Abstract
Background:
There are few contemporary cohorts of Trypanosoma cruzi-seropositive individuals, and the basic clinical epidemiology of Chagas disease is poorly understood. Herein, we report the incidence of cardiomyopathy and death associated with T. cruzi seropositivity.
Methods:
Participants were selected in blood banks at 2 Brazilian centers. Cases were defined as T. cruzi–seropositive blood donors. T. cruzi-seronegative controls were matched for age, sex, and period of donation. Patients with established Chagas cardiomyopathy were recruited from a tertiary outpatient service. Participants underwent medical examination, blood collection, electrocardiogram, and echocardiogram at enrollment (2008 to 2010) and at follow-up (2018 to 2019). The primary outcomes were all-cause mortality and development of cardiomyopathy, defined as the presence of a left ventricular ejection fraction <50% and/or QRS complex duration ≥ 120 ms. To handle loss to follow-up, a sensitivity analysis was performed using inverse probability weights for selection.
Results:
We enrolled 499 T. cruzi–seropositive donors (age 48 ± 10 years, 52% male), 488 T. cruzi-seronegative donors (age 49 ± 10 years, 49% male), and 101 patients with established Chagas cardiomyopathy (age 48 ± 8 years, 59% male). The mortality in patients with established cardiomyopathy was 80.9 deaths/1000 person-years (py) (54/101, 53%) and 15.1 deaths/1000py (17/114, 15%) in T. cruzi–seropositives with cardiomyopathy at baseline. Among T. cruzi–seropositive donors without cardiomyopathy at baseline mortality was 3.7 events/1000py (15/385, 4%), which was no different from T. cruzi–seronegative donors with 3.6 deaths/1000py (17/488, 3%). The incidence of cardiomyopathy in T. cruzi-seropositive donors was 13.8 (95% CI 9.5-19.6) events/1000py (32/262, 12%) compared with 4.6 (95% CI 2.3-8.3) events/1000 py (11/277, 4%) in seronegative controls, with an absolute incidence difference associated with T. cruzi seropositivity of 9.2 (95% CI 3.6 - 15.0) events/1000py. T. cruzi antibody level at baseline was associated with development of cardiomyopathy (adjusted OR of 1.4, 95% CI 1.1-1.8).
Conclusions:
We present a comprehensive description of the natural history of T. cruzi seropositivity in a contemporary patient population. The results highlight the central importance of anti-T. cruzi antibody titer as a marker of Chagas disease activity and risk of progression.
Keywords: Chagas disease, cardiomyopathy, Trypanosoma cruzi seropositivity, anti-T. cruzi antibody, progression, mortality
INTRODUCTION
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, is the most common cause of infectious cardiomyopathy worldwide1, 2. Despite substantial progress toward its control, Chagas disease remains a major public health problem in Latin America3, 4. Over the past several decades, migration has spread the disease to non-endemic countries, becoming a global health concern. Current estimates of 6 million T.cruzi seropositive persons and 1.2 million cases of cardiomyopathy make Chagas disease the highest burden parasitic disease in the Americas 5.
Chagas disease is often a lifelong infection in which most T.cruzi- seropositive persons remain asymptomatic but at risk of progression to cardiac damage6, 7. It is often quoted that one-third of seropositive individuals will develop Chagas cardiomyopathy over a lifetime8. This figure likely comes from early studies of the natural history of Chagas disease from hyperendemic rural populations with acute infections or electrocardiographic (ECG) findings, but without the additional sensitivity of modern echocardiography to identify cardiac involvement9-12. Current transmission control, making new T. cruzi infection increasingly rare5, has produced a cohort effect whereby most individuals with Chagas disease are now in their fourth decade of life or older 13, 14. Therefore, the life-time risk of Chagas cardiomyopathy, its incidence in a contemporary aging patient population, and risk factors for progression to cardiomyopathy remain poorly understood.
Two methodological issues make it challenging to study the natural history of Chagas disease. First, the protracted time frame over which cardiac damage accumulates, which can run into decades, necessitates many years of follow-up in order to detect incident cases of disease progression. Second, the current definition of Chagas cardiomyopathy based on the presence of typical ECG changes in a T. cruzi-seropositive patient15 is insufficient epidemiologically, as findings considered typical of Chagas disease are also prevalent in older adults without T. cruzi infection13, 16. As such, to determine the T. cruzi-attributable incidence of cardiomyopathy parallel and optimally blinded follow-up assessments of a group of matched seronegative controls is required.
Herein we present the 10-year follow-up results of the National Institutes of Health Recipient Epidemiology and Donor Evaluation Study (Brazilian NIH REDS) cohort, made up of 499 T. cruzi–seropositive blood donors identified in routine donor screening, 488 age- and sex-matched T. cruzi–seronegative blood donors, and 101 patients with established Chagas cardiomyopathy recruited from a tertiary outpatient service. Blood donors were further stratified according to the presence of cardiomyopathy, based on ECG and echocardiographic findings. The present study aimed to determine the incidence of cardiomyopathy and death associated with T. cruzi seropositivity. Furthermore, based on preliminary reports of the value of anti-T. cruzi antibody level and T. cruzi polymerase chain reaction (PCR) positivity in predicting cardiomyopathy, we also investigated the prognostic value of these parameters in a population of T. cruzi–seropositive blood donors.
To address these aims we conducted four main analyses. First, we compared all-cause mortality between T. cruzi-seronegative blood donors and the three other study groups (T. cruzi–seropositive blood donors with and without cardiomyopathy at baseline, and patients with established Chagas cardiomyopathy). Second, we built a model to assess the association between total anti-T. cruzi antibody level and T. cruzi PCR positivity with mortality in the T. cruzi–seropositive blood donor group. Third, we estimated the incidence of new-onset cardiomyopathy associated with T. cruzi seropositivity among participants initially free of cardiomyopathy at the baseline visit. Fourth, we built a model to assess the independent associations between baseline antibody level and PCR positivity and development subsequent cardiomyopathy.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for the sole purpose of reproducing the study results.
Study Design and Data Collection
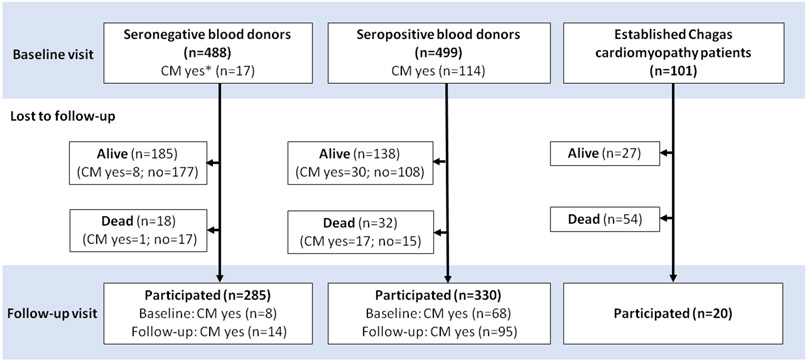
The REDS study has been described in detail elsewhere17. Briefly, healthy blood donors were recruited between 1996 and 2002 from two donation centers in Brazil (Fundação Pró-Sangue in São Paulo and Hemominas blood center in Montes Claros). Participants were selected based on the results of routine T. cruzi serology screening performed at the time of blood donation, confirmed by contemporary ELISA, hemaglutination, and immunofluorescence. A total of 1327 seropositive and 1887 seronegative blood donors were invited to participate in the study. Of those blood donors who were initially eligible, 499 T. cruzi–seropositive and 488 T. cruzi-seronegative blood donors matched by age, sex, and period of donation had complete clinical, ECG, and echocardiography data and were enrolled in the study (Figure 1).
Figure 1: Study population flow chart.
*CM = Cardiomyopathy, which was defined as left ventricular ejection fraction < 50% and/or QRS complex duration ≥ 120 ms
Additionally, 101 T. cruzi-seropositive participants with established Chagas cardiomyopathy, defined by the presence of left ventricular dilatation with systolic dysfunction were recruited from a tertiary cardiology outpatient service (InCor Hospital das Clínicas, São Paulo) for management of heart failure. This group was included to compare mortality rates with asymptomatic blood donors identified through serologic screening.
The baseline visit was conducted between 2008 and 2010. All participants underwent a medical history and physical examination, 12-lead electrocardiogram (ECG), and echocardiogram. Baseline characteristics of this population have been described previously17. The follow-up study visit was performed between 2018 and 2019. All participants were invited for a second cardiovascular evaluation, including blood collection, ECG and echocardiographic assessments.
Cardiomyopathy among T. cruzi seropositive and seronegative blood donors was defined as the presence of a left ventricular ejection fraction <50% and/or QRS complex duration ≥ 120 ms18. All patients with established cardiomyopathy recruited from the outpatient service had left ventricular systolic dysfunction.
Clinical and Laboratory Evaluation
All individuals underwent a clinical examination by a cardiologist, and demographic data were recorded. Cardiovascular history and risk factors, including hypertension, dyslipidemia, diabetes, and previous history of ischemic heart disease or revascularization procedures was also recorded. New York Heart Association (NYHA) functional class was assessed based on symptoms and physical activity questionnaire. Serum lipids and other biochemical blood measurements were determined in the local laboratories using standard laboratory procedures. Cardiac injury markers including troponin I, CK-MB, and myoglobin were measured at the central laboratory. B-type natriuretic peptide (NT-ProBNP) was also measured.
For T. cruzi–seropositive participants, parasite detection in blood by PCR and the evaluation of semiquantitative antibody results by ELISA were obtained. Levels of antibodies were reported as the ratio of signal-to-cutoff (S/CO) which is a function of the amount of anti-T. cruzi antibody present in the test sample (Ortho T.cruzi ELISA test system, Raritan, NJ).
Electrocardiographic and Echocardiographic Examination
ECGs were recorded at both sites during the two visits using standardized procedures17, 19. All ECGs were interpreted by trained cardiologists who were blinded to study group at a central reading center, and were classified according to the Minnesota code criteria13.
Comprehensive Doppler-echocardiographic examinations were performed at enrollment and at follow-up using a commercially available ultrasound system at each site. All images were stored digitally and analyzed offline by central reading centers. The echocardiographic measurements were performed according to the recommendations of the American Society of Echocardiography by independent investigators who were blinded to study group20. Left ventricular ejection fraction (LVEF) was calculated according to the modified Simpson method. A comprehensive examination from multiple windows was performed to detect wall motion abnormalities and apical aneurysms.
Diastolic function was assessed by pulsed-wave Doppler examination of mitral inflow, and by tissue Doppler imaging. Early diastolic velocity (e') at septal and lateral mitral annulus was obtained and the ratio between peak mitral E and e' (E/e') was calculated20. Left atrial volume was assessed by the biplane area-length method from the apical 4- and 2-chamber views.
Outcome definition and analytic groups
The main outcomes were all-cause mortality and development of cardiomyopathy at follow-up visit. The date and occurrence of death was determined first by direct interview with participants’ relatives when contact was possible. We also conducted a probabilistic linkage with the Brazilian National Mortality System (Sistema de Informação sobre Mortalidade - SIM) using full name, date of birth, mother’s name and municipality of residence as matching variables. The linkage algorithm has been previously validated with a sensitivity and specificity of 94% (95% CI 90-97%) and 91% (95% CI 86-95%), respectively21. Patients not identified in the mortality database were censured at June 1, 2020, the date the linkage was performed. As such, vital status was determined for all participants irrespective of whether contact was possible at the follow-up visit.
New-onset cardiomyopathy was defined as the presence of left ventricular systolic dysfunction (LVEF < 50%) and/or QRS complex duration ≥ 120 ms in participants undergoing cardiovascular assessment at the follow-up visit, but in whom cardiomyopathy was absent at the baseline visit 18. Using this definition, our study design is at risk of a survivorship bias, whereby participants with new-onset cardiomyopathy were more likely to die and thus not attend the follow-up visit. This would lead to an underestimate of the true incidence of cardiomyopathy because of differential loss to follow-up. As such, we conducted a sensitivity analysis in which we assumed all deaths represented cardiomyopathy cases, and as such we used a combined outcome of death or new-onset cardiomyopathy.
Statistical analysis
Continuous variables were expressed as medians with interquartile ranges, and categorical variables were presented as numbers and percentages proportions. Clinical characteristics were compared across the groups using the chi-square test, unpaired Student’s t-test, Mann-Whitney test, one-way ANOVA or Kruskal–Wallis tests, according to the pattern of variable distributions.
The mortality rate, incidence of new-onset cardiomyopathy and the incidence of the combined outcome (death or new-onset cardiomyopathy) were calculated by dividing the number of incident events by the person-years of follow-up calculated from the date of visit 1 until either the date of death or the date of cardiovascular assessment at the follow-up visit. Absolute incidence differences were calculated with the T. cruzi-seronegative group as reference. Exact Poisson 95% confidence intervals were calculated.
A Cox proportional hazards regression model was performed to identify the predictors of mortality in the T. cruzi seropositive group. Separate multivariable models were built to assess the association between mortality and the two predictors of interest: anti-T. cruzi antibody level and T. cruzi PCR result (positive or negative). PCR and antibody level were not included in the same model for statistical and theoretical reasons, as they are highly collinear and both are an indirect indicator of parasite burden. Covariates tested in the model included age, sex, prior benznidazole treatment and traditional cardiovascular risk factors.
We built a multivariable logistic regression model including T. cruzi seropositive and seronegative blood donors without cardiomyopathy at baseline to determine the independent contribution of T. cruzi serostatus to the development of new-onset cardiomyopathy. C-statistic and the integrated discrimination improvement (IDI) were used to determine the added value of serostatus in predicting new-onset cardiomyopathy. Subsequently, we developed a multivariable logistic regression model including only T. cruzi seropositive blood donors without cardiomyopathy at baseline to determine the independent contribution of anti-T. cruzi antibody level and T. cruzi PCR result in predicting new-onset cardiomyopathy.
To handle loss to follow-up, we assumed missing data are missing at random (MAR) which is a requirement for the validity of the maximum likelihood-based regression methods applied 22. In order to identify the variables that make the MAR assumption plausible we built logistic regression models to predict loss to follow-up. These predictive models included demographic and clinical characteristics that could influence missingness. Main effects and interaction terms were tested. Variables that were found to predict the missingness pattern were included in the regression models predicting new-onset cardiomyopathy, to minimize potential bias due to differential loss to follow-up. Additionally a sensitivity analysis for new-onset cardiomyopathy was performed using inverse probability weights for selection. The weights were obtaining from the logistic model using all variables found to be significantly associated with loss to follow-up.
Statistical analysis was performed in the Statistical Package for Social Sciences for Windows, version 22.0 (SPSS Inc., Chicago, Illinois) and R for Statistical Computing version 4.0.3 (R Foundation, Vienna, Austria) using the packages tidyverse (data manipulation), survey (weighted analysis), (survival (survival analysis), epiR (incidence calculations, and PredictABEL (risk reclassification analysis).
Ethics
The study was approved by the Brazilian National Institutional Review Board (CONEP), number 179.685/2012. In this study, written informed consent was obtained from all participants at baseline visit.
RESULTS
Study population characteristics
The study population flow chart is shown in Figure 1. After initial evaluation, 114 T. cruzi-seropositive blood donors met criteria for cardiomyopathy (23%). Sixty-six percent (330/499) of T. cruzi-seropositive blood donors and 58% (285/488) of T. cruzi-seronegative blood donors participated in the follow-up visit. Among the 101 patients with established Chagas cardiomyopathy who were in heart failure treatment, 20 (19.8%) underwent the second cardiovascular evaluation at follow-up. The median [IQR] time between visit 1 and visit 2 was 8.7 [8.3 - 9.2] years. Demographic and clinical characteristics of the cohort at baseline and follow-up visits are shown in Table 1.
Table 1:
Clinical characteristics of the 1088 individuals enrolled in the cohort study stratified according to T. cruzi-serologic tests and the presence of Chagas cardiomyopathy at baseline and at follow-up.
| Time | Visit 1 (Baseline) | Visit 2 (Follow-up) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant characteristics |
T. cruzi- seronegative (n=488) |
T. cruzi– seropositive (n=499) |
Chagas cardiomyopathy (n=101) |
P-value |
T. cruzi- seronegative (n=285) |
T. cruzi– seropositive (n=330) |
Chagas cardiomyopathy (n=20) |
P-value | |
| Age, years | 49 (42-58) | 48 (40-57) | 48 (42-54) | 0.237 | 59 (52-66) | 56 (50-65) | 55 (50-61) | 0.054 | |
| Male sex | 241 (49.4) | 261 (52.3) | 60 (59.4) | 0.172 | 140 (49.1) | 159 (48.2) | 13 (65.0) | 0.344 | |
| Clinical history | |||||||||
| Diabetes | 24 (4.9) | 27 (5.4) | 6 (5.9) | 0.870 | 38 (13.3) | 42 (12.7) | 3 (15) | 0.504 | |
| Hypertension | 119 (24.4) | 113 (22.6) | 36 (35.6) | 0.072 | 102 (35.8) | 128 (38.8) | 9 (45) | 0.648 | |
| Chronic kidney disease | 15 (3.1) | 15 (3.0) | 10 (9.9) | 0.009 | 15 (5.3) | 21 (6.4) | 2 (10) | 0.793 | |
| Suspected CAD* | 5 (1.0) | 3 (0.6) | 12 (11.9) | 0.001 | 10 (3.5) | 11 (3.3) | 2 (10) | 0.039 | |
| Symptoms - NYHA functional class | |||||||||
| Class I | 469 (96.1) | 461 (92.4) | 60 (59.3) | <0.001 | 262 (91.9) | 298 (90.3) | 9 (45.0) | <0.001 | |
| Class II | 18 (3.7) | 35 (7.0) | 27 (26.7) | 20 (6.9) | 24 (7.3) | 8 (40.0) | |||
| Class III/IV | 1 (0.2) | 3 (0.6) | 14 (13.9) | 3 (1.2) | 8 (2.4) | 3 (15.0) | |||
| Smoking history | Never | 255 (52.2) | 283 (56.7) | 47 (46.5) | 0.014 | 165 (57.9) | 215 (65.2) | 9 (45.0) | 0.119 |
| Past | 158 (32.4) | 161 (32.3) | 46 (45.5) | 77 (27.0) | 65 (19.7) | 6 (30.0) | |||
| Current | 75 (15.4) | 55 (11.0) | 8 (8.0) | 43 (15.1) | 50 (15.2) | 5 (25.0) | |||
| BMI, kg/m2 | 27 (25-30) | 26 (24-29) | 26 (23-28) | <0.001 | 27 (25-31) | 27 (25-30) | 26 (23-31) | 0.307 | |
| Obesity (BMI>30) | 127 (26.0) | 94 (18.8) | 14 (13.9) | 0.002 | 85 (29.8) | 80 (24.2) | 5 (25.0) | 0.274 | |
| Heart rate, bpm | 70 (60-75) | 65 (60-70) | 60 (58-70) | <0.001 | 73 (65-80) | 70 (63-78) | 67 (59-72) | 0.002 | |
| SBP, mmHg | 125 (115-140) | 125 (114-140) | 122 (107-134) | 0.003 | 130 (120-145) | 130 (116-143) | 110 (102-118) | <0.001 | |
| DBP, mmHg | 79 (65-88) | 76 (65-85) | 80 (69-90) | 0.088 | 80 (70-88) | 80 (70-85) | 71 (61-80) | 0.004 | |
| NT-ProBNP, pg/mL | 38 (23-65) | 48 (27-90) | 746 (335-2267) | <0.001 | 37 (23-61) | 46 (24-84) | 360 (129-550) | <0.001 | |
Chest pain suggestive of ischemic heart disease.
BMI: body mass index; CAD: coronary artery disease; DBP: diastolic blood pressure; NYHA: New York Heart Association; SBP: systolic blood pressure
Overall mortality
The greatest mortality was observed in patients with established cardiomyopathy at the baseline visit, with 54 deaths (53%) and a mortality rate of 80.9 deaths/1000 person-years follow-up (Figure 2, Table 2). There were 17 deaths (15%) among seropositive blood donors meeting the definition of cardiomyopathy at baseline visit (114/499), equating to a mortality rate of 15.1 deaths/1000 person-years follow-up. There was no difference in the mortality between seropositive donors without cardiomyopathy at the baseline visit and seronegative controls, with incidence rates of 3.7 and 3.6 events/1000 person-years, respectively.
Figure 2: Long-term survival curves according to the presence of cardiomyopathy and T.cruzi seropositivity.
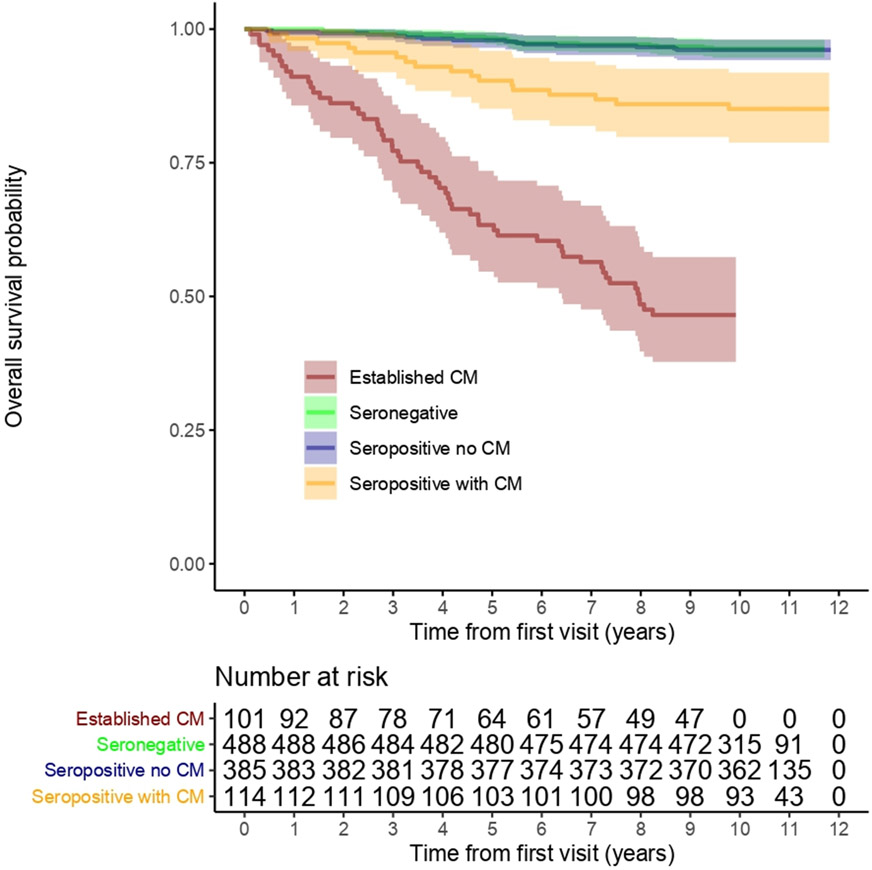
The greatest mortality was found among patients with established cardiomyopathy at baseline, with a mortality rate of 80.9 deaths/1000 person-years follow-up (red line). The mortality rate among T. cruzi–seropositive blood donors without cardiomyopathy at baseline and T. cruzi–seronegative blood donors was similar (light green and dark blue lines are overlapping).
CM = Cardiomyopathy
Table 2:
Overview of mortality and disease progression among the overall study population.
| Definition of event and participants at risk at baseline visit |
Number of participants at risk at baseline visit |
Follow-up time* (Person-years) |
Number of events N (n deaths, n new-onset CM) |
Incidence events/1000-person- years (95% CI) |
Absolute incidence difference /1000 person-years (95% CI) |
|---|---|---|---|---|---|
| Overall mortality (all participants at baseline visit) | |||||
| Seronegative donors | 488 | 4,977 | 18 (18, 0) | 3.62 (2.14 to 5.72) | Reference |
| Seropositive donors without cardiomyopathy | 385 | 4,091 | 15 (15, 0) | 3.67 (2.05 to 6.04) | 0.05 (−2.45 to 2.54) |
| Seropositive donors with cardiomyopathy | 114 | 1,128 | 17 (17, 0) | 15.1 (8.79 to 24.1) | 11.5 (4.1 to 18.8) |
| Chagas cardiomyopathy patients | 101 | 667 | 54 (54, 0) | 80.9 (60.8 to 105.6) | 77.3 (55.7 to 98.9) |
| New-onset cardiomyopathy or death (participants without cardiomyopathy at baseline visit) | |||||
| Seronegative donors | 294 | 2,465 | 28 (17, 11) | 11.4 (7.6 to 16.5) | Reference |
| T. cruzi-seropositive donors | 277 | 2,393 | 47 (15, 32) | 19.6 (14.5 to 26.3) | 8.2 (1.3 to 15.4) |
| New-onset cardiomyopathy (participants without cardiomyopathy at baseline visit who underwent the second cardiovascular evaluation) | |||||
| Seronegative donors | 277 | 2,376 | 11 (0, 11) | 4.6 (2.3 to 8.3) | Reference |
| T. cruzi-seropositive donors | 262 | 2,326 | 32 (0, 32) | 13.8 (9.5 to 19.6) | 9.2 (3.6 to 15.0) |
For overall mortality analysis follow-up time was contributed by each participant evaluated at baseline visit (visit 1) until the date of death or until the date of linkage with the national mortality system (Sistema de Informação sobre Mortalidade - SIM) on the 1st June 2020. For new-onset cardiomyopathy follow-up time was contributed either until the date of death or until the date of assessment at follow-up visit (visit 2).
Cardiomyopathy was defined as left ventricular ejection by echocardiography less than 50% and/or QRS complex duration ≥ 120 ms)
CI: confidence interval; CM: cardiomyopathy.
The predictors of death among T. cruzi–seropositive blood donors are shown in Table 3. Among 499 participants, 32 died at a median follow-up of 10.8 years (range 5.3 months to 11.8 years). After adjusting for age and sex, positive T. cruzi PCR at baseline was associated with greater mortality (hazard ratio [HR] of 2.4, 95% confidence interval [CI] of 1.1 - 5.3). Similarly, higher anti-T. cruzi antibody level detected by the semi-quantitative ELISA at baseline was associated with greater mortality (HR of 1.5 for each unit increase, 95% CI 1.1 - 2.0). T. cruzi–seropositive individuals with low anti-T. cruzi antibody level had identical mortality rate to T. cruzi-seronegative controls (Table I in the Data Supplement). As antibody T. cruzi titers increased, mortality rate also increased.
Table 3:
Univariate and age-sex adjusted associations with overall mortality among 499 T. cruzi seropositive participants.
| Variable* | Alive (n= 467) |
Died (n= 32) |
Unadjusted | Adjusted for age and sex | Adjusted for age, sex, antibody†, and cardiomyopathy |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Sex | Female | 225 (48.2) | 13 (40.6) | Reference | Reference | Reference | |||
| Male | 242 (51.8) | 19 (59.4) | 1.35 (0.67-2.74) | 0.399 | 1.53 (0.75 -3.11) | 0.242 | 1.57 (0.72 - 3.39) | 0.250 | |
| Age, years | 47 (40-56) | 56 (44-64) | 1.05 (1.02-1.09) | 0.005 | 1.05 (1.02 -1.09) | 0.004 | 1.05 (1.01- 1.09) | 0.009 | |
| <40 | 122 (26.1) | 5 (15.6) | Reference | Reference | |||||
| 40-49 | 142 (30.4) | 8 (25.0) | 1.36 (0.44-4.14) | 0.594 | NA | ||||
| 50-59 | 135 (28.9) | 7 (21.9) | 1.25 (0.39-3.94) | 0.703 | |||||
| ≥60 | 68 (14.6) | 12 (37.5) | 4.08 (1.44-11.59) | 0.008 | |||||
| BMI, kg/m2 | <24.9 | 159 (34.0) | 13 (40.6) | Reference | Reference | ||||
| 25-29.9 | 218 (46.7) | 15 (46.9) | 0.81 (0.39-1.71) | 0.586 | 0.77 (0.37-1.63) | 0.498 | |||
| ≥30 | 90 (19.3) | 4 (12.5) | 0.53 (0.17-1.64) | 0.273 | 0.59 (0.19-1.82) | 0.354 | |||
| Benznidazole use | 46 (9.9) | 3 (9.4) | 0.90 (0.28-3.04) | 0.899 | 1.11 (0.34-3.65) | 0.868 | |||
| Diabetes | 25 (5.4) | 2 (6.3) | 1.09 (0.26-4.55) | 0.908 | 0.85 (0.20-3.62) | 0.828 | |||
| Hypertension | 106 (22.7) | 7 (21.9) | 1.21 (0.52-.80) | 0.655 | 0.79 (0.33-1.88) | 0.587 | |||
| NYHA FC | I | 424 (90.8) | 26 (81.3) | Reference | Reference | ||||
| II | 28 (6) | 6 (18.8) | 2.18 (1.02-4.66) | 0.045 | 2.34 (1.06-5.18) | 0.035 | |||
| Smoking | Never | 272 (58.2) | 11 (34.4) | Reference | Reference | ||||
| Past | 144 (30.8) | 17 (53.1) | 2.78 (1.30-5.94) | 0.008 | 2.17 (0.98-4.80) | 0.055 | |||
| Current | 51 (10.9) | 4 (12.5) | 1.89 (0.60-5.94) | 0.275 | 1.84 (0.57-5.97) | 0.308 | |||
| T. cruzi DNA detected by PCR‡ | |||||||||
| Negative | 215 (96.4) | 8 (3.6) | Reference | Reference | Reference | ||||
| Positive | 246 (91.1) | 24 (8.9) | 2.56 (1.15-5.70) | 0.021 | 2.38 (1.07-5.31) | 0.035 | NA | ||
| Antibody against T. cruzi | |||||||||
| EIA (S/C) | 6.3 (5.2-6.9) | 6.9 (5.8-7.3) | 1.45 (1.07-1.97) | 0.017 | 1.48 (1.08-2.02) | 0.015 | 1.40 (1.01-1.95) | 0.047 | |
| Antibody EIA quartiles | 1st | 116 (24.8) | 6 (18.8) | Reference | Reference | Reference | |||
| 2nd | 125 (26.7) | 4 (12.5) | 0.63 (0.18-2.21) | 0.467 | 0.54 (0.15-1.94) | 0.348 | 0.55 (0.15-1.99) | 0.363 | |
| 3rd | 118 (25.2) | 8 (25.0) | 1.29 (0.45-3.72) | 0.638 | 1.26 (0.44-3.65) | 0.667 | 1.19 (0.40-3.54) | 0.750 | |
| 4th | 108 (23.3) | 14 (43.8) | 2.39 (0.92-6.21) | 0.075 | 2.44 (0.93-6.42) | 0.070 | 2.06 (0.74-5.73) | 0.165 | |
| Cardiomyopathy at baseline visit | |||||||||
| No | 370 (79.2) | 15 (46.9) | Reference | Reference | Reference | ||||
| Yes | 97 (20.8) | 17 (53.1) | 4.07 (2.03-8.15) | <0.001 | 3.73 (1.83-7.57) | <0.001 | 3.01 (1.45- 6.21) | 0.003 | |
Data are expressed as the absolute numbers (percentage) or median (interquartile range-IQR)
Continuous or stratified as quartiles.
PCR was not included simultaneously with antibody in the multivariable model.
CM: Cardiomyopathy; FC: functional class; S/C: absorbance/cut off
This association between antibody level and mortality was attenuated after additional adjustment for the presence of cardiomyopathy at baseline, which was the strongest predictor of death in seropositive individuals (unadjusted HR of 4.1, 95% CI 2.0 - 8.2). Multivariable analysis identified age (HR = 1.1 per year), antibody level (HR = 1.4 per unit increase in S/CO) and cardiomyopathy (HR=3.0) as independent predictors of mortality. PCR positivity lost statistical significance in the fully adjusted model.
Cardiomyopathy development
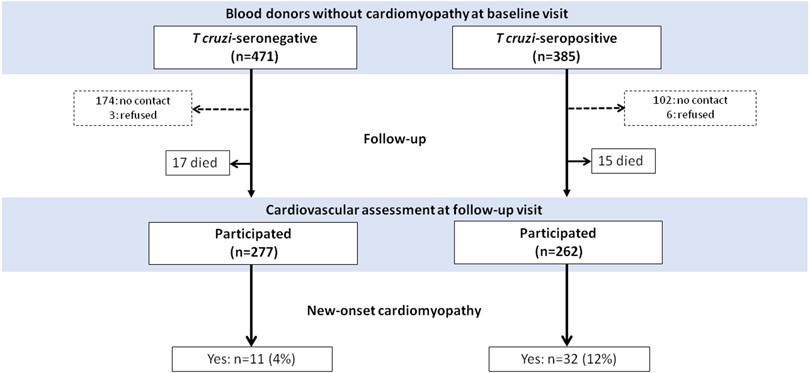
For the purpose of assessing the incidence and risk factors for new-onset cardiomyopathy, only individuals in whom no cardiomyopathy was detected at baseline visit were included. After initial evaluation, 114 T cruzi–seropositive individuals (23%), and 17 seronegative controls (3.5%) were excluded from the analysis of progression (Figure 3). The baseline characteristics of the 385 T. cruzi-seropositive and 471 T. cruzi seronegative blood donors without cardiomyopathy at the baseline visit are shown in Table 4. The clinical characteristics of these participants were not different. Of these participants, 262 of the seropositive donors and 277 of the seronegative donors attended the follow-up visit for cardiovascular assessment, meaning that 108 (28.1%) seropositive and 177 (37.6%) seronegative donors could not be classified for this outcome (Figure 3). For assessment of new-onset cardiomyopathy, some strategies were employed to deal with loss to follow-up (Text I in the Data Supplement).
Figure 3: Disease progression according to T. cruzi serological tests among blood donors without cardiomyopathy at the baseline visit.
After initial evaluation, 471 T. cruzi–seronegative and 385 T. cruzi–seropositive blood donors were included. Disease progression was defined as either new-onset cardiomyopathy or death.
Table 4:
Characteristics of seropositive and seronegative blood donors without cardiomyopathy at the baseline visit.
|
T. cruzi-seronegative donors (n=471) |
T. cruzi-seropositive donors (n=385) |
p-value | ||
|---|---|---|---|---|
| Age, years* | 49 (42-58) | 48 (40-56) | 0.128 | |
| Male sex | 231 (49.0) | 187 (48.6) | 0.890 | |
| Body Mass Index, Kg/m2 | 26.9 (25-30) | 26.6 (24-29) | 0.014 | |
| NYHA functional class | I | 454 (96.4) | 357 (92.8) | 0.020 |
| II/III | 17 (3.6) | 28 (7.2) | ||
| Heart rate (bpm) | 68 (60-75) | 65 (60-72) | 0.005 | |
| Systolic blood pressure (mmHg) | 125 (114-140) | 125 (114-140) | 0.943 | |
| Diastolic blood pressure (mmHg) | 79 (65-88) | 76 (68-86) | 0.740 | |
| Laboratory measurements | ||||
| Low-density lipoprotein, mg/dL) | 124 (100-151) | 118 (96-145) | 0.087 | |
| 46 (38-55) | 48 (41-58) | 0.015 | ||
| High-density lipoprotein, mg/dL | ||||
| Triglycerides, mg/dL | 125 (89-176) | 116 (80-167) | 0.024 | |
| Glycemia, mg/dL | 86 (80-97) | 87 (79-95) | 0.676 | |
| Myoglobin, ng/ml | 35.5 (30.0-50.0) | 36.2 (29.3 - 44.3) | 0.913 | |
| Troponin-I, ng/dl | 0.01 (0.01 -0.01) | 0.01 (0.01 -0.01) | 0.195 | |
| CK-MB, ng/dl | 0.68 (0.41-1.18) | 0.78 (0.47-1.25) | 0.043 | |
| NT-ProBNP, pg/mL | 36.6 (23 - 61) | 42.9 (24 - 72) | 0.009 | |
| ECG parameters | ||||
| QRS duration (ms) | 88 (82-94) | 86 (80-94) | 0.209 | |
| PR duration (ms) | 156 (142-168) | 158 (142-174) | 0.252 | |
| QTc calculated (ms) | 427 (410-441) | 425 (409-441) | 0.564 | |
| Low QRS amplitude | 9 (1.9) | 15 (3.9) | 0.080 | |
| First degree AV block | 3 (0.6) | 10 (2.6) | 0.020 | |
| Sinus bradycardia ≥40 bpm | 133 (28.2) | 125 (32.5) | 0.176 | |
| Minor isolated ST-T abnormalities | 37 (7.9) | 47 (12.2) | 0.033 | |
| Isolated ventricular premature beats | 3 (0.6) | 2 (0.5) | 0.826 | |
| Echocardiographic data | ||||
| LV end-diastolic diameter (mm) | 45 (41-49) | 45 (42-49) | 0.639 | |
| LV end-systolic diameter (mm) | 29 (27-32) | 30 (27-33) | 0.993 | |
| LV ejection fraction (%) | 63 (60-65) | 63 (60-65) | 0.274 | |
| LA diameter, mm | 34 (32-37) | 35 (32-37) | 0.201 | |
| LA volume, mL/m2 | 26.5 (22.9-30.6) | 28.7 (22.9-30.6) | 0.001 | |
| LV mass, g/m2 | 76 (64-90) | 80 (67-89) | 0.013 | |
| Mitral inflow E, cm/s | 70 (58-80) | 68 (56-82) | 0.818 | |
| Mitral inflow A, cm/s | 57 (48-70) | 59 (48-71) | 0.394 | |
| Deceleration time, ms | 197 (166-233) | 194 (159-227) | 0.220 | |
| E/A ratio | 1.2 (1.0-1.5) | 1.2 (1.0-1.4) | 0.672 | |
| E/e' ratio | 6 (5-8) | 6 (5-7) | 0.352 | |
| Right atrial area, cm2 | 14 (12-15) | 14 (12-15) | 0.554 | |
Age at the time of the cardiovascular assessment (baseline visit).
Data are expressed as absolute numbers (percentage) or median (interquartile range)
Abbreviations: CK-MB: creatine kinase isoenzyme MB; E/e’: ratio of the early diastolic transmitral flow velocity to early diastolic mitral annular velocity (average at septal and lateral mitral annulus); LA: left atrial; LV: left ventricular; NT-ProBNP: B-type natriuretic peptide; NYHA: New York Heart Association
There were 32 new-onset cardiomyopathy cases among 262 seropositive blood donors that attended the follow-up visit, equating to an incidence of 13.8 (95% CI 7.6-16.5) events/1000 person-years. Among the 277 seronegative blood donors there were 11 new-onset cardiomyopathy, or 4.6 (95% CI 2.3 to 8.3) events/1000 person-years. The absolute incidence difference associated with T. cruzi seropositivity was 9.2 (95% CI 3.6 to 15.0) events/1000 person-years follow-up (p=0.001) (Table 2). We next conducted a sensitivity analysis assuming all deaths to represent incident cardiomyopathy cases. There were 47 progression events (15 deaths, 32 new-onset cardiomyopathy) among 277 T cruzi-seropositive blood donors attending the second visit or dying during follow-up, equating to 19.6 (95% CI 14.5 to 26.3) events/1000 person-years (Figure 3). Among the 294 seronegative donors there were 28 events (17 deaths, 11 new-onset cardiomyopathy), giving an incidence of 11.4 (95% CI 7.6-16.5) events/1000 person-years with absolute incidence difference associated with seropositivity of 8.2 events/1000 person-years (95% CI 1.3 to 15.4) (Table 2).
In the overall population (seropositive and seronegative blood donors) without cardiomyopathy at baseline visit, the predictors of new-onset cardiomyopathy are shown in Table 5. After adjusting for age, sex, comorbidities, and variables associated with loss to follow-up, T.cruzi-seropositive blood donors carried twice the odds of new-onset cardiomyopathy compared to seronegative blood donors. Specifically, the inclusion of a positive serologic test in a model with traditional risk factors for cardiovascular disease, resulted in significant improvement in model performance with the integrated discrimination improvement (IDI) of 0.014 (95% CI 0.001 – 0.026; p=0.031). The C-statistic increased from 0.611 (95% CI 0.570-0.651) to 0.678 (95% CI 0.619-0.736).
Table 5:
Predictors of new-onset cardiomyopathy or death at long-term follow-up
| Disease status at follow-up |
Did not progress (n = 496) |
Progressed (n = 75) |
Unadjusted | Adjusted for age and sex | Adjusted for age and sex, and risk factors* |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Overall participants without cardiomyopathy at baseline visit (n = 571) | |||||||||
| Male sex | 227 (45.8) | 43 (57.3) | 1.59 (0.98-2.60) | 0.063 | 1.73 (1.05-2.85) | 0.031 | 1.89 (1.13 - 3.16) | 0.016 | |
| Age, years | 49 (42-56) | 53 (44-61) | 1.04 (1.01-1.06) | 0.004 | 1.05 (1.02-1.07) | 0.002 | 1.05 (1.02-1.08) | 0.001 | |
| T. cruzi serological test | |||||||||
| Negative | 266 (53.6) | 28 (37.3) | Reference | Reference | Reference | ||||
| Positive | 230 (46.4) | 47 (62.7) | 1.94 (1.18 - 3.20) | 0.009 | 2.25 (1.34 - 3.76) | 0.002 | 2.24 (1.33-3.77) | 0.002 | |
| Centers | MOC | 274 (55.2) | 30 (40.0) | Reference | Reference | Reference | |||
| SP | 222 (44.8) | 45 (60.0) | 1.85 (1.13 - 3.03) | 0.015 | 1.47 (0.87 - 2.48) | 0.144 | 1.47 (0.86 - 2.50) | 0.156 | |
| T. cruzi seropositive donors without cardiomyopathy at baseline visit (n = 277) | |||||||||
| (n=230) | (n=47) | ||||||||
| Male sex | 99 (43.0) | 26 (55.3) | 1.63 (0.87-3.08) | 0.125 | 1.73 (0.91-3.28) | 0.094 | 1.98 (1.02-3.84) | 0.043 | |
| Age, years | 48 (41-56) | 50 (41-59) | 1.02 (0.99-1.05) | 0.257 | 1.02 (0.99-1.05) | 0.185 | 1.03 (0.99-1.06) | 0.153 | |
| Benznidazole use† | 23 (10.0) | 5 (10.6) | 1.07 (0.38-2.98) | 0.895 | 1.26 (0.44-3.58) | 0.664 | 1.51 (0.48-4.69) | 0.480 | |
| T. cruzi DNA detected by PCR | |||||||||
| Negative | 129 (56.1) | 23 (48.9) | Reference | Reference | Reference | ||||
| Positive | 101 (43.9) | 24 (51.1) | 1.34 (0.71-2.51) | 0.367 | 1.32 (0.70-2.49) | 0.387 | 1.42 (0.74- 2.69) | 0.291 | |
| Centers | MOC | 123 (53.5) | 23 (48.9) | Reference | Reference | Reference | |||
| SP | 107 (46.5) | 24 (51.1) | 1.20 (0.64 - 2.25) | 0.570 | 0.98 (0.50-1.91) | 0.955 | 0.96 (0.49-1.89) | 0.916 | |
| Antibody against T. cruzi | |||||||||
| EIA (S/C) | 6.1 (4.8-6.8) | 6.5 (5.2-7.3) | 1.34 (1.06-1.70) | 0.015 | 1.38 (1.08-1.77) | 0.010 | 1.37 (1.07-1.76) | 0.011 | |
| Antibody EIA quartiles | 1st | 72 (31.3) | 10 (21.3) | Reference | Reference | Reference | |||
| 2nd | 60 (26.1) | 12 (25.5) | 1.44 (0.58-3.57) | 0.430 | 1.41 (0.56-3.53) | 0.464 | 1.39 (0.55-3.47) | 0.486 | |
| 3rd | 58 (25.2) | 9 (19.1) | 1.12 (0.43-2.93) | 0.822 | 1.16 (0.44-3.06) | 0.770 | 1.11 (0.42-2.96) | 0.829 | |
| 4th | 40 (17.4) | 16 (34.0) | 2.88 (1.20-6.94) | 0.018 | 3.23 (1.31-7.94) | 0.011 | 3.18 (1.29-7.85) | 0.012 | |
Data are expressed as the absolute numbers (percentage) or median (interquartile range-IQR)
Risk factors: Diabetes, hypertension, dyslipidemia, and body mass index. S/C: absorbance/cut off
Benznidazole was the antitrypanosomal medication
Abbreviations: MOC: Montes Claros; SP: São Paulo
Predictors of new-onset cardiomyopathy in T. cruzi seropositive blood donors
In the subset of T. cruzi-seropositive blood donors without cardiomyopathy at baseline, total anti-T. cruzi antibody level was associated with new-onset cardiomyopathy, with an adjusted OR of 1.4 (95% CI 1.1-1.8; p=0.011) per unit increase in assay signal-to-cutoff and an adjusted OR of 3.2 (95% CI 1.3-7.9; p=0.012) comparing the highest and lowest antibody quartiles (Table 5). We also found a strong association between antibody levels and the presence of cardiomyopathy using the cross-sectional data at visit 1 (Table II in the Data Supplement). PCR status was not retained in the final adjusted model. When we performed the sensitivity analysis adjusting for missing data, the results remained unchanged. We found an association of new-onset cardiomyopathy with antibody level (adjusted OR of 2.9, 95% CI 1.1-7.6; p=0.035).
DISCUSSION
We have reported the long-term follow-up of the NIH REDS Chagas disease cohort. The main findings were as follows. First, the mortality rate among T. cruzi-seropositive blood donors with cardiomyopathy was 15.1 deaths/1000 person-years, whereas seropositive blood donors without heart involvement had four-times lower mortality, dying at the same rate as T. cruzi-seronegative individuals. Second, anti-T. cruzi serum antibodies level, a marker of parasite burden, was associated with both new-onset cardiomyopathy and mortality. Third, the rate of incident cardiomyopathy associated with T. cruzi seropositivity was 9.2 (95% CI 3.6 to 15.0) events/1000 person-years. The risk was two-times higher compared to seronegative individuals, after adjusting for age, sex, and other cardiovascular risk factors. The odds of incidence cardiomyopathy were three-fold higher in T. cruzi-seropositive individuals with the highest antibody titres. Taken together, our study findings reinforce the concept that the presence of cardiomyopathy in chronically seropositive individuals is a major determinant of survival in Chagas disease. Subclinical cardiac dysfunction induced by T. cruzi infection precedes the development of heart failure and death. Additionally, our results corroborate growing evidence that the degree of parasite burden, as indirectly measured by quantitative serology, plays a major role in the pathogenesis of Chagas cardiomyopathy.
We previously estimated that the incidence of cardiomyopathy associated with T. cruzi seropositivity was 18.5 cases/1000-person years. 17 However, this estimation was based only on cross-sectional data from the baseline visit. The key assumption was that all seropositive blood donors were free of cardiomyopathy at their index donation, approximately 10 years prior the baseline visit. Although this was the best approximation at the time, it is likely that some participants with cardiomyopathy were prevalent at index donation, thus causing an overestimation of the true incidence. Now, given two time points with comprehensive cardiovascular assessment (baseline and follow-up visits), and also including seronegative blood donors as a control group, we were able to exclude all prevalent cases, resulting in the lower incidence estimate associated with T. cruzi seropositivity of 9.2 cases/1000-person years, approximately half of our previous estimate.
Previous studies of Chagas cardiomyopathy have mostly relied on clinical and ECG markers of cardiac involvement with a paucity of information regarding progression of ventricular dysfunction10, 23, 24. In a systematic review and meta-analysis of 23 studies, the estimated annual rate of cardiomyopathy was 1.9% among patients with the indeterminate form9. Most of these studies were conducted in Brazil or Argentina between 1960 and 2005, with a mean participant age of 31 years. More recently, a study showed an annual progression rate of 1.48%25 but without comparing with a control group. A key methodological strength of our cohort, compared to these other studies, was the inclusion of a matched T. cruzi-seronegative control group drawn from a similar population (blood donors), with a blinded parallel clinical, ECG and echocardiographic assessment of all participants at the baseline visit to rule out subclinical ventricular dysfunction. This allowed all prevalent cases to be excluded at baseline and the T. cruzi-attributable incidence of cardiomyopathy to be calculated. It is likely for these reasons that the value of 9.2 cases/1000-person years (0.92% annual rate) is lower than these existing estimates.
Previous studies assessing the risk of cardiomyopathy in T-cruzi seropositive individuals and its predictors had several limitations9. The main criterion used to determine the onset of cardiomyopathy was typical ECG abnormalities, which may overestimate the risk of progression as ECG changes can occur in the general population not infected with T. cruzi due to aging and comorbidities 16, 25, 26. On the other hand, other studies that considered disease progression were based on the development of heart failure symptoms or the presence of complications associated with advanced cardiomyopathy, which reflects late disease process9, 27, 28. Additionally, in earlier studies in endemic areas with persistent exposure to vectors that showed high progression rates may have been confounded by recurrent infections which could play a major role in cardiomyopathy development10-12, 23, 24, 29. Finally, previous studies were conducted in different epidemiologic settings and included small samples, younger populations, and variable follow-up durations, which could have accounted for differences in the rates of progression among studies9.
We defined Chagas cardiomyopathy as the presence of prolonged QRS duration and/or left ventricular systolic dysfunction on echocardiography. This definition reproduced, with 95% accuracy, the diagnosis of cardiomyopathy in participants at the baseline visit, which was made by a panel of expert cardiologists19. QRS duration ≥ 120 ms encompasses right bundle branch block (RBBB) which is the most typical finding associated with T. cruzi seropositivity13, 19. We have shown that T. cruzi-seropositive individuals with normal QRS duration and left ventricular ejection fraction have an identical 10-year mortality compared to T. cruzi-seronegative individuals. In contrast, seropositive cases meeting this definition of cardiomyopathy have a relative mortality risk of 4.2 compared to seronegative controls (15.1 versus 3.62 deaths/1000-person years). The strong association between patient-outcome validates these criteria as a simple definition of cardiomyopathy for use in epidemiologic studies of Chagas disease. Furthermore, the presence of these ECG and echocardiographic findings can be used for risk stratification and patient counseling.
Predictors of Chagas disease progression
Various factors have been reported to be associated with cardiomyopathy onset, including the T. cruzi-genotype, persistent tissue and/or blood parasitism, abnormal immune responses, male sex, oral acquisition of infection, and recurrent infections17, 30-32. There is a growing consensus that parasite persistence is required for the development of cardiomyopathy and consequently antiparasitic treatment may have impact on the clinical course of the disease33-36. The disappearance of T. cruzi antibodies is considered as the best evidence for a parasitological cure. However, there is still no robust evidence that seroreversion is a surrogate of clinical outcome, or halting the progression of the disease 37, 38.
There was a biological gradient whereby the prevalence of cardiomyopathy increased at higher antibody levels (Table I in the Data Supplement). Individuals with higher antibody level at baseline were more likely to develop cardiomyopathy or to die during the follow-up period. Those with antibody levels in the highest quartile carried odds of 3.2, compared to the lowest quartile, for this outcome. Serology assays that detect total antibody against T. cruzi cell lysate reflect the overall humoral immune response against T. cruzi. In patients with greater parasite burden, experiencing more frequent parasitemia, antigenic immune stimulation by T. cruzi is thought to be greater, resulting in higher total antibody levels39, 40. As the development of cardiomyopathy seems to be driven by parasite persistence, the association between antibody levels, a marker of parasite burden, and incident cardiomyopathy is expected mechanistically, but has not previously been demonstrated. This observation may be useful in counselling patients about their long-term prognosis and risk of cardiomyopathy. It may also be a useful criterion for enrollment in clinical trials of antitrypansomal treatment. By selecting individuals without cardiac disease but with high antibody titres, the event rate, and therefore power, will be higher. Furthermore, patients with higher parasite burden, as assessed by antibody level, may stand to benefit the most from etiologic treatment, although this is unproven.
Parasite detection in blood by PCR has also been used to assess parasite load and treatment effectiveness. Previous studies demonstrated the role of PCR in predicting progression12, 40. However, a negative PCR does not mean absence of parasite as false negatives occur due to fluctuations in parasitemia, the intrinsic limit of detection of PCR, qPCR techniques, and other factors that contribute to the overall performance of PCR assays41. Therefore, due to frequent false-negatives, PCR is a relatively poor indicator of parasite burden and the association with disease outcome would be expected to be biased towards the null. It makes sense therefore that PCR status was not retained within the fully adjusted models, but antibody level, which is a more stable indicator of parasite burden, did remains significantly associated with death and new onset cardiomyopathy.
Study limitations
Loss to follow-up may lead to bias, which may affect the inferences drawn from the study. To ensure that those lost to follow-up did not have higher mortality rate than those who complete the study, the vital status of each participant lost to follow-up was determined from the National Mortality System. The linkage method used has high sensitivity and specificity to detect deaths. To assess new-onset of cardiomyopathy among participants who were alive at the time of the linkage, we compared well-established risk factors for cardiovascular diseases between those lost to follow-up with those who returned for follow-up visit to complete the study (Text I, Figure I and Table III in the Data Supplement). Additionally, a model to predict loss to follow-up was built including variables that predict either loss to follow-up and progression (Table IV in the Data Supplement). Finally, a sensitivity analysis for new-onset cardiomyopathy was performed using inverse probability weights for selection (Table V in the Data Supplement) with no important changes in inferences. The effect of the variables that we previously found to be associated with new-onset cardiomyopathy (Table 5), including antibody levels, remained statistically significant.
Our approach was intended to be explorative, and may be useful for inferences about possible selection bias in new-onset of cardiomyopathy measured after loss to follow-up has occurred. After careful evaluation, we assumed that loss to follow-up depended on a MAR mechanism, in which the probability of a participant remaining in the study depends on the exposure or confounders, but not on non-observed outcomes42. The MAR mechanisms provide an unbiased estimate of effect because collected variables can explain the potential bias by controlling for the covariates that are associated with loss to follow-up in multivariable analysis42.
To assess the impact of changes in life expectancy observed in T. cruzi- seropositive individuals, other associated comorbidities must be considered to increase the risk of cardiovascular events and death26, 43. Over the past few decades, the migration from rural areas to large urban centers exposes these individuals to a lifestyle that predispose to atherosclerosis and cardiovascular diseases. Comorbidities become increasingly more frequent as Chagas disease population ages43. As such, in an aging cohort of individuals with Chagas disease, the incidence of cardiomyopathy associated with T. cruzi is expected to fall, while cardiac disease due to other causes is expected to increase. However, after controlling for age and comorbidities by including seronegative individuals, T. cruzi seropositivity remained an important determinant of cardiomyopathy even among elderly in our study.
The pathophysiology of myocardial damage in Chagas cardiomyopathy is complex and multifactorial. Although we found an association between antibody levels and cardiomyopathy, pathogenic differences in T. cruzi strains and host genetic and immunological susceptibility factors are also likely to play roles in disease progression44. Therefore, the progression to cardiomyopathy likely results from multiple factors linked to the parasite, the host and the interaction between them, which cannot be determined from our study.
Finally, we recommend caution in extrapolating these findings to T. cruzi- seropositive individuals living in nonendemic countries. It is possible that in nonendemic settings, which present little or no risk of reinfection and higher socioeconomic conditions, the progression to cardiomyopathy is delayed.
CONCLUSIONS
We have reported a comprehensive description of the natural history of T. cruzi seropositivity in a large contemporary cohort of blood donors with detailed cardiovascular evaluation. T. cruzi seropositivity was a strong determinant of new-onset cardiomyopathy and death. Levels of T. cruzi antibody level, an indirect measure of parasite burden, was associated with disease progression. Our data highlight the central importance of total anti-T. cruzi antibody titer as a marker of Chagas disease activity and risk of progression.
Supplementary Material
Clinical Perspective.
What Is New?
The incidence of cardiomyopathy associated with T. cruzi seropositivity has been steadily declining over the past decade with effective vector control.
T. cruzi-seropositive individuals with normal left ventricular ejection fraction and QRS duration have identical mortality to T. cruzi-seronegative controls.
T. cruzi antibody levels play a major role in predicting progression to cardiomyopathy.
What Are the Clinical Implications?
T. cruzi seropositivity is a strong determinant of new-onset cardiomyopathy and death.
Identification of T. cruzi-seropositive individuals at risk of developing cardiomyopathy would be useful to inform patient counselling, intensity of follow-up, and designing clinical trials of treatment.
Patients with high levels of anti-T. cruzi serum antibodies may be considered for anti-trypanosomal therapy.
Sources of Funding
This work was funded by the NIH, FAIN: U19AI098461. MCPN, ALPR and ECS are National Council for Scientific and Technological Development (CNPq) scholarship recipients.
Non-standard Abbreviations and Acronyms
- IDI
integrated discrimination improvement
- LVEF
left ventricular ejection fraction
- MAR
missing at random
- NYHA
New York Heart Association
- PCR
polymerase chain reaction
- RBBB
right bundle ranch block
- REDS
Recipient Epidemiology and Donor Evaluation Study
- S/CO
signal-to-cutoff
Footnotes
Disclosures
None.
REFERENCES
- 1.Moolani Y, Bukhman G, Hotez PJ. Neglected tropical diseases as hidden causes of cardiovascular disease. PLoS Negl Trop Dis. 2012;6:e1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverria LE, Dutra WO, Gascon J, Morillo CA, Oliveira-Filho J. Chagas cardiomyopathy: An update of current clinical knowledge and management: A scientific statement from the american heart association. Circulation. 2018;138:e169–e209. [DOI] [PubMed] [Google Scholar]
- 3.WHO/PAHO. Neglected infectious diseases in the americas: Success stories and innovation to reach the neediest. 2016
- 4.Bern C, Messenger LA, Whitman JD, Maguire JH. Chagas disease in the united states: A public health approach. Clin Microbiol Rev. 2019;33: e00023–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. Chagas disease in latin america: An epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90:33–43. [PubMed] [Google Scholar]
- 6.Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL, Council on Chagas Disease of the Interamerican Society of C. Chagas disease: An overview of clinical and epidemiological aspects. J Am Coll Cardiol. 2013;62:767–776. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. Diagnosis and management of chagas disease and cardiomyopathy. Nat Rev Cardiol. 2012;9:576–589. [DOI] [PubMed] [Google Scholar]
- 8.Prata A. Clinical and epidemiological aspects of chagas disease. Lancet Infect Dis. 2001;1:92–100. [DOI] [PubMed] [Google Scholar]
- 9.Chadalawada S, Sillau S, Archuleta S, Mundo W, Bandali M, Parra-Henao G, Rodriguez-Morales AJ, Villamil-Gomez WE, Suárez JA, Shapiro L. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of chagas disease: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire JH, Hoff R, Sherlock I, Guimaraes AC, Sleigh AC, Ramos NB, Mott KE, Weller TH. Cardiac morbidity and mortality due to chagas' disease: Prospective electrocardiographic study of a brazilian community. Circulation. 1987;75:1140–1145. [DOI] [PubMed] [Google Scholar]
- 11.Coura JR, Abreu LLd, Pereira JB, Willcox HP. Morbidity in chagas' disease: Iv. Longitudinal study of 10 years in pains and iguatama, minas gerais, brazil. Mem Inst Oswaldo Cruz. 1985;80:73–80. [DOI] [PubMed] [Google Scholar]
- 12.Basquiera A, Sembaj A, Aguerri A, Omelianiuk M, Guzmán S, Barral JM, Caeiro T, Madoery R, Salomone O. Risk progression to chronic chagas cardiomyopathy: Influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart. 2003;89:1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro AL, Marcolino MS, Prineas RJ, Lima-Costa MF. Electrocardiographic abnormalities in elderly chagas disease patients: 10-year follow-up of the bambui cohort study of aging. J Am Heart Assoc. 2014;3:e000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizzoni AG, Varela MC, Sangenis LHC, Hasslocher-Moreno AM, do Brasil PEAA, Saraiva RM. Ageing with chagas disease: An overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors. 2018;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dias JC, Ramos AN Jr., Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, Torres RM, Melo JR, Almeida EA, Oliveira W Jr., et al. 2 nd Brazilian Consensus on Chagas Disease, 2015. Rev Soc Bras Med Trop. 2016;49 Suppl 1:3–60. [DOI] [PubMed] [Google Scholar]
- 16.Marcolino MS, Palhares DMF, Alkmim MBM, Ribeiro AL. Prevalence of normal electrocardiograms in primary care patients. Rev Assoc Med Bras. 2014;60:236–241. [DOI] [PubMed] [Google Scholar]
- 17.Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, Ianni BM, Nastari L, Fernandes F, Patavino GM, et al. Ten-year incidence of chagas cardiomyopathy among asymptomatic trypanosoma cruzi-seropositive former blood donors. Circulation. 2013;127:1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buss LF, Bes TM, Pereira A, Natany L, Oliveira CDL, Ribeiro ALP, Sabino EC. Deriving a parsimonious cardiac endpoint for use in epidemiological studies of chagas disease: Results from the retrovirus epidemiology donor STUDY-II (REDS-II) cohort. Rev Inst Med Trop Sao Paulo. 2021;63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro AL, Sabino EC, Marcolino MS, Salemi VM, Ianni BM, Fernandes F, Nastari L, Antunes A, Menezes M, Oliveira CDL. Electrocardiographic abnormalities in trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013;7:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr, 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 21.Capuani L, Bierrenbach AL, Abreu F, Takecian PL, Ferreira JE, Sabino EC. Accuracy of a probabilistic record-linkage methodology used to track blood donors in the mortality information system database. Cad Saude Publica. 2014;30:1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter J, Kenward M. Multiple imputation and its application. John Wiley & Sons; 2012. [Google Scholar]
- 23.Espinosa R, Carrasco HA, Belandria F, Fuenmayor AM, Molina C, González R, Martinez O. Life expectancy analysis in patients with chagas' disease: Prognosis after one decade (1973–1983). Int J Cardiol. 1985;8:45–56. [DOI] [PubMed] [Google Scholar]
- 24.Mota EA, Guimarães AC, Santana OO, Sherlock Í, Hoff R, Weller TH. A nine year prospective study of chagas' disease in a defined rural population in Northeast Brazil. Am J Trop Med Hyg. 1990;42:429–440.. [DOI] [PubMed] [Google Scholar]
- 25.Hasslocher-Moreno AM, Xavier SS, Saraiva RM, Sangenis LHC, Holanda MT, Veloso HH, Costa ARD, Mendes F, Brasil P, Silva G, Mediano MFF, Sousa AS. Progression rate from the indeterminate form to the cardiac form in patients with chronic chagas disease: Twenty-two-year follow-up in a brazilian urban cohort. Trop Med Infect Dis. 2020;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa MLe, Barreto S, Guerra H, Firmo J, Uchoa E, Vidigal P. Ageing with trypanosoma cruzi infection in a community where the transmission has been interrupted: The bambuí health and ageing study (BHAS). Int J Epidemiol. 2001;30:887–893. [DOI] [PubMed] [Google Scholar]
- 27.Nunes MC, Barbosa MM, Ribeiro AL, Colosimo EA, Rocha MO. Left atrial volume provides independent prognostic value in patients with chagas cardiomyopathy. J Am Soc Echocardiogr. 2009;22:82–88. [DOI] [PubMed] [Google Scholar]
- 28.Nunes MC, Carmo AA, Rocha MO, Ribeiro AL. Mortality prediction in chagas heart disease. Expert Rev Cardiovasc Ther. 2012;10:1173–1184. [DOI] [PubMed] [Google Scholar]
- 29.Laranja F, Dias E, Nobrega G, Miranda A. Chagas' disease: A clinical, epidemiologic, and pathologic study. Circulation. 1956;14:1035–1060. [DOI] [PubMed] [Google Scholar]
- 30.Jones EM, Colley DG, Tostes S, Lopes ER, Vnencak-Jones CL, McCurley TL. Amplification of a trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg. 1993;48:348–357. [DOI] [PubMed] [Google Scholar]
- 31.Benvenuti LA, Roggerio A, Freitas HF, Mansur AJ, Fiorelli A, Higuchi ML. Chronic american trypanosomiasis: Parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol. 2008;102:481–487. [DOI] [PubMed] [Google Scholar]
- 32.Bustamante JM, Novarese M, Rivarola HW, Lo Presti MS, Fernandez AR, Enders JE, Fretes R, Paglini-Oliva PA. Reinfections and trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. J Parasitol Res. 2007;100:1407–1410. [DOI] [PubMed] [Google Scholar]
- 33.Viotti R, de Noya BA, Araujo-Jorge T, Grijalva M, Guhl F, Lopez M, Ramsey J, Ribeiro I, Schijman A, Sosa-Estani SN. Latin american network for chagas disease. Antimicrob Agents Chemother. 2014;58:635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro V, Dias N, Paiva T, Hagstrom-Bex L, Nitz N, Pratesi R, Hecht M. Current trends in the pharmacological management of chagas disease. Int J Parasitol Drugs Drug Resist. 2020;12:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. Long-term cardiac outcomes of treating chronic chagas disease with benznidazole versus no treatment: A nonrandomized trial. Ann Int Med. 2006;144:724–734. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, Silva JLP, Colosimo EA, Ferreira JE, Lee T-H. Beneficial effects of benznidazole in chagas disease: Nih sami-trop cohort study. PLoS Negl Trop Dis. 2018;12:e0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Lancheros E, Chatelain E, Ndao M. Chagas disease treatment efficacy biomarkers: Myths and realities. Chagas disease. Springer; 2019:323–349. [Google Scholar]
- 38.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C. Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. New Engl J Med. 2015;373:1295–1306. [DOI] [PubMed] [Google Scholar]
- 39.Buss LF, de Oliveira-da Silva LC, Moreira CH, Manuli ER, Sales FC, Morales I, Di Germanio C, de Almeida-Neto C, Bakkour S, Constable P. Declining antibody levels to trypanosoma cruzi correlate with polymerase chain reaction positivity and electrocardiographic changes in a retrospective cohort of untreated brazilian blood donors. PLoS Negl Trop Dis. 2020;14:e0008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georg I, Hasslocher-Moreno AM, Xavier SS, Holanda MTd, Roma EH, Bonecini-Almeida MdG. Evolution of anti-trypanosoma cruzi antibody production in patients with chronic chagas disease: Correlation between antibody titers and development of cardiac disease severity. PLoS Negl Trop Dis. 2017;11:e0005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Britto CC. Usefulness of pcr-based assays to assess drug efficacy in chagas disease chemotherapy: Value and limitations. Mem Inst Oswaldo Cruz. 2009;104:122–135. [DOI] [PubMed] [Google Scholar]
- 42.Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: How much is too much? Eur J Epidemiol. 2004;19:751–760 [DOI] [PubMed] [Google Scholar]
- 43.Martins-Melo FR, Ramos AN Jr.., Alencar CH, Heukelbach J. Prevalence of chagas disease in brazil: A systematic review and meta-analysis. Acta Trop. 2014;130:167–174. [DOI] [PubMed] [Google Scholar]
- 44.Nogueira PM, Ribeiro K, Silveira AC, Campos JH, Martins-Filho OA, Bela SR, Campos MA, Pessoa NL, Colli W, Alves MJ. Vesicles from different trypanosoma cruzi strains trigger differential innate and chronic immune responses. J Extracell Vesicles. 2015;4:28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.