Key Points
cffDNA induces innate inflammatory responses in maternal monocytes.
This response activates T and endothelial cells and causes myometrial contraction.
cffDNA may have an important role in the initiation of parturition.
Visual Abstract
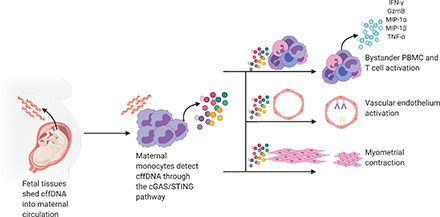
Abstract
Throughout gestation, the maternal immune system is tightly modulated to allow growth of a semiallogeneic fetus. During the third trimester, the maternal immune system shifts to a proinflammatory phenotype in preparation for labor. What induces this shift remains unclear. Cell-free fetal DNA (cffDNA) is shed by the placenta and enters maternal circulation throughout pregnancy. Levels of cffDNA are increased as gestation progresses and peak before labor, coinciding with a shift to proinflammatory maternal immunity. Furthermore, cffDNA is abnormally elevated in plasma from women with complications of pregnancy, including preterm labor. Given the changes in maternal immunity at the end of pregnancy and the role of sterile inflammation in the pathophysiology of spontaneous preterm birth, we hypothesized that cffDNA can act as a damage-associated molecular pattern inducing an inflammatory cytokine response that promotes hallmarks of parturition. To test this hypothesis, we stimulated human maternal leukocytes with cffDNA from primary term cytotrophoblasts or maternal plasma and observed significant IL-1β and CXCL10 secretion, which coincides with phosphorylation of IFN regulatory factor 3 and caspase-1 cleavage. We then show that human maternal monocytes are crucial for the immune response to cffDNA and can activate bystander T cells to secrete proinflammatory IFN-γ and granzyme B. Lastly, we find that the monocyte response to cffDNA leads to vascular endothelium activation, induction of myometrial contractility, and PGE2 release in vitro. Our results suggest that the immune response to cffDNA can promote key features of the parturition cascade, which has physiologic consequences relevant to the timing of labor.
Introduction
Trophoblast cells of the placenta release cell-free fetal DNA (cffDNA) into maternal circulation as early as 5 wk after conception. The concentration of fetal-specific DNA in maternal circulation increases with gestational age, reaching a peak at the onset of labor and dropping to undetectable levels hours after delivery (1). From the placenta, cffDNA enters maternal circulation, where it can be isolated and used clinically for noninvasive prenatal screening (2, 3). This has revolutionized the diagnosis of inherited diseases by eliminating the need for invasive procedures to obtain fetal or placental tissue for genetic analysis. Although the use of cffDNA as a diagnostic tool has been widely accepted, the biologic role of cffDNA in the physiology of pregnancy and labor remains unclear (4, 5).
Parturition is characterized by proinflammatory cytokine release, cervical ripening, and myometrial contractions; however, in humans, the inducer of these reactions remains a mystery. Current hypotheses concerning the role of cffDNA in parturition propose that cffDNA detection by the innate immune system could lead to inflammatory cytokine release, which stimulates endothelial activation and the recruitment of additional leukocytes (6). The influx and activation of immune cells into uterine tissue could then promote myometrial contractions leading to labor. This suggests that cffDNA can induce sterile inflammation, which is characterized by an inflammatory response in the absence of infection. Specifically, cffDNA may act as a damage-associated molecular pattern that activates pattern recognition receptors (PRRs) leading to cytokine secretion (7).
This hypothesis is further supported by the fact that increased levels of cffDNA in sera of women with preterm labor have been observed (8). Interestingly, immune activation plays a large role in the pathophysiology of spontaneous preterm birth, which accounts for a majority of the fetal mortality and morbidity seen in industrialized countries (9). In the United States, 1 in 10 babies is born before 37 wk, and there are very few effective tools to predict or prevent it (10).
Pregnancy requires significant changes in maternal physiology and immunology to support the establishment of a placenta and maintenance of a haploidentical fetus. Healthy pregnancy induces an overall immunotolerant state. Significant inflammation is required, however, for implantation of the embryo, placentation, and finally, labor. At the end of pregnancy, the maternal immune system shifts from an immunotolerant state to a more activated, proinflammatory environment (11). What induces this shift in the maternal immune profile remains unknown. Because cffDNA is shed from the placenta, it may act as a damage-associated molecular pattern and initiate inflammatory responses through innate PRRs. Many physiologic events at term suggest that cffDNA may promote sterile inflammation. For example, placental senescence results in apoptosis, which increases levels of cffDNA just before labor (12). The increase in cffDNA concentration coincides with the shift in maternal immunity away from tolerance and the immune infiltration of uterine tissues observed with labor (13). Considering the wide expression of cytoplasmic and endosomal DNA-detecting PRRs in immune and epithelial cells, cffDNA that enters maternal circulation may be able to activate intracellular DNA signaling cascades leading to the production of inflammatory cytokines and even a shift in the maternal immune profile.
DNA-detection pathways, such as cyclic GMP–AMP synthase/stimulator of IFN genes (cGAS/STING), absent in melanoma 2 (AIM2), and TLR9 have proved critical in innate responses to DNA viruses and even immunotherapy and radiation for cancer treatment (14). Activation of any of these pathways results in a proinflammatory cytokine response leading to acute inflammation and immune infiltration. In human tissues, AIM2 and cGAS/STING are widely expressed in monocytes and macrophages, where they detect dsDNA in the cytoplasm (15, 16). This leads to activation of the AIM2 or NLRP3 inflammasome and release of IL-1β and IL-18. In addition, the cGAS/STING pathway induces a type I IFN response through phosphorylation and dimerization of IFN regulatory factor 3 (IRF3). Endosomal TLR9 is present in human dendritic cells, B cells, and mucosal epithelium, where it detects unmethylated CpG-rich sequences predominant in bacterial and viral genomes (17), leading to a type I IFN response. Analysis of cffDNA demonstrates that it is hypomethylated and is typically much shorter in length than other circulating DNA fragments (18). These differences may result in maternal immune recognition through intracellular DNA signaling pathways. The recent identification of cffDNA as a constituent of microparticles shed by the placenta provides a packaging medium by which cffDNA could leave fetal tissue and enter maternal circulation protected from circulating DNases (18–21). These microparticles could then fuse with or be endocytosed by the cell membrane of an immune or epithelial cell introducing their cffDNA cargo to be detected by PRRs in the cytoplasm.
Although cffDNA increases in concentration as gestation progresses, the potential inflammatory consequences of cffDNA detection, and whether it has a role in the shift in maternal immunity to a proinflammatory state and the initiation of parturition, remain to be determined. Here, we use primary maternal immune cells to determine whether cffDNA from term cytotrophoblasts (CTBs) activates a proinflammatory cytokine response. We then describe the mechanism of cffDNA detection and address the effect of inflammation on bystander immune cell activation, vascular endothelial activation, and myometrial contractility.
Materials and Methods
Patient selection
Patients were recruited and consented for collection of term placenta and peripheral blood samples after approval from the Mayo Clinic Institutional Review Board (18-003184). Inclusion criteria for placenta and blood collection included age (≥18 and ≤35 y) at study entry, ability to provide written informed consent, and weight >110 pounds (50 kg). Exclusion criteria included known immunodeficiency, chronic viral infections (i.e., HIV, human T cell leukemia virus, hepatitis B virus, hepatitis C virus), known autoimmune disease, transplant recipient, current smoker (last use within 30 d of registration), multiple current gestations, gestational diabetes not controlled by diet, and conception from assisted reproductive technology (prior clomiphene use was allowed).
Primary CTB isolation and cffDNA purification
All placental tissues were collected within 4 h of an uncomplicated, term delivery. The basal and chorionic plates were carefully removed, and the remaining villous tissue was washed thoroughly in PBS to remove blood. Tissue was minced well and washed three times with PBS before undergoing 30-min HBSS digestions at 37°C in a water bath. The digestion solution consists of 0.4 mg/ml DNase I (Alfa Aesar, Haverhill, MA), 2.5% trypsin (Thermo Fisher, Waltham, MA), 1 mM CaCl2 (MilliporeSigma, Burlington, MA), 1 mM MgSO4 (Millipore Sigma), and 25 mM HEPES (ThermoFisher). Cell suspensions from the digested tissue were layered over FBS, pelleted, and strained through a 70-µm filter before being layered on top of a 50/45/35/30% Percoll gradient (MilliporeSigma). After a 20-min centrifugation step (1200 × g without the brake), cells in the 35–45% fraction were removed and washed in HBSS with 25 mM HEPES. Cells were counted using a hemocytometer, and purity was tested by flow cytometry using an anti–cytokeratin-7 Ab (clone CAM5.2; D Pharmingen, Franklin Lakes, NJ). CTBs with a purity >95% were then cultured at 15–18 × 106 cells in a T75 flask in IMDM (Thermo Fisher) with 10% FBS and 1× penicillin/streptomycin/amphotericin B (Thermo Fisher) at 37°C and 5% CO2. Conditioned media (CM) from CTBs were harvested every 3 d for isolation of cffDNA using Qiagen QIAamp MinElute ccfDNA MinElute kit (Qiagen, Germantown, MD) following the manufacturer’s instructions. Concentrations were measured using Qubit high-sensitivity dsDNA kit (Thermo Fisher).
Primary CTB staining
After supernatants were used for three rounds of cffDNA isolation, CTBs were harvested and stained for viability (Invitrogen LIVE/DEAD Fixable Yellow Dead Cell Stain Kit, for 405 nm excitation) and cytokeratin-7 expression (clone EPR1619Y; Abcam, Cambridge, UK). After staining, cells were washed and resuspended in 50 μl of PBS and imaged using the ImageStream X MKII (Luminex, Austin, TX), which provides confocal images and flow cytometry analysis. Events were gated on live, single cells as determined by size and appearance. A total of 10,000 single cells were collected from each sample per experiment for analysis using ImageStream IDEAS software (Luminex).
Cell-free DNA isolation from maternal plasma
A total of 30 ml of maternal blood was collected at 36–38 wk gestation and 6 wk postpartum in EDTA tubes to obtain plasma. A total of 30 ml of maternal blood yielded ∼10 ml of maternal plasma from the third trimester (36–38 wk gestation, “3rd trimester”) and 6 wk postpartum (“Postpartum”). Plasma samples were used to isolate cell-free DNA using the Qiagen QIAamp MinElute ccfDNA MinElute kit (Qiagen, Germantown, MD) following the manufacturer’s instructions. Concentrations were measured using Qubit high-sensitivity dsDNA kit (Thermo Fisher).
Oligonucleotide stimulation
PBMCs from one female donor of reproductive age were plated at 7 × 105 cells/well and stimulated with different concentrations of a synthetic, double-stranded, 200-bp oligonucleotide (Integrated DNA Technologies, Coralville, IA) using the Effectene (Qiagen) per the manufacturer’s instructions. After 18-h incubation, ELISA was used to measure IL-1β in PBMC supernatants.
DNA stimulation protocol
A total of 60 ml of maternal blood was collected between 36 and 38 wk gestation in citrate tubes. Blood samples were processed to isolate PBMCs using Ficoll gradient, and PBMCs were stored in FBS+10% DMSO in liquid nitrogen until use. Monocytes were negatively isolated from maternal PBMCs using Miltenyi Human Classic Monocyte Isolation Kit (Cat. No. 130-117-337; Miltenyi, Auburn, CA) per the manufacturer’s instructions. Monocyte-depleted PBMCs were negatively isolated from whole PBMCs using Miltenyi CD14 MicroBeads (Cat. No. 130-050-201) per the manufacturer’s instructions. After negative isolation, cells were plated in a 24-well plate at 7 × 105 cells/well. cffDNA stimulation complexes were produced using the Qiagen Effectene kit (Qiagen) and 375 ng of cffDNA following the manufacturer’s instructions for a final concentration of 750 ng/ml cffDNA. Effectene mixture without cffDNA was used as negative control (labeled “Media” in the figures). Maternal PBMCs, monocytes, or monocyte-depleted PBMCs were incubated in a 24-well plate at 7 × 105 cells/well with the cffDNA–Effectene reaction and complete media for 18 h.
Protein quantification
All ELISA reagents were purchased from R&D Systems (Minneapolis, MN) and were resuspended and stored using the manufacturer’s instructions. ELISAs of cell supernatants were performed according to the manufacturer’s instructions. The following proteins were quantified by ELISA: IL-1β (Cat. No. DY201), CXCL10 (Cat. No. DY2660), granzyme B (GzmB; Cat. No. DY2906), IFN-γ (Cat. No. DY285B), and TNF-α (Cat. No. DY210). Absorbance at 450 nm was measured using an 800 TS plate reader (BioTek Instruments, Winooski, VT). A seven-point standard curve was constructed and used to calculate protein concentrations in each sample. In addition, multiplex analysis was performed using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel Assay (MilliporeSigma) according to the manufacturer’s instructions. For both assays, all samples were run in duplicate and averaged to get the final concentration. Samples with a variation between replicates >20% were repeated.
Caspase-1 activity
Maternal monocytes were stimulated with Effectene alone (“Media”) or Effectene with cffDNA (“cffDNA”) as described earlier for 4 h. Cells and supernatants were then harvested for measurement of caspase-1 activity using Caspase-Glo 1 Inflammasome Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Caspase-1 activity was determined by detection of luminescence using a Varioskan LUX Multimode Microplate Reader (ThermoFisher). Blank samples were used as a negative control and contained Effectene reagent without monocytes.
Cell sorting
PBMCs from one female donor of reproductive age were used to sort cells into different populations based on surface expression of CD3 (T cells, clone UCHT1; BD Pharmingen, Franklin Lakes, NJ), CD14 (monocytes, clone M5E2; BD Pharmingen), CD20 (B cells, clone 2H7; BD Pharmingen), and CD56 (NK cells, clone B159; BD Pharmingen). Cells were then stimulated for 18 h using Effectene and cffDNA as described earlier.
Bystander activation assays
Maternal monocytes were stimulated with cffDNA isolated from CTBs for 18 h using Effectene reagent. After stimulation, monocyte supernatants were harvested and centrifuged at 1800 rpm for 5 min to pellet cells and debris. Supernatants (CM) were used as culture media for same-donor PBMCs plated in a 24-well plate at 7 × 105 cells/well. Supernatant from one monocyte well was used for each PBMC well (1:1 ratio). Maternal PBMCs were incubated in same-donor Effectene (“CM”) or Effectene+cffDNA-stimulated monocyte CM (“cffDNA CM”) for 18 h. After incubation, ELISAs were used to determine the concentration of cytokines in the PBMC supernatants.
As described earlier, maternal monocytes were stimulated with Effectene reagent alone or Effectene+cffDNA from CTBs for 18 h before monocyte supernatants (CM) were harvested. CM were used to culture same-donor T cells negatively isolated from maternal PBMCs using Miltenyi Pan T cell isolation kit (Cat. No. 130-096-535). After isolation, T cells were plated in a 24-well plate at 7 × 105 cells/well and incubated in matched-donor Effectene (CM) or Effectene+cffDNA-stimulated monocyte supernatants (“cffDNA CM”) for 18 h. Supernatant from one monocyte well was used for each PBMC well (1:1 ratio). After overnight incubation, ELISAs were used to determine the concentration of cytokines in the T cell supernatants. Neutralizing Abs targeting IL-1β (clone: 2805R, 5 µg/ml; R&D Systems) or CXCL10 (clone: 33036, 10 µg/ml; R&D Systems) were added along with the monocyte CM in the overnight incubation to block the effects of these cytokines on PBMCs or T cells.
Western blotting
Cells were lysed on ice in radioimmunoprecipitation assay buffer (ThermoFisher) with protease inhibitors, and protein concertation was determined by BCA assay (ThermoFisher). A total of 30 µg of total protein was mixed with 4× sample buffer containing 10% 2-ME and incubated at 65°C for 15 min. Samples were loaded onto a 4–15% TGX gel (Bio-Rad, Hercules, CA) and then transferred to a polyvinylidene difluoride membrane. Blocking was carried out in TBST with 1% fish gelatin and 1.5% polyvinylpyrrolidone. Primary Abs used included IRF3 (clone: D83B9, 1:100 dilution; Cell Signaling), p-IRF3 (clone: 4D4G, 1:1000 dilution; Cell Signaling), and GAPDH (1:6000 dilution; Novus Biologicals). Primary Abs were incubated with the membrane overnight at 4°C with rocking. Membranes were then incubated for 1 h in secondary Abs at room temperature before exposure to Clarity Western ECL substrate (Bio-Rad) and imaging on the Azure Imaging System (Azure Biosystems, Dublin, CA). Membranes are then stripped and reprobed with anti-GAPDH. Signal intensity was quantified by densitometry using AzureSpot Analysis Software (Azure Biosystems), with ratios of the band of interest/housekeeping gene used to normalize variability in protein loading.
HUVEC stimulation and imaging
HUVECs (CRL-1730) were purchased directly from ATCC, seeded at 4 × 105 cells/well, and cultured for 18 h to adhere in 24-well plates. After adherence, HUVEC media were removed and replaced with CM from Effectene (CM) or Effectene+cffDNA-stimulated monocytes or PBMCs (“cffDNA CM”). For each well of HUVECs, CM from two wells of stimulated monocytes or PBMCs was used (2:1 ratio). For select HUVEC wells, neutralizing Abs for IL-1β (clone: 2805R, 5 µg/ml; R&D Systems) or TNF-α (clone: 28401, 10 µg/ml; R&D Systems) were added to monocyte or PBMC CM, respectively. HUVECs were incubated for 18 h in monocyte or PBMC CM.
After incubation with monocyte or PBMC CM, HUVECs were harvested for staining with fluorescent Abs for E-selectin (clone: HCD62E; BioLegend, San Diego, CA) and ICAM-1 (clone: HA58; BioLegend). Cells were stained in 100 μl of FACS buffer using 2 μl of each Ab per reaction for 30 min at 4°C. After staining, cells were washed and resuspended in 50 μl of PBS and imaged using the ImageStream X MKII (Luminex), which provides confocal images and flow cytometry analysis. Events were gated on single cells as determined by size and appearance. A total of 10,000 single cells were collected from each sample per experiment for analysis using ImageStream IDEAS software (Luminex).
PHM1-41 in vitro contraction
PHM1-41 cells (ATCC: CRL-3046) were cultured in a 24-well plate at 1 × 104 cells per well using CytoSelect 24-well Cell Contraction Assay Kit (Cell BioLabs, San Diego, CA) per the manufacturer’s instructions. Cells were embedded in the collagen matrix, which was solidified at 37°C for 1 h. After an hour, CM from PBMCs or monocytes stimulated with Effectene alone (“CM”) or Effectene with cffDNA (“cffDNA CM”) were added to the solidified matrix and incubated for 18 h. For each well of PHM1-41 cells embedded in a collagen matrix, CM from monocyte or PBMC well were used (1:1 ratio). For selected contraction wells, neutralizing Abs for IL-1β (clone: 2805R, 5 µg/ml; R&D Systems) or TNF-α (clone: 28401, 10 µg/ml; R&D Systems) were added to the cffDNA-stimulated monocyte or PBMC CM, respectively. After an 18-h incubation, measurements were made using a metric ruler in millimeters to determine the distance between the wall of the well and the contracted matrix edge. Images were obtained using a dissecting microscope (Zeiss, White Plains, NY) at 8× magnification.
PHM1-41 PGE2 secretion
PHM1-41 cells were adhered in a 24-well plate overnight at 2 × 105 cells/ml. After overnight adherence, media were removed, and PHM1-41 cells were incubated with CM collected from maternal PBMCs or monocytes treated with either Effectene alone (“CM”) or Effectene and cffDNA (“cffDNA CM”). Neutralizing Abs targeting TNF-α (clone: 28401, 5 µg/ml; R&D Systems) or IL-1β (clone: 2805R, 5 µg/ml; R&D Systems) were added to wells treated with PBMC or monocyte cffDNA CM, respectively. PHM1-41 cells were incubated overnight, and levels of PGE2 were measured using PGE2 Parameter Assay Kit (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.3. Data are presented as medians with interquartile ranges (IQRs). All figures are representative of at least three independent experiments with duplicates, using unique patient samples from 20 donors. For determination of significant comparisons between two groups, Mann–Whitney U tests were used. Significance was defined as p ≤ 0.05. For comparisons between three or more groups, Kruskal–Wallis tests were performed for unpaired data and Friedman test for paired data. For both, multiple comparisons were made by comparing the mean rank of each treatment group with the mean rank of every other group and controlling the false discovery rate by the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. Significance was defined as p ≤ 0.05.
Results
Maternal mononuclear cells respond to cffDNA by secreting IL-1β and CXCL10
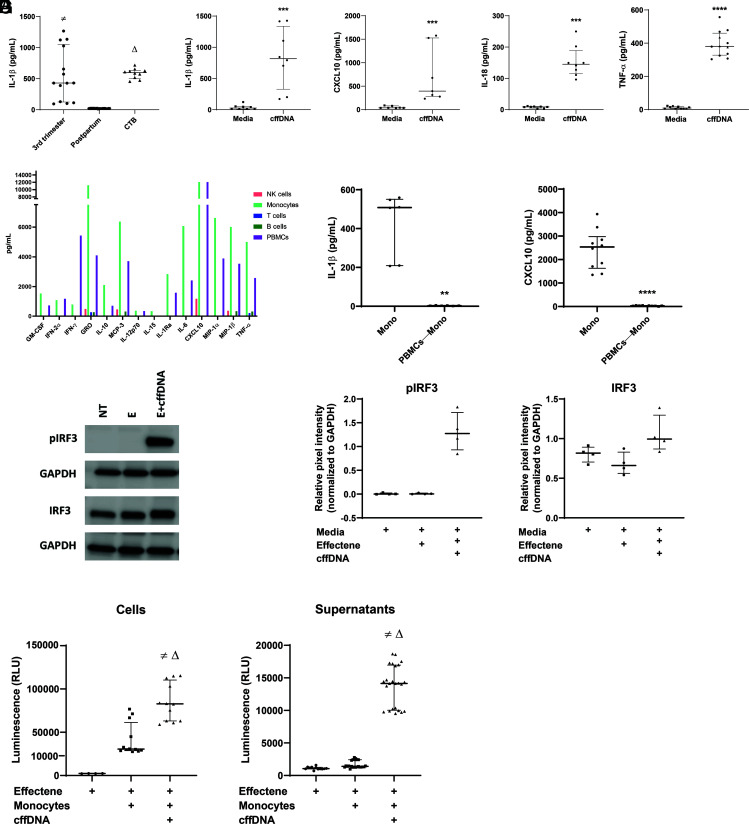
To determine the potential inflammatory nature of cffDNA, we stimulated maternal PBMCs with cell-free DNA isolated from maternal plasma samples collected at 36–38 wk gestation and 6 wk postpartum. Plasma at 36–38 wk gestation is a mixture of both maternal- and fetal-derived cell-free DNA fragments, while the postpartum sample contains only maternal cell-free DNA. Because PRRs in innate immune cells respond to cytoplasmic DNA detection with activation of the inflammasome (14, 15), we measured levels of IL-1β. Testing various concentrations of cffDNA, we determined that 750 ng/ml DNA is necessary to induce cytokine secretion by PBMCs (Supplemental Fig. 1A) and only when the DNA is packaged in a lipid-based transfection reagent versus as naked DNA (Supplemental Fig. 1B). Our data demonstrate that cell-free DNA from blood collected at 36–38 wk gestation can induce IL-1β secretion by maternal PBMCs, while cell-free DNA from blood collected postpartum fails to do the same (430.3 ± 1173 versus 18.74 ± 14.67 pg/ml IL-1β; p < 0.0001) (Fig. 1A).
FIGURE 1.
Monocytes are necessary and sufficient for the detection of cffDNA through activation of the cGAS/STING pathway. (A) Maternal PBMCs collected at 36–38 wk gestation were incubated with 750 ng/ml cffDNA isolated from maternal plasma samples collected at 36–38 wk gestation (“3rd trimester”), 6 wk postpartum (“Postpartum”), or from term CTB supernatants (“CTB”). After overnight incubation, IL-1β was measured in PBMC supernatants by ELISA (n = 5–7 PBMC donors). Data are displayed as medians with IQRs. The p values were compared by Kruskal–Wallis test, with correction using false discovery rate for multiple comparison testing and two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. Significant comparisons (p ≤ 0.05) are indicated by third trimester versus postpartum (≠) and postpartum versus CTB (Δ). (B) Maternal PBMCs were stimulated overnight with 750 ng/ml cffDNA isolated from term CTBs. After overnight incubation with Effectene alone (“Media”) or Effectene with cffDNA (“cffDNA”), IL-1β, CXCL10, IL-18, and TNF-α levels were measured in PBMC supernatants (n = 4–5 PBMC donors). Data were presented as medians and IQRs, and p values were calculated using Mann–Whitney U test with ***p < 0.0005 and ****p < 0.0001. (C) PBMCs from a healthy female donor were sorted into populations of B cells (CD20+), NK cells (CD56+), monocytes (CD14+), and T cells (CD3+). Each cell type was stimulated with cffDNA overnight, and cytokine levels were quantified in duplicate using multiplex analysis. (D) Maternal PBMCs isolated from blood collected at 36–38 wk gestation were used to isolate monocytes (Mono) or monocyte-depleted PBMCs (PBMCs—Mono), then stimulated with cffDNA overnight. ELISA was used to measure IL-1β and CXCL10 concentrations in supernatants (n = 3–5 donors). Data are presented as medians and IQRs, and p values were calculated using Mann–Whitney tests: **p < 0.005, ****p < 0.0001. (E) Monocytes from three healthy female donors and one pregnant donor were incubated for 4 h with media (NT), Effectene reagent (E), or Effectene reagent with cffDNA DNA (E+cffDNA). After incubation, cells were harvested and lysed for Western blot analysis of IRF3, p-IRF3, and GAPDH expression (n = 4 donors). (F) Densitometry analysis was performed using Azure software and normalized to GAPDH expression. (G) Maternal monocytes were stimulated with either Effectene reagent alone or Effectene with cffDNA for 4 h. After incubation, cells (n = 3 donors, 4 replicates each) and supernatants (n = same 3 donors, 8 replicates each) were harvested to measure levels of active caspase-1. Media alone containing no cells was used as a blank. Data are represented as medians and IQRs, with p values calculated using Kruskal–Wallis tests. Significant comparisons (p ≤ 0.05) are indicated by Effectene (Media Blank) versus Monocytes+Effectene+cffDNA (≠) and Monocytes+Effectene versus Monocytes+Effectene+cffDNA (Δ).
We next wanted to determine whether cffDNA shed from CTBs, the primary source of cffDNA in vivo, had the same effect as cell-free DNA from maternal plasma collected during the third trimester. Term placenta from uncomplicated pregnancies was collected within 4 h of delivery. Samples were then digested and processed for the isolation of primary CTBs. Purity of the isolated CTBs was determined by cytokeratin-7 staining, and results demonstrated 95% positivity for this CTB marker. CTBs were then cultured and supernatants harvested for the isolation of cffDNA (Supplemental Fig. 2). Stimulation of maternal PBMCs with cffDNA isolated from primary term CTBs resulted in increased IL-1β secretion, and the levels were comparable with the concentration of IL-1β released by maternal PBMCs in response to cell-free DNA from plasma samples collected at 36–38 wk gestation (430.3 ± 1173 versus 598.7 ± 262.7 pg/ml IL-1β; p = 0.5023; (Fig. 1A). Furthermore, cffDNA from term CTBs induced significantly more IL-1β secretion than cell-free DNA from postpartum plasma (598.7 ± 262.7 versus 18.74 ± 14.67 pg/ml IL-1β; p < 0.0001; (Fig. 1A). This suggests that cffDNA shed from the placenta, and isolated from either maternal plasma or primary CTBs, is detected by maternal PBMCs, which respond by secreting proinflammatory IL-1β. Because the composition and quantity of cffDNA in maternal circulation is highly heterogenous, we used cffDNA isolated from term CTB cultures for the remaining experiments.
In addition to IL-1 family cytokines, CXCL10 and IL-18 are also involved in the inflammatory response of parturition (22, 23) and are secreted after detection of cytoplasmic DNA by PRRs and activation of the inflammasome (15). To determine whether maternal PBMCs can respond to cffDNA by secreting both IL-1β and CXCL10, we stimulated cells overnight and measured cytokine levels in the supernatants. Our results demonstrate that maternal PBMCs respond to cffDNA by secreting IL-1β, CXCL10 (39.60 ± 66.10 versus 395.8 ± 1344 pg/ml CXCL10; p = 0.0001), and IL-18 (8.891 ± 7.944 versus 145.2 ± 153.4 pg/ml IL-18; p = 0.0002), suggesting that cffDNA induces the secretion of proinflammatory cytokines and chemokines from maternal PBMCs (Fig. 1B). Lastly, because TNF-α secretion is also involved in hallmarks of labor, including myometrial contraction (24, 25), and is secreted in response to a variety of stimuli, we measured TNF-α secretion in response to cffDNA. Our results demonstrate that maternal PBMCs release TNF-α after stimulation with cffDNA (9.136 ± 19.79 versus 381.0 ± 253.1 pg/ml TNF-α; p < 0.0001). Thus, peripheral maternal immune cells respond to cffDNA shed from the placenta by secreting a robust proinflammatory cytokine milieu that includes IL-1β, CXCL10, IL-18, and TNF-α.
Maternal monocytes are necessary and sufficient for the IL-1β/CXCL10 response to cffDNA through cGAS/STING and caspase-1
To determine which cells in the PBMC population are responsible for the release of inflammatory cytokines in response to cffDNA, we first cell-sorted PBMCs from an adult female donor at reproductive age into separate populations of CD20+ B cells, CD3+ T cells, CD56+ NK cells, and CD14+ monocytes, each of which were stimulated with cffDNA for 18 h. Using a nonpregnant donor allowed us to obtain sufficient PBMCs for cell sorting into multiple populations. After overnight incubation, we measured levels of cytokines in the culture supernatants through multiplex bead analysis. The results demonstrate that mainly monocytes secrete proinflammatory cytokines, including CXCL10, MIP-1α, and MIP-1β, in response to stimulation with cffDNA (Fig. 1C). We then compared the secretion of IL-1β and CXCL10 between maternal monocytes and monocyte-depleted PBMCs exposed to cffDNA to validate our multiplex findings. Our results demonstrate that monocytes, but not monocyte-depleted PBMCs, respond to cffDNA stimulation by secreting IL-1β (508.3 ± 351.8 versus 2.674 ± 3.222 pg/ml; p = 0.0022) and CXCL10 (2534 ± 2587 versus 27.14 ± 46.98 pg/ml; p < 0.0001) (Fig. 1D). This suggests that within the maternal PBMC pool, monocytes are responsible for the secretion of IL-1β and CXCL10 induced by cffDNA.
In human innate immune cells, two PRRs induce inflammasome activation downstream of cytoplasmic DNA detection resulting in IL-1β and CXCL10 secretion: AIM2 and cGAS/STING (15). Previous studies have determined that cGAS/STING, not AIM2, is the default pathway of DNA detection and inflammasome activation in human monocytes (15). Robust secretion of IL-1β and CXCL10 from monocytes in response to cffDNA suggests activation of the cGAS/STING. Detection of DNA in the cytoplasm induces activation of cGAS, which binds to and activates STING in the endoplasmic reticulum membrane. Activation of STING has two consequences: (1) phosphorylation of the transcription factor IRF3, which induces CXCL10 secretion; and (2) activation of the NLRP3 inflammasome, which activates caspase-1 leading to cleavage and secretion of IL-1β and IL-18. To determine whether the cGAS/STING pathway is responsible for cffDNA detection, we stimulated monocytes from healthy women of reproductive age or pregnant donors with cffDNA and analyzed levels of p-IRF3. Our results demonstrate that only monocytes treated with cffDNA have robust levels of p-IRF3 that can enter the nucleus and promote transcription of IFN response genes, including CXCL10 (Fig. 1E, 1F). To determine whether inflammasome activation is responsible for the levels of IL-1β and IL-18 secretion we observed in response to cffDNA, we used a bioluminescence assay to measure levels of activated caspase-1. Our results demonstrate high levels of active caspase-1 in both monocytes (30,530 ± 49,040 versus 82,790 ± 56,330 relative luminescence units; p = 0.0034) and monocyte supernatants (1413 ± 1803 versus 14,150 ± 9167 relative luminescence units; p < 0.0001) after cffDNA activation compared with untreated controls (Fig. 1G). Taken together, our results suggest that maternal monocytes respond to cffDNA through the cGAS/STING pathway, which leads to activation of the inflammasome downstream of caspase-1 and activation of transcription downstream of p-IRF3, resulting in the secretion of IL-1β and CXCL10, respectively.
The monocyte response to cffDNA can activate bystander T cells to secrete IFN-γ and GzmB
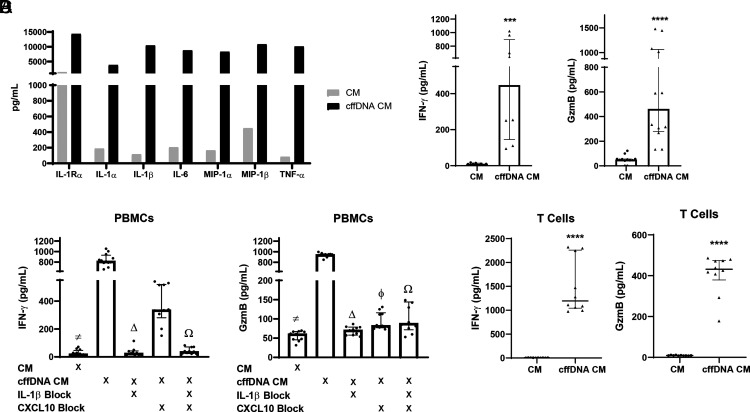
Given that the fetal fraction composes only an average of 13% of the total cell-free DNA pool in maternal circulation during pregnancy (26, 27), we hypothesized that the monocyte response to cffDNA could activate bystander cells, which themselves have not detected cffDNA, resulting in the amplification of proinflammatory responses. To test this hypothesis broadly, we stimulated maternal monocytes from one donor with cffDNA overnight and harvested CM for coculture with same-donor (autologous) PBMCs. We then analyzed cytokine concentrations in PBMC supernatants by multiplex. Our results demonstrated that the monocyte response to cffDNA induces further inflammatory cytokine secretion from PBMCs (Fig. 2A). Because the majority of these cytokines were characteristic of a Th1, proinflammatory response (TNF-α, MIP, IL-1, IL-6), we repeated these experiments focusing on IFN-γ and GzmB, which are classically secreted during a Th1-predominant, cytotoxic response. Interestingly, we observed that PBMCs treated with cffDNA CM secrete significant IFN-γ (447.1 ± 926.3 versus 10.58 ± 18.09 pg/ml; p = 0.0002) and GzmB (461.4 ± 1345 versus 50.17 ± 121.7 pg/ml; p < 0.0001) compared with CM from untreated monocytes (Fig. 2B). To further demonstrate that the PBMC response is specifically induced by the cytokines released from cffDNA-activated monocytes, we used neutralizing Abs for IL-1β and/or CXCL10 in the coculture experiments to block these cytokines in monocyte cffDNA CM. Blocking IL-β significantly reduced the secretion of IFN-γ (819.5 ± 393.3 versus 26.95 ± 103.0 pg/ml; p < 0.0001) and GzmB (944.5 ± 147.6 versus 53.83 ± 32.71 pg/ml; p < 0.0001) from PBMCs (Fig. 2C). Likewise, treatment with the CXCL10 blocking Ab also decreased IFN-γ (819.5 ± 393.3 versus 336.9 ± 388.9 pg/ml; p = 0.0615) and GzmB (944.5 ± 147.6 versus 82.76 ± 58.13 pg/ml; p = 0.0039) secretion from PBMCs, although this effect was not statistically significant for IFN-γ. Lastly, treatment with both IL-1β and CXCL10 neutralizing Abs together inhibited secretion of IFN-γ (819.5 ± 393.3 versus 38.90 ± 59.81 pg/ml; p < 0.0001) and GzmB (944.5 ± 147.6 versus 88.31 ± 209.6 pg/ml; p = 0.0039) compared with no treatment with neutralizing Abs (Fig. 2C).
FIGURE 2.
The monocyte response to cffDNA activates bystander PBMCs and T cells to secrete IFN-γ and GzmB. (A) Maternal monocytes were stimulated with Effectene alone or Effectene with cffDNA overnight. CM were harvested from both groups, and autologous maternal PBMCs were cultured in same-donor monocyte supernatants (CM or cffDNA CM) for 18 h before cytokine levels were measured using Multiplex analysis (n = 1 donor). (B) Third trimester maternal monocytes were stimulated with cffDNA. After overnight stimulations, CM and cffDNA CM were harvested and added to maternal PBMCs from the same donor. Levels of IFN-γ and GzmB (n = 4–6 donors) were measured in duplicate by ELISA. Data are presented as median and IQR. The p values were calculated using Mann–Whitney U test: ***p < 0.0005, ****p < 0.0001. (C) As in (B), monocytes were stimulated overnight with either Effectene alone or Effectene with cffDNA. Supernatants from these cultures (termed CM and cffDNA CM, respectively) were used as media for same-donor PBMCs with and without neutralizing Abs specific for IL-1β and CXCL10. After overnight incubation, levels of IFN-γ and GzmB (n = 6 donors) were measured in duplicate by ELISA. Data are displayed as medians and IQRs. The p values were compared by Kruskal–Wallis test, with correction using false discovery rate for multiple comparison testing and two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. Significant comparisons (p ≤ 0.05) are indicated by CM versus cffDNA CM (≠), cffDNA CM versus IL-1β block (Δ), cffDNA CM versus CXCL10 block (ϕ), and cffDNA CM versus IL-1β and CXCL10 block (Ω). (D) Maternal T cells were cultured overnight in CM from same-donor monocytes treated with either Effectene reagent alone (CM) or Effectene and cffDNA (cffDNA CM). Levels of IFN-γ and GzmB were measured in duplicate T cell supernatants after overnight incubation (n = 5 donors). Data are presented as median and IQR, with p values calculated by Mann–Whitney U test: ****p < 0.0001.
Because T cells are a classic source of both IFN-γ and GzmB secretion, we repeated the monocyte CM experiments with T cells. Maternal monocytes were stimulated with cffDNA overnight, and supernatants were harvested for CM culture with same-donor CD3+ T cells (both CD4+ and CD8+). We observed that T cells secrete significant amounts of IFN-γ (1195 ± 1349 versus 4.446 ± 4.554 pg/ml; p < 0.0001) and GzmB (431.9 ± 308.0 versus 9.061 ± 3.786 pg/ml; p < 0.0001) after coculture with CM from monocytes stimulated with cffDNA (cffDNA CM), but they do not respond to CM from untreated monocytes (Fig. 2D). This suggests that the maternal monocyte response to cffDNA leads to proinflammatory cytokine release, which can further induce release of IFN-γ and GzmB from maternal T cells, which have not interacted with cffDNA directly.
The cytokine response to cffDNA activates vascular endothelium
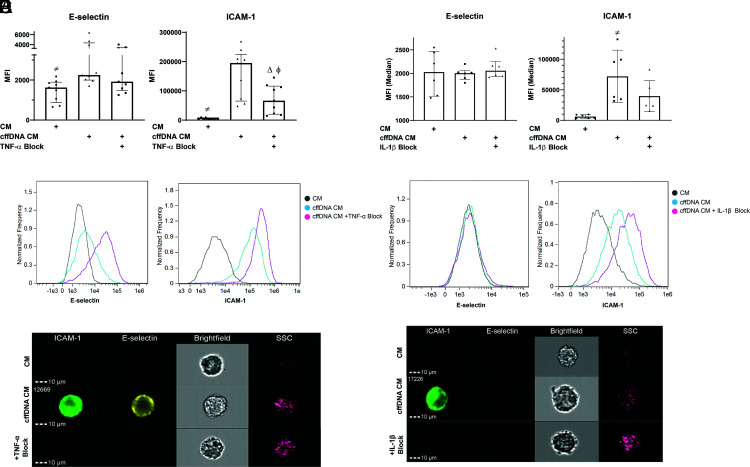
For cffDNA to induce infiltration of leukocytes into the uterus, which would aid in cervical ripening at term, we hypothesized that the cytokine response to cffDNA must activate the vascular endothelium because this is the first step in the infiltration of any tissue by immune cells. To test this hypothesis, we stimulated maternal PBMCs with cffDNA overnight and harvested the supernatants (CM) for coculture with HUVECs. After coculture with PBMC CM, HUVECs were harvested and stained for expression of E-selectin and ICAM-1. In response to supernatants from PBMCs stimulated with cffDNA (cffDNA CM), HUVEC expression of ICAM-1 (195,183 ± 221,232 versus 4096 ± 8872 median fluorescence intensity [MFI]; p < 0.0001) and E-selectin (2245 ± 4700 versus 1622 ± 1525 MFI; p = 0.0046) were significantly increased when compared with HUVECs treated with CM from untreated PBMCs (Fig. 3A–C). To determine whether activation of the endothelium by CM from cffDNA-treated PBMCs is cytokine dependent, we repeated these experiments with a neutralizing Ab to block TNF-α, a well-known activator of vascular endothelium (28), which is increased with cffDNA stimulation. Our results demonstrate that both E-selectin (1916 ± 2768 MFI; p = 0.0593) and ICAM-1 (66,474 ± 130,869 MFI; p = 0.0339) expression decreased after neutralizing TNF-α in the PBMC supernatant, although the effect on E-selectin was not statistically significant. This suggests that endothelial activation in response to cffDNA may be cytokine dependent.
FIGURE 3.
The cytokine response to cffDNA can induce endothelial activation. (A) Third trimester maternal PBMCs were stimulated overnight with Effectene alone or Effectene with cffDNA. CM from stimulated PBMCs (either CM or cffDNA CM) were harvested for overnight incubation with HUVECs. For designated wells, neutralizing Ab blocking TNF-α was added to supernatants from PBMCs treated with cffDNA CM. (B) Histograms from ICAM-1 and E-selectin staining of HUVECs from one representative experiment in which 10,000 single cells were collected for analysis. (C) Representative images of stained HUVECs with each treatment using ImageStream. (D) Third trimester maternal monocytes stimulated overnight with Effectene alone or Effectene with cffDNA. Supernatants from stimulated monocytes (either CM or cffDNA CM) were harvested for overnight incubation with HUVECs. For designated wells, neutralizing Ab blocking IL-1β was added to supernatants from monocytes treated with cffDNA CM. (E) Histograms from ICAM-1 and E-selectin staining of HUVECs from one representative experiment in which 10,000 single cells were collected for analysis. (F) Representative cell images of stained HUVECs using ImageStream. For all experiments, 10,000 single cells were collected and analyzed per replicate from each experiment (n = 2–3 donors). (A) and (D) represent median and IQR of the data. The p values were compared by Friedman test, with correction using false discovery rate for multiple comparison testing and two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. Significant comparisons (p ≤ 0.05) are indicated by CM versus cffDNA CM (≠), cffDNA CM versus TNF-α or IL-1β block (Δ), and CM versus TNF-α or IL-1β block (ϕ).
Because monocytes are necessary and sufficient for the secretion of IL-1β and CXCL10, we next determined whether the monocyte response alone could activate the endothelium. cffDNA may play an important role in the clinically observed infiltration of the maternal–fetal interface at term and during labor (29–33). Therefore, we cultured HUVECs in CM from monocytes treated with cffDNA and observed an increase in ICAM-1 expression compared with HUVECs treated with CM from untreated monocytes (67,079 ± 100,860 versus 5929 ± 6344 MFI; p = 0.0005) (Fig. 3D–F). E-selectin expression, however, did not change in response to monocyte cffDNA CM (2005 ± 401.4 versus 2028 ± 1073 MFI; p = 0.7728). In addition to TNF-α, IL-1 family cytokines are also known inducers of endothelial activation (28). Therefore, to determine whether the increased ICAM-1 expression observed in response to cffDNA CM is dependent on IL-1β, we used an IL-1β neutralizing Ab to block this effect in monocyte CM. Although we observed a decrease in ICAM-1 expression in HUVECs cocultured with monocyte cffDNA CM and IL-1β blocking Ab, the decrease was not significant (31,717 ± 67,832 MFI; p = 0.0833). Our results demonstrate that the cytokine response to cffDNA can induce endothelial activation through upregulation of ICAM-1, which, along with secretion of the chemokine CXCL10, could be hypothesized to promote infiltration of leukocytes into the uterine compartment.
The cytokine response to cffDNA induces myometrial contraction
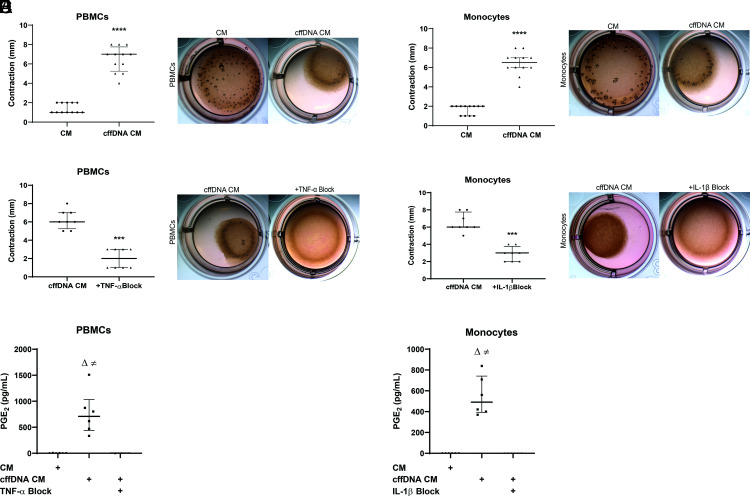
Previous studies have demonstrated how proinflammatory cytokines can induce myometrial contractility (25, 34); thus, we hypothesized that the PBMC and monocyte responses to cffDNA could induce myometrial contraction in vitro. To test this hypothesis, we embedded PHM1-41 myometrial cells in a collagen matrix that would allow us to observe contraction as a size change in the collagen–cell matrix. We then added CM from cffDNA-stimulated PBMCs or monocytes (cffDNA CM) to the matrices and measured the distance between the edge of the well and the collagen matrix (0 mm at the start of the coculture) as a surrogate for contraction. Our results demonstrate that cffDNA CM from PBMCs treated with Effectene + cffDNA, but not PBMCs treated with Effectene alone (CM), can induce contraction of PHM1-41 cells (7.0 ± 4.0 versus 1.0 ± 1.0 mm; p < 0.0001) (Fig. 4A). Likewise, supernatants from monocytes treated with Effectene reagent and cffDNA (cffDNA CM), but not Effectene reagent alone (CM), can stimulate PHM1-41 contraction (6.5 ± 4.0 versus 2.0 ± 1.000 mm; p < 0.0001) (Fig. 4D).
FIGURE 4.
The inflammatory response to cffDNA induces myometrial contraction in vitro. (A) Maternal PBMCs from 36 to 38 wk gestation were stimulated overnight with Effectene reagent alone or Effectene reagent with cffDNA (n = 6). Supernatants (CM or cffDNA CM) were then added to PHM1-41 cells embedded in a collagen matrix, and contraction was measured after overnight incubation as the distance between the edge of the well and the collagen–cell matrix. Representative wells are shown. (B) Third trimester maternal PBMCs were stimulated overnight with Effectene reagent alone or Effectene reagent with cffDNA (n = 4 donors) as in (A). Resulting supernatants (CM or cffDNA CM) were then added to PHM1-41 cells embedded in a collagen matrix with or without TNF-α neutralizing Abs for 18 h, and contraction was measured as the distance between the edge of the well and the collagen–cell matrix. Representative wells are shown. (C) Maternal PBMCs (n = 3 donors) were stimulated with Effectene or Effectene+cffDNA overnight. PBMC supernatants (CM and cffDNA CM) were harvested for coculture with PHM1-41 cells in a 24-well plate. Levels of PGE2 were measured in PHM1-41 supernatants after overnight incubation with cffDNA CM supernatants with or without TNF-α blocking Ab. The p values were calculated using Kruskal–Wallis test. Significant comparisons (p ≤ 0.05) are indicated by CM versus cffDNA CM (≠) and cffDNA CM versus TNF-α block (Δ). (D) Maternal monocytes from blood collected at 36–38 wk gestation were stimulated overnight with Effectene reagent alone or Effectene reagent with cffDNA (n = 6 donors). Resulting monocyte supernatants (CM or cffDNA CM) were added to PHM1-41 cells embedded in a collagen matrix. Contraction was measured after overnight coculture of CM with the PHM1-41 collagen matrix. (E) Maternal monocytes from 36–38 wk gestation were stimulated overnight with Effectene reagent alone or Effectene reagent with cffDNA (n = 4 donors). CM or cffDNA CM were then added to PHM1-41 cells embedded in a collagen matrix with or without neutralizing Abs for IL-1β. For all data in this figure, the matrices were imaged and contraction was measured after overnight (18 h) incubation as the distance between the edge of the well and the collagen–cell matrix. The p values were calculated using Mann–Whitney tests, ****p < 0.0001; graphs display median and IQR. (F) Maternal monocytes (n = 3 donors) were stimulated with Effectene or Effectene and cffDNA overnight. Monocyte supernatants (CM or cffDNA CM) were harvested for overnight coculture with PHM1-41 cells with or without IL-1β blocking Abs in a 24-well plate. Levels of PGE2 were measured by ELISA. The p values were calculated using Kruskal–Wallis test. Significant comparisons (p ≤ 0.05) are indicated by CM versus cffDNA CM (≠) and cffDNA CM versus IL-1β block (Δ).
To determine whether the increased contractility in response to CM from cffDNA-stimulated PBMCs and monocytes is cytokine dependent, we used neutralizing Abs to block TNF-α in PBMC cffDNA CM and IL-1β in monocyte cffDNA CM. We observed a significant decrease in myometrial contractility in response to PBMC (6.000 ± 3.000 versus 2.000 ± 2.000 mm; p = 0.0002) and monocyte (6.000 ± 3.000 versus 3.000 ± 2.000 mm; p = 0.0002) cffDNA CM when TNF-α or IL-1β blocking Abs were added, respectively (Fig. 4B, 4E). Together, our results demonstrate that blocking these cytokines decreases myometrial contractility, suggesting that cffDNA can induce cytokine-dependent contractions, which is a key hallmark of parturition.
Lastly, to determine a possible mechanism by which supernatants from PBMCs and monocytes stimulated with cffDNA could induce myometrial contraction, we measured levels of PGE2 in the supernatants of PHM1-41 myometrial cells, because PGE2 is a known uterotonic and calcium ionophore (24). Our results demonstrate that PHM1-41 cells treated with cffDNA-stimulated PBMCs (cffDNA CM) secrete PGE2 in a TNF-α–dependent manner (Fig. 4C) (709.5 ± 1175 versus 1.867 ± 1.724 pg/ml PGE2; p = 0.0058). In addition, PHM1-41 cells treated with cffDNA-stimulated monocyte supernatants (cffDNA CM) secrete PGE2 in an IL-1β–dependent manner (Fig. 4F) (490.9 ± 468.1 versus 0.02879 ± 0.02952 pg/ml PGE2; p = 0.0007). This suggests that the inflammatory cytokine response to cffDNA may lead to myometrial contraction through PGE2.
Discussion
Initiation of labor requires a physiologic response that results in cervical ripening, membrane rupture, and phasic uterine contractions (6). Although hormonal changes can prepare the myometrium and cervix for labor, data suggest that inflammation plays a major role in this process (9, 33, 35–38). In fact, labor is characterized by an inflammatory infiltrate into the cervix, myometrium, and choriodecidua (23, 29, 33, 39, 40). TNF-α, CXLC10, and IL-1β recruit leukocytes to the maternal–fetal interface (23), and higher levels of CXCL10 in the membrane rupture zone are associated with premature rupture of membranes (22). IL-1β, IL-6, and IL-8 increase PG and matrix metalloprotease expression, resulting in the weakening of fetal membranes and ripening of the cervix (33, 41–46). Choriodecidual leukocytes at term also express significantly higher levels of proinflammatory TNF-α and IL-1β (47). Although higher levels of IL-1, IL-6, and TNF-α are observed in plasma samples from laboring women and an inflammatory infiltrate in the uterine compartment can be observed in labor (33), the origin of this sterile inflammation in normal pregnancy remains unclear. Several previous studies suggest that cffDNA may have a role in establishing sterile inflammation that helps to activate the labor process. First, the concentration of cffDNA is known to increase in maternal blood as gestation progresses, peaking just before labor (1). In addition, cffDNA levels have been found to be abnormally high in plasma samples from women with pregnancy complications, including preterm labor (8, 48, 49). Furthermore, murine studies demonstrate significant IL-6 secretion and fetal resorption in response to human cffDNA injection in the uterine compartment (50). Yet, there is limited evidence on the inflammatory potential of cffDNA in humans (5). We hypothesized that cffDNA could induce proinflammatory cytokine secretion from maternal immune cells, which promotes systemic maternal immune activation and physiologic changes associated with labor onset.
We tested this hypothesis by using both cell-free DNA from third trimester maternal plasma samples and cffDNA isolated from primary term CTBs to stimulate late third trimester maternal PBMCs and monocytes. Our data demonstrate that maternal monocytes are necessary and sufficient for the secretion of significant levels of IL-1β and CXCL10 in response to cffDNA. These results conflict with previous studies that suggest that cffDNA does not induce the secretion of cytokines, including TNF-α and CXCL10 from maternal PBMCs (51). This may be because of differences in the amount of cffDNA used and the method of PBMC stimulation. We hypothesize that there may be a critical threshold of cffDNA concentration that must be reached before an appreciable inflammatory response is induced. This threshold may be reached at term when placental senescence results in trophoblast apoptosis and increased release of cffDNA (4, 12, 52).
By measuring levels of p-IRF3 and activated caspase-1 in cffDNA-stimulated monocytes, we observed that the cGAS/STING pathway of dsDNA detection is most likely responsible for the IL-1β and CXCL10 response to cffDNA. Our results agree with previous findings that demonstrate that the cGAS/STING pathway, not AIM2, is responsible for activation of the NLRP3 inflammasome and IL-1β secretion downstream of DNA detection in human monocytes (15). Activation of STING by the second messenger cyclic GMP–AMP results in both phosphorylation of IRF3 and lysosomal disruption. Although p-IRF3 induces a type I IFN response leading to CXCL10 secretion, lysosomal disruption induces potassium efflux, which activates the NLRP3 inflammasome resulting in IL-1β secretion downstream of activated caspase-1. Thus, the cGAS/STING pathway of NLRP3 activation has been proved to be responsible for inflammasome activity and IL-1β secretion in human myeloid cells, including monocytes (15). Although current hypotheses suggest that TLR9 and STING may be involved in an inflammatory response to cffDNA (5, 50), our results contrast with previous studies in mice that have demonstrated IL-6 secretion in response to human cffDNA downstream of TLR9 signaling and NF-κB activation. This can best be explained by differences in PRR expression between human and murine tissues. Specifically, murine monocytes express high levels of TLR9, while expression of this PRR is restricted to B cells, dendritic cells, and epithelial cells in humans (53). Studies in humans demonstrate increased AIM2 expression in women with preeclampsia compared with women with healthy pregnancies (54). Stimulation of HTR/SVneo cells and placenta villous explants with poly(dA:dT) or DNA from necrotic JEG3 cells increases proinflammatory release of soluble endoglin, sFlt-1, CXCL10, IL-6, IL-8, and MCP-1, which can be partially attenuated by knocking down AIM2 (54). Furthermore, Li et al. (54) demonstrated a decrease in soluble endoglin, sFlt-1, and CXCL10 production in IFI16 knockdown HTR/SVneo trophoblast cells. IFI16 binds cytoplasmic and nucleic viral dsDNA and interacts with STING to induce a type I IFN response. In addition, IFI16 inhibits AIM2 and independently activates the caspase-1 inflammasome to induce IL-1β secretion (55). Thus, although our results demonstrate activation of maternal innate immunity through the cGAS/STING pathway, we cannot dismiss the possibility that trophoblasts may respond to cffDNA through both AIM2 and STING, resulting in inflammation.
Although levels of cffDNA increase as gestation progresses, the fetal fraction accounts for only up to 30% of total cell-free DNA in maternal circulation by the third trimester (26, 27). Therefore, amplification of the inflammatory response to cffDNA through activation of bystander cells may be an important and necessary mechanism in generating robust inflammation and promoting changes in maternal immunity observed at parturition (11). Thus, we aimed to determine whether the monocyte cytokine response can activate bystander cells that have not been exposed to cffDNA. Our data demonstrate that, in response to the inflammatory cytokines produced by monocytes after cffDNA detection, PBMCs secrete proinflammatory cytokines that are characteristic of a Th1 cytotoxic phenotype (IFN-γ and GmzB). Previous studies have demonstrated that CXCL10 recruits T cells to fetal membranes during labor (22, 47, 56). Furthermore, increased concentrations of CTLs, which express perforin and GzmB, are found at the maternal–fetal interface at term (57–59). Our data suggest that the observed inflammatory response to cffDNA may recruit T cells to the maternal–fetal interface through endothelial activation and CXCL10-driven chemotaxis, while inducing T cells to secrete IFN-γ and GzmB once they enter the tissue. IFN-γ from uterine NK cells is necessary during implantation for spiral artery formation; however, increased levels of IFN-γ later in pregnancy can induce MHC class II expression, CTB death, and pregnancy loss in mouse and rabbit models (60, 61). Although we did not appreciate a cytokine response to cffDNA by peripheral NK cells, we did observe a T cell response to supernatants from cffDNA-stimulated monocytes. Our results demonstrate increased IFN-γ and GzmB secretion from bystander T cells in response to IL-1β and CXCL10 secreted by cffDNA-activated monocytes. Although these cytokines are characteristic of a Th1 response and may contribute to the observed shift in maternal immunity toward an inflammatory phenotype at the end of pregnancy, they may have aberrant effects in conditions in which cffDNA is abnormally elevated. Lastly, this observed bystander effect suggests that although cffDNA may comprise only a fraction of the total cell-free DNA in maternal circulation, the monocyte response to cffDNA detection can induce cytokine secretion by other maternal leukocytes, thus perpetuating inflammation.
Current studies demonstrate maternal immune cell infiltration, specifically with macrophages, neutrophils, and T cells, into the uterine compartment in laboring women (33). However, the inflammatory source(s) that activates the endothelium and recruits’ leukocytes to the tissue has not been identified. We hypothesized that the cytokine response to cffDNA could induce expression of endothelial markers necessary for leukocyte recruitment. This hypothesis was supported by our finding that detection of cffDNA by maternal PBMCs and monocytes results in the secretion of TNF-α and IL-1β, respectively. These cytokines are well-characterized activators of vascular endothelium. In response to TNF-α and IL-1 family cytokines, vascular endothelial cells increase expression of E-selectin and ICAM-1, which are necessary for leukocyte rolling and firm adhesion along the endothelium (28). Because endothelial activation is a necessary first step for the infiltration of any tissue with leukocytes, we analyzed E-selectin and ICAM-1 expression on HUVECs in response to cffDNA stimulation of maternal PBMCs and monocytes to determine whether cffDNA may be involved in promoting inflammation, which would recruit circulating leukocytes to the uterus. We observed increased expression of ICAM-1 induced by the cytokine response to cffDNA from both maternal PBMCs and monocytes. Previous studies have demonstrated increased ICAM-1 expression in fetal membranes after labor, which was induced by leukocytes (32) and in the cervix and myometrium (62). Our data suggest that cffDNA detected by leukocytes at the fetal–maternal interface may promote infiltration of uterine tissue by maternal immune cells, as observed in these previous studies.
Given the established role of IL-1β and TNF-α in myometrial contraction, we hypothesized that cffDNA may be further involved in labor by promoting an inflammatory response that can induce phasic uterine contractions. Previous studies have demonstrated the effect of inflammation on myometrial contractility (25, 63). Specifically, IL-1β has been shown to promote contraction by increasing calcium entry into smooth muscle and helping to generate a robust contractile force (34). In addition, both TNF-α and IL-1β induce synthesis and release of PGE2 from the myometrium (42, 64, 65). PGE2 is a strong activator of contraction and is given clinically as misoprostol to induce labor. Although a mechanistic role has been established for myometrial contractility in response to TNF-α and IL-1β (24, 34, 42, 63), a source of sterile inflammation that could induce cytokine secretion in labor has not been identified. Our data demonstrate that cffDNA induces an inflammatory response characterized by IL-1β and TNF-α secretion, which can promote myometrial contraction in vitro. Furthermore, IL-1β and TNF-α secreted by cffDNA-stimulated monocytes or PBMCs, respectively, can induce PGE2 secretion from PHM1-41 myometrial cells. The inflammation and increased contractility we observe in response to cffDNA may explain the role of inflammatory cytokines in mediating preterm labor (63).
Our data are strengthened by the fact that we used primary human blood and placental tissues from pregnant mothers to complete these studies. In addition, we used autologous stimulations to measure bystander effects to minimize allogenicity-mediated inflammation. These studies warrant further investigation into the epigenetic modifications and structural differences unique to cffDNA to determine why the fetal fraction is uniquely inflammatory. Previous studies have demonstrated that fetal DNA is hypomethylated in comparison with adult DNA, which would make it a suitable ligand for TLR9 (66). Because our data do not implicate TLR9 in the response to cffDNA, studies will be necessary to determine characteristics of cffDNA that make it a possible stimulatory ligand of cGAS or AIM2 pathways. Factors such as the mitochondrial fraction of cffDNA and whether cffDNA is enriched for telomeric or centromeric regions may be relevant. Considering the increase in maternal serum levels of DNases during pregnancy (67), a packaging medium such as extracellular vesicles or syncytial knots, which could envelop and protect cffDNA while mediating its entry into monocytes, must also be characterized. Indeed, our experiments required packaging of cffDNA using Effectene transfection reagent because the use of cffDNA alone had no impact in cytokine release, suggesting vesicles likely have an important role in cellular uptake and signaling. The effect of cffDNA detection on the differentiation of monocytes into macrophage subsets and the role of monocyte activation in the modulation of T cell activity toward a Th1 phenotype should also be further characterized. Specifically, the secretion of IFN-γ and GzmB must be investigated to determine whether this is an Ag-independent response mediated by monocyte-derived IL-1β and CXCL10 and which T cell subset(s) is responsible. Given our observation that neutralizing Abs targeting IL-β and CXCL10 can diminish IFN-γ and GzmB secretion, γδ T cells mediating an Ag-independent response may well be implicated and are the focus of our ongoing work. We were limited by access to primary uterine tissues and the decidual immune compartment for our work; however, future studies could use immune cells from uterine samples to model the nature of inflammation at the maternal–fetal interface more relevantly than cell lines. Specifically, uterine NK cells should be studied to determine whether their proximity to the source of cffDNA and ability to secrete IFN-γ can contribute to a localized inflammatory response.
In conclusion, we observed that cffDNA induces a significant proinflammatory response mediated by monocytes through the cGAS/STING pathway, which can perpetuate inflammation by inducing IFN-γ and GzmB secretion from bystander T cells. cffDNA may also have a role in labor by activating the endothelium to promote cell trafficking to the uterine compartment and by inducing myometrial contractility through PGE2. Further elucidation into the role of cffDNA in parturition may lead to the development of novel strategies to control the timing of labor and delivery more effectively.
Supplementary Material
This work was supported by U.S. Department of Health and Human Services, National Institutes of Health Grant HD065987 (to E.A.L.E.).
The online version of this article contains supplemental material.
- cffDNA
- cell-free fetal DNA
- cGAS/STING
- cyclic cGMP–AMP synthase/stimulator of IFN genes
- CM
- conditioned media
- CTB
- cytotrophoblast
- GzmB
- granzyme B
- IQR
- interquartile range
- IRF3
- IFN regulatory factor 3
- PRR
- pattern recognition receptor
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Lo Y. M., Corbetta N., Chamberlain P. F., Rai V., Sargent I. L., Redman C. W., Wainscoat J. S.. 1997. Presence of fetal DNA in maternal plasma and serum. Lancet 350: 485–487. [DOI] [PubMed] [Google Scholar]
- 2. Lo Y. M., Tein M. S., Lau T. K., Haines C. J., Leung T. N., Poon P. M., Wainscoat J. S., Johnson P. J., Chang A. M., Hjelm N. M.. 1998. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 62: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang J., Li J., Saucier J. B., Feng Y., Jiang Y., Sinson J., McCombs A. K., Schmitt E. S., Peacock S., Chen S., et al. 2019. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. [Published erratum appears in 2019 Nat. Med. 25: 701–702.] Nat. Med. 25: 439–447. [DOI] [PubMed] [Google Scholar]
- 4. Phillippe M. 2015. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod. Sci. 22: 1186–1201. [DOI] [PubMed] [Google Scholar]
- 5. van Boeckel S. R., Davidson D. J., Norman J. E., Stock S. J.. 2018. Cell-free fetal DNA and spontaneous preterm birth. Reproduction 155: R137–R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillippe M. 2014. Cell-free fetal DNA—a trigger for parturition. N. Engl. J. Med. 370: 2534–2536. [DOI] [PubMed] [Google Scholar]
- 7. Nadeau-Vallée M., Obari D., Palacios J., Brien M. E., Duval C., Chemtob S., Girard S.. 2016. Sterile inflammation and pregnancy complications: a review. Reproduction 152: R277–R292. [DOI] [PubMed] [Google Scholar]
- 8. Farina A., LeShane E. S., Romero R., Gomez R., Chaiworapongsa T., Rizzo N., Bianchi D. W.. 2005. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am. J. Obstet. Gynecol. 193: 421–425. [DOI] [PubMed] [Google Scholar]
- 9. Christiaens I., Zaragoza D. B., Guilbert L., Robertson S. A., Mitchell B. F., Olson D. M.. 2008. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 79: 50–57. [DOI] [PubMed] [Google Scholar]
- 10. Kassebaum N. J., Bertozzi-Villa A., Coggeshall M. S., Shackelford K. A., Steiner C., Heuton K. R., Gonzalez-Medina D., Barber R., Huynh C., Dicker D., et al. 2014. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Förger F., Villiger P. M.. 2020. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. [Published erratum appears in 2020 Nat. Rev. Rheumatol. 16: 184.] Nat. Rev. Rheumatol. 16: 113–122. [DOI] [PubMed] [Google Scholar]
- 12. Athapathu H., Jayawardana M. A., Senanayaka L.. 2003. A study of the incidence of apoptosis in the human placental cells in the last weeks of pregnancy. J. Obstet. Gynaecol. 23: 515–517. [DOI] [PubMed] [Google Scholar]
- 13. Houben M. L., Nikkels P. G., van Bleek G. M., Visser G. H., Rovers M. M., Kessel H., de Waal W. J., Schuijff L., Evers A., Kimpen J. L., Bont L.. 2009. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PLoS One 4: e6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu J., Chen Z. J.. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32: 461–488. [DOI] [PubMed] [Google Scholar]
- 15. Gaidt M. M., Ebert T. S., Chauhan D., Ramshorn K., Pinci F., Zuber S., O’Duill F., Schmid-Burgk J. L., Hoss F., Buhmann R., et al. 2017. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171: 1110–1124.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lugrin J., Martinon F.. 2018. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol. Rev. 281: 99–114. [DOI] [PubMed] [Google Scholar]
- 17. Müller T., Hamm S., Bauer S.. 2008. TLR9-mediated recognition of DNA. Handb. Exp. Pharmacol. 183: 51–70. [DOI] [PubMed] [Google Scholar]
- 18. Bischoff F. Z., Lewis D. E., Simpson J. L.. 2005. Cell-free fetal DNA in maternal blood: kinetics, source and structure. Hum. Reprod. Update 11: 59–67. [DOI] [PubMed] [Google Scholar]
- 19. Colucci G., Pesenti E., Molteni E., Lobbiani A., De Andreis C., Pariani S., Rossella F., Semprini A. E., Simoni G.. 1993. Applicability of DNA isolated from syncytiotrophoblast vesicles to gene amplification and molecular analysis. Prenat. Diagn. 13: 335–340. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A. K., Holzgreve W., Huppertz B., Malek A., Schneider H., Hahn S.. 2004. Detection of fetal DNA and RNA in placenta-derived syncytiotrophoblast microparticles generated in vitro. Clin. Chem. 50: 2187–2190. [DOI] [PubMed] [Google Scholar]
- 21. Orozco A. F., Jorgez C. J., Horne C., Marquez-Do D. A., Chapman M. R., Rodgers J. R., Bischoff F. Z., Lewis D. E.. 2008. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am. J. Pathol. 173: 1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Lopez N., Hernandez-Santiago S., Lobb A. P., Olson D. M., Vadillo-Ortega F.. 2013. Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod. Sci. 20: 276–284. [DOI] [PubMed] [Google Scholar]
- 23. Gomez-Lopez N., Estrada-Gutierrez G., Jimenez-Zamudio L., Vega-Sanchez R., Vadillo-Ortega F.. 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol. 80: 122–131. [DOI] [PubMed] [Google Scholar]
- 24. Egarter C. H., Husslein P.. 1992. Biochemistry of myometrial contractility. Baillieres Clin. Obstet. Gynaecol. 6: 755–769. [DOI] [PubMed] [Google Scholar]
- 25. Frascoli M., Coniglio L., Witt R., Jeanty C., Fleck-Derderian S., Myers D. E., Lee T. H., Keating S., Busch M. P., Norris P. J., et al. 2018. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci. Transl. Med. 10: eaan2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu H., Liu H., Peng C., Deng T., Fu X., Chung C., Zhang E., Lu C., Zhang K., Liang Z., Yang Y.. 2016. Clinical experience of non-invasive prenatal chromosomal aneuploidy testing in 190,277 patient samples. Curr. Mol. Med. 16: 759–766. [DOI] [PubMed] [Google Scholar]
- 27. Hu P., Liang D., Chen Y., Lin Y., Qiao F., Li H., Wang T., Peng C., Luo D., Liu H., Xu Z.. 2019. An enrichment method to increase cell-free fetal DNA fraction and significantly reduce false negatives and test failures for non-invasive prenatal screening: a feasibility study. J. Transl. Med. 17: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pober J. S., Sessa W. C.. 2007. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7: 803–815. [DOI] [PubMed] [Google Scholar]
- 29. Gomez-Lopez N., Guilbert L. J., Olson D. M.. 2010. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukoc. Biol. 88: 625–633. [DOI] [PubMed] [Google Scholar]
- 30. Gomez-Lopez N., Laresgoiti-Servitje E., Olson D. M., Estrada-Gutiérrez G., Vadillo-Ortega F.. 2010. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol. Reprod. 82: 809–814. [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Lopez N., Tanaka S., Zaeem Z., Metz G. A., Olson D. M.. 2013. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy Childbirth 13(Suppl. 1): S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osman I., Crawford M., Jordan F., Young A., Norman J., Thomson A.. 2004. Expression and localization of cell adhesion molecules in human fetal membranes during parturition. J. Reprod. Immunol. 63: 11–21. [DOI] [PubMed] [Google Scholar]
- 33. Thomson A. J., Telfer J. F., Young A., Campbell S., Stewart C. J., Cameron I. T., Greer I. A., Norman J. E.. 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum. Reprod. 14: 229–236. [PubMed] [Google Scholar]
- 34. Tribe R. M., Moriarty P., Dalrymple A., Hassoni A. A., Poston L.. 2003. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol. Reprod. 68: 1842–1849. [DOI] [PubMed] [Google Scholar]
- 35. Arck P. C., Hecher K.. 2013. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 19: 548–556. [DOI] [PubMed] [Google Scholar]
- 36. Bollopragada S., Youssef R., Jordan F., Greer I., Norman J., Nelson S.. 2009. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol. 200: 104.e1–11. [DOI] [PubMed] [Google Scholar]
- 37. Kelly R. W. 1996. Inflammatory mediators and parturition. Rev. Reprod. 1: 89–96. [DOI] [PubMed] [Google Scholar]
- 38. Norman J. E., Bollapragada S., Yuan M., Nelson S. M.. 2007. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 7(Suppl. 1): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halgunset J., Johnsen H., Kjøllesdal A. M., Qvigstad E., Espevik T., Austgulen R.. 1994. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur. J. Obstet. Gynecol. Reprod. Biol. 56: 153–160. [DOI] [PubMed] [Google Scholar]
- 40. Osman I., Young A., Ledingham M. A., Thomson A. J., Jordan F., Greer I. A., Norman J. E.. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 41. Elliott C. L., Loudon J. A., Brown N., Slater D. M., Bennett P. R., Sullivan M. H.. 2001. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am. J. Reprod. Immunol. 46: 260–267. [DOI] [PubMed] [Google Scholar]
- 42. Oger S., Méhats C., Dallot E., Ferré F., Leroy M. J.. 2002. Interleukin-1beta induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E2- and cyclic adenosine 3',5'-monophosphate-dependent pathway. J. Clin. Endocrinol. Metab. 87: 5524–5531. [DOI] [PubMed] [Google Scholar]
- 43. Osmers R., Rath W., Adelmann-Grill B. C., Fittkow C., Kuloczik M., Szeverényi M., Tschesche H., Kuhn W.. 1992. Origin of cervical collagenase during parturition. Am. J. Obstet. Gynecol. 166: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 44. Roh C. R., Oh W. J., Yoon B. K., Lee J. H.. 2000. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol. Hum. Reprod. 6: 96–102. [DOI] [PubMed] [Google Scholar]
- 45. Stygar D., Wang H., Vladic Y. S., Ekman G., Eriksson H., Sahlin L.. 2002. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol. Reprod. 67: 889–894. [DOI] [PubMed] [Google Scholar]
- 46. Young A., Thomson A. J., Ledingham M., Jordan F., Greer I. A., Norman J. E.. 2002. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol. Reprod. 66: 445–449. [DOI] [PubMed] [Google Scholar]
- 47. Gomez-Lopez N., Vega-Sanchez R., Castillo-Castrejon M., Romero R., Cubeiro-Arreola K., Vadillo-Ortega F.. 2013. Evidence for a role for the adaptive immune response in human term parturition. Am. J. Reprod. Immunol. 69: 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Darghahi R., Mobaraki-Asl N., Ghavami Z., Pourfarzi F., Hosseini-Asl S., Jalilvand F.. 2019. Effect of cell-free fetal DNA on spontaneous preterm labor. J. Adv. Pharm. Technol. Res. 10: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dugoff L., Barberio A., Whittaker P. G., Schwartz N., Sehdev H., Bastek J. A.. 2016. Cell-free DNA fetal fraction and preterm birth. Am. J. Obstet. Gynecol. 215: 231.e1–7. [DOI] [PubMed] [Google Scholar]
- 50. Scharfe-Nugent A., Corr S. C., Carpenter S. B., Keogh L., Doyle B., Martin C., Fitzgerald K. A., Daly S., O’Leary J. J., O’Neill L. A.. 2012. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J. Immunol. 188: 5706–5712. [DOI] [PubMed] [Google Scholar]
- 51. van Boeckel S. R., Macpherson H., Norman J. E., Davidson D. J., Stock S. J.. 2020. Inflammation-mediated generation and inflammatory potential of human placental cell-free fetal DNA. Placenta 93: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kazemi N. Y., Fedyshyn B., Yelsa I., Fedyshyn Y., Ruano R., Markovic S. N., Chakraborty R., Enninga E. A. L.. 2021. Increased cell-free fetal DNA release after apoptosis and sterile inflammation in human trophoblast cells. Am. J. Reprod. Immunol. 2021: e13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdörfer B., Giese T., Endres S., Hartmann G.. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168: 4531–4537. [DOI] [PubMed] [Google Scholar]
- 54. Li N., He F., Gao H., Ge Y., Fan X., Zhang J., Qi H., Ren L.. 2020. Elevated cell-free fetal DNA contributes to placental inflammation and antiangiogenesis via AIM2 and IFI16 during pre-eclampsia. J. Cell. Physiol. 235: 9577–9588. [DOI] [PubMed] [Google Scholar]
- 55. Jin T., Perry A., Jiang J., Smith P., Curry J. A., Unterholzner L., Jiang Z., Horvath G., Rathinam V. A., Johnstone R. W., et al. 2012. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gomez-Lopez N., Vadillo-Perez L., Hernandez-Carbajal A., Godines-Enriquez M., Olson D. M., Vadillo-Ortega F.. 2011. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am. J. Obstet. Gynecol. 205: 235.e15–24. [DOI] [PubMed] [Google Scholar]
- 57. Tilburgs T., Roelen D. L., van der Mast B. J., van Schip J. J., Kleijburg C., de Groot-Swings G. M., Kanhai H. H., Claas F. H., Scherjon S. A.. 2006. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta 27(Suppl. A): S47–S53. [DOI] [PubMed] [Google Scholar]
- 58. Tilburgs T., Scherjon S. A., Roelen D. L., Claas F. H.. 2009. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum. Immunol. 70: 96–100. [DOI] [PubMed] [Google Scholar]
- 59. Tilburgs T., Schonkeren D., Eikmans M., Nagtzaam N. M., Datema G., Swings G. M., Prins F., van Lith J. M., van der Mast B. J., Roelen D. L., et al. 2010. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J. Immunol. 185: 4470–4477. [DOI] [PubMed] [Google Scholar]
- 60. Liu H. Y., Liu Z. K., Chao H., Li Z., Song Z., Yang Y., Peng J. P.. 2014. High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization. J. Interferon Cytokine Res. 34: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang L., Zhao M., Jiao F., Xu X., Liu X., Jiang Y., Zhang H., Ou X., Hu X.. 2015. Interferon gamma is involved in apoptosis of trophoblast cells at the maternal-fetal interface following Toxoplasma gondii infection. Int. J. Infect. Dis. 30: 10–16. [DOI] [PubMed] [Google Scholar]
- 62. Ledingham M. A., Thomson A. J., Jordan F., Young A., Crawford M., Norman J. E.. 2001. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet. Gynecol. 97: 235–242. [DOI] [PubMed] [Google Scholar]
- 63. Sadowsky D. W., Adams K. M., Gravett M. G., Witkin S. S., Novy M. J.. 2006. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am. J. Obstet. Gynecol. 195: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 64. Goldenberg R. L., Hauth J. C., Andrews W. W.. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 65. Pollard J. K., Mitchell M. D.. 1996. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. Am. J. Obstet. Gynecol. 174: 682–686. [DOI] [PubMed] [Google Scholar]
- 66. de Jong S. D., Basha G., Wilson K. D., Kazem M., Cullis P., Jefferies W., Tam Y.. 2010. The immunostimulatory activity of unmethylated and methylated CpG oligodeoxynucleotide is dependent on their ability to colocalize with TLR9 in late endosomes. J. Immunol. 184: 6092–6102. [DOI] [PubMed] [Google Scholar]
- 67. Ershova E., Sergeeva V., Klimenko M., Avetisova K., Klimenko P., Kostyuk E., Veiko N., Veiko R., Izevskaya V., Kutsev S., Kostyuk S.. 2017. Circulating cell-free DNA concentration and DNase I activity of peripheral blood plasma change in case of pregnancy with intrauterine growth restriction compared to normal pregnancy. Biomed. Rep. 7: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.