Abstract
Black people in the US experience greater atopic dermatitis (AD) prevalence, severity, and persistence when compared to White people. While very little published literature describes AD in the Latinx population, additional differences in severity, persistence, and age of onset exist in contrast to White people. Thus far, genetic polymorphisms associated with increased risk and/or severity of AD are less common among Black people, so should confer reduced, rather than the observed increased AD risk, among Black people. Little is known regarding genetic risk factors in Latinx people. In contrast, there is consistent evidence that socioeconomic, environmental, and health care factors influence AD prevalence, severity, and/or persistence, and these same risk factors are more common among racial and ethnic minority populations as a result of racism. Researchers too often pursue genetic explanations for racial and ethnic AD disparities when the evidence points to the importance of contextual, rather than genetic, causes of these disparities. Reframing the prevailing view that innate differences among racial groups are responsible for these disparities by emphasizing the role of racism and its downstream effects on contextual factors will be a critical first step towards shrinking these disparities.
Keywords: Atopic dermatitis, Eczema, Disparities, Race, Ethnicity, Racism, Social Determinants of Health
Introduction
Atopic dermatitis (AD, eczema) is the skin disease with the greatest global disease burden (1). Up to 25% of children and 8% of adults have a current or past diagnosis of AD (2). Atopic dermatitis contributes negatively to quality of life (QoL) and often leads to hours of lost sleep at night due to itch (3, 4). In addition to asthma and allergies, some AD comorbidities include behavior problems in children, depression, anxiety, low self-esteem, parent-child conflict, cardiovascular disease, and cognitive dysfunction (4–9).
Black and Latinx people with AD experience greater disease burden than White people. The bulk of the evidence supporting the existence of these disparities is from secondary data analyses identifying associations between sociodemographic characteristics or exposures and AD (10–14). While these studies are an important foundation for future research, conclusions may be difficult to draw from them as contextual and upstream factors that play large parts in the disparities are not usually captured.
The influences on AD are multifactorial, with genetics, biology, socioeconomic, environment, and health care access/quality factors contributing. Due to rapid advances in biomedical science, success of biologic drugs, and identification of genetic risk factors, researchers have primarily emphasized a biologic lens for the study of racial and ethnic disparities in AD (2, 15–20). Although genetic risk factors, such as filaggrin mutations, explain some of the variation in AD within populations, they do not explain differences in AD risk between racial/ethnic populations, so that racial and ethnic AD disparities are more likely the result of contextual factors such as socioeconomic status (SES), physical environment, and health care access and quality (19, 21–24). These risk factors are determined in large part, but not exclusively, by the more powerful sources of inequity: structural and individual racism (25). In this review we describe the risk factors that drive racial and ethnic AD disparities and discuss the implications for research, clinical practice, and policy.
Conceptual framework
When examining racial and ethnic health disparities, consideration of the contextual factors that contribute to AD disparities and their inter-relationships is essential (26, 27). We propose a framework for conceptualizing racial and ethnic disparities in AD (Figure 1), which is based on documented AD prevalence, severity, and persistence risk factors and associations and an understanding of how racism can influence them.
Figure 1.
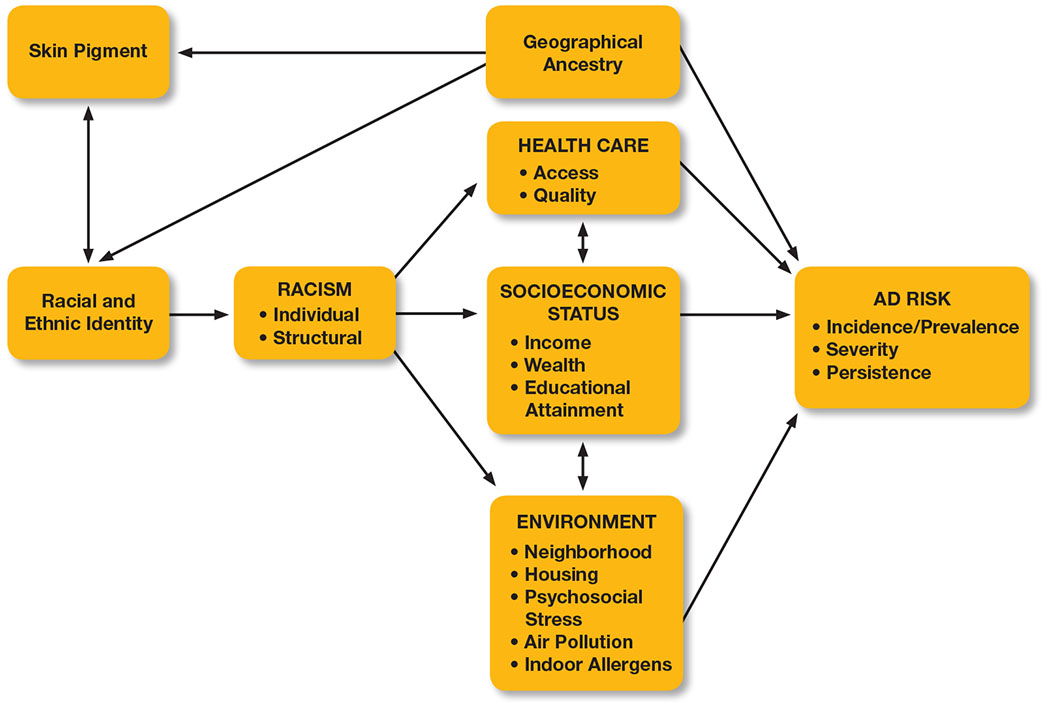
Proposed framework for conceptualizing racial and ethnic disparities in atopic dermatitis. Geographical ancestry often correlates with skin pigment, self-identified racial/ethnic identity, and genetic variation that may increase the risk of prevalent or persistent AD in some populations. Racism is arguably the largest shared health risk factor among racial and ethnic minority populations. Health care, socioeconomic status, and environment are linked to features of AD and are therefore conceptualized as mediators of the effects of racism on AD incidence/prevalence, severity, and persistence.
Geographical ancestry is often correlated with skin pigment, self-identified racial (e.g. Black, White, etc.) and ethnic (e.g. African, Mexican, etc.) identity, and genetic variation; but identified genetic factors that contribute to the risk of AD diagnosis, persistence, and severity are not known to be tied to darker skin pigment or Black race or Latinx ethnicity (21, 28–30). Instead, arguably the largest shared health risk factor among racial and ethnic minority populations is the experience of racism (structural and individual), which is inflicted upon people based on their racial and/or ethnic identity (31). In fact, racism has resulted in racial and ethnic inequities in health care access/quality, SES, and environmental exposures, all of which have been linked to features of AD, so that these factors can be conceptualized as mediators of the effects of racism on AD prevalence, severity, and persistence (32–38). Research, patient care, and policy interventions that involve AD in racial and ethnic minority populations should be framed in such a way that these factors are measured or observed directly when possible and considered in the interpretation of results (26, 39).
Racial and ethnic differences
Differences in AD incidence/prevalence, severity, and persistence exist across racial and ethnic groups in the US (40, 41). The published literature primarily describes children, with limited epidemiological data on adults with AD (42). A limitation of many secondary data analyses describing the epidemiology of AD is that some are designed to specifically identify atopic dermatitis while others more broadly identify eczema (12, 43). However, the preponderance of eczema in children is due to atopic dermatitis and we therefore included both eczema and atopic dermatitis in our review (12). Here we focus on Black and Latinx communities in the United States because there is more literature describing how racism shapes social determinants of health (SDOH), which in turn influence AD risk, for these racial and ethnic populations than others. There are certainly other racial and ethnic groups that are disproportionately affected by AD, such as Asian Americans, but there is less published literature spanning the concepts of racism, SDOH, and AD for these other groups. Varying methods of determining and/or documenting race and ethnicity may affect these studies’ results. Additionally, information about both the racial and ethnic identities of study participants is often not collected, limiting our understanding of AD burden among some populations, such as those who are both Latinx and Black (e.g. Afro-Cuban, Dominican, etc.). The sparsity of published scientific literature for certain racial/ethnic groups highlights the importance of pursuing studies that address this gap among all minority races and ethnicities not covered in this review.
The excess AD burden is better studied for Black people than Latinx people, but has been described in both populations. Black children are approximately 1.7 to 2.1 times more likely to receive an AD diagnosis than White children (2, 11, 43). In one analysis, Black children with AD were more than twice as likely and Latinx children were more than 1.5 times as likely to have severe AD than White children (12). Black and Latinx children are more than 3 times more likely to have persistent AD compared to White children, and persistent AD is associated with increased AD severity, odds of an asthma diagnosis, greater AD burden, and poorer overall health (11, 13). In one study, Latinx children had approximately half the AD prevalence compared to White children at 5 years of age (8.7% vs. 15.6%), but more than 1.5 times higher prevalence at 15 years of age (14.2% vs. 9.5%); and this observation may be partially explained by Latinx children being more likely to have late childhood-onset or persistent AD, and/or reporting bias due to presenting later to a health care provider for their AD (2, 10, 11, 13, 44).
Black, Latinx, and White children born outside of the US, or whose parents were born outside of the US (country not specified), were all found to be much less likely to have an AD diagnosis (OR 0.43) in one study, but this protective effect disappeared after 10 years in the country (45). Additionally, analyses specifically considering AD across different Latinx ethnicities and races are lacking, and these groups are often included in a single Latinx/Hispanic group. This approach obscures heterogeneity among these subgroups, but some studies have reported that AD incidence is much higher in Puerto Rican children and much lower in Mexican children than incidence rates reported for Latinx children in the US (46, 47).
Genetic and biological influences
Some genetic factors have been implicated in AD, though the genetics of AD are complex and a person can (and often does) develop the disease in absence of identified genetic risk factors (21). There is also discussion surrounding several AD biomarkers, whether they vary across race and ethnicity, and if so, whether variations are innate or acquired over time due to exposures (16, 19, 20).
Genetic analyses that attempt to explain racial and ethnic health disparities are fraught with limitations, including lumping genetically heterogeneous groups of people into categories defined by race, a social construct (24, 39). Although genetics are clearly implicated in AD for some people, there is scant evidence that genetic differences contribute to the disparities observed among racial/ethnic groups (48, 49). For instance, authors of one study found increased African ancestry was not associated with increased AD risk in people who self-identify as African American (21). In contrast, there is growing evidence implicating contextual factors, so that research should focus on potential influences such as racism and its downstream effects on SDOH (12–14, 32). It is also important to improve the racial/ethnic representativeness in studies examining the genetic underpinnings of AD since genetic risk factors may be important in Black and Latinx populations even if they do not explain differences in AD risk among racial/ethnic groups. Because these populations are severely underrepresented in genetic studies, whether and to what extent genetic risk factors contribute to AD risk within these populations is unclear (50, 51).
Filaggrin mutations
The most well described genetic risk factors for AD are loss-of-function (LOF) mutations in the genes that produce filaggrin (FLG and FLG2), an essential protein for skin barrier function (2, 48). A sample of children (n=857) with a history of AD underwent genetic testing for the four most common FLG mutations and Black children were nearly six times less likely than White children to have any FLG null mutation (48). When present, however, FLG and FLG2 LOF mutations or non-synonymous variants associated with AD persistence have similar implications for persistent disease in Black or White children (17, 41). Authors of another study describe the use of array-based sequencing which identified novel FLG LOF mutations in a small, Black population with moderate-to severe AD (18). Overall, known FLG mutations appear to be less important with respect to population cause and attributal risk in Black people with AD when compared with White people and we are unaware of any papers describing prevalence of FLG mutations in Latinx people (2). While work should continue in order to identify genes implicated in the differences in AD risk within racial and ethnic groups, the preponderance of evidence points to contextual factors being the major factor in AD disparities among racial and ethnic minority groups.
Transepidermal water loss
Transepidermal water loss (TEWL) is a biomarker that when increased is associated with increased AD severity due to inadequate skin hydration (52). It is affected by exogenous factors such as humidity and exposure to chemicals (52, 53). Previous studies suggesting TEWL may be increased in darker skin involved small sample sizes of participants with healthy skin whereas another study of participants with AD found lower TEWL in Black people when compared with White people in spite of greater disease severity (41, 54). Fewer data exist regarding Latinx people, but TEWL performance appears to be similar to that observed among White people (55). Because of these conflicting findings and the potential for exogenous influence, TEWL has significant limitations when applied to ethnically diverse populations or studies of racial or ethnic AD disparities.
Immunologic phenotypes
Additional phenotypic differences reported across race and ethnicity in AD include Th2 cytokine signaling pathways and serum immunoglobulin E (IgE) (16, 41). Though a few studies have reported differences in cytokine activation in skin and/or IgE in blood across race and ethnicity in people with AD, it is important to note that immunologic phenotypes – such as those based on cytokine profiles and/or IgE concentrations – can be influenced by contextual factors that affect their activation or expression (16, 41, 56, 57).
Racism
Given the scant evidence implicating genetic differences in AD disparities, it is important to carefully consider contextual factors as causes of these disparities and one contextual factor that is shared by minority communities is the experience of racism. Racism is a system within which a dominant group racializes and categorizes social groups and devalues and disempowers them based on their perceived inferiority across levels that range from individual to entire systems (36, 58). Structural racism occurs within and is perpetuated by systems such as housing, health care, labor, criminal justice, education, and economic systems in which certain groups (typically White) are provided numerous advantages while other groups (such as Black and Latinx) are denied access (58). Racism permeates and can powerfully impact nearly all aspects of life and is known to be a strong negative influence on health (36).
In the only published study addressing AD and structural racism, Black children in North Carolina had greater AD severity if they lived in racially segregated neighborhoods than Black children who did not (59). Severity was not moderated by neighborhood segregation for White children in the same study (59). This study population was a convenience sample from a dermatology practice in one geographical area, but their results generally align with other studies showing lower SES correlates with increased disease severity. This study is an important first step in more directly examining the relationship between racism and AD. No studies to our knowledge have investigated the relationship between individual racism or other aspects of structural racism and AD. However, robust public health and epidemiological evidence suggest individual and structural discriminatory forces shape many aspects of life that are associated with AD prevalence, severity, and persistence, including socioeconomic status (SES), environment, and health care quality and access (35, 60). This link can also be inferred based on what has been reported more thoroughly in asthma, a similar, comorbid diagnosis (45, 59, 60). Therefore, understanding the context of AD risk factors across race and ethnicity, and acknowledging the significant role that racism plays in the development of these risk factors, will help reframe their implications and provide the basis for developing and testing interventions aimed at shrinking AD disparities (26).
Socioeconomic status
Socioeconomic status – which broadly describes a person’s or family’s relative position in society and their access or capacity to consume/amass resources, wealth, and power – is well established as contributing to disease rates, severity, and access to health care for many health conditions (61). Researchers attempt to capture SES using measures such as income, wealth, education, occupation, housing type, and location of residence (61). Minority race and ethnicity are strongly correlated with lower SES due to centuries of racism and discrimination in the US (35). Therefore, Black and Latinx people are more likely to experience negative disease outcomes that are associated with lower SES.
The relationship between SES and AD is complex as the effect of SES varies depending on the population and outcome being studied. Higher SES tends to be associated with increased AD prevalence whereas lower SES is associated with increased severity and persistence (11–13, 34, 43). These mixed results might be explained by higher SES being a risk factor for developing AD, but protective against severity because of fewer barriers to accessing good medical care and implementing consistent and time-intensive home management. Because people with higher SES have greater access to medical care, they may also be overrepresented in studies attempting to characterize this association (32, 34).
While we would expect controlling for SES to attenuate the difference in AD risk between racial and ethnic minority populations and their White counterparts, few studies have directly examined the effect of SES on racial or ethnic AD disparities (11, 13, 14, 44). Furthermore, it appears that no study of AD racial or ethnic disparities has simultaneously controlled for the many dimensions of SES, such as income, wealth, educational attainment, occupation, and material hardship in order to determine what remaining excess risk for Black and Latinx people, if any, remains. When adjustment for SES variables does not fully explain racial or ethnic differences in AD, adjustment for SES may be inadequate given its multi-dimensional nature. Alternatively, the remaining disparity may be explained by non-SES contextual factors. One study, surprisingly, found controlling for maternal education and neighborhood income strengthened the relationship between Black race and AD incidence and it is important to further understand whether this is an outlier or repeatable (11).
Environmental exposures
The environment can influence AD through different pathways including air pollution, chemical exposures, allergens, psychosocial stress, and other exposures collectively known as the exposome (33, 62). Exposure to allergens, chemicals, and pollutants in people with an already impaired skin barrier due to AD may lead to increased AD severity and increased risk of related allergic diseases (63). Maternal psychosocial stress, which is associated with increased likelihood of AD in offspring, is not an environmental factor itself but can be caused by certain neighborhood features (64). Black and Latinx people have a greater burden of harmful environmental exposures when compared with White people because of longstanding structural racism, specifically environmental racism and housing discrimination (37, 65).
Neighborhood and housing
Metropolitan living is a proxy for other exposures, such as increased proximity to roads and certain air pollutants, and perhaps exposure to violence or crime and the stress that occurs as a result. Urban/inner-city living was associated with approximately 1.7 times increased odds of an AD diagnosis, in an analysis across 20 large US cities (43). Other studies found dilapidated housing, older homes, and communities with garbage on the street were associated with increased AD severity and unsafe and unsupportive neighborhoods were associated with increased AD prevalence and severity (12, 14, 59). Alternatively, studies in more homogeneous populations with respect to race/ethnicity and geographical ancestry, such as an almost entirely Black population in South Africa, have found reduced AD risk in rural-residing children in spite of generally lower SES (66). One hypothesis is that this is due to farm animal exposure. This highlights the potential role of exposures (e.g. environmental, animals) on incident AD. These initial observations are intriguing but large gaps remain in our understanding of the relationship between urban environments and AD risk, and future work should focus on understanding what about these settings are true risk factors versus associations, and which groups of people within those environments are most likely to be affected.
Air pollution
The relationship between air pollution and skin health is not fully understood but emerging evidence suggests that there is an association. The link between pollution and skin health is biologically plausible as pollutants may trigger innate and adaptive immune responses (53, 67). Airborne exposures to nitrogen oxides, tobacco smoke, benzene, particulate matter, and other outdoor airborne pollutants are associated with higher AD prevalence and severity, and concentrations of air pollution vary across race/ethnicity and lower SES people (38, 62, 68). Whether and how the composition of air pollution exposures differs across race and ethnicity, and also whether/how these differences might contribute to overall disparities in AD risk remains unclear.
Indoor allergens
Indoor allergen exposures, such as pets, house dust mites (HDM), mice, cockroaches, and mold that are implicated in asthma risk may also be associated with AD prevalence and severity (69–73). There is a substantial body of literature addressing HDM exposure among HDM-sensitized people and increased risk of developing AD (73, 74). Early dog and other pet exposures may protect against an AD diagnosis, while later pet exposures in sensitized people can exacerbate AD (72, 75). A few studies have shown positive associations between mold exposures and development or worsening of AD (71, 76). In contrast to pets and HDM, there is surprisingly scant data regarding mice, cockroaches, and mold exposures and their associations with AD in spite of their importance in asthma being well-described (70, 77).
We do not have enough information to understand the role of most indoor allergen exposures in AD, nor whether differences in exposures contribute to racial and ethnic AD disparities, but some (i.e. pests and mold) are more common in low SES settings and this is another understudied area in which the upstream factor of housing discrimination may contribute to particular indoor exposures that may increase AD risk among racial and ethnic minority populations (70).
Chemical exposures
Topical chemical exposures, such as those that occur through personal care products, can alter the skin pH and trigger inflammation, which may contribute to either onset or exacerbation of skin conditions such as AD (53). There is evidence, typically via biomarkers excreted in urine, that Black and Latinx people in the US are disparately exposed to certain chemicals when compared to White people (78). Black women specifically appear to have higher exposure to endocrine disrupting chemicals (EDCs), such as phthalates, parabens, and environmental phenols. Although there are multiple sources of EDCs, this disparity appears to be due in part to hair care and feminine hygiene products marketed specifically to this population. Some studies suggest these products may contribute to higher EDC exposure among Black individuals because they are used at a greater frequency and/or contain potentially higher concentrations of EDCs. (79) Though the relationship is not well established with AD, EDCs are implicated in higher incidence and worse asthma in general populations and the proposed mechanisms include endocrine disruption, altered immune response, oxidative stress, and/or alterations in the gut microbiome (79).
Staphylococcus aureus
Colonization with Staphylococcus aureus (S. aureus AD) is a risk factor for AD severity, allergic sensitization, and barrier dysfunction (80). Some emerging data suggests S. aureus may be more common in low-income urban communities (81). Black children had the highest rates of positive S. aureus skin cultures in one AD study, which could be due to different exposures and may explain or be due to, in part, greater disease severity and persistence within the Black population (80, 82).
Psychosocial stress
Several studies have found positive associations between many types of maternal stress and increased AD risk in offspring, and a proposed though unconfirmed mechanism is that stress leads to changes in the hypothalamic-pituitary axis that affect the infant immune system, resulting in the inflammation seen in AD (33, 64). Upstream factors that may contribute to stress include individual level poverty and other aspects of neighborhood poverty that lead to stress such as barriers to accessing quality health care and transportation, exposure to violence, and having access to a high quality grocery store (33). Maternal stress is also associated with increased IgE levels in children who develop AD, and Black children have the highest IgE levels (2, 64). Additionally, stress has been implicated in increased AD severity in adults, and the pruritus and sleep disruption AD causes can in turn further exacerbate existing stressors (83, 84). Studies focused on understanding the role of psychosocial stressors experienced by Black and Latinx people and their effects on AD outcomes are needed to better understand mechanisms and targets for intervention.
Health care
Health care quality and access for AD differ across race and ethnicity. Black children are significantly less likely to see a dermatologist for AD but are three times more likely to receive an AD diagnosis in that visit, suggesting missed primary care diagnoses (41, 44, 85). One suggested explanation is different disease presentation, but Black people have some key AD physical exam findings which may even warrant increased likelihood of specialty referral, suggesting there are other factors at play in delayed diagnoses (41). Differences in skin findings across race, ethnicity, and skin tone can easily be taught and numerous representative textbooks exist to address this, but medical, nursing schools, and other health science schools must include them in their curricula (86–89).
More work is necessary to develop AD instruments that are reliable and valid for diverse racial/ethnic populations as evidenced by a dearth of AD validation studies specifically considering race, ethnicity, and skin tone (41). Erythema may be more difficult to assess in darker skin and AD instruments often underestimate severity in darker skin, with Black children having up to six times the risk of severe AD than White counterparts after adjusting for their erythema scores (41).
Across all levels of disease control, Black and Latinx children have higher rates of emergency department (ED) utilization and are nearly three times as likely to have any type of health care visit for AD than White children (44, 85). The excess ED use may be due to several factors including decreased access to specialist care, inadequate primary care access or management, or poor disease control requiring additional care to achieve and maintain AD control. More research is needed to determine barriers and preferences regarding health care utilization across race and ethnicity, and whether PCP bias or other health care system inequities lead to this disparity.
Many published studies for AD do not report race/ethnicity or only include this information in a supplement, though the US Food and Drug Administration (FDA) requires representation and reporting of minority races and ethnicities for clinical trials intended to lead to drug licensure. Often, the proportion of Black and Latinx participants in studies does not reflect AD or societal demographics, which is necessary to draw conclusions about a subpopulation (41). For studies about the general US population, representation of racial/ethnic groups should be proportionate to reflect the US population whereas studies that ask race- or ethnicity-specific questions (which are sparse) should be designed and sufficiently powered to investigate the specific race or ethnicity being studied.
Research, Clinical & Policy Implications
Though not enough research is dedicated to this topic, there is a clear disparity in AD rates, severity, and persistence between Black and White people and early evidence suggests there are disparities between Latinx and non-Latinx White populations. There is a time-urgent need to correct health inequities globally, including those that are experienced by Black and Latinx people with AD in the US. More research that is specifically designed to study disparities and contextual factors in AD is needed and it should tether data to historical context, and continued efforts to determine what matters and how to measure it will be essential for maximizing the impact of research in this area (25, 26). Race is an external marker of many other variables – such as where you live, environmental exposures, SES, and access to health care – and these must all be accounted for in any analysis attempting to draw conclusions based on race, ethnicity, and AD. Self-reported race and ethnicity should be collected and subsequently reported in all publications. Both structural and individual racism should be measured using survey questions and instruments designed to capture individual experiences of discrimination and the domains that are influenced by racism (90, 91).
Health care providers should appreciate that there are different presentations of AD and learn how to recognize AD in all skin tones. Understanding that Black and Latinx people may have more severe and/or persistent AD warrants appropriate management by the PCP, increased surveillance when necessary, and appropriate referral to dermatology specialists to help achieve disease control whenever applicable.
If, in fact, what really contributes most to Black – and Latinx to some extent – people bearing disproportionate AD burden in the US are the contextual factors rooted in structural racism, ample opportunities for policy advocacy abound. As new evidence emerges regarding the role of systemic contextual factors in AD disparities, policy approaches in economic, health care, and environmental and housing policy could be effective approaches for reducing AD disparities.
Our review highlights disparities in Black and Latinx populations with AD, gaps in the literature, and the need to have greater focus on SDOH (and how SDOH may influence the expression of genetics) to understand and meaningfully intervene on these disparities. There is much work to be done, but by reframing how we conceptualize the importance of certain AD risk factors, we will be able to make more progress in understanding and dismantling the mechanisms that lead to racial and ethnic disparities in AD.
Table 1.
Gaps in knowledge and future research directions to improve equity in atopic dermatitis for Black, Latinx, and other racial and ethnic minority populations.
| General AD Research Questions | Racial/Ethnicity Disparities Research Questions | Intervention Research Questions | |
|---|---|---|---|
| Individual/personal Racism | -What is the role of individual racism and AD in prevalence, severity, and/or persistence? -What mechanism(s)? |
-To what extent does individual racism explain differences in AD risk between racial/ethnic groups? | -What interventions are effective for individual racism? -Do interventions reduce the impact of individual racism on AD risk? |
| Health care | -Are there health care provider knowledge gaps that lead to delayed AD diagnoses across different skin types? | -To what extent do delayed diagnoses and/or differences in treatment or referral patterns explain differences in disease severity, persistence, and/or control between racial/ethnic groups? -Are there differences in barriers/preferences towards accessing health care for AD across race and ethnicity? -To what extent do barriers/preferences towards accessing health care for AD explain delayed diagnoses or differences in AD severity/persistence/control between racial/ethnic groups? |
-What interventions are effective for increasing diagnostic accuracy across all skin types? -What culturally tailored interventions are effective for increasing access to health care for AD within racial/ethnic groups? |
| SES – income, educational status, wealth | -What aspects of SES contribute to AD risk and by what mechanisms? | -How much of the association between race and/or ethnicity and AD risk is mediated by SES? | -Does intervening on SES factors reduce AD risk disparities? |
| Neighborhood – segregation, poverty/deprivation, urbanicity | -Which risk factors associated with urban/metropolitan living contribute to increased AD incidence, persistence, and/or severity? -What are the associations between neighborhood socioeconomic deprivation and AD incidence, severity, and/or persistence? -What are the associations between neighborhood segregation and AD risk? |
-Does urban living explain racial and/or ethnic differences in AD risk? -Does neighborhood socioeconomic deprivation explain racial and/or ethnic differences in AD risk? -Does neighborhood segregation explain racial and/or ethnic differences in AD risk? |
-Do neighborhood interventions – such as relocating to areas that are rural, less socioeconomically deprived, or less segregated – reduce AD risk? |
| Psychosocial stress – including upstream contributors | -What is the association between childhood psychosocial stress and AD incidence, severity, and /or prevalence? -What are the upstream factors that contribute to psychosocial stress that is associated with incident AD? |
-Do psychosocial stressors explain racial and/or ethnic differences in AD risk? | -Do culturally tailored interventions aimed at reducing parental and/or child stressors reduce AD risk? |
| Indoor and outdoor air pollution | -Does air pollution cause and/or exacerbate AD? -If so, which air pollutants? -Are some air pollutant mixtures worse than others with respect to AD? |
-Do differences in air pollution concentrations, types, or mixtures explain differences in AD incidence, severity, and/or persistence among racial/ethnic groups? | -What interventions within and outside the home improve air pollution exposures for people with AD, and does the AD improve? |
| Indoor allergens – pests (cockroaches, mice), dust mites, fungi (mold) | -What is the association between pest allergen exposure and AD? -What is the association between dust mite allergen exposure and AD? -What is the association between pet allergen exposure and AD? -What is the association between exposure to fungi and AD? |
-Are there differences in exposures to pest, dust mite, and pet allergens and exposure to fungi across race and ethnicity among people with AD? -Do rates of sensitization to pest, dust mite, and pet allergens, and fungi differ across race and ethnicity among people with AD? |
-Do household interventions that reduce pest, dust mite, and fungi exposures result in improvement in AD? |
Funding declaration:
Emily Croce is supported by the National Institute of Nursing Research of the National Institutes of Health (T32NR019035) and a Pfizer Dermatology Research Fellowship. Dr. Adamson is supported by the Dermatology Foundation, the National Institutes of Health (UL1 TR002645), the American Cancer Society, and the Robert Wood Johnson Foundation. Dr. Matsui is supported by the National Institutes of Health (K24AI114769, R01ES023447, R01ES026170). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AD
atopic dermatitis
- ADD, ADHD
attention deficit disorder, attention deficit hyperactivity disorder
- EDC
endocrine disrupting chemicals
- FLG
filaggrin
- GWAS
genome-wide association study
- LOF
loss-of-function
- SES
socioeconomic status
- QoL
quality of life
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have no relevant conflicts of interest to report.
Contributor Information
Emily Croce, The University of Texas at Austin, School of Nursing, Austin, TX 78712; Dell Children’s Medical Group, Austin, TX 78723.
Moise L. Levy, The University of Texas at Austin Dell Medical School, Austin, TX 78712; Dell Children’s Medical Group, Austin, TX 78723.
Adewole S. Adamson, The University of Texas at Austin Dell Medical School, Austin, TX 78712.
Elizabeth C. Matsui, The University of Texas at Austin Dell Medical School, Austin, TX 78712; Dell Children’s Medical Group, Austin, TX 78723.
References
- 1.Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153(5):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):449–55. [DOI] [PubMed] [Google Scholar]
- 3.Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134(6):e1735–44. [DOI] [PubMed] [Google Scholar]
- 4.Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: A review. Pediatric Dermatology. 2019;36(1):66–71. [DOI] [PubMed] [Google Scholar]
- 5.Horii KA, Simon SD, Liu DY, Sharma V. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120(3):e527–34. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Association of atopic dermatitis severity with cognitive function in adults. J Am Acad Dermatol. 2020;83(5):1349–59. [DOI] [PubMed] [Google Scholar]
- 7.Jackson-Cowan L, Cole EF, Silverberg JI, Lawley LP. Childhood atopic dermatitis is associated with cognitive dysfunction: A National Health Interview Survey study from 2008 to 2018. Ann Allergy Asthma Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 8.Wan J, Mitra N, Hooper SR, Hoffstad OJ, Margolis DJ. Association of Atopic Dermatitis Severity With Learning Disability in Children. JAMA Dermatol. 2021;157(6):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt SAJ, Mailhac A, Darvalics B, Mulick A, Deleuran MS, Sørensen HT, et al. Association Between Atopic Dermatitis and Educational Attainment in Denmark. JAMA Dermatol. 2021;157(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan J, Margolis DJ, Mitra N, Hoffstad OJ, Takeshita J. Racial and ethnic differences in atopic dermatitis-related school absences among US children. JAMA Dermatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Blomberg M, Rifas-Shiman SL, Camargo CA Jr., Gold DR, Thyssen JP, et al. Racial/Ethnic Differences in Incidence and Persistence of Childhood Atopic Dermatitis. J Invest Dermatol. 2019;139(4):827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25(3):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Annals of Allergy, Asthma,and Immunology. 2019;123(2):173–8.e1. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie C, Silverberg JI. Associations of unsafe, unsupportive, and underdeveloped neighborhoods with atopic dermatitis in US children. Ann Allergy Asthma Immunol. 2019;122(2):198–203.e3. [DOI] [PubMed] [Google Scholar]
- 15.Leung DYM. The effect of being African American on atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(1):1. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N, et al. Atopic dermatitis in African American patients is T(H)2/T(H)22-skewed with T(H)1/T(H)17 attenuation. Ann Allergy Asthma Immunol. 2019;122(1):99–110.e6. [DOI] [PubMed] [Google Scholar]
- 17.Margolis DJ, Gupta J, Apter AJ, Ganguly T, Hoffstad O, Papadopoulos M, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2014;133(3):784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathyer ME, Quiggle AM, Wong X, Denil S, Kumar MG, Ciliberto HM, et al. Tiled array-based sequencing identifies enrichment of loss-of-function variants in the highly homologous filaggrin gene in African-American children with severe atopic dermatitis. Exp Dermatol. 2018;27(9):989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta J, Johansson E, Bernstein JA, Chakraborty R, Khurana Hershey GK, Rothenberg ME, et al. Resolving the etiology of atopic disorders by using genetic analysis of racial ancestry. J Allergy Clin Immunol. 2016;138(3):676–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29(4):395–402. [DOI] [PubMed] [Google Scholar]
- 21.Abuabara K, You Y, Margolis DJ, Hoffmann TJ, Risch N, Jorgenson E. Genetic ancestry does not explain increased atopic dermatitis susceptibility or worse disease control among African American subjects in 2 large US cohorts. J Allergy Clin Immunol. 2020;145(1):192–8.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol. 2020;124(1):36–43. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Chang C, Lu Q. The Genetics and Epigenetics of Atopic Dermatitis-Filaggrin and Other Polymorphisms. Clin Rev Allergy Immunol. 2016;51(3):315–28. [DOI] [PubMed] [Google Scholar]
- 24.Yudell M, Roberts D, DeSalle R, Tishkoff S. SCIENCE AND SOCIETY. Taking race out of human genetics. Science. 2016;351(6273):564–5. [DOI] [PubMed] [Google Scholar]
- 25.Givens ML, Gennuso KP, Pollock EA, Johnson SL. Deconstructing Inequities - Transparent Values in Measurement and Analytic Choices. N Engl J Med. 2021;384(19):1861–5. [DOI] [PubMed] [Google Scholar]
- 26.Matsui EC, Perry TT, Adamson AS. An Antiracist Framework for Racial and Ethnic Health Disparities Research. Pediatrics. 2020;146(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diez Roux AV. Conceptual approaches to the study of health disparities. Annu Rev Public Health. 2012;33:41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60. [DOI] [PubMed] [Google Scholar]
- 29.Teteh DK, Dawkins-Moultin L, Hooker S, Hernandez W, Bonilla C, Galloway D, et al. Genetic ancestry, skin color and social attainment: The four cities study. PLoS One. 2020;15(8):e0237041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonham VL, Green ED, Pérez-Stable EJ. Examining How Race, Ethnicity, and Ancestry Data Are Used in Biomedical Research. Jama. 2018;320(15):1533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landor AM, Simons LG, Simons RL, Brody GH, Bryant CM, Gibbons FX, et al. Exploring the impact of skin tone on family dynamics and race-related outcomes. J Fam Psychol. 2013;27(5):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegfried EC, Paller AS, Mina-Osorio P, Vekeman F, Kaur M, Mallya UG, et al. Effects of variations in access to care for children with atopic dermatitis. BMC Dermatol. 2020;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(4):360–6. [DOI] [PubMed] [Google Scholar]
- 35.Gee GC, Ford CL. Structural racism and health inequities: Old issues, new directions. Du Bois Review. 2011;8(1):115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–63. [DOI] [PubMed] [Google Scholar]
- 37.Dey T, Dominici F. COVID-19, Air Pollution, and Racial Inequity: Connecting the Dots. Chem Res Toxicol. 2021;34(3):669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui EC, Adamson AS, Peng RD. Time’s up to adopt a biopsychosocial model to address racial and ethnic disparities in asthma outcomes. J Allergy Clin Immunol. 2019;143(6):2024–5. [DOI] [PubMed] [Google Scholar]
- 40.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–8. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27(4):340–57. [DOI] [PubMed] [Google Scholar]
- 42.Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605. [DOI] [PubMed] [Google Scholar]
- 43.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer AH, Shin DB, Margolis DJ, Takeshita J. Racial and ethnic differences in health care utilization for childhood eczema: An analysis of the 2001-2013 Medical Expenditure Panel Surveys. J Am Acad Dermatol. 2017;77(6):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverberg JI, Simpson EL, Durkin HG, Joks R. Prevalence of allergic disease in foreign-born American children. JAMA Pediatr. 2013;167(6):554–60. [DOI] [PubMed] [Google Scholar]
- 46.Maymí MA, Somolinos AL, Nazario CM, Sánchez JL. The prevalence of atopic dermatitis in Puerto Rican school children^ien. P R health sci j. 2007;26(2):127–33. [PubMed] [Google Scholar]
- 47.Ramírez-Soto M, Bedolla-Barajas M, González-Mendoza T. [Prevalence of asthma, allergic rhinitis and atopic dermatitis in school children of the Mexican Bajío region]. Rev Alerg Mex. 2018;65(4):372–8. [DOI] [PubMed] [Google Scholar]
- 48.Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130(4):912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polcari I, Becker L, Stein SL, Smith MS, Paller AS. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31(4):489–92. [DOI] [PubMed] [Google Scholar]
- 50.Kumar R, Seibold MA, Burchard EG. Atopic dermatitis, race, and genetics. J Allergy Clin Immunol. 2020;145(1):108–10. [DOI] [PubMed] [Google Scholar]
- 51.Daya M, Barnes KC. African American ancestry contribution to asthma and atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.du Plessis J, Stefaniak A, Eloff F, John S, Agner T, Chou TC, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.David Boothe W, Tarbox JA, Tarbox MB. Atopic Dermatitis: Pathophysiology. Adv Exp Med Biol. 2017;1027:21–37. [DOI] [PubMed] [Google Scholar]
- 54.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121(3):725–30.e2. [DOI] [PubMed] [Google Scholar]
- 55.Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003;4(12):843–60. [DOI] [PubMed] [Google Scholar]
- 56.Dave ND, Xiang L, Rehm KE, Marshall GD Jr. Stress and allergic diseases. Immunol Allergy Clin North Am. 2011;31(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113(6):1051–7. [DOI] [PubMed] [Google Scholar]
- 58.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tackett KJ, Jenkins F, Morrell DS, McShane DB, Burkhart CN. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37(1):142–6. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan K, Thakur N. Structural and Social Determinants of Health in Asthma in Developed Economies: a Scoping Review of Literature Published Between 2014 and 2019. Curr Allergy Asthma Rep. 2020;20(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–23. [PMC free article] [PubMed] [Google Scholar]
- 62.Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narla S, Silverberg JI. The Role of Environmental Exposures in Atopic Dermatitis. Curr Allergy Asthma Rep. 2020;20(12):74. [DOI] [PubMed] [Google Scholar]
- 65.Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One. 2014;9(4):e94431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levin ME, Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, et al. Environmental factors associated with allergy in urban and rural children from the South African Food Allergy (SAFFA) cohort. J Allergy Clin Immunol. 2020;145(1):415–26. [DOI] [PubMed] [Google Scholar]
- 67.Fadadu RP, Grimes B, Jewell NP, Vargo J, Young AT, Abuabara K, et al. Association of Wildfire Air Pollution and Health Care Use for Atopic Dermatitis and Itch. JAMA Dermatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Penard-Morand C, Charpin D, Raherison C, Kopferschmitt C, Caillaud D, Lavaud F, et al. Long-term exposure to background air pollution related to respiratory and allergic health in schoolchildren. Clin Exp Allergy. 2005;35(10):1279–87. [DOI] [PubMed] [Google Scholar]
- 69.Roul S, Léauté-Labréze C, Perromat M, Ducombs G, Taïeb A. [Sensitization to cockroach allergens evaluated by skin tests in children with atopic dermatitis]. Ann Dermatol Venereol. 2001;128(2):115–7. [PubMed] [Google Scholar]
- 70.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132(4):830–5. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee E, Choi KY, Kang MJ, Lee SY, Yoon J, Cho HJ, et al. Prenatal mold exposure is associated with development of atopic dermatitis in infants through allergic inflammation. J Pediatr (Rio J). 2020;96(1):125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelucchi C, Galeone C, Bach JF, La Vecchia C, Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: a meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2013;132(3):616–22.e7. [DOI] [PubMed] [Google Scholar]
- 73.Miller JD. The Role of Dust Mites in Allergy. Clin Rev Allergy Immunol. 2019;57(3):312–29. [DOI] [PubMed] [Google Scholar]
- 74.Liu L, Chen J, Xu J, Yang Q, Gu C, Ni C, et al. Sublingual immunotherapy of atopic dermatitis in mite-sensitized patients: a multi-centre, randomized, double-blind, placebo-controlled study. Artif Cells Nanomed Biotechnol. 2019;47(1):3540–7. [DOI] [PubMed] [Google Scholar]
- 75.Bufford JD, Gern JE. Early exposure to pets: good or bad? Curr Allergy Asthma Rep. 2007;7(5):375–82. [DOI] [PubMed] [Google Scholar]
- 76.Kim HJ, Lee E, Lee SH, Kang MJ, Hong SJ. Mold elicits atopic dermatitis by reactive oxygen species: Epidemiology and mechanism studies. Clin Immunol. 2015;161(2):384–90. [DOI] [PubMed] [Google Scholar]
- 77.Ahluwalia SK, Matsui EC. The indoor environment and its effects on childhood asthma. Curr Opin Allergy Clin Immunol. 2011;11(2):137–43. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen VK, Kahana A, Heidt J, Polemi K, Kvasnicka J, Jolliet O, et al. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999-2014. Environ Int. 2020;137:105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raley E, Quirós-Alcalá L, Matsui EC. Chemical Exposures via Personal Care Products and the Disproportionate Asthma Burden Among the U.S. Black Population. J Allergy Clin Immunol Pract. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson EL, Villarreal M, Jepson B, Rafaels N, David G, Hanifin J, et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J Invest Dermatol. 2018;138(10):2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis MF, Ludwig S, Brigham EP, McCormack MC, Matsui EC. Effect of home exposure to Staphylococcus aureus on asthma in adolescents. Journal of allergy and clinical immunology. 2018;141(1):402–5.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Briscoe CC, Reich P, Fritz S, Coughlin CC. Staphylococcus aureus antibiotic susceptibility patterns in pediatric atopic dermatitis. Pediatr Dermatol. 2019;36(4):482–5. [DOI] [PubMed] [Google Scholar]
- 83.Zeiser K, Hammel G, Kirchberger I, Traidl-Hoffmann C. Social and psychosocial effects on atopic eczema symptom severity - a scoping review of observational studies published from 1989 to 2019. J Eur Acad Dermatol Venereol. 2021;35(4):835–43. [DOI] [PubMed] [Google Scholar]
- 84.Barilla S, Felix K, Jorizzo JL. Stressors in Atopic Dermatitis. Adv Exp Med Biol. 2017;1027:71–7. [DOI] [PubMed] [Google Scholar]
- 85.Wan J, Oganisian A, Spieker AJ, Hoffstad OJ, Mitra N, Margolis DJ, et al. Racial/Ethnic Variation in Use of Ambulatory and Emergency Care for Atopic Dermatitis among US Children. J Invest Dermatol. 2019;139(9):1906–13.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diao JA, Adamson AS. Representation and misdiagnosis of dark skin in a large-scale visual diagnostic challenge. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 87.Kelly APTS, Lim HC, Serrano AMA. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York: McGraw-Hill Medical; 2015. [Google Scholar]
- 88.Alexis ABV. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York: Springer; 2014. [Google Scholar]
- 89.Silverberg ND-MC, Kwang Tay Y. Pediatric Skin of color. New York: Springer; 2015. [Google Scholar]
- 90.Dougherty GB, Golden SH, Gross AL, Colantuoni E, Dean LT. Measuring Structural Racism and Its Association With BMI. Am J Prev Med. 2020;59(4):530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576–96. [DOI] [PubMed] [Google Scholar]


