Abstract
To examine the role of estradiol in hippocampal-dependent spatial memory in women, 86 female undergraduates were tested in a virtual Morris water task (VMWT), a virtual radial arm maze (VRAM), and a mental rotation task (MRT) within a single daily session. The VMWT and RAM were also administered 24 h later to examine the effects of estradiol on memory consolidation. Women on oral contraceptives (OCs) or those who were naturally cycling and exhibited low estradiol (LE) or high estradiol (HE), as determined by salivary assays, were included. At the start of day two, the HE group showed superior spatial reference memory on the VMWT relative to the LE group, as evidenced by significantly shorter distances navigating to the hidden platform. The LE group also had the poorest probe trial performance at the start of day two compared to both other groups. There were no group differences in performance on the RAM or MRT. These results provide support for estradiol’s role in the consolidation of spatial reference memory in women, and emphasize the differential sensitivities of various virtual memory tasks in assessing spatial memory function in women.
Keywords: Estrogen, Estradiol, Contraceptives, Spatial memory, Memory consolidation, Hippocampus, Morris water task, Radial arm maze
1. Introduction
Numerous studies have examined the role of estrogen hormones on hippocampal structure and function in nonhumans. Experiments conducted with female rodents and monkeys have found that 17β-estradiol, the major estrogen hormone, can significantly alter hippocampal morphology and function. For example, endogenous and exogenous estradiol significantly increases CA1 dendritic spine density, neurogenesis, synaptic plasticity, neurotransmission, and gene expression [10, 22,39,46,55,61,64,69]. Additionally, these changes coincide with enhanced long-term potentiation and some hippocampal-dependent cognitive functions, such as spatial working and reference memory [12,21,22,41]. Physiologically, it has been demonstrated that estradiol can facilitate hippocampal cell signaling, structural changes in dendritic properties, and protein synthesis that facilitate memory consolidation [18]. Behaviorally, a negative impact of lowered levels of estradiol has been observed in both animal and human studies. In rats, bilateral ovariectomies have been associated with diminished abilities to discriminate in object recognition memory tasks [67], and administration of estradiol to ovariectomized female rats improves hippocampal-dependent spatial memory [43,50,57], working memory [7,43], and object and place recognition memory [18,36,42]. Thus, in rodents with artificially lowered levels of estrogens, administration of estradiol tends to improve hippocampal-dependent memory, particularly spatial memory.
The rodent data are consistent with findings in women indicating that low estrogen levels are associated with poorer cognition. For example, low estrogen levels in post-menopausal women have been associated with an increased risk of dementia [59]. In women aged 70–79 years, low estradiol levels were predictive of faster cognitive decline compared to women with high levels [71]. Among young normally cycling women, those in the late-follicular phase, during which estradiol levels are at peak elevation, had better memory on a verbal declarative task than women in the late-luteal phase, during which estradiol levels are relatively low [53]. Similarly, other researchers have found that women in the mid-luteal phase, during which estradiol levels are medium to high, performed better on the Stroop task compared to women during menses/early follicular phase [32]. Others have seen that women in the ovulatory phase, during which estradiol levels are relatively high, had enhanced performance on a visuospatial task compared to women in the late-luteal and menstrual phases of their cycles [60]. Additionally, those in the low estrogen phase of the cycle exhibited significantly more working memory errors on a spatial working memory task compared to women in the high estrogen phase [30]. Another study found that with administration of estradiol via a transdermal skin patch for 31 days, performance on a spatial working memory version of an N-back task and verbal recollection were enhanced in the estradiol-treated group compared to the non-treated group [4]. However, there was no effect of the estradiol treatment on verbal fluency, attention on the Stroop task, or information processing [4]. These data suggest that natural reductions in estrogens during the menstrual cycle may result in cognitive impairments. However, the effects of estradiol across the menstrual cycle seem to be task dependent. Studies have found that women during the early follicular phase, when estradiol levels are low, perform significantly better on tasks that measure mental rotation ability compared to women in the mid-luteal phase, when estradiol levels are higher [33,44,45]. Given this discrepancy in the literature, it is vital to further examine how cognition is impacted by changes of estradiol across the menstrual cycle on various types of spatial memory domains.
In addition to natural fluctuations of estradiol, it is also important to consider whether synthetic estradiol in oral contraceptive (OC) impact cognition. According to the Centers for Disease Control, 61% of women aged 15–44 used OC during the period from 2011 to 2013, and these percentages remained similarly high in subsequent years [14]. Furthermore, 88% of US women have used a hormonal contraceptive, and 85% of women typically use an OC for around five years at some point in their life [9]. Considering the widespread use of OCs, it is important to examine whether a synthetic form of estradiol has this detrimental effect on other types of cognitive domains or whether the effect is specific to emotional memory. The most common types of OCs inhibit conception by decreasing the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH; [54]). In a natural menstrual cycle, LH and FSH are secreted concurrently to stimulate a rise in estradiol that induces ovulation. Thus, OCs prevent ovulation by inhibiting gonadotropin release, decreasing estradiol levels, and altering the physical lining of the uterus to prevent implantation [54]. OCs contain different forms of synthetic estradiol (e.g., ethinyl estradiol) and progesterone (progestin) that bind to the same estrogen and progesterone receptors as endogenous hormones [24,28,54]. These synthetic hormones also have different pharmacokinetic properties. For example, ethinyl estradiol has greater bioavailability and is slower to be metabolized than 17β-estradiol [38]. Given that these synthetic forms of hormones are taken exogenously into the system, the effects on hippocampal cognition may be different than endogenous estradiol that is synthesized internally [5,24,28,48]. One study by Graham and Milad [26] indicated that women using OCs had impaired memory for the extinction of fear compared to women with high estradiol levels. Another study found that OC users had impaired verbal fluency and mental rotation than naturally cycling women [28]. These findings suggest that OCs do have an unfavorable effect on certain types of memory processes. However, others have found no differences in verbal, visual, object, or working memory between OC users and non-users [35, 47]. Meanwhile, one study found that while there were no differences between OC users and non-users for the total recall on an emotional memory task, results indicated that OC users had better memory for the gist of an emotional story whereas, non-users had better memory for specific details of the story [49]. Taken together, it is unclear based on prior work how OCs may impact learning and memory.
The present study was designed to test hippocampal-dependent spatial memory in female undergraduates taking OCs or who were in the high or low estradiol phases of the menstrual cycle. To assess spatial memory, we employed two virtual hippocampal-dependent tasks, the Morris water task (VMWT) and radial arm maze (VRAM), which are 3-dimensional computer versions of tasks commonly used to test spatial memory in rodents. In humans, these tasks activate the hippocampus in functional neuroimaging studies [2,58], and damage to the hippocampus impairs spatial learning and memory in these tasks [2,25]. Both tasks are used regularly to assess spatial memory, but they differ in the degree to which they constrain behavior. In the water task, there are almost an infinite number of swim path trajectories that could be used, while in the radial arm task, the number of trajectories is limited. Additionally, in the radial arm task, alternative strategies are more severely discouraged, because traveling down incorrect arms often results in long delays in solving the task. On the other hand, a sub-optimal strategy in the Morris water task may only add a few seconds to the time that it takes to reach the goal. Hence, there might be differential consequences that motivate which strategies one might decide to adopt in solving these tasks. Moreover, the radial arm maze has a spatial working memory component that is not present in the virtual pool. Previously, we have published that even within the same participants, there is a sex difference in the virtual pool, but not in the radial arm maze, suggesting that each task has a unique contribution in deciphering spatial strategies [3]. Accordingly, we are using both of these virtual spatial navigation tasks in addition to a spatial mental rotation task to have converging and complementary tasks to optimize our ability to observe the effects of estradiol levels on spatial memory.
Furthermore, based on findings demonstrating that estradiol facilitates memory consolidation in female rodents [8,16,17,37], testing was conducted over two consecutive days to examine the impact of estradiol on memory consolidation rather than acquisition. A paper-and-pencil mental rotation task (MRT) was also used for further assessment of spatial abilities [65]. Based on studies showing that high levels of estradiol facilitate memory in rodents, it was hypothesized that women with high endogenous levels of estradiol would outperform women with low endogenous levels of estradiol and women on OCs on the VMWT and VRAM, particularly at the beginning of the day two, which would reflect estradiol’s role in memory consolidation in humans. In contrast, given the results in previous research testing the effects of menstrual cycle phase and mental rotation ability [33,44,45], it was hypothesized that women with low levels of estradiol would perform best on the MRT.
2. Method
2.1. Participants
A total of 251 female undergraduates (M = 19.03 years, SD = 1.05) were recruited from the University of Connecticut. Of the participants who initially consented, 89 were excluded for various reasons (cyber-sickness, irregular menstrual cycles, use of intrauterine devices, or incomplete data sets), leaving 162 participants for analysis.
A total of 76 participants were categorized as naturally cycling because they were not taking an OC at the time of testing. Of those, salivary data was not available for ten participants. The remaining 66 participants were divided into high and low estradiol groups by salivary assay. Those with estradiol levels in the upper 40% were assigned to the high estradiol (HE) group (M = 3.89 pg/mL) and those with levels in the lower 40% were assigned to the low estradiol (LE) group (M = 1.19 pg/mL). Twelve participants were excluded for having estradiol levels that fell within the middle 20%. For the HE group, participants were approximately 11 days after the start of menstruation, corresponding to the late-follicular phase. Estradiol levels are high during this phase, whereas progesterone levels are low. For the LE group, participants were approximately 17 days after the start of menstruation, corresponding to the mid-luteal phase. During this phase, estradiol levels are lower than the late-follicular phase but higher than during menstruation. Progesterone levels are also elevated during this phase.
Participants were placed into the OC group if they indicated that they were taking an OC at the time of testing. Of the 86 participants on OCs at the time of testing, 32 were on a monophasic type with ethinyl estradiol levels that ranged between 0.020 and 0.035 mg and progestin (noreth-indrone) levels that ranged between 1.00 and 1.25 mg. Common OCs that met these criteria included Gildess Fe, Junel Fe, Lo Loestrin Fe, Lomedia 24 Fe, Minastrin 24, Alyacen, Zovia, and Microgestin. Of the remaining participants on OCs, 5 were on biphasics, 12 were on triphasics, and 17 used other types or were unknown. We chose to analyze data from the 32 monophasic OC users for a more homogenous sample of OC users. Within this group, the length of OC treatment ranged from six months to seven years.
After exclusions and categorizations, the sample sizes were as follow: HE (n = 27), LE (n = 27), and OC (n = 32). None of the 86 participants were pregnant at the time of testing. Subjects received class credit for their participation. Approval for this study was obtained from the University of Connecticut Institutional Review Board.
2.2. Apparatus
An IBM-compatible computer with a SVGA color monitor was used for testing. Participants seated at the computer navigated through the virtual environments by manipulating a joystick. Headphones connected to the computer were used to provide auditory feedback.
2.3. Procedure
Participants were required to sign up for two consecutive days of testing. Testing was completed between the hours of 11:00 am and 5:00 pm EST. On day one, participants completed a general questionnaire after consent was obtained, and then completed the VMWT, VRAM, and MRT. On day two, participants completed a retest of the VMWT and VRAM. After completion of the tasks on day two, saliva was collected to assess estradiol levels. Participants were then debriefed and thanked for their participation.
2.4. Virtual Morris water task
Participants were informed that they would find themselves in a virtual pool, and that their goal was to escape from the water as quickly as possible by swimming to a hidden goal platform (Fig. 1A). Within the virtual world, the participant was 1.8 m tall, and the room was 20 × 16 × 4 m. The virtual pool had a diameter of 12 m. They were told that the platform would be visible for the first four trials, during which the platform was slightly raised out of the water and visible from the water’s surface. These trials served as practice and to control for any visual, sensory, or motor differences between participants. Participants were told that they would receive hidden platform trials following the visible platform trials. Trials started from four different locations (north, south, east, and west) three times each for a total of 12 trials with a three second inter-trial interval (ITI). If the participant swam over the area where the platform was located, a tone sounded, the platform rose slightly out of the water, and a message saying “Congratulations” was displayed. If the platform was not found within 60 s, then a message appeared saying “Time has expired. Please swim to the red platform”, a repetitive thumping noise was played, and the platform rose out of the water so that it was visible. The participant then swam to the now-visible platform. Following the hidden platform trials, a probe trial was given in which the platform was removed from the pool, and the participant was allowed to search for the platform for 20 s, after which the trial terminated. There was no indication to the participant that the probe trial was in any way different than the previous trials until the probe trial was already completed. On day two, participants first received a probe trial to test for memory retention of the platform location learned the previous day, and then were given eight hidden platform trials and one final probe trial.
Fig. 1.

Pictured above are third-person views of the virtual Morris water task (A) and radial arm maze (B). Salient cues are present throughout the room to distinguish each wall. Participants were in first person view during all tasks.
2.5. Virtual radial arm maze
Participants were informed that they would find themselves in a virtual room that had eight runways extending out from a round middle area (Fig. 1B). Within the virtual world, the participant was 1.8 m tall, and the room was 22 × 18 × 4 m. The radial arm maze had a 14 m diameter. They were further instructed that a well was located at the end of each runway, and that four of wells contained rewards whereas four did not. They were told to retrieve all four rewards as quickly as possible. Upon discovering a reward, a pleasant tone sounded and the word “Reward” was displayed for two seconds. After discovering all four rewards, a pleasant chord played, and the following congratulatory message was displayed: “Congratulations. You have found all the rewards.” The screen then went blank, and the next trial restarted from the middle area after an intertrial interval of three seconds. If five minutes elapsed and the four rewards were not found, the trial was terminated. Five trials were administered per day. The same configuration of rewarded arms was used for all subjects. A reference memory error was scored if the participant entered into an arm that was never rewarded. A working memory error was scored if the participant entered an arm that was previously entered during that trial, regardless of whether that arm was rewarded.
2.6. Mental rotation task
A pen and paper mental rotation task (shortened form) adapted from Vandenberg & Kuse [65] was used. Specifically, participants were given a target object and four choices, two of which matched the target object but were rotated relative to its initial position. The other two choices did not match the target. Subjects were required to pick the two matching rotated choices. Subjects were given four minutes to complete 12 of these problems. One point was given for each correct response and participants were instructed to work as quickly as possible without compromising accuracy.
2.7. Saliva collection
For naturally cycling participants, saliva was collected in individually marked 2 mL test tubes via a passive drool method and stored in a freezer and kept at − 25 °C until shipped. Saliva was collected once at the end of the session on day two. Samples were packed in dry ice and shipped to Salimetrics (State College, PA) and assayed for estradiol levels using a highly sensitive enzyme immunoassay. All estradiol assays were performed in duplicate and the average value was used as the participants’ value. We did not assay the HC group for estradiol levels because Mordecai (2008) has published that estradiol levels are markedly reduced in participants on hormonal contraceptives (e.g. less than 1/3 of normal levels), and we were confident that they had reduced estradiol levels. Also, because of financial limitations, we were unable to assay for progesterone levels although that is a goal for future studies.
2.8. Analyses
For all analyses, a Type I error alpha level of 0.5 was used. A between-group ANOVA indicated no significant differences among the three groups in age or video game experience as quantified by a question about hours playing video games per week.
2.8.1. Virtual Morris water task
Distance to the platform was chosen as the main dependent variable because it is less affected by joystick navigation skill than escape time. For day one, a 3 × 3 repeated measures ANOVA was used with Block (1–3) as the repeated factor and Group (HE, LE, and OC) as the between-subjects factor. Block was an average of four consecutive trials in which participants started from each of the four locations. For day two, a one-way ANOVA for Group was analyzed for the first block as a measure of memory consolidation. Furthermore, to directly compare the low and high estradiol groups on memory consolidation, a t-test was conducted for the first block of day two. A 2 (Block) × 3 (Group) ANOVA was also conducted to examine participants’ distance to find the platform.
For probe trial performance, the percent distance in the quadrant where the platform was previously located was used as the dependent variable. A one-way ANOVA with Group as the between-subjects factor was used to determine the effect of estradiol on probe trial performance for the probe on day one and both probes on day two. For between group comparisons, Fisher’s LSD post-hoc analyses were conducted.
2.8.2. Virtual radial arm maze
For days one and two, a 5 × 3 repeated measures ANOVA was used with Trial (1–5) as the repeated factor and Group as the between-subjects factor. Distance, working memory errors, and reference memory errors were used as dependent variables.
2.8.3. Mental rotation task
A one-way ANOVA was conducted to examine differences between the three groups (HE, LE, and OC) for total MRT score.
3. Results
3.1. Salivary estradiol levels
There was a significant difference in salivary estradiol levels between the HE and the LE group, F(1, 52) = 26.55, p < 0.001. Specifically, the HE group had significantly higher estradiol levels than the LE group: HE (M=3.73 pg/mL, SD=2.54), LE (M=1.20 pg/mL, SD=0.24).
3.2. Virtual Morris water task
Distance to Platform. On day one, there was a significant effect of Block, F(2, 82) = 14.18, p < 0.001 (Fig. 2), such that participant’s distance to find the platform was reduced across Blocks. However, the main effect of Group was not significant, F(2, 83) = 0.75, non-significant (n. s.), nor was the Group by Block interaction, F(2, 166) = 1.24, n.s. Together, these data suggest hormone status did not affect task acquisition on day one.
Fig. 2.
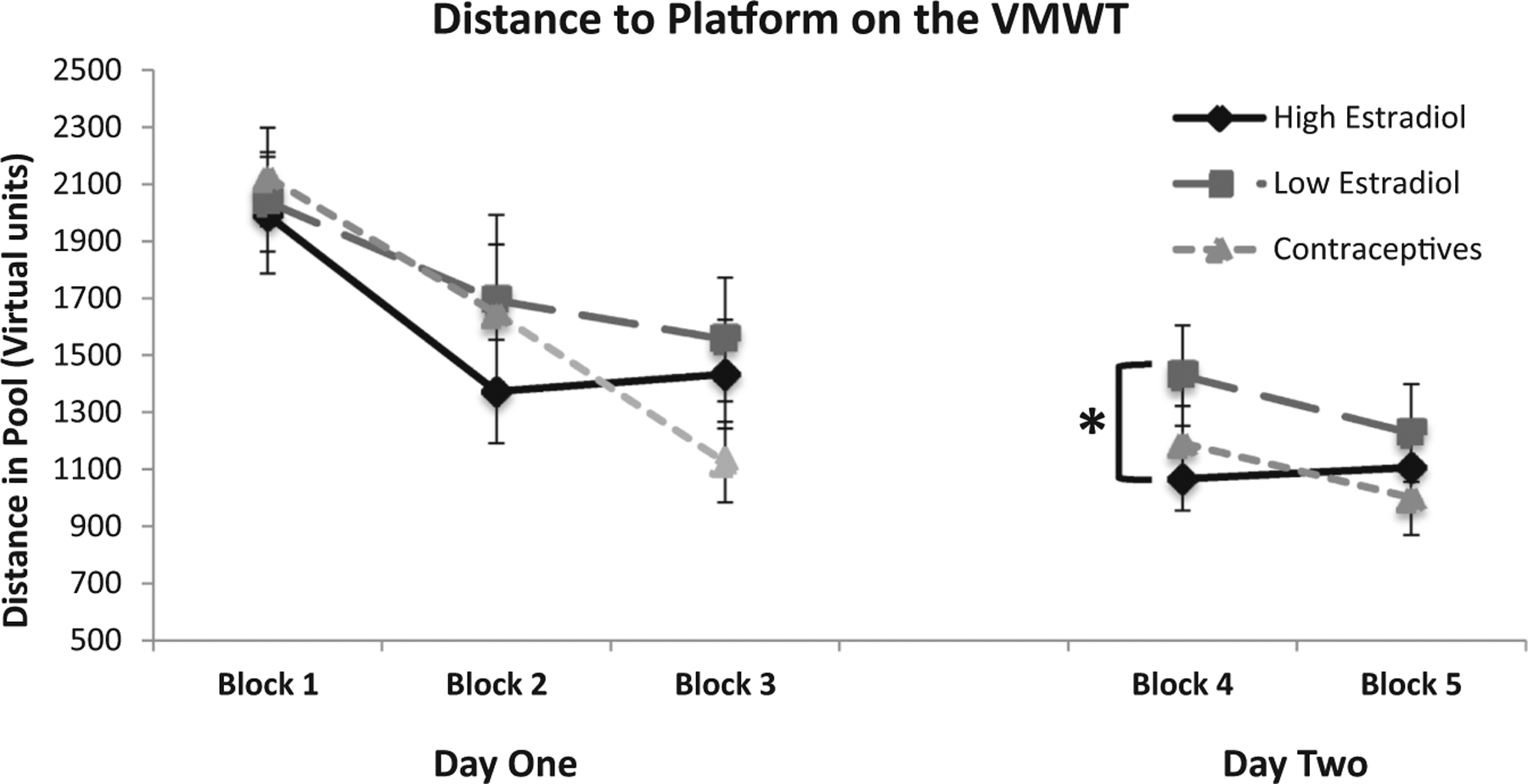
Mean distance to platform on the virtual Morris water task across days. Shorter distances indicate more direct paths (better performance). Error bars indicate standard error of the mean (SEM). In Block 4, the high estradiol group exhibited significantly shorter distances to the platform than the low estradiol group (p = 0.01). The high estradiol and contraceptive groups did not differ on any blocks across days.
On day two, there was no longer a main effect of Block, F(1, 83) = 1.58, n.s., likely indicating that participants were at ceiling performance. As on day one, there also was no effect of Group, F(2, 83) = 1.61, n.s., nor a Group by Block interaction, F(2, 83) = 0.69, n.s. However, we also ran an a priori analysis on block four data from the naturally cycling groups to measure retention from day 1 and found that the distance to find the platform was significantly lower for the HE group than the LE group, t(52) = 1.74, p = 0.01 (Fig. 2). These data suggest that among naturally cycling women, high endogenous estradiol levels benefit spatial reference memory consolidation relative to low naturally cycling levels. Additionally, to examine performance across days, we conducted a Group X Day repeated measures ANOVA with Block 3 and Block 4 as the repeating factor and the three groups as the between subjects factor for distance to the platform. There was no effect of Day (F(1, 157) = 2.14, n.s.) or Group (F(1, 157) = 0.85, n.s.) or a Group × Day interaction (F(3, 157) = 1.13, n.s.). However, as suggested by a reviewer, when looking at each group individually, we observed that the HC and LE groups did not show a change in performance from Block 3 to Block 4 (t(84) = −0.28, n.s.; t(26) = 0.71, n.s., respectively), but the HE group showed a significant decrease in path length from Block 3 to Block 4, t(26) = 2.04, p < 0.05.
Probe Trial Performance. Participants experienced probe trials at three points during testing: at the end of day one, at the beginning of day two, and at the end of day two. A significant main effect of Group was observed for the probe trial at the beginning of day two, F(2, 137) = 3.69, p = 0.03. Post-hoc tests revealed that the HE group spent significantly more distance in the correct quadrant compared to the LE group (42% vs. 29%, respectively), p = 0.01 (Fig. 3). The OC group (38%) also exhibited significantly greater distance in the correct quadrant compared to the LE group, p = 0.04 (Fig. 3). Consistent with the distance measure from the platform trials, these data suggest that low estrogens in naturally cycling women are detrimental to spatial reference memory consolidation relative to high endogenous or exogenous estradiol. In contrast to the first probe on day 2, the main effect of Group was not significant for the probe trials at the end of day one, F(2, 135) = 0.36, n.s., or day two, F(2, 137) = 0.15, n.s. (Fig. 3), suggesting no effect of estrogens on acquisition within a session.
Fig. 3.
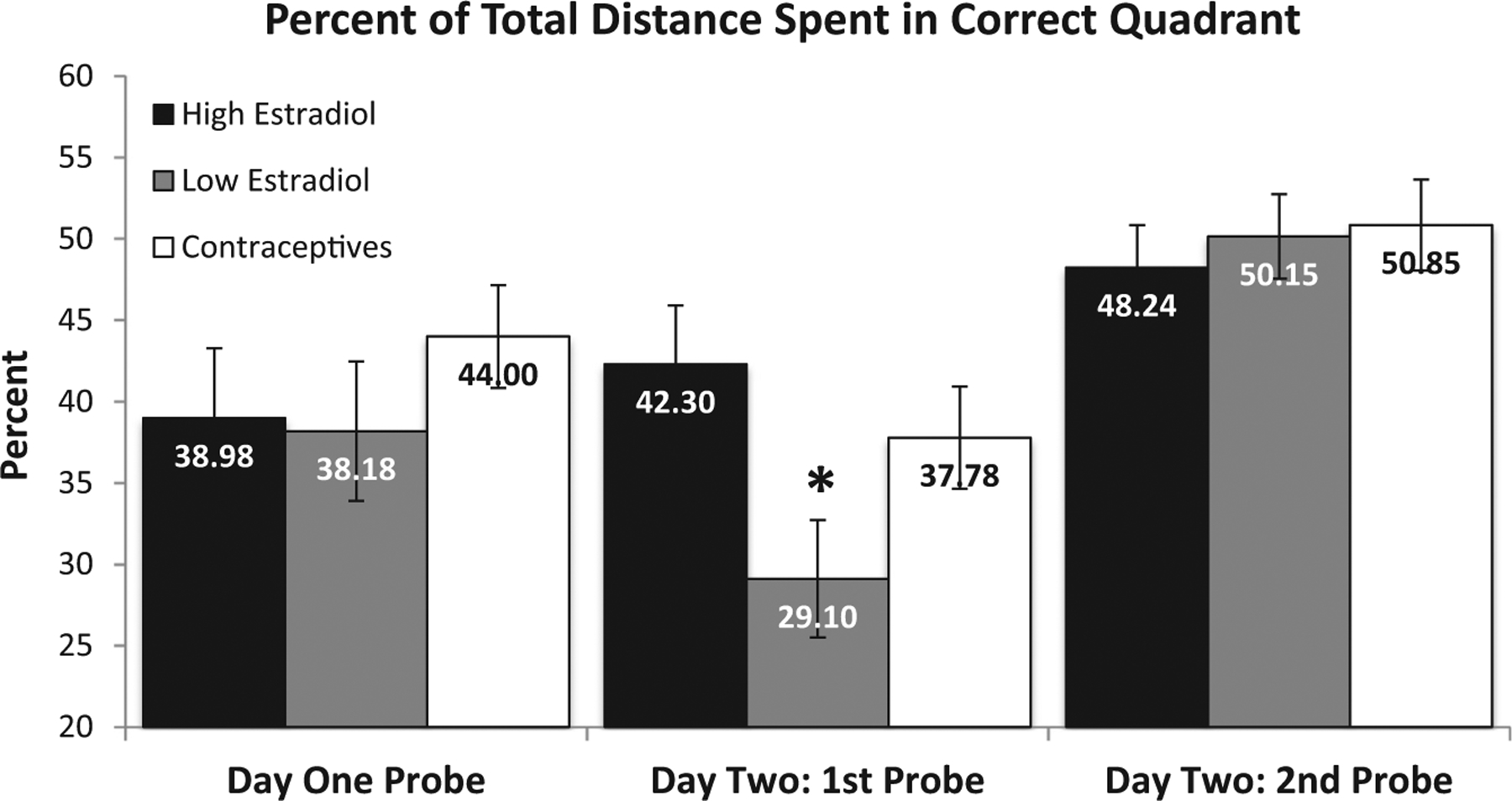
Percent total distance (± SEM) in the quadrant that contained the platform during hidden trials for all probe trials on the virtual Morris water task. During the first probe on day two, the percent distance of the low estradiol group was significantly lower in the correct quadrant compared to the high estradiol group (p = 0.01) and the contraceptive group (p = 0.04).
3.3. Virtual radial arm maze
The main effect of Trial was significant for all measures on day one, such that the distance across trials, F(4, 75) = 47.63, p < 0.001, number of working memory errors, F(4, 76) = 8.44, p < 0.001, and number of reference memory errors, F(4, 76) = 108.1, p < 0.001, decreased with training (Fig. 4). The same was true for distance across trials, F(4, 65) = 13.98, p < 0.001, and number of reference memory errors, F(4, 65) = 7.03, p < 0.001, on day two (Fig. 5). The main effect of Trial was not significant on day two for number of working memory errors, F(4, 65) = 0.89, n.s., for the HE (M = 0.17, SD = 0.34), LE (M = 0.06, SD = 0.16), and OC (M = 0.35, SD = 0.77) groups, likely due to a floor effect (Fig. 5). However, there were no significant effects of Group on day one, F(2, 78) = 1.10, n.s., or day two, F(2, 117) = 0.30, p > 0.10, nor Trial by Group interactions on day one, F(2, 312) = 0.12, n.s., or day two, F(2, 272) = 0.70, n.s., suggesting that spatial working memory and spatial reference memory in the VRAM were not affected by exogenous or endogenous estradiol levels.
Fig. 4.
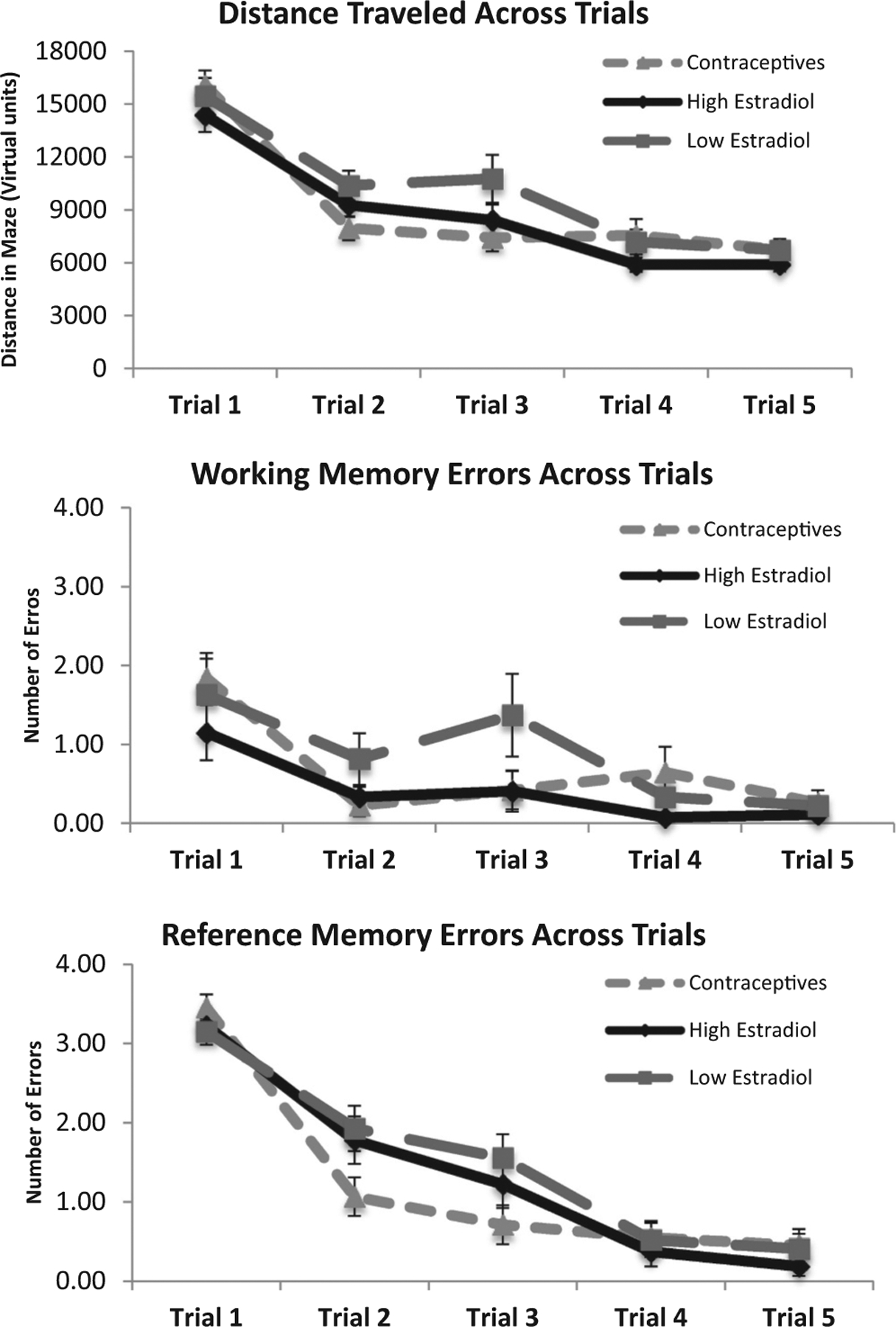
Mean distance, number of working memory errors, and number of reference memory errors across trials on day one. Shorter distances and lower errors scores indicate better performance. Error bars indicate standard error of the mean (SEM). There was a significant main effect of Trial for all measures on day one, in that distance (p < 0.001), number of working memory errors (p < 0.001), and number of reference memory errors (p < 0.001), decreased with training.
Fig. 5.
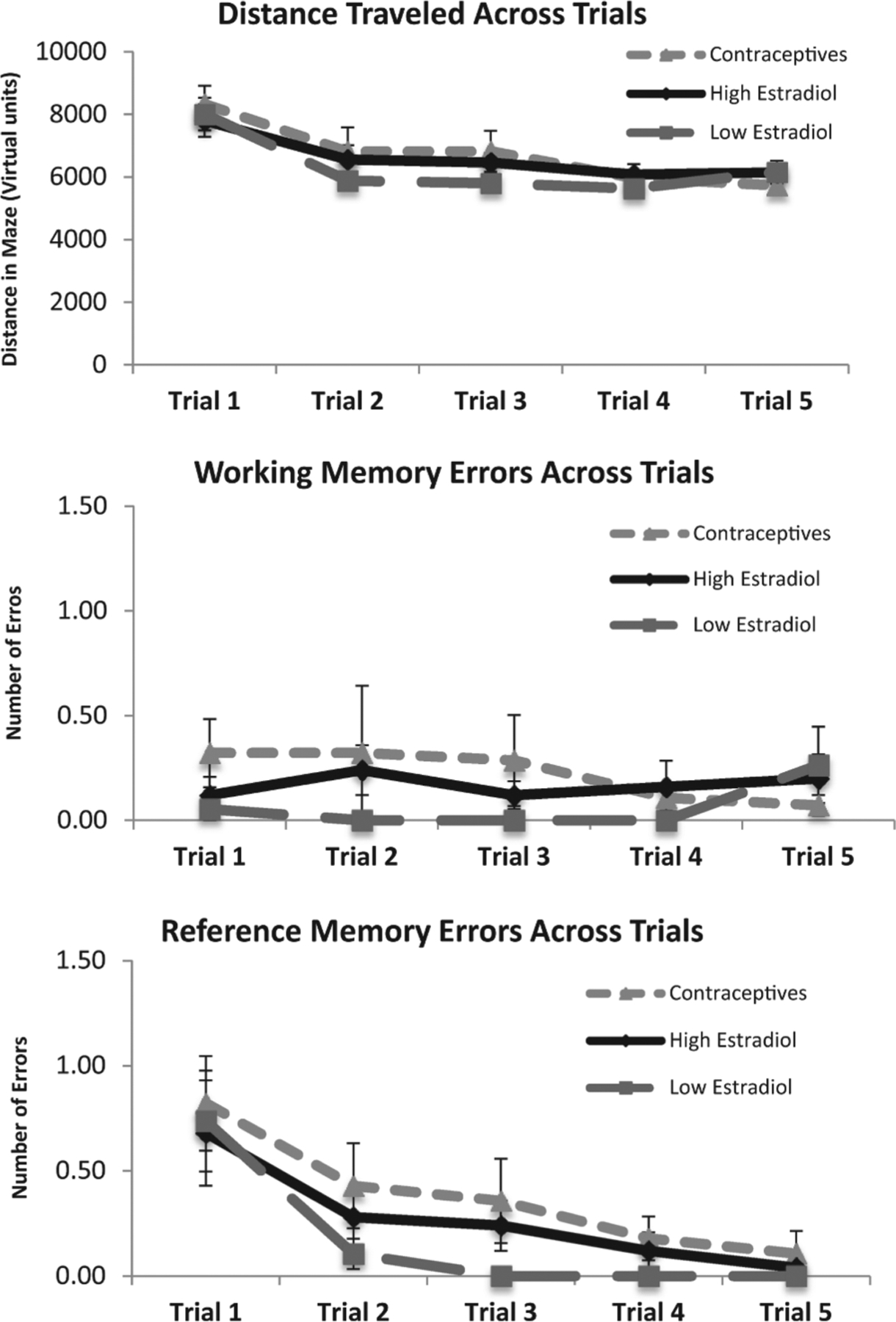
Mean distance, number of working memory errors, and number of reference memory errors across trials on day two. Shorter distances and lower errors scores indicate better performance. Error bars indicate standard error of the mean (SEM). There was a significant main effect of Trial for distance (p < 0.001) and number of reference memory errors (p < 0.001), in that both decreased with training on day two. The effect of Trial was not significant for working memory errors on day two (p > 0.05).
3.4. Mental rotation task
The main effect of Group was not significant, F(2, 83) = 0.93, n.s., indicating no differences in mental rotation abilities among the HE (M=10.89, SD=3.56), LE (M=9.63, SD=2.87), and OC (M=10.41, SD=3.72) groups.
3.5. Regression analyses
To include all participants in the analysis, we conducted a linear regression with MRT errors, vMWT probe trial performance, and radial arm maze path length as outcomes and regressed them to predict estradiol levels. The overall regression did not explain a significant amount of variance in estradiol levels, F(3, 49) = 1.04, p = 0.38, R2 = 0.06, R2adjusted = 0.003. The regression coefficients were not significant (BMRT = 0.19; BVMWT = 0.14; BRAM = 0.09) suggesting that there is not a linear relationship between estradiol levels and spatial skills as used in this experiment.
4. Discussion
The present findings suggest that low endogenous estradiol levels hindered spatial reference memory consolidation in a virtual navigation task relative to high levels of endogenous estradiol or exogenous synthetic estradiol. Our use of virtual reality versions of common rodent tasks provides translational insight into how estradiol affects spatial memory in humans relative to rodents. The results of the VMWT indicated better memory for the HE group compared to the LE group at the start of day two. The LE group also had the poorest probe trial performance at the start of day two. Together, these multiple measurements suggest a significant disadvantage of low estradiol for spatial memory consolidation. In contrast, however, estradiol did not affect memory in other spatial tasks, the VRAM and MRT, suggesting an effect specific to the VMWT. The discrepancy between the VMWT and VRAM supports our previous reports that these virtual spatial navigation tasks do not tap into identical spatial memory processes [1,3]. The present data suggest that the effects of estradiol on spatial memory in women may be limited to more complex spatial memory tasks such as the VMWT. This task is more ambiguous, in that that there are no direct paths to the goal location and participants are free to move in any direction. In contrast, the VRAM forces the participants to navigation within specific arms which could make it easier to complete.
4.1. Virtual Morris water task
The lack of differences among the groups on day one indicates that all three groups could successfully acquire the task, and that estradiol levels may not be critical in women for the acquisition of spatial information. However, on day two, the HE group demonstrated better memory consolidation than the LE group as indicated by initial day two performance. Specifically, the HE group had significantly shorter distances to find the platform for the first block of day two compared to the LE group. At the end of day one training, these two groups performed equally in terms of distance to find the platform and probe trial performance. The fact that 24 h later, the HE group initially found the platform with shorter distances than the LE group suggests that some consolidation process seemed to improve their performance. Whereas it is beyond the scope of the current design to disambiguate consolidation on the part of the HE group from forgetting by the LE group, the findings in Fig. 2 suggests a consolidation process. Only, the HE group shows an improvement at the onset of day two, even without additional training, suggesting that a consolidation process is responsible for this improvement. Furthermore, on the initial probe trial on day two, the HE and OC groups performed better than the LE group, once again demonstrating a deficit for the LE group.
Our VMWT results support previous findings in which naturally cycling young women with high levels of estradiol displayed significantly fewer working memory errors compared to a low estradiol group across various learning conditions including immediate and delayed spatial memory performance [30]. Our data are also consistent with those from a fear-conditioning study in which OC, LE, and HE groups of women exhibited similar day one acquisition and extinction, but the HE group displayed the best extinction memory on day two [26]. Rodent data also support the notion that high estradiol levels enhance memory consolidation. For example, gonadally intact mice and rats in proestrus (high estradiol levels) exhibit better spatial memory than rats in estrus or diestrus (low estradiol levels) in the Morris water maze [19] and on an object location task ([20,52]; although see [6]). In addition, systemic exogenous estradiol treatment in ovariectomized rodents enhances memory consolidation in a variety of memory tasks including the Morris water maze, object placement, and object recognition [27,34,51]. In particular, ovariectomized rats and mice treated with a single systemic injection of 0.2 mg/kg 17β-estradiol immediately after eight Morris water maze training trials exhibited enhanced spatial memory relative to controls or rodents treated with 0.1 or 0.4 mg/kg estradiol [27,51]. However, the 0.2 and 0.4 mg/kg doses both enhanced consolidation of object recognition memories in ovariectomized mice [16,27], suggesting that different levels of estradiol may be optimal for different tasks or types of memory. These studies illustrate the importance of establishing an optimal dose of estradiol for memory enhancement. For spatial reference memory as tested in the VMWT, it would appear that high estradiol during the menstrual cycle is optimal.
However, there are some alternative explanation to this behavioral decrement for the low E group. Rather than superior consolidation for the high E group, it may be that the design of the study affected the groups differentially. Specifically, Day 1 ended with a probe trial, and it may be that the probe trial functioned as an extinction trial whereby participants realized that the platform was no longer in the same location, and they started to search elsewhere in the pool for the platform. Looking at the data, we see that all group performed similarly on the Day 1 probe trial, which suggests that they searched for the platform for equivalent times in the platform location, but also, that they searched elsewhere in the pool for an equivalent amount of time. So, it seems unlikely that the groups differentially switched strategies during the probe trial. Moreover, it is important to note that extinction is generally facilitated when rodent or human females are in high estrogen phases relative to low estrogen phases [68,72,73] which suggests that it would be the high E group that would have been more likely to switch strategies and show a decrement in performance on the Day 2 probe trial rather than the low E group. Hence, the literature suggests that extinction would not be facilitated in a low E group.
However, there may be other alternatives instead of extinction to explain this decrement in performance, such as an increased sensitivity to interference of the probe trial, or enhanced forgetting in the low E group. Each of these could be investigated more deeply in future studies designed to tease apart the various mechanisms. For example, it might be worthwhile to have a few refresher trials after the probe trial at the end of Day 1 which would minimize any extinction component. Or, sessions might be conducted 12 h apart with half of the sessions being 12 hrs apart without sleep (e.g. 8 am and 8 pm) compared to 12 hr apart with sleep (e.g. 8 pm and 8 am) which might tap into consolidation components that occur during sleep. Lastly, some studies might use various interference or distractor tasks to examine whether the groups differ in their sensitivity to distractors, which might contribute to this decrement in spatial memory across days. Clearly, there are a number of studies that can be conducted in the future to understand these performances more deeply and help elucidate the role of estrogens in spatial memory.
4.2. Virtual radial arm maze
There were no group differences on the VRAM in analyses of distance, reference memory errors, or working memory errors on day one or two, indicating that all groups were able to successfully learn and retain the task. Working memory errors were defined as entering an arm that had already been visited within a trial, these errors are not direct measures of spatial memory, per se. Rather, these errors reflect within-session memory components rather than across-session spatial memory information. The observed lack of estradiol effects in working memory errors is consistent with previous work in gonadally-intact rats [62], but not with prior research in rats in which systemic estradiol treatment improved spatial working memory in a radial arm maze among ovariectomized rats [13,15]. Our null effects on spatial reference memory are consistent with data from ovariectomized rats treated with exogenous estradiol [15]. Thus, the current data are consistent with some VRAM findings from the rodent literature. However, our findings are not consistent across tasks; that is, the lack of estradiol effects in the VRAM were inconsistent with the beneficial effects of high estradiol on spatial memory consolidation observed in VMWT. We have previously shown that virtual spatial navigation tasks do not always produce similar performance across tasks. Specifically, we previously reported a male advantage in the virtual Morris water task, but no sex differences in the radial arm maze among the same participants [3]. This discrepancy lends support to the notion that these tasks are not interchangeable in terms of spatial memory ability, and hence, are differentially sensitive to memory processes such as reference and working memory. Future research should examine whether parametric manipulations (e.g. number of arms in the maze, number of rewards provided, delays, etc.) of the radial arm maze task might increase sensitivity to estradiol levels.
4.3. Mental rotation task
Our results yielded no significant group differences on the mental rotation task. Males typically outperform women on the MRT [3,29,40]. Furthermore, as seen in previous studies, women in phases with low estradiol levels typically perform better than women in phases with high estradiol levels [33,44,45]. Although this result was expected in the current study, other studies have also found no difference in performance on the MRT between OC users and naturally cycling women [48, 56]. Thus, this task may be generally less sensitive to changes in estradiol level in females. Given that the mean scores for all three groups ranged from 9.6 to 11 out a potential score of 24, it is likely that the task was similarly difficult for all groups. Perhaps if task difficulty was decreased or the time limit to complete questions was lengthened, group differences may have been more apparent.
4.4. Influence of oral contraceptives
Interestingly, across both virtual tasks, there were no significant behavioral impairments observed in the OC group. In fact, in most cases, the OC group performed similarly to the HE group, which is in contrast to some prior research. For example, one study found that women on OCs performed worse than naturally cycling women overall and naturally cycling women with low estradiol levels performed significantly better than OC women [26]. Moreover, the same study reported that the deficit induced by OCs was reversed by terminating OC use or administering estrogen receptor agonists [26]. In contrast, the OC group in the present study was not impaired relative to the LE group, even when we adopted a very similar testing design. Based on prior research, it was expected that the OC group would show some deficit, given that most OCs typically reduce the overall level of estradiol and progesterone even with the consumption of synthetic forms these hormones [28,48]. However, our results suggest that high levels of estradiol may be more beneficial for women in a natural cycle than low levels. This conclusion is supported by other results indicating that naturally cycling women experienced no change in verbal memory on the California Verbal Learning Test across the menstrual cycle but that OC users exhibited enhanced memory during the active pill phase compared to the inactive phase [48]. Other studies have shown that naturally cycling women with high estradiol levels and OC users perform the best on verbal memory tasks [56] and on immediate and delayed memory for verbal lists of words [23] compared to naturally cycling women with low estradiol levels. Additionally, some studies have shown no differences between OC users and naturally cycling women in working memory [66].
One distinction between the naturally cycling groups and the OC groups other than level of estradiol is the type of estrogen hormone. Although OC groups typically have a reduced level of estradiol [28,48], a steady dose of synthetic estradiol in the form of ethinyl estradiol is taken exogenously into the system, whereas in naturally cycling women, estradiol is synthesized and released endogenously, and this may result in some differences in how estradiol is processed [5,24,28,48]. Furthermore, in ovariectomized mice, estradiol synthesized in and released by the hippocampus is necessary for spatial and object recognition memory consolidation [64], indicating a potential impact of local synthesis on learning and memory. Thus, it is possible that estradiol synthesized within the hippocampus might differ in functionality from ethinyl estradiol derived from OCs. These issues notwithstanding, the OC group in the current study did not display any spatial memory impairments, suggesting that these differences are not critical in our current spatial navigation tasks.
4.5. Limitations and future directions
4.5.1. Menstrual cycle phases
Because we did not specifically recruit participants in any particular phase in their cycle, it is difficult to ascertain whether factors other than estradiol impacted the findings of this study. For example, by including women in the luteal phase of their cycle, progesterone levels may have influenced behavior in these tasks. Given that the average day for our LE group was 17 days post the start of menstruation, there is a high probability that progesterone mediated behavioral performance in these women. It is well established that progesterone plays an important regulatory role in enhancing estradiol’s effects in altering dendritic spine structure in the CA1 region of the hippocampus [70]. Behavioral studies have shown that progesterone levels impact human mental rotation performance [11] and rodent performance in object recognition [63] and object placement [20,63]. Furthermore, large doses of progesterone were shown to counteract the benefits provided by estradiol among ovariecomized mice tested in the Morris water maze [31]. Although it would have been ideal to assay progesterone levels in the present study, doing so was beyond the resources of this current investigation and thus, remains a topic for future investigation.
Future studies will expand upon these results by examining whether a single dose of estradiol can improve consolidation in humans as is seen in rodents. General intelligence and general spatial ability were not measured and could have impacted the results of this study and will be considered in future experiments. Furthermore, no data were obtained about whether participants were taking any hormone-altering medications other than OCs that could have affected both performance and grouping of the participants. Additionally, future studies will more carefully examine the type of OCs being used and include factors such as age of first use and duration of OC treatment as variables. This information should prove useful in more fully understanding how OCs may affect memory processing and will help provide a more thorough understanding of the role of estradiol in spatial memory in humans.
4.6. Conclusion
Collectively, these data provide support for the role of estradiol in consolidating spatial information in humans. Specifically, we observed facilitation of spatial memory by the HE group compared to the LE group across the 24-hour delay between sessions on the VMWT. The superior memory observed at the start of the second day by the HE group is particularly indicative of consolidation being a central process affected by estradiol. Additionally, the OC group was not impaired relative to the HE group on any of the tasks, indicating that these women performed just as well as the naturally cycling groups and in some cases, better than the LE group. Overall, the outcomes of this study indicate that naturally low estradiol levels are specifically associated with poor spatial reference memory consolidation in young women.
Acknowledgements
We would like to thank Adriana Racki, Ambica Mehndiratta, Emily Errante, Franchesca Kuhney, Lauren Masayda, Rachel Niezrecki, and Stephen Friedland for their assistance with the study. Funding was provided through Astur Lab funds and The Connecticut Institute for the Brain and Cognitive Sciences (IBACS) undergraduate research award. Dr. Frick’s contributions to this work were supported by National Institutes of Health grant R01MH107886, Alzheimer’s Association grant SAGA-17-419092, and the University of Wisconsin-Milwaukee College of Letters & Science.
Footnotes
CRediT authorship contribution statement
Robert Astur, Soniya Assudani Patel, Karyn Frick, Paul New-house: Conceptualization, Methodology, Visualization, Writing – review & editing. Robert Astur, Soniya Assudani Patel: Data collection, Data curation, Writing – original drafts. Robert Astur, Karyn Frick: Software, Supervision, Formal analysis, Resources.
References
- [1].Astur RS, Ortiz ML, Sutherland RJ, A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference, Behav. Brain Res 93 (1) (1998) 185–190. [DOI] [PubMed] [Google Scholar]
- [2].Astur RS, Germain SAS, Baker EK, Calhoun V, Pearlson GD, Constable RT, fMRI hippocampal activity during a virtual radial arm maze, Appl. Psychophysiol. Biofeedback 30 (3) (2005) 307–317. [DOI] [PubMed] [Google Scholar]
- [3].Astur RS, Tropp J, Sava S, Constable RT, Markus EJ, Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation, Behav. Brain Res 151 (1) (2004) 103–115. [DOI] [PubMed] [Google Scholar]
- [4].Bartholomeusz CF, Wesnes KA, Kulkarni J, Vitetta L, Croft RJ, Nathan PJ, Estradiol treatment and its interaction with the cholinergic system: effects on cognitive function in healthy young women, Horm. Behav 54 (5) (2008) 684–693. [DOI] [PubMed] [Google Scholar]
- [5].Hampson Beltz, Berenbaum, Oral contraceptives and cognition: a role for ethinyl estradiol, Horm. Behav 74 (2015) 209–217. [DOI] [PubMed] [Google Scholar]
- [6].Berry B, McMahan R, Gallagher M, Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task, Behav. Neurosci 111 (2) (1997) 267–274. [DOI] [PubMed] [Google Scholar]
- [7].Bimonte HA, Denenberg VH, Estradiol facilitates performance as working memory load increases, Psychoneuroendocrinology 24 (2) (1999) 161–173. [DOI] [PubMed] [Google Scholar]
- [8].Boulware MI, Heisler JD, Frick KM, The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling, J. Neurosci 33 (38) (2013) 15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chadwick KD, Burkman RT, Tornesi BM, Mahadevan B, Fifty years of “the pill”: risk reduction and discovery of benefits beyond contraception, reflections, and forecast, Toxicol. Sci 125 (1) (2012) 2–9. [DOI] [PubMed] [Google Scholar]
- [10].Cooke BM, Woolley CS, Gonadal hormone modulation of dendrites in the mammalian CNS, J. Neurobiol 64 (1) (2005) 34–46. [DOI] [PubMed] [Google Scholar]
- [11].Courvoisier DS, Renaud O, Geiser C, Paschke K, Gaudy K, Jordan K, Sex hormones and mental rotation: an intensive longitudinal investigation, Horm. Behav 63 (2) (2013) 345–351. [DOI] [PubMed] [Google Scholar]
- [12].Daniel JM, Effects of oestrogen on cognition: what have we learned from basic research? J. Neuroendocrinol 18 (10) (2006) 787–795. [DOI] [PubMed] [Google Scholar]
- [13].Daniel JM, Fader AJ, Spencer AL, Dohanich GP, Estrogen enhances performance of female rats during acquisition of a radial arm maze, Horm. Behav 32 (3) (1997) 217–225. [DOI] [PubMed] [Google Scholar]
- [14].Daniels K, Daugherty J, Jones J, Current contraceptive status among women aged 15–44: United States, 2011–2013, NCHS Data Brief. 173 (173) (2014) 1–8. [PubMed] [Google Scholar]
- [15].Fader AJ, Johnson PE, Dohanich GP, Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze, Pharmacol. Biochem. Behav 62 (4) (1999) 711–717. [DOI] [PubMed] [Google Scholar]
- [16].Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors, J. Neurosci 28 (35) (2008) 8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Frankfurt M, Luine V, The evolving role of dendritic spines and memory: Interaction(s) with estradiol, Horm. Behav 74 (2015) 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frick KM, Molecular mechanisms underlying the memory-enhancing effects of estradiol, Horm. Behav 74 (2015) 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Frick KM, Berger-Sweeney J, Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice, Behav. Neurosci 115 (1) (2001) 229–237. [DOI] [PubMed] [Google Scholar]
- [20].Frye CA, Duffy CK, Walf AA, Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task, Neurobiol. Learn. Mem 88 (2) (2007) 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gibbs RB, Gabor R, Estrogen and cognition: applying preclinical findings to clinical perspectives, J. Neurosci. Res 74 (5) (2003) 637–643. [DOI] [PubMed] [Google Scholar]
- [22].Gillies GE, McArthur S, Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines, Pharmacol. Rev 62 (2) (2010) 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gogos A, Natural and synthetic sex hormones: effects on higher-order cognitive function and prepulse inhibition, Biol. Psychol 93 (1) (2013) 17–23. [DOI] [PubMed] [Google Scholar]
- [24].Gogos A, Wu YC, Williams AS, Byrne LK, The effects of ethinylestradiol and progestins (“the pill”) on cognitive function in pre-menopausal women, Neurochem. Res 39 (12) (2014) 2288–2300. [DOI] [PubMed] [Google Scholar]
- [25].Goodrich-Hunsaker NJ, Hopkins RO, Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage, Behav. Neurosci 124 (3) (2010) 405–413. [DOI] [PubMed] [Google Scholar]
- [26].Graham BM, Milad MR, Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women, Biol. Psychiatry 73 (4) (2013) 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gresack JE, Frick KM, Post-training estrogen enhances spatial and object memory consolidation in female mice, Pharmacol. Biochem. Behav 84 (1) (2006) 112–119. [DOI] [PubMed] [Google Scholar]
- [28].Griksiene R, Ruksenas O, Effects of hormonal contraceptives on mental rotation and verbal fluency, Psychoneuroendocrinology 36 (8) (2011) 1239–1248. [DOI] [PubMed] [Google Scholar]
- [29].Halpern DF. Sex Differences in Cognitive Abilities, Psychology Press,, New York, NY, 2013. [Google Scholar]
- [30].Hampson E, Morley EE, Estradiol concentrations and working memory performance in women of reproductive age, Psychoneuroendocrinology 38 (12) (2013) 2897–2904. [DOI] [PubMed] [Google Scholar]
- [31].Harburger LL, Bennett JC, Frick KM, Effects of estrogen and progesterone on spatial memory consolidation in aged females, Neurobiol. Aging 28 (4) (2007) 602–610. [DOI] [PubMed] [Google Scholar]
- [32].Hatta T, Nagaya K, Menstrual cycle phase effects on memory and Stroop task performance, Arch. Sex. Behav 38 (5) (2009) 821–827. [DOI] [PubMed] [Google Scholar]
- [33].Hausmann M, Slabbekoorn D, Van Goozen SH, Cohen-Kettenis PT, Güntürkün O, Sex hormones affect spatial abilities during the menstrual cycle, Behav. Neurosci 114 (6) (2000) 1245–1250. [DOI] [PubMed] [Google Scholar]
- [34].Inagaki T, Gautreaux C, Luine V, Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas, Horm. Behav 58 (3) (2010) 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Islam F, Sparkes C, Roodenrys S, Astheimer L, Short-term changes in endogenous estrogen levels and consumption of soy isoflavones affect working and verbal memory in young adult females, Nutr. Neurosci 11 (6) (2008) 251–262. [DOI] [PubMed] [Google Scholar]
- [36].Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V, Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines, Neurobiol. Learn. Mem 94 (4) (2010) 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim J, Szinte J, Boulware M, Frick K, 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms, J. Neurosci 36 (11) (2016) 3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuhl H, Pharmacology of estrogens and progestogens: influence of different routes of administration, Climacteric 8 (sup1) (2005) 3–63. [DOI] [PubMed] [Google Scholar]
- [39].Leranth C, Petnehazy O, MacLusky NJ, Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats, J. Neurosci 23 (5) (2003) 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Linn MC, Petersen AC, Emergence and characterization of sex differences in spatial ability: a meta-analysis, Child Dev. 56 (1985) 1479–1498. [PubMed] [Google Scholar]
- [41].Luine VN, Sex steroids and cognitive function, J. Neuroendocrinol 20 (6) (2008) 866–872. [DOI] [PubMed] [Google Scholar]
- [42].Luine VN, Estradiol and cognitive function: past, present and future, Horm. Behav 66 (4) (2014) 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Luine VN, Richards ST, Wu VY, Beck KD, Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters, Horm. Behav 34 (2) (1998) 149–162. [DOI] [PubMed] [Google Scholar]
- [44].Mäntylä T, Gender differences in multitasking reflect spatial ability, Psychol. Sci 24 (4) (2013) 514–520. [DOI] [PubMed] [Google Scholar]
- [45].McCormick CM, Teillon SM, Menstrual cycle variation in spatial ability: relation to salivary cortisol levels, Horm. Behav 39 (1) (2001) 29–38. [DOI] [PubMed] [Google Scholar]
- [46].McEwen BS, Milner TA, Hippocampal formation: shedding light on the influence of sex and stress on the brain, Brain Res. Rev 55 (2) (2007) 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mihalik JP, Ondrak KS, Guskiewicz KM, McMurray RG, The effects of menstrual cycle phase on clinical measures of concussion in healthy college-aged females, J. Sci. Med. Sport 12 (3) (2009) 383–387. [DOI] [PubMed] [Google Scholar]
- [48].Mordecai KL, Rubin LH, Maki PM, Effects of menstrual cycle phase and oral contraceptive use on verbal memory, Horm. Behav 54 (2) (2008) 286–293. [DOI] [PubMed] [Google Scholar]
- [49].Nielsen SE, Ertman N, Lakhani YS, Cahill L, Hormonal contraception usage is associated with altered memory for an emotional story, Neurobiol. Learn. Mem 96 (2) (2011) 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Packard MG, Posttraining estrogen and memory modulation, Horm. Behav 34 (2) (1998) 126–139. [DOI] [PubMed] [Google Scholar]
- [51].Packard MG, Teather LA, Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism, Neurobiol. Learn. Mem 68 (2) (1997) 172–188. [DOI] [PubMed] [Google Scholar]
- [52].Paris JJ, Frye CA, Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks, Reproduction 136 (1) (2008) 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen BS, Silbersweig D, Stern E, Hippocampal structural changes across the menstrual cycle, Hippocampus 18 (10) (2008) 985–988. [DOI] [PubMed] [Google Scholar]
- [54].Rivera R, Yacobson I, Grimes D, The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices, Am. J. Obstet. Gynecol 181 (5) (1999) 1263–1269. [DOI] [PubMed] [Google Scholar]
- [55].Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS, Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha, Neuroendocrinology 81 (6) (2005) 391–399. [DOI] [PubMed] [Google Scholar]
- [56].Rosenberg L, Park S, Verbal and spatial functions across the menstrual cycle in healthy young women, Psychoneuroendocrinology 27 (7) (2002) 835–841. [DOI] [PubMed] [Google Scholar]
- [57].Sandstrom NJ, Williams CL, Spatial memory retention is enhanced by acute and continuous estradiol replacement, Horm. Behav 45 (2) (2004) 128–135. [DOI] [PubMed] [Google Scholar]
- [58].Shipman SL, Astur RS, Factors affecting the hippocampal BOLD response during spatial memory, Behav. Brain Res 187 (2) (2008) 433–441. [DOI] [PubMed] [Google Scholar]
- [59].Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial, JAMA 289 (20) (2003) 2651–2662. [DOI] [PubMed] [Google Scholar]
- [60].Solís-Ortiz S, Corsi-Cabrera M, Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women, Psychoneuroendocrinology 33 (7) (2008) 989–998. [DOI] [PubMed] [Google Scholar]
- [61].Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS, Uncovering the mechanisms of estrogen effects on hippocampal function, Front. Neuroendocrinol 29 (2) (2008) 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stackman RW, Blasberg ME, Langan CJ, Clark AS, Stability of spatial working memory across the estrous cycle of Long–Evans rats, Neurobiol. Learn. Mem 67 (2) (1997) 167–171. [DOI] [PubMed] [Google Scholar]
- [63].Tuscher JJ, Fortress AM, Kim J, Frick KM, Regulation of object recognition and object placement by ovarian sex steroid hormones, Behav. Brain Res 285 (2015) 140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tuscher JJ, Luine V, Frankfurt M, Frick KM, Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus, J. Neurosci 36 (5) (2016) 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vandenberg SG, Kuse AR, Mental rotations, a group test of three-dimensional spatial visualization, Percept. Mot. Skills 47 (2) (1978) 599–604. [DOI] [PubMed] [Google Scholar]
- [66].Vranić A, Hromatko I, Content-specific activational effects of estrogen on working memory performance, J. Gen. Psychol 135 (3) (2008) 323–336. [DOI] [PubMed] [Google Scholar]
- [67].Wallace M, Luine V, Arellanos A, Frankfurt M, Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex, Brain Res. 1126 (1) (2006) 176–182. [DOI] [PubMed] [Google Scholar]
- [68].Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH, Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life, Neurobiol. Learn Mem 116 (2014) 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Woolley CS, Effects of estrogen in the CNS, Curr. Opin. Neurobiol 9 (3) (1999) 349–354. [DOI] [PubMed] [Google Scholar]
- [70].Woolley CS, McEwen BS, Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat, J. Comp. Neurol 336 (2) (1993) 293–306. [DOI] [PubMed] [Google Scholar]
- [71].Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR, Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort, Neurobiol. Aging 28 (2) (2007) 171–178. [DOI] [PubMed] [Google Scholar]
- [72].Yousuf H, Smies CW, Hafenbreidel M, Tuscher JJ, Fortress AM, Frick KM, Mueller D, Infralimbic estradiol enhances neuronal excitability and facilitates extinction of cocaine seeking in female rats via a BDNF/TrkB mechanism, Front Behav. Neurosci 13 (2019) 168. July 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR, Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats, Biol. Psychiatry 70 (2011) 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]


