Abstract
Background
Among chronic opioid users, the association between decreasing or increasing preoperative opioid utilization and postoperative outcomes is unknown. We hypothesized that decreasing utilization would be associated with improved outcomes and increasing utilization with worsened outcomes.
Methods
Using commercial insurance claims, we identified 57,019 chronic opioid users (≥10 prescriptions or ≥120 days supplied during the preoperative year), aged 18-89 undergoing one of ten surgeries between 2004 and 2018. Patients with a ≥20% decrease or increase in opioid utilization between preoperative days 7-90 and 91-365 were compared to patients with <20% change (stable utilization). The primary outcome was opioid utilization during postoperative days 91-365. Secondary outcomes included alternative measures of postoperative opioid utilization (filling a minimum number of prescriptions during this period), postoperative adverse events, and healthcare utilization.
Results
The average age was 63 (SD 13), with 38,045 (66.7%) female patients. Preoperative opioid utilization was decreasing for 12,347 (21.7%) patients, increasing for 21,330 (37.4%) patients, and stable for 23,342 (40.9%) patients. Patients with decreasing utilization were slightly less likely to fill an opioid prescription during postoperative days 91-365 compared to stable patients (89.2% vs. 96.4%, OR 0.323, 95% CI 0.296 to 0.352, p<0.001), though the average daily doses were similar among patients who continued to utilize opioids during this timeframe (46.7 vs. 46.5 morphine milligram equivalents, difference 0.2, 95% CI −0.8 to 1.2, P=0.684). 93.6% of patients with increasing utilization filled opioid prescriptions during this period (OR 0.57, 95% CI 0.52 to 0.62, p<0.001), with slightly lower average daily doses (44.3 morphine milligram equivalents, difference −2.2, 95% CI −3.1 to −1.3, P<0.001). Except for alternative measures of persistent postoperative opioid utilization, there were no clinically significant differences for the secondary outcomes.
Conclusions
Changes in preoperative opioid utilization were not associated with clinically significant differences for several postoperative outcomes including postoperative opioid utilization.
INTRODUCTION
Opioid use remains a challenging public health crisis in the United States. While progress has been made to reduce opioid prescribing, the rate remains high, with 51.4 opioid prescriptions filled per 100 persons in the US in 2018.1 Many patients who present for surgery utilize opioids on a chronic basis, with studies reporting rates of chronic preoperative use between 23.8% to 65.1% among patients undergoing orthopedic surgery.2,3 Chronic preoperative opioid utilization has been associated with worse perioperative outcomes, including higher mortality, costs, rate of surgical complications, longer hospital length of stay, and more frequent readmissions.4-6 In addition, postoperative pain in chronic opioid users is often difficult to control, relating to pharmacological tolerance and opioid-induced hyperalgesia.7,8,9 The resulting resistance to opioid analgesic effects and heightened susceptibility to pain can perpetuate a cycle of inadequate pain control and persistent opioid requirements.
The preoperative period is an ideal time to optimize patients for surgery, and is the focus of efforts such as the Perioperative Surgical Home and Enhanced Recovery After Surgery programs.10,11 For example, smokers are often counseled to cease smoking preoperatively, as doing so is associated with improved outcomes.12,13 Along these lines, it remains unknown if changes in the amount of opioid utilized in the weeks to months leading up to surgery can affect postoperative outcomes among chronic opioid users. Several smaller studies have suggested preoperative opioid weaning may be beneficial,14-16 and some institutions are undertaking substantial initiatives to taper patients prior to surgery due to the hypothesized benefits.17 However, evidence supporting opioid tapering remains limited. Furthermore, it is unknown if escalation of opioid doses in the immediate preoperative period leads to worsened perioperative outcomes. This study used a national database of healthcare claims to examine whether decreasing or increasing patterns in opioid utilization before surgery are associated with differences in opioid utilized during postoperative days 91-365, as well as postoperative adverse events, number of days admitted, and healthcare costs within the first 30 days. We hypothesized that a decreasing pattern may be associated with improved outcomes, while an increasing pattern may be associated with worsened outcomes.
MATERIALS AND METHODS
Data
This study used a retrospective cohort analysis of administrative health claims data. The data was provided by Optum’s Clinformatics® Data Mart Database (CDM), a statistically de-identified database of administrative health claims for members of a large national managed care company affiliated with Optum. Patients in the data receive private insurance coverage, often through their employer. In addition, the database also contains claims for elderly (age 65 and older) patients who receive private insurance coverage through the Medicare Advantage program. The data are detailed and include clinical data such as diagnosis and procedure codes, as well as socioeconomic data. Pharmacy claims data were used to identify prescriptions filled for the following opioids: codeine, fentanyl, hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, and tramadol by matching against the generic name of the drug provided in the data. Prescriptions were converted to oral morphine milligram equivalents and the daily dose of opioid was calculated for each patient using the unit strength, number of units prescribed, and days supplied from the data.18 Importantly, while the data describes prescriptions filled, the actual amount and timing of opioid consumed by patients may significantly differ and remains unknown.19,20
This study was included in the umbrella Institutional Review Board protocol for de-identified data managed by the Center for Population Heath Sciences at Stanford University (PHS-40974), which included a waiver of consent. A data analysis and statistical plan was written, date-stamped, and recorded in the investigators’ files before data were accessed.
Sample
The initial sample included 2,261,766 surgical procedures between January 1, 2004 and June 30, 2018 for patients 18 to 89 years old undergoing one of the following: primary total knee arthroplasty, primary total hip arthroplasty, laparoscopic cholecystectomy, open cholecystectomy, laparoscopic appendectomy, open appendectomy, cesarean section, functional endoscopic sinus surgery, transurethral resection of the prostate, or simple mastectomy. Procedures were identified using current procedural terminology (CPT) codes using previously described methods (eTable 1 in the Supplement).21
We excluded patients who underwent any other surgical procedure in the year before or after the surgery of interest (N=185,471), except for postoperative days 0-30 to measure postoperative complications. We then excluded patients who did not have continuous enrollment in their insurance plan during this 2-year period (N= 1,107,135) and hip arthroplasties associated with fracture within the previous 30 days (N=4,192).22 From the remaining 964,968 patients with 3,993,731 opioid prescriptions (585 of which were excluded due to invalid strength data), we excluded patients who did not meet our definition of chronic preoperative opioid utilization, which we defined as having ≥10 opioid prescriptions filled or 120 days of opioid prescribed in the year prior to surgery (N=907,373).21 Finally, to avoid the influence of extreme outliers, we excluded patients with the top 1% of opioid utilized during the year before surgery (N=576, >458 morphine milligram equivalents per day). The final sample consisted of 57,019 patients (eFigure 1 in the Supplement). No statistical power calculation was conducted prior to the study as the study used all available data.
Outcomes
The primary outcome was the amount of opioid prescribed during postoperative days 91-365. Secondary outcomes included the incidence of persistent postoperative opioid utilization during days 91-365, the average daily dose of opioid during preoperative day 7 to postoperative day 30 (including the 7 days before surgery to account for patients who prefilled their postoperative prescriptions), the incidence of adverse events, healthcare costs, and the number of days admitted within the first 30 days. Persistent postoperative opioid utilization was modeled using a range of 8 definitions based upon the number of prescriptions filled and days with an opioid prescribed (at least 4/60, 5/70, 6/80, 7/90, 8/100, 9/110, 10/120, 11/130 prescriptions filled/days with an opioid prescribed) during postoperative days 91-365.21 Postoperative adverse events included: surgical site infection, urinary tract infection, pneumonia, sepsis, thromboembolic event, myocardial infarctions, and narcotic overdose. All adverse events were identified by diagnosis codes using previously described methods.23,24 Healthcare costs were calculated as the sum of all charges requested to be reimbursed by the insurance plan.25,26
Exposure
Our independent variable of interest was decreasing or increasing opioid utilization prior to surgery, assessed by comparing the average daily opioid dose between preoperative days 91-365 and preoperative days 7-90. A ≥20% decrease, ≥20% increase, or remaining within ±20% were classified as decreasing, increasing, and stable utilization, respectively. Opioid prescriptions in the 7 days prior to surgery were not included since patients may fill their postoperative opioid prescriptions during this period.
Other Variables
Variables captured as potential confounders included age, sex, type, and year of surgery. Using previously described methods, medical comorbidities were measured using the Elixhauser index based upon relevant diagnosis codes.27,28 In addition, we included variables for the average daily opioid dose from preoperative days 7-90 and 91-365 to adjust for effects on our outcomes attributable to the specific dose rather than a change in utilization. For sensitivity analyses we obtained socioeconomic variables (race, household income, and education level), as well as the National Provider Identifier of the surgeon.
Statistical Analysis
Demographic and comorbidity data were reported as means and 95% confidence intervals. Independent samples t-tests for continuous variables and Chi-squared tests for categorical variables were used to assess differences between patient cohorts, with Hedges’ g provided as a measure of effect size.29,30 Two-tailed hypothesis testing was used for all analyses in the study.
We estimated the associations between preoperative opioid utilization patterns and our outcomes using multivariable regression models which included adjustments for the potential confounders shown in Table 1 and eTable 2 in the supplement. In the case of the primary outcome, average daily morphine milligram equivalents prescribed during postoperative days 91-365, a significant percentage of patients did not fill any prescription for an opioid during this period (N=4,920, 8.6%). Therefore, a simple regression which included all patients (including those with no opioid prescribed) would be downward-biased.31 To mitigate this issue, we modeled postoperative opioid utilization using a two-step analysis.32-34 In the first step, a logistic regression was used to assess the association between preoperative opioid utilization patterns and whether the patient was prescribed any opioid at all during postoperative days 91-365. In the second step, a linear regression was used to assess the association preoperative opioid utilization patterns and average daily morphine milligram equivalents and was restricted to patients who were prescribed some opioid during postoperative days 91-365.
Table 1.
Patient characteristics
| Variable | Patients with stable opioid utilization N=23,342 (40.9%) |
Patients with decreasing opioid utilization N=12,347 (21.7%) |
Patients with increasing opioid utilization N=21,330 (37.4%) |
||||
|---|---|---|---|---|---|---|---|
| P-value | Hedges’ g | P-value | Hedges’ g | ||||
| Demographics | |||||||
| Age, mean, years [SD] | 64 [12] | 62 [15] | <0.001 | 0.147 | 63 [14] | <0.001 | 0.097 |
| Female, N (%) | 15,382 (65.9%) | 8,526 (69.1%) | <0.001 | −0.067 | 14,137 (66.3%) | 0.398 | −0.008 |
| Opioid utilization in preoperative year, mean [SD] | |||||||
| Number of opioid prescriptions | 14 [8] | 12 [7] | <0.001 | 0.343 | 12 [7] | <0.001 | 0.251 |
| Number of days with opioid prescription | 290 [78] | 214 [82] | <0.001 | 0.948 | 218 [85] | <0.001 | 0.877 |
| Average daily morphine milligram equivalents utilized in preoperative days 365-91 | 59.9 [77.3] | 43.5 [65.1] | <0.001 | 0.224 | 27.4 [42.2] | <0.001 | 0.516 |
| Average daily morphine milligram equivalents utilized in preoperative days 90-7 | 60.5 [78.5] | 20.1 [37.1] | <0.001 | 0.602 | 49.7 [69.1] | <0.001 | 0.145 |
| Type of surgery, N (%) | |||||||
| Total knee arthroplasty | 8,204 (35.1%) | 3,982 (32.3%) | <0.001 | 0.061 | 7,066 (33.1%) | <0.001 | 0.043 |
| Total hip arthroplasty | 3,650 (15.6%) | 1,695 (13.7%) | <0.001 | 0.054 | 5,584 (26.2%) | <0.001 | −.0263 |
| Laparoscopic cholecystectomy | 6,253 (26.8%) | 3,399 (27.5%) | 0.136 | −0.017 | 4,761 (22.3%) | <0.001 | 0.104 |
| Open cholecystectomy | 421 (1.8%) | 220 (1.8%) | 0.884 | 0.002 | 340 (1.6%) | 0.087 | 0.016 |
| Laparoscopic appendectomy | 793 (3.4%) | 445 (3.6%) | 0.309 | −0.011 | 623 (2.9%) | 0.004 | 0.018 |
| Open appendectomy | 150 (0.6%) | 86 (0.7%) | 0.549 | −0.007 | 106 (0.5%) | 0.042 | 0.019 |
| Cesarean section | 228 (1.0%) | 565 (4.6%) | <0.001 | −0.246 | 300 (1.4%) | <0.001 | −0.040 |
| Functional endoscopic sinus surgery | 1,636 (7.0%) | 968 (7.8%) | 0.004 | −0.032 | 1,264 (5.9%) | <0.001 | 0.044 |
| Transurethral resection of the prostate | 667 (2.9%) | 344 (2.8%) | 0.668 | 0.004 | 438 (2.1%) | <0.001 | 0.052 |
| Simple mastectomy | 1,340 (5.7%) | 643 (5.2%) | 0.037 | 0.023 | 848 (4.0%) | <0.001 | 0.082 |
P-values reflect the comparison between the decreasing or increasing cohort and patients with stable utilization and were computed using Chi-squared and independent samples t-tests for categorical and continuous variables respectively. Hedge’s g measures effect size as a standardized difference between cohorts, with values less than 0.2 representing small differences, values between 0.2 and 0.5 representing moderate differences, and values greater than 0.5 representing large differences.
For the secondary outcomes, multivariable linear regressions were used for continuous outcomes and multivariable logistic regressions were used for binary outcomes with the same set of covariates described above. We also applied a Bonferroni-corrected threshold for significance to adjust for multiple comparisons for our 18 reported secondary outcomes (ɑ = 0.002).35 Our analyses were performed using MATLAB, version R2015a (MathWorks, Inc.; Natick, MA) and STATA 14/MP (StataCorp; College Station, TX).
Subgroup Analyses
To gain additional insight into how the association between preoperative opioid utilization patterns and our primary outcome varies for different surgical situations, we analyzed several subgroups of procedures. First, we compared elective procedures, defined as procedures where preoperative optimization is possible, to nonelective procedures, where optimization is generally not possible. Elective procedures included primary total knee/hip arthroplasty, cesarean section, transurethral resection of the prostate, and simple mastectomy. Nonelective procedures included laparoscopic/open appendectomy. Laparoscopic and open cholecystectomies were classified as elective or nonelective based upon the presence of a diagnosis code for acute cholecystitis.36 We also compared procedures related to chronic pain (total knee and hip arthroplasties) to procedures unrelated to chronic pain (the remaining procedures) and analyzed each procedure as an independent subgroup.
Sensitivity Analyses
We considered the robustness of our findings to several sensitivity analyses. First, since our main analysis measured the pattern of preoperative opioid utilized using a 90-day cutoff, we repeated the analysis using 30 and 180 day-cutoffs. Second, our main analysis assigned cohorts using a ≥20% change in opioid dose, so we repeated our main analyses using a ≥50% change. Third, to assess the influence of socioeconomic variables, we repeated our analyses on the subset of patients for whom socioeconomic data were available (N=45,764, 80.3%) to adjust for race, household income, and education level. Finally, to adjust for provider-specific effects, we added clustering based upon the surgeon’s National Provider Identifier when available (N=54,659, 95.9%).
Revisions to Analysis Plan
The following analyses were not included in the original analysis plan and were added during peer review: secondary analyses for measures of persistent postoperative opioid utilization, subgroup analyses for elective vs. non-elective procedures and procedures related vs. unrelated to chronic pain, and sensitivity analyses for socioeconomic status and provider-specific effects. Furthermore, while the original analysis included data for surgeries up to December 31, 2016, during review additional data for procedures through June 30, 2018 became available which were included in the final version of the manuscript.
RESULTS
Patient Characteristics
The average age was 63 years (SD 13 years), with 38,045 (66.7%) female patients. Preoperative opioid utilization was decreasing for 12,347 patients (21.7%), increasing for 21,330 (37.4%) patients, and stable for 23,342 (40.9%) patients. Patient characteristics are shown by cohort in Table 1 and eTable 2 in the supplement.
Main Analysis
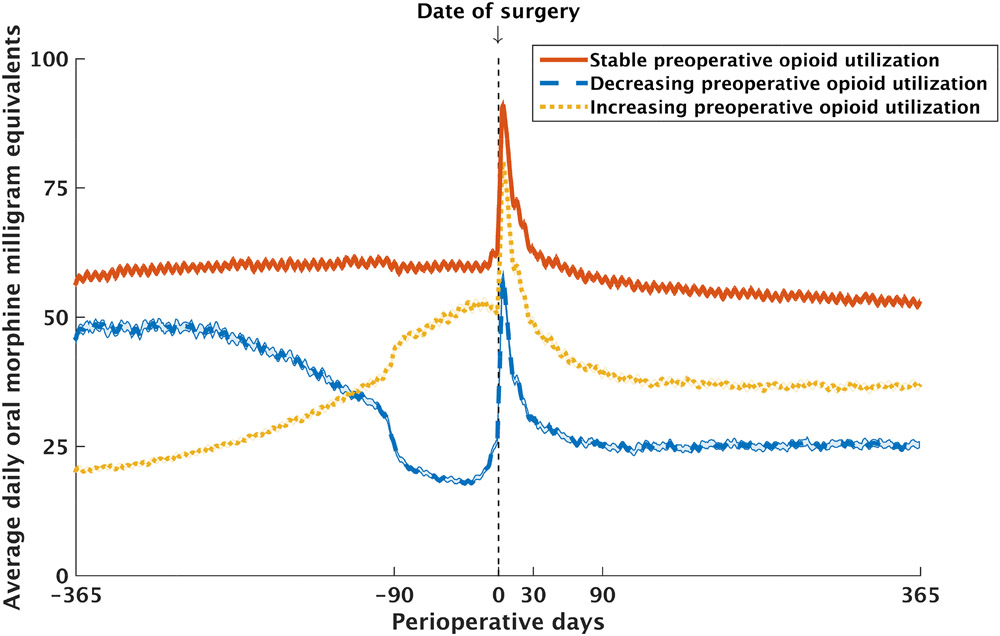
Overall, 52,099 (91.4%) of patients had at least one prescription for an opioid during postoperative days 91-365. Prior to adjusting for confounders, the incidence of having any opioid prescribed in this period was lower for patients with both decreasing (85.1%, 95% CI 84.8 to 85.3%, odds ratio (OR) 0.272, 95% CI 0.251 to 0.294, P<0.001) and increasing (90.6%, 95% CI 90.3% to 90.8%, OR 0.46, 95% CI 0.42 to 0.50, P<0.001) opioid utilization compared to patients with stable utilization (95.4%, 95% CI 95.2% to 95.7%). The average daily dose of opioid during postoperative days 91-365 was also lower for patients with both decreasing (29.7 morphine milligram equivalents, 95% CI 28.5 to 30.8, difference −27.7, 95% CI −29.2 to −26.1, P<0.001) and increasing (41.0 morphine milligram equivalents, 95% CI 40.0 to 42.0, difference −16.3, 95% CI −17.8 to −14.9, P<0.001) opioid utilization compared to patients with stable utilization (57.3 morphine milligram equivalents, 95% CI 56.3 to 58.4) (Table 2, Figure 1).
Table 2.
Outcomes
| Patients with stable opioid utilization N=23,342 (40.9%) |
Patients with decreasing opioid utilization N=12,347 (21.7%) |
Patients with increasing opioid utilization N=21,330 (37.4%) |
|||||
|---|---|---|---|---|---|---|---|
| Primary Outcomes | |||||||
| Incidence of utilizing any opioid during postoperative days 91-365, % | Unadjusted | ||||||
| 95.4% (95.2 to 95.7%) | 85.1% (84.4 to 85.7%) | Odds ratio | 90.6% (90.2 to 91.0%) | Odds ratio | |||
| 0.272 (0.251 to 0.294) | P<0.001 | 0.46 (0.42 to 0.50) | P<0.001 | ||||
| Adjusted | |||||||
| 96.2% (96.0 to 96.5%) | 89.2% (88.5 to 89.9%) | 0.323 (0.296 to 0.352) | P<0.001 | 93.6% (93.2 to 93.9%) | 0.57 (0.52 to 0.62) | P<0.001 | |
| Average daily opioid dose utilized during postoperative days 91-365, morphine milligram equivalents | Unadjusted | ||||||
| 57.3 (56.3 to 58.4) | 29.7 (28.5 to 30.8) | Difference | 41.0 (40.0 to 42.0) | Difference | |||
| −27.7 (−29.2 to −26.1) | P<0.001 | −16.3 (−17.8 to −14.9) | P<0.001 | ||||
| Adjusted | |||||||
| 46.5 (45.9 to 47.0) | 46.7 (45.8 to 47.6) | 0.2 (−0.8 to 1.2) | P=0.684 | 44.3 (43.6 to 44.9) | −2.2 (−3.1 to −1.3) | P<0.001 | |
| Secondary Outcomes a | |||||||
| Average daily opioid dose utilized during preoperative day 7 to postoperative day 30, morphine milligram equivalents | Unadjusted | ||||||
| 74.4 (72.7 to 76.1) | 41.1 (39.7 to 42.5) | Difference | 62.1 (60.4 to 63.8) | Difference | |||
| −33.3 (−35.5 to −31.1) | P<0.001 | −12.3 (−14.7 to −9.9) | P<0.001 | ||||
| Adjusted | |||||||
| 62.8 (62.1 to 63.6) | 67.4 (66.2 to 68.6) | 4.6 (3.1 to 6.0) | P<0.001 | 60.6 (59.7 to 61.5) | −2.3 (−3.5 to −1.0) | P<0.001 | |
| Incidence of narcotic overdose, % | Unadjusted | ||||||
| 0.4% (0.3 to 0.5%) | 0.3% (0.1 to 0.4%) | Odds ratio | 0.4% (0.2 to 0.5%) | Odds ratio | |||
| 0.66 (0.36 to 1.20) | P=0.037 | 0.85 (0.53 to 1.34) | P=0.276 | ||||
| Adjusted | |||||||
| Regression model unable to converge | |||||||
| Total healthcare costs, US$ | Unadjusted | ||||||
| $48,632 (47,754 to 49,511) | $45,987 (44,785 to 47,189) | Difference | $51,430 (50,500 to 52,359) | Difference | |||
| −$2,645 (−4,134 to −1,156) | P<0.001 | $2,797 (1,518 to 4,076) | P<0.001 | ||||
| Adjusted | |||||||
| $48,732 (47,937 to 49,528) | $49,054 (47,884 to 50,224) | Difference | $49,544 (48,657 to 50,431) | Difference | |||
| $322 (−1,084 to 1,727) | P=0.494 | $812 (−412 to 2,035) | P=0.047 | ||||
| Number of days admitted | Unadjusted | ||||||
| 0.7 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | Difference | 0.8 (0.7 to 0.9) | Difference | |||
| 0.0 (−0.1 to 0.1) | P=0.314 | 0.2 (0.1 to 0.3) | P<0.001 | ||||
| Adjusted | |||||||
| 0.7 (0.6 to 0.8) | 0.7 (0.6 to 0.8) | Difference | 0.8 (0.7 to 0.8) | Difference | |||
| 0.0 (−0.1 to 0.1) | P=0.610 | 0.1 (−0.0 to 0.2) | P=0.087 | ||||
The incidences, average daily dose of opioid, healthcare costs and number of days admitted are reported with both unadjusted and adjusted values. Adjustment modeled outcomes controlling for age, sex, type and year of surgery, and medical comorbidities. For the average daily dose of opioid, patients who were not prescribed any opioid in the relevant period were excluded to prevent downward biasing of the results. Primary outcomes are reported with 95% confidence intervals.
Secondary outcomes applied a Bonferroni correction for multiple comparisons. With 18 reported outcomes (4 in this table, 6 in eTable 2 in the supplement, and 8 definitions for persistent postoperative utilization shown in Figure 2), the threshold for significance is α = 0.05 / 18 = 0.002. Thus, 99.8% confidence intervals are reported. Except where otherwise specified, secondary outcomes were measured during postoperative days 0-30.
Figure 1. Average daily dose of opioid prescribed during the 2-year perioperative window.
Compared to preoperative days 365 to 91, patients with stable utilization maintained their average daily opioid dose within ±20% during preoperative days 90 to 7, while patients with decreasing utilization reduced their average daily dose by at least 20%, and patients with increasing utilization escalated their average daily dose by at least 20% in the same period.
After adjusting for potential confounders, the incidence of having any opioid prescribed during postoperative days 91-365 remained lower for patients with both decreasing (89.2%, 95% CI 88.5% to 89.9%, OR 0.323, 95% CI 0.296 to 0.352, P<0.001) and increasing opioid utilization (93.6%, 95% CI 93.2% to 93.9%, OR 0.57, 95% CI 0.52 to 0.62, P<0.001) compared to patients with stable utilization (96.2%, 95% CI 96.0% to 96.5%). Among patients who continued to utilize opioids in this period, the average daily dose of opioid was not different for patients with decreasing utilization (46.7 morphine milligram equivalents, 95% CI 45.8 to 47.6, difference 0.2, 95% CI −0.8 to 1.2, P=0.684), but was slightly lower for patients with increasing utilization (44.3 morphine milligram equivalents, 95% CI 43.6 to 44.9, difference −2.2, 95% CI −3.1 to −1.3, P<0.001) compared to patients with stable utilization (46.5 morphine milligram equivalents, 95% CI 45.9 to 47.0) (Table 2).
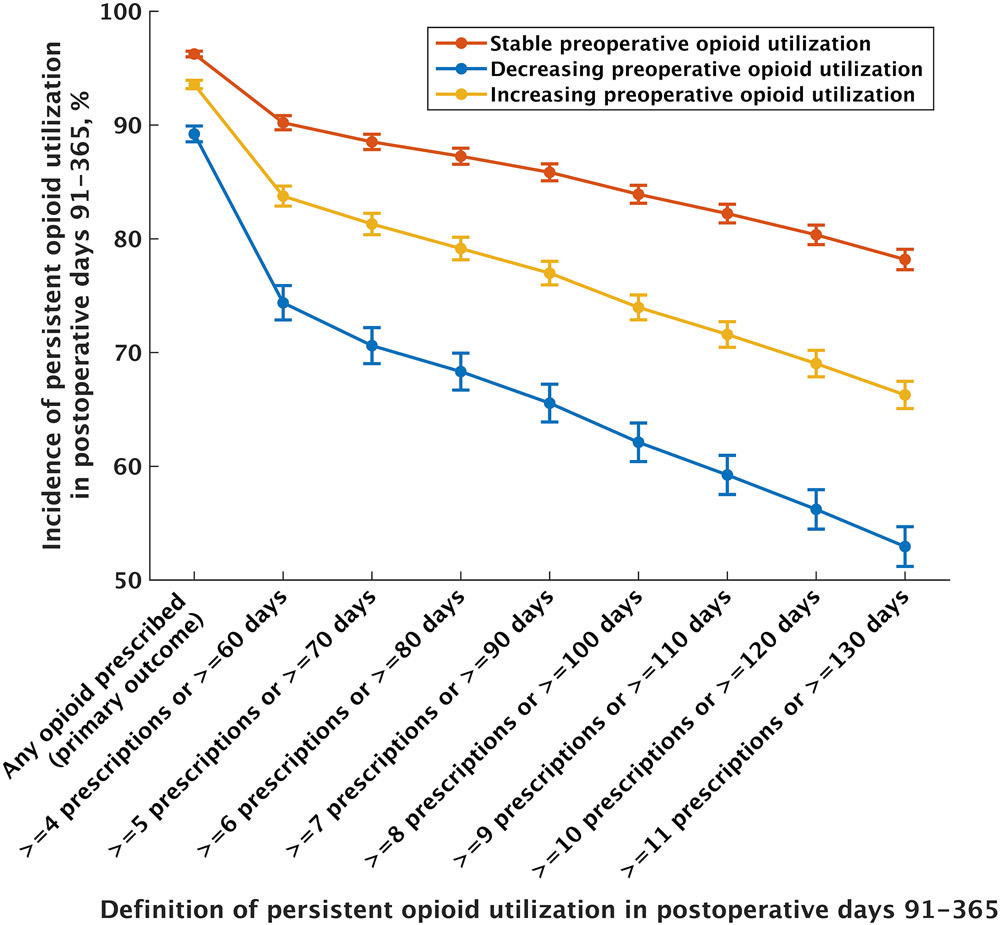
For our secondary outcomes, we modeled a range of 8 definitions for persistent postoperative opioid utilization which consistently demonstrated lower rates of incidence for patients with both decreasing and increasing preoperative opioid utilization. For example, using a definition for persistent postoperative opioid utilization of ≥10 prescriptions filled or ≥120 days supplied during postoperative days 91-365, the adjusted incidence was 56.2% (99.8% CI 54.5% to 57.9%, OR 0.314, 99.8% CI 0.289 to 0.341, P<0.001) for patients with decreasing utilization and 69.0% (99.8% CI 67.9% to 70.2%, OR 0.55, 95% CI 0.51 to 0.59, P<0.001) for patients with increasing utilization compared to patients with stable utilization (80.3%, 99.8% CI 77.3% to 79.1%) (Figure 2).
Figure 2. The association between patterns of preoperative opioid utilization and persistent postoperative opioid utilization.
The adjusted incidence of persistent postoperative opioid utilization for a range of 8 definitions are shown. Patients with both decreasing and increasing preoperative opioid utilization had reduced incidence of persistent postoperative opioid utilization compared to patients with stable utilization. Values are shown with 99.8% confidence intervals, which use the Bonferroni correction for multiple comparisons as described in the methods section.
The adjusted average daily dose of opioid during preoperative day 7 to postoperative day 30 was higher for patients with decreasing utilization (67.4 morphine milligram equivalents, 99.8% CI 66.2 to 68.6, difference 4.5, 99.8% CI 3.1 to 6.0, P<0.001) and lower for patients with increasing utilization (60.6 morphine milligram equivalents, 99.8% CI 59.7 to 61.5, difference −2.3, 99.8% CI −3.5 to −1.0, P<0.001) compared to patients with stable utilization (62.8 morphine milligram equivalents, 99.8% CI 62.1 to 63.6) among patients who filled a prescription for opioids in this period. No differences were found in the rate of postoperative adverse events, healthcare costs, or the number of days admitted for patients with either decreasing or increasing preoperative opioid utilization compared to patients with stable utilization (Table 2, eTable 3 in the supplement).
Subgroup and sensitivity analyses
Subgroup analyses stratifying patients by procedure urgency, relation to chronic pain treatment, and type of surgery (Table 3), as well sensitivity analyses which varied the timing and magnitude of changes in dose required to classify patterns in opioid utilization and added additional adjustments for socioeconomic and surgeon-related factors (eTable 4 in the Supplement) yielded qualitatively similar results to our main analysis.
Table 3.
Subgroup analyses by type of procedure
| Subgroup | Number of patients with preoperative chronic opioid use (% of full sample) |
Number of patients with any opioid prescribed in postoperative days 91-365 (% of subgroup) |
Patients with stable opioid utilization | Patients with decreasing opioid utilization | Patients with increasing opioid utilization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients (%) |
Adjusted incidence of any opioid prescribed in postoperative days 91-365 |
Adjusted average daily dose of opioid in postoperative days 91-365 (morphine milligram equivalents) |
Number of patients (%) |
Adjusted incidence and odds ratio of any opioid prescribed in postoperative days 91-365 |
Adjusted amount and difference of average daily opioid dose in postoperative days 91-365 (morphine milligram equivalents) |
Number of patients (%) |
Adjusted incidence and odds ratio of any opioid prescribed in postoperative days 91-365 |
Adjusted amount and difference of average daily opioid dose in postoperative days 91-365 (morphine milligram equivalents) |
|||
| Procedure urgency a | |||||||||||
| Elective procedures | 48,199 (84.5%) | 43,837 (91.0%) | 19,438 (40.3%) | 95.8% (95.5 to 96.1) | 45.6 (45.0 to 46.2) | 10,232 (21.2%) | 88.8% (88.0 to 89.5%) | 46.0 (45.0 to 46.9) | 18,529 (38.4%) | 91.8% (91.4 to 92.2%) | 42.2 (41.6 to 42.9) |
| 0.348 (0.317 to 0.381) P<0.001 | 0.3 (−0.7 to 1.5) P=0.540 | 0.49 (0.45 to 0.54) P<0.001 | −3.3 (−4.3 to −2.4) P<0.001 | ||||||||
| Nonelective procedures | 8,820 (15.5%) | 8,262 (93.7%) | 3,904 (44.3%) | 97.6% (97.0 to 98.0%) | 52.8 (51.5 to 54.2) | 2,115 (24.0%) | 90.1% (88.2 to 91.6%) | 53.0 (50.9 to 55.1) | 2,801 (31.8%) | 96.0% (95.2 to 96.7%) | 52.2 (50.5 to 54.0) |
| 0.227 (0.177 to 0.291) P<0.001 | 0.2 (−2.4 to 2.7) P=0.905 | 0.60 (0.46 to 0.78) P<0.001 | −0.6 (−2.8 to 1.7) P=0.610 | ||||||||
| Pain-related procedures b | |||||||||||
| Procedure treats chronic pain | 30,181 (52.9%) | 26,844 (88.9%) | 11,854 (39.3%) | 94.3% (93.9 to 94.7%) | 42.1 (41.4 to 42.8) | 5,677 (18.8%) | 86.3% (85.3 to 87.3%) | 41.0 (39.9 to 42.2) | 12,650 (41.9%) | 90.3% (89.7 to 90.9%) | 39.0 (38.2 to 39.8) |
| 0.380 (0.341 to 0.423) P<0.001 | −1.1 (−2.4 to 0.2) P=0.111 | 0.56 (0.51 to 0.62) P<0.001 | −3.1 (−4.2 to −2.0) P<0.001 | ||||||||
| Procedure does not treat chronic pain | 26,838 (47.1%) | 25,255 (94.1%) | 11,488 (42.8%) | 97.7% (97.4 to 98.0%) | 51.1 (50.3 to 52.0) | 6,670 (24.9%) | 91.5% (90.5 to 92.3%) | 53.3 (52.1 to 54.6) | 8,680 (32.3%) | 96.0% (95.5 to 96.4%) | 49.5 (48.4 to 50.5) |
| 0.252 (0.218 to 0.292) P<0.001 | 2.2 (0.6 to 3.7) P=0.005 | 0.56 (0.48 to 0.66) P<0.001 | −1.7 (−3.1 to −0.3) P=0.018 | ||||||||
| Individual surgical procedures | |||||||||||
| Primary total knee arthroplasty | 19,252 (33.8%) | 17,799 (92.5%) | 8,204 (42.6%) | 96.5% (96.1 to 96.9%) | 42.0 (41.2 to 42.9) | 3,982 (20.7%) | 90.8% (89.6 to 91.9%) | 43.4 (42.1 to 44.7) | 7,066 (36.7%) | 94.3% (93.7 to 94.9%) | 40.3 (39.3 to 41.3) |
| 0.359 (0.308 to 0.419) P<0.001 | 1.3 (−0.2 to 2.9) P=0.088 | 0.60 (0.52 to 0.69) P<0.001 | −1.7 (−3.0 to −0.44) P=0.009 | ||||||||
| Primary total hip arthroplasty | 10,929 (19.2%) | 9,045 (82.8%) | 3,650 (33.4%) | 89.6% (88.5 to 90.6%) | 41.6 (40.3 to 43.0) | 1,695 (15.5%) | 79.7% (77.5 to 81.8%) | 36.9 (34.7 to 39.2) | 5,584 (51.1%) | 85.1% (84.0 to 86.2%) | 37.0 (35.8 to 38.2) |
| 0.46 (0.39 to 0.54) P<0.001 | −4.7 (−7.2 to −2.2) P<0.001 | 0.66 (0.58 to 0.76) P<0.001 | −4.6 (−6.5 to −2.7) P<0.001 | ||||||||
| Laparoscopic cholecystectomy | 14,413 (25.3%) | 13,588 (94.3%) | 6,253 (43.4%) | 97.8% (97.4 to 98.1%) | 53.1 (52.0 to 54.2) | 3,399 (23.6%) | 92.2% (91.0 to 93.3%) | 55.0 (53.4 to 56.7) | 4,761 (33.0%) | 96.3% (95.7 to 96.8%) | 50.5 (49.1 to 51.8) |
| 0.268 (0.219 to 0.328) P<0.001 | 1.9 (0.0 to 3.9) P=0.055 | 0.58 (0.47 to 0.72) P<0.001 | −2.7 (−4.4 to −0.9) P=0.003 | ||||||||
| Open cholecystectomy | 981 (1.7%) | 901 (91.8%) | 421 (42.9%) | N/A | 58.9 (53.8 to 63.9) | 220 (22.4%) | N/A | 59.0 (50.8 to 67.1) | 340 (34.7%) | N/A | 56.6 (50.4 to 62.8) |
| 0.1 (−9.5 to 9.8) P=0.978 | −2.2 (−10.4 to 6.0) P=0.592 | ||||||||||
| Laparoscopic appendectomy | 1,861 (3.3%) | 1,742 (93.6%) | 793 (42.6%) | N/A | 49.8 (46.7 to 53.0) | 445 (23.9%) | N/A | 47.2 (42.2 to 52.2) | 623 (33.5%) | N/A | 54.2 (50.3 to 58.0) |
| −2.6 (−8.6 to 3.3) P=0.387 | 4.3 (−0.8 to 9.4) P=0.095 | ||||||||||
| Open appendectomy | 342 (0.6%) | 318 (93.0%) | 150 (43.9%) | N/A | N/A | 86 (25.1%) | N/A | N/A | 106 (31.0%) | N/A | N/A |
| Cesarean section | 1,093 (1.9%) | 1,000 (91.5%) | 228 (20.9%) | N/A | N/A | 565 (51.7%) | N/A | N/A | 300 (27.4%) | N/A | N/A |
| Functional endoscopic sinus surgery | 3,868 (6.8%) | 3,667 (94.8%) | 1,636 (42.3%) | 98.8% (98.2 to 99.2%) | 51.2 (48.4 to 53.9) | 968 (25.0%) | 93.2% (90.5 to 95.1%) | 53.2 (49.1 to 57.2) | 1,264 (32.7%) | 97.2% (96.2 to 98.0%) | 49.4 (46.1 to 52.8) |
| 0.162 (0.102 to 0.258) P<0.001 | 2.0 (−2.9 to 6.9) P=0.418 | 0.42 (0.257 to 0.68) P<0.001 | −1.7 (−6.2 to 2.8) P=0.454 | ||||||||
| Transurethral resection of the prostate | 1,449 (2.5%) | 1,351 (93.2%) | 667 (46.1%) | N/A | 44.0 (41.8 to 46.3) | 344 (23.7%) | N/A | 46.5 (42.9 to 50.1) | 438 (30.2%) | N/A | 44.8 (41.7 to 47.8) |
| 2.4 (−1.8 to 6.7) P=0.277 | 0.8 (−3.1 to 4.6) P=0.703 | ||||||||||
| Simple mastectomy | 2,831 (5.0%) | 2,688 (94.9%) | 1,340 (47.3%) | N/A | 43.7 (41.6 to 45.7) | 643 (22.7%) | N/A | 43.3 (39.8 to 46.7) | 848 (30.0%) | N/A | 45.4 (42.6 to 48.2) |
| −0.4 (−4.4 to 3.6) P=0.837 | 1.7 (−1.8 to 5.3) P=0.337 | ||||||||||
Subgroup analyses modeled the primary outcomes of the study stratifying patients by surgical context and specific procedures. Fields marked “N/A” represent regression models which were unable to converge due to limited sample size of the subgroup.
“Elective” is defined as surgeries for which preoperative optimization is typically feasible, and includes primary total knee arthroplasty, primary total hip arthroplasty, laparoscopic and open cholecystectomy without associated acute cholecystitis, cesarean section, transurethral resection of the prostate, and simple mastectomy, while “Nonelective” includes laparoscopic and open cholecystectomy with acute cholecystitis, and laparoscopic and open appendectomy.
Procedures related to chronic pain treatment include: total knee arthroplasty and total hip arthroplasty, while procedures not intended to treat chronic pain include laparoscopic and open cholecystectomy, laparoscopic and open appendectomy, cesarean section, functional endoscopic sinus surgery, transurethral resection of the prostate, and simple mastectomy
DISCUSSION
In this retrospective analysis of 57,019 chronic opioid users aged 18-89 undergoing one of 10 common surgeries, either a decrease or an increase in the daily dose of opioid by at least 20% in the 90 days before surgery was associated with slightly less long-term opioid utilization, with the adjusted incidence of any opioid prescribed during postoperative days 91-365 being 89.2% for patients with decreasing utilization and 93.6% for patients with increasing utilization, compared to 96.4% for patients with stable utilization. However, for patients continuing to utilize opioids, the adjusted average daily dose prescribed for patients with decreasing utilization was not different from patients with stable utilization. Surprisingly, patients with increasing preoperative opioid utilization continuing opioids during postoperative days 91-365 had a slightly lower adjusted average daily dose (44.3 morphine milligram equivalents per day) compared to patients with stable utilization (46.7 morphine milligram equivalents per day), though this likely represents a clinically insignificant difference. For example, several studies in chronic pain patients used a threshold of 8-30 morphine milligram equivalents per day to define a clinically significant reduction in opioid use.37,38 We hypothesize that increasing preoperative utilization may be attributable to worsening pain that was improved by surgery, allowing cessation or further reduction in opioid utilization postoperatively.
Importantly, while the relative reduction in the odds of filling a prescription for an opioid during postoperative days 91-365 was substantial for patients with both decreasing (adjusted odds ratio 0.323) and increasing (adjusted odds ratio 0.57) preoperative opioid utilization, the absolute reductions were small (−7.0% and −2.6% respectively). This suggests that preoperative changes in opioid utilization may have limited clinical associations with several measures of long-term postoperative opioid utilization. However, a secondary analysis which defined persistent postoperative opioid utilization using a minimum number of opioid prescriptions and/or days supplied did find large absolute reductions in the incidence of persistent postoperative opioid utilization for both decreasing and increasing patterns (56.2% and 69.0% respectively, compared to 80.3% for stable patterns using ≥10 prescriptions filled or ≥120 days supplied), indicating further study is warranted.
For our secondary outcomes, both decreasing and increasing preoperative opioid utilization were associated with small, likely clinically insignificant differences in opioid doses prescribed during preoperative day 7 to postoperative day 30. Additionally, no difference in postoperative adverse events, number of days admitted, or healthcare costs in either cohort were observed.
This study has several important limitations. First, by limiting this study to patients with no other surgical interventions in a 2-year period, the sample population may be biased toward healthier patients who are less likely to have complications with shorter length of stay and lower total costs. This, along with the relatively short duration that preoperative changes in dose were sustained, may explain why no significant associations were detected despite previous literature suggesting that chronic preoperative opioid utilization negatively correlates with these outcomes.4-6
Second, due to the limited nature of claims data, we cannot assess why patients altered their opioid dose prior to surgery, and the possibility of hidden confounders associated with changes in opioid utilization is real. For example, patients with decreasing utilization may have received preoperative guidance from surgeons, pain specialists, or primary care physicians, and this management approach may have been continued postoperatively. These patients may also have been self-motivated, and success with reducing their opioid requirements preoperatively may facilitate postoperative cessation. Patients with decreasing utilization may also have had unrelated improvements in chronic pain preoperatively, which could lead to reduced pain and opioid requirements postoperatively. Conversely, patients with increasing utilization may have experienced worsening of their underlying pain conditions, which was then improved by surgery leading to decreased pain and a reduced postoperative opioid requirement. However, one source of reassurance against hidden confounding is that our results held for the subgroup of nonelective procedures, as any preoperative changes in opioid utilization could not relate to the preparation for surgery. An additional source of confounding could be regression artifacts such as “regression to the mean”, which may be of particular concern in our study since it defined exposure groups based upon preoperative opioid use and modeled postoperative opioid utilization based on these same groups.39 However, these regression artifacts would tend to bias our results upwards (i.e. bias toward finding a larger effect than the actual effect). Since our adjusted results were generally small in magnitude, this suggests that regression to the mean has minimal actual influence on our findings. Third, claims data measures drug utilization (i.e., the fulfillment of prescriptions), but not drug use (the actual amount and timing of opioid consumed). While utilization and use would generally correlate, studies have suggested that many patients do not actually consume the entire amount of opioid that is prescribed.19,20
While it has been suggested that preoperative opioid weaning may be beneficial,15 there have been few studies on this topic to date.14,16 In this context, our study has mixed findings. On the one hand, our results suggest that preoperative changes in opioid utilization are not associated with statistically and/or clinically significant differences for a broad variety of perioperative outcomes. However, a secondary analysis did demonstrate a clinically and statistically significant association between changes in preoperative opioid utilization and a lower incidence of persistent postoperative opioid utilization (e.g. the adjusted incidence of at least 10 opioid prescriptions or 120 days of prescription coverage in postoperative days 91-365 was 56.2% for patients with decreasing utilization, 69.0% for patients with increasing utilization, compared to 80.4% for patients with stable utilization), suggesting potential benefit for more meaningful measures of persistent postoperative opioid use. Our results may also provide cautious reassurance for the management of patients who experience worsening pain prior to surgery, as we found no evidence that preoperative escalation of opioid was associated with worsened outcomes. Ultimately, further study in the form of randomized trials may be necessary to clarify whether efforts to impact preoperative opioid utilization can improve perioperative outcomes.
Supplementary Material
Funding Statement:
Dr. Rishel was supported by funding from the National Institute of Health Anesthesia Training Grant in Biomedical Research (NIH T32 GM 089626). Dr. Angst is supported by the National Institute of Health (NS114926, AG058417, HL139844, DE027728), the Bill and Melinda Gates Foundation and The March of Dimes. Dr. Sun was supported by funding from the National Institute on Drug Abuse (K08DA042314). Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest: Dr. Sun reports consulting fees unrelated to this work from the Analysis Group, the Mission Lisa Foundation, and Lucid Lane, LLC.
Prior Presentations: International Anesthesia Research Society Annual Meeting 2020 (Conference cancelled)
References
- 1.Centers for Disease Control and Prevention: 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes. 2019. at <https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf>
- 2.Jiang X, Orton M, Feng R, Hossain E, Malhotra NR, Zager EL, Liu R: Chronic Opioid Usage in Surgical Patients in a Large Academic Center. Ann Surg 2017; 265:722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilliard PE, Waljee J, Moser S, Metz L, Mathis M, Goesling J, Cron D, Clauw DJ, Englesbe M, Abecasis G, Brummett CM: Prevalence of Preoperative Opioid Use and Characteristics Associated With Opioid Use Among Patients Presenting for Surgery. JAMA Surg 2018; 153:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menendez ME, Ring D, Bateman BT: Preoperative Opioid Misuse is Associated With Increased Morbidity and Mortality After Elective Orthopaedic Surgery. Clin Orthop Relat Res 2015; 473:2402–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waljee JF, Cron DC, Steiger RM, Zhong L, Englesbe MJ, Brummett CM: Effect of preoperative opioid exposure on healthcare utilization and expenditures following elective abdominal surgery. Ann Surg 2017; 265:715–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozell JC, Courtney PM, Dattilo JR, Wu CH, Lee GC: Preoperative Opiate Use Independently Predicts Narcotic Consumption and Complications After Total Joint Arthroplasty. J Arthroplasty 2017; 32:2658–62 [DOI] [PubMed] [Google Scholar]
- 7.Smith SR, Bido J, Collins J, Yang H, Katz J, Elena L: Impact of preoperative opioid use on total knee arthroplasty outcomes 2017; 99:pp 803–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll I, Barelka P, Wang CKM, Wang BM, Gillespie MJ, McCue R, Younger JW, Trafton J, Humphreys K, Goodman SB, Dirbas F, Whyte RI, Donington JS, Cannon WB, MacKey SC: A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg 2012; 115:694–702 [DOI] [PubMed] [Google Scholar]
- 9.Angst MS, Clark JD: Opioid-induced Hyperalgesia A Qualitative Systematic Review. Anesthesiology 2006; 104:570–87 [DOI] [PubMed] [Google Scholar]
- 10.Walters TL, Mariano ER, Clark JD: Perioperative Surgical Home and the Integral Role of Pain Medicine 2015; 16:pp 1666–72 [DOI] [PubMed] [Google Scholar]
- 11.Ljungqvist O, Scott M, Fearon KC: Enhanced Recovery After Surgery: A Review. JAMA Surg 2017; 152:292. [DOI] [PubMed] [Google Scholar]
- 12.Møller AM, Villebro N, Pedersen T, Tønnesen H: Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. Lancet 2002; 359:114–7 [DOI] [PubMed] [Google Scholar]
- 13.Lindström D, Azodi OS, Wladis A, Tønnesen H, Linder S, Nåsell H, Ponzer S, Adami J: Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Ann Surg 2008; 248:739–45 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen L-CL, Sing DC, Bozic KJ: Preoperative Reduction of Opioid Use Before Total Joint Arthroplasty. J Arthroplasty 2016; 31:282–7 [DOI] [PubMed] [Google Scholar]
- 15.McAnally H: Rationale for and approach to preoperative opioid weaning: a preoperative optimization protocol. Perioper Med 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hah JM, Trafton JA, Narasimhan B, Krishnamurthy P, Hilmoe H, Sharifzadeh Y, Huddleston JI, Amanatullah D, Maloney WJ, Goodman S, Carroll I, Mackey SC: Efficacy of motivational-interviewing and guided opioid tapering support for patients undergoing orthopedic surgery (MI-Opioid Taper): A prospective, assessor-blind, randomized controlled pilot trial. EClinicalMedicine 2020; 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preoperative Opioid Taper Initiative at <https://anes-conf.med.umich.edu/opioidtaper/opioidtaper.htm>
- 18.CMS: Opioid Oral Morphine Milligram Equivalent ( MME ) Conversion Factors 2018:p 2 at <https://www.cdc.gov/drugoverdose/resources/data.html>
- 19.Kharasch ED, Clark JD: Persistent Postoperative Opioid Use: Perception, Progress, and Promise 2020; 132:pp 1304–6 [DOI] [PubMed] [Google Scholar]
- 20.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL: Prescription opioid analgesics commonly unused after surgery: A systematic review 2017; 152:pp 1066–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun EC, Darnall BD, Baker LC, Mackey S: Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016; 176:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, Goltzman D, Kreiger N, Prior J, Leslie WD: Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health 2012; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, Rapp M, Ko CY: A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg 2012; 256:973–81 [DOI] [PubMed] [Google Scholar]
- 24.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M: Overdose and prescribed opioids: Associations among chronic non-cancer pain patients. Ann Intern Med 2010; 152:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbaugh CM, Cooper JN: Administrative databases. Semin Pediatr Surg 2018; 27:353–60 [DOI] [PubMed] [Google Scholar]
- 26.Sun EC, Mello MM, Moshfegh J, Baker LC: Assessment of Out-of-Network Billing for Privately Insured Patients Receiving Care in In-Network Hospitals. JAMA Intern Med 2019; 179:1543–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9 [DOI] [PubMed] [Google Scholar]
- 29.Hedges L: Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J Educ Stat 1981; 6:107–28 [Google Scholar]
- 30.Sawilowsky SS: New Effect Size Rules of Thumb. J Mod Appl Stat Methods 2009; 8:597–9 [Google Scholar]
- 31.Amemiya T: Regression Analysis when the Dependent Variable Is Truncated Normal. Econometrica 1973; 41:997 [Google Scholar]
- 32.O’Donnell O, van Doorslaer E, Wagstaff A, Lindelow M: Analyzing Health Equity Using Household Survey Data. Washington DC, The World Bank, 2008. doi: 10.1596/978-0-8213-6933-3 [DOI] [Google Scholar]
- 33.Sun EC, Dexter F, Miller TR, Baker LC: “Opt Out” and Access to Anesthesia Care for Elective and Urgent Surgeries among U.S. Medicare Beneficiaries. Anesthesiology 2017; 126:461–71 [DOI] [PubMed] [Google Scholar]
- 34.Rishel CA, Zhang Y, Sun EC: Association Between Preoperative Benzodiazepine Use and Postoperative Opioid Use and Health Care Costs. JAMA Netw Open 2020; 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG: Multiple significance tests: The Bonferroni method. BMJ 1995; 310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadhwa V, Jobanputra Y, Garg SK, Patwardhan S, Mehta D, Sanaka MR: Nationwide trends of hospital admissions for acute cholecystitis in the United States. Gastroenterol Rep 2017; 5:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goudman L, De Smedt A, Forget P, Moens M: Determining the Minimal Clinical Important Difference for Medication Quantification Scale III and Morphine Milligram Equivalents in Patients with Failed Back Surgery Syndrome. J Clin Med 2020; 9:3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez HR, Buonora M, Cunningham CO, Heo M, Starrels JL: Opioid Taper Is Associated with Subsequent Termination of Care: a Retrospective Cohort Study. J Gen Intern Med 2020; 35:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linden A: Assessing regression to the mean effects in health care initiatives. BMC Med Res Methodol 2013; 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.