Abstract
Tumor growth is associated with metabolic reprogramming of various organs including the liver. This metabolic reprogramming is responsible for the development of behavioral fatigue represented by decreased voluntary wheel running in a murine model of lung cancer.
To determine whether interleukin (IL-)6 induced by the tumor is responsible for the metabolic reprogramming, mice injected with Lewis lung carcinoma cells in the flank were treated with an anti-mouse IL-6 monoclonal neutralizing antibody using a 2 × 2 factorial design (+/− tumor and +/− anti-IL-6 antibody). Endpoints were represented by behavioral, metabolic and immune phenotypes. Despite its ability to abrogate the increase in plasma levels of IL-6 that was apparent in tumor-bearing mice and decrease inflammatory signaling in the liver, immunoneutralization of IL-6 had no effect on voluntary wheel running and did not modify the tumor-induced alterations in hepatic gene expression of inflammatory cytokines and metabolic factors. These negative results indicate that IL-6 does not mediate the communication between tumor and host in mice implanted with Lewis lung carcinoma
Keywords: Cancer, Lewis lung carcinoma, Mouse, Interleukin-6, Fatigue, Liver metabolism, Inflammation
Fatigue is the most common non-specific symptom presented by cancer patients (Berger et al. 2015; Mortimer et al. 2010). While it is often present at the time of diagnosis. Its prevalence and intensity increase dramatically during cancer therapy. Fatigue does not always wane off at the completion of treatment and persists in about 30 percent of patients. The negative impact of fatigue on personal and professional quality of life and functionality is considerable. Despite this, there is no FDA-approved preventive or curative treatment. As a palliative measure, patients are counseled to spare their energy, exercise when possible, and administer the psychostimulant methylphenidate in very severe cases.
Among the several putative mechanisms that have been proposed to account for the development of fatigue, inflammation is certainly the one that has received the most attention (Bower 2014; Saligan et al. 2015). Inflammation is associated with the development, progression and dissemination of the tumor and it becomes more severe in response to treatment-induced cellular damage. It would induce fatigue by propagating throughout the body and recruiting signaling pathways in the central nervous system. Interleukin-6 (IL-6) is one of the candidate inflammatory cytokines for the communication between the tumor and the rest of the body. This cytokine has the ability to act at distance when released in the general circulation, in contrast to other cytokines that act in a paracrine or autocrine manner (Kishimoto 2005). Cancer is often associated with increased circulating levels of IL-6. In addition, certain modalities of cancer treatment also increase circulating levels of IL-6 at the same time that they increase fatigue. This is the case for androgen deprivation therapy in prostate cancer patients (Hoogland et al. 2020). Increased IL-6 is associated with fatigue in breast cancer patients (Cohen et al. 2020; Saligan and Kim 2012; Zick et al. 2014), head and neck cancer patients (Xiao et al. 2020) and patients with acute myeloid leukemia (Khosravi et al. 2018), ovarian cancer (Schrepf et al. 2013) or colorectal cancer (Himbert et al. 2019). However, increased IL-6 is not a constant in cancer. Several studies report no differences in circulating IL-6 levels between cancer patients and controls (Cameron et al. 2012; Dirksen et al. 2014; Kim et al. 2020; Pertl et al. 2013). For this reason, the association between IL-6 levels and fatigue is not sufficient to infer causality as IL-6 can vary independently of fatigue. Additionally, the psychological stress experienced by fatigued cancer patients can itself increase IL-6 levels (Rohleder et al. 2012).
Experimental studies in animal models of cancer provide a way to test causality as they allow monitoring and manipulation of IL-6 production during tumor growth. There is plenty of evidence in favor of a key role for tumor-secreted IL-6 and STAT3 signaling in the metabolic changes that develop during cancer (Mauer et al. 2015). In particular, tumor-induced IL-6 reprograms host metabolism in murine models of colon cancer and pancreatic ductal adenocarcinoma (Flint et al. 2016). In the same manner, inflammatory STAT3 signaling that is activated downstream of IL-6 alters hepatic insulin signaling and lipid metabolism and glucose tolerance in a genetic murine model of lung adenocarcinoma (Masri et al. 2016). Based on these findings, a few recent studies have examined whether IL-6 mediates the relationship between tumor-associated metabolic changes and cancer-related symptoms. However, the results have been essentially negative as immunoneutralization of IL-6 had no effect on both the metabolic phenotype and the behavioral alterations associated with tumor growth in a murine model of breast cancer and a murine model of human papilloma virus-related head and neck cancer (Borniger et al. 2018; Grossberg et al. 2020). Whether these negative results generalize to inflammatory tumors, in which IL-6 plays a role in the progression and dissemination of the tumor, is not known. We undertook the present study to determine whether immunoneutralization of IL-6 has any effect on the relationship between metabolic reprogramming and tumor-induced fatigue in a tumor model characterized by higher dependence on IL-6 than breast cancer or HPV-related head and neck cancer. We selected Lewis lung carcinoma cells for this purpose as they produce and release IL-6 at the same time they potently induce the expression of IL-6 in the host (Demers et al. 2018; Graves et al. 2006; Hu et al. 2019; Hung et al. 2020; Kim et al. 2009; Zhang et al. 2017).
Animals and methods
Animals
The experiments were conducted on male C57BL/6J mice purchased from Jackson Laboratories and housed in a standard laboratory environment in a 12 h dark/light cycle with lights off from 7:00 PM to 7:00 AM on the next day. Mice were 10-week old at the beginning of the experiments and were individually housed with ad libitum access to food and water.
Tumor model
The tumor model was based on heterotopic implantation of a cell line established from the lung of a C57BL mouse bearing a tumor resulting from an implantation of primary Lewis lung carcinoma (CRL-1642, ATCC, Manassas, VA). This cell line is highly tumorigenic and weakly metastatic. The tumor cells were injected subcutaneously into the right flank at a concentration of 5 × 106 cells in 50 μl. Tumors were dissected at the end of the experiment and weighed.
IL-6 neutralizing antibody treatment
Mice were randomly allocated to 4 treatment groups (n=6 mice/group) counterbalanced by baseline wheel running using a 2 × 2 factorial design (+/− LLC versus +/− IL-6na). Starting 15 days after injection of tumor cells, mice received daily intraperitoneal injections of IL-6na (200 μg in 100 μl PBS, BioXcell, BE0046) or placebo (100 μl PBS) for 7 days. One mouse assigned to the LLC-IL-6na experimental group died before initiation of IL-6na injection. One mouse assigned to the LLC-placebo group died on day 17 after initiation of the placebo. The data from these two mice were not included in the statistical analysis. Four mice assigned to the LLC-IL-6na group had to be euthanized on day 22 because of the size and ulceration of their tumors. Tissues and plasma from these 4 mice were collected for biochemistry analyses. Data from these 4 mice were included in the statistical analysis.
Measurement of behavioral fatigue
Mice were individually housed with in-cage low-profile running wheels, wirelessly connected to an activity counter (Wheel Manager, Med Associates, Inc, Fairfax, VT). The experiment started after stabilization of wheel running which required 14 days. As wheel running activity occurs mostly during the night portion of the cycle, wheel running activity was measured by the total number of counts during the 12-hour nights.
Tissue collection
At the end of the experiment mice were anesthetized by CO2 inhalation. Blood was collected in EDTA coated tubes by cardiac puncture and plasma was separated by centrifugation before being stored at −80°C until analysis. Mice were perfused intracardially with phosphate buffered saline. Liver was collected, snap frozen in liquid nitrogen and kept at −80°C until analysis.
Quantitative real time polymerase chain reaction and ELISA
RNA was extracted from the liver using the Trizol (Invitrogen, Carlsbad, CA) chloroform method. cDNA was generated using a high capacity cDNA reverse transcription kit (Applied Biosystems). PCR reactions were run on a CFX384 (Biorad) using SYBR Green Mastermix (Biorad) with the gene expression assays from Integrated DNA Technologies (IDT) except for Pkm1 and Pkm2 for which the SYBR green primer-based assays and SYBR Green Master Mix were used. EIF3 and RPS3 were used as housekeeping genes after checking their lack of variation between the experimental conditions under test. Function of the target genes is presented in supplementary material. Primers sequences are listed in Supplementary Table I. Relative expression to the control mice without any tumor and injected with the placebo was calculated using the ΔΔCt method.
Plasma concentrations of IL-6 were measured by ELISA (BioLegend) following manufacturer’s instructions. Concentrations of IL-6 lower than the smaller standard value (4.68 pg/ml) were replaced by this value.
Statistical analysis
All data are presented as mean+/− standard error of the mean and analyzed using SPSS (version 10, Chicago, IL). Two-way analyses of variance (control vs. tumor, placebo vs. IL-6na) with time as a repeated factor were performed to analyze nightly counts of wheel running before and during IL-6na treatment in the two experiments. Two-way analyses of variance (control vs. tumor, placebo vs. IL-6na) were used to analyze gene expression in liver and plasma levels of IL-6. Post hoc comparisons were adjusted for multiple comparisons using Bonferroni correction. Statistical significance was set at p<=0.05.
Results
In the present experiment, IL-6na was injected daily for 7 days starting 15 days after injection of LLC cells, when nightly wheel running had decreased by about 50% in tumor-bearing mice. During the first 15 days after injection of tumor cells, wheel running activity decreased in tumor bearing mice but not in control mice (tumor × day interaction: F(14,252)=2.24, p<0.01) with this decrease being significant on Days 13, 14 and 15 (p<0.05) (Fig. 1A). Administration of IL-6na and the control injection resulted in a decrease in wheel running activity independently of the treatment (day 15 to day 16: F(1,19)=30.6, p<0.001) and this decrease persisted in all groups during the 7 days of IL-6na treatment. However, tumor-bearing mice were still less active than control mice (F(1,14)=4.76, p<0.05) and this difference was not modified by IL-6na treatment.
Fig. 1 –
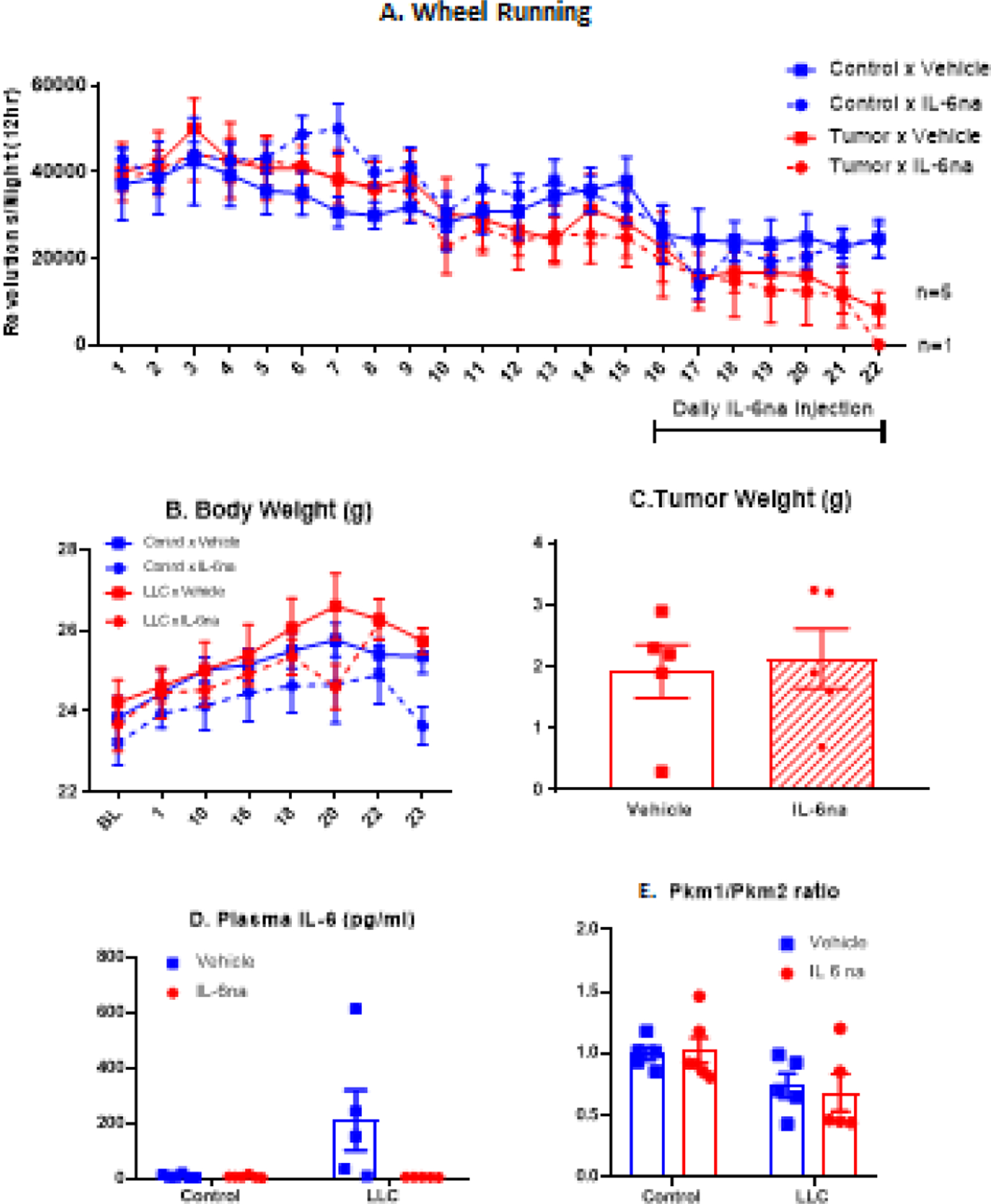
LLC-induced fatigue, inflammation and metabolic reprogramming are independent of IL-6 signaling. (A) Time course of nightly wheel running counts for each experimental group (mean +/− SEM). For the tumor bearing mice, the symbol change to a triangle means the data that are represented correspond to the survivors (days 18 to 22 for Tumor × Vehicle and day 22 for Tumor × IL-6na). (B) Time course of body weights (grams) of mice over the duration of the experiment (mean +/− SEM). (C) Tumor weight (grams) measured at the end of the experiment (mean +/− SEM); points represent individual data. (D) Plasma IL-6 levels (pg/ml) measured at the end of the experiment (mean +/− SEM); points represent individual data. (F) Liver Pkm1/Pkm2 ratio (mean +/− SEM); points represent individual data. N=6 per group for Control × Vehicle and Control × IL-6na groups; N=5 per group for LLC × Vehicle and LLC × IL-6na groups
Measurement of IL-6 levels in the plasma confirmed that IL-6na fully blocked circulating 6 in tumor bearing mice as it was no longer detectable in LLC tumor bearing mice treated with IL-6na (LLC × IL-6na interaction, F(1,18)=5.17, p<0.05) (Fig. 1B). The inhibitory effect of IL-6na on IL-6 signaling was confirmed by a significant decrease in the expression of genes associated with IL-6 signaling (Socs3, Stat3, p<0.001) in the liver of IL-6na-treated mice (Fig. 2). IL-6na treatment had no effect on body weight expressed as percent of body weight before injection of LLC cells and tumor-bearing mice did not differ in their body weight from control mice (Fig. 1C). IL-6na treatment had no effect on tumor weight measured at the end of the experiment (Fig. 1D). Analysis of gene expression in the liver by qRT-PCR confirmed that tumor growth was associated with significantly higher expression of metabolic genes (Hk1, Hk2, Ldha, Mct4, Ucp2), inflammatory genes (Rela, Il1, Tnf, Nlrp3) and genes associated with IL-6 signaling (Socs3, Stat3) (Fig. 1E). Tumor growth was also associated with decreased Pkm1/Pkm2 ratios (p<0.05) (Fig. 1E). In terms of inflammation, administration of IL-6na abrogated LLC-induced increase in Il1 (p<0.05). IL-6na also decreased the expression of Nfkb1 and Rela in the liver but had no effect on other inflammatory genes and on metabolic genes.
Fig. 2 -.
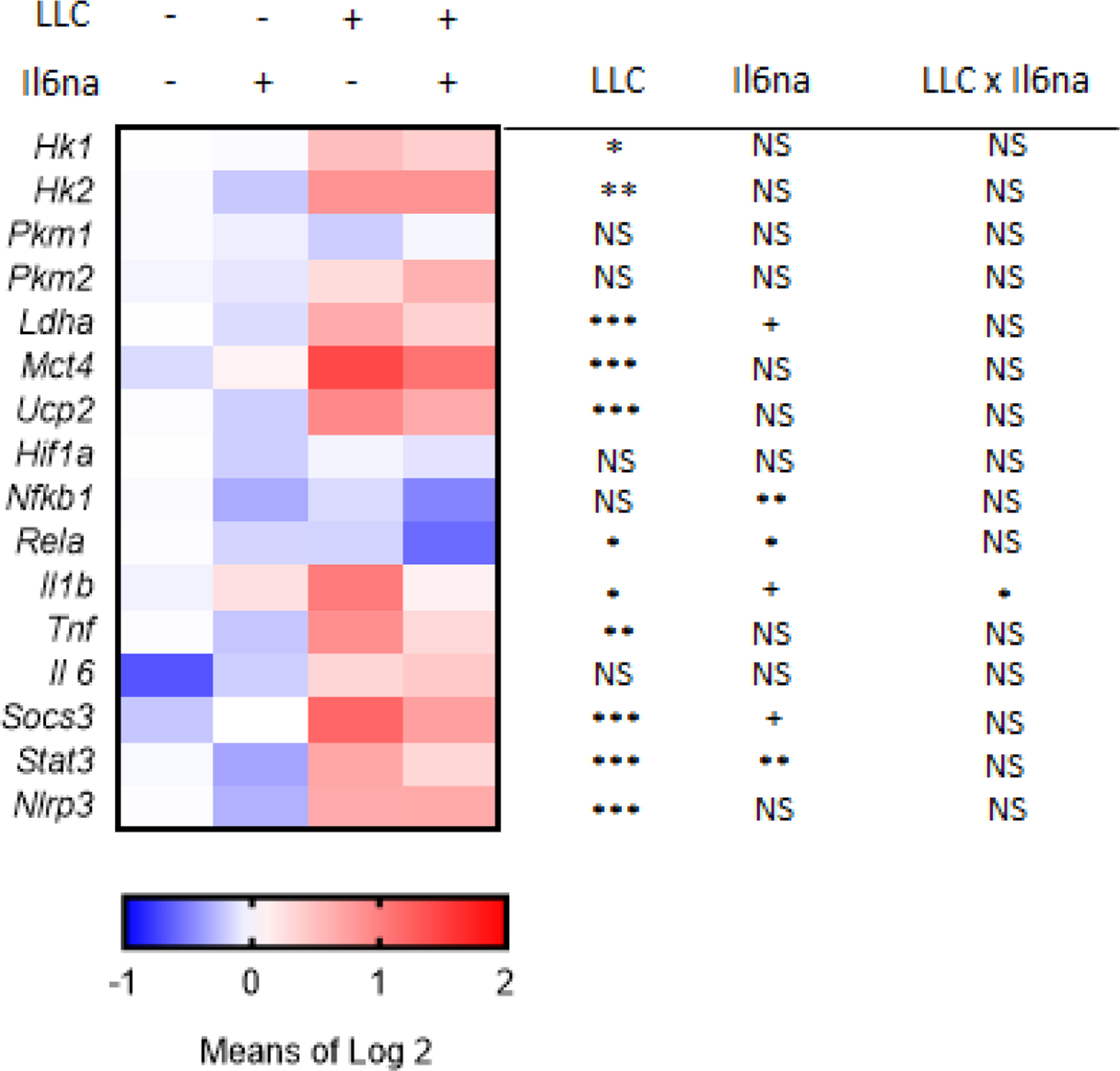
Heat map of liver gene expression for metabolic genes (Hk1, Hk2, Ldha, Mct4, Ucp2, Hif1a), inflammatory genes (Nfkb1, Rela, Il1, Tnf, Nlrp3) and IL-6 signaling (Il6, Socs3, stat3). Values represent averages of individual log2 transform of relative expression. N=6 per group for Control × Vehicle and Control × IL-6na groups; N=5 per group for LLC × Vehicle and LLC × IL-6na groups. Significance levels for the effects of main factors and their interaction are presented in the figure (+ p,0.10, * p<0.05, ** p<0.01, *** p<0.001).
Discussion
The results of the present experiment show that despite an increase in circulating levels of IL-6 in tumor bearing mice, immunoneutralization of IL-6 does not treat tumor-induced fatigue as measured by wheel running. In addition, this treatment has no effect on tumor-induced alterations in hepatic expression of inflammatory and metabolic genes.
Most authors consider voluntary wheel running activity in mice as equivalent to voluntary exercise in humans and home cage activity as equivalent to spontaneous physical activity (Kostrzewa and Kas 2014). However, this view is somewhat simplistic as the intense running wheel activity that is deployed by mice when they are provided with a wheel in their home cage is out of proportion with any form of physical exercise in humans. Based on its excessive nature, other authors view wheel running activity as a form of abnormal stereotypic behavior that is triggered by the impoverished environment provided by cage housing. Contrasting with this view is the observation that wheel running activity also occurs in wild mice, with bouts lengths of running matching those for captive mice (Meijer and Robbers 2014). It is more appropriate to view wheel running as a remnant of foraging activity to obtain food. This view is consistent with the observation that voluntary running mice increase their food intake by increasing their meal size and that mice increase their already high levels of activity when running is required to access food (Atalayer and Rowland 2011). In addition, food-restricted mice run more than ad libitum fed mice, which is compatible with the foraging interpretation of this behavior (Vaanholt et al. 2007). We have already reported that voluntary wheel running activity is much more sensitive to tumor growth than spontaneous physical activity (Grossberg et al. 2018). This can be explained by the competition between the tumor and skeletal muscles for the hepatic Cori cycle that normally recycles lactate from exercising muscles into glucose (Grossberg et al. 2020).
The observation that LLC increases circulating levels of IL-6 agrees with the results of a number of studies in tumor bearing mice as mentioned in the introduction section. As strenuous physical exercise increases the production of IL-6 by skeletal muscles (Pedersen and Febbraio 2012), it could be argued that at least part of the increased plasma levels of IL-6 observed in LLC tumor bearing mice was due to wheel running. However, this cannot explain the large increase seen in LLC tumor bearing mice as these mice were running less than control mice without a tumor. In addition, the increase in plasma levels of IL-6 in response to voluntary wheel running reported in studies on this myokine in B16 tumor bearing mice (from 4.3 to 29.3 pg/ml in the study by (Pedersen et al. 2016)) is much smaller than the one observed in the present study (from 10 to 210 pg/ml on average, Fig. 1D).
A number of data support a role for IL-6 in the local environment in which it is produced and at distance. At the local level, production of IL-6 by LLC tumor cells promotes cancer proliferation and dissemination (Mauer et al. 2015). At distance, tumor-induced IL-6 mediates also the metabolic reprogramming of the liver in tumor-bearing mice and the glucocorticoid-dependent immune suppression within the tumor microenvironment (Flint et al. 2016). Immunoneutralization of IL-6 abrogated these effects in pre-cachectic mice implanted with colon cancer cells or with pancreatic cancer cells (Flint et al. 2016). However, these findings cannot be generalized to other conditions. Immunoneutralization of IL-6 did not block tumor-induced metabolic reprogramming of the liver in two other murine models that do not induce cachexia, a model of human papilloma virus (HPV)-positive head and neck cancer (Grossberg et al. 2020) and a model of non-metastatic breast cancer (Borniger et al. 2018). The present findings obtained in an inflammatory murine model of cancer agree with the results reported in these two last studies and confirm that IL-6 does not mediate the inflammatory and metabolic phenotypes that develop in the livers of tumor-bearing mice. Furthermore, IL-6 plays no role in the behavioral alterations that develop in LLC tumor-bearing mice; this finding supports the negative results reported in mice with HPV-related head and neck cancer and in mice with breast cancer (Borniger et al. 2018; Grossberg et al. 2020). However, it must be noted that IL-6 produced by voluntary wheel running impede tumor growth by contributing to mobilization and redistribution of natural killer cells (Pedersen et al. 2016). This antagonistic effect of IL-6 on tumor growth could explain why in the present study the mortality due to the tumor was higher in LLC tumor bearing mice treated with IL-6na.
The present experiment has a number of limitations. We inferred metabolism from gene expression data in the liver but did not measure plasma and tissue levels of metabolites. However, our metabolic gene expression data agrees with metabolite data in similar paradigms which utilize IL-6 immunoneutralization in tumor bearing mice (Borniger et al. 2018; Grossberg et al. 2020). The observation that tumor weights were not modified by IL-6na treatment could be due to the fact that IL-6na did not modify IL-6 signaling in the tumor, which was not assessed. Because all mice had access to running wheels, we cannot speculate on the possible role of IL-6 as a myokine (Pedersen et al. 2016). Despite these limitations, we can still safely conclude that IL-6 does not contribute to LLC-induced decrements in voluntary wheel running.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Kiersten Scott: Methodology, Investigation, Data analysis, Draft presentation. Thien Phan Trong: Methodology, Investigation, Data analysis, Draft Presentation. A Phillip West: Conceptualization, Reviewing and Editing. Cullen M. Taniguchi: Conceptualization, Reviewing and Editing. Robert Dantzer: Conceptualization, Methodology, Writing
References
- Atalayer D, Rowland NE (2011) Comparison of voluntary and foraging running wheel activity on food demand in mice. Physiol Behav 102: 22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C, National comprehensive cancer n (2015) Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw 13: 1012–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniger JC, Walker WH Ii, Surbhi, Emmer KM, Zhang N, Zalenski AA, Muscarella SL, Fitzgerald JA, Smith AN, Braam CJ, TinKai T, Magalang UJ, Lustberg MB, Nelson RJ, DeVries AC (2018) A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer. Cell Metab 28: 118–129 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE (2014) Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron BA, Bennett B, Li H, Boyle F, deSouza P, Wilcken N, Friedlander M, Goldstein D, Lloyd AR (2012) Post-cancer fatigue is not associated with immune activation or altered cytokine production. Ann Oncol 23: 2890–2895. [DOI] [PubMed] [Google Scholar]
- Cohen M, Levkovich I, Katz R, Fried G, Pollack S (2020) Low physical activity, fatigue and depression in breast cancer survivors: Moderation by levels of IL-6 and IL-8. Int J Psychophysiol 158: 96–102. [DOI] [PubMed] [Google Scholar]
- Demers M, Suidan GL, Andrews N, Martinod K, Cabral JE, Wagner DD (2018) Solid peripheral tumor leads to systemic inflammation, astrocyte activation and signs of behavioral despair in mice. PLoS One 13: e0207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen SR, Kirschner KF, Belyea MJ (2014) Association of symptoms and cytokines in prostate cancer patients receiving radiation treatment. Biol Res Nurs 16: 250–7. [DOI] [PubMed] [Google Scholar]
- Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT (2016) Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab 24: 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves E, Ramsay E, McCarthy DO (2006) Inhibitors of COX activity preserve muscle mass in mice bearing the Lewis lung carcinoma, but not the B16 melanoma. Res Nurs Health 29: 87–97. [DOI] [PubMed] [Google Scholar]
- Grossberg AJ, Vichaya EG, Christian DL, Molkentine JM, Vermeer DW, Gross PS, Vermeer PD, Lee JH, Dantzer R (2018) Tumor-Associated Fatigue in Cancer Patients Develops Independently of IL1 Signaling. Cancer Res 78: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg AJ, Vichaya EG, Gross PS, Ford BG, Scott KA, Estrada D, Vermeer DW, Vermeer P, Dantzer R (2020) Interleukin 6-independent metabolic reprogramming as a driver of cancer-related fatigue. Brain Behav Immun 88: 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himbert C, Ose J, Lin T, Warby CA, Gigic B, Steindorf K, Schrotz-King P, Abbenhardt-Martin C, Zielske L, Boehm J, Ulrich CM (2019) Inflammation- and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur J Cancer Care (Engl) 28: e13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland AI, Jim HSL, Gonzalez BD, Small BJ, Gilvary D, Breen EC, Bower JE, Fishman M, Zachariah B, Jacobsen PB (2020) Systemic inflammation and symptomatology in patients with prostate cancer treated with androgen deprivation therapy: Preliminary findings. Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Ru Z, Zhou Y, Xiao W, Sun R, Zhang S, Gao Y, Li X, Zhang X, Yang H (2019) Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 1091–1102. [DOI] [PubMed] [Google Scholar]
- Hung CC, Zhen YY, Niu SW, Hsu JF, Lee TH, Chuang HH, Wang PH, Lee SC, Lin PC, Chiu YW, Wu CH, Huang MS, Hsiao M, Chen HC, Yang CJ (2020) Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi M, Taghvaye Masoumi H, Gholami K, Vaezi M, Hadjibabaei M, Ghavamzadeh A (2018) The Relationship between Fatigue and Cytokine Levels in Patients with Acute Myeloid Leukemia. Int J Hematol Oncol Stem Cell Res 12: 318–321. [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457: 102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Park H, Lee H, Kim S (2020) Pro-inflammatory Cytokine Levels and Cancer-related Fatigue in Breast Cancer Survivors: Effects of an Exercise Adherence Program. J Breast Cancer 23: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T (2005) Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol 23: 1–21. [DOI] [PubMed] [Google Scholar]
- Kostrzewa E, Kas MJ (2014) The use of mouse models to unravel genetic architecture of physical activity: a review. Genes Brain Behav 13: 87–103. [DOI] [PubMed] [Google Scholar]
- Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, Jacks T, Sassone-Corsi P (2016) Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell 165: 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Denson JL, Bruning JC (2015) Versatile functions for IL-6 in metabolism and cancer. Trends Immunol 36: 92–101. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Robbers Y (2014) Wheel running in the wild. Proc Biol Sci 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JE, Barsevick AM, Bennett CL, Berger AM, Cleeland C, DeVader SR, Escalante C, Gilreath J, Hurria A, Mendoza TR, Rugo HS (2010) Studying cancer-related fatigue: report of the NCCN scientific research committee. J Natl Compr Canc Netw 8: 1331–9. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–65. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P (2016) Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab 23: 554–62. [DOI] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Harkin A, Kennedy MJ, Connor TJ (2013) C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun 34: 108–19. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Aringer M, Boentert M (2012) Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci 1261: 88–96. [DOI] [PubMed] [Google Scholar]
- Saligan LN, Kim HS (2012) A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun 26: 830–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, Escalante CP, del Giglio A, Kober KM, Kamath J, Palesh O, Mustian K, Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working G (2015) The biology of cancer-related fatigue: a review of the literature. Support Care Cancer 23: 2461–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA 3rd, Ganjei-Azar P, Mendez L, Markon K, Lubaroff DM, Thaker PH, Slavich GM, Sood AK, Lutgendorf SK (2013) Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun 30 Suppl: S126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaanholt LM, De Jong B, Garland T Jr., Daan S, Visser GH (2007) Behavioural and physiological responses to increased foraging effort in male mice. J Exp Biol 210: 2013–24. [DOI] [PubMed] [Google Scholar]
- Xiao C, Eldridge RC, Beitler JJ, Higgins KA, Chico CE, Felger JC, Wommack EC, Knobf T, Saba NF, Shin DM, Bruner DW, Miller AH (2020) Association Among Glucocorticoid Receptor Sensitivity, Fatigue, and Inflammation in Patients With Head and Neck Cancer. Psychosom Med 82: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP (2017) Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep 7: 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick SM, Zwickey H, Wood L, Foerster B, Khabir T, Wright B, Ichesco E, Sen A, Harris RE (2014) Preliminary differences in peripheral immune markers and brain metabolites between fatigued and non-fatigued breast cancer survivors: a pilot study. Brain Imaging Behav 8: 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


