Abstract
Studies have demonstrated antinociceptive synergy between morphine and delta-9-tetrahydrocannabinol (THC) in animals, but whether such synergy occurs against all types of pain and in humans is unclear. Because a majority of chronic pain patients are women, and sex differences in morphine and THC potencies have been observed in rodents, the present study examined sex-specific effects of morphine and THC given alone and in combination, in rats with persistent inflammatory pain. On Day 1, baseline mechanical and thermal response thresholds, hindpaw weight-bearing, locomotor activity, and hindpaw thickness were determined. Inflammation was then induced via hindpaw injection of complete Freund’s adjuvant (CFA). Three days later, morphine (s.c.), THC (i.p), or a morphine-THC combination (1:1, 3:1, and 1:3 dose ratios) was administered, and behavioral testing was conducted at 30-240 min post-injection. Morphine alone was anti-allodynic and anti-hyperalgesic, with no sex differences, but at some doses increased weight-bearing on the CFA-treated paw more in males than females. THC alone reduced mechanical allodynia with similar potency in both sexes, but reduced thermal hyperalgesia and locomotor activity with greater potency in females than males. All morphine-THC combinations reduced allodynia and hyperalgesia, but isobolographic analysis of mechanical allodynia data showed no significant morphine-THC synergy in either sex. Additionally, whereas morphine alone was antinociceptive at doses that did not suppress locomotion, morphine-THC combinations suppressed locomotion and did not increase weight-bearing on the inflamed paw. These results suggest that THC is unlikely to be a beneficial adjuvant when given in combination with morphine for reducing established inflammatory pain.
Keywords: Cannabis, Opioids, Morphine, THC, Drug Interactions, Inflammatory Pain, Sex Differences
Introduction
Opioids are widely used for the treatment of acute and chronic pain (Kuo et al., 2015; Sverrisdottir et al., 2015). Approximately 1.7 million Americans had an opioid use disorder in 2017 (Center for Behavioral Health Statistics and Quality, 2018), leading the U.S. Department of Health and Human Services to declare the opioid epidemic a “public health emergency” (Hargan, 2017), and creating an urgent need for alternative analgesics. Because it has been shown that cannabinoids may enhance opioid-induced antinociception without enhancing side-effects of opioids in animal studies, it has been proposed that adjunctive treatment with cannabinoids may allow for use of lower doses of opioids in pain patients, thus decreasing the risk of opioid overdose and addiction (Maguire et al., 2013; Altun et al., 2015; Kazantzis et al., 2016; Grenald et al., 2017; Alsalem et al., 2020). Current evidence in humans does not show a consistent benefit of opioid-cannabinoid combinations, but few well-controlled studies have tested the analgesic efficacy of this drug combination (for reviews, see Babalonis and Walsh, 2020; Le Foll, 2020). Two potentially important gaps between animal and human studies are related to pain chronicity and sex differences. The primary clinical interest in opioid-cannabinoid analgesic interactions is for chronic pain patients, who are predominately women, whereas animal studies of opioid-cannabinoid interactions have primarily examined acute antinociception in healthy males (Unruh, 1996; Keefea et al., 2000; Blyth et al., 2001).
Opioids and cannabinoids each produce antinociception in both male and female rodents, although sex differences in antinociceptive potency have been reported. When sex differences are observed, opioids are typically more potent in male compared to female rats (Romero et al., 1988; Baamonde et al., 1989; Kepler et al., 1989; Candido et al., 1992; Bartok and Craft, 1997; Cook et al., 2000; Baker and Ratka, 2002; Cook and Nickerson, 2005). For example, morphine was a more potent anti-hyperalgesic agent in males compared to females with complete Freund’s adjuvant (CFA)-induced hindpaw inflammation (Wang et al., 2006). Two studies in humans suggest greater morphine efficacy in women (Niesters et al., 2010; Averitt et al., 2019), yet other studies suggest no sex differences (Fillingim et al., 2005; Comer et al., 2010), and in one study more morphine was required in women than in men to produce similar analgesia (Cepeda and Carr, 2003). Thus, sex differences in opioid analgesia in humans remain unclear.
In regard to sex differences in cannabinoid effects, THC was more potent in healthy female than male rats tested on acute pain assays (e.g., Tseng and Craft, 2001; Britch et al., 2017) and in a CFA model of inflammatory pain (Craft et al., 2013). Studies in humans have yielded mixed results; a recent meta-analysis suggested that sex did not affect cannabinoid analgesia using experimental pain procedures in healthy humans (De Vita et al., 2018).
Several studies have reported opioid-cannabinoid synergy on measures of acute pain in healthy male rodents (Smith et al., 1998; Cichewicz et al., 1999; Cichewicz and McCarthy, 2003; Finn et al., 2004; Wilson-Poe et al., 2013). Furthermore, the synthetic cannabinoid JWH-015 acted synergistically with morphine to reduce post-operative pain, acute inflammatory pain, and neuropathic pain in male rodents (Grenald et al., 2017). Morphine and THC were also synergistic against mechanical hyperalgesia in arthritic male rats (Cox et al., 2007). Despite evidence of sex differences in both opioid and cannabinoid-induced antinociception and evidence of synergy between opioids and cannabinoids in rodent models of pain, morphine-THC interactions have never been investigated in female rodents with persistent pain, nor have morphine-THC interactions been compared between the sexes, to our knowledge. We addressed this critical gap by characterizing morphine-THC interactions in male and female rats with CFA-induced hindpaw inflammation. To this end, we employed dose addition analysis, a commonly used approach for statistically testing for drug synergy (Tallarida, 2001), to compare morphine-THC interactions on the von Frey test in males vs. females. The von Frey test of mechanical allodynia is one of the most common tests of pain in rodent chronic pain models, although its predictive validity as a primary measure of pain has been questioned (Cobos and Portillo-Salido, 2013; Abboud et al., 2021). Thus, for a broader perspective, we also examined the effects of the same morphine-THC combinations on response to noxious heat, and on a functional measure of pain, weight-bearing on the inflamed paw. Finally, in the same rats, we assessed the effect of morphine-THC combinations on locomotor activity, since decreases in general activity may contribute to delayed responding on reflexive pain tests (Negus, 2019).
Methods
Animals
All experiments were completed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Male and female Sprague-Dawley rats aged 60-90 days were used (bred in-house from Envigo stock, Livermore, CA). They were housed in same-sex groups of 2-3, under a 12:12 hr light:dark cycle (lights on at 0700). Each home cage contained soft, absorbent bedding made from wood pulp (TEK-Fresh®, Envigo), and a 12- to 15-cm long x 10-cm diameter PVC tube. The room was maintained at 21±2°C with approximately 30-35% humidity. Food (LabDiet 5001; Animal Specialities & Provisions, Quakertown, PA) and water were available ad libitum except during testing. Rats were randomly assigned to treatment groups with the exception that same-sex siblings generally were not assigned to the same treatment group.
Drugs
Morphine was purchased from Millipore Sigma (St. Louis, MO). THC was obtained from the National Institute on Drug Abuse (Bethesda, MD). Drugs were administered by s.c. (morphine) or i.p. (THC) injection in volumes of 1 ml/kg. Morphine was dissolved in saline, which served as the vehicle for morphine. When morphine was given alone, doses were 0.032, 0.1, 0.32, 1.0, or 3.2 mg/kg. THC was dissolved in a 1:1:18 ethanol:cremophor:saline solution, which served as the vehicle for THC. When THC was given alone, doses were 0.5, 1.0, 2.0, or 4.0 mg/kg. Morphine:THC dose equivalency ratios at 1:1, 3:1, and 1:3 were calculated based on von Frey data (see Data Analysis section below for additional details), and several dilutions (5, 10, 20, 40, or 80%) of each equivalency ratio were administered (Table 1). Experimenters were blind to dose. CFA (1 mg/ml heat-killed Mycobacterium tuberculosis) was purchased from Millipore Sigma (St. Louis, MO). Isoflurane was purchased from Patterson Veterinary Supply (Everett, WA).
Table 1.
Doses of morphine and THC administered to male and female rats in the 1:1, 1:3, and 3:1 dose ratios.
| Dose Ratio (Morphine:THC) |
Males | Females | ||
|---|---|---|---|---|
| Morphine (mg/kg) | THC (mg/kg) | Morphine (mg/kg) | THC (mg/kg) | |
| 1:1 | 0.05 | 0.36 | -- | -- |
| 0.1 | 0.73 | 0.1 | 0.26 | |
| 0.2 | 1.46 | 0.2 | 0.51 | |
| 0.4 | 2.91 | 0.4 | 1.02 | |
| 3:1 | 0.05 | 0.12 | -- | -- |
| 0.1 | 0.24 | 0.1 | 0.08 | |
| 0.2 | 0.49 | 0.2 | 0.17 | |
| 0.4 | 0.97 | 0.4 | 0.34 | |
| -- | -- | 0.8 | 0.68 | |
| 1:3 | 0.05 | 1.09 | 0.05 | 0.38 |
| 0.1 | 2.19 | 0.1 | 0.77 | |
| 0.2 | 4.37 | 0.2 | 1.53 | |
Apparatus
Mechanical sensitivity was assessed using an electronic von Frey aesthesiometer (IITC Inc., Woodland Hills, CA). Thermal sensitivity was assessed using a Hargreaves apparatus (Ugo Basile Plantar Test, Model 7371, Collegeville, PA). Hindpaw weight-bearing was measured using an incapacitance meter (Columbus Instruments, Columbus, OH). Horizontal locomotor activity was measured using a photobeam apparatus (Opto-varimex, Columbus Instruments, Columbus, OH): 15 photobeams cross the width of a 20 x 40 x 23-cm clear plastic rodent cage, with photobeams spaced 2.5 cm apart and 6.5 cm high. Paw edema was quantified by measuring maximal dorsal-ventral hindpaw thickness with calipers.
Behavioral procedures
On Day 1 at approximately 0800, rats were weighed and baseline measurements were taken. First, rats were placed in hanging wire cages to habituate for approximately 15 min. The baseline threshold at which the rat responded when the von Frey probe was applied to the plantar surface of the right hindpaw was then recorded in g. Three assessments were made over approximately 2 min with 30 sec between tests. The Hargreaves test was completed next: latency to withdraw the right hindpaw from an infrared beam was recorded to the nearest 0.1 sec with a cutoff of 20 sec. Three assessments were made over approximately 2 min with 30 sec between tests. Next, the maximal dorsal-ventral thickness of each hind paw was measured in mm. Rats were then placed in a standing position into a plexiglass chamber and weight-bearing (in g) on each hindpaw was recorded for 15 sec, three times over approximately 1 min. Finally, rats were placed into locomotor chambers, and the number of photobeam breaks in 10 min was recorded. Immediately after baseline measures were taken, rats were briefly anesthetized with isoflurane, and 0.1 mL CFA was injected into the plantar surface of the right hindpaw.
To determine dose-effect curves for each drug alone in each sex, 3 days after CFA injection, at approximately 0800, vehicle, 0.032, 0.1, 0.32, 1.0, or 3.2 mg/kg morphine, or 0.5, 1.0, 2.0, or 4.0 mg/kg THC was injected (separate rats tested at each dose). In the second experiment, 3 days after CFA injection, morphine (or saline) and THC (or vehicle) were injected consecutively. At 30, 60, 120, and 240 min post-injection, von Frey, Hargreaves, incapacitance (weight-bearing), and locomotor tests were conducted as described on Day 1. Paw thickness was measured at 240 min post-injection.
Data Analysis
The mean of the 3 trials conducted for each assay (von Frey, Hargreaves, and incapacitance) was calculated to yield single baseline and drug test scores at each time point, for each rat. Baseline data were then analyzed using a one-way ANOVA, to determine if there were sex differences in responses on each assay. To quantify drug potency, raw drug data were transformed to percent maximum possible effect (%MPE) for each rat at each time point: [(drug effect– minimum effect)/(maximum effect-minimum effect) × 100]; the minimum effect was the mean of the vehicle control group at a given time point, and the maximum effect was the mean baseline (before CFA) for all animals, except for Hargreaves, where the maximum was 20 sec (the cutoff value). Missing baseline locomotor data (<1% of cases, due to equipment malfunction) were replaced by the mean baseline for that treatment group. Peak effects for morphine alone and THC alone occurred between 30 -120 min post-injection, and thus, dose-response curves were generated by calculating the mean of the 30-, 60-, and 120-min time point %MPE data for von Frey, Hargreaves, weight-bearing, and locomotor tests. Mean 30-120 min time point %MPE data (von Frey, Hargreaves, weight-bearing, locomotor tests) and paw thickness %MPE data were analyzed using a 2-way ANOVA to determine the effects produced by each drug alone and in combination (morphine dose: 6 levels, THC dose: 5 levels, 1:1 dose ratio: 3 levels, 3:1 dose ratio: 3 levels, or 1:3 dose ratio, 3 levels) in each sex (Sex: 2 levels). Follow up ANOVAs (e.g., within each sex, when sex differences were significant) plus Tukey’s HSD or Dunnett’s t-test (to compare multiple drug doses to vehicle) were used for post-hoc determination of significance. When drug efficacy was sufficient to allow determination of ED50 values (von Frey, Hargreaves, and locomotor activity tests) in both sexes, a sum-of-squares F test was conducted on Hill slope and ED50 values to compare THC potency between males and females, using GraphPad Prism version 8.2.0 for Mac (GraphPad Software, La Jolla California USA, www.graphpad.com). Significance level was p≤ 0.05 for all statistical tests. Partial eta squared (ηp2) was used to estimate effect size. Although doses in the morphine-THC combinations were sex-specific (Table 1), analysis of sex differences in THC-morphine combinations was possible because the dose ratios were equivalent between the sexes.
To specifically test for morphine-THC synergy, an isobolographic analysis was conducted on the von Frey data (Tallarida, 2001). ED50 values on the von Frey assay were calculated from %MPE data for morphine and THC in males and females separately using GraphPad Prism version 8.2.0 for Mac (GraphPad Software, La Jolla California USA, www.graphpad.com). Using the ED50 values for each drug alone, a dose-equivalence analysis was conducted, allowing for determination of predicted morphine+THC effects at 1:1, 3:1, and 1:3 dose ratios. Actual doses of morphine and THC given to rats of each sex are shown in Table 1. ED50 values obtained on the von Frey assay were calculated for each dose ratio in males and females separately, and a two-way ANOVA was used to determine if there were significant differences between the predicted (Eadd) and observed (Eobs) effects of combined morphine and THC, and if these effects differed between the sexes (Tallarida, 2001). Dunnett’s test was used for post-hoc determination of significance. Significance level was p≤ 0.05 for all statistical tests.
Results
Baseline Data
Analysis of baseline data revealed sex differences on all assays. Males had higher mechanical thresholds than females on the von Frey assay (78.2 ± 2.0 vs. 58.2 ± 2.1 g, respectively; Sex, F1,193=47.21, p<0.001). Latency to respond to noxious heat was longer in males than females on the Hargreaves test (11.01 ± 0.38 vs. 9.98 ± 0.39 s, respectively; Sex, F1,193=4.02, p=0.047). Males put more weight on their right hindpaw than females (122.86 ± 2.53 vs. 87.80 ± 2.54 g, respectively; Sex, F1,193=95.77, p<0.001). Baseline dorsal-ventral right hindpaw thickness was greater in males than females (5.31 ± 0.05 vs. 4.71 ± 0.05 mm, respectively; Sex, F1,193=85.96, p<0.001). Finally, males locomoted less than females (985 ± 24 vs. 1085 ± 24 photobeam breaks, respectively; Sex, F1,193=8.46, p=0.004). Given these baseline sex differences, data obtained after drug (or vehicle) injection were transformed to %MPE on each measure prior to analysis of drug effect.
Morphine Alone
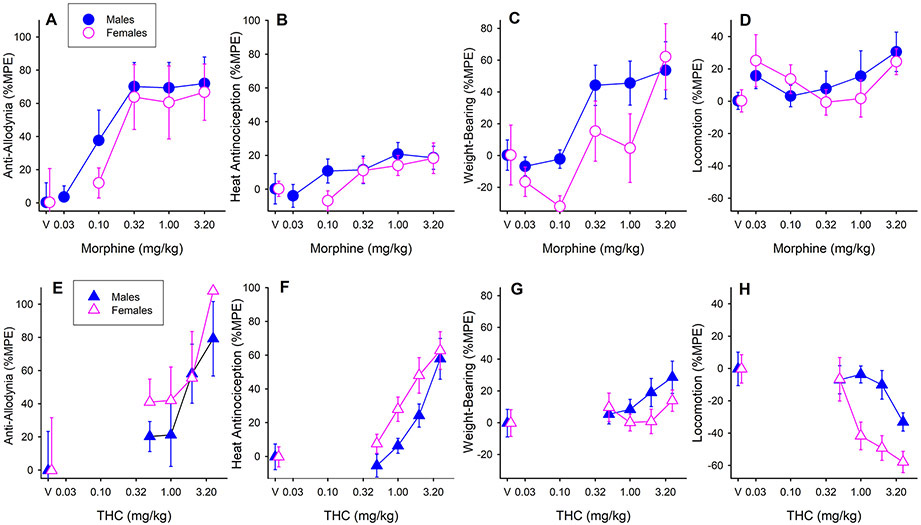
Figure 1 (A-D) shows the effects of morphine when administered alone, in male and female rats. Morphine dose-dependently increased mechanical threshold (Fig. 1A; Morphine dose, F5,90=6.98, p<0.001, ηp2=0.28), and this effect did not differ significantly between the sexes. Post-hoc analysis revealed that compared to saline, 0.32,1.0, and 3.2 mg/kg morphine all increased mechanical threshold (all p’s≤0.001). Although the morphine ED50 was lower in males than females (Table 2), statistical comparison of ED50 values indicated that morphine potency did not significantly differ between the sexes on the von Frey test. On the Hargreaves test, morphine lengthened latency to respond to noxious heat in a dose-dependent manner (Fig. 1B; Morphine dose, F5,90=5.54, p<0.001, ηp2=0.24), with no sex differences. Post-hoc tests showed significant effects at 1.0 and 3.2 mg/kg morphine (p=0.029 and p=0.025, respectively). Morphine also increased weight-bearing on the CFA-injected hindpaw (Fig. 1C; Morphine dose, F5,90=7.59, p<0.001, ηp2=0.30), and this effect was greater in males than females at most doses (Sex, F1,90=4.51, p=0.036, ηp2=0.05). Analysis in only males revealed that morphine increased hindpaw weight-bearing (F5,46=6.09, p<0.001), specifically at 0.32, 1.0, and 3.2 mg/kg (all p’s ≤0.032). Analysis in only females revealed that morphine increased hindpaw weight-bearing (F5,46=3.16, p=0.016), but this effect was not significant at any specific dose in post-hoc analysis. The morphine ED50 was 1.58 ±0.26 mg/kg for males on the weight-bearing test, but could not be determined for females. Morphine did not significantly affect locomotor activity (Fig. 1D) or paw thickness (data not shown) in either sex.
Figure 1.
The effects of morphine alone (A-D) and THC alone (E-H) in male and female CFA-treated rats. On the von Frey test (A, E), both morphine and THC were anti-allodynic. On the Hargreaves test (B, F), both morphine and THC were anti-hyperalgesic, and THC was more potent in females than males. On the test of hindpaw weight-bearing (C, G), morphine increased weight-bearing on the inflamed paw, whereas THC had no effect on hindpaw weight-bearing. On the locomotor activity test (D, H), morphine had no significant effect on locomotor activity in either sex, whereas THC decreased locomotor activity and was more potent in females than males. “V” = vehicle control groups; saline was the vehicle for morphine and 1:1:18 ethanol:cremophor:saline was the vehicle for THC. Each symbol is the mean ± 1 S.E.M. of 8-10 males or females.
Table 2.
ED50 values for males and females on the von Frey test.
| Dose Ratio (Morphine:THC) |
Males | Females | ||
|---|---|---|---|---|
| Morphine (mg/kg) | THC (mg/kg) | Morphine (mg/kg) | THC (mg/kg) | |
| Morphine alone | 0.24 ±0.11 | -- | 0.46 ±0.30 | -- |
| THC alone | -- | 1.72 ±0.51 | -- | 1.20 ±0.51 |
| 1:1 | 0.12 ±0.04 | 0.89 ±0.33 | 0.16 ±0.05 | 0.42 ±0.13 |
| 3:1 | 0.18 ±0.11 | 0.44 ±0.28 | 0.24 ±0.12 | 0.21 ±0.11 |
| 1:3 | 0.05 ±0.02 | 1.13 ±0.40 | 0.06 ±0.03 | 0.45 ±0.24 |
THC Alone
Figure 1 (E-H) shows the effects of THC alone in each sex on the four behavioral measures. THC dose-dependently increased mechanical threshold, similarly in both sexes (Fig. 1E; THC dose, F4,83=4.63, p=0.002, ηp2=0.18); post-hoc analysis showed that 2 and 4 mg/kg were anti-allodynic (p=0.033 and p<0.001, respectively). The THC ED50 was slightly but not significantly higher in males than females (Table 2), on the von Frey test. THC also lengthened latency to withdraw from noxious heat (Fig. 1F; THC dose, F4,83=18.80, p<0.001, ηp2=0.48), and this THC effect was greater in females than males (Sex, F1,83=6.14, p=0.015, ηp2=0.07). Analysis in only females revealed that THC increased the thermal threshold (F4,41=9.55, p<0.001, ηp2=0.48); this effect was significant at 2 and 4 mg/kg (p’s ≤0.001). Analysis in only males revealed that THC increased the thermal threshold (F4,42=10.04, p<0.001, ηp2=0.48), but this effect was significant at only the highest dose, 4 mg/kg (p<0.001). ED50 values for THC on the Hargreaves test were 4.40 ±0.65 mg/kg in males and 3.30±0.65 mg/kg in females; statistical comparison of ED50 values showed that THC was significantly more potent in females than males against noxious heat (Fig 1F; Sex, F2,68=3.23, p=0.046). THC did not significantly alter weight-bearing on the inflamed paw in either sex (Fig. 1G). THC-induced decreases in locomotor activity were greater in females than males at the higher doses tested (Fig. 1H; Sex x THC dose, F4,83=2.60, p=0.042, ηp2=0.11). Subsequent analysis within each sex revealed that THC decreased locomotor activity in females (p<0.001), but not in males. Post-hoc analysis in females revealed that 1, 2, and 4 mg/kg THC decreased locomotor activity (all p’s ≤0.005). The THC ED50 was 5.90 ±1.85 mg/kg in males and 2.19±0.53 mg/kg in females; statistical comparison showed that THC was significantly more potent in females than males in decreasing locomotor activity (Fig. 1H; Sex, F2,68=12.17, p<0.001). THC did not significantly alter paw thickness in either sex (data not shown).
Morphine-THC Combinations
Figure 2 shows the dose-effect functions for morphine and THC administered in 1:1, 3:1, or 1:3 dose ratio combinations, in males (Fig. 2A-D) and females (Fig. 2E-H). The effects of morphine alone are replotted from Figure 1 to provide a visual comparison between the effects of morphine alone and in combination with THC, on each test. All three dose ratio combinations increased mechanical threshold, with no sex differences (Fig. 2A, E; 1:1 ratio, F4,95=5.39, p=0.001, ηp2=0.19; 3:1 ratio, F5,119=3.51, p=0.006, ηp2=0.13; 1:3 ratio, F3,98=8.98, p<0.001, ηp2=0.22). Additionally, all dose ratio combinations increased latency to respond to noxious heat, with no sex differences (Fig. 2B, F; 1:1 ratio, F4,95=7.19, p<0.001, ηp2=0.23; 3:1 ratio, F5,119=4.96, p<0.001, ηp2=0.17; 1:3 ratio, F3,98=12.99, p<0.001, ηp2=0.28). The 1:1 and 1:3 dose ratio combinations decreased locomotor activity with no sex difference, and the 3:1 ratio did not alter locomotor activity (Fig. 2D, H; 1:1 ratio, F4,95=4.94, p<0.001, ηp2=0.23; 1:3 ratio, F3,98=7.27, p<0.001, ηp2=0.18). Morphine-THC combinations had no significant impact on weight-bearing on the inflamed paw (Fig. 2C, G) or on paw thickness (data not shown) in either sex.
Figure 2.
The effects of morphine alone and morphine-THC combinations in males (A-D) and females (E-H). Specific doses administered in each combination were based on each drug’s potency in each sex on the von Frey test (see Table 1). A, D: morphine-THC combinations were anti-allodynic at all dose ratios, with no sex differences. B, F: morphine-THC combinations were anti-hyperalgesic at all dose ratios. C, G: no dose combination affected weight-bearing on the inflamed paw. D, H: The 1:1 and 1:3 morphine:THC combinations decreased locomotor activity, similarly in both sexes. The 3:1 morphine:THC dose combination did not alter locomotor activity, likely because this dose combination contained the smallest proportion of THC. “V” = vehicle control groups; saline was the vehicle for morphine and 1:1:18 ethanol:cremophor:saline + saline was the vehicle for the morphine-THC combinations. Each symbol is the mean ± 1 S.E.M. of 10-14 males or females.
Drug Synergy Analysis
Isobolographic analysis was conducted on von Frey data only; this analysis can only be conducted using a single pain measure unless drug potencies are exactly the same on every pain test, which did not occur in the present study. Figure 3 shows that 1:1, 3:1, and 1:3 dose ratio combinations of morphine and THC produced additive effects in both males and females. That is, the observed ED50 was not significantly different from the predicted ED50 for any of the three dose ratio combinations in either sex (ED50, F1,921=0.47, n.s.; ED50 x Sex, F2,921=0.27, n.s.). Although several combinations trended toward a synergistic interaction in females, these effects were below the level of significance.
Figure 3.
Isobolographic analysis of von Frey data: Anti-allodynic effects produced by 1:1, 3:1, and 1:3 morphine:THC dose ratios did not differ significantly from predicted additivity in either males or females. Each symbol is the ED50 ± 1 S.E.M.; ED50 values were derived from dose-effect data obtained in 38-53 males or 36-48 females (see Table 2).
Discussion
When administered alone, morphine was anti-allodynic and anti-hyperalgesic in the present study. Morphine also increased weight-bearing on the inflamed paw, and all antinociceptive effects were observed at doses that did not alter locomotor activity. The only sex difference in morphine effect was on the weight-bearing test: at most doses tested, morphine increased weight-bearing on the inflamed paw more in males than females. Although numerous studies have reported that morphine has greater acute antinociceptive potency in male than female rats, some animal studies have reported no sex differences in morphine potency, and sex comparisons in human studies are particularly equivocal (for reviews, see Craft, 2008; Niesters et al., 2010; Nasser and Afify, 2019). In rats, sex differences in morphine antinociception are most likely to be observed when females are in estrus (Stoffel et al., 2003; Terner et al., 2005). Thus, it is possible – perhaps likely, given the brevity of estrus – that most females in the present study were not in estrus at the time of testing, which would decrease the likelihood of observing sex differences in the antinociceptive potency of morphine.
Like morphine, THC largely produced the expected antinociceptive effects when it was administered alone to rats with CFA-induced hindpaw inflammation. THC was more potent in females than males in its anti-hyperalgesic and locomotor suppressant effects, whereas the anti-allodynic effect of THC did not differ significantly between the sexes. The sex difference on the Hargreaves test aligns with results from previous studies that report greater acute antinociceptive potency of THC against noxious heat in female rats compared to males, whether CFA-treated (Craft et al., 2013; Britch et al., 2020) or healthy (for reviews, see Cooper and Craft, 2018; Blanton et al., 2021). Furthermore, greater potency of THC in females in regard to locomotor suppression has been reported previously (for review, see Cooper and Craft, 2018), and systemically administered THC has not been observed to differ in its anti-allodynic potency in male vs. female CFA-treated rats (Craft et al., 2013; Britch et al., 2020). In the present study, THC did not increase weight-bearing on the inflamed paw, nor did it significantly reduce hindpaw thickness, an index of inflammation. Cannabinoids are widely accepted to be anti-inflammatory, largely through their immunosuppressant effects (Nagarkatti et al., 2009). However, previous studies suggest that systemic administration of THC is less effective than local administration in reducing CFA-induced hindpaw edema in rats (Craft et al., 2013), that administering THC repeatedly is more effective than a single injection for increasing weight-bearing in CFA-treated rats (Britch et al., 2020), and that treating with cannabinoids before rather than after inducing inflammation is also more effective (Rock et al., 2018). Thus, the fact that rats in the present study were treated with THC systemically, only once, several days after CFA administration, likely contributed to the lack of significant THC effect on weight-bearing and hindpaw inflammation.
Sex-specific drug combination doses were determined from morphine and THC dose-effect functions on the von Frey test, and an isobolographic approach was used to test whether drug combinations had additive, sub-additive, or synergistic effects against CFA-induced allodynia. Morphine-THC combinations at all three dose ratios (1:1, 3:1, and 1:3) were effective against CFA-induced mechanical allodynia in both males and females, but no synergy was observed in either sex. That is, the potencies of morphine-THC combinations were not statistically different from predicted additivity based on the potency of each drug alone. We did not conduct a similar (isobolographic) analysis of morphine-THC effects on the other two measures of pain (heat hyperalgesia and weight-bearing tests), since drug potencies on these measures differed from those on the von Frey test, and sex differences also varied among measures. However, visual inspection of Figure 2 shows that morphine-THC combinations were more effective than morphine alone against noxious heat pain in both sexes (Fig. 2B, F). In contrast, the same morphine-THC combinations did not increase weight-bearing on the inflamed paw, whereas morphine alone did (Fig.2C, G). Thus, THC – which by itself did not increase weight-bearing at doses that were anti-allodynic and anti-hyperalgesic (Fig. 1) – either did not affect or blocked morphine’s effect on weight-bearing. Finally, morphine-THC combinations decreased locomotor activity in both sexes, whereas morphine alone did not (Fig. 2D, H). Therefore, it is possible that the enhancement of morphine’s effects by THC against heat hyperalgesia – in which antinociception is defined as longer latency to respond – is secondary to sedative effects of the drug combination, as measured by locomotor activity. Indeed, the more predominant THC was in the morphine-THC combination (i.e., 1:3 vs. 1:1 or 3:1 morphine:THC), the more locomotion was suppressed, and the more likely the drug combination was to enhance morphine’s anti-allodynic and anti-hyperalgesic effects. Taken together, these results suggest that combining morphine and THC would not acutely enhance pain relief without also enhancing an adverse side-effect, as suggested by several previous studies that have characterized motor side-effects (Finn et al., 2004; Kazantzis et al., 2016) or abuse-related effects (Cooper et al., 2018; Babalonis et al., 2019) of opioid-cannabinoid combinations.
The current finding of additive antinociceptive interactions between THC and morphine agrees with some but not all previous studies. Using primarily males and acute pain measures in healthy animals, many previous studies report enhancement of opioid antinociception by a cannabinoid agonist (Cichewicz et al., 1999; Finn et al., 2004; Maguire et al., 2013; Maguire and France, 2014). Several studies have employed isobolographic approaches to distinguish between additive and synergistic interactions, and unlike the present study, these typically report synergistic antinociception between opioid and cannabinoid agonists (Cichewicz and McCarthy, 2003; Cox et al., 2007; Kazantzis et al., 2016; Grenald et al., 2017; Maguire and France, 2018). Although none of these previous studies used the hindpaw CFA model that we used, several studies reporting opioid-cannabinoid synergy used other chronic pain models with nociceptive tests similar to the ones we used, to assess mechanical and heat hypersensitivity (hindpaw incision model, sciatic nerve injury model: (Grenald et al., 2017); chronic constriction injury model: (Kazantzis et al., 2016)). To our knowledge, only one study has examined morphine-THC combinations using a model of persistent inflammatory pain. Cox and colleagues reported that morphine and THC were synergistic in male rats with CFA-induced arthritis, using a paw pressure test of mechanical hyperalgesia (Cox et al., 2007). Although it is possible that the different tests used in our study vs. that used by Cox and colleagues contribute to the discrepant findings, numerous other methodological differences may be important. For example, we administered CFA locally and tested rats 3 days later, whereas Cox et al. administered CFA systemically and tested rats 19 days after CFA injection. Given the dramatic changes in behavior and immune response that occur over time after CFA administration (Nagarkatti et al., 2009; Wu et al., 2017; Gajtkó et al., 2020), as well as μ-opioid type 1 receptor and cannabinoid receptor 1 upregulation that occur after induction of chronic pain (Lim et al., 2003; Amaya et al., 2006; Wang et al., 2007; Ni et al., 2013), opioid and cannabinoid potency and opioid-cannabinoid interactions may change over time after induction of chronic pain. To our knowledge, the trajectory of opioid-cannabinoid interactions over time after onset of inflammation has not been examined.
In contrast to animal studies commonly demonstrating cannabinoid enhancement of opioid antinociception, laboratory studies in humans report only slight or no enhancement of opioid analgesia with cannabinoids, across a range of heat, cold, pressure, and other pain tests (Naef et al., 2003; Roberts et al., 2006; Cooper et al., 2018; Babalonis et al., 2019; Dunn et al., 2021). However, in chronic pain patients maintained on opioids, the addition of vaporized cannabis (Abrams et al., 2011) or oral THC (Narang et al., 2008: a placebo-controlled trial) significantly decreased pain, suggesting that cannabinoids may be a useful adjuvant for chronic pain patients. Whether or not the analgesic effect reported in these latter studies reflects opioid-cannabinoid synergy, however, is not known.
Conclusion
In summary, the present study demonstrates that when given acutely 3 days after induction of persistent inflammation, morphine and THC each dose-dependently reduced mechanical allodynia when given alone, and the two drugs produced additive anti-allodynic effects when co-administered, in both male and female rats. However, in males, THC appeared to block morphine-induced increases in weight-bearing on the inflamed paw, and in both sexes, THC-induced enhancement of morphine’s antinociceptive effect observed at some dose combinations was accompanied by enhanced locomotor suppression. These results contrast with those of some previous animal studies reporting opioid-cannabinoid synergy, but are largely consistent with studies in humans (for review, see Babalonis and Walsh, 2020), suggesting that THC may not be a particularly beneficial adjuvant to opioid treatment for chronic pain.
Acknowledgements
The authors thank C. Hsiao, A. Pondelick, and G. Rosales for excellent technical assistance.
Conflicts of Interest and Sources of Funding:
The authors report no conflicts of interest. This work was funded by the National Institute on Drug Abuse (T32DA035200 to S.B.), and by funds provided by a Herbert L. Eastlick Professorship (to R.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abboud C, Duveau A, Bouali-Benazzouz R, Massé K, Mattar J, Brochoire L, et al. (2021). Animal models of pain: Diversity and benefits. J Neurosci Methods 348:108997. [DOI] [PubMed] [Google Scholar]
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL (2011). Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90:844–51 [DOI] [PubMed] [Google Scholar]
- Alsalem M, Altarifi A, Haddad M, Azab B, Kalbouneh H, Imraish A, et al. (2020). Analgesic Effects and Impairment in Locomotor Activity Induced by Cannabinoid/Opioid Combinations in Rat Models of Chronic Pain. Brain Sci 10:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun A, Yildirim K, Ozdemir E, Bagcivan I, Gursoy S, Durmus N (2015). Attenuation of morphine antinociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. J Physiol Sci 65:407–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, et al. (2006). Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124:175–83 [DOI] [PubMed] [Google Scholar]
- Averitt DL, Eidson LN, Doyle HH, Murphy AZ (2019). Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 44:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baamonde AI, Hidalgo A, Andres-Trelles F (1989). Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology 28:967–70 [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Sloan PA, Nuzzo PA, Fanucchi LC, Walsh SL (2019). Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans. Psychopharmacology (Berl) 236:3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalonis S, Walsh S L (2020). Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence. Eur Neuropsychopharmacol 36:206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L, Ratka A (2002). Sex-specific differences in levels of morphine, morphine-3-glucuronide, and morphine antinociception in rats. Pain 95:65–74 [DOI] [PubMed] [Google Scholar]
- Bartok R E, Craft R M (1997). Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–78 [PubMed] [Google Scholar]
- Blanton H L, Barnes R C, McHann M C, Bilbrey J A, Wilkerson J L, Guindon J (2021). Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav 202:173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth FM, March LM, Brnabic AJM, Jorm LR, Williamson M, Cousins MJ (2001). Chronic pain in Australia: a prevalence study. Pain 89:127–134 [DOI] [PubMed] [Google Scholar]
- Britch S, Goodman A, Wiley J, Pondelick A, Craft R (2020). Antinociceptive and Immune Effects of Delta-9-tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. J Pharmacol Exp Ther 373:416–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM (2017). Cannabidiol-Delta(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Inturrisi CE, et al. (1992). Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav 42:685–92 [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB (2003). Women Experience More Pain and Require More Morphine Than Men to Achieve a Similar Degree of Analgesia. Anesth Analg 97:1464–1468 [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP (1999). Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther 289:859–67 [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA (2003). Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther 304:1010–5 [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Portillo-Salido E (2013). “Bedside-to-Bench” Behavioral Outcomes in Animal Models of Pain: Beyond the Evaluation of Reflexes. Curr Neuropharmacol 11:560–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Cooper ZD, Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, et al. (2010). Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology (Berl) 208:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ (2000). Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the μ opioid receptor. Psychopharmacology (Berl) 150:430–442 [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD (2005). Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther 313:449–59 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M (2018). Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 43:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM (2018). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 43:34–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP (2007). Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 567:125–30 [DOI] [PubMed] [Google Scholar]
- Craft RM (2008). Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol 16:376–85 [DOI] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM (2013). Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Delta(9)-tetrahydrocannabinol in the rat. Pain 154:1709–17 [DOI] [PubMed] [Google Scholar]
- De Vita MJ, Moskal D, Maisto SA, Ansell EB (2018). Association of Cannabinoid Administration With Experimental Pain in Healthy Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 75:1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Bergeria CL, Huhn AS, Speed TJ, Mun CJ, Vandrey R, et al. (2021). Within-subject, double-blinded, randomized, and placebo-controlled evaluation of the combined effects of the cannabinoid dronabinol and the opioid hydromorphone in a human laboratory pain model. Neuropsychopharmacology 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, et al. (2005). Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. The Journal of Pain 6:116–124 [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SRG, Roe CH, Madjd A, Fone KCF, Kendall DA, et al. (2004). Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19:678–686 [DOI] [PubMed] [Google Scholar]
- Gajtkó A, Bakk E, Hegedűs K, Ducza L, Holló K (2020). IL-1β Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front Physiol 11:543331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenald S A, Young M A, Wang Y, Ossipov M H, Ibrahim M M, Largent-Milnes T M, et al. (2017). Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 116:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantzis N P, Casey S L, Seow P W, Mitchell V A, Vaughan C W (2016). Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br J Pharmacol 173:2521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefea FJ, Lefebvrea JC, Egert JR, Affleck G, Sullivand MJ, Caldwell DS (2000). The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain 87: [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ (1989). Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav 34:119–27 [DOI] [PubMed] [Google Scholar]
- Kuo A, Wyse BD, Meutermans W, Smith MT (2015). In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol 172:532–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B (2020). Opioid-sparing effects of cannabinoids: Myth or reality? Prog Neuropsychopharmacol Biol Psychiatry 110065. [DOI] [PubMed] [Google Scholar]
- Lim G, Sung B, Ji RR, Mao J (2003). Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 105:275–83 [DOI] [PubMed] [Google Scholar]
- Maguire DR, France CP (2014). Impact of efficacy at the mu-opioid receptor on antinociceptive effects of combinations of mu-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther 351:383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP (2018). Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur J Pharmacol 819:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP (2013). Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther 345:354–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R (2003). The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 105:79–88 [DOI] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder S A, Hegde VL, Nagarkatti M (2009). Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1:1333–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. (2008). Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain 9:254–64 [DOI] [PubMed] [Google Scholar]
- Nasser SA, Afify EA (2019). Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sci 237:116926. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC:The National Academies Press [Google Scholar]
- Negus SS (2019). Core Outcome Measures in Preclinical Assessment of Candidate Analgesics. Pharmacol Rev 71:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Gao Y, Gong S, Guo S, Hisamitsu T, Jiang X (2013). Regulation of μ-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur J Pain 17:313–23 [DOI] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, et al. (2010). Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 151:61–8 [DOI] [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M (2006). Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol 530:54–8 [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Parker LA (2018). Effect of cannabidiolic acid and (9)-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology (Berl) 235:3259–3271 [DOI] [PubMed] [Google Scholar]
- Romero MT, Kepler KL, Bodnar RJ (1988). Gender determinants of opioid mediation of swim analgesia in rats. Pharmacol Biochem Behav 29:705–9 [DOI] [PubMed] [Google Scholar]
- Smith FL, Chichewicz D, Martin ZL, Welch SP (1998). The Enhancement of Morphine Antinociception in Mice by delta-9-Tetrahydrocannabinol. Pharmacology Biochemistry and Behavior 60:559–566 [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM (2003). Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain 103:285–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverrisdottir E, Lund TM, Olesen AE, Drewes AM, Christrup LL, Kreilgaard M (2015). A review of morphine and morphine-6-glucuronide's pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci 74:45–62 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ (2001). Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–72 [PubMed] [Google Scholar]
- Terner J M, Lomas L M, Picker M J (2005). Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain 6:372–83 [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM (2001). Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430:41–47 [DOI] [PubMed] [Google Scholar]
- Unruh AM (1996). Gender variations in clinical pain experience. Pain 65:123–67 [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Mao J, Sung B, Yang L, Mao J (2007). Central glucocorticoid receptors regulate the upregulation of spinal cannabinoid-1 receptors after peripheral nerve injury in rats. Pain 131:96–105 [DOI] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ (2006). Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol 291:R300–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM (2013). The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol Biochem Behav 103:444–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yao X, Jiang Y, He X, Shao X, Du J, et al. (2017). Pain aversion and anxiety-like behavior occur at different times during the course of chronic inflammatory pain in rats. J Pain Res 10:2585–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]