to chondrocyte-specific enhancer regions in the Col2a1 and Col11a2 genes (22). L-Sox5 and Sox6, which are highly similar to each other, are coexpressed with Sox9 during chondrogenesis. In cotransfection experiments, they cooperate with Sox9 in activating the Col2a1 gene (22). We thus hypothesized that L-Sox5 and Sox6 act together with Sox9 to control chondrocyte differentiation.
In the present study, in order to identify possible SOX9-interacting proteins that could control SOX9 activity, we screened a primary chondrocyte cDNA library using a yeast two-hybrid method and found specific interactions between SOX9 and the catalytic subunit of protein kinase A (PKA-Cα). In cultured chondrocytes, cyclic AMP (cAMP) enhances the expression of several markers of chondrocyte differentiation, such as Col2a1, link protein, and aggrecan (14, 23, 28); cAMP also mediates the effects of the parathyroid hormone-related peptide (PTHrP), a known modulator of chondrocyte differentiation in growth plates (12, 29). Hence, we postulated that the interactions between SOX9 and PKA-Cα may be physiologically relevant and asked whether SOX9 might be a target for PKA phosphorylation and whether such phosphorylation may affect the activity of SOX9. We found that SOX9 can be phosphorylated by PKA and that this phosphorylation increases SOX9 binding to a Col2a1 enhancer element and stimulates SOX9 transcriptional activity. The finding that Sox9 phosphorylated at one of the two PKA phosphorylation site was mainly localized to the prehypertrophic zone of the growth plate in vivo suggests the hypothesis that Sox9 may be a target for PTHrP signaling.
MATERIALS AND METHODS
cDNA library construction and SRS screening.
Total RNA was extracted from primary chondrocytes isolated from the ribs of newborn mice and cultured for 48 h. After polyadenylated RNA purification, synthesis of double-stranded cDNA with random priming and ligation of adapters were performed with the Superscript Choice system from Gibco-BRL (Gaithersburg, Md.). The cDNA was digested with EcoRI and inserted into the pYES-2 plasmid vector (1) 3′ to the DNA for a v-Src myristoylation sequence, which was to anchor the library-derived polypeptides to the cytoplasmic membrane. Full-length human SOX9 coding sequence was cloned in the pADNS vector (1) in frame with and 3′ to the carboxy terminus of the Son of Sevenless (SOS) cDNA sequence, with six glycine codons inserted between the SOS and SOX9 sequences. SOS recruitment system (SRS) screening was performed as described by Aronheim et al. (1) with some modifications. The cdc25-2 Saccharomyces cerevisiae strain was first transformed with plasmid pADNS-SOS-SOX9 and subsequently transformed with the pYES-2 cDNA library plasmids. Transformants were plated on galactose plates coated with a thin layer of solution containing glucose (67 mg/ml). Plates were incubated at 25°C for 2 days and at 37°C for 4 more days, after which colonies were picked and tested for galactose-dependent growth at 37°C. Plasmids were extracted from surviving colonies and amplified in Escherichia coli for further retransformation into yeast cells and DNA sequence analysis. The specificity of the interactions between SOX9 and library-derived polypeptides was tested by cotransforming the cdc25-2 cells with screening-positive cDNA library plasmids and either empty pADNS, pADNS-p110-SOS (1), or pADNS-SOS-SOX9.
Mutagenesis and production of bacterially expressed GST-SOX9 and mutant SOX9 proteins.
Serine-to-alanine substitution mutations were introduced into the two PKA phosphorylation consensus sites of SOX9 by using the Transformer mutagenesis kit (Clontech, Palo Alto, Calif.). The full-length SOX9 coding sequence was cloned into the pGEX4T3 bacterial expression vector (Pharmacia Biotech, Piscataway, N.J.) to generate a glutathione S-transferase (GST) fusion polypeptide. The recombinant proteins were produced in bacterial strain BL21(DE3). After cell growth to an optical density at 600 nm (OD600) of 0.6, 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to induce protein synthesis, and cells were grown for another 4 h at 37°C. Recombinant polypeptides were purified on a GST-glutathione affinity column, and GST moieties were cleaved by thrombin as described elsewhere (30). Purified proteins were stored at −80°C.
In vitro phosphorylation.
Recombinant wild-type and mutant SOX9 proteins (100 ng) were added to a PKA reaction buffer containing 20 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM dithiothreitol, 1 mM ATP, 100 mM NaCl, and 1 mM [γ-32P]ATP (0.5 Ci/mmol) and incubated with 50 U of PKA-Cα (Sigma) at 30°C for 30 min in a total volume of 50 μl (6). To test for the specificity of the reaction for PKA, 1 μg of PKA inhibitor (PKI) (Sigma) was added to the reaction buffer to specifically inhibit the activity of PKA.
Cell culture and transfection experiments.
All cell types were cultured as described previously (22). Cells were then transfected with luciferase reporter plasmids containing an 89-bp Col2a1 promoter without (p89Luc) or with (4x48-p89Luc) four copies of a 48-bp chondrocyte-specific Col2a1 enhancer element and the pSV2-β-gal plasmid (an internal control for transfection efficiency) in a ratio of 3:1 as described previously (20). All transfections were done with FuGene6 (Roche Molecular Biochemicals, Indianapolis, Ind.) according to the manufacturer's instructions. Expression plasmids for wild-type and mutant SOX9 proteins (100 ng) and PKA-Cα (400 ng) were transfected as indicated in Fig. 7 and 8. Luciferase and β-galactosidase activities were assayed in cell lysates prepared as described previously (20). 8-Bromo-cAMP (1 mM) and 10 or 20 μM H8 (PKA inhibitor) were added 4 h after transfection, and the cells were incubated for 8 h before being lysed. Reporter activities are reported as the average of triplicate cultures in one of several representative experiments as previously described (20).
FIG. 7.
Mutations in the PKA phosphorylation sites of SOX9 decrease the ability of PKA to enhance SOX9 activation of the Col2a1 enhancer. COS-7 cells were transfected with the 4x48-p89Luc reporter construct and plasmids encoding wild-type SOX9 (lanes 3 and 7) or one of the SOX9 mutants (m1, lanes 4 and 8; m2, lanes 5 and 9; m1+2, lanes 6 and 10), and PKA-Cα (lanes 2 and 7 to 10). Lane 1 is a transfection with the 4x48-p89Luc reporter plasmid alone. Promoter activity was determined 12 h later and is shown as the average plus standard deviation of triplicate transfections. A Western blot of cell lysates was probed with SOX9 antibody to show the relative amounts of each of the SOX9 proteins. In cells cotransfected with PKA-Cα, the levels of SOX9 were 1.5 to 1.8 times higher than in cells not cotransfected with PKA-Cα. Promoter activities were normalized relative to the levels of SOX9 protein.
FIG. 8.
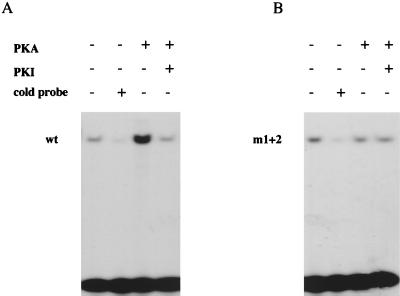
Phosphorylation of SOX9 by PKA increases its efficiency of binding to the 18-bp Col2a1 enhancer element. Bacterially expressed and purified wild-type (wt) (A) or m1+2 mutant (B) SOX9 protein was incubated in vitro with PKA-Cα. Nonphosphorylated and phosphorylated proteins were then tested in an EMSA with the 32P-labeled 18-bp Col2a1 enhancer element as a probe. A 100-fold excess of unlabeled 18-bp oligonucleotide competitor and PKI were added to the phosphorylation reactions, as indicated. The positions of wild-type and m1+2 mutant SOX9 complexes with the 18-bp probe are marked.
In vivo phosphorylation and immunoprecipitations.
RCS cells grown in monolayer culture for 2 days were labeled with [32P]orthophosphate (0.5 mCi/ml) for 4 h and then collected in phosphate-buffered saline (PBS) containing 0.5% NP-40 and 1% sodium dodecyl sulfate (SDS). COS-7 cells were cotransfected with expression plasmids for wild-type and m1+2 mutant SOX9 and PKA-Cα for 8 h as indicated in Fig. 5. Cells were then labeled with [32P]orthophosphate for 4 h and collected. Next, 25 μl of the cell lysates was incubated with 15 μl of SOX9 antibody (22) or 15 μl of SOX9 preimmune antiserum under gentle agitation at 4°C for 2 h. A 2.5-μl volume of protein A-Sepharose beads (Sigma) was added and incubated for another 2 h at 4°C. The beads were washed three times with 1 ml of buffer A (0.5% NP-40 in PBS), resuspended in 10 μl of SDS-polyacrylamide gel electrophoresis (PAGE) sample loading buffer, and boiled for 2 min. Half the volume of each sample was resolved by electrophoresis on an SDS–10% polyacrylamide gel, and the gel was dried and autoradiographed. The other halves of the samples were used for Western blotting to visualize the amount of SOX9 that was immunoprecipitated.
FIG. 5.
PKA phosphorylation of SOX9 does not affect its nuclear localization. COS-7 cells were transfected with expression plasmids for wild-type SOX9, m1+2 mutant SOX9, and PKA-Cα, as indicated. The top panels (A to D) show the nuclear localization of the wild-type and m1+2 mutant SOX9 proteins (yellow signal) visualized by immunofluorescence with SOX9 antibodies. The lower panels represent the same fields in which cell nuclei were stained with DAPI.
Immunofluorescence.
COS-7 cells were cotransfected with plasmids expressing wild-type or mutant SOX9 and PKA-Cα or empty vector. Twenty-four hours after transfection, the cell monolayers were fixed with methanol for 10 min and incubated with blocking buffer (PBS with 5% goat serum and 3% bovine serum albumin) for 30 min. SOX9 rabbit antibody (diluted 1:500 with blocking buffer) was added, and the mixture was incubated for 1 h at room temperature. A secondary antibody (Jackson Immunoresearch, West Grove, Pa.) consisting of goat fluorescein-conjugated anti-rabbit immunoglobulin G (diluted 1:200 in blocking buffer) was added, and the mixture was incubated for another 1 h. Cells were then washed three times with cold PBS, the last time supplemented with 5 μg of the DNA dye 4′,6-diamidino-2 phenylindole (DAPI; Sigma) per ml. Slides were mounted with Aqua-Poly Mount (PolyScience, Warrington, Pa.).
Western blotting.
Cell lysates were prepared and separated by electrophoresis on SDS–10% polyacrylamide gels as described previously (20). Western blotting was done with the enhanced chemiluminescence ECL kit from Amersham (Piscataway, N.J.). SOX9 antibodies were used at a 1:1,000 dilution. To reprobe the membrane with another antibody, the previous antibodies were stripped off and the blots were reprobed with a new antibody.
EMSAs.
For electrophoretic mobility shift assays (EMSAs), the preparation of the 18-bp Col2a1 enhancer probe and the composition of the EMSA incubation buffer were described previously (20). Full-length SOX9 or m1+2 mutant SOX9 made in E. coli (100 ng) was phosphorylated with 50 U of PKA-Cα in a volume of 50 μl. After phosphorylation, the proteins were incubated with the 18-bp probe in the presence of 1 μg of poly(dG-dC) · poly(dG-dC) and 25 μg of bovine serum albumin at room temperature for 30 min. Unlabeled 18-bp probe was added in 100-fold excess compared with the labeled probe. The samples were then fractionated by electrophoresis through a nondenaturing 5% polyacrylamide gel in 0.5× Tris-borate-EDTA at 150 V for 3 h, and the gel was used for autoradiography.
Generation of SOX9.P antibody.
A phosphopeptide derived from the SOX9 sequence in which serine 211 was phosphorylated (CYQPRRRKS211VKNGQA) was used to immunize rabbits (Quality Controlled Biochemical, Hopkinton, Mass.). Serum from the rabbits was preadsorbed on an affinity column containing the nonphosphorylated peptide to remove antibodies that were not phosphospecific and then affinity purified through a phosphopeptide column. The final yield of phosphospecific SOX9 antibody (SOX9.P) was about 0.5 mg/ml.
Immunohistochemical analysis.
Mouse embryo hind legs (16.5 days postcoitum) were fixed in 4% paraformaldehyde in PBS for 16 h. After dehydration, tissues were embedded in paraffin and sectioned. Immunohistochemical staining was done with the DAKO EnVision+ system (Dako Corp, Carpinteria, Calif.). After rehydration, the slides were incubated in 3% hydrogen peroxide in methanol for 15 min at room temperature. Sections were then digested with 1% hyaluronidase for 45 min at 37°C and subsequently heat treated at 95 to 99°C in citrate buffer (10 mM, pH 6). Sections were incubated for 45 min at room temperature with either SOX9 or SOX9.P antibodies at a dilution of 1:30, then with the peroxidase-labeled polymer for 30 min, and subsequently with the substrate chromogen for 30 min at room temperature. After being washed with PBS, slides were mounted with the Crystal Mount aqueous mounting medium (Biomeda Corp., Foster City, Calif.).
RESULTS
PKA-Cα interacts with SOX9 in a yeast two-hybrid assay.
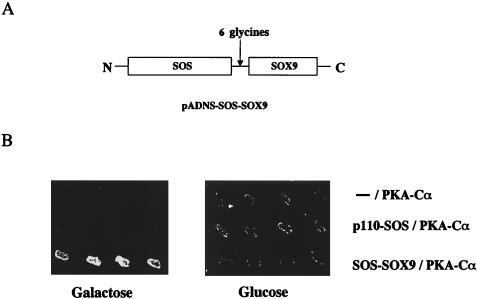
To identify proteins that interact with Sox9 in chondrocytes, we used a yeast two-hybrid method based on the Son of Sevenless recruitment system, called SRS (1), and screened a primary chondrocyte cDNA library using full-length SOX9 as the bait. In this system, interactions between a bait polypeptide fused to SOS and a cDNA-derived prey polypeptide anchored to the membrane suppress the temperature-sensitive phenotype of an S. cerevisiae cdc-25 mutant grown on galactose. For this screen, we generated a recombinant SOS-SOX9 fusion polypeptide consisting of SOS at the amino terminus followed by a linker of six glycine residues and the SOX9 sequence at the carboxyl terminus (Fig. 1A). The linker was used to avoid possible conformational interference between the two protein moieties. Among 20 positive clones, 2 independent cDNAs were identified as coding for PKA-Cα; one of them encoded the full-length sequence of this polypeptide. To assess specificity, yeast cells containing either an empty bait vector, a nonrelevant bait (p110-SOS) (1), or the original bait (SOS-SOX9) were transformed together with the cDNA for full-length PKA-Cα, grown at 25°C, replicated on galactose and glucose plates, and incubated at 37°C (Fig. 1B). Suppression of the cdc25-2 temperature-sensitive phenotype occurred only in cells that expressed the SOS-SOX9 fusion polypeptide and PKA-Cα on galactose plates. These results indicated that PKA-Cα specifically interacts with SOX9 in the yeast SRS system.
FIG. 1.
PKA-Cα interacts with SOX9 in yeast cells. (A) Bait plasmid used in the SRS screening. The full-length coding sequence of SOX9 was cloned into pADNS in frame with and carboxy-terminal to the SOS coding sequence. Six glycine residues were inserted between the SOX9 and SOS sequences. (B) Complementarity of the cdc25-2 temperature-sensitive mutation through specific interactions of SOX9 with PKA-Cα. The plasmid isolated from a yeast colony, which was positive upon primary library screening and encoded PKA-Cα, was retransformed into the cdc25-2 mutant strain together with either empty pADNS, pADNS-p110-SOS, or pADNS-SOS-SOX9. Four independent colonies were isolated for each plasmid combination, grown at 25°C, replica plated onto galactose and glucose (control) plates, and grown at 37°C. Only the colonies that expressed SOS-SOX9 and PKA-Cα grew on the galactose plates.
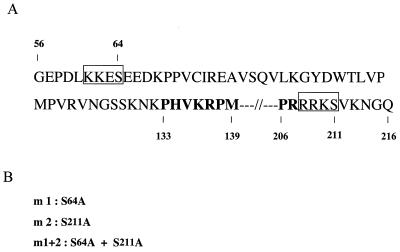
Two PKA phosphorylation consensus sites are present in the SOX9 protein sequence.
The amino acid sequence R/K R/K N S/T is the consensus PKA recognition site, and either S or T is the phosphorylation site (4). Two putative PKA phosphorylation sites containing serine 64 (S64) and serine 211 (S211) are present in the human SOX9 sequence, one on each side of the HMG DNA-binding domain (Fig. 2A). These two PKA recognition sites are conserved in mouse and chicken Sox9. To test the role of PKA phosphorylation in SOX9 function, we introduced serine-to-alanine substitution mutations in these two sites of the SOX9 sequence to generate the SOX9 mutants m1 (S64A), m2 (S211A), and m1+2 (S64A and S211A) (Fig. 2B).
FIG. 2.
Mutations of the two PKA phosphorylation sites of SOX9. (A) The amino acid sequence of human SOX9 is shown from amino acids 56 to 216, with the HMG domain highlighted in bold and the two PKA phosphorylation consensus sites shown in boxes. (B) Serine 64 and serine 211 in the PKA phosphorylation consensus sites were replaced with alanine in SOX9 mutant m1 and SOX9 mutant m2, respectively. SOX9 mutant m1+2 contained alanine substitutions at both sites.
SOX9 is phosphorylated in vitro by PKA.
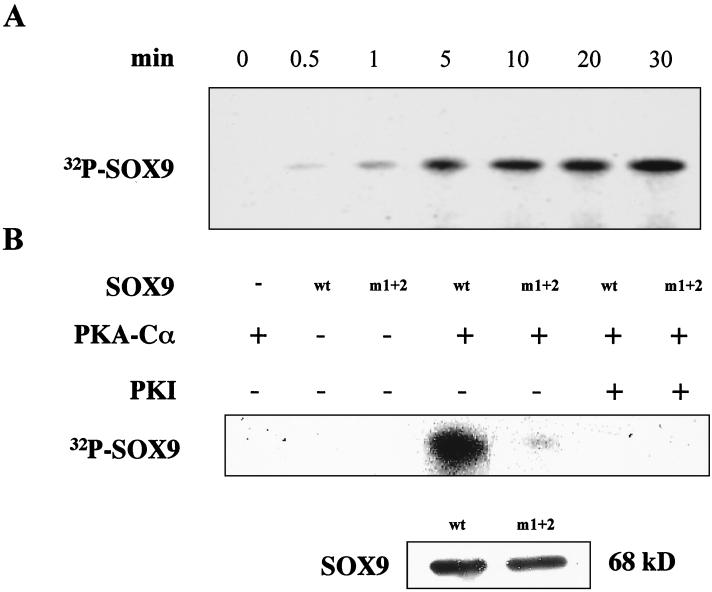
GST fusion polypeptides, with either the wild-type SOX9 or the m1+2 SOX9 mutant, were generated in E. coli and purified by glutathione affinity chromatography. The purified proteins were digested with thrombin to remove the GST moiety and incubated with PKA-Cα and [γ-32P]ATP. Incubation of wild-type SOX9 resulted in the appearance of a 32P-labeled species of 68 kDa (the expected apparent molecular mass of SOX9), the amount of which increased over time (Fig. 3A). This phosphorylation was completely inhibited by PKI, a specific inhibitor of PKA (Fig. 3B). With a mutant SOX9 in which the two consensus PKA phosphorylation sites were mutated (m1+2), phosphorylation by PKA was virtually abolished (Fig. 3B), although a Western blot showed that the same amounts of SOX9 protein were present in the reactions containing wild-type and mutant SOX9. Incubation of either mutant m1 or mutant m2 with PKA and [γ-32P]ATP resulted in phosphorylation of SOX9 (data not shown), indicating that each site can be phosphorylated by PKA. These results also indicated that the two consensus phosphorylation sites of SOX9 are the only sites to be phosphorylated by PKA in vitro.
FIG. 3.
Phosphorylation of SOX9 by PKA in vitro. (A) Time course. Bacterially expressed SOX9 was incubated with PKA-Cα in the presence of [γ-32P]ATP for 0 to 30 min, as indicated, before PKI was added to terminate the reactions. Reaction mixtures were separated by SDS-PAGE, and the gels were autoradiographed. A time-dependent increase in phosphorylated protein was obtained at the characteristic migration level of SOX9. Two faster-migrating products of PKA-Cα phosphorylation were detected that presumably correspond to incomplete or degraded recombinant SOX9 polypeptides (not shown). (B) Specific phosphorylation of sites 1 and 2. Bacterially expressed wild-type (wt) or double-mutant (m1+2) SOX9 proteins were incubated with (+) or without (−) PKA-Cα and PKI for 30 min, as indicated above the lanes. Phosphorylation of SOX9 was determined as described for panel A. A Western blot of wild-type and m1+2 mutant SOX9 proteins shows that the same amount of each SOX9 protein species was present in all reactions.
SOX9 is phosphorylated by PKA-Cα in intact cells.
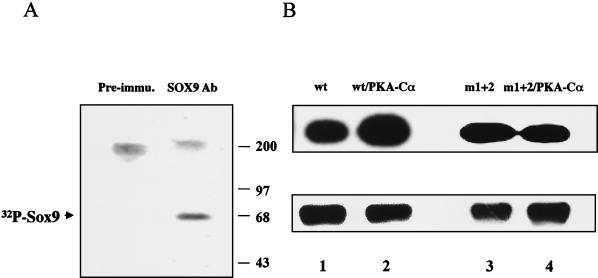
To determine whether SOX9 is phosphorylated in intact cells, we labeled with [32P]orthophosphate a well-differentiated rat chondrosarcoma cell line (RCS) that contains high levels of Sox9 protein and immunoprecipitated Sox9 with SOX9-specific antibodies from cell lysates. A single 68-kDa, 32P-labeled protein with a gel electrophoretic mobility corresponding to that of Sox9 was immunoprecipitated from RCS cell lysates (Fig. 4A), whereas no corresponding signal was observed when preimmune antiserum was used. Thus, phosphorylated Sox9 can be detected in intact cells.
FIG. 4.
Phosphorylation of Sox9 in intact cells. (A) Endogenous Sox9 is phosphorylated in RCS cells. RCS cells in culture were incubated with 32P-labeled inorganic phosphate for 4 h. Sox9 was immunoprecipitated from cell lysates with SOX9 antibody (Ab) and visualized by autoradiography after SDS-PAGE (lane SOX9 Ab). A unique 32P-labeled band migrated with the characteristic 68-kDa molecular mass of SOX9. The specificity of the immunoprecipitation was assessed with SOX9 preimmune (Pre-immu.) serum. (B) PKA-Cα phosphorylates SOX9 at sites 1 and 2 in COS-7 cells. COS-7 cells were transfected with plasmids expressing wild-type SOX9 (wt), double-mutant SOX9 (m1+2), and PKA-Cα, as indicated. Cells were incubated with 32P, and lysates were immunoprecipitated with SOX9 antibody. Autoradiographs of phosphorylated SOX9 proteins recovered after immunoprecipitation and SDS-PAGE are shown in the top panel. PKA increased the level of phosphorylation of wild-type SOX9 but not that of m1+2 mutant SOX9. A Western blot showing the amount of SOX9 protein in each sample after immunoprecipitation is presented in the bottom panel.
To determine whether PKA could phosphorylate SOX9 in intact cells, we cotransfected plasmids expressing either wild-type SOX9 or the m1+2 SOX9 mutant together with a PKA-Cα expression plasmid into COS-7 cells, which do not contain endogenous Sox9, labeled the cells with [32P]orthophosphate, and immunoprecipitated SOX9 from cell lysates with a SOX9-specific antibody. In intact COS-7 cells, phosphorylation of wild-type SOX9 was increased by cotransfection with PKA-Cα (Fig. 4B). The m1+2 mutant SOX9 was phosphorylated in COS-7 cells, although to a lesser degree than wild-type SOX9, but cotransfection with PKA-Cα failed to increase phosphorylation of the mutant SOX9. This finding suggests that SOX9 contains other sites that can be phosphorylated by kinases other than PKA. In summary, these results indicated that SOX9 is a phosphoprotein and a target for PKA phosphorylation in intact cells.
Phosphorylation does not affect the subcellular localization of SOX9.
The PKA phosphorylation site containing serine 211 is included in a nuclear localization signal in the sequence PRRRKS211 (32). To determine whether PKA phosphorylation might change the subcellular localization of SOX9 and whether the S211A substitution might affect the subcellular localization of SOX9, we transfected COS-7 cells with a plasmid expressing either wild-type SOX9 or the m1+2 mutant SOX9 with or without a PKA-Cα-expressing plasmid and examined the subcellular localization of SOX9 by immunofluorescence. Both wild-type SOX9 and m1+2 mutant SOX9 were nuclear (Fig. 5A and B), findings that agree with those of an earlier study (20). Hence, the PKA phosphorylation site mutations did not affect the nuclear localization of SOX9. In addition, no change in the nuclear localization of the wild-type and m1+2 mutant SOX9s was seen in cells cotransfected with PKA-Cα (Fig. 5C and D).
cAMP increases the activity of a Sox9-dependent chondrocyte-specific enhancer.
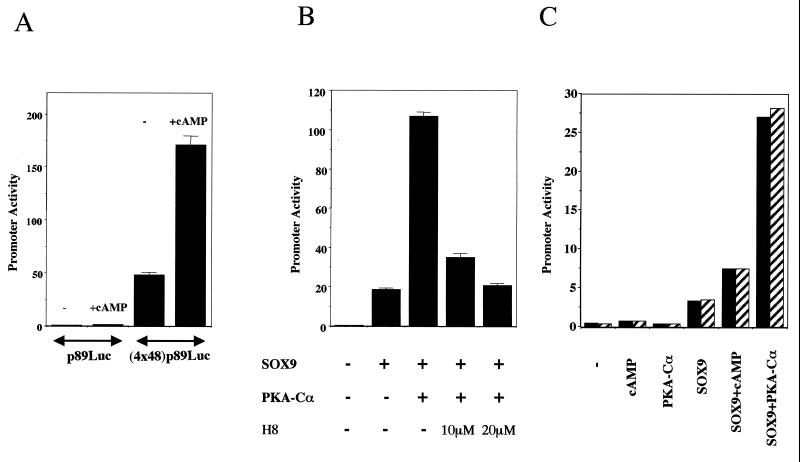
To determine if PKA phosphorylation affects the activity of Sox9, we first tested whether cAMP signaling in RCS cells could increase the activity of a 48-bp Col2a1 enhancer that was previously shown to be dependent on Sox9 (20). Indeed, this 48-bp enhancer, which is chondrocyte specific in both DNA transfections and transgenic mice, binds Sox9 and becomes strongly active upon cotransfection of SOX9 in nonchondrocytic cells; in addition, a mutant 48-bp enhancer, which has lost the ability to bind SOX9, is inactive in chondrocytes and is not activated by SOX9. In this experiment, we used the activity of a 48-bp Col2a1 enhancer construct to conduct a functional assay of the transcriptional activity of SOX9. Treatment of RCS cells with 1 mM 8-bromo-cAMP increased the activity of this Col2a1 chondrocyte-specific enhancer more than threefold (Fig. 6A). No increase in activity occurred in the presence of 8-bromo-cAMP when only the 89-bp Col2a1 promoter, without the 48-bp enhancer element, was used. Hence, the Sox9-dependent 48-bp Col2a1 enhancer seems to be a target for cAMP signaling in RCS cells.
FIG. 6.
PKA and cAMP stimulate the activity of chondrocyte-specific Col2a1 enhancers. (A) 8-Bromo-cAMP increased the activity of the 48-bp Col2a1 enhancer in RCS cells. A luciferase reporter plasmid containing no (p89Luc) or four copies of a 48-bp chondrocyte-specific enhancer element (4x48-p89Luc) was transfected into RCS cells, and 4 h later, 1 mM 8-bromo-cAMP was added (+cAMP) or not (−) to the culture medium for 8 h. Promoter activities were shown as the average plus standard deviation of triplicate transfections for A and B. (B) PKA-Cα increases the SOX9-dependent activation of the 48-bp Col2a1 enhancer in 10T1/2 fibroblasts. Expression plasmids for SOX9 and PKA-Cα were either transfected individually or cotransfected in a 1:4 ratio for a total of 16 h. Where indicated, the PKA inhibitor H8 was added to the medium for 12 h beginning 4 h after the start of transfection. (C) Cotransfected PKA-Cα increases the SOX9-dependent activation of the 100-bp Col2a1 enhancer in 10T1/2 fibroblasts. A luciferase reporter plasmid containing two copies of the 100-bp enhancer was cotransfected without (−) or with the expression plasmids for SOX9 and PKA-Cα into 10T1/2 fibroblasts for a total of 16 h. Eight hours after the start of transfection, 1 mM 8-bromo-cAMP was added as indicated, and the mixture was incubated for an additional 8 h. Solid and hatched columns show the results of separate duplicate transfections.
PKA-Cα increases the activity of Sox9-dependent Col2a1 chondrocyte-specific enhancers.
To further examine the molecular basis for the increase in activity of the 48-bp Col2a1 enhancer that is produced by cAMP in chondrocytes, we cotransfected the expression plasmids for SOX9 and PKA-Cα into 10T1/2 cells along with the 4x48-p89Luc Col2a1 construct (Fig. 6B). In agreement with previous results (20), SOX9 stimulated the activity of the Col2a1 48-bp enhancer in these cells, and cotransfection of PKA-Cα and SOX9 further increased this activity five- to sixfold. This increase was inhibited by H8, a PKA-specific inhibitor (18), in a dose-dependent manner (Fig. 6B). Moreover, cotransfection with PKA-Cα also increased the Sox9-dependent activities of two larger chondrocyte-specific Col2a1 enhancer constructs containing either two copies of a 100-bp sequence (Fig. 7C) or two copies of a 231-bp sequence (data not shown), each including the 48-bp element. Hence, the activities of the 48-bp and the larger Col2a1 Sox9-dependent enhancers were stimulated by PKA.
SOX9 mutations in each of the two PKA phosphorylation consensus sites inhibit the activation of SOX9 by PKA.
To examine whether the increase in Sox9-dependent Col2a1 enhancer activity caused by PKA was directly due to phosphorylation of SOX9 by PKA, we tested the m1, m2, and m1+2 SOX9 mutants in cotransfection experiments of COS-7 cells with the 4x48-p89Luc enhancer construct and a PKA-Cα expression plasmid. Western blotting indicated that similar levels of SOX9 were present in the lysates of cells transfected with wild-type and mutant forms of SOX9, but in all cells cotransfected with PKA-Cα the levels of SOX9 were approximately 1.5 to 1.8 times higher than in cells not cotransfected with PKA-Cα. It is possible that the vector expressing SOX9 contains a promoter element that is responsive to the forced expression of PKA-Cα. We thus normalized the promoter activities relative to the levels of SOX9 proteins. In cells not transfected with PKA-Cα, the levels of activation of the Col2a1 enhancer by wild-type and mutant SOX9 were similar (Fig. 7). In contrast, mutations in each PKA phosphorylation site of SOX9 inhibited the increase in Sox9-dependent Col2a1 enhancer activity produced by PKA. The extent of this inhibition was more pronounced with the S211A mutation (m2 mutant), and the m1+2 mutant SOX9 had roughly equivalent activity to that without cotransfected PKA-Cα. In summary, these experiments indicate that direct phosphorylation of SOX9 by PKA enhances SOX9's ability to transactivate Col2a1 chondrocyte-specific enhancers. Although the two PKA phosphorylation consensus sites in SOX9 are important, the S211 site adjacent to the carboxyl-terminal end of the HMG DNA-binding domain seems to be more crucial in mediating the effects of PKA on transactivation of Col2a1 enhancers by SOX9.
Phosphorylation of SOX9 by PKA increases SOX9's DNA-binding activity to 18-bp and 48-bp Col2a1 enhancer elements.
The DNA-binding activity of SRY, which contains an HMG DNA-binding domain with 50% identity to that of SOX9, was previously shown to be increased by PKA phosphorylation (6). We tested whether DNA binding of SOX9 to either the 48-bp Col2a1 enhancer element or an 18-bp subsegment of this enhancer (21), which includes the binding site for SOX9 (20), was increased after PKA phosphorylation. Phosphorylation of SOX9 by PKA-Cα in vitro increased its DNA binding to an 18-bp Col2a1 enhancer element (Fig. 8A). This increased binding was strongly inhibited by including PKI in the phosphorylation reaction. In contrast, DNA binding of the m1+2 mutant SOX9 was unchanged by incubation with PKA-Cα (Fig. 8B). The same result was obtained when a smaller segment of recombinant SOX9 that included the HMG domain and the two PKA phosphorylation sites was used in DNA-binding experiments involving both the 48-bp and the 18-bp Col2a1 enhancer elements (data not shown). Thus, the PKA-dependent increase in DNA binding of SOX9 may account for the observed PKA-dependent increase in transcriptional activation of SOX9-dependent Col2a1 enhancers.
8-Bromo-cAMP mediates phosphorylation of Sox9 at serine 211 in rat chondrosarcoma cells.
The specificity of a phosphospecific antibody for a SOX9 peptide in which S211 was phosphorylated is illustrated in Fig. 9A. In extracts of COS-7 cells transfected with a wild-type SOX9 expression plasmid, the phosphospecific antibody showed a very weak signal. The intensity of this signal increased markedly when extracts of COS-7 cells that had been cotransfected with SOX9 and PKA-Cα expression plasmids were tested. No signal was seen in extracts of cells cotransfected with the m2 SOX9 mutant carrying the S211A mutation and PKA-Cα, demonstrating the complete specificity of the antibody. Extracts of cells transfected with the m1 SOX9 mutant and PKA-Cα showed the same signal intensity as extracts of cells transfected with wild-type SOX9, whereas those of cells transfected with the m1+2 mutant and PKA-Cα showed no signal. In addition, the SOX9 phosphospecific antibody did not recognize SOX9 made in E. coli. Thus, the antibody specifically reacted with SOX9 that was phosphorylated at S211. Our experiments demonstrate that cotransfection with PKA-Cα led to phosphorylation of SOX9 at S211. Moreover, adding 8-bromo-cAMP to RCS cells produced a marked increase in signal intensity (Fig. 9B), directly demonstrating that signaling by cAMP results in phosphorylation at S211 of endogenous Sox9.
FIG. 9.
Phosphorylation of Sox9 at S211 in intact cells. (A) Recombinant SOX9 protein made in E. coli and extracts from COS-7 cells that were transfected with plasmids expressing wild-type (wt) SOX9 or mutant SOX9 proteins and a plasmid expressing PKA-Cα, as indicated, were resolved by SDS–10% PAGE followed by Western blotting with SOX9.P antibody (Ab) at a dilution of 1:1,000. The antibodies were stripped off the blots, and the membranes were reprobed with another SOX9 antibody as described previously (20). In A and B, the 68-kDa mobility of Sox9 is indicated by an arrow. (B) Cell extracts from RCS cells, untreated or treated with two different concentrations of 8-bromo-cAMP for 8 h, were used for Western blotting.
Sox9 phosphorylated at S211 is present in the prehypertrophic zone of the growth plate.
In order to determine whether Sox9 was phosphorylated at S211 in the growth plate in vivo during chondrocyte differentiation, we used the SOX9 phosphospecific antibody to perform an immunohistochemical analysis of the growth plate of 16.5 dpc mouse embryo hind legs. Immunohistochemistry with the SOX9 antibody directed against an epitode located at the carboxyl terminus of SOX9 (22) showed that Sox9 was evenly distributed in the cells along the growth plate, including the resting, proliferative, and prehypertrophic zones, but was absent in the hypertrophic zone (Fig. 10A). These results are in perfect agreement with those of previous RNA in situ hybridizations (27, 37). In contrast, Sox9 phosphorylated at S211 localized mainly in the prehypertrophic zone (Fig. 10B).
FIG. 10.
Sox9 is phosphorylated at S211 in the prehypertrophic zone of the growth plate in vivo. (A) Immunohistochemistry of a hind limb section of E 16.5 mouse embryo with SOX9 antibodies. Sox9 is evenly distributed in the cells of the resting, proliferative (P), and prehypertrophic (Pre-hy) zones of the growth plate. No Sox9 protein was observed in the hypertrophic (hy) zone. (B) Immunohistochemistry of a parallel section with SOX9.P antibodies. Sox9 phosphorylated at S211 was present in the prehypertrophic zone of the growth plate. Bars, 50 μm.
DISCUSSION
The results of our yeast two-hybrid screen indicating interactions between SOX9 and PKA-Cα prompted us to hypothesize that SOX9 might be a target for PKA phosphorylation. Several lines of evidence show that cAMP is an important signaling molecule in chondrocyte differentiation. For example, PTHrP, which inhibits the transition from prehypertrophic chondrocytes to hypertrophic chondrocytes (12, 16), signals by increasing the intracellular concentration of cAMP, since the PTH/PTHrP receptor is a G protein-coupled receptor that activates the adenylate cyclase enzyme (11). In addition, in cell culture cAMP increases the expression of several chondrocyte marker genes, including Col2a1, that are targets of Sox9 in chondrocytes (3, 14). Since PKA has also been shown to modulate signaling by sonic hedgehog (7, 9), one can postulate that, by analogy, cAMP might also be important in signaling by indian hedgehog (Ihh), which has a key role in controlling the expression of PTHrP (16). Moreover, signaling by bone morphogenetic proteins, which have the ability to induce the whole cascade of chondrocyte differentiation in vivo and in vitro, activates PKA in cultured chondrocytes (18). Because Sox9 has an essential role in chondrocyte differentiation, its phosphorylation by PKA might provide a mechanism to control its activity. We therefore investigated the potential role of PKA phosphorylation in modulating Sox9 activity.
SOX9 contains two consensus PKA phosphorylation sites that are conserved in humans, mice, and chickens. We showed that each site can be phosphorylated by PKA-Cα in vitro, because each of the single SOX9 mutants m1 and m2 could be phosphorylated by PKA-Cα, whereas the double mutant m1+2 could not. We next determined that SOX9 is also phosphorylated by PKA in intact cells. Indeed, cotransfection of wild-type SOX9 with PKA-Cα produced an increase in SOX9 phosphorylation that was not observed with the m1+2 mutant SOX9. Our finding that the m1+2 mutant SOX9 was phosphorylated suggests that SOX9 can be phosphorylated at other sites in intact cells, presumably by kinases other than PKA. Consensus phosphorylation sites for PKC, PKG, and casein kinase II are present in the SOX9 sequence, as determined by computer analysis (data not shown). Evidence that SOX9 is phosphorylated at S211 in intact cells was obtained by using a phosphospecific antibody. This site became highly phosphorylated in cells cotransfected with SOX9 and PKA-Cα but not in cells transfected with SOX9 only. Moreover, the addition of 8-bromo-cAMP to RCS cells induced a marked increase in the phosphorylation of endogenous Sox9 at S211.
Since S211 is part of a Sox9 nuclear localization signal (32), the phosphorylation status of Sox9 may be important for nuclear translocation. Our immunofluorescence experiments demonstrated that the nuclear localization of the m1+2 SOX9 mutant was identical to that of wild-type SOX9 in transfected COS-7 cells. Moreover, the nuclear localization of SOX9 was not different in cells that were cotransfected with PKA-Cα. We therefore concluded that PKA phosphorylation has no effect on the nuclear localization of SOX9.
We used the activity of Sox9-dependent Col2a1 enhancer elements in DNA transfection experiments as a functional assay to test whether cAMP signaling increased the transcriptional activity of Sox9. We found that treating RCS cells with 8-bromo-cAMP increased the activity of a construct carrying four copies of the 48-bp Col2a1 enhancer; similarly, cotransfection of SOX9 with PKA-Cα in COS-7 cells and other fibroblasts also stimulated severalfold the Sox9-dependent activity of this and two larger Col2a1 enhancer elements. Moreover, adding a PKA inhibitor to these cotransfection experiments strongly inhibited this stimulation. Cotransfection of mutant forms of SOX9 together with PKA-Cα resulted in a lesser degree of stimulation than that produced by PKA and wild-type SOX9. Although both mutation 1 (S64) and mutation 2 (S211) decreased the PKA-enhanced activity, mutation 2 did so to a greater extent, suggesting that S211 has a greater role than S64 in mediating the effect of PKA phosphorylation. With SOX9 containing both mutations, there was practically no stimulation by PKA. The presence of these mutations did not affect SOX9 activity in the absence of PKA, since wild-type SOX9 and the three different SOX9 mutants activated the Col2a1 48-bp enhancer to similar levels.
Together, these experiments strongly suggest that phosphorylation of SOX9 by PKA increases the transcriptional activity of SOX9. This increase in SOX9 transcriptional activity is unlikely to be due to stabilization of SOX9, because similar levels of SOX9 were found in cells expressing wild-type and mutant SOX9 proteins. The increased transcriptional activity of SOX9 could be accounted for by the increased efficiency of DNA binding of SOX9 that was observed upon PKA-Cα phosphorylation. It is also possible that phosphorylated SOX9 interacts more efficiently with components of the transcriptional machinery. In addition, active PKA also results in phosphorylation of several other transcription factors (4), some of which may interact more efficiently with phosphorylated SOX9 and increase the observed activity of Sox9-dependent Col2a1 enhancers.
The PTH/PTHrP receptor is expressed in a relatively narrow band in the prehypertrophic zone of growth plate cartilages. In situ hybridization experiments have shown that the levels of Col2a1 mRNA are higher in this zone than in other areas of the growth plate (17). Since cAMP signaling is one the major signaling pathways of the PTH/PTHrP receptor, we speculate that PKA phosphorylation of Sox9 may increase the transcriptional activity of Sox9 in this zone, which would result in higher levels of Col2a1 mRNA. Our immunohistochemical analysis using the SOX9 phosphospecific antibody showed a strong signal for Sox9 phosphorylated at S211 in the prehypertrophic zone of the growth plate where the PTH/PTHrP receptor is expressed (17). In a control experiment, an antibody against the carboxyl terminus of SOX9 showed that Sox9 was evenly distributed throughout the resting, proliferative, and prehypertrophic zones. Our results therefore strongly suggest that PTHrP might be a physiological signal for the PKA-mediated phosphorylation of Sox9 in the growth plate. Since the major function of PTHrP is to inhibit further differentiation of chondrocytes into hypertrophic chondrocytes, we speculate that the increase in activity of the master chondrogenic factor Sox9 by PKA phosphorylation could mediate at least some of the effects of PTHrP in the growth plate by maintaining the cells as nonhypertrophic chondrocytes.
ACKNOWLEDGMENTS
This work was funded by NIH grants R01 AR42909 and P01 AR 42919-02 to Benoit de Crombrugghe. Véronique Lefebvre was supported by the Arthritis Foundation. DNA sequencing was performed by the University of Texas M. D. Anderson Cancer Center core sequencing facility, which is supported by NCI grant CA 16672.
We thank James H. Kimura for the RCS cells, Ami Aronheim for the SRS plasmids and the cdc25-2 yeast strain, and Michael Uhler for the PKA-Cα cDNA plasmid. We are grateful to Sankar N. Maity and Kazuhisa Nakashima for valuable advice throughout the work and to Shane Zhao for help in computer analysis of SOX9 protein sequences. We also thank Heidi Eberspaecher and Gerald Pinero for their help in the immunohistochemistry and Patricia Arubaleze for help in typing the manuscript.
REFERENCES
- 1.Aronheim A, Zandi E, Hennemann H, Elledge S J, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interaction. Mol Cell Biol. 1997;17:3094–3102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D M, Leung K K H, Whearley S C, Ng L J, Zhou S, Ling K W, Sham M H, Koopman P, Tam P P L, Cheah K S E. Sox9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 3.Bi W, Deng J, Zhang Z, Behringer R R, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 4.Boulikas T. Phosphorylation of transcription factors and control of the cell cycle. Crit Rev Eukaryot Gene Expression. 1995;5:1–77. [PubMed] [Google Scholar]
- 5.Bridgewater L C, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem. 1998;273:14998–15006. doi: 10.1074/jbc.273.24.14998. [DOI] [PubMed] [Google Scholar]
- 6.Desclozeaux M, Poulat F, de Santa Barbara P, Capony J P, Turowski P, Jay P, Mejean C, Moniot B, Boizet B, Berta P. Phosphorylation of an N-terminal motif enhances DNA-binding activity of the human SRY protein. J Biol Chem. 1998;273:7988–7995. doi: 10.1074/jbc.273.14.7988. [DOI] [PubMed] [Google Scholar]
- 7.Fan C, Porter J A, Chiang C, Chang D T, Beachy P A, Tessier-Lavigne M. Long-range sclerotome induction by Sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 8.Foster J W, Dominguez-Steglich M A, Guioli S, Kwok C, Weller P A, Stevanovic M, Weissenbach J, Mansour S, Young I D, Goodfellow P N, Brook J D, Schafer A J. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 9.Hammerschmidt M, Bitgood M J, McMahon A P. Protein kinase A is a common negative regulator of hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;15:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 10.Houston C S, Opitz J M, Spranger J W, Macpherson R I, Reed M H, Gilbert E F, Herrman J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al. in 1971. Am J Med Genet. 1993;15:2–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- 11.Jüppner H, Abou-Samra A, Freeman M, Kong X, Schipani E, Richards J, Kolakowski L F, Hock J, Potts J T, Kronenberg H M, Segre G V. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 12.Kapaplis A C, Luz A, Glowacki J, Bronson R T, Tybulewicz V L J, Kronenberg H M, Mulligan R C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 13.Kent J, Wheatley S C, Andrews J E, Sinclair A H, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 14.Kosher R A, Gay S W, Kamanitz J R, Kulyk W M, Rodger B J, Sai S, Tanaka T, Tanzer M L. Cartilage proteoglycan core protein gene expression during limb cartilage differentiation. Dev Biol. 1986;118:112–117. doi: 10.1016/0012-1606(86)90078-3. [DOI] [PubMed] [Google Scholar]
- 15.Kwok C, Weller P A, Guioli S, Foster J W, Mansour S, Zuffardi O, Punett H H, Dominguez-Steglich M A, Brook J D, Young I D, Goodfellow P N, Schafer A J. Mutations in SOX9, the gene responsible for campomelic dysplasia and autosomal sex reversal. Am J Hum Genet. 1995;57:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 16.Lanske B, Karaplis A C, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defice L H K, Ho C, Mulligan R C, Alou-Samra A, Juppner H, Segre G V, Kronenberg H M. PTH/PTHrP receptor in early development and indian-hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 17.Lee K, Lanske B, Karaplis A C, Deeds J D, Kohno H, Nissenson R A, Kronenberg H M, Segre G V. Parathyroid hormone-related peptide delays terminal differentiation of chondrocytes during endochondral bone development. Endocrinology. 1996;137:5109–5118. doi: 10.1210/endo.137.11.8895385. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y S, Chuong C M. Activation of protein kinase A is involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J Cell Physiol. 1997;170:153–165. doi: 10.1002/(SICI)1097-4652(199702)170:2<153::AID-JCP7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre V, Huang W, Harley V R, Goodfellow P N, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the proα1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefebvre V, Zhou G, Mukhopadhyay K, Smith C N, Zhang Z, Eberspaecher H, Zhou X, Sinha S, Maity S N, de Crombrugghe B. An 18-base-pair sequence in the mouse proα1(II) collagen gene is sufficient for cartilage expression and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996;16:4512–4523. doi: 10.1128/mcb.16.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malemud C J, Mills T M, Shuckett R, Papay R S. Stimulation of sulfated-proteoglycan synthesis by forskolin in monolayer cultures of rabbit articular chondrocytes. J Cell Physiol. 1986;129:51–59. doi: 10.1002/jcp.1041290108. [DOI] [PubMed] [Google Scholar]
- 24.Mansour S, Hall C M, Pembrey M E, Young I D. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995;32:415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer J, Südbeck P, Held M, Wagner T, Schmitz M L, Bricarelli F D, Eggermont E, Friedrich U, Haas O A, Kobelt A, Leroy J G, Van Maldergem L, Michel E, Mitulla B, Pfeiffer R A, Schinzel A, Schmidt H, Scherer G. Mutational analysis of the SOX9 gene in campomelic dysplasia and autosomal sex reversal: lack of genotype/phenotype correlations. Hum Mol Genet. 1997;6:91–98. doi: 10.1093/hmg/6.1.91. [DOI] [PubMed] [Google Scholar]
- 26.Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;13:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 27.Ng L-J, Wheatley S, Muscat G E O, Conway-Campbell J, Bowles J, Wright E, Bell D M, Tam P P L, Cheah K S E, Koopman P. Sox9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers B J, Kulyk W M, Kosher R A. Stimulation of limb cartilage differentiation by cyclic AMP is dependent on cell density. Cell Differ Dev. 1989;28:179–187. doi: 10.1016/0922-3371(89)90003-8. [DOI] [PubMed] [Google Scholar]
- 29.Schipani E, Lanske B, Hunzelman J, Luz A, Koracs C S, Lee K, Pirro A, Kronenberg H M, Jüppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci USA. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Maity S N, Lu J, de Crombrugghe B. Recombinant rat CBF-C, the third subunit of CBF/NFY, allows formation of a protein-DNA complex with CBF-A and CBF-B and with yeast HAP2 and HAP3. Proc Natl Acad Sci USA. 1995;92:1624–1628. doi: 10.1073/pnas.92.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Südbeck P, Schmitz L, Baeuerle P A, Scherer G. Sex reversal by loss of the C-terminal transactivation domain of human SOX9. Nat Genet. 1996;13:230–232. doi: 10.1038/ng0696-230. [DOI] [PubMed] [Google Scholar]
- 32.Südbeck P, Scherer G. Two independent nuclear localization signals are present in the DNA-binding high-mobility-group domains of SRY and SOX9. J Biol Chem. 1997;272:27848–27852. doi: 10.1074/jbc.272.44.27848. [DOI] [PubMed] [Google Scholar]
- 33.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Dagna Bricarelli F, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 34.Wirth J, Wagner T, Meyer J, Pfeiffer R A, Tietze H-U, Schempp W, Scherer G. Translocation breakpoints in three patients with campomelic dysplasia and autosomal sex reversal map more than 130 kb from SOX9. Hum Genet. 1996;97:186–193. doi: 10.1007/BF02265263. [DOI] [PubMed] [Google Scholar]
- 35.Wright E M, Snopek B, Koopman P. Seven new members of the SOX gene family expressed during mouse development. Nucleic Acids Res. 1993;21:744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright E, Hargrave M R, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Q, Eberspaecher H, Lefebvre V, de Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]