Abstract
The antibacterial activity and mechanisms of Australian propolis ethanol extract (APEE) against methicillin-resistant Staphylococcus aureus (MRSA) were investigated herein. The diameter of inhibition zones (DIZ) of APEE was 19.7 mm, while the minimum inhibition concentration (MIC) and minimum bactericide concentration (MBC) of APEE were both 0.9 mg/mL against the tested strain of MRSA. Nucleic acid leakage and propidium iodide (PI) staining assays showed that APEE can stimulate the release of intracellular nucleic acids by disrupting the integrity of the cell wall and cytoplasmic membrane. Scanning electron microscopy (SEM) further confirmed that APEE could depress cellular activities via damaging the cell structure, including the cell wall and membrane. Western blot analysis and β-lactamase activity assay showed that APEE could inhibit the expression of PBP2a and reduce the activity of β-lactamase, suggesting that APEE is able to reverse the drug resistance of MRSA. XTT and crystal violet (CV) assays indicated that APEE had the capacity to prevent the formation of biofilms through decreasing cellular activities and biomass. Bacterial adhesion assay revealed that APEE could reduce the adhesive capacity of the strain, belonging to its antibiofilm mechanisms. Furthermore, nine main compounds of APEE were identified and quantified by HPLC–DAD/Q-TOF–MS. The results above all verified that the antibacterial activity of APEE against MRSA was mainly due to disrupting cell structure, reversing resistance, and resisting biofilm formation, which indicates that APEE is expected to be an efficient functional ingredient with great potential application in the field of medicine and food.
Keywords: Australian propolis, Methicillin-resistant Staphylococcus aureus, Cell structure, Drug resistance, Biofilm
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most prominent human pathogens, and can cause soft tissue infection, septicemia, pneumonia, and other malignant diseases. The high mortality of MRSA infections has made it a serious hazardous pathogen that threatens hospitalized patients worldwide [1, 2]. Unfortunately, the resistant capability and range of MRSA have increased significantly due to the abuse of antibiotics and other antibacterial drugs in recent years. To this day, MRSA has become resistant to β-lactams, macrolides, tetracyclines, aminoglycosides, fluoroquinolones, sulfanilamide, and other broad-spectrum antibacterial drugs [3]. In addition to the resistance of planktonic cells to antibiotics, the formation of a biofilm is another key factor for the survival and persistence of MRSA in its hosts [4, 5]. Many reports have shown that mature biofilm formation is a major virulence factor during infection, providing defense against the host’s immune response and protecting bacterial cells from antibacterial agents [6, 7]. The ability to form a biofilm gives MRSA a dual drug resistance and further reduces the effectiveness of antibiotics and other antibacterial drugs.
However, the more serious problem is that the rate of detection and separation of MRSA is increasing year by year, and the epidemic scope is also obviously expanding [8]. As early as 2013, the China Antimicrobial Surveillance Network (CHINET) data showed that Staphylococcus aureus accounted for 9.61% of the total number of pathogens, of which the average detection rate of MRSA was 45.2%, and the highest reached 72% in hospital and community infections. The situation abroad is also serious; for example, in the USA in 2010 and Europe in 2015, the detection rate of MRSA accounted for 51.3% and 16.8%, respectively, of Staphylococcus aureus that caused human infection, and the proportion was still increasing [9]. The serious multidrug resistance and high detection rate of MRSA have made the research and development for novel potential antagonists against MRSA urgent [10].
Previous studies have demonstrated that propolis is an important natural product with diverse biological activities. It is a resinous substance collected by honeybees from secretions and buds of multiple plants mixed with wax and enzymes from the bees [11]. From the perspective of pharmacy, propolis has been widely used in human and animal medicines in China and various types of propolis have been included in the “Pharmacopoeia of the People’s Republic of China.” In regard to its chemical composition, propolis contains more than 600 natural ingredients belonging to over 20 categories, which are highly complex and variable [12]. Propolis can be classified into six main types: poplar propolis, birch propolis, Brazilian green propolis, red propolis, pacific propolis, and Canarian propolis [13]. Until now, propolis has exhibited a broad spectrum of biological properties, such as antibacterial, antioxidation, antitumor, and anti-inflammatory activities, and regulation of immunity, hypoglycemia, hypolipidemia, etc. [14–16]. Of these, the significant antibacterial activity of various types of propolis is especially notable for its potential medical properties [17–19]. However, Australian propolis has not been thoroughly investigated in the antibacterial field, and the antibacterial activity and mechanism of Australian propolis against MRSA still remain unclear. Hence, a study on the anti-MRSA activity of Australian propolis could provide scientific theoretical guidance for its potential applications in the fields of medicine and food.
Considering the mentioned aspects above, this work was mainly focused on the antibacterial activity of Australian propolis ethanol extract (APEE) against MRSA. After evaluating the antibacterial activity of APEE against MRSA, this study further elucidated the mechanisms of APEE against MRSA in terms of its interaction with the integrity of the cell wall and membrane, the vitally important bacterial barrier serving as a defense against antibacterial drugs. Then, the effect of APEE on PBP2a and β-lactamase of MRSA, which is the key resistance mechanisms of MRSA, was also determined. Subsequently, the effect of APEE on biofilm formation, which endows MRSA much more resistance against antibacterial agents, was investigated. Finally, the chemical composition of APEE was analyzed by HPLC–DAD/Q-TOF–MS. To the best of our knowledge, the antibacterial activity and mechanisms of APEE against MRSA have not yet been reported.
Materials and methods
Propolis materials and bacterial strain
The propolis was collected from New South Wales in southeast Australia in 2018. The main plant origin was xanthorrhoea. Methicillin-resistant Staphylococcus aureus (MRSA; ATCC 43,300) was purchased from Guangdong Microbiology Culture Center of China and maintained in trypticase soy agar (TSA) slants at 4 °C.
Preparation of Australian propolis ethanol extract (APEE)
The extraction method of propolis was based on the published literature [20]. Briefly, the Australian propolis sample was extracted with 95% (v/v) ethanol for 24 h. The extracting solution was filtered and concentrated in a rotary evaporator at 40 °C until it reached a constant weight. Then, the samples were filtered through 0.22 μm filter membranes to remove bacterial cells and conserved at − 20 °C.
Antibacterial activity assessments
Determination of inhibition zone diameter (DIZ)
The method for measuring DIZ was based on the published report [21]. Briefly, 100 μL of logarithmic phase bacterial cell suspension of MRSA (105 CFU/mL) was spread on the TSA plate. Sterile filter paper disks (6 mm) impregnated with 1 mg/disk APEE (dissolved in TSB containing 2% tween 80) were dried and placed on the surface of TSA and incubated at 37 °C for 24 h. The DIZs of control (TSB), 2% tween 80, and gentamicin (1 mg/disk) against MRSA were also detected.
Determination of minimal inhibitory concentration (MIC)
The MIC of APEE against MRSA was measured by resazurin microdilution staining assay, as recommended by a previous study with minor modifications [22]. Briefly, MRSA was cultured in trypticase soy broth (TSB) overnight at 37℃ to acquire logarithmic phase bacterial suspension. Then, 180 μL of APEE (dissolved in TSB) at different concentrations was mixed with 20 µL of bacterial suspension (105 CFU/mL) of MRSA in a 96-well plate, giving the final concentrations of APEE from 0.05625 to 1.8 mg/mL. The plate was incubated again at 37℃ for 24 h. Finally, 20 µL of resazurin sodium (1 mg/mL) was added per well and incubated in the dark at 37℃ for 3 h. Tween 80 was used in every well to a final concentration of 2% to dissolve the APEE. TSB only containing 2% tween 80 (control) or gentamicin (50 μg/mL) was also observed. The MIC was the lowest concentration of APEE that prevented the solution turning from purple to orange.
Determination of minimum bactericidal concentration (MBC)
Bacterial suspensions (100 μL) of MRSA treated with APEE (0.9 and 1.8 mg/mL) where there was no bacterial growth in the above wells were spread on TSA plates and sub-cultured. The surviving colonies were observed. MBC was the lowest concentration at which there was no growth after sub-culturing using fresh TSA plates.
Growth curves
The effects of APEE on the growth of MRSA were detected according to the reported literature with slight modifications [23]. In brief, the logarithmic phase cells of MRSA were collected and added into a 24-well plate containing 200 μL of sterile TSB to keep the bacterial concentration of 105 CFU/mL. Afterwards, the bacterial suspensions were exposed to different concentrations of APEE (control, 1/4 MIC, 1/2 MIC, MIC, and 2 MIC) and incubated at 37 °C for 24 h. The growth of bacterial cells was measured every 2 h at OD600nm using a microplate reader (ELX808, BioTek, USA). Samples without APEE but with 2% tween 80 were used as the controls.
Cell damage assessments
Leakage of nucleic acid assay
The leaking of nucleic acids was assessed using the relevant method according to previous literature [24]. In brief, logarithmic phase bacterial cells of MRSA (107 CFU/mL) were collected, washed, and resuspended in phosphate-buffered saline (PBS, pH 7.4). The above suspension was treated with different concentrations of APEE (control, MIC, and 2 MIC). Afterwards, the mixed suspension was filtered through 0.22-μm filter membranes and the supernatant was centrifuged at 12,000 rpm for 10 min. Then, the above precipitate was resuspended in PBS (pH 7.4) and detected at 260 nm by a microplate reader. The controls were without APEE but with 2% tween 80.
Propidium iodide (PI) staining assay
The PI staining assay method was based on the published literature with slight modifications [25]. In brief, logarithmic phase bacterial cells of MRSA (107 CFU/mL) were pre-collected, washed, and resuspended in PBS (pH 7.4). Then, the suspensions were incubated with different levels of APEE (control, MIC, and 2 MIC). After this, PI was added into the suspension to a final concentration of 2.5 μg/mL and incubated in the dark for 10 min. Then, 100 μL of stained suspension was transferred to special dishes for confocal laser scanning microscopy (CLSM). Finally, the dishes were observed and photographed using a CLSM (FV1200, Olympus, Japan).
Scanning electron microscopy (SEM) observation
The detection method of SEM was based on our published paper [26].
Resistance reversal assessments
Western blotting analysis
Western blotting assay was performed as previously described [20]. In brief, the pre-cultured bacterial cells were pre-collected, washed, and resuspended in PBS (pH 7.4). Then, the suspensions were incubated with different levels of APEE (control, 1/2 MIC, MIC, and 2 MIC). After that, lysozyme and protease inhibitors were added to the suspensions. The protein concentration was measured by a BCA protein assay kit. Twenty micrograms of protein was separated by 12% SDS-PAGE and electro-blotted to a PVDF membrane using a semi-dry blotting apparatus (Bio-Rad, USA). The bands were visualized using an enhanced chemiluminescence detection kit (Thermo Electron Corporation, USA).
Determination of β-lactamase activity
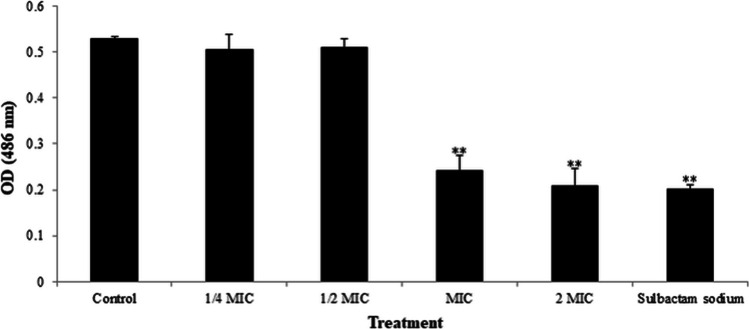
The detection method of APEE on the activity of β-lactamase produced by MRSA was based on the published literature with a few modifications [27]. This assay was divided into three parts: (1) preparation of bacterial suspension. MRSA were cultured in trypticase soy broth (TSB) overnight at 37℃ and logarithmic phase bacterial suspensions were collected and diluted to 107 CFU/mL. (2) Induction and extraction of β-lactamase. Oxacillin sodium was added to the above bacterial suspension to keep the final concentration of 128 μg/mL, and the bacterial cells were incubated again. After this, the bacterial suspension was harvested by centrifugation at 3000 rpm for 15 min to obtain the cells of MRSA. Next, the cells were washed three times and resuspended in 0.9% NaCl solution. Finally, the cells were broken by ultrasound using an ultrasonic cleaning machine and the bacterial suspensions were centrifuged again to obtain the supernatants. (3) Determination of β-lactamase activity. The above supernatants were treated with different concentrations of APEE (control, 1/4 MIC, 1/2 MC, MIC, and 2 MIC) and 2 mL of the treated supernatants was transferred to the sterilized centrifuge tubes and incubated at 37 °C for 30 min. Then, 200 μL nitrocefin (0.5 mg/mL) was added to each tube and incubated at 37 °C for another 10 min. The absorbance was measured at 486 nm. The supernatant containing 1 mg/mL sulbactam sodium was used as the positive control and the negative control was supernatant with 2% tween 80 without APEE.
Biofilm formation inhibition assessments
Biofilm formation and treatment
The formation method of MRSA biofilms was based on the published literature [28]. Briefly, 180 μL of TSB with different concentrations of APEE (control, 1/4 MIC, 1/2 MC, MIC, and 2 MIC) and 20 μL of bacterial suspensions of MRSA per well were added into 96-well microplates and incubated at 37 °C for 24 h. Meanwhile, sucrose was added to all wells to a final concentration of 1% to promote biofilm formation. After that, planktonic cells were removed and all wells were washed three times with PBS (pH 7.4). The controls were MRSA in TSB without APEE but with 2% tween 80.
XTT reduction assay
The activities of cells in MRSA biofilms were evaluated according to our previous literature [25].
Crystal violet (CV) assay
The total biofilm biomasses of MRSA were assessed using the CV assay according to our pertinent literature [26].
Effect of APEE on bacterial adhesion
The experiment was carried out according to relevant literature [28]. In brief, 100 μL of MRSA overnight culture in TSB supplemented with 2% glucose was added to the wells of a 96-well plate, followed by the equal volume of TSB with different concentrations of APEE (control, 1/4 MIC, 1/2 MC, MIC, and 2 MIC).The plates were incubated at 37 °C for 4 h for the bacteria adhesion. Then, the plates were washed by sterile water to discard the planktonic cells and the optical density of the wells was measured at 600 nm to quantify the amount of the cells adhered to the plates.
HPLC–DAD/Q-TOF–MS analysis
The chemical constituents of APEE were analyzed as previously described by using HPLC–DAD/Q-TOF–MS (UItive Triple Quad LC/MS G6465A, Agilent, USA) [11]. Briefly, InfinityLab Poroshell SB-C18 (3.0 × 100 mm, 2.7 µm) was used in this chromatographic test. The mobile phases were water (containing 0.1% methanol, A) and methanol (B). The gradient elution procedure was set as follows: 0–1 min 5% B; 1–6 min 5–55% B; 6–20 min 55–95% B; 20–21.5 min 95–5% B. The sample concentration, injection volume, column temperature, and flow rate were 1 g/L, 2 μL, 35 °C, and 0.25 mL/min, respectively. The optimal MS condition was as follows: electrospray ionization (ESI); the temperature, flow rate, and nebulizer pressure were 270 °C, 12.0 L/min, and 25 psi, respectively.
Statistical analysis
All assays were performed in triplicate at least and all data were expressed as means ± standard error. The statistical analysis was executed by one-way ANOVA. Experiments were considered significant at p < 0.05.
Results
Antibacterial activity
The antibacterial activity of APEE against MRSA was mainly indicated by DIZ, MIC, and MBC. The values of DIZ were qualitatively assessed for the anti-MRSA activity of APEE, while the values of MIC and MBC were quantitatively evaluated. The DIZ of APEE (1 mg/disk) determined by the filter paper disk diffusion method is shown in Table 1. The DIZ values (19.7 mm) indicated that APEE exhibited significant antibacterial activity against MRSA. And 2% tween 80 (negative control) and gentamicin (1 mg/disk) had no antibacterial effect on the strain tested.
Table 1.
The DIZ, MIC, and MBC of APEE against MRSA
| Strain | DIZ (mm)a | MIC (mg/mL) | MBC (mg/mL) | |||
|---|---|---|---|---|---|---|
| Control | 2% tween 80 | APEE | Gentamicin | |||
| MRSA | 6 | 6 | 19.7 ± 1.2 | 6 | 0.9 | 0.9 |
aDIZ: the value indicated as an average of six replicates ± standard error. DIZ diameter of inhibition zone, MIC minimum inhibition concentration, MBC minimum bactericide concentration
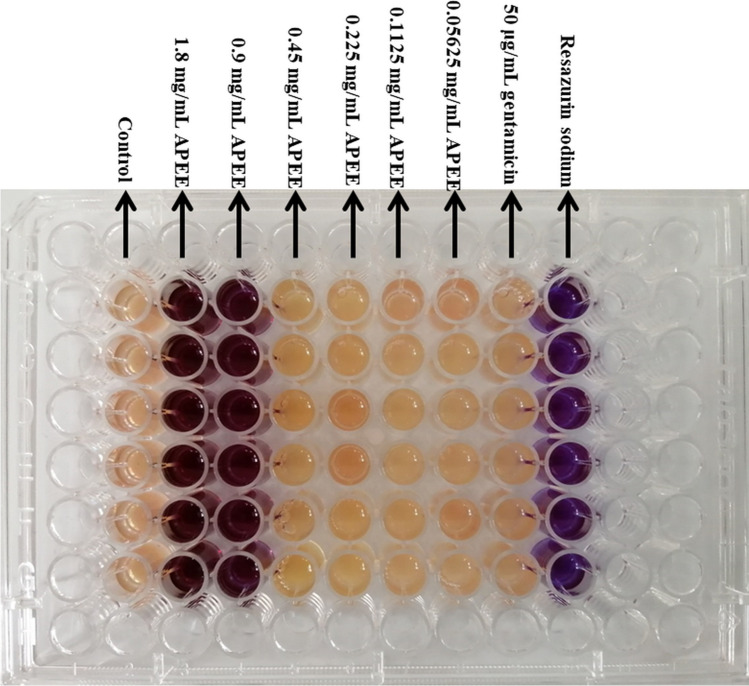
The MIC was determined using the method of resazurin microdilution staining, which can react with live bacterial cells and change the culture color from purple to orange. As shown in Fig. 1 and Table 1, the MIC value of APEE against MRSA was 0.9 mg/mL. The working concentration of gentamicin (50 μg/mL) did not show any inhibitory effect on MRSA, confirming the effective resistance of MRSA, which was consistent with the result of the above assay. The MBC value of APEE detected by the agar dilution method was also 0.9 mg/mL, which was shown to be the same as its MIC (Table 1).
Fig. 1.
Detection of MIC for APEE against MRSA. The lowest concentration that could prevent the change in the color of the solution from purple to orange was detected as the MIC. The control was TSB with bacterial cells of MRSA and 2% tween 80; the groups of APEE were TSB with bacterial cells, 2% tween 80, and different concentrations of APEE; the group of gentamicin was TSB with bacterial cells and 50 μg/mL gentamicin; the group of resazurin sodium only contained the resazurin sodium solution without bacterial cells
To further confirm the anti-MRSA activity of APEE, the growth curves of MRSA in the presence or absence of APEE were plotted. As shown in Fig. 2A, the number of MRSA cells did not increase at all with the levels of MIC and 2 MIC of APEE during the incubation period. Meanwhile, the 1/4 MIC and 1/2 MIC of APEE were unable to inhibit the increase of MRSA cells. Moreover, other results showed that the control and all treatments had a lag phase in the first 2 h, and MRSA treated with the levels of 1/4 MIC and 1/2 MIC of APEE showed delayed growth into the phases of logarithmic, stabilization, and decline compared with the control, which suggests that the low concentrations of APEE could delay the growth cycle of MRSA in a dose-dependent manner. These results indicated that APEE exhibited significant inhibitory activity against MRSA.
Fig. 2.
Effect of different concentrations of APEE on A cell growth of MRSA and B release of nucleic acids from MRSA. Error bars indicate standard error of the mean; where error bars are not visible, they are smaller than the symbol
Cell damage studies
The release of cellular components, especially macromolecular components, can reveal the integrity of bacterial cells, including the cell wall and cytoplasmic membrane. Nucleic acids, such as DNA and RNA, are macromolecules, and the extravasation of nucleic acids is a good method to evaluate the permeability and the integrity of the cellular wall and membrane. In our study, the phenomenon of leakage of nucleic acids was obvious after treatment with APEE at levels of MIC and 2 MIC, while there was no apparent nucleic acid leak in the group treated with 1/4 MIC APEE compared with the control (Fig. 2B). The absorbance values (OD260nm) increased from 0.019 (control) to 0.239 (MIC) and 0.301 (2 MIC) at 12 h. In the interval between 12 and 21 h, the absorbance values of all treatments stopped increasing and remained stable, and there was a slight drop in the last 3 h, indicating that nucleic acids began to degrade. These results clearly suggested that the integrities of the cell wall and membrane of MRSA were disrupted in the presence of APEE, which can consequently bring death to bacterial cells.
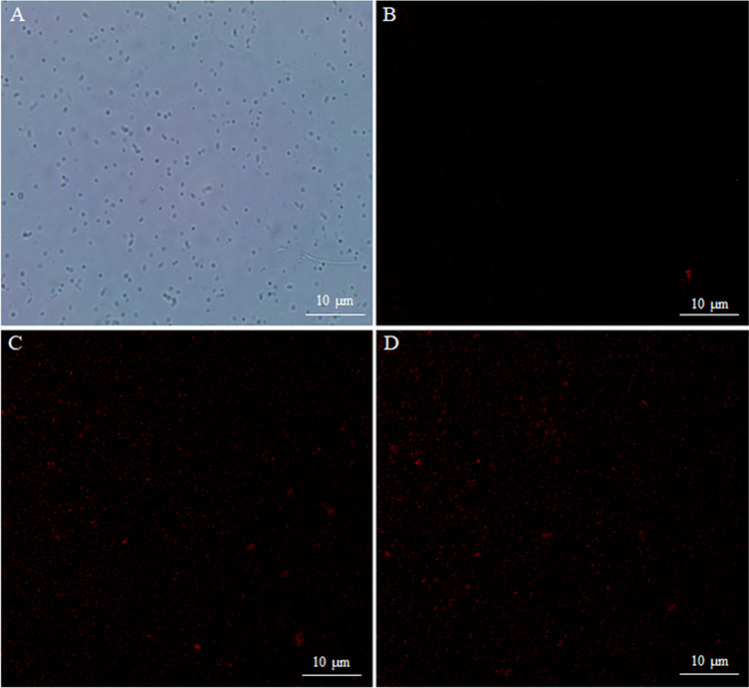
Propidium iodide (PI) is a highly sensitive fluorescent dye that can combine with DNA and release red fluorescence. However, PI can only pass through the damaged cell membrane but not the integral cell membrane. Therefore, the assay of PI fluorescence staining is used to confirm the integrity of the cell membrane [29]. In our study, bacterial cells of MRSA in the logarithmic phase were collected and stained in the presence and absence of APEE. As shown in Fig. 3, the notable phenomenon of red fluorescence effects was observed from the cells treated with the MIC and 2 MIC of APEE (Fig. 3C, D) compared with the control (Fig. 3B). Meanwhile, the fluorescence photographs (Fig. 3B) and optical microscopy (Fig. 3A) proved that most experimental bacterial cells were alive. These results also indicated that APEE could effectively destroy the structure and function of bacterial cells, which was very similar to the results of the nucleic acid leakage test.
Fig. 3.
Fluorescence microscopy photos of MRSA treated with different concentrations of APEE. The photo of A is bacteria untreated with APEE observed under an optical microscope; B–D were bacterial cells treated with different concentrations of APEE observed under a CLSM: B control, C MIC, D 2 MIC
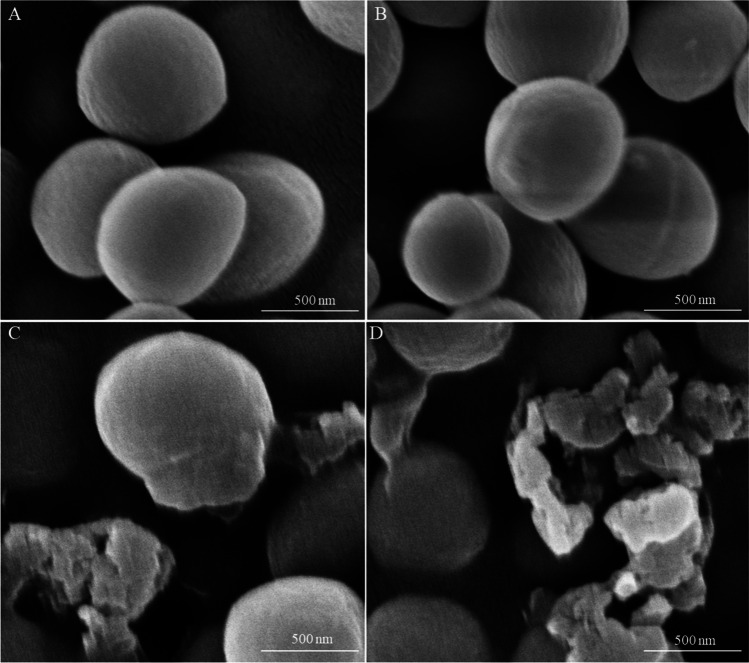
In order to display the morphological changes of bacterial cells intuitively, the cells of the tested strain in the presence and absence of APEE were observed by SEM. As shown in Fig. 4, the photos of MRSA treated with APEE (MIC and 2 MIC) showed that acute changes of morphology had taken place (Fig. 4C, D). For the control and cells treated with 1/2 MIC of APEE, the MRSA cells showed a regular shape, smooth surface, and distinctive characteristics (Fig. 4A, B). However, the surface of cells became wrinkled and irregular, and some cells even appeared to have extensive fractures that resulted in the leaking of contents after treatment with APEE at levels of MIC and 2 MIC. These results were consistent with the above experiments on the integrity of cellular structure.
Fig. 4.
SEM photos of MRSA treated with different concentrations of APEE. A Control. B 1/2 MIC. C MIC. D 2 MIC
Resistance reversal study
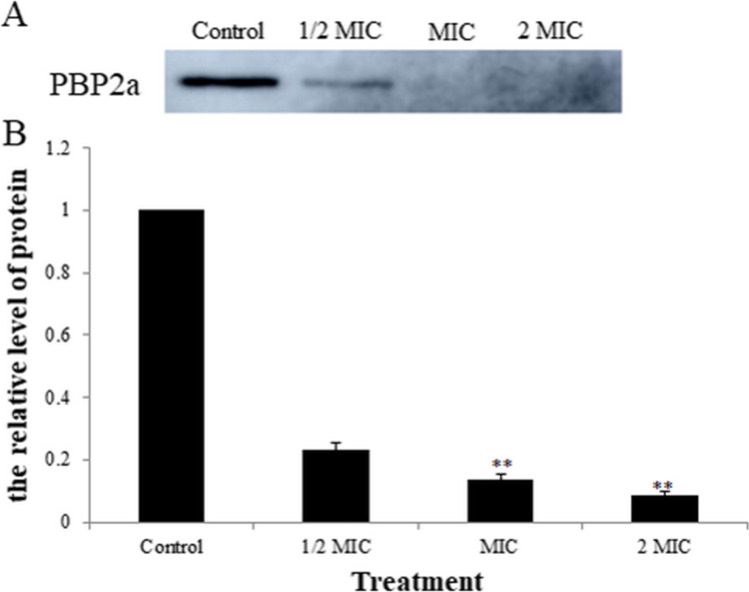
The most important mechanisms of MRSA drug resistance are the high expression of PBP2a and β-lactamase. In this part, the effect of APEE on the expression of PBP2a of MRSA was analyzed by western blot assay and the results are shown in Fig. 5. The expression levels of PBP2a in the groups treated with APEE (1/2 MIC, MIC, and 2 MIC) obviously decreased compared with the control, which implied that APEE significantly inhibited the expression of PBP2a in MRSA. The effect of APEE on β-lactamase produced by MRSA was studied by the method of competition inhibition and sulbactam sodium was used as an inhibitor. As shown in Fig. 6, the OD values of β-lactamase extraction liquid treated with APEE at levels of MIC and 2 MIC decreased significantly and were similar with the results of the sulbactam sodium treatment group, while the OD values of β-lactamase extract treated with APEE at levels of 1/4 MIC and 1/2 MIC were in accordance with the control. These results indicated that APEE was capable of inhibiting the expression of PBP2a and decreasing the activity of β-lactamase of MRSA.
Fig. 5.
Effect of different concentrations of APEE on the expression of PBP2a of MRSA. A The expression of PBP2a. B Quantification of relative quantity of PBP2a. Data are presented as the mean ± standard error based on three individual tests (**p < 0.01 vs. control)
Fig. 6.
Effect of different concentrations of APEE on β-lactamase produced by MRSA. Data are presented as the mean ± standard error based on three individual tests (**p < 0.01 vs. control)
Antibiofilm studies
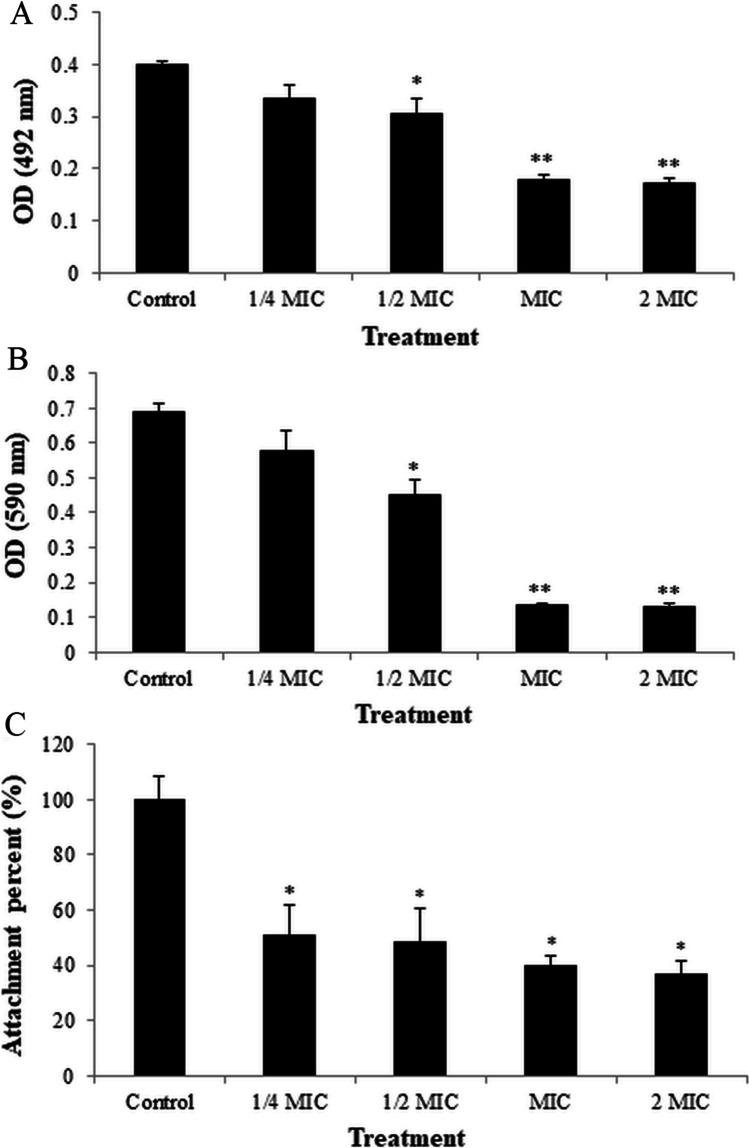
As shown in Fig. 7A, the activities of cells in MRSA biofilms were remarkably different among the treatments measured by the XTT reduction test. APEE at levels of 1/2 MIC, MIC, and 2 MIC decreased the activities of cells in the biofilm significantly, while there was no obvious decrease in cellular activities of the groups treated with APEE (1/4 MIC) compared with the control.
Fig. 7.
Effect of different concentrations of APEE on A cellular activities in MRSA biofilms determined by XTT reduction assay, B biofilm biomass of MRSA quantified by CV assay, C attachment percent of MRSA detected by attachment assay (the control was set to 100%). Data are presented as the mean ± standard error based on three individual tests (*p < 0.05, **p < 0.01 vs. control)
The change in biofilm biomass of MRSA detected using the crystal violet test is shown in Fig. 7B. The biofilm biomass of the groups treated with APEE at levels of 1/2 MIC, MIC, and 2 MIC decreased significantly compared with control, while there was no apparent decrease in other groups (1/4 MIC) treated with APEE, which agreed with the results of the XTT reduction assay. These results indicated that APEE caused a distinct inhibitory effect on MRSA biofilm formation.
As shown in Fig. 7C, the results indicated that APEE significantly inhibited the adhesion of MRSA to solid surface ranging from 1/4 MIC to 2 MIC. It was shown that APEE not only inhibited cell growth but also affected the adhesion on solid surface, which may be its initial mechanism against MRSA biofilm.
Major chemical composition of APEE
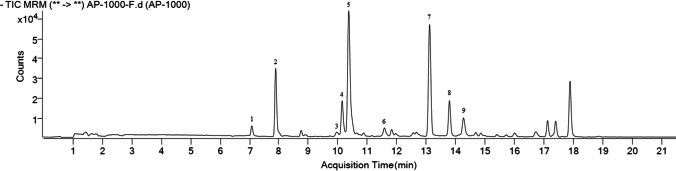
To better understand the chemical composition of APEE, the major polyphenolic constituents were identified by HPLC–DAD/Q-TOF–MS. The main compounds and their relative concentrations in APEE are presented in Fig. 8 and Table 2. As shown in the figure and table, the nine major components were caffeic acid, p-coumaric acid, quercetin, naringenin, pinobanksin, apigenin, pinocembrin, caffeic acid phenethyl ester (CAPE), and galangin. To detect the bioactive components of APEE on antibacterial activity against MRSA, we determined the effects of the nine main polyphenolic compounds (caffeic acid, p-coumaric acid, quercetin, naringenin, pinobanksin, apigenin, pinocembrin, CAPE, and galangin) from APEE on MRSA. The results showed that the MICs of these compounds against MRSA all exceeded the maximum concentration of the detection gradient (3.6 mg/mL), which indicated that none of these compounds exhibited a significant inhibitory effect against MRSA. The results also suggested that the antibacterial activity of APEE may be not the efficacy of a single component, but the comprehensive effect of multiple components.
Fig. 8.
HPLC–DAD/Q-TOF–MS analysis of APEE. Major peaks (compounds 1–9) were caffeic acid, p-coumaric acid, quercetin, naringenin, pinobanksin, apigenin, pinocembrin, CAPE, and galangin
Table 2.
Major compounds identified from APEE by HPLC–DAD/Q-TOF–MS
| Peak number | Retention time (min) | Name of the compounds | Molecular formula | Molecular weight | Content ( mg/g) |
|---|---|---|---|---|---|
| 1 | 7.077 | Caffeic acid | C9H8O4 | 180 | 0.3326 |
| 2 | 7.892 | P-Coumaric acid | C9H8O3 | 164 | 1.6208 |
| 3 | 9.970 | Quercetin | C15H10O7 | 302 | 0.5585 |
| 4 | 10.142 | Naringenin | C15H12O5 | 272 | 0.8519 |
| 5 | 10.373 | Pinobanksin | C15H12O5 | 272 | 20.1341 |
| 6 | 11.585 | Apigenin | C15H10O5 | 270 | 0.4972 |
| 7 | 13.121 | Pinocembrin | C15H12O4 | 256 | 14.9326 |
| 8 | 13.796 | CAPE | C17H16O4 | 284 | 0.5849 |
| 9 | 14.275 | Galangin | C15H10O5 | 270 | 11.0414 |
CAPE caffeic acid phenethyl ester
Discussion
This research was carried out in view of the harmfulness of MRSA and the versatility of the biological activity of propolis. In our study, the three indexes DIZ, MIC, and MBC and the growth curves assay were used to assess the antibacterial capacity against MRSA of APEE. The results show its effective inhibitory activity against MRSA. Until now, there is no report on the antibacterial activity against MRSA of Australian propolis, so we cannot compare the anti-MRSA activity of Australian propolis in the present work with other propolis from Australia directly. Despite the lack of research on the anti-MRSA activity of Australian propolis, several other propolis types have been studied in the field of anti-MRSA. El-Guendouz et al. showed that Moroccan propolis exhibited a strong inhibitory effect on MRSA; Chen et al. reported that Taiwanese green propolis also displayed significant antibacterial property against MRSA [19, 30]. These results were consistent with our results showing the remarkable anti-MRSA activity of APEE. Furthermore, we found that APEE showed weak antibacterial ability to Escherichia coli (ATCC 25,922) and Salmonella typhimurium (ATCC 14,028) (data not shown), which is quite different with the inhibitory effect on MRSA. This result may be caused by the structural difference between the cell wall of Gram-positive and Gram-negative bacteria. The cell wall of Gram-positive bacteria has a single peptidoglycan layer structure, which only weakly prevents the invasion of foreign molecules. The cell wall structure of Gram-negative bacteria including the outer membrane, outer membrane protein, peptidoglycan, and other structural components is more complicated. Among the components of the cell wall of Gram-negative bacteria, the lipopolysaccharide in the outer membrane layer has been reported to express significant resistance to antibacterial materials [24, 31–33]. Therefore, the difference in the structure of the cell wall may be one of the reasons for the different antibacterial capacities of APEE against E. coli, S. typhimurium, and MRSA.
The cell wall and membrane both play vital roles in bacterial cells. When the cellular structure, permeability, or integrity is damaged, the micromolecules and macromolecules will be leaked out of the cells in sequence [34]. The small molecules are mainly inorganic ions such as H+, K+, and Na+, which play enormously important roles in facilitating the function of the cytoplasmic membrane, and maintaining the activities of multiple enzymes and the normal operation of metabolism [35]. In addition, the homeostasis of these small molecular ions is crucial for maintaining intracellular energy stabilization as it is essential for energy-related processes such as solute transport and metabolic control [36]. Hence, leaking of these micromolecules could easily cause the death of cells in multiple ways [37]. The macromolecules are principally proteins and nucleic acids that exist throughout the membrane and cytoplasm of bacterial cells. Proteins provide the structural information of bacterial cells, except for those acting as enzymes, while the core function of nucleic acids is to carry genetic information. Furthermore, proteins and nucleic acids both also participate in the transmission of genetic information including DNA replication, transcription, and translation [38]. In our study, we detected and analyzed the leakage of nucleic acids of MRSA when exposed to different concentrations of APEE. The leak of nucleic acids after treatment with APEE was obvious and it also implies that micromolecules had been released at an earlier time. The above results proved that the permeability and integrities of the cell wall and cytoplasmic membrane were disrupted by APEE which would cause the death of bacterial cells. These results were in accordance with the conclusion of the PI staining experiment and a previous study of bacterial cell damage caused by Brazil green propolis [39]. SEM was also performed in this work to exhibit structural changes of bacterial cells intuitively and the result once again confirmed that APEE could destroy the integrity of the structure and function of the cells. The SEM result was in agreement with the above assays and some other antibacterial materials against pathogens [37, 40].
Due to the effective drug resistance of MRSA, domestic and foreign researchers have studied its mechanisms of resistance, which include the following aspects: (1) producing a specific drug-resistant protein named penicillin binding protein 2a (PBP2a) expressed by mec A, which has low affinity for β-lactam antibiotics and can function as a transpeptidase to maintain the synthesis of the cell wall [41]; (2) secreting large amounts of β-lactamase to promote the hydrolysis of β-lactam antibiotics [42]; and (3) decreasing drug accumulation by enhancing the expression of efflux pump-related genes to promote drugs efflux [43]. In our study, the ability of APEE to inhibit the expression PBP2a and decrease the β-lactamase activity was found, which meant our tested material could reverse the resistance of MRSA. This result revealed that APEE could be combined with ampicillin, methicillin, and other β-lactam antibiotics to give more significant effects.
The results of XTT and CV assays showed that APEE can prevent the formation of MRSA biofilms at levels of 1/2 MIC, MIC, and 2 MIC. In fact, almost all antimicrobial agents have much weaker inhibition of bacteria in biofilms than that of planktonic bacteria, indicating that bacterial cells in biofilm have better resistance to antimicrobials [44, 45]. Therefore, antibacterial materials with excellent inhibitory effect to biofilms will have greater application potential. Up to now, the mechanisms of biofilm resistance mainly include the following aspects: (1) osmotic restriction theory: Extracellular polymers such as glycoproteins (EPS) produced by bacteria surround biofilm cells, which are natural barriers to prevent antimicrobial penetration [45]. (2) Nutrition restriction theory: According to this theory, most bacteria grow slowly due to a nutrient deficiency in the biofilm, and the slower growth rate endows them better resistance than planktonic bacterial cells [46–48]. (3) Other theories include the degradation of antimicrobial agents and structural modification of antibacterial materials [45]. Unfortunately, most pathogenic bacteria including MRSA can spread diseases through biofilms, and bacterial infection mediated by biofilms has become more difficult in clinical treatment [49]. Fortunately, APEE in this study possessed significant inhibitory activity against planktonic bacterial cells and biofilm of MRSA, meaning that APEE has extremely high application value in the field of anti-MRSA. At the end of this work, the results of bacterial adhesion assay indicated that APEE weakened the adhesion of MRSA at all tested levels. The ability of bacteria to adhere is a key factor in the formation and maintenance of their biofilm. Furthermore, the bacterial adhesion is mainly related to polysaccharide intercellular adhesion (PIA) and extracellular adherence protein, regulated by ica operon (ica ADBCR) and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) [50]. The expression of these regulatory genes will be the focus of our next research.
This study suggests that APEE has prominent anti-MRSA activity, and the mechanisms were clarified by analyzing the integrities of the cell wall and membrane, the reversal of drug resistance, and the inhibitory effect on MRSA biofilms. The assays of the integrities of the cell wall and membrane revealed that APEE disrupted the cell wall and membrane of MRSA, causing a leak of nucleic acids which was further clarified by PI staining and SEM. The decrease of PBP2a expression level and β-lactamase activity confirmed the ability of APEE to reverse the drug resistance of MRSA. XTT and CV assays revealed that APEE possessed significant antibiofilm ability via the reduction of cellular activity in the biofilm and biofilm biomass. Bacterial adhesion assay revealed that the APEE was able to reduce the adhesive capacity of the tested strain, which was attributed to its antibiofilm mechanisms. All of these results indicate that APEE might be an attractive candidate in future clinical treatment.
Abbreviations
- APEE
Australian propolis ethanol extract
- CAPE
Caffeic acid phenethyl ester
- CV
Crystal violet
- DIZ
Diameter of inhibition zone
- MBC
Minimum bactericide concentration
- MIC
Minimum inhibition concentration
- MRSA
Methicillin-resistant Staphylococcus aureus
- PBS
Phosphate-buffered saline
- PI
Propidium iodide
- SEM
Scanning electron microscopy
- TSA
Trypticase soy agar
- TSB
Trypticase soy broth
- XTT
2,3-Bis (2-methyloxy 4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
Funding
This work was supported by grants from the following foundations: the National Natural Science Foundation of China (No. 31672499), the Modern Agricultural Technology System of Shandong Province (SDAIT-24–05), and the Doctoral Research Foundation of Liaocheng University (No. 318051826).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Wang and Hui Liu contributed equally to this work.
Contributor Information
Bin Jiang, Email: jiangbin1117@126.com, Email: hongzhuanxuan@163.com.
Hongzhuan Xuan, Email: jiangbin1117@126.com, Email: hongzhuanxuan@163.com.
References
- 1.Flora M, Perrotta F, Nicolai A, Maffucci R, Pratillo A, Mollica M, et al. Staphylococcus aureus in chronic airway diseases: an overview. Respir Med. 2019;155:66–71. doi: 10.1016/j.rmed.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Mera RM, Suaya JA, Amrine-Madsen H, Hogea CS, Miller LA, Lu EP, et al. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb Drug Resist. 2011;17(2):321–328. doi: 10.1089/mdr.2010.0193. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Wang XL, Zhang L, Shen W. Distribution and drug resistance of pathogenic bacteria in children with lower respiratory tract infection from Chengdu Children’s Hospital between 2001 and 2006. Chinese Journal of Contemporary Pediatrics. 2008;10(1):17–20. [PubMed] [Google Scholar]
- 4.Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014;4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azmi K, Qrei W, Abdeen Z. Screening of genes encoding adhesion factors and biofilm production in methicillin resistant strains of Staphylococcus aureus isolated from Palestinian patients. BMC Genomics. 2019;20(1):578. doi: 10.1186/s12864-019-5929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vancraeynest D, Herman K, Haesebrouck F. Genotypic and phenotypic screening of high and low virulence Staphylococcus aureus isolates from rabbits for biofilm formation and MSCRAMMs. Vet Microbiol. 2004;103(3–4):241–247. doi: 10.1016/j.vetmic.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Resch A, Rosenstein R, Nerz C, Gotz F. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol. 2005;71(5):2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Back KT, Grundling A, Mogensen RG, Thegersen L, Petersen A, Paulander W, et al. Beta-Lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob Agents Cemother. 2014;58(8):4593–4603. doi: 10.1128/AAC.02802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao XY, Cullen PJ, Liu DH, Muhammad AI, Chen SG, Ye XQ, et al. Combating Staphylococcus aureus and its methicillin resistance gene (mecA) with cold plasma. Sci Total Environ. 2018;645:1287–1295. doi: 10.1016/j.scitotenv.2018.07.190. [DOI] [PubMed] [Google Scholar]
- 10.Qin N, Tan XJ, Jiao YM, Liu L, Zhao WS, Yang S, et al. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci Rep. 2014;4:5467. doi: 10.1038/srep05467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan WW, Chang HS, Liu XY, Wang SQ, Liu H, Xuan HZ (2019) Brazilian green propolis inhibits Ox-LDL-stimulated oxidative stress in human umbilical vein endothelial cells partly through PI3K/Akt/mTOR-mediated Nrf2/HO-1 pathway. Evidence-based Complementary and Alternative Medicine 2019 5789574 [DOI] [PMC free article] [PubMed]
- 12.Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19(12):19610–19632. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133(2):253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Hu FL, Hepburn HR, Xuan HZ, Chen ML, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005;51(2):147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Braakhuis A. Evidence on the health benefits of supplemental propolis. Nutrients. 2019;11(11):2705. doi: 10.3390/nu11112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gezgin Y, Kazan A, Ulucan F, Yesil-Celiktas O. Antimicrobial activity of propolis and gentamycin against methicillin-resistant Staphylococcus aureus in a 3D thermo-sensitive hydrogel. Industrial Crops and Products. 2019;139:111588. [Google Scholar]
- 17.Ambi A, Vera C, Parikh N, Perez N, Rojas AL, Kumar S, et al. Plasma-initiated graft polymerization as an immobilization platform for metal free Russian propolis ethanol extracts designed specifically for biomaterials. Biofouling. 2019;34(5):557–568. doi: 10.1080/08927014.2018.1471467. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kim YG, Khadke SK, Yamano A, Woo JT, Lee J. Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine. 2019;63:153033. doi: 10.1016/j.phymed.2019.153033. [DOI] [PubMed] [Google Scholar]
- 19.Chen YW, Ye SR, Ting C, Yu YH. Antibacterial activity of propolins from Taiwanese green propolis. J Food Drug Anal. 2018;26(2):761–768. doi: 10.1016/j.jfda.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan HZ, Yuan WW, Chang HS, Liu MM, Hu FL. Antiinflammatory effects of Chinese propolis in lipopolysaccharide-stimulated human umbilical vein endothelial cells by suppressing autophagy and MAPK/NF-κB signaling pathway. Inflammopharmacology. 2019;27(3):561–571. doi: 10.1007/s10787-018-0533-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZF, He B, Zhou J, He DH, Deng JD, Zeng RH. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind Crops Prod. 2016;83:607–613. [Google Scholar]
- 22.Liu YC, Xu YJ, Song QH, Wang F, Sun LG, Liu L, et al. Anti-biofilm activities from Bergenia crassifolia leaves against Streptococcus mutans. Front Microbiol. 2017;8:1738. doi: 10.3389/fmicb.2017.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YP, Bai JR, Zhong K, Huang YN, Gao H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R, 3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017;218:463–470. doi: 10.1016/j.foodchem.2016.07.090. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YB, Liu XY, Wang YF, Jiang PP, Quek SY. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. [Google Scholar]
- 25.Yan FL, Dang QF, Liu CS, Yan JQ, Wang T, Fan B, et al. 3,6-O-[N-(2-Aminoethyl)-acetamide-yl]-chitosan exerts antibacterial activity by a membrane damage mechanism. Carbohydrate Polymer. 2016;149:102–111. doi: 10.1016/j.carbpol.2016.04.098. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Wei FY, Song CX, Jiang B, Tian SY, Yi JW, et al. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind Crops Prod. 2017;109:358–366. [Google Scholar]
- 27.Catteau L, Reichmann NT, Olson J, Pinho MG, Nizet V, Bambeke FV, et al. Synergy between ursolic and oleanolic acids from Vitellaria paradoxa leaf extract and–lactams against methicillin-resistant Staphylococcus aureus: in vitro and in vivo activity and underlying mechanisms. Molecules. 2017;22(12):2245. doi: 10.3390/molecules22122245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Li W, Zhu XY, Zhao HZ, Lu YJ, Zhang C, et al. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl Microbiol Biotechnol. 2019;103(11):4565–4574. doi: 10.1007/s00253-019-09808-w. [DOI] [PubMed] [Google Scholar]
- 29.Novo DJ, Perlmutter NG, Hunt RH, Shapiro HM. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob Agents Chemother. 2000;44(4):827–834. doi: 10.1128/aac.44.4.827-834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Guendouz S, Aazza S, Lyoussi B, Bankova V, Popova M, Neto L, et al. Moroccan propolis: a natural antioxidant, antibacterial, and antibiofilm against Staphylococcus aureus with no induction of resistance after continuous exposure. Evidence-based Complementary and Alternative Medicine. 2018;2018:9759240. doi: 10.1155/2018/9759240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salton MR (1953) Studies of the bacterial cell wall: IV. The composition of the cell walls of some gram-positive and gram-negative bacteria. Biochimica et Biophysica Acta 10(4) 512-523 [DOI] [PubMed]
- 32.Sikkema J, De Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Mol Biol Rev. 1995;59(2):201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Microbiol. 2011;148(1):66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23(16):3359–3368. doi: 10.1016/s0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 35.Diao WR, Hu QP, Zhang H, Xu JG. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill) Food Control. 2014;35:109–116. [Google Scholar]
- 36.Cox SD, Mann CM, Markham JL, Gustafson JE, Warmington JR, Wyllie SG. Determining the antimicrobial actions of tea tree oil. Molecules. 2001;6(2):87–91. [Google Scholar]
- 37.Sharma A, Bajpai VK, Baek KH. Determination of antibacterial mode of action of allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J Food Saf. 2013;33(2):197–208. [Google Scholar]
- 38.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Campos JV, Garrido Assis OB, Bernardes-Filho R. Atomic force microscopy evidences of bacterial cell damage caused by propolis extracts on E. coli and S. aureus. Food Science and Technology. 2020;40(1):55–61. [Google Scholar]
- 40.Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22:725–731. [Google Scholar]
- 41.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 42.Tazi A, Chapron J, Touak G, Longo M, Hubert D, Collobert G, et al. Rapid emergence of resistance to linezolid and mutator phenotypes in Staphylococcus aureus isolates from an adult cystic fibrosis patient. Antimicrob Agents Chemother. 2013;57(10):5186–5188. doi: 10.1128/AAC.01392-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nannini E, Murray BE, Arias CA. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol. 2010;10(5):516–521. doi: 10.1016/j.coph.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Melchior MB, Vaarkamp H, Fink-Gremmels J. Biofilms: a role in recurrent mastitis infections? Veterinary Journal. 2006;171:398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol. 2010;50(1):30–35. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 46.Miladi H, Mili D, Slama RB, Zouari S, Ammar E, Bakhrouf A. Antibiofilm formation and anti-adhesive property of three Mediterranean essential oils against a foodborne pathogen Salmonella strain. Microb Pathog. 2016;93:22–31. doi: 10.1016/j.micpath.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mah TC, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 49.Aiassa V, Zoppi A, Becerra MC, Albesa I, Longhi MR. Enhanced inhibition of bacterial biofilm formation and reduced leukocyte toxicity by chloramphenicol: β-cyclodextrin:N-acetylcysteine complex. Carbohyd Polym. 2016;152:672–678. doi: 10.1016/j.carbpol.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Kot B, Sytykiewicz H, Sprawka L, Witeska M. Effect of Manuka honey on biofilm-associated genes expression during methicillin-resistant Staphylococcus aureus biofilm formation. Sci Rep. 2020;10:13552. doi: 10.1038/s41598-020-70666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]