Abstract
Background:
Prenatal air pollution exposure is associated with reductions in self-regulation and academic achievement. Self-regulation has been separately linked with academic achievement. Understudied, however, are the contributions of pollution exposure to inhibitory control, a facet of self-regulation, and whether pollution-related inhibitory control deficits are associated with impairment in academic achievement.
Methods:
Participants were recruited from a prospective birth cohort. Measures of prenatal airborne polycyclic aromatic hydrocarbons (PAH) during the third trimester of pregnancy, inhibitory control (NEPSY Inhibition) at mean age = 10.4 years, and Woodcock-Johnson Tests of Achievement-III at mean age = 13.7 were available for N = 200 participants. Multiple linear regression examined sex-dependent and sex independent associations among prenatal PAH, childhood inhibitory control, and academic achievement during adolescence, and whether childhood inhibitory control mediated associations between prenatal PAH and academic achievement during adolescence, controlling for ethnicity, maternal country of birth, language of prenatal interview, maternal marital status, maternal years of education, material hardship, quality of home caregiving environment, and early life stress.
Results:
Across all participants, higher prenatal PAH was significantly associated with worse spelling skills (WJ-III Spelling, β = −0.16, 95%Confidence Interval [CI]: 0.30, −0.02, p = .02). Trend level associations between higher prenatal PAH and worse reading comprehension (WJ-III Passage Comprehension, β = −0.13, 95%CI: 0.28, 0.01, p = .07) and math skills (WJ-III Broad Math, β = −0.11, 95%CI: 0.25, 0.03, p = .11) were detected. Across all participants, higher PAH was significantly associated with worse inhibitory control (β = −0.15, 95%CI: 0.29, −0.01 p = .03). Better inhibitory control was significantly associated with better reading comprehension (WJ-III Passage Comprehension, β = 0.22, 95%CI: 0.09, 0.36, p < .002) and math skills (WJ-III Broad Math Index, β = 0.32, 95%CI: 0.19, 0.45, p < .001), and trend level associations with better spelling skills (WJ-III Spelling, β = 0.12, 95%CI: 0.02, 0.26, p = .10). Inhibitory control significantly mediated PAH-related achievement effects for Passage Comprehension (β = −0.61, 95%CI: 1.49, −0.01) and Broad Math Index (β = −1.09, 95%CI: 2.36, −0.03).
Conclusions:
Higher prenatal PAH exposure and lower childhood inhibitory control were associated with worse spelling, passage comprehension, and math in adolescence. Notably, childhood inhibitory control mediated PAH exposure-related effects on achievement in adolescents. Identifying these potential exposure-related phenotypes of learning problems may promote interventions that target inhibitory control deficits rather than content specific deficits.
Keywords: Air pollution, Inhibitory control, Academic achievement, Environmental exposure, Learning disorder, Reading, Math
Air pollutants, including compounds such as polycyclic aromatic hydrocarbons (PAH), are neurotoxic carcinogens produced during incomplete combustion of fossil fuels, tobacco, and other organic material (Boström et al., 2002). While all humans are exposed to PAH through air and dietary sources, differential placement of outdoor pollution sources increases risk for exposure among low-income, urban, and minority communities (Heritage, 1992; Metzger et al., 1995; Olden and Poje, 1995; Pirkle et al., 1996; Wagenknecht et al., 1993; Wernette and Nieves, 1992). Emerging evidence suggests that prenatal PAH exposure has deleterious effects on child health and development (Grandjean and Landrigan, 2006; Perera et al., 2005; Perera et al., 2006). During the fetal period and early childhood years, the brain is developing rapidly and thus is vulnerable to neurotoxic insults that may subsequently manifest as adverse physical and mental health outcomes in childhood and adulthood (Shonkoff et al., 2012; Stein et al., 2002).
Prenatal and postnatal exposure to air pollutants have been linked to lowered academic achievement in both males and females (Grineski et al., 2020; Lett et al., 2017; Stingone, McVeigh, & Claudio, 2016, 2017), highlighting the costs of high levels of air pollution to society. The developmental pathways linking exposure to achievement, however, remain understudied. Prior findings suggest strong links between self-regulation and academic achievement in both males and females (Matthews et al., 2009; McClelland and Cameron, 2011; McClelland and Wanless, 2012). One possibility is that air pollution operates on academic achievement through its effects on self-regulation. One prior study showed associations between prenatal exposure to fine particulate matter and children’s self-regulation as measured by a response inhibition task (Guxens et al., 2018). Our previous work has demonstrated an association between prenatal PAH exposure and impaired development of parent-reported self-regulation of thought, behavior, and emotion across childhood (Margolis et al., 2016). To date, some studies have also identified sex-specific effects of prenatal air pollutant exposure on child executive functions that contribute to self-regulatory capacity in boys (Chiu et al., 2013, 2016; Cowell et al., 2015; Rivas et al., 2019). Moreover, both human studies and animal models have documented effects of prenatal PAH exposure on brain structures that support self-regulatory processes and behavioral outcomes, such as hyperactivity (Lapole et al., 2007; Patten et al., 2020; Peterson et al., 2015), underscoring the effects of exposure to PAH on self-regulation.
Studies have not yet examined the effects of prenatal exposure to PAH on child performance using an inhibitory control task, specifically. Inhibitory control is commonly operationalized by tasks that measure an individual’s ability to inhibit a prepotent response in favor of a less habitual response (Diamond, 2013). Although it is known that air pollution exposure and poor inhibitory control are each associated with worse academic performance, and that air pollution exposure is associated with reduced executive functions, almost nothing is known about the shared versus distinct contributions of prenatal air pollution exposure and inhibitory control to academic achievement. Leveraging a large prospective birth cohort, we examined associations between prenatal exposure to PAH and performance on tests of reading and math skills as well as performance on a measure of inhibitory control in late childhood. We further examined whether these effects were sex-specific, given previously observed sex-dependent neurodevelopmental effects of prenatal air pollution exposure. First, consistent with prior studies we hypothesized that effects of PAH exposure on reading and math would be detected in both males and females. We further hypothesized that effects of PAH exposure on inhibitory control would be detected only in males. Third, we hypothesized that childhood inhibitory control would be positively associated with performance on measures of math and reading skill during adolescence. Last, we hypothesized that inhibitory control would mediate associations between prenatal exposure and academic achievement.
1. Method
1.1. Sample
Participants in this study were recruited from the Mothers and Newborns prospective birth cohort (Perera et al., 2006) followed by the Columbia Center for Children’s Environmental Health (N = 727). The cohort enrolled pregnant women recruited from obstetrics and gynecology clinics at New York Presbyterian Hospital and Harlem Hospital between 1998 and 2006. Participants were self-reported Black and Hispanic/Latinx women who resided in Washington Heights, Central Harlem, or the South Bronx. Exclusion criteria included active maternal smoking during current pregnancy and documented or reported substance abuse, gestational diabetes, hypertension, or human immunodeficiency virus. The study was approved by the Institutional Review Board of Columbia University and each parent and child provided written consent and assent, respectively.
Of the 727 participants in Mothers and Newborns, 683 had complete prenatal airborne PAH data (four participants with PAH scores greater than 3.5 standard deviations from the mean were excluded from analyses; Supplementary Fig. S1), 356 had complete PAH and inhibitory control data derived from the NEPSY Inhibition task, and 200 of this subset had complete data from the Woodcock-Johnson Tests of Achievement-III (Fig. S1). With the exception of being younger, the subset of children with available achievement data did not differ demographically from the larger set with inhibitory control data (Table 1).
Table 1.
Characteristics of sample participants (N = 683) with prenatal air pollution data. Comparisons are provided based on availability of outcome measures (inhibitory control and academic achievement).
| N = 683 | Participants with PAH/NEPSY (n = 356) | Participants with PAH but without NEPSY (n = 327) | Difference between samples |
|---|---|---|---|
| Mean (SD)/N (%) | |||
| Sex (Female) | 193 (54.2%) | 158 (48.3%) | χ = 2.37, p = .12 |
| Maternal years of education | 11.8 (2.2) | 11.9 (2.1) | t = −0.46, p = .64 |
| HOME | 39.56 (5.4) | 38.3 (1.35) | t = 0.82, p = .42 |
| Material hardship | 0.62 (0.74) | 0.63 (0.20) | t = −0.23, p = .81 |
| Ethnicity (Dominican) | 223 (62.6) | 215 (66.4) | χ = 0.87, p = .35 |
| Log-transformed PAH | 0.83 (0.68) | 0.87 (0.76) | t = −0.75, p = .45 |
| Age at NEPSY | 10.4 (1.2) | – | – |
| NEPSY INN SS | 7.5 (3.5) | – | – |
| NEPSY INI SS | 6.8 (3.2) | – | – |
| N = 356 | Participants with PAH/NEPSY/WJ (n = 200) | Participants with PAH/NEPSY but without WJ (n = 156) | Difference between samples |
| Mean (SD)/N (%) | |||
| Sex (Female) | 118 (59.0%) | 75 (55.1%) | χ = 0.49, p = .48 |
| Maternal years of education | 11.8 (2.3) | 11.8 (2.1) | t = −0.35, p = .73 |
| HOME | 39.7 (5.4) | 39.4 (5.5) | t = 0.50, p = .61 |
| Material hardship | 0.58 (0.58) | 0.68 (0.68) | t = −1.2, p = .22 |
| Ethnicity (Dominican) | 136 (68.0) | 87(55.8) | χ = 5.6, p = .02 |
| Log-transformed PAH | 0.85 (0.65) | 0.81 (0.71) | t = 0.47, p = .64 |
| Age at NEPSY (years) | 10.3 (1.0) | 10.7 (1.4) | t = −3.29, p = .001 |
| NEPSY INN SS | 7.7 (3.5) | 7.1 (3.5) | t = −1.6, p = .11 |
| NEPSY INI SS | 6.8 (2.9) | 6.8 (3.6) | t = 0.06, p = .95 |
| Age at WJ (years) | 13.7 (0.6) | – | – |
| WJ Basic Reading SS | 95.1 (12.1) | – | – |
| WJ Passage Comp SS | 86.8 (13.0) | – | – |
| WJ Spelling | 98.8 (14.5) | ||
| WJ Broad Math SS | 89.5 (15.3) | – | – |
Notes. From N = 356: 2 participants (0.6%) were missing maternal education data, 57 (16%) were missing HOME, 57 (16%) were missing material hardship data. From N = 327: 3 participants (0.9%) were missing for ethnicity. To address potential outliers, one Broad Math score (SS = 39), 2 Basic Reading Index scores (SS = 16, 36), 4 Passage Comprehension scores (SS = 14, 18, 30, 41), and 2 Spelling scores (SS = 22, 38) were Winsorized because they were more than 3 standard deviations from the mean. PAH = polycyclic aromatic hydrocarbon; HOME=Home Observation for Measurement of the Environment; INN= Inhibition Naming; INI=Inhibition-Inhibition; Passage Comp = Passage Comprehension; SS = scaled/standard score; WJ= Woodcock-Johnson.
1.2. PAH assessment
During the third trimester of pregnancy, maternal air data were acquired and analyzed as previously described (Perera et al., 2006; Tonne et al., 2004). Briefly, mothers received an air monitoring backpack that collected airborne vapors, aerosols, and Particulate Matter 2.5 (PM2.5). Particle bound and volatile and semi-volatile PAH were extracted from the filter and PUF via a Soxhlet Extractor and extracts were assayed by GC-MS for pyrene and eight non-volatile, carcinogenic PAH at Southwest Research Institute: (benz[a]anthracene, benzo[a]pyrene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, indeno-[1,2,3-cd]pyrene, disbenz[a,h]anthracene, and benzo[g,h,i]perylene). Exposure was computed as the log transformed sum of these eight carcinogenic PAHs (see Supplementary Methods).
1.3. Inhibitory control
Inhibitory control was measured during late childhood (range 8–14 years old, mean = 10.4 years) by the NEPSY-II Inhibition subtest (Korkman et al., 2007), which assesses a participant’s ability to inhibit automatic responses and instead activate a novel response. First, the child is instructed to name shapes (squares and circles) or the direction of arrows as fast as possible (Inhibition Naming; INN). Next, the child is instructed to name the other shape or arrow direction instead, i.e. saying “square” for each circle and “circle” for each square (Inhibition; INI). Raw scores were converted to age-adjusted scaled scores based on the NEPSY-II normative sample.
1.4. Academic achievement
Reading and math skills were measured using the Woodcock-Johnson Tests of Achievement-III (WJ-III) during adolescence (range 13–15 years old, mean = 13.7), on average 3.4 years (standard deviation = 1.1 years) and always after inhibitory control was measured. Raw scores were converted to age-adjusted standard scores based on the WJ-III normative sample (Woodcock et al., 2000).
Components of reading skills including decoding, encoding, and comprehension were measured by the Basic Reading Index, a standard score that combines performance across Letter-Word Identification and Word Attack subtests, and by the Spelling and Passage Comprehension subtests, analyzed separately. Letter-Word Identification requires the participant to read words aloud, testing single word reading. Word Attack requires the participant to read pseudowords (nonsense) words aloud, testing the ability to apply phonetic rules to decode unknown words. These subtests are combined in a weighted normed Basic Reading Index that reflects an individual’s ability to decode words. The Spelling subtest measures encoding by requiring the child write out the spelling of words spoken by the examiner. Passage Comprehension measures the ability to read a short passage and provide a missing word that completes the sentence.
Components of math skills including problem solving, calculating, and fluency were measured by the Broad Math Index. The Index combines performance across three subtests, Applied Problems, Calculation, and Math Facts Fluency. Applied Problems measures the participant’s ability to solve word problems; the problems are simultaneously read aloud by the examiner and presented on a stimulus book, reducing memory requirements. Calculation presents the participant with computation problems of increasing complexity in a paper-and-pencil format. Math Facts Fluency challenges participants to solve as many one- and two-digit addition, subtraction, and multiplication problems as possible in 3 min.
1.5. Statistical analyses
The distribution of prenatal PAH exposure was examined and log-transformed as in previous studies (Perera et al., 2012) (Fig. S2). To minimize the influence of potential outliers, academic performance scores more than three standard deviations from the mean (z-score>3) were Winsorized to the next most extreme non-outlier value (Table 1).
To address our first hypothesis, multiple linear regression analyses were used to examine sex-dependent effects of prenatal airborne PAH exposure on academic achievement (WJ-III Basic Reading, Passage Comprehension, Spelling, Broad Math) during adolescence. Next, we tested sex-dependent effects of PAH on childhood inhibitory control (NEPSY-II Inhibition-Inhibition (INI) scaled score), additionally controlling for baseline naming speed (NEPSY-II Inhibition-Naming (INN) scaled score). To address our third hypothesis, multiple linear regression tested the sex-dependent effects of childhood inhibitory control on academic achievement during adolescence. Non-significant sex interaction terms (p < .05) were dropped from final models. Exploratory analyses examined associations between PAH or inhibitory control and individual achievement subtests that contributed to any significant composite scores.
Finally, we used a nonparametric bootstrapping method (5000 simulations) implemented in the R (version 4.0.0) mediation package (version 4.5.0) to test the third hypothesis that childhood inhibitory control would serve as a mediator between prenatal PAH exposure and academic achievement in adolescence. The indirect effect of PAH on achievement through a mediator (inhibitory control) was defined as the average causal mediation effect (ACME). The ACME equals the product, ab, of the regression coefficient, a, relating the independent variable (PAH) to the mediator, and the regression coefficient, b, relating the mediator to the dependent variable (WJ-III achievement) with the independent variable as a covariate. Whether the ACME was significantly different from zero was tested using a bootstrapping method with 5000 resamples (Tingley et al., 2014). We tested mediation for academic skills that were significantly associated with inhibitory control whether or not there was a significant total effect (PAH on achievement). This criterion was selected because mediation can occur in the absence of a significant total effect (Hayes and Rockwood, 2017).
All analyses controlled for ethnicity (Hispanic/Latinx vs. non-Hispanic/Latinx Black), and commonly included potential confounds of PAH exposure (Pagliaccio et al., 2020) or predictors of academic achievement: maternal country of birth (USA vs. other), language of prenatal interview (English vs. other), maternal marital status (married or live with partner >7 yrs vs. other) at prenatal visit, material hardship at prenatal visit (see Supplementary Methods), maternal years of education at prenatal visit, quality of home caregiving environment at child age 3 (Home Observation for Measurement of the Environment [HOME (Bradley, 1994);]), and early life stress at child age 5 (Pagliaccio et al., 2020). Age was not included as a covariate as all dependent variables were norm-referenced standardized, age-adjusted scores. Missing values were multiple imputed with the MICE package in R (van Buuren &Groothuis-Oudshoorn 2011). We report standardized regression coefficient estimates, significance tests were two-tailed, and alpha was set at p < .05.
2. Results
2.1. Participants
Children with available PAH and NEPSY data (n = 356) did not differ systematically from those without NEPSY-II data (n = 327, Table 1). Among children with available PAH exposure and inhibitory control data, those with academic achievement data (n = 200) were younger at NEPSY-II completion than those without achievement data (n = 156) but did not differ on other measures (Table 1).
2.2. Prenatal PAH and academic achievement
There were no significant prenatal PAH-by-sex interaction effects on any measure of achievement (interaction p-values > .31) and, thus, this term was dropped from all models. Higher prenatal PAH exposure was associated with worse Spelling (β = −0.16, 95%CI: 0.30,−0.02, p = .02; Fig. 1A). Trend level findings were observed for Passage Comprehension (β = −0.13, 95%CI: 0.28, 0.01, p = .07), and Broad Math Index (β = −0.11, 95%CI: 0.25, 0.03, p = .11). No significant associations were detected between prenatal PAH and Basic Reading (β = −0.06, 95% CI: 0.21, 0.08, p = .40).
Fig. 1.
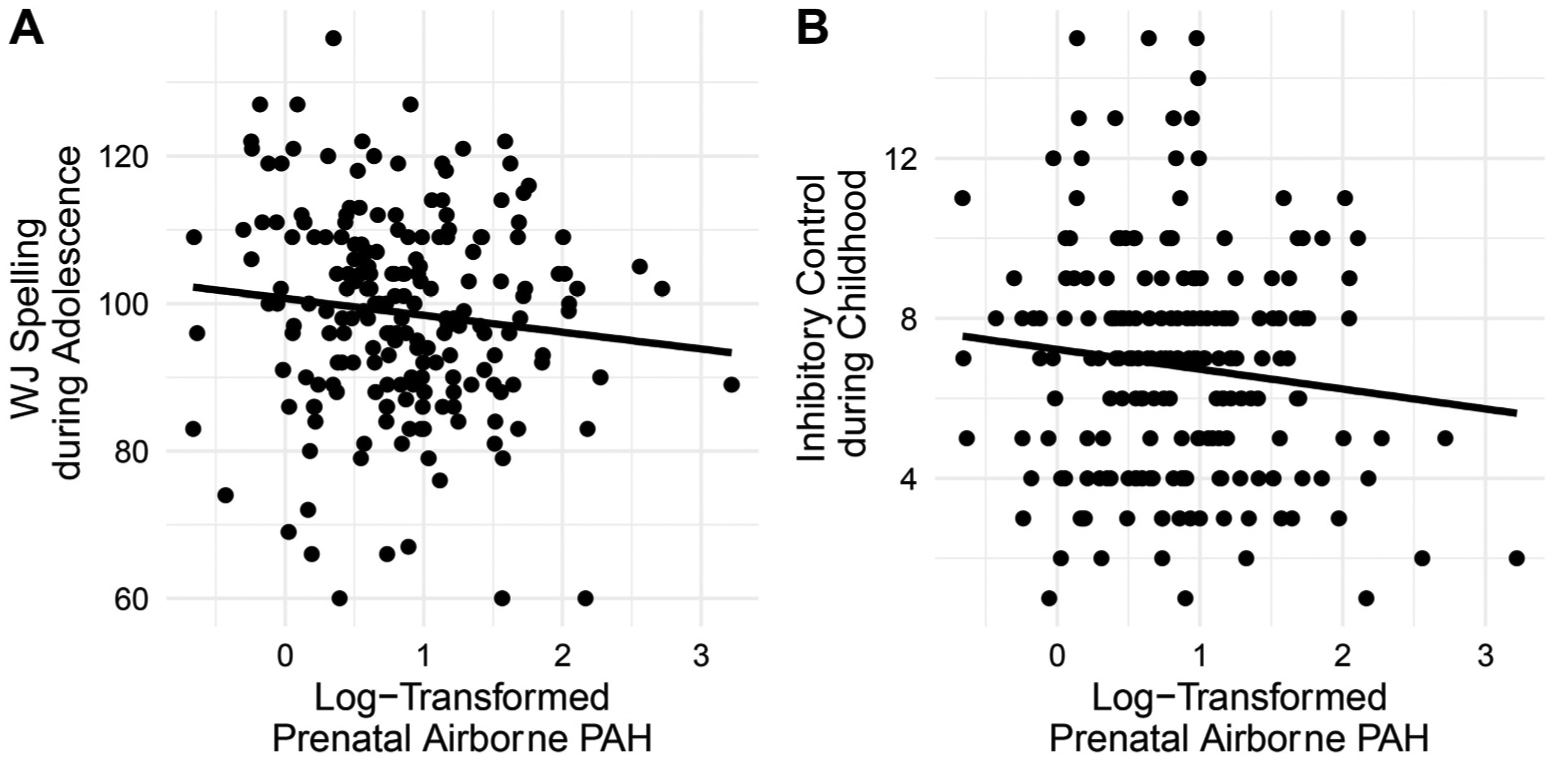
Higher prenatal airborne PAH is associated with A) worse spelling across all participants and B) worse inhibitory control, as measured with the NEPSY Inhibitory Control task. Figures present unresidualized analyses. PAH = polycyclic aromatic hydrocarbon.
2.3. Prenatal PAH and inhibitory control
Higher prenatal PAH exposure was associated with worse performance on the inhibitory control task (β = −0.15, 95%CI: 0.29, −0.01, p < .03, Fig. 1B). The prenatal PAH-by-sex interaction was not significant (β = −0.0007, 95%CI: 0.30, 0.30, p > .9).
2.4. Inhibitory control and academic achievement
There were no significant inhibitory control-by-sex interaction effects on any measure of achievement (interaction p-values > .30) and, thus, this term was dropped from all models. Better performance on the inhibitory control task was associated with better performance on the Passage Comprehension subtest (β = 0.22, 95%CI: 0.09, 0.36, p < .002) and the Broad Math Index (β = 0.32, 95%CI: 0.19, 0.45, p < .001 Fig. 2; Table 2) as well as a trend level association between inhibitory control and the Spelling subtest (β = 0.12, 95%CI: 0.02, 0.25, p = .10). Inhibitory control was not associated with the Basic Reading Index score (β = 0.03, 95% CI: 0.12, 0.17, p = .72). Exploratory analyses of the separate subtests that comprise the Broad Math Index were each also associated with between inhibitory control (Supplementary Results).
Fig. 2.
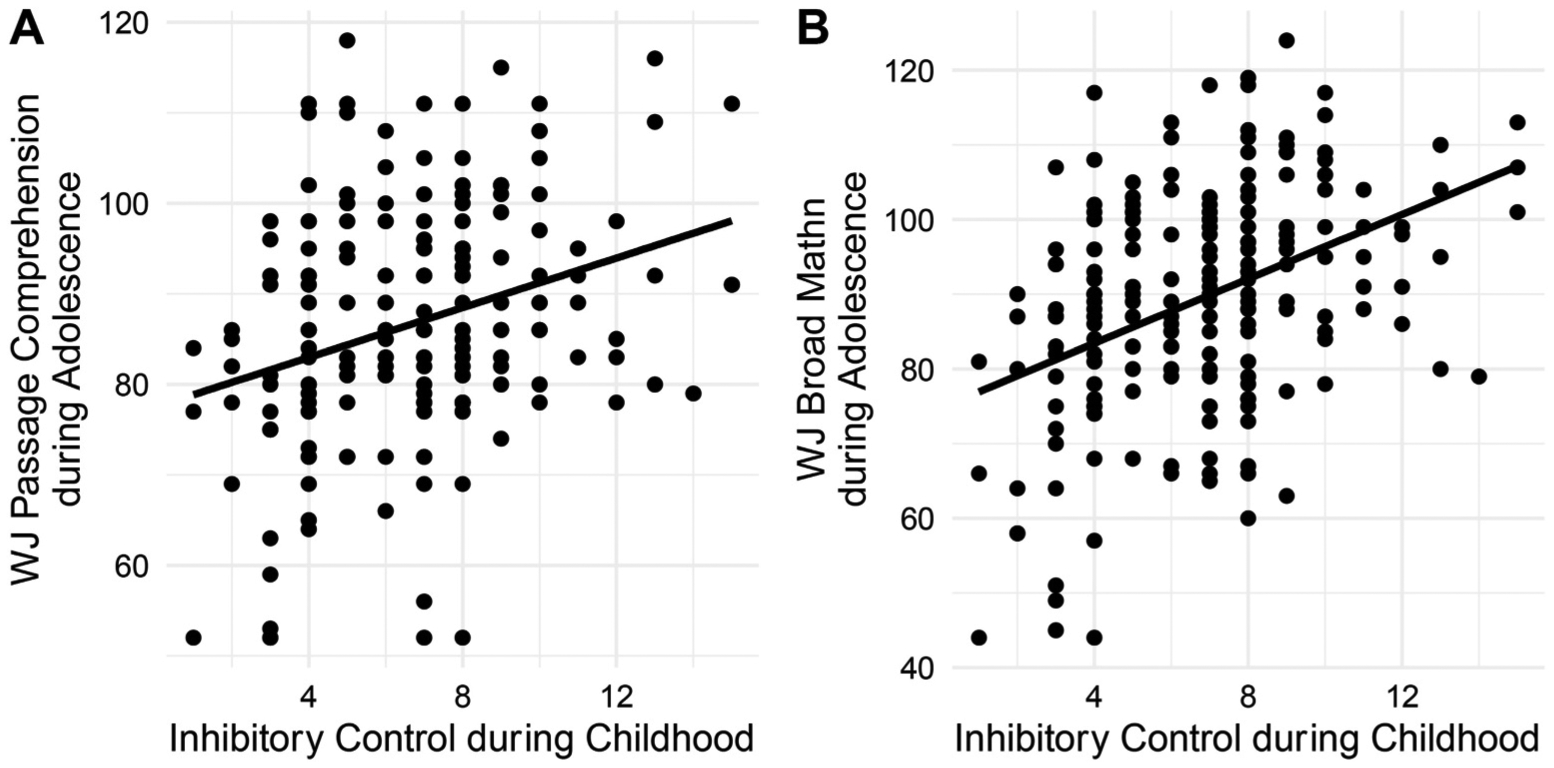
Childhood inhibitory control, as measured with the NEPSY Inhibitory Control task, is positively associated with adolescent A) passage comprehension and B) broad math scores. Figures present unresidualized analyses. WJ=Woodcock-Johnson-III.
Table 2.
Effects of inhibitory control on achievement skills in N = 200 participants.
| N = 200 | Effect of NEPSY INI | |||
|---|---|---|---|---|
| β | t | 95% CI | p | |
| WJ Basic Reading | 0.03 | 0.36 | −0.12, 0.17 | .71 |
| WJ Passage Comprehension | 0.22 | 3.18 | 0.09, 0.36 | <.001 |
| WJ Spelling | 0.12 | 1.68 | −0.02, 0.26 | .10 |
| WJ Broad Math | 0.32 | 4.82 | 0.19, 0.45 | <.001 |
Basic Reading, Spelling, Passage Comprehension, and Broad Math served as the dependent variables in our main analyses. All models control for sex, ethnicity, NEPSY baseline naming performance, maternal country of birth (USA vs. other), language of prenatal interview (English vs. other), maternal marital status (married or live with partner >7 yrs vs. other) at prenatal visit, material hardship at prenatal visit, maternal years of education at prenatal visit, quality of home caregiving environment at child age 3 (Home Observation for Measurement of the Environment [HOME (Bradley, 1994);]), and early life stress at child age 5 (Pagliaccio et al., 2020), reported betas are standardized. WJ=Woodcock-Johnson-III; INI= NEPSY Inhibition-Inhibition; CI = confidence interval.
2.5. Mediation models
Inhibitory control significantly mediated associations between prenatal PAH and Passage Comprehension (β = −0.61, 95%CI: 1.5, −0.01) as well as Broad Math Index (β = −1.1, 95%CI: 2.4, −0.03, Table 3).
Table 3.
Effects of prenatal airborne pollution on early adolescent academic performance through childhood inhibitory control in N = 200 participants.
| Outcome | Estimate | 95% CI |
|---|---|---|
| WJ Passage Comprehension | ||
| ACME | −0.61 | −1.49, −0.01 |
| ADE | −2.30 | −5.05, 0.50 |
| Total Effect | −2.91 | −5.62, −0.09 |
| Proportion Mediated | .13 | −0.07, 1.15 |
| WJ Broad Math | ||
| ACME | −1.09 | −2.36, −0.03 |
| ADE | −1.87 | −4.97, 1.31 |
| Total Effect | −2.96 | −6.20, 0.41 |
| Proportion Mediated | −0.03 | −0.82, 2.18 |
Passage Comprehension and Broad Math served as the dependent variables in our main analyses. All models control for sex, ethnicity, NEPSY baseline naming performance, maternal country of birth (USA vs. other), language of prenatal interview (English vs. other), maternal marital status (married or live with partner >7 yrs vs. other), material hardship at prenatal visit, maternal years of education at prenatal visit, quality of home caregiving environment at child age 3 (Home Observation for Measurement of the Environment [HOME (Bradley, 1994);]), and early life stress at child age 5 (Pagliaccio et al., 2020). The average causal mediation effect is defined as the product of a) the regression coefficient predicting inhibitory control by prenatal PAH exposure and b) the regression coefficient predicting academic skill by inhibitory control, controlling for the effects of prenatal PAH exposure. Confidence intervals and p-values were generated using 5000 bootstrapping simulations in the “mediation” package in R. WJ=Woodcock-Johnson-III; INI= NEPSY Inhibition-Inhibition; CI = confidence interval.
3. Discussion
Using a large longitudinal birth cohort, we provide evidence for a pathway by which prenatal exposure to PAH affects academic achievement through impaired inhibitory control. Specifically, we found that higher prenatal exposure to PAH was associated with worse inhibitory control during late childhood and worse academic skills in early adolescence, regardless of sex. Next, we show that more difficulty with inhibitory control in late childhood was associated with more problems with reading comprehension and math achievement in adolescence. Finally, we provide evidence that late childhood inhibitory control mediates associations between prenatal PAH exposure and early adolescent reading comprehension and math skills, elucidating cognitive processes which link prenatal PAH exposure and behavioral outcomes. Our findings suggest that exposure-related phenotypes of learning problems are important to consider when evaluating student’s learning problems and formulating treatment plans. Problems in academic skills related to environmental exposures may require intervention focused on inhibitory control problems, as shown here, rather than on content related skill deficits as is typical in interventions designed to address learning disabilities.
We detected significant associations between prenatal PAH and early adolescent achievement including spelling, reading comprehension, and math skills. Our findings are consistent with prior studies showing associations between prenatal exposure to air pollutants and achievement (Grineski et al., 2020; Lett et al., 2017; Stingone, McVeigh, & Claudio, 2016, 2017). Childhood inhibitory control was also associated with prenatal PAH exposure as well as with early adolescent academic achievement. Our findings are again consistent with prior studies showing associations between prenatal exposure to PAH and self-regulation (Margolis et al., 2016), and between inhibitory control and reading comprehension (Kieffer et al., 2013) as well as math skills (Blair and Razza, 2007). Herein, we show how prenatal environmental exposures may catalyze such developmental cascades. PAH exposure may compromise childhood inhibitory control, altering the foundation upon which later academic skills are built. Alternatively, inhibitory control problems could be stable over time, exerting differential effects on academic skills depending on the developmental stage of the particular skill. For example, our test of math achievement assessed presumably newly acquired skills that require multistep problem solving, which might tap inhibitory control. Similarly, reading comprehension requires monitoring one’s responses and maintaining goal directed activity, both of which require a sufficient level of inhibitory control. Thus, these two domains of academic achievement are likely vulnerable to inhibitory control deficits during early adolescence. In contrast, we did not observe associations between inhibitory control and word reading, perhaps because word reading has already become automatized (routinized or well-established) by age 13–15 years (Margolis et al., 2019). Consistent with this interpretation, one study in a younger sample did find associations between inhibitory control and word reading (van der Schoot et al., 2004). Notably, inhibitory control was associated with math fluency (rapid access to math facts) in our study, which could be seen as a parallel skill to single word reading, thereby contradicting our theory. Alternatively, math fluency may not be as automatized as word reading at this age, supporting our theory. Future studies should examine the modulatory role of automaticity, expertise and/or age on associations between inhibitory control and multiple dimensions of achievement.
Contrary to our hypothesis, we did not detect sex-specific effects of prenatal PAH on inhibitory control. Although other studies have reported sex specific effects of black carbon and PM2.5 on attention problems in males, e.g., increased omission errors, commission errors, reaction time and reaction variability during the Conners’ Continuous Performance Test (Chiu et al., 2013, 2016), we did not detect sex-specific effects on inhibitory control. Our findings align in part with our prior study showing prenatal PAH DNA adducts were associated with altered development of self-regulatory capacity as measured by parent report regardless of sex (Margolis et al., 2016). In combination, these extant findings fit within the larger epidemiologic picture that prenatal exposure to air pollution, as measured by PAH or PM, is associated with increased levels of behaviors associated with Attention Deficit Hyperactivity Disorder (ADHD) symptoms (Min and Min, 2016; Perera et al., 2014; Perera et al., 2012). ADHD symptoms of impulsivity may derive from deficits in inhibitory control as detected herein, whereas symptoms of inattention may derive from deficits in sustained attention. More fine-grained analyses of components of air pollution on neurocognitive capacities and behaviors associated with distinct ADHD subtypes are needed to design more targeted interventions.
The biological mechanism underlying the sex-specific effects of exposure to PAH remains unknown as animal models have not examined effects of prenatal PAH exposure on inhibitory control. One study showed no effects of prenatal elemental carbon exposure on inhibitory control in rodents suggesting that the neurotoxicity of air pollution exposure derives from other constituent species (Morris-Schaffer et al., 2019). Sex-specific effects of air pollutants do appear to be brain region specific (Klocke et al., 2018), providing potential mechanisms through which exposure may differentially affect the neural circuitry underlying attention problems in males and females, but yield similar behavioral outcomes. However, human neuroimaging studies of prenatal PAH exposure have been conducted with small sample sizes that were underpowered to detect sex-specific effects (Peterson et al., 2015). Larger studies are needed to examine sex-specific effects of exposure on brain and behavioral outcomes.
Our study has some limitations such as our time-limited measurement of PAH and inability to control for postnatal or concurrent PAH exposure. Although we controlled for many factors known to be associated with performance on tests of academic achievement including psychosocial stressors, mother’s nativity, mother’s preferred language during the prenatal interview, and marital status, other unmeasured factors, including postnatal exposure, may contribute to the variance in test scores. Nevertheless, a growing body of literature shows the deleterious health effects of prenatal exposure to air pollution on child health outcomes, including academic achievement (Lu, 2020). Given effects of environmental injustice, by enrolling women from low-income and race/ethnic minority backgrounds living in Northern Manhattan and Washington Heights in NYC, the cohort was enriched for PAH exposure and potential biological vulnerability. This strategy has allowed us to examine the effects of these inequitably distributed exposures on health disparities and now academic disparities in our sample, providing valuable information on the origins of learning challenges in understudied groups. Our findings should be viewed as preliminary and requiring replication, as the primary analyses are not corrected for multiple comparisons and some are at trend level. Nevertheless our findings add to the growing literature on the detrimental effects of air pollution on development with fine-grained, direct measurement of exposure, academic skills, and a mediating neurocognitive factor.
In conclusion, identification of exposure-related phenotypes of learning problems may help develop more targeted interventions for these academic difficulties. For example, based on our findings, intervention may need to target inhibitory control deficits rather than, or in conjunction with, content specific deficits. Such findings underscore the need for neuropsychological interventions that are designed specifically for individuals with academic problems, distinct from existing educational interventions targeting academic content areas. Last, reducing levels of air pollution may also lead to improvement in children’s academic achievement.
Supplementary Material
Acknowledgements
Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA). NIEHS K23ES026239, R01 ES030950, R01ES014393, RC2ES018784, R01ES13163, R01ES08977, 5P50ES009600NIEHS/EPA, P01ES09600/RD82702701, NIEHS/EPA P01ES09600/RD832141, NIEHS/EPA P01ES09600/RD834509, NIEHS/EPA P50ES09600/RD83615401, the New York Community Trust, Trustees of the Blanchette Hooker Rockefeller Fund, and the John and Wendy Neu Foundation.
Abbreviations:
- PAH
Polycyclic Aromatic Hydrocarbons
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111570.
References
- Blair C, Razza RP, 2007. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 78, 647–663. 10.1111/j.1467-8624.2007.01019.x, 0009–3920 (Print). [DOI] [PubMed] [Google Scholar]
- Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Westerholm R, 2002. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect 110, 451–488. 10.1289/ehp.110-1241197. Suppl 3(Suppl 3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, 1994. The Home Inventory: review and reflections. Adv. Child Dev. Behav 25, 241–288. [DOI] [PubMed] [Google Scholar]
- Chiu Yueh-Hsiu Mathilda, Bellinger David C., Coull Brent A., Anderson Shawn, Barber Rachel, Wright Robert O., Wright Rosalind J., 2013. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ. Health Perspect 121 (7), 859–864. 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Yueh-Hsiu Mathilda, Hsu Hsiao-Hsien Leon, Coull Brent A., Bellinger David C., Kloog Itai, Schwartz Joel., Wright Rosalind J., 2016. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ. Int 87, 56–65. 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Bellinger DC, Coull BA, Gennings C, Wright RO, Wright RJ, 2015. Associations between prenatal exposure to black carbon and memory domains in urban children: modification by sex and prenatal stress. PloS One 10 (11), e0142492. 10.1371/journal.pone.0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond Adele, 2013. Executive functions. Annu. Rev. Psychol 64 (1), 135–168. 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ, 2006. Developmental neurotoxicity of industrial chemicals. Lancet 368 (9553), 2167–2178. 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grineski Sara E., Collins Timothy W., Adkins Daniel E., 2020. Hazardous air pollutants are associated with worse performance in reading, math, and science among US primary schoolchildren. Environ. Res 181, 108925. 10.1016/j.envres.2019.108925. [DOI] [PubMed] [Google Scholar]
- Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, El Marroun H, 2018. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatr 84, 295–303, 1873–2402 (Electronic). [DOI] [PubMed] [Google Scholar]
- Hayes Andrew F., Rockwood Nicholas J., 2017. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav. Res. Ther 98, 39–57. 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Heritage J, 1992. Environmental protection—has it been fair? EPA J. 18. [Google Scholar]
- Kieffer Michael J., Vukovic Rose K., Berry Daniel, 2013. Roles of attention shifting and inhibitory control in fourth-grade reading comprehension. Read. Res. Q 48 (4), 333–348. [Google Scholar]
- Klocke C, Sherina V, Graham UM, Gunderson J, Allen JL, Sobolewski M, CorySlechta DA, 2018. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal. Toxicol 30 (9–10), 381–396. 10.1080/08958378.2018.1533053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S, 2007. NEPSY, second ed. Harcourt Assessment, San Antonio, TX. [Google Scholar]
- Lapole D, Rychen G, Grova N, Monteau F, Le Bizec B, Feidt C, 2007. Milk and urine excretion of polycyclic aromatic hydrocarbons and their hydroxylated metabolites after a single oral administration in ruminants. J. Dairy Sci 90 (6), 2624–2629. 10.3168/jds.2006-806. [DOI] [PubMed] [Google Scholar]
- Lett LA, Stingone JA, Claudio L, 2017. The combined influence of air pollution and home learning environment on early cognitive skills in children. Int. J. Environ. Res. Publ. Health 14 (11). 10.3390/ijerph14111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Jackson G., 2020. Air pollution: a systematic review of its psychological, economic, and social effects. Curr. Opin. Psychol 32, 52–65. 10.1016/j.copsyc.2019.06.024. [DOI] [PubMed] [Google Scholar]
- Margolis AE, Herbstman JB, Davis KS, Thomas VK, Tang D, Wang Y, Rauh VA, 2016. Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J. Child Psychol. Psychiatry Allied Discip 57 (7), 851–860. 10.1111/jcpp.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Davis KS, Thomas L, Banker SM, Cyr M, Marsh R, 2019. Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging Behav. 10.1007/s11682-019-00083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JS, Ponitz Claire Cameron, Morrison Frederick J., 2009. Early gender differences in self-regulation and academic achievement. J. Educ. Psychol 101 (3), 689–704. 10.1037/a0014240. [DOI] [Google Scholar]
- McClelland Megan M., Cameron Claire E., 2011. Self-regulation and academic achievement in elementary school children. N. Dir. Child Adolesc. Dev 2011 (133), 29–44. 10.1002/cd.302. [DOI] [PubMed] [Google Scholar]
- McClelland Megan M., Wanless Shannon B., 2012. Growing up with assets and risks: the importance of self-regulation for academic achievement. Res. Hum. Dev 9 (4), 278–297. 10.1080/15427609.2012.729907. [DOI] [Google Scholar]
- Metzger R, Delgado JL, Herrell R, 1995. Environmental health and Hispanic children. Environ. Health Perspect 103 (Suppl. 6), 25–32. 10.1289/ehp.95103s625. Suppl 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Jin-young, Min Kyoung-bok, 2016. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int 99 10.1016/j.envint.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Morris-Schaffer Keith, Merrill Alyssa, Jew Katrina, Wong Candace, Conrad Katherine, Harvey Katherine, Slechta Cory, Deborah A, 2019. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Part. Fibre Toxicol 16 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K, Poje J, 1995. Environmental justice and environmental health. Bull. Soc. Occup. Environ. Health (4), 3–4. [Google Scholar]
- Pagliaccio D, Herbstman JB, Perera F, Tang D, Goldsmith J, Peterson BS, Margolis AE, 2020. Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and Thought Problems in late childhood. J. Child Psychol. Psychiatry Allied Discip 10.1111/jcpp.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten KT, Gonźalez EA, Valenzuela A, Berg E, Wallis C, Garbow JR, Lein PJ, 2020. Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl. Psychiatry 10 (1), 166. 10.1038/s41398-020-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Rauh V, 2014. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PloS One 9 (11), e111670. 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Kinney PL, 2005. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology 26 (4), 573–587. 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Kinney P, 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect 114 (8), 1287–1292. 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Rauh V, 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ. Health Perspect 120 (6), 921–926. 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Perera F, 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatr. 72 (6), 531–540. 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR, 1996. Exposure of the US population to environmental tobacco smoke: the third national health and nutrition examination survey, 1988 to 1991. J. Am. Med. Assoc 275 (16), 1233–1240. [PubMed] [Google Scholar]
- Rivas Ioar, Basagaña Xavier, Cirach Marta, López-Vicente Mónica, Suades-González Elisabet, Garcia-Esteban Raquel,., Sunyer Jordi, 2019. Association between early life exposure to air pollution and working memory and attention. Environ. Health Perspect 127 (5) 10.1289/EHP3169, 57002-57002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, 2012. The lifelong effects of early childhood adversity and toxic stress. Committee on Psychosocial Aspects of, Child, Family, Health, Committee on Early Childhood, Adoption, Dependent, Care, … Behavioral, Pediatrics Pediatrics 129 (1), e232–246. 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Stein J, Schettler T, Wallinga D, Valenti M, 2002. In harm’s way: toxic threats to child development. J. Dev. Behav. Pediatr 23 (1 Suppl. l), S13–S22. [DOI] [PubMed] [Google Scholar]
- Stingone JA, McVeigh KH, Claudio L, 2017. Early-life exposure to air pollution and greater use of academic support services in childhood: a population-based cohort study of urban children. Environ. Health 16 (1), 2. 10.1186/s12940-017-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone Jeanette A., McVeigh Katharine H., Claudio Luz, 2016. Association between prenatal exposure to ambient diesel particulate matter and perchloroethylene with children’s 3rd grade standardized test scores. Environ. Res 148, 144–153. 10.1016/j.envres.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley Dustin, Yamamoto Teppei, Hirose Kentaro, Keele Luke, Imai Kosuke, 2014. Mediation: R package for causal mediation analysis. J. Stat. Software. [Google Scholar]
- Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL, 2004. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ. Health Perspect 112 (6), 754–759. 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. MICE: multivariate imputation by chained equations in R. J. Stat. Software 45 (3), 1–67. [Google Scholar]
- van der Schoot M, Licht R, Horsley TM, Aarts LT, van Koert B, Sergeant JA, 2004. Inhibitory control during sentence reading in dyslexic children. Child Neuropsychol. 10 (3), 173–188. 10.1080/09297040409609808. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Manolio TA, Sidney S, Burke GL, Haley NJ, 1993. Environmental tobacco smoke exposure as determined by cotinine in black and white young adults: the CARDIA Study. Environ. Res 63 (1), 39–46. S0013–9351(83) 71124–2 [pii] 10.1006/enrs.1993.1124. [DOI] [PubMed] [Google Scholar]
- Wernette D, Nieves L, 1992. Breathing polluted air. EPA J. 18, 16–17. [Google Scholar]
- Woodcock R, McGrew M, Mather N, 2000. Woodcock Johnson-III Tests of Achievement. Riverside, Rolling Meadows, IL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


