Abstract
The Carolina chickadee, Poecile carolinensis Audubon is a relatively small songbird belonging to the tit and chickadee family Paridae. Feces from three P. carolinensis from Polk County, Arkansas, USA, and a single P. carolinensis in McCurtain County, Oklahoma, USA, were collected and examined for coccidia; the latter bird was found to be passing a new species of Isospora. Oöcysts of Isospora oklahomaensis n. sp. are subspheroidal to ovoidal with a smooth to slightly-pitted bi-layered wall, measure (L × W) 32.1 × 28.3 μm, and have a length/width (L/W) ratio of 1.1; a micropyle and oöcyst residuum were absent but a bilobed and refractile polar granule is present. Sporocysts are ellipsoidal and measure 18.4 × 11.8 μm, L/W 1.6; a prominent Stieda body is present as well as a distinct sub-Stieda body. The sporocyst residuum is composed of an irregular mass of granules lying between and dispersed among the sporozoites. This is the first coccidian described from the Carolina chickadee and, most importantly, only the second described from a member of the Paridae, worldwide.
Introduction
The Carolina chickadee or Mésange de Caroline, Poecile carolinensis (Audubon) is a relatively small passerine belonging to the tit and chickadee family Paridae. It possesses a black cap and bib with white cheeks and formerly belonged to the genus Parus (L.) but was transferred to Poecile Kaup by AOU (1998). This bird ranges (as a permanent resident) from southern Kansas east to central Indiana, southern Pennsylvania, and central New Jersey south to southern Texas, the Gulf Coast, and the northern peninsular of Florida. Four subspecies are recognized (Avibase, 2021), and in Oklahoma, P. carolinensis atricapilloides (Lunk) is found statewide except for the Panhandle. It seldom decends to the ground and prefers valleys and foothills in open deciduous woodland, forest clearings and edge, swamps, thickets, second-growth woodland, parks, brushy areas,and suburban areas. Carolina chickadees eat insects, especially moths and caterpillars, insect eggs, spiders, fruits, and seeds (Terres, 1980).
Although there are many species of coccidian parasites reported from other related passerines (see Duszynski et al., 2000; Berto et al. 2011), there are no reports of coccidia from this bird. Here, we describe a new species of Isospora from a P. carolinensis from Oklahoma, USA.
Materials and methods
Fresh feces were collected from 3 P. carolinensis taken with a mist net in March and May 2021 from the Ouachita Mountains Biological Station (OMBS), Polk County, Arkansas, USA (34° 27′ 43.4484″N, −93° 59′ 54.3264″W). In addition, fresh feces were collected from a single (nesting female) P. carolinensis taken from a bird box in April 2021 in Hochatown, McCurtain County, Oklahoma, USA (34°10′17.0286″N, −94°45′05.7414″W); after defecation, all birds were released unharmed. Fecal samples were placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7). After flotation in Sheather’s sugar solution (specific gravity = 1.30), they were examined for coccidia using an Olympus BX53 light microscope (Olympus Corporation, Center Valley, Pennsylvania, USA). One bird was found to be passing unsporulated and partially sporulated oöcysts and the sample was placed in a Petri dish containing a small layer of K2Cr2O7 for 48‒72 h to allow sporulation. It was further examined using Olympus© cellSens 1.14 digital software (https://www.olympus-lifescience.com/en/software/cellsens/) and all morphological measurements are reported in micrometers (μm) with the means followed by the ranges in parentheses. Photographs were taken using Nomarski interference-contrast optics at ×1,000 magnification. Oöcysts were ca. 30 days old when measured and photographed.
Descriptions of oöcysts and sporocysts follow the standard guidelines of Wilber et al. (1998). The host photovoucher was accessioned into the Eastern Oklahoma State College (EOSC) Collection, Idabel, Oklahoma, USA. Photosyntypes of sporulated oöcysts were accessioned into the Harold W. Manter Laboratory of Parasitology (HWML), Lincoln, Nebraska, USA.
Results
A single Carolina chickadee was found to be passing a coccidian that we describe herein as new.
Eimeriidae Minchin, 1903
Isospora Schneider, 1881
Isospora oklahomaensis n. sp.
Type species:
Isospora rara Schneider, 1881 by monotypy.
Type-and only host:
Poecile carolinensis (Audubon, 1834) (Aves: Passeriformes: Paridae), adult female collected 15 April 2021.
Type-and only locality:
Hochatown, McCurtain County, Oklahoma, USA (34°10′17.0286″N, −94°45′05.7414″W).
Type-material:
Photosyntypes of sporulated oöcysts are deposited as HWML 216542.
Prevalence:
1 of 4 (25%) overall; 1/1 McCurtain County, Oklahoma, USA; 0/3 (0%) Polk County, Arkansas, USA.
Sporulation time:
All oöcysts were fully sporulated within 48‒72 hrs.
Site of infection:
Unknown; oöcysts were passed in feces.
ZooBank registration:
To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Isospora oklahomaensis n. sp. is urn:lsid:zoobank.org:act:BD2187AA-9ED5–4DE3–8ABF-F0B4470814BB.
Etymology:
The specific name is derived from the US state locality for the coccidian, Oklahoma, the 46th state to enter the union on November 16, 1907. The state’s name is derived from the Choctaw words okla and humma, meaning “honored people”. The new name is formed as an adjective, feminine.
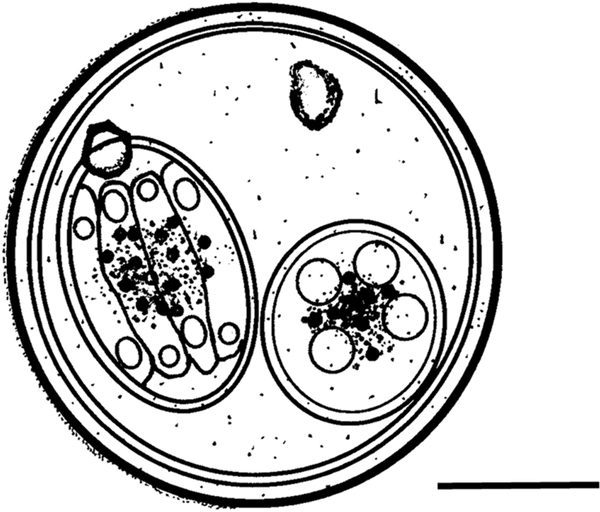
Fig. 1.
Composite line drawing of Isospora oklahomaensis n. sp. Scale bar: 10 μm
Fig. 2.
A-D Nomarski-interference contrast photomicrographs of sporulated oocysts of Isospora oklahomaensis n. sp. (A) View showing sporocyst residuum (SR), Stieda body (SB), and subStieda body (SSB). (B) Another view showing anterior refractile body (ARB), posterior refractile body (PRB), and SB. (C) Oocyst showing polar granule (PG) and SB. (D) Oocyst showing oocyst wall (OW) and sporozoites (SZ). Scale bars: 10 μm.
Sporulated oöcyst
Oöcyst (n = 15) subspheroidal to ovoidal; (32.1 × 28.3) 29–36 × 27–30, length/width (L/W) ratio 1.1–1.2. Wall smooth to slightly-pitted, thick, bilayered, tan to yellow, c.2.0 (1.3–2.1), outer c. 1.2 (0.8‒1.4), inner 0.7 (0.5‒0.8). Micropyle and oöcyst residuum absent but single bilobed and refractile polar granule (3 × 2.7) present.
Sporocyst and sporozoite
Sporocysts (n = 15) 2, ovoidal 18.4 × 11.8 (16–20 × 9–13); L/W ratio 1.6 (1.4–1.7); wall smooth, thin and uni-layered, light brown, c.0.5 thick. Prominent Stieda and distinct sub-Stieda bodies present, para-Stieda body absent; sporocyst residuum spheroidal granules of various sizes dispersed between sporozoites. Sporozoites (4), vermiform 12.7 × 3.6; L/W ratio 3.3; subspheroidal anterior and ellipsoidal posterior refractile bodies, nucleus in centre of sporozoites.
Remarks
There are seven species of the genus Poecile Kaup currently recognized in the tit family Paridae Vigors (Gosler and Clement, 2007; AOS, 2020). Surprisingly, only a single coccidian, Isospora parusae Ray, Shivnani, Ommen, and Bhaskaran, 1952 from grey-crested tit, Lophophanes dichrous (Blyth) from India has been described worldwide from birds of this family (Ray et al., 1952) (Table 1). Furthermore, to our knowledge, there are no coccidian taxa fully described from any member of the Paridae in North America. However, there are several isosporans from this family but none have been described beyond being reported as an Isospora sp. (see Table 2).
Table 1.
Comparison of the sporulated oocysts of Isospora spp. from Old and New World passerine birds of the Superfamily Sylvioidea (Hirundinidae, Paridae, Timaliidae, Zosteropidae).
| Isospora spp. | Type host (Describer) Type locality | Oocyst shape, size, featuresa,b | Sporocyst shape, size, featuresa,b | References |
|---|---|---|---|---|
|
| ||||
| I. brayi | Zosterops japonicus Temminck & Schlegel Hawaii, USA | Subspheroidal 27 × 26; L/W 1.0 26–28 × 25–27 M, OR, PG: all ‒ | Ovoidal to pyriform 19 × 12; L/W 1.6 18–21 × 11–13 SB, SSB, SR: all + PSB: – | Upton et al. (1988) |
| I. leiothrixi | Leiothrix lutea (Scopoli) Hawaii, USAc | Ellipsoidal 28.0 × 16.6; L/W 1.5 24–32 × 15–18 M, OR: both – PG: + | Ovoidal 15.5 × 10.3; L/W 1.5 12–20 × 7–12 SB, SSB SR: all + PSB: – | McQuistion et al. (1996) |
| I. manoaensis | Z. japonicus Hawaii, USA | Subspheroidal 28.0 × 26.5; L/W 1.1 25–31 × 22–29 M, OR: both ‒ PG: + | Ovoidal 18.5 × 12.0; L/W 1.5 16–20 × 10–14 SB, SSB, SR: all + PSB: – | Upton et al. (1988) |
| Isospora mejiro | Z. japonicus Hawaii, USA | Subspheroidal 28.5 × 27.0; L/W 1.1 25–32 × 25–30 M, OR: both ‒ PG: + | Ovoidal 17 × 11; L/W 1.5 16–19 × 10–12 SB, SSB, SR: all + PSB: – | Upton et al. (1988) |
| I. oklahomaensis n. sp. | Poecile carolinensis (Audubon) Oklahoma, USA | Subspheroidal to ovoidal 32.1 × 28.3; L/W 1.1 29–36 × 27–30 M, OR: both ‒ PG: + | Ovoidal 18.4 × 11.8; L/W 1.6 16–20 × 9–13 SB, SSB, SR: all + PSB: ‒ | This report |
| I. parusae | Lophophanes dichrous (Blyth) India | Subspheroidal 24.2 × 20.8; L/W 1.2 23–28 × 20–23 M, PG: both + OR: ‒ | Pyriform 15 × 10; L/W 1.5 10–18 × 10 SB, SR: both + SSB, PSB: both – | Ray et al. (1952) |
| I. petrochelidon | Petrochelidon pyrrhonota (Vieillot) Colorado, USA | Subspheroidal to ovoidal 25.2 × 22.2; L/W 1.1 23–30 × 19–25 M, OR: both ‒ PG: + | Lemon-shaped 18.4 × 10.8; L/W 1.7 16–22 × 10–12 SB, SSB, SR: all + PSB: – | Stabler & Kitzmiller (1972) |
Measurements in μm.
Descriptions of oöcysts and sporocysts follow guidelines of Wilber et al. (1998) as follows: oocyst length (L) and width (W), their ranges and ratios (L/W), micropyle (M), oöcyst residuum (OR), polar granule(s) (PG), sporocyst (SP) length (L) and width (W), their ratio (L/W), Stieda body (SB), substieda body (SSB), parastieda body (PSB), and sporocyst residuum (SR).
Fecal samples were collected from native birds that originated from Hawaii and captives housed at the Dallas Zoo, Texas, USA.
Table 2.
Isosporans (as Isospora sp.)a reported from members of the family Paridae.
| Host (Describer) & Common Name | Locality | References |
|---|---|---|
|
| ||
| Baeolophus inornatus (Gambel) (Oak Titmouse) | California, USA | Boughton et al. (1938) |
| Cyanistes cyanus (Pallus) (Azure Tit) | France | Labbé (1893, 1896, 1899)b |
| Cyanistes coeruleus (L.) (Eurasian Blue Tit) | France; Czech Republic | Labbé (1893, 1896)b; Ryšavý (1954)c; Boughton et al. (1938); Svobodová (1994)d |
| Great Britain | Brown et al. (2010) e | |
| Parus major (L.) (Great Tit) | Germany; Czech Republic | Wasielewski (1904) f |
| Czech Republic | Ryšavý (1954)c; Schwalbach (1959)g | |
| Czech Republic | Svobodová (1994) d,h | |
| Great Britain | Brown et al. 2010)e | |
| Peripatus ater (L.) (Coal Tit) | Czech Republic | Ryšavý (1954)c |
| Poecile atricapilla (L.) (Black-capped chickadee) | Czech Republic | Ryšavý (1954)c |
| Poecile montanus (von Baldenstein) (Willow Tit) | Great Britain | Brown et al. (2010)e |
All of these isosporans are considered species inquirendae.
Synonym: Diplospora rivoltae Labbe, 1893, pro parte.
non Diplospora lacazei of Ryšavý, 1954.
Isospora sp. 25 of Svobodová, 1994.
Isospora sp. (Brown et al., 2010).
Synonym: Diplospora lacazei of Wasielewski, 1904.
non Diplospora sylvianthina Schwalback, 1959.
Isospora sp. 26 of Svobodová, 1994.
Nearly all of the older descriptions of bird coccidians (isosporans) from passeriform birds are problematic as they were classified as Isospora lacazei (Labbé, 1893), some other combination, or simply as Isospora sp. (Table 2). In addition, others have reported oöcyst measurements and photomicrographs of unnamed isosporans from two species of parid birds, Eurasian blue tit, Cyanistes caeruleus (L.) and great tit, Parus major (L.) from the Czech Republic (Svobodová, 1994; designated types 25 and 26, see her figs. 25‒26, Table 2) and oöcyst and sporocyst measurements of C. caeruleus, P. major, and willow tit, Parus montanus (von Baldenstein) from Great Britain (Brown et al., 2010, Table 2). However, these authors have simply designated these “forms” as Isospora sp. in these two documents, so there were no type specimens, line drawings, photosyntypes, stages in tissue sections, and/or oöcysts in preservative, so these isosporans must be considered species inquirendae. Furthermore, Svobodová (1994) and Brown et al. (2010) thought that these isosporans were “probably or indicated” new species but, unfortunately, they were never described.
When the new species is compared to I. parusae, there are three major differences: (1) oöcysts of the new species are considerably larger (32.1 × 28.3 vs. 24.2 × 20.8), (2) I. parusae possesses a micropyle that the new species clearly does not, and (3) the hosts are from different, widely-separated continents. In addition, when compared to coccidians reported from New World passerine birds of the Superfamily Sylvioidea (Hirundinidae, Paridae, Timaliidae, Zosteropidae) (Berto et al. 2011), oöcysts of the new species are the largest known from this taxon (Table 1). Therefore, we believe it is clear that I. oklahomaensis is a new species.
Discussion
Svobodová (1994) surveyed 13 additional parid birds, including six marsh tits, Parus palustris (L.), six P. montanus, and a single coal tit, Parus ater (L.) from the Czech Republic but none were passing coccidians at the time they were examined. It is obvious that novel coccidian parasites have been rarely described as novel species among members of the Paridae. This is an enigma because this family is a large and widespread group of 64 species of small passerine birds which mainly occur in Europe, Asia, North America, and Africa (AOS, 2020). Therefore, in the past, there should have been plenty of opportunity for these birds to be surveyed for coccidia and be reported as hosts compared to other passeriform species with fewer species serving as hosts of coccidians. Herein, we document the first coccidian from P. carolinensis as well as the first described from a parid bird in the Western Hemisphere.
Acknowledgements
We thank Drs. Scott L. Gardner and Gabor Racz (HWML) for expert curatorial assistance and Bruno P. Berto (Universidade Federal Rural do Rio de Janeiro, Brazil) for helpful advice on bird coccidians. CTM thanks Dr. Laurence M. Hardy (OMBS) for providing gratis housing and laboratory space.
Funding This study was funded, in part, by a grant from the National Institute of General Medical Sciences (2P20GM103432), National Institutes of Health (NIH) to RSS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with ethical standards
Ethical approval All applicable institutional, national and international guidelines for the care and use of animals were followed. The Arkansas Game and Fish Commission and Oklahoma Department of Wildlife Conservation provided Scientific Collecting Permits to CTM.
Data availability The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub:91487788-5767-4583-9427-1DAF555E6FD7
Conflict of interest The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Contributor Information
Chris T. McAllister, Science and Mathematics Division, Eastern Oklahoma State College, Idabel, OK 74745, USA.
R. Scott Seville, Department of Zoology and Physiology, University of Wyoming, Casper, WY 82601, USA.
References
- American Ornithological Society (AOS). 2020. Checklist of North and Middle America birds: Family Paridae. http://checklist.americanornithology.org/taxa/133. Accessed 20 May 2021. [Google Scholar]
- American Ornithologists’ Union (AOU). (1998). Check-list of North American birds. Seventh edition. American Ornithologists’ Union, Washington, D.C. http://www.aou.org/. Accessed 20 May 2021. [Google Scholar]
- Avibase. (2021). The World bird database. Carolina chickadee. https://avibase.bsc-eoc.org/species.jsp?avibaseid=F941AAD38D7BA54D. Accessed 20 May 2021. [Google Scholar]
- Berto BP, Flausino W, McIntosh D, Teixeira-Filho WL, & Lopes CWG (2011). Coccidia of New World passerine birds (Aves: Passeriformes): A review of Eimeria Schneider, 1875 and Isospora Schneider, 1881 (Apicomplexa: Eimeriidae). Systematic Parasitology 80, 159–204. 10.1007/s11230-011-9317-8. [DOI] [PubMed] [Google Scholar]
- Boughton DC, Boughton RB, & Volk J. (1938). Avian hosts of the genus Isospora (Coccidiida). Ohio Journal of Science 38, 149–163. [Google Scholar]
- Brown MA, Ball SJ, & Snow KR (2010). Coccidian parasites of British wild birds. Journal of Natural History, 44, 2669–2691. 10.1080/00222933.2010.501531 [DOI] [Google Scholar]
- Duszynski DW, Couch L, & Upton SJ (2000). The coccidia of Passeriformes (Isospora). Available at: https://www.k-state.edu/parasitology/worldcoccidia/PASSER01? Accessed 20 May 2021. [Google Scholar]
- Gosler A, & Clement P. (2007). “Family Paridae (Tits and Chickadees)”. In del Hoyo J, Elliott A, & Christie D. (eds.). Handbook of the Birds of the World. Volume 12: Picathartes to Tits and Chickadees. Barcelona: Lynx Edicions, pp. 662–709. [Google Scholar]
- ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé A. (1893). Sur les coccidies des oiseaux. Comptes Rendus de l’Academie des Sciences, Series 3 116, 1300–1303. [Google Scholar]
- Labbé A. (1896). Recherches zoologiques, cytologiques et biologiques sur les coccidies. Archives de Zoologie Experimentale et Generale 24, 517–654. [Google Scholar]
- Labbé A. (1899). Sporozoa. In Schulz FE & Bütschli O. (eds.). Das Tierreich. Eine Zusammenstellung und Kennzeichnung der rezenten Tierformen, Verlag von R. Friedländer und Sohn, Berlin, Germany, 180 p. [Google Scholar]
- McQuistion TE, McAllister CT, & Buice RE (1996). A new species of Isospora (Apicomplexa) from captive Pekin robins, Leiothrix lutea (Passeriformes: Sylviidae), from the Dallas Zoo. Acta Protozoologica 35, 73–75. [Google Scholar]
- Ray DK, Shivnani GA, Oommen M, & Bhaskaran R. (1952). A study on the coccidia of some Himalayan birds. Proceedings of the Zoological Society, Bengal, 5, 141–147. [Google Scholar]
- Ryšavý B. (1954). Pripevek k poznani kokcidii nasich i dovezenych obratlovcu. Ceskoslovenska Parasitologie 1, 131–134. [Google Scholar]
- Schneider A. (1881). Sur les psorospermies oviformes ou coccidies. Espèces novelles ou peu connues. Archives de Zoologie Expérmentale et Générale 9, 387–404. [Google Scholar]
- Schwalbach G. (1959). Untersuchungen und Beobachtungen an Coccidien der Gattungen Eimeria, Isospora und Caryospora bei Vogeln mit einer Beschreibung von sechzehn neuen Arten. Archiv für Protistenkunde 104, 431–494. [Google Scholar]
- Stabler RM, & Kitzmiller NJ (1972). Isospora petrochelidon sp. n. (Protozoa: Eimeriidae) from the cliff swallow, Petrochelidon pyrrhonota. Journal of Protozoology 19, 248–251. 10.1111/j.1550-7408.1972.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Svobodová M. (1994). Isospora, Caryospora and Eimeria (Apicomplexa: Eimeriidae) in passeriform birds from Czech Republic. Acta Protozoologica 33, 101–108. [Google Scholar]
- Terres JK (1980). The Audubon Society encyclopedia of North American birds (p. 109). Alfred A. Knopf, New York. [Google Scholar]
- Upton SJ, Marchiondo AA, & Williams RN (1988). New species of Isospora (Apicomplexa: Eimeriidae) from passeriform birds of Hawaii. Systematic Parasitology 12, 81–85. 10.1007/bf00000141. [DOI] [Google Scholar]
- Wasielewski Th. (1904). Studien und Mikrophotogramme sur Kenntnis der pathogenen Protozoen. 1. Heft. III. Uber den Erreger einer Coccidienseuche bei Vogeln (Diplospora lacazei) Leipzig, pp. 69–88. [Google Scholar]
- Wilber PG, Duszynski DW, Upton SJ, Seville RS, & Corliss JO (1998). A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae). Systematic Parasitology, 39, 113–135. 10.1023/a:1005914010087. [DOI] [Google Scholar]