Abstract
Neurosecretory protein VGF (non-acronymic) belongs to the granin family of neuropeptides. VGF and VGF-derived peptides have been repeatedly identified in well-powered and well-designed multi-omic studies as dysregulated in neurodegenerative and psychiatric diseases. New therapeutics is urgently needed for these devastating and costly diseases, as are new biomarkers to improve disease diagnosis and mechanistic understanding. From a list of 537 genes involved in Alzheimer’s disease pathogenesis, VGF was highlighted by the Accelerating Medicines Partnership in Alzheimer’s disease as the potential therapeutic target of greatest interest. VGF levels are consistently decreased in brain tissue and CSF samples from patients with Alzheimer’s disease compared to controls, and its levels correlate with disease severity and Alzheimer’s disease pathology. In the brain, VGF exists as multiple functional VGF-derived peptides. Full-length human VGF1–615 undergoes proteolytic processing by prohormone convertases and other proteases in the regulated secretory pathway to produce at least 12 active VGF-derived peptides. In cell and animal models, these VGF-derived peptides have been linked to energy balance regulation, neurogenesis, synaptogenesis, learning and memory, and depression-related behaviours throughout development and adulthood. The C-terminal VGF-derived peptides, TLQP-62 (VGF554–615) and TLQP-21 (VGF554–574) have differential effects on Alzheimer’s disease pathogenesis, neuronal and microglial activity, and learning and memory. TLQP-62 activates neuronal cell-surface receptors and regulates long-term hippocampal memory formation. TLQP-62 also prevents immune-mediated memory impairment, depression-like and anxiety-like behaviours in mice. TLQP-21 binds to microglial cell-surface receptors, triggering microglial chemotaxis and phagocytosis. These actions were reported to reduce amyloid-β plaques and decrease neuritic dystrophy in a transgenic mouse model of familial Alzheimer’s disease. Expression differences of VGF-derived peptides have also been associated with frontotemporal lobar dementias, amyotrophic lateral sclerosis, Lewy body diseases, Huntington’s disease, pain, schizophrenia, bipolar disorder, depression and antidepressant response. This review summarizes current knowledge and highlights questions for future investigation regarding the roles of VGF and its dysregulation in neurodegenerative and psychiatric disease. Finally, the potential of VGF and VGF-derived peptides as biomarkers and novel therapeutic targets for neurodegenerative and psychiatric diseases is highlighted.
Keywords: VGF, neuropeptide, biomarker, therapy, disease
Quinn et al. summarizes the physiological role of the neuropeptide VGF and VGF-derived peptides in brain and behaviour alongside their dysregulation and role in neurodegenerative and psychiatric disease. Finally, the potential use of VGF and VGF-derived peptides as biomarkers and therapeutic targets of neurodegenerative and psychiatric diseases is highlighted.
<Please insert Graphical abstract here>
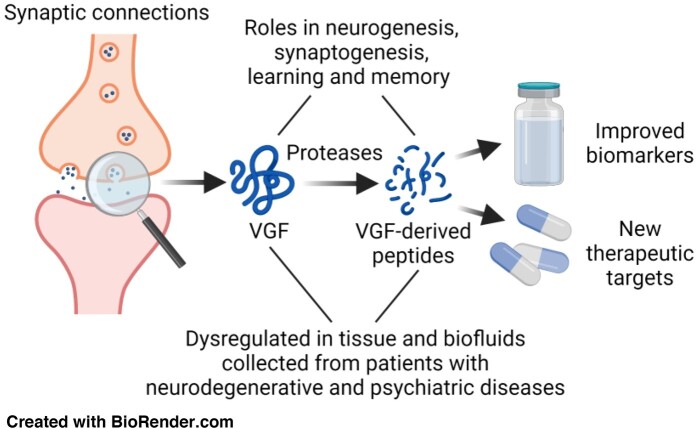
Graphical Abstract
Graphical Abstract.
Introduction
The neurosecretory protein VGF (non-acronymic, also known as secretogranin VII), belongs to the granin family of neuropeptides, distinctively composed of acidic secretory proteins.1 VGF was first identified as a nerve growth factor (NGF)-response gene by cloning of the nervous system-specific mRNA, VGF8a, from PC12 cells treated with NGF.2VGF mRNA is potently upregulated by NGF and other neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in cortical and hippocampal neurons.3,4 Epidermal growth factor (EGF), fibroblast growth factor, interleukin-6 (IL-6) and insulin also increase VGF mRNA levels but to a lesser extent compared to NGF, BDNF and NT-3.4–8 Full-length human VGF1–615 is predominantly synthesized in neurons and neuroendocrine cells.5,9 The human VGF gene comprising two exons totalling 2551 bp, with a protein coding region of 1848 bp was identified in chromosome 7q22.10 Full-length murine VGF1–617 is 84.33% homologous to human VGF1–615.11
VGF undergoes proteolytic processing in the regulated secretory pathway to produce at least 12 active VGF-derived peptides.12 The largest known active VGF-derived peptide, NAPP-129 (VGF485–615), consists of 131 amino acids in humans (129 amino acids in murine species).13 Smaller active C-terminal VGF-derived peptides also exist including the 21 amino acid peptide, TLQP-21 (VGF554–574).14 The VGF signal peptide (VGF1–22) promotes self-sorting into the regulated secretory pathway where the signal peptide is removed, mediating VGF secretion.15 Functional VGF-derived peptides are conserved among humans, rats, mice, opossums, chimpanzees, macaques, cows, horses and zebrafish, highlighting their crucial role in normal physiology throughout evolution.16
VGF and VGF-derived peptides are critical regulators of energy balance, synaptogenesis, neurogenesis, learning and memory during development and throughout adulthood.5,12,17 VGF and VGF-derived peptides are also implicated in neurodegenerative and psychiatric disease pathology, raising interest in their potential as biomarkers of disease or even as novel targets for therapies. C-terminal VGF-derived peptides, including AQEE-30 (murine VGF588–617) and LQEQ-19 (murine VGF599–617) are protective in in vivo and in vitro Huntington’s disease models.18 Administration of murine AQEE-30 into mice and rats have antidepressant-like effects, while Vgf+/− haploinsufficient mice show pro-depressant phenotypes.19 VGF and AQEE-30 have been linked to exercise-mediated increase in BDNF and VGF expression and the regulation of synaptic plasticity genes by VGF through a positive feedback cycle.19 Furthermore, the binding of TLQP-21 to microglial cell-surface receptors triggers microglial chemotaxis and phagocytosis resulting in a reduction in amyloid-β (Aβ) plaques and neuritic dystrophy in the 5xFAD transgenic mouse model of Alzheimer’s disease.20,21 Administration of the C-terminal VGF-derived peptide TLQP-62 (murine VGF556–617) in 5xFAD mice reduces Aβ plaque burden, disease-associated microglial activation and dystrophic neurites.22 Intracerebroventricular injection of murine TLQP-62 into mice prior to lipopolysaccharide-induced innate immune response activation prevents memory deficits, depression-like and anxiety-like behaviours through BDNF/tropomyosin receptor kinase B (TrkB) signalling.23 Finally, VGF and VGF-derived peptides in biofluids differ from controls in numerous neurodegenerative and psychiatric diseases.16
In this review, we summarize recent studies that identify the physiological roles of VGF and VGF-derived peptides, their dysregulation in and potential use as neurodegenerative and psychiatric disease biomarkers and novel therapeutic targets.
Cellular and regional expression of VGF
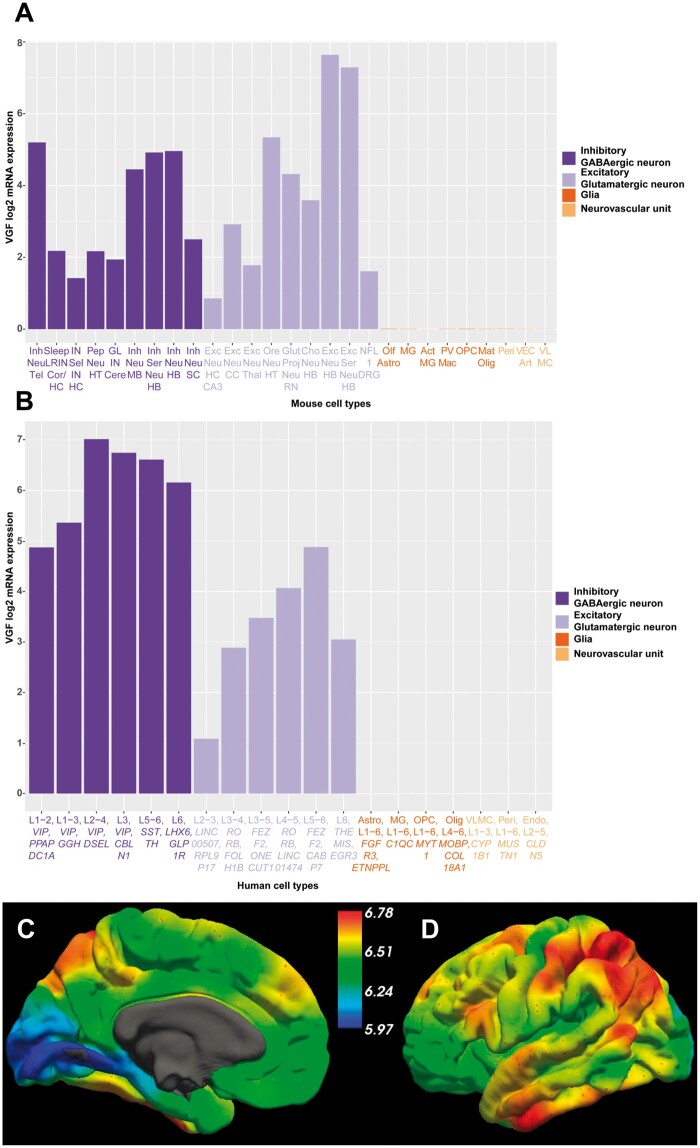
One challenge to understanding VGF function in humans is the important differences in mouse and human VGF mRNA expression in both neurons and glia (Fig. 1). Data from the Allen mouse brain atlas sorted by VGF mRNA expression in 27 representative cell types are portrayed in Fig. 1A. The top three VGF expressing neurons were excitatory and glutamatergic from the hindbrain and hypothalamus while glial and neurovascular unit cells had low or negligible VGF mRNA expression. Rat VGF353–372 expression increases in the rat cerebellum during the first postnatal week suggesting a potential role of VGF in cerebellar development before decreasing with age.24 In a single transgenic line of VGF-overexpressing mice, cerebellar development and motor function were both impaired.25 This highlights the possibility that excessive VGF expression might impair normal brain development, although overexpression experiments may not recapitulate normal physiology and the location of transgene insertion in this line could have impacted the phenotype.
Figure 1.
Comparison of human and mouse VGF mRNA expression from the Allen Brain Atlas. (A) VGF mRNA expression data were downloaded from the Allen Mouse Brain Atlas; https://celltypes.brain-map.org/rnaseq/mouse (Accessed 03/30/2020). These data represent log2 VGF mRNA expression intensity in 265 different cell types across the whole mouse cortex and hippocampus obtained through single cell RNA-sequencing. Data were sorted by VGF expression and 27 representative cell types were included based on the following criteria: those with the highest VGF expression across a range of brain regions, different neuronal subtypes, glial cells and neurovascular unit cells to ensure a representative sample. (B) VGF mRNA expression data were downloaded from the Allen Human Brain Atlas; https://celltypes.brain-map.org/rnaseq/human/cortex (Accessed 03/30/2020). These data represent log2 VGF mRNA expression intensity in 120 different cell types across multiple human cortical regions (middle temporal gyrus, anterior cingulate gyrus, primary visual cortex, primary motor cortex, primary somatosensory cortex and primary auditory cortex) obtained through single nucleus RNA-sequencing. Data were sorted by VGF expression and 19 representative cell types were included based on the same criteria from the mouse atlas to ensure a representative sample. For each cell type examined, L refers to the layer(s) of the cortex where that cell type was identified along with their associated marker genes in italics. (C) Medial view and (D) lateral view of the predicted whole-brain mRNA expression of VGF using microarray data from the Allen Human Brain Atlas on the surface of the left cortical hemisphere. Colour scales represent log2 mRNA expression intensity and data were accessed from http://www.meduniwien.ac.at/neuroimaging/mRNA.html (Accessed 04/10/2020). Act = activated; Art = arterial; Astro = astrocyte; Cere = cerebellum; CC = cerebral cortex; Cho = cholinergic; Cor = cortex; DRG = dorsal root ganglion; Endo = endothelial cell; Exc = excitatory; Glut proj = glutamatergic projection; GL = granular layer; HB = hindbrain; HC = hippocampus; HT = hypothalamus; Inh = inhibitory; IN = interneuron; LRIN = long-range interneurons; Mat = mature; MG = microglia; MB, midbrain; NFL1 = neurofilament 1; Neu = neuron; Olf = olfactory; Olig = oligodendrocyte; OPC = oligodendrocyte progenitor cell; Ore = orexin-producing; Pep = peptidergic; Peri = pericyte; PV mac = perivascular macrophages; Sel = selective; Ser = serotonergic; SC = spinal cord; Tel = telencephalon; Thal = thalamus; VEC = vascular endothelial cells; VLMC = vascular and leptomeningeal cell.
Data from the Allen human brain atlas were sorted by VGF mRNA expression in 19 representative cell types, depicted in Fig. 1B. The highest VGF mRNA expression levels were detected in the inhibitory GABAergic neurons spread across Layers 1–6 of the cortex (Fig. 1B). VGF mRNA expression in excitatory glutamatergic neurons was lower compared to the inhibitory neurons, and while present in all neuronal layers of the cortex, it was mainly expressed in glutamatergic neurons of Layers 4–6 (Fig. 1B). Overall, this suggests higher VGF mRNA expression in inhibitory neurons in humans, contrasting with the mouse data, which showed an even distribution between inhibitory and excitatory neurons. VGF mRNA expression was negligible in glial and neurovascular unit cells, as in mice (Fig. 1B). Single cell RNA-sequencing in induced pluripotent stem cells (iPSCs) differentiated into medium spiny neurons identified VGF RNA in dopamine receptor Drd1-expressing medium spiny neurons, Drd2-expressing medium spiny and cholinergic striatal neurons, Drd1- and Drd2-expressing spiny neurons, neural stem cells and neural progenitor cells (NPCs).26 In human, pig and rat brains, VGF419–427 and VGF607–615 were more abundant compared to VGF23–31 and VGF298–306,27 highlighting that VGF-derived peptides that cover the mid to C-terminus are more abundant in the brain compared to those that cover the mid to N-terminus.
In human brain, the hypothalamus has the highest VGF expression, which is critical for the established role of VGF in energy homeostasis and maintenance of body weight.28,29 Regional VGF mRNA expression throughout the cerebral cortex was visualized using software established by Gryglewski et al.30 Medial (Fig. 1C) and lateral views (Fig. 1D) of the predicted whole-brain VGF mRNA expression were mapped on the surface of the left cortical hemisphere; however, this software does not permit the examination of subcortical regions, such as the hypothalamus. The medial view shows elevated expression of VGF in the medial frontal gyrus (Fig. 1C), which has been associated with depression-related behaviours and the response to ketamine administration.31 The lateral view shows high expression in the inferior frontal gyrus, inferior temporal gyrus, angular gyrus and surrounding parietotemporal association areas and postcentral gyrus (Fig. 1D). It has been suggested that high VGF expression in the parietal cortex may help integrate sensory function and spatial processing.27 However, as this is exclusively mRNA data, further work should determine the brain-region-specific localization of VGF and VGF-derived peptides during development, ageing and disease pathogenesis.
Physiological function of VGF
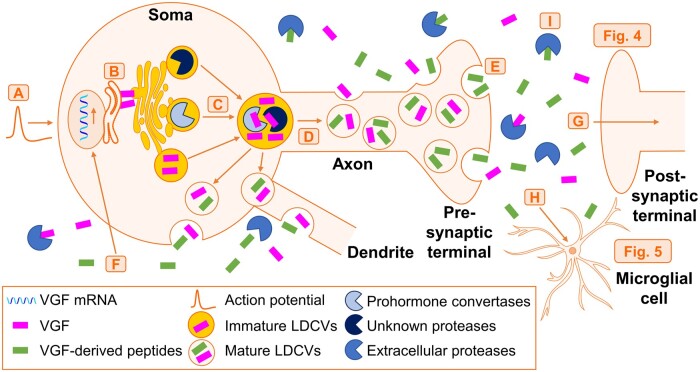
VGF mRNA synthesis is stimulated by conventional cell depolarization,8 the antidepressant imipramine,32 exercise,19 EGF,8,33 IL-6,5 insulin,8 NGF,5,7,8 BDNF and NT3 (Fig. 2A).3,4
Figure 2.
Regulated processing and secretion of VGF and its resulting downstream effects on microglia and neurons. (A) Action potential-mediated VGF mRNA synthesis is triggered by different factors, including conventional cell depolarization, the antidepressant imipramine, exercise, EGF, IL-6, insulin, NGF, BDNF and NT3. (B) VGF is translated in the rough endoplasmic reticulum, where the signal peptide is removed to promote its sorting into the regulated secretory pathway. VGF promotes the biogenesis of immature LDCVs and targets itself into LDCVs in the Golgi apparatus. (C) Immature LDCVs containing VGF, PCs and other proteases are released from the Golgi apparatus and undergo homotypic fusion with other immature LDCVs. Inside the fused immature LDCVs, VGF is cleaved into a variety of active VGF-derived peptides by different proteases including PCs. (D) Anterograde axonal transport to the pre-synaptic terminal and local transport within the soma and to dendrites promotes condensation and maturation of LDCVs. (E) Mature LDCVs containing VGF and VGF-derived peptides undergo regulated secretion by exocytosis from the soma, dendrites, the axon and the pre-synaptic terminal. (F) VGF and VGF-derived peptides locally diffuse after regulated secretion where they bind to and activate different receptors on the soma, triggering action potentials and inducing VGF mRNA synthesis to rapidly replace VGF protein levels. (G) VGF and VGF-derived peptides, such as TLQP-62, diffuse across the synaptic cleft where they activate neuronal cell-surface receptors (Fig. 4). (H) VGF-derived peptides, such as TLQP-21 bind and activate microglial cell-surface receptors (Fig. 5). (I) Extracellular proteases cleave VGF and VGF-derived peptides, which reduces their active diffusion and is the only known mechanism for inhibiting their function. BDNF = brain-derived neurotrophic factor; EGF = epidermal growth factor; IL-6 = Interleukin-6; LDCV = large dense core vesicles; NGF = nerve growth factor; NT3 = neurotrophin-3; PC = prohormone convertase.
Post protein translation, VGF undergoes proteolytic processing in the regulated secretory pathway to produce active VGF-derived peptides (Fig. 3). VGF is translated in the rough endoplasmic reticulum, where the signal peptide is removed, which promotes its sorting into the regulated secretory pathway (Fig. 2B).15,34 In the Golgi apparatus,35 neuropeptides including VGF help promote both the biogenesis of immature large dense core vesicles (LDCVs) and the localization of VGF inside the LDCVs (Fig. 2B).15,36 Immature LDCVs containing VGF, prohormone convertases (PCs) and other proteases are released from the Golgi apparatus37 and undergo homotypic fusion with other immature LDCVs (Fig. 2C).38 Inside the fused immature LDCVs, VGF is cleaved by different proteases including cathepsins, metalloproteases and PCs39 to produce active VGF-derived peptides (Fig. 2C).12,7
Figure 3.
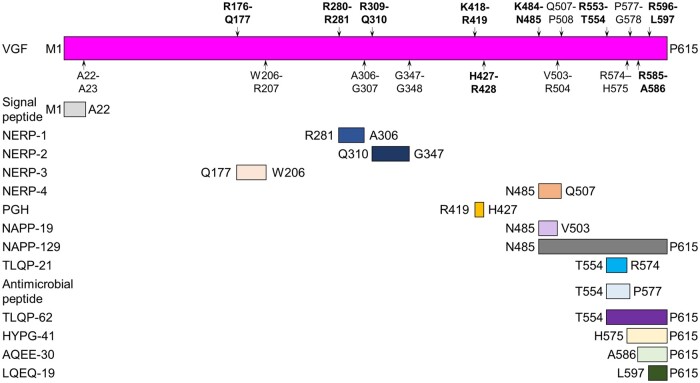
Proteolytic processing of VGF. Schematic of human VGF1–615 showing the known proteolytic cleavage sites from the literature as indicated by the P1–P1’ nomenclature of Schechter and Berger to indicate the amino acids N-terminal and C-terminal to the peptide bond that is cleaved.54 Putative PC cleavage sites are highlighted in bold while the other cleavage sites do not have a known protease responsible for their cleavage as determined by literature searches. Letters refer to the single amino acid code while the number refers to the position along the length of VGF. NERP = neuroendocrine regulatory peptide-1, -2, -3 and -4.
Local transport within the neuronal soma and anterograde axonal transport to the pre-synaptic terminal promotes LDCV condensation and maturation (Fig. 2D).38 Mature LDCVs containing VGF and VGF-derived peptides undergo regulated secretion by exocytosis from the soma, axon, pre-synaptic terminal and dendrites (Fig. 2E).38 VGF and VGF-derived peptides also diffuse locally by axonal, somatic and dendritic exocytosis into the extracellular space where they activate receptors on the soma to trigger intracellular action potentials, which also induce VGF mRNA synthesis to rapidly replace intracellular VGF protein levels (Fig. 2F).15,36,40–42 The specific receptors that VGF and VGF-derived peptides bind and activate requires further characterization. TLQP-62 activates the TrkB receptor, NMDA receptor (NMDAR) and metabotropic glutamate receptor 5 (mGluR5) on neurons via an unknown mechanism (Fig. 4). TLQP-21 binds and activates complement 3a receptor 1 (C3aR1) and globular head of complement component 1q receptor (gC1qR) on microglia (Fig. 5). Both TLQP-21 and TLQP-62 were reviewed in detail because they are the most studied VGF-derived peptides based on their physiological function and role in neurodegenerative and psychiatric diseases.
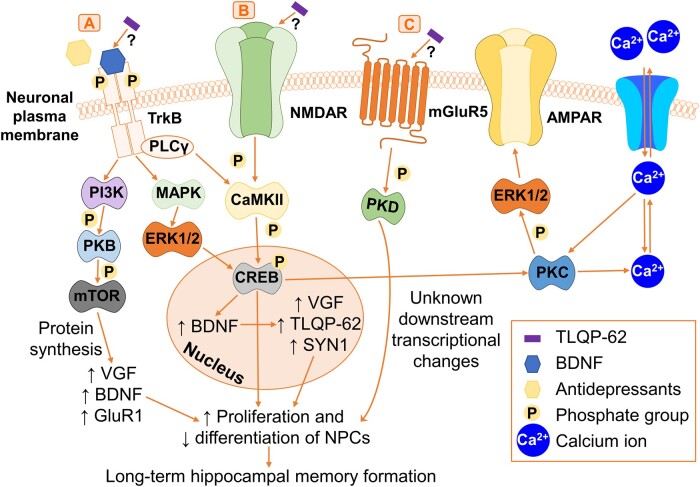
Figure 4.
Activation of neuronal tyrosine kinase receptors, G-protein-coupled receptors and ionotropic glutamate receptors by TLQP-62. (A) Activation of the BDNF/TrkB pathway by TLQP-62 or antidepressants through TrkB phosphorylation initiates PI3K activation, which subsequently phosphorylates and activates PKB followed by mTOR. This induces VGF, BDNF and GluR1 protein synthesis, promoting NPC proliferation and decreasing NPC differentiation, which modulates long-term hippocampal memory formation. Activation of BDNF/TrkB sequentially activates MAPK followed by ERK1/2. This triggers CREB activation in the nucleus and leads to increased mRNA expression of BDNF followed by VGF and SYN1. This results in increased synapsin-1 and VGF protein synthesis, followed by TLQP-62 production, together promoting NPC-mediated long-term hippocampal memory formation. Activation of BDNF/TrkB triggers PLCγ on the plasma membrane to activate CaMKII, which converges with pathway B. (B) TLQP-62 binds via an unknown mechanism and activates the ionotropic glutamate receptor, NMDAR, which triggers the sequential phosphorylation and activation of CaMKII followed by CREB. CREB subsequently activates PKC, which regulates intracellular Ca2+ levels and phosphorylates and activates ERK1/2 to promote AMPAR formation on the plasma membrane. This contributes to long-term hippocampal memory formation. Activation of CREB also directly induces gene transcription to trigger NPC-mediated hippocampal memory formation. (C) TLQP-62 binds via an unknown mechanism and activates the GPCR, mGluR5, which induces the phosphorylation and activation of PKD. PKD induces downstream transcriptional changes that contribute to NPC-mediated long-term memory formation by an unknown mechanism. AMPAR = AMPA receptor; BDNF = brain-derived neurotrophic factor; CaMKII = calcium/calmodulin-dependent protein kinase II; CREB = cAMP response element-binding protein; ERK1/2 = extracellular-signal-regulated kinase 1/2; GluR1 = glutamate receptor 1; MAPK = mitogen-activated protein kinase; mGluR5 = metabotropic glutamate receptor 5; mTOR = mammalian target of rapamycin; NMDAR = NMDA receptor; NPCs = neural progenitor cells; PLCγ = phospholipase C-γ; PI3K = phosphoinositide 3-kinase; PKB = protein kinase B; PKC = protein kinase C; PKD = protein kinase D; TrkB = tropomyosin receptor kinase B.
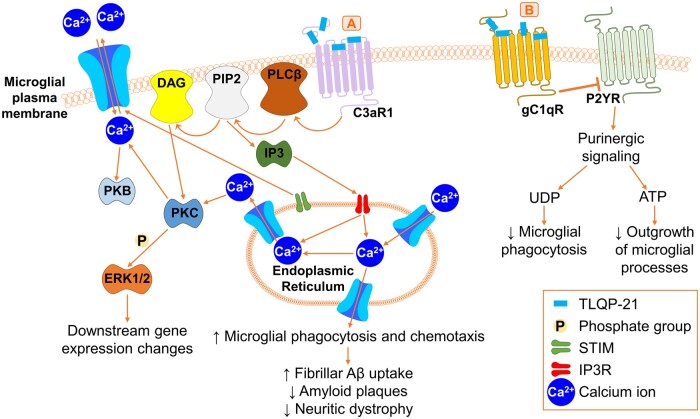
Figure 5.
Binding of TLQP-21 to microglial G-protein-coupled receptors. (A) TLQP-21 binds and activates microglial C3aR1, which sequentially activates PLCβ, PIP2 and DAG on the microglial plasma membrane. DAG activates PKC, which subsequently phosphorylates ERK1/2 and triggers downstream changes in gene expression. PIP2 activates IP3, which subsequently binds to IP3R on the surface of the endoplasmic reticulum. IP3R triggers an increase in the endoplasmic reticulum Ca2+ levels, which regulate intracellular and extracellular Ca2+ levels through STIM. Intracellular Ca2+ levels also regulate PKC and PKB activity. Ca2+ release from the endoplasmic reticulum increases microglial phagocytosis and chemotaxis. This triggered increased fibrillar Aβ uptake in vitro, while in 5xFAD mice, this decreased Aβ plaques and neuritic dystrophy. (B) TLQP-21 binds microglial gC1qR, which inhibits P2YR on the microglial plasma membrane. This inhibits UDP- and ATP-mediated purinergic signalling, which results in a reduction in microglial phagocytosis and a reduction in the outgrowth of microglial processes, respectively. C3aR1 = complement 3a receptor 1; DAG = diacylglycerol; ERK1/2 = extracellular-signal-regulated kinase 1/2; gC1qR = globular head of complement component 1q receptor; IP3 = inositol trisphosphate; IP3R = inositol trisphosphate receptor; PIP2 = phosphatidylinositol 4,5-bisphosphate; PLCβ = phospholipase C-β; PKB = protein kinase B; PKC = protein kinase C; P2YR = P2Y purinergic receptor; STIM = stromal interaction molecule; UDP = uridine diphosphate.
VGF and VGF-derived peptides also diffuse across the synaptic cleft where they bind and activate different neuronal and microglial cell-surface receptors (Fig. 2G and H).15,36,40 These receptors are also present on astrocytes and oligodendrocytes, however, the potential for VGF-derived peptides to bind these other cell types requires further investigation. VGF-derived peptides activate neuronal receptors to mediate synaptogenesis, neurogenesis, learning and memory (Figs 2G and 4). VGF-derived peptides also bind receptors on microglia to modulate microglial phagocytosis, outgrowth and chemotaxis (Figs 2Hand 5). As there is no cellular re-uptake of neuropeptides, extracellular proteases are the only known mechanism for stopping VGF activity and diffusion (Fig. 2I).43 These pathways require further characterization in both normal physiology and disease pathophysiology.
VGF processing by proteases
The processing pathways and proteases that cleave VGF to produce VGF-derived peptides remain incompletely characterized. However, the strongest evidence for proteases that cleave VGF exists for PCs.5,12,14,15,44–47 Cathepsins and metalloproteases were predicted to cleave VGF,39 however, this has not yet been experimentally validated. VGF-derived peptides can be classified by their cleavage sites in VGF1–615, the responsible protease, their mechanism of action and cellular function, their physiological function and how they were identified as illustrated in Table 1.
Table 1.
Physiological VGF-derived peptides; production, mechanism of action and cellular function, physiological function and peptide identification method
| Human VGF peptide | Human VGF cleavage sites and protease if known | Mechanism of action and cellular function | Physiological function | Peptide identification method | References |
|---|---|---|---|---|---|
| VGF1–22 (signal peptide) | A22-A23 | The signal peptide promotes self-sorting of VGF into the regulated secretory pathway where removed; mediates VGF secretion | Mediates plethora of VGF physiological functions | Predicted as signal peptide based on MALDI-TOF-MS in human CSF and program SignalP (signal peptide database) | 15 |
| VGF177–206 (NERP-3/QQET-30) | R176-Q177 (PC1/3 or PC2); W206-R207 | Intracellular Ca2+ regulation in the hypothalamus and pituitary; regulates body fluid homeostasis | Wistar rats: injecting NERP-3 ↑ arginine-vasopressin release in the posterior pituitary | Immunohistochemistry and radioimmunoassay in hypothalamus, hippocampus, amygdaloid and accumbens nuclei and prefrontal cortex in rat brains | 55 |
| VGF281–306 (NERP-1) | R280-R281 (PC1/3 or PC2); A306-G307 | Unknown | Wistar rats: intracerebroventricular injection of NERP-1 potently suppressed vasopressin release to modulate body fluid homeostasis | Immunohistochemistry in parietal, frontal and temporal cortex | 50 , 56 |
| VGF310–347 (NERP-2) | R309-Q310 (PC1/3 or PC2); G347-G348 | Modulates gastric acid secretion and function through stimulation of orexin release |
|
MS analysis and reversed phase-high performance LC-radioimmunoassay analysis of rat brains and human medullary thyroid carcinoma TT (non-acronymic) cells | 50 , 56–58 |
| VGF351–370 | G350-L351; E370-R371 | Unknown | Wistar rats: ↑ during the first postnatal week but ↓ with age in the rat cerebellum | LC-MS/MS and targeted-MS/MS in rat cerebellum | 24 |
| VGF419–427 (TPGH) | K418-R419 (PC1/3 or PC2); H427-R428 (PC1/3 or PC2) | Unknown | Sprague-Dawley rats: expressed in different brain regions and in the ghrelin cells of the stomach, however, this was unaffected by fasting or feeding status | Immunohistochemistry in parietal, frontal, and temporal cortex | 27 , 59 |
| VGF485–503 (NAPP-19) | K484-N485 (PC1/3 or PC2); V503-R504 | Unknown |
|
ELISA in plasma | 60 |
| VGF485–507 (NERP-4) | K484-N485 (PC1/3 or PC2); Q507-P508 | Unknown | Administration of NERP-4 to hypothalamus and pituitary tissue sections induced a transient intracellular Ca2+ concentration response in mice with a transgenic Ca2+ sensor | Immunohistochemistry in hypothalamus, hippocampus, amygdaloid and accumbens nuclei, prefrontal cortex in rat brains | 55 |
| VGF485–615 (NAPP-129) | K484-N485 (PC1/3 or PC2) | Unknown | Sprague-Dawley rats and INS-1 cell line: most abundant VGF-derived peptide in different brain regions and enriched in neuronal and pancreatic islet cell secretory granules and released upon stimulation | Gel chromatography and MS in plasma | 13 |
| VGF554–574 (TLQP-21) | R553-T554 (PC1/3); R574-H575 | Microglial cell-surface GPCRs, ERK1/2 signalling cascade, ATP- and UDP-mediated purinergic signalling cascade, HSPA8 activation leads to intracellular Ca2+ regulation; microglial activation, phagocytosis and chemotaxis; neuronal survival |
|
Immunohistochemistry in parietal, frontal and temporal cortex; gel chromatography and MS in plasma | 14 , 20 , 21 , 61 , 62 |
| VGF554–577 (antimicrobial peptide) | R553-T554 (PC1/3); P577-G578 | Unknown | Bactericidal activity against Micrococcus luteus, and antifungal activity against Pichia pastoris | Electron transfer dissociation LC-MS of secretory granules of a human endocrine cell line | 52 |
| VGF554–615 (TLQP-62) | R553-T554 (PC1/3) | BDNF/TrkB pathway through activation of GPCRs and ionotropic glutamate receptors, MAPK-ERK1/2 signalling cascade, NMDAR signalling cascade leads to neuron development, maintenance and signalling; hypothalamus: energy homeostasis; hippocampus: learning and long-term memory; insulin regulation and glucose homeostasis; modulation of pain response |
|
Immunohistochemistry in parietal, frontal, and temporal cortex | 3 , 14 , 22 , 23 , 28 , 31 , 32 , 44 , 63–68 |
| VGF575–615 (HYPG-41) | R574-H575 | Unknown |
|
MALDI-TOF-MS in rat brain | 59 , 69 |
| VGF586–596 (AQEE-11) | R585-A586 (PC1/3 or PC2); R596-L597 (PC1/3 or PC2) | Unknown | SOD1-G93A NSC-34 cells: treatment with AQEE-11 did not prevent neuronal apoptosis Sprague-Dawley rats: stimulates sympathetic outflow and facilitates penile erection | MALDI-TOF-MS in rat brain | 62 , 69 |
| VGF586–615 (AQEE-30) | R585-A586 (PC1/3 or PC2) | ERK1/2 signalling cascade leads to neuroprotection against neuronal apoptosis |
|
Gel chromatography and MS in plasma, ELISA in CSF | 18 , 19 , 62 , 69 |
| VGF597–615 (LQEQ-19) | R596-L597 (PC1/3 or PC2) | MAPK-ERK1/2 signalling cascade, phosphorylation of MAPK p38 leads to microglial activation; induction of thermal hyperalgesia; neuroprotection against neuronal apoptosis |
|
MALDI-ToF-MS in rat brain | 69 , 70 |
MALDI-TOF-MS = matrix assisted laser desorption ionization-time of flight-mass spectrometry.
At least 12 VGF-derived peptides have been identified to date (Fig. 3). For this review the human VGF1–615 sequences are listed, based on data from Uniprot (Accession: O15240), and murine VGF sequences are listed where relevant. VGF was first identified to be cleaved by PCs using ectopic expression of the neuroendocrine-specific PC1/3 or PC2 in pituitary-derived GH3 cells.44 PC1/3 and PC2 cleave at the paired basic amino acids, arginine, lysine and histidine; VGF contains many conserved stretches of these amino acids.44 PC1/3 or PC2 cleaves VGF at K484-N485 to produce NAPP-129 (VGF485–615). PC1/3 cleaves VGF at R553-T554 to produce TLQP-62 (VGF554–615),14,44 which is further cleaved at R574-H575 by unknown proteases to produce TLQP-21 (VGF554–574).14
Murine VGF1–617 is predominantly synthesized and cleaved in the hypothalamus, midbrain, thalamus and pituitary gland of the CNS, with lower levels also identified in the gastrointestinal tract.47 Chromatographic and mass spectrometry (MS) analysis identified that murine VGF1–617 is cleaved by PCs into a plethora of different murine VGF-derived peptides,47 validating prior work.44 VGF-derived peptides identified by analysing the secretopeptidome in human endocrine cells activated with an exocytosis stimulator48 match the murine VGF-derived peptides.47
VGF-derived peptides have varying roles in energy balance regulation, pain response, feeding response and sexual behaviour.12,49–51 However, additional physiological functions have been recently identified, including VGF-derived peptides acting as antimicrobial peptides,52 contributing towards cerebral cortex development24 and thermal hyperalgesia and nociceptive processing (Table 1).53
Physiological function of TLQP-62
TLQP-62 is one of the most extensively studied VGF-derived peptides. Its best described roles are in hypothalamic energy homeostasis17,28,67 and hippocampal long-term memory formation.3,23,68 TLQP-62 represents an autocrine, endocrine or paracrine factor that contributes towards energy homeostasis maintenance by modulating insulin secretion and glucose homeostasis.63 This review is focussed on the most relevant roles of VGF and VGF-derived peptides to neurodegenerative and psychiatric diseases. Excellent reviews have been published on the roles of VGF and VGF-derived peptides in energy balance maintenance17 and the response to pain.71
TLQP-62 and diverse antidepressants including ketamine, imipramine and fluoxetine activate the BDNF/TrkB pathway via neuronal cell-surface tyrosine kinase receptors, G-protein coupled receptors (GPCRs) and ionotropic glutamate receptors (Fig. 4). However, the mechanism of TLQP-62 binding to and activating these receptors currently remains unknown.72 These receptors activate converging signalling pathways, triggering action potentials through an influx of intracellular Ca2+, resulting in downstream gene expression leading to neurogenesis and synaptogenesis (Fig. 4).64,68,73
Triggering TrkB phosphorylation leads to the sequential phosphorylation and activation of phosphoinositide 3-kinase (PI3K), protein kinase B (PKB) and mammalian target of rapamycin (mTOR), inducing VGF, BDNF and glutamate receptor 1 (GluR1) protein synthesis (Fig. 4A).31,74–76 This promotes NPC proliferation and halts NPC differentiation contributing to long-term hippocampal memory formation (Fig. 4A).73 Activation of BDNF/TrkB initiates the mitogen-activated protein kinase-extracellular-signal-regulated kinase 1/2 (MAPK-ERK1/2) signalling cascade.77 This activates cAMP response element-binding protein (CREB) in the nucleus (Fig. 4A).68 Increased BDNF, VGF and SYN1 mRNA expression results in increased synapsin-1 and VGF protein production and subsequent increases in VGF-derived peptides, including TLQP-62 (Fig. 4A).68 Synapsin-1 is crucial for synaptic communication and vesicle formation,78 together with VGF and TLQP-62 they promote NPC-mediated long-term memory formation (Fig. 4A).68 Activation of BDNF/TrkB also triggers phospholipase C-γ (PLCγ) to activate calcium/calmodulin-dependent protein kinase II (CaMKII)31,74 and converges with pathway B (Fig. 4A and B).
TLQP-62 also binds and activates the ionotropic glutamate receptors, NMDAR and mGluR5 via unknown mechanisms (Fig. 4B).73 NMDAR-mediated phosphorylation and activation of CaMKII73 and CREB induces direct gene transcription which contributes to NPC-mediated hippocampal memory formation (Fig. 4B).68 CREB also activates protein kinase C (PKC) to regulate intracellular Ca2+ levels (Fig. 4B).68 PKC also phosphorylates and activates ERK1/2 promoting the formation AMPA receptors (AMPAR) on the plasma membrane, further contributing to long-term hippocampal memory formation (Fig. 4B).31,74 Via mGluR5, VGF leads to phosphorylation and activation of protein kinase D (PKD) and downstream transcriptional changes that contribute to NPC-mediated hippocampal memory formation (Fig. 4C).73
Through these pathways, TLQP-62 increases glutamatergic and BDNF signalling resulting in enhanced hippocampal proliferation of early NPCs, neurogenesis, synaptogenesis and memory formation. TLQP-62 has also been found to prevent lipopolysaccharide-mediated induction of memory deficits as well as depression-like and anxiety-like behaviours in mice.23 Additional observations in mice include TLQP-62 modulating long-term memory as assessed by freezing behaviour after bilateral administration of murine TLQP-62 to the hippocampus.68 TLQP-62 was administered into embryonic day 18 hippocampi obtained from Sprague-Dawley rats using adeno-associated viruses (AAV). This resulted in dendritic branching, dendritic maturation and neurite extension of developing hippocampal neurons observed between four and seven days in vitro.64 This is reduced by three non-synonymous single nucleotide polymorphisms (SNPs) in VGF64; however, they are not currently linked to any disease as yet. This highlights a potential role for genetic variation of VGF in its functions.
Physiological function of TLQP-21
TLQP-21 has also been extensively studied, particularly for its role in microglial activity. TLQP-21 binds and activates the microglial cell-surface GPCRs, C3aR1 and gC1qR.79–82 Based on experiments performed treating a murine macrophage cell line and Chinese hamster ovary cells with TLQP-21 and its derivate JMV5656,81,82 C3aR1 likely activates phospholipase C-β (PLCβ), phosphatidylinositol 4,5-bisphosphate (PIP2) and diacylglycerol (DAG) on the microglial plasma membrane (Fig. 5A).81,82 DAG activation of PKC subsequently phosphorylates ERK1/2 and triggers downstream changes in gene expression (Fig. 5A).81,82 PIP2 activates inositol trisphosphate (IP3), which binds to IP3 receptors on the endoplasmic reticulum (Fig. 5A).81,82 IP3 receptors trigger an increase in the endoplasmic reticulum Ca2+ levels which regulate intracellular and extracellular Ca2+ levels through stromal interaction molecules (STIMs) (Fig. 5A).81,82 Intracellular Ca2+ levels also regulate PKC and PKB activity (Fig. 5A).81,82 Ca2+ release from the endoplasmic reticulum increases microglial phagocytosis and chemotaxis (Fig. 5A).20,62
TLQP-21 binding to microglial cell-surface receptors, presumably via activation of the above signalling cascades, was found to decrease Aβ plaque load and neuritic dystrophy in in vitro and in vivo models of Alzheimer’s disease (Fig. 5).20,21,62 In particular, TLQP-21 increased fibrillar Aβ uptake in immortalized mouse microglial BV2 cells (Fig. 5A).62 This was not seen in BV2 cells treated with AQEE-11 (VGF586–596), LQEQ-19 (VGF597–615) or AQEE-30 (VGF586–615),62 again highlighting the diverse functions of VGF-derived peptides. Infusion of murine TLQP-21 into 5xFAD mice reduced Aβ plaques, decreased neuritic dystrophy and rescued expression of Alzheimer’s disease-associated microglial activation genes (Fig. 5A).20
TLQP-21 binding to gC1qR in rat macrophages and mouse microglia regulates intracellular Ca2+ levels, which may promote macrophage activation in chronic pain.83 TLQP-21 activation of gC1qR inhibits the microglial P2Y purinergic receptor (P2YR) in acute brain slices (Fig. 5B).21 This inhibits ATP- and uridine diphosphate (UDP)-mediated purinergic signalling (Fig. 5B).21 ATP-induced K+ conductance activation was inhibited which reduced outgrowth of microglial processes in response to laser-induced lesions and the inhibition of UDP-induced microglial phagocytic activity (Fig. 5B).21 Finally, TLQP-21 also bound heat shock 71 kDa protein 8 (HSPA8) on the surface of SH-SY5Y human neuroblastoma cells84 and to unknown neuronal receptors on the surface of cerebellar granule cells, which promoted neuronal survival.40 These studies suggest that TLQP-21 has differential receptor-mediated effects on microglial activity, and a potential role in modulating neuronal activity; however, further work is required to identify the neuronal cell-surface receptors involved.
VGF is dysregulated in different forms of neurodegenerative diseases
The following sections will describe the available evidence for VGF and VGF-derived peptide dysregulation in these neurodegenerative diseases at the genetic, RNA, protein, network, tissue-specific and biofluid level. The potential of VGF and VGF-derived peptides as novel diagnostic biomarkers and therapeutic targets for neurodegenerative diseases is also highlighted (Table 2).
Table 2.
Biomarker VGF-derived peptides; production, peptide identification method and biomarker of disease pathology
| Human VGF peptide | Human VGF cleavage sites and protease if known | Peptide identification method | Biomarker of disease pathology | References |
|---|---|---|---|---|
| VGF23–62 (APPG-40) | A22-A23; A62-R63 | SELDI-TOF-MS in CSF | Schizophrenia/psychosis: ↑ in CSF from first-onset drug-naïve patients compared to controls; ↑ in CSF compared to Alzheimer’s disease, MDD, and OCD; ↑ in CSF from patients with psychotic depression compared to schizophrenia | 85 , 86 |
| VGF23–174 (APPG-152) | A22-A23; A174-K175 | ELISA in serum | MDD: ↓ in serum compared to controls, rescued with 8 weeks of treatment with antidepressants; associated with ↑ risk for suicide | 87 , 88 |
| VGF26–59 (GRPE-34) | P25-G26; V59-R60 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF26–62 (GRPE-37) | P25-G26; A62-R63 | SELDI-TOF-MS, MS/MS in CSF | FTD: ↓ in CSF compared to controls Schizophrenia/psychosis: stable in CSF from first-onset drug-naïve patients compared to controls | 85 , 90 |
| VGF41–52 (EPVA-12) | K40-E41; K52-D53 | PRM-MS in CSF | FTD: ↓ in CSF from symptomatic GRN mutation carriers compared to non-carriers and pre-symptomatic carriers | 91 |
| VGF53–60 (DGSA-8) | K52-D53; R60-G61 | DIA-MS in CSF | Parkinson’s disease: ↓ in CSF compared to controls | 92 |
| VGF64–80 (NSEP-17) | R63-N64; R80-A81 | LC-MS in CSF; DIA-MS in CSF |
|
92–96 |
| VGF78–340 (DPRA-263) | V77-D78; G340-A341 | ELISA in CSF and plasma | ALS: ↓ with muscle weakness in CSF compared to controls; ↓ in CSF and plasma from pre-symptomatic SOD1-G93A mice compared to wild-type mice | 97 |
| VGF168–176 (DFSP-9) | R167-D168; R176-Q177 | LC-ESI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF195–205 (VNLE-11) | R194-V195; V205-W206 | SWATH-MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls, correlates with cognitive decline and dementia severity | 98 |
| VGF208–216 (ASWG-9) | R207-A208; R216-V217 | LC-MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls, predicts conversion from MCI to Alzheimer’s disease | 95 , 99 |
| VGF235–247 (MPDS-13) | R234-M235; F247-G248 | SWATH-MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls, correlates with cognitive decline and dementia severity | 98 |
| VGF256–267 (THLG-12) | K255-T256; K267-A268 | LC-MS and PRM-MS in CSF |
|
91 , 95 , 99 |
| VGF268–278 (AYQG-11) | K267-A268; K278-A279 | Tandem Mass Tag-MS and PRM-MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls; ↓ in CSF compared to MCI | 96 , 99 |
| VGF268–280 (AYQG-13) | K267-A268; R280-R281 | LC-ESI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF281–295 (RPES-15) | R280-R281 (PC1/3 or PC2); R295-L296 | SWATH-MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls, correlates with cognitive decline and dementia severity | 98 |
| VGF281–306 (NERP-1) | R280-R281 (PC1/3 or PC2); A306-G307 | Immunohistochemistry in parietal, frontal and temporal cortex |
|
27 |
| VGF296–309 (LLQQ-14) | R295-L296; R309-Q310 (PC1/3 or PC2) | DIA-MS in CSF | Parkinson’s disease: ↓ in CSF compared to controls | 92 |
| VGF350–367 (GLQE-18) | R349-G350; A367-E368 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF350–370 (GLQE-21) | R349-G350; E370-R371 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF351–370 (LQEA-20) | G350-L351; E370-R371 | LC-MS/MS and targeted-MS/MS in rat cerebellum | Potential biomarker of ageing | 24 |
| VGF373–397 (GGEE-25) | R372-G373; R397-A398 | DIA-MS in CSF | Parkinson’s disease: ↓ in CSF compared to controls | 92 |
| VGF373–402 (GGEE-30) | R372-G373; A402-L403 | LC-ESI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF373–415 (GGEE-43) | R372-G373; A415-E416 | LC-ESI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF373–417 (GGEE-45) | R372-G373; D417-K418 | ELISA in CSF | Lewy body disease: ↓ in CSF compared to Alzheimer’s disease or controls | 100 , 101 |
| VGF398–411 (ARQN-14) | R397-A398; G411-E412 | SELDI-TOF-MS in CSF | ALS: ↓ in CSF compared to controls | 97 , 102 |
| VGF419–427 (TPGH) | K418-R419 (PC1/3 or PC2); H427-R428 (PC1/3 or PC2) | Immunohistochemistry in parietal, frontal and temporal cortex |
|
27 |
| VGF485–495 (NAPP-11) | K484-N485 (PC1/3 or PC2); R495-A496 | DIA-MS in CSF | Parkinson’s disease: ↓ in CSF compared to controls | 92 |
| VGF485–503 (NAPP-19) | K484-N485 (PC1/3 or PC2); V503-R504 | LC-ESI MS, LC-MALDI MS and ELISA in CSF |
|
89 , 100 |
| VGF485–507 (NERP-4) | K484-N485 (PC1/3 or PC2); Q507-P508 | ELISA in plasma | Parkinson’s disease: ↓ in plasma compared to controls, rescued with >6 years of levodopa treatment; negatively correlates with odour discrimination tests | 103 |
| VGF485–615 (NAPP-129) | K484-N485 (PC1/3 or PC2) | Gel chromatography, MS and ELISA in plasma |
|
13 , 101 |
| VGF486–495 (APPE-10) | N485-A486; R495-A496 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF486–503 (APPE-18) | N485-A486; V503-R504 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF496–504 (APPE-9) | R495-A496; R504-S505 | DIA-MS in CSF | Parkinson’s disease: ↓ in CSF compared to controls | 92 |
| VGF507–522 (QPPP-16) | P506-Q507; D522-W523 | LC-ESI MS and LC-MALDI MS in CSF | Alzheimer’s disease: ↓ in CSF compared to controls | 89 |
| VGF514–610 (APAR-97) | P513-A514; V610-L611 | Antibody suspension bead arrays in CSF | FTD: ↓ in bvFTD CSF compared to pre-symptomatic mutation carriers and non-carriers | 104 |
| VGF554–574 (TLQP-21) | R553-T554 (PC1/3); R574-H575 | Immunohistochemistry in parietal, frontal and temporal cortex; gel chromatography and MS in plasma |
|
13 , 27 , 61 , 105 |
| VGF554–615 (TLQP-62) | R553-T554 (PC1/3) | Immunohistochemistry in parietal, frontal and temporal cortex; gel chromatography and MS in plasma |
|
13 , 27 , 61 , 105 |
| VGF586–595 (AQEE-10) | R585-A586 (PC1/3 or PC2); R595-R596 | SRM-MS in CSF | DLB: ↓ in CSF compared to Alzheimer’s disease or controls | 101 |
| VGF586–615 (AQEE-30) | R585-A586 (PC1/3 or PC2) | Gel chromatography and MS in plasma, ELISA in CSF | ALS: ↓ in plasma and cultured fibroblasts compared to controls; ↓ with muscle weakness in CSF compared to controls; ↓ in CSF and plasma from pre-symptomatic SOD1-G93A mice compared to wild-type mice | 13 |
| VGF591–610 (EAEE-20) | A590-E591; V610-L611 | ELISA in serum | MDD: ↓ in serum compared to controls BD: ↑ in serum compared to controls |
106 |
| VGF607–615 (C-terminus) | Y606-I607 | Immunohistochemistry in parietal, frontal, and temporal cortex | Alzheimer’s disease: ↓ in parietal cortex compared to controls | 27 |
LC-ESI = liquid chromatography-electrospray ionization; LC-MALDI = liquid chromatography matrix assisted laser desorption ionization-time of flight-mass spectrometry.
Alzheimer’s disease
Alzheimer’s disease represents ∼60–80% of all dementia cases.107 Typical symptoms of Alzheimer’s disease include memory loss, behavioural issues and progressive cognitive impairment.107 Neuropathological diagnosis of Alzheimer’s disease108 is defined by the accumulation of Aβ109 and the microtubule-associated protein tau.110
Bulk-RNA-sequencing showed decreased VGF RNA in the temporal and dorsolateral prefrontal cortex of patients with Alzheimer’s disease compared to controls.111 Multiscale causal network modelling showed that VGF RNA and VGF protein were the most decreased gene and protein in brain tissue from Alzheimer’s disease patients compared to controls in two large independent cohorts.22 Low VGF expression was associated with the downregulation of a cluster of genes, which correlated with Alzheimer’s disease clinical severity in three model systems of Alzheimer’s disease and was associated with a higher Alzheimer’s disease-polygenic risk score (AD-PRS) in humans.22 VGF was determined to be an important regulator of genes and proteins predicted by Bayesian network analysis to modulate the state of the Alzheimer’s disease network, classifying it as an Alzheimer’s disease key driver gene and was the only one identified across RNA, protein and RNA/protein networks.22
VGF protein levels partially mediated the effects of an AD-PRS composed of 457 independent SNPs.112 VGF protein levels contributed ∼30% of the effect of AD-PRS on global cognitive decline when endophenotypes were assessed alone.112 This was an effect size similar to those shown by Aβ, tau-tangles and hippocampal sclerosis.112 The optimal combination of the different endophenotypes contribution to the effect of the AD-PRS on global cognitive decline and the potential overlap between different endophenotypes was not examined.112 The relationship between genetics and different Alzheimer’s disease traits was examined; here, VGF protein levels clustered with the hormone and neurotransmitter calcium-dependent exocytosis and endocytosis gene, syntaxin 1A and the microRNA involved in cognition and nervous system function, MIR-132.112
Two members of the secretory neurotrophic factor signalling network, VGF and BDNF, were decreased in human brain and CSF samples from patients with Alzheimer’s disease compared to controls in two independent cohorts using multilayer brain proteomics and transcriptomics.113 VGF and BDNF levels were stable between 5xFAD and wild-type mice,113 suggesting that alternative models are required to study VGF dysregulation in Alzheimer’s disease. Hierarchical clustering of differentially abundant proteins between controls, patients with Alzheimer’s disease and mild cognitive impairment (MCI) showed that VGF clustered with other proteins involved in neurotrophic factor signalling and mitochondrial function.113 This cluster was decreased in patients with Alzheimer’s disease compared to controls but no changes were detected in patients with high amyloid pathology with and without MCI.113 No changes in VGF or BDNF were seen in another tauopathy, progressive supranuclear palsy.113 VGF progressively increased with cognitive stability independent of Alzheimer’s disease neuropathological burden in human brain tissue, highlighting that VGF acts independently of Aβ and tau, while potentially negating their role in cognitive decline.114 These studies indicate that decreases in VGF and other proteins involved in neurotrophic factor signalling and mitochondrial function may be specific to late-stage Alzheimer’s disease but not MCI, amyloid pathology or progressive supranuclear palsy.
A 4.8 kDa VGF-derived peptide was identified as a novel CSF biomarker for Alzheimer’s disease using surface enhanced laser desorption/ionization-time of flight-MS (SELDI-TOF-MS) and strong anionic exchange chromatography.115 However, the sequence of this VGF-derived peptide remains unknown. Recent work has further examined VGF-derived peptides as potential biomarkers for Alzheimer’s disease. Levels of VGF-derived peptides (sequence not listed) as detected by ELISA and MS were decreased in Alzheimer’s disease, MCI and frontotemporal dementia (FTD) compared with controls.116 These VGF-derived peptides were increased in stable MCI compared with Alzheimer’s disease, but remained unchanged between those with stable MCI and those who converted from MCI to Alzheimer’s disease.116 These VGF-derived peptides correlated with the conventional CSF Alzheimer’s disease biomarkers; Aβ1–42, total tau and phosphorylated tau.116
Utilizing systematic meta-analysis, VGF was highlighted as the second most identified protein and was shown in 15 of 47 independent proteomic studies to be decreased in CSF samples from Alzheimer’s disease patients compared to controls.117 From 17 of these 47 studies; nine tryptic VGF CSF peptides were repeatedly found to decrease in CSF samples from Alzheimer’s disease patients compared to controls,117 likely representing a reliable diagnostic biomarker. However, further work is required to test their potential use as a diagnostic biomarker in larger well-characterized studies.
Many VGF-derived peptides have been identified as potential CSF biomarkers of MCI and Alzheimer’s disease using untargeted and targeted proteomics, western blots and ELISAs. The proteomic experiments mentioned throughout this review have mainly used tryptic digestion upstream of quantification, therefore it is important to highlight that the peptides identified by these studies do not directly reflect endogenous VGF-derived peptides. For naming purposes and ease of comparison between diseases, we named each VGF-derived peptide with its 4-letter N-terminal amino acid sequence followed by their total amino acid length, for example, VGF64–80 (NSEP-17). For example, the strongest evidence for a potential CSF biomarker exists for (NSEP-17) which decreased by 21% in CSF samples from Alzheimer’s disease patients compared to controls at baseline.93 NSEP-17 likely represents VGF1–615 as it derives from a region of VGF from which no endogenous peptides have been identified to date (Fig. 3). Using linear mixed models in a longitudinal cohort identified NSEP-17 decreasing at ∼10.9% annually.93 Targeted proteomics identified decreased NSEP-17, VGF208–216 (ASWG-9), VGF256–267 (THLG-12) and VGF268–278 (AYQG-11) in CSF samples from Alzheimer’s disease patients compared with controls.94–96,99 Measuring CSF NSEP-17, ASWG-9 and THLG-12 together with CSF Aβ1–42, CSF phosphorylated tau and hippocampal volume improved the prediction of the conversion from MCI to Alzheimer’s disease over 3 years.95 NSEP-17, AYQG-11 and other synaptic proteins were altered in CSF samples from controls, and patients with MCI and Alzheimer’s disease.96,113 Differences in NSEP-17 and AYQG-11 were also identified between those with stable MCI and those who converted from MCI to Alzheimer’s disease. However, this contrasts with the data from Khoonsari et al.116 who showed that VGF-derived peptides remained unchanged between those with stable MCI and those who converted from MCI to Alzheimer’s disease. This suggests that VGF could be a prognostic indicator; however, studies in larger patient cohorts are warranted. A prognostic indicator would be useful for monitoring of patients and could allow clinical trials to target the early stages of Alzheimer’s disease. Sequential window acquisition of all theoretical mass spectra (SWATH-MS), western blots and targeted-MS identified decreased VGF195–205 (VNLE-11), VGF235–247 (MPDS-13) and VGF281–295 (RPES-15) in CSF samples from Alzheimer’s disease patients compared to controls in two independent cohorts, correlating with cognitive decline and dementia severity.98
To our knowledge, the only study to quantify endogeously cleaved peptides (i.e. non-tryptic peptides) that included VGF, was performed by Hölttä et al.89 Endogenous peptides were separated from the CSF using 30 kDa molecular weight filters while proteins that remained on the filter were analysed by tryptic digestion. Endogenous VGF-derived peptides VGF26–59 (GRPE-34), VGF350–367 (GLQE-18), VGF350–370 (GLQE-21), VGF373–402 (GGEE-30), VGF373–415 (GGEE-43), VGF485–503 (NAPP-19), VGF486–495 (APPE-10), VGF486–503 (APPE-18) and VGF507–522 (QPPP-16) decreased between 19% and 55% in CSF samples from patients with Alzheimer’s disease compared to controls.89 Within the endogenous peptides examined, NAPP-19 was identified (Fig. 3), highlighting that VGF-derived peptides with a known physiological function are detectable and altered in the CSF of patients with Alzheimer’s disease compared to controls.89 The tryptic VGF-derived peptides, VGF168–176 (DFSP-9) and VGF268–280 (AYQG-13), decreased by 30% in CSF samples from patients with Alzheimer’s disease compared to controls.89 Further work is needed to examine endogenous neuropeptide-derived peptides in larger cohorts and to translate this into improved biomarkers for neurodegenerative and psychiatric diseases.
Given the multi-level evidence suggesting a functional role for VGF and VGF-derived peptides in Alzheimer’s disease pathogenesis, preliminary investigations are examining the potential for VGF to protect against Alzheimer’s disease. Germline overexpression of murine VGF1–617, AAV-VGF injection or chronic intracerebroventricular injection of murine TLQP-62 in 5xFAD mice partially rescued Aβ-mediated synaptic degeneration, while also reducing Aβ plaques, microglial activation and tau pathology with partial restoration of spatial learning and memory impairments.22 Improved neurogenesis in the subgranular zone of the hippocampus was also observed.22 It is important to consider that even though VGF is not actively dysregulated in these mouse models, overexpression, or injection of VGF and VGF-derived peptides has potential therapeutic benefit.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), the most common motor neuron disease, is characterized by motor neuron degeneration, progressive muscle weakness and eventual paralysis, with additional cognitive and behavioural symptoms in some people.118 Accumulations of transactive response DNA binding protein 43 kDa (TDP-43) occur in ∼97% of ALS cases.
In situ hybridization showed VGF mRNA co-localizing with neurofilament heavy chain, an intermediate filament protein highly enriched in motor neurons and a decreased number of motor neurons expressing VGF in ALS patients compared to controls.119 This was also detected in the anterior horn but not the dorsal horn of cervical and lumbar spinal cords from ALS patients compared to controls. VGF was not detected in the white matter.119 In ALS patients with longer-term survival, intracellular and extracellular VGF protein expression levels were maintained.119 These findings suggest an association of VGF with ALS pathophysiology and that VGF may possibly protect against ALS progression.
Novel C-terminal specific VGF antibodies were developed to assess VGF-derived peptide changes in ALS patients and the superoxide dismutase type 1 (SOD1)-G93A mouse model (SOD1 mutations cause ∼0.7% of all ALS cases).13 C-terminal VGF-derived peptides were reduced in grey matter structures from pre-symptomatic SOD1-G93A mice compared to wild-type mice, in plasma samples, and to a greater degree in the spinal cord from late-symptomatic SOD1-G93A mice compared to wild-type mice.13 Gel chromatography and MS analysis showed that VGF1–615 and C-terminal VGF-derived peptides containing the start of the NAPP-19/-129, AQEE-30 and TLQP-21/-62 sequences were decreased in plasma samples and cultured fibroblast cells from patients with ALS compared to controls.13 TLQP peptides were also decreased in plasma samples from both early- and late-disease stage ALS patients compared to controls.61 These C-terminal VGF-derived peptides contrast with the N-terminal VGF-derived peptides identified in Alzheimer’s disease. Reductions in TLQP peptides were also identified in SOD1-G93A mice prior to muscle weakness onset and in ALS patient-derived fibroblasts that were treated with sodium arsenite to induce oxidative stress.61
Using SELDI-TOF-MS, a 4.8 kDa VGF-derived peptide was reduced in CSF samples from ALS patients compared to controls by 32.8% in two separate cohorts with an accuracy of 72%, sensitivity of 48% and specificity of 86%.102 The 4.8 kDa peptide was claimed to represent VGF398–411 (ARQN-14)97,102; however, the molecular weight did not match the amino acid sequence length and so these data were unclear. Performing an ELISA using antibodies against VGF78–340 (DPRA-263) and VGF588–617 (AQEE-30), VGF levels progressively decreased with muscle weakness in CSF samples from patients with ALS compared to controls and in CSF and plasma samples from pre-symptomatic SOD1-G93A mice compared to wild-type mice.97 Further work analysing VGF levels in biofluid and brain tissue samples of patients with ALS is required to examine their potential as a novel diagnostic biomarker of ALS.
Overexpression of murine VGF1–617 in primary mixed spinal cord neuron cultures protected against excitotoxic injury induced with the glutamate receptor agonists 5 μM AMPA and 20 μM NMDA for 48 h.97 This could attenuate excitotoxic injury in the spinal cord of patients with ALS and other neurodegenerative diseases. Administration of murine TLQP-21 into the medium of mouse motor neuron NSC-34 cells rescued sodium arsenite-induced apoptosis and oxidative stress.61 The authors posited that TLQP-21 binds gC1qR on the nucleus of NSC-34 cells to exert its positive effects on cell viability61 based on work showing TLQP-21 binding to gC1qR on mouse microglia and macrophages.83 AQEE-30 and LQEQ-19, but not TLQP-21, prevented neuronal apoptosis in SOD1-G93A-expressing NSC-34 cells.70 LQEQ-19, which contains the shortest sequence of VGF required for survival also promoted neuroprotective effects that were blocked by PI3K or mitogen-activated protein kinase (MEK)/ERK inhibitors.70 A systematic meta-analysis of preclinical ALS models also identified that VGF decreases over time during ALS progression.120 Overall, this work indicates that VGF and C-terminal VGF-derived peptides are potential therapeutic targets for ALS.
Dementia with Lewy bodies
Dementia with Lewy bodies (DLB) is an age-associated synucleinopathy present in ∼20% of all dementia cases.121 Symptoms include fluctuating cognition, visual hallucinations, sleep behaviour disorder and varying features of parkinsonism.122 DLB is characterized neuropathologically by α-synuclein accumulations in Lewy bodies and Lewy neurites, classifying it as a Lewy body disease alongside Parkinson’s disease.121 Previously, DLB was predominantly diagnosed as Alzheimer’s disease or Parkinson’s disease due to their overlapping clinical manifestations, with mixed diagnoses identified in ∼50% of all DLB cases.122
RNA-sequencing and quantitative PCR identified that VGF was decreased in the anterior cingulate cortex and the dorsolateral prefrontal cortex from patients with DLB and Parkinson’s disease compared to controls.123 This decrease may compromise neuronal survival and energy homeostasis in the brains of patients with DLB and Parkinson’s disease.123 Differences between DLB and Parkinson’s disease were not examined.
CSF VGF373–417 (GGEE-45) and NAPP-19 quantified by ELISA and VGF586–595 (AQEE-10) by selected reaction monitoring-MS (SRM-MS) were found to be decreased in patients with DLB compared to patients with Alzheimer’s disease or controls.100 In DLB, VGF correlated with progressive cognitive decline, CSF total tau and CSF α-synuclein but not with CSF Aβ1–42.100 These results were replicated using label-free liquid chromatography (LC)/tandem MS, ELISA and SRM-MS in three cohorts.101 CSF GGEE-45 and AQEE-10 correlated with lower mini-mental state examination scores when CSF was collected.101 Using random forest analysis, the optimal CSF biomarker panel for distinguishing DLB from controls, Alzheimer’s disease, FTD and Parkinson’s disease included VGF, secretogranin-2 and the PC inhibitor, proenkephalin-B.101 This biomarker panel needs validating in larger cross-sectional, longitudinal and post-mortem confirmed cohorts to enable the use of VGF and other proteins as potential novel biomarkers of DLB. This work highlights the importance of VGF and synaptic health in DLB, warranting further investigation into targeting VGF therapeutically.
Frontotemporal dementia
FTD is a heterogenous disorder with several major clinical presentations spanning behaviour, language, attention, executive control and motor symptoms.124 FTDs are also neuropathologically heterogeneous. Predominant frontal and anterior temporal lobe neuron loss can be associated with TDP-43 pathology alone or in association with motor neuron disease, or tau pathology due to Pick’s disease, progressive supranuclear palsy, corticobasal degeneration or frontal variant Alzheimer’s disease.124 Behavioural variant FTD (bvFTD) accounts for ∼60% of cases, and progressive non-fluent/agrammatic, semantic and logopenic variants of primary progressive aphasia (PPA) accounts for 40%.124
So far, research has only focussed on the potential of VGF as a CSF biomarker of FTD, warranting further investigation into its potential dysregulation in FTD pathogenesis. Five novel CSF protein biomarkers for sporadic FTD were identified using SELDI-TOF-MS and validated using tandem MS including VGF26–62 (GRPE-37) and secretogranin-1446–493, which were both decreased in CSF samples from patients with FTD compared to controls.90
Using unbiased MS, 20 novel CSF protein biomarkers, including VGF, in progranulin mutation-associated FTD were identified. VGF levels were decreased in symptomatic GRN mutation carriers compared to non-carriers but were stable between symptomatic and pre-symptomatic GRN mutation carriers.91 VGF and five other proteins were further validated using parallel reaction monitoring-MS (PRM-MS).91 Here, VGF41–52 (EPVA-12) and THLG-12 were decreased in CSF samples from symptomatic GRN mutation carriers compared to non-carriers and pre-symptomatic GRN mutation carriers, while levels were stable between non-carriers and pre-symptomatic GRN mutation carriers.91 No differences in VGF levels were identified in MAPT and C9orf72 mutation carriers.91 Antibody suspension bead arrays were used to analyse 328 proteins in CSF samples, 26 of which, including VGF514–610 (APAR-97), discriminated between patients with FTD, pre-symptomatic mutation carriers and non-carriers.104 APAR-97 decreased in CSF samples from patients with bvFTD but not PPA compared to pre-symptomatic mutation carriers and non-carriers, which was validated in an independent cohort.104 However, no differences in APAR-97 were seen between CSF samples from patients with FTD and Alzheimer’s disease,104 highlighting that VGF alone may not aid in their differential diagnosis. Overall, this work suggests that VGF and other protein biomarkers identified using unbiased MS may help separate symptomatic FTD from pre-symptomatic mutation carriers and non-carriers. However, further studies in larger patient cohorts are required across the FTD disease spectrum to confirm whether VGF represents a diagnostic biomarker of FTD.
Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disease derived from the HTT mutation that causes an expanded polyglutamine repeat within the huntingtin protein, promoting huntingtin aggregation and the degeneration of striatal medium spiny neurons.125,126 Patients with more than 40 polyglutamine repeats develop Huntington’s disease in their adulthood.125 Clinical presentations include movement disorders, psychiatric disturbances and progressive cognitive impairment resulting in death within 10–15 years of symptom onset.125
New therapeutic strategies for Huntington’s disease were identified by studying the anti-oxidant Na+/Ca2+ channel inhibitor SUN N8075 ((2S)-1–(4-amino-2,3,5-trimethylphenoxy)-3-{4-[4–(4-fluorobenzyl)phenyl]-1-piperazinyl}-2-propanol-dimethanesulfonate) administration in in vivo and in vitro models of Huntington’s disease.18 SUN N8075 administration upregulated VGF mRNA 4-fold via the phosphorylation of ERK1/2.18 In striatal cells derived from Huntington’s disease mice expressing 7 or 111 polyglutamine repeats, SUN N8075 dose-dependently inhibited cell death induced by serum deprivation.18 Treatment with AQEE-30 but not TLQP-21 inhibited striatal cell death in cells expressing 111 polyglutamine repeats.18 AQEE-30 inhibited mutant huntingtin aggregation akin to SUN N8075.18 Inhibition of MEK suppressed the upregulation of VGF mRNA and the neuroprotective effects of SUN N8075.18 Treating R6/2 transgenic Huntington’s disease mice (human HTT with ∼120 polyglutamine repeats) with SUN N8075 prolonged survival, recovered the clasping response and prevented the degeneration of striatal neurons, although, motor performance in the rotarod task was not recovered.18 This suggests that treatments targeting VGF in Huntington’s disease may represent a novel therapeutic strategy. However, further work is needed to examine the effect of VGF-derived peptides on additional models of Huntington’s disease, including iPSCs26 where no differences were identified in VGF RNA expression levels in iPSCs derived from Huntington’s disease patients compared to controls.26 Investigations into the use of VGF-derived peptides as a biomarker of Huntington’s disease are warranted.
Parkinson’s disease
Parkinson’s disease is characterized by neurodegeneration in the substantia nigra, dopamine deficiency and the intraneuronal accumulation and aggregation of α-synuclein into Lewy bodies and neurites.127,128 Parkinson’s disease is diagnosed clinically by the presence of motor and non-motor symptoms including bradykinesia, resting tremor and gait impairment. Cognitive, olfactory and behavioural abnormalities are common.128 Some symptoms can be treated with dopamine replacement (e.g. with levodopa).
Using RNA-sequencing in posterior cingulate cortex post-mortem tissue. Immune response genes were upregulated in Parkinson’s disease and Parkinson’s disease with dementia compared to controls while cytoskeletal component and signal transduction genes, including VGF, were downregulated.129VGF was the sixth most downregulated gene with an almost 4- and six-fold decrease in Parkinson’s disease and Parkinson’s disease with dementia, respectively, compared to controls.129
The presence and localization of VGF-derived peptides in Parkinson’s disease and other neurodegenerative diseases were examined using antisera generated against human VGF-derived peptides,27 these antibodies recognize multiple species of VGF-derived peptides, which contain the listed VGF sequence. VGF-derived peptides containing VGF419–427 (TPGH) and VGF298–306 [neuroendocrine regulatory peptide-1 (NERP-1) C-terminus] were decreased in the parietal cortex of Parkinson’s disease patients compared to controls, while VGF-derived peptides containing VGF23–31 (N-terminus) and VGF607–615 (C-terminus) remained stable in all regions examined.27 In the parietal cortex of Alzheimer’s disease patients, all antibodies recognizing these VGF-derived peptides had decreased signal compared to controls.27 No changes in VGF were shown in parietal cortex sections from patients with ALS, multiple sclerosis or Pick’s disease.27 VGF expression was reduced by 6-hyroxydopamine-induced lesions to mimic Parkinson’s disease and was rescued after levodopa treatment in Sprague-Dawley rat brain samples.103 This highlights that VGF and VGF-derived peptides are dysregulated in the pathophysiology of Parkinson’s disease and are responsive to treatment with levodopa. However, these antibodies examined can also bind VGF1–615 and other VGF-derived peptides, further work utilizing targeted proteomics is required to examine changes in specific VGF-derived peptides.
To identify novel biomarkers, data-independent acquisition-mass spectrometry (DIA-MS) was performed on CSF samples from Parkinson’s disease patients and controls from two independent cross-sectional cohorts and a longitudinal cohort.92 VGF and secretogranins-1 and -3 were decreased in CSF samples from patients with Parkinson’s disease compared to controls in both cross-sectional cohorts, alongside 10 other differentially expressed proteins.92 VGF53–60 (DGSA-8), NSEP-17, VGF296–309 (LLQQ-14), VGF373–397 (GGEE-25) and VGF485–495 (NAPP-11) were decreased in one cohort while VGF496–504 (APPE-9) was decreased in both cohorts.92 Plasma VGF levels were further examined using an ELISA against the region that includes NAPP-129 (VGF485–615) and NAPP-19 (VGF485–507).103 The sequence of NAPP-19 diverges from other publications (VGF485–503), whereas the sequence examined refers to NERP-4 (VGF485–507).103 Plasma NAPP-19 and NERP-4 were reduced after Parkinson’s disease diagnosis but were comparable with controls in Parkinson’s disease patients with more than 6 years of levodopa treatment.103 Plasma NAPP-19 and NERP-4 negatively correlated with combined odour tests, specifically odour discrimination but not with odour threshold and odour identification tests.103 This work suggests that plasma and CSF VGF levels may represent an early diagnostic biomarker of Parkinson’s disease that is sensitive to brain dopamine levels and treatment response in clinical trials. However, formal testing in treatment response studies and in larger neuropathologically confirmed cohorts with a focus on assessing the sensitivity, specificity and accuracy of VGF levels are required to further develop the potential of VGF as a biomarker for Parkinson’s disease.
VGF is dysregulated in different forms of psychiatric diseases
Psychiatric disease classification is complex due to overlapping symptoms, overlapping genetics and unknown pathology and pathophysiology. As yet, the field lacks biomarkers to aid disease diagnosis, select and manage patient medications and optimize patient care. The following sections will describe the available evidence for VGF and VGF-derived peptide dysregulation in major psychiatric diseases at the genetic, RNA, protein, network, tissue-specific and biofluid level. Finally, the potential of VGF and VGF-derived peptides as novel diagnostic biomarkers and therapeutic targets for psychiatric diseases is highlighted (Table 2).
Mood disorders
Major depressive disorders (MDD) are the most common major psychiatric disorder, characterized by persistent (at least 2 weeks of) pervasive impairments in mood, energy, interest, self-esteem, sleep, appetite and pain without evident cause. MDD may be episodic, recurrent or chronic with a lifetime prevalence >20% and is frequently treatment-resistant.130 Bipolar disorder (BD) affects ∼1% of the global population131 and is characterized by the presence of recurrent or chronic hyperenergetic and euphoric states, most commonly interspersed with recurrent or chronic dysphoric mood states.132 Cognitive impairments are frequent in both MDD and BD patients, while both disorders are lifetime risk factors for dementia in late life compared to non-psychiatric controls.133
Using in situ hybridization, VGF mRNA expression in the pyramidal cell layer of the hippocampus and the dorsolateral prefrontal cortex was reduced in patients with BD, but not in patients with MDD or schizophrenia, compared with controls.65 This was due to a reduced amount of VGF per pyramidal cell while the VGF positive cell count remained stable between patients with BD and controls.65 Human VGF mRNA levels were reduced in post-mortem hippocampus samples66 and Brodmann area 2531 from both unmedicated and medicated male and female MDD subjects compared to controls. VGF and BDNF mRNA were reduced in leukocytes from drug-free patients with MDD compared with non-depressed controls.134 After 8–12 weeks of treatment with the antidepressant, escitalopram, VGF and BDNF mRNA levels increased compared to controls only in the patients whose depressive symptoms had responded to escitalopram.134
An ELISA targeting the region of VGF that includes VGF591–610 (EAEE-20) was examined in serum, EAEE-20 levels were decreased in MDD patients and increased in BD patients compared with controls.106 EAEE-20 levels did not correlate with the 17-item Hamilton Depression Rating Scale nor age of first onset, level of education and number of psychotic events.106 EAEE-20 levels discriminated between BD and MDD patients with 95% sensitivity, 100% specificity and 95% accuracy.106 EAEE-20 levels may accurately differentiate BD and MDD patients; however, detailed examinations of VGF-derived peptides in serum, plasma, CSF and brain samples from larger patient cohorts is required. Serum VGF23–174 (APPG-152) levels as assessed by ELISA were decreased in patients with MDD compared with controls, and restored following 8 weeks of treatment with antidepressants.87 Reduced serum APPG-152 levels were also associated with an increased risk for suicide.88 These results, alongside those studying MDD and BD,106 suggest that measuring serum VGF levels may represent a novel diagnostic biomarker for MDD.
Studies using murine models to examine the effects of global or regional modulation of VGF on depression-like behaviours were recently reviewed.135 Heterozygous germline Vgf+/− mice show pro-depressant phenotypes, memory impairment and increased susceptibility to chronic social defeat stress.19,25,66,136 Germline overexpression of VGF137 and floxed overexpression of VGF in the mouse dorsal hippocampus31 caused pro-depressant and antidepressant phenotypes, respectively. Exogenous administration of VGF-derived peptides in mice mimicked the effects of antidepressants in a BDNF-dependent manner.31 Intracerebroventricular or intra-hippocampal administration of human or murine TLQP-62 into mice had antidepressant-like efficacy in the forced swim test.31,32 This was dependent on BDNF expression, GluR1 insertion, mTOR and AMPAR activation.31 Intracerebroventricular injection of TLQP-62, prior to activation of the innate immune response using lipopolysaccharide prevented induction of memory deficits as well as depression-like and anxiety-like behaviours through BDNF/TrkB signalling.23 Administration of AQEE-30 (murine VGF588–617), into both male C57BL/6J mice and Sprague-Dawley rats induced an antidepressant-like effect.19 These studies highlight the importance of the maintenance of endogenous VGF and VGF-derived peptide levels in regulating depression-like behaviours.
Lithium chloride (LiCl) was the first mood stabilizing medication with anti-suicidal properties approved for BD, however, its mechanism of action remains incompletely characterized.132 The contribution of VGF and VGF-derived peptides to the behavioural response of mice to LiCl injection was investigated using novelty-induced hypophagia, a behavioural outcome measure sensitive to chronic antidepressant treatment.65 Intracerebroventricular administration of TLQP-62 elicited antidepressant-like effects and activated signalling pathways resembling treatment with LiCl.65 Furthermore, in heterozygous Vgf+/− null mice, LiCl failed to reduce both depressive behaviours and amphetamine-induced hyperlocomotion as compared to wild-type mice.65 This suggests that normal levels of VGF and TLQP-62 may be necessary for the behavioural effects of LiCl administration.
Both exercise and peripheral antidepressant administration increased hippocampal VGF expression in murine models.19,32 VGF expression was increased in mouse hippocampal neuronal cultures following the administration of serotonin and imipramine.32 Ketamine administration increased VGF levels in the mouse ventromedial prefrontal cortex, here, VGF-deficiency resulted in reduced ketamine efficacy.31 Exercise-induced stimulation of VGF and BDNF increased the expression of growth factor-stimulated MEK-ERK signalling pathway genes in harvested mouse hippocampal cell RNA.19 This signalling pathway further regulates synaptic plasticity genes.19 Conversely, stress induced by learned helplessness, the forced swim test and chronic social defeat reduced hippocampal VGF expression; this is relevant as chronic stress is a known risk factor for MDD in humans.31,32
How VGF modulates depression-like behaviours and the response of VGF to treatments remains unknown. This link may be associated with neurogenesis, as clinically effective antidepressants increase hippocampal neurogenesis.138 Increased VGF-mediated hippocampal neurogenesis was identified both in vivo and in vitro.32 This suggests a pathway where VGF mediates antidepressant-induced neurogenesis, possibly through its regulation by serotonin and BDNF signalling.
Schizophrenia and psychosis
Schizophrenia is a complex cognitive and behavioural condition with a mean lifetime prevalence of 1%.139 Using immunohistochemistry, a reduction in VGF-immunoreactive neurons in the hypothalamus of patients with schizophrenia compared to controls was identified, especially in non-obese patients.140 However, VGF mRNA expression was similar in hippocampal, dorsolateral and medial prefrontal cortex sections from patients with schizophrenia compared with controls.65 Further studies are required to understand the clinical relevance of these findings for schizophrenia.
Using SELDI-TOF-MS CSF levels of VGF23–62 (APPG-40) were increased in first-onset drug-naive schizophrenic patients compared to controls, while CSF levels of GRPE-37 remained stable.85 CSF APPG-40 contributed towards distinguishing patients with schizophrenia from patients with Alzheimer’s disease, depression and obsessive-compulsive disorder (OCD), with a sensitivity of 88% and specificity of 95%.85 CSF APPG-40 also contributed towards a model distinguishing between patients with depression and controls and between patients with depression with psychosis and patients with schizophrenia.85 However, measurements of APPG-40 and other CSF proteins did not distinguish between patients with OCD and controls and between psychotic and non-psychotic depression subtypes.85
Proteomic profiling was performed on CSF from patients with the initial prodromal state of psychosis, representing patients with an early disease stage prior to psychosis, with minimal or unspecific psychiatric symptoms.86 About 36% of prodromal patients had proteomic profiles, including APPG-40, akin to schizophrenia, while 29% of prodromal patients had metabolic profiles akin to schizophrenia.86 However, no correlations were identified between these metabolic and proteomic profiles regarding the transition from prodrome to schizophrenia.86 APPG-40 and other proteins may represent an important biomarker of schizophrenia85,86; however, validation in larger cohorts and studies to uncover the mechanism behind dysregulated VGF in schizophrenia are required.
Conclusions and future directions in VGF research
This review highlights the myriad roles of VGF and VGF-derived peptides in normal physiology and how these pathways are dysregulated in neurodegenerative and psychiatric diseases. VGF-derived peptides have diverse mechanisms of action; for example, TLQP-62 binds neuronal cell-surface receptors to modulate neurogenesis while TLQP-21 binds microglial cell-surface receptors to modulate microglial activity. However, the mechanism of action, cell-surface binding and underlying biology of the other VGF-derived peptides remain largely unknown. To further study these VGF-derived peptides, the following avenues of investigation are warranted: (i) examine the proteases responsible for the production of VGF-derived peptides using proteolysis studies combined with MS, for example as highlighted by this review, cathepsins and metalloproteases; (ii) examine factors that stimulate production and activation of VGF-derived peptides and whether they are co-regulated; (iii) perform in vitro and in vivo studies to examine the physiological function of these VGF-derived peptides; and (iv) examine which receptors and on which cell types are bound by VGF-derived peptides and their mechanism of action. The functions of VGF-derived peptides are extremely diverse; therefore, all potential roles warrant further investigation.
To improve the potential of VGF-derived peptides as biomarkers or therapeutics for neurodegenerative and psychiatric diseases, we need to understand whether they represent either full-length VGF or VGF-derived peptides with a known physiological function. This is timely since the endogenous VGF-derived peptide NAPP-19, with a potential role in diabetes and obesity, was also identified to be decreased in the CSF of patients with Alzheimer’s disease. Therefore, it will be important to distinguish tryptic VGF-derived peptides from endogenous VGF-derived peptides. More work is required to identify what these tryptic VGF-derived peptides are representing in biofluids from patients with neurodegenerative and psychiatric diseases. As some tryptic VGF-derived peptides, such as NSEP-17, are decreased in the CSF across diseases, including Alzheimer’s disease and Parkinson’s disease compared to controls and between Alzheimer’s disease and MCI. Others, such as GRPE-37, are decreased in FTD compared to controls but remained stable in patients with schizophrenia. It will also be important to study VGF and VGF-derived peptide levels in a variety of different longitudinal and diverse cohorts to help in the transition of this biomarker into the clinic.
VGF is dysregulated in both neurodegenerative and psychiatric diseases but whether these changes merely represent the smoke emanating from the fire or the actual fire requires further study to determine the causality of VGF. Do stable levels of VGF just represent normal neuronal and microglial functioning? Are changes in VGF levels in both neurodegenerative and psychiatric diseases because of dysregulated neuronal and microglial functioning or are disruptions to VGF directly dysregulating normal cell functioning? Based on the multiple VGF knockdown and knockout studies, VGF overexpression and VGF administration studies and multiscale causal network analyses described in the review, it is likely that disruptions to VGF directly dysregulate normal cell functioning but further work in this area is warranted.
We need a concerted effort to establish a comprehensive model of VGF and VGF-derived peptides akin to other proteins, such as tau and TDP-43, unravelling the role of VGF in the pathogenesis of neurodegenerative and psychiatric disease to enable the development of novel biomarkers and potential therapeutic targets.
Acknowledgements
We thank Elizabeth Ethier for constructive criticism on this manuscript.
Funding
This work was supported by grants from the National Institute of Aging (AG062306, AG062421 and AG059856) and the BrightFocus foundation (A2019128S).
Competing interest
The authors report no competing interests.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed.
Glossary
- AAV =
adeno-associated viruses
- AD-PRS =
Alzheimer’s disease-polygenic risk score
- ALS =
amyotrophic lateral sclerosis
- AMPAR =
AMPA receptors
- Aβ =
amyloid-β
- BD =
bipolar disorder
- BDNF =
brain-derived neurotrophic factor
- bvFTD =
behavioural variant frontotemporal dementia
- C3aR1 =
complement 3a receptor 1
- CAMKII =
calcium/calmodulin-dependent protein kinase II
- CREB =
cAMP response element-binding protein
- DAG =
diacylglycerol
- DIA-MS =
data-independent acquisition-mass spectrometry
- DLB =
dementia with Lewy bodies
- EGF =
epidermal growth factor
- ERK1/2 =
extracellular-signal-regulated kinase 1/2
- FTD =
frontotemporal dementia
- gC1qr =
globular head of complement component 1q receptor
- GluR1 =
glutamate receptor 1
- GPCRs =
G-protein coupled receptors
- HSPA8 =
heat shock 71 kDa protein 8
- IL-6 =
Interleukin-6
- IP3 =
inositol trisphosphate
- IP3R =
inositol trisphosphate receptor
- iPSCs =
induced pluripotent stem cells
- LC =
liquid chromatography
- LDCVs =
large dense core vesicles
- LiCl =
lithium chloride
- MAPK =
mitogen-activated protein kinase
- MAPK-ERK1/2 =
mitogen-activated protein kinase-extracellular-signal-regulated kinase 1/2
- MCI =
mild cognitive impairment
- MDD =
major depressive disorder
- MEK =
mitogen-activated protein kinase
- mGluR5 =
metabotropic glutamate receptor 5
- MS =
mass spectrometry
- mTOR =
mammalian target of rapamycin
- NERP =
neuroendocrine regulatory peptide
- NGF =
nerve growth factor
- NMDAR =
NMDA receptor
- NPCs =
neural progenitor cells
- NT-3 =
neurotrophin-3
- OCD =
obsessive-compulsive disorder
- P2YR =
P2Y purinergic receptor
- PC =
prohormone convertase
- PI3K =
phosphoinositide 3-kinase
- PIP2 =
phosphatidylinositol 4,5-bisphosphate
- PKB =
protein kinase B
- PKC =
protein kinase C
- PKD =
protein kinase D
- PLCβ =
phospholipase C-β
- PLCγ =
phospholipase C-γ
- PPA =
primary progressive aphasia
- PRM =
parallel reaction monitoring
- SELDI-TOF-MS =
surface enhanced laser desorption/ionization-time of flight-mass spectrometry
- SNP =
single nucleotide polymorphism
- SRM-MS =
selected reaction monitoring-mass spectrometry
- STIM =
stromal interaction molecule
- SWATH-MS =
sequential window acquisition of all theoretical mass spectra
- TDP-43 =
transactive response DNA binding protein 43 kDa
- TrkB =
tropomyosin receptor kinase B
- UDP =
uridine diphosphate
References
- 1. Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: Comparative and functional aspects. Biol Rev Camb Philos Soc. 2004;79(4):769–794. [DOI] [PubMed] [Google Scholar]
- 2. Levi A, Eldridge JD, Paterson BM.. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985;229(4711):393–395. [DOI] [PubMed] [Google Scholar]
- 3. Alder J, Thakker-Varia S, Bangasser DA, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23(34):10800–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonni A, Ginty DD, Dudek H, Greenberg ME.. Serine 133-Phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6(2):168–183. [DOI] [PubMed] [Google Scholar]
- 5. Ferri GL, Possenti R.. vgf: A neurotrophin-inducible gene expressed in neuroendocrine tissues. Trends Endocrinol Metab. 1996;7(7):233–239. [DOI] [PubMed] [Google Scholar]
- 6. Possenti R, Di Rocco G, Nasi S, Levi A.. Regulatory elements in the promoter region of vgf, a nerve growth factor- inducible gene. Proc Natl Acad Sci USA. 1992;89(9):3815–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawley RJ, Scheibe RJ, Wagner JA.. NGF induces the expression of the VGF gene through a cAMP response element. J Neurosci. 1992;12(7):2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salton SR, Fischberg DJ, Dong KW.. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol Cell Biol. 1991;11(5):2335–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salton SRJ, Ferri GL, Hahm S, et al. VGF: A novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21(3):199–219. [DOI] [PubMed] [Google Scholar]
- 10. Canu N, Possenti R, Ricco AS, Rocchi M, Levi A.. Cloning, structural organization analysis, and chromosomal assignment of the human gene for the neurosecretory protein Vgf. Genomics. 1997;45(2):443–446. [DOI] [PubMed] [Google Scholar]
- 11. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. [DOI] [PubMed] [Google Scholar]
- 12. Levi A, Ferri GL, Watson E, Possenti R, Salton SRJ.. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol. 2004;24(4):517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brancia C, Noli B, Boido M, et al. VGF protein and its C-terminal derived peptides in amyotrophic lateral sclerosis: Human and animal model studies. PLoS One. 2016;11(10):e0164689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartolomucci A, La Corte G, Possenti R, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci USA. 2006;103(39):14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia AL, Han SK, Janssen WG, et al. A prohormone convertase cleavage site within a predicted α-helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway. J Biol Chem. 2005;280(50):41595–41608. [DOI] [PubMed] [Google Scholar]
- 16. Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SRJ.. The extended granin family: Structure, function, and biomedical implications. Endocr Rev. 2011;32(6):755–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis JE, Brameld JM, Jethwa PH.. Neuroendocrine role for VGF. Front Endocrinol (Lausanne). 2015;6(3):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noda Y, Shimazawa M, Tanaka H, et al. VGF and striatal cell damage in in vitro and in vivo models of Huntington’s disease. Pharmacol Res Perspect. 2015;3(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunsberger JG, Newton SS, Bennett AH, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13(12):1476–1482. [DOI] [PubMed] [Google Scholar]
- 20. El Gaamouch F, Audrain M, Lin WJ, et al. VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice. Mol Neurodegener. 2020;15(1):4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elmadany N, de Almeida Sassi F, Wendt S, et al. The VGF-derived peptide TLQP21 impairs purinergic control of chemotaxis and phagocytosis in mouse microglia. J Neurosci. 2020;40(17):3320–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beckmann ND, Lin WJ, Wang M, et al. Multiscale causal networks identify VGF as a key regulator of Alzheimer’s disease. Nat Commun. 2020;11(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C, Li M, Yu H, et al. Neuropeptide VGF C-terminal peptide TLQP-62 alleviates lipopolysaccharide-induced memory deficits and anxiety-like and depression-like behaviors in mice: The role of BDNF/TrkB signaling. ACS Chem Neurosci. 2017;8(9):2005–2018. [DOI] [PubMed] [Google Scholar]
- 24. Corbière A, Walet-Balieu ML, Chan P, Basille-Dugay M, Hardouin J, Vaudry D.. A peptidomic approach to characterize peptides involved in cerebellar cortex development leads to the identification of the neurotrophic effects of nociceptin. Mol Cell Proteomics. 2018;17(9):1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizoguchi T, Shimazawa M, Ohuchi K, Kuse Y, Nakamura S, Hara H.. Impaired cerebellar development in mice overexpressing VGF. Neurochem Res. 2019;44(2):374–387. [DOI] [PubMed] [Google Scholar]
- 26. Smith-Geater C, Hernandez SJ, Lim RG, et al. Aberrant development corrected in adult-onset Huntington’s disease iPSC-derived neuronal cultures via WNT signaling modulation. Stem Cell Reports. 2020;14(3):406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cocco C, D'Amato F, Noli B, et al. Distribution of VGF peptides in the human cortex and their selective changes in Parkinson’s and Alzheimer’s diseases. J Anat. 2010;217(6):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis JE, Brameld JM, Hill P, et al. Hypothalamic over-expression of VGF in the Siberian hamster increases energy expenditure and reduces body weight gain. PLoS One. 2017;12(2):e0172724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahm S, Mizuno TM, Wu TJ, et al. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23(3):537–548. [DOI] [PubMed] [Google Scholar]
- 30. Gryglewski G, Seiger R, James GM, et al. Spatial analysis and high resolution mapping of the human whole-brain transcriptome for integrative analysis in neuroimaging. Neuroimage. 2018;176:259–267. [DOI] [PubMed] [Google Scholar]
- 31. Jiang C, Lin WJ, Labonté B, et al. VGF and its C-terminal peptide TLQP-62 in ventromedial prefrontal cortex regulate depression-related behaviors and the response to ketamine. Neuropsychopharmacology. 2019;44(5):971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thakker-Varia S, Krol JJ, Nettleton J, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27(45):12156–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petrocchi Passeri P, Biondini L, Mongiardi MP, et al. Neuropeptide TLQP-21, a VGF internal fragment, modulates hormonal gene expression and secretion in GH3 cell line. Neuroendocrinology. 2013;97(3):212–224. [DOI] [PubMed] [Google Scholar]
- 34. Stark M, Danielsson O, Griffiths WJ, Jörnvall H, Johansson J.. Peptide repertoire of human cerebrospinal fluid: Novel proteolytic fragments of neuroendocrine proteins. J Chromatogr B Biomed Sci Appl. 2001;754(2):357–367. [DOI] [PubMed] [Google Scholar]
- 35. Russo AF. Overview of neuropeptides: Awakening the senses? Headache. 2017;57(Suppl 2):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fargali S, Garcia AL, Sadahiro M, et al. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J. 2014;28(5):2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephens SB, Edwards RJ, Sadahiro M, et al. The prohormone VGF regulates β cell function via insulin secretory granule biogenesis. Cell Rep. 2017;20(10):2480–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merighi A. Costorage of high molecular weight neurotransmitters in large dense core vesicles of mammalian neurons. Front Cell Neurosci. 2018;12:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wegrzyn JL, Bark SJ, Funkelstein L, et al. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J Proteome Res. 2010;9(10):5002–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Severini C, Ciotti MT, Biondini L, et al. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem. 2008;104(2):534–544. [DOI] [PubMed] [Google Scholar]
- 41. Leal G, Comprido D, de Luca P, et al. The RNA-binding protein hnRNP K mediates the effect of BDNF on dendritic mRNA metabolism and regulates synaptic NMDA receptors in hippocampal neurons. eNeuro. 2017;4(6):ENEURO.0268-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hökfelt T, Barde S, Xu ZQD, et al. Neuropeptide and small transmitter coexistence: Fundamental studies and relevance to mental illness. Front Neural Circuits. 2018;12:106–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trani E, Giorgi A, Canu N, et al. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem. 2002;81(3):565–574. [DOI] [PubMed] [Google Scholar]
- 45. Pan H, Nanno D, Che FY, et al. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry. 2005;44(12):4939–4948. [DOI] [PubMed] [Google Scholar]
- 46. Pan H, Che FY, Peng B, Steiner DF, Pintar JE, Fricker LD.. The role of prohormone convertase-2 in hypothalamic neuropeptide processing: A quantitative neuropeptidomic study. J Neurochem. 2006;98(6):1763–1777. [DOI] [PubMed] [Google Scholar]
- 47. Mishiro-Sato E, Sasaki K, Matsuo T, et al. Distribution of neuroendocrine regulatory peptide-1 and -2, and proteolytic processing of their precursor VGF protein in the rat. J Neurochem. 2010;114(4):1097–1106. [DOI] [PubMed] [Google Scholar]
- 48. Sasaki K, Satomi Y, Takao T, Minamino N.. Snapshot peptidomics of the regulated secretory pathway. Mol Cell Proteomics. 2009;8(7):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartolomucci A, Possenti R, Levi A, Pavone F, Moles A.. The role of the vgf gene and VGF-derived peptides in nutrition and metabolism. Genes Nutr. 2007;2(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Toshinai K, Nakazato M.. Neuroendocrine regulatory peptide-1 and -2: Novel bioactive peptides processed from VGF. Cell Mol Life Sci. 2009;66(11-12):1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bartolomucci A, Pasinetti GM, Salton SRJ.. Granins as disease-biomarkers: Translational potential for psychiatric and neurological disorders. Neuroscience. 2010;170(1):289–297. [DOI] [PubMed] [Google Scholar]
- 52. Sasaki K, Osaki T, Minamino N.. Large-scale identification of endogenous secretory peptides using electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2013;12(3):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Riedl MS, Braun PD, Kitto KF, et al. Proteomic analysis uncovers novel actions of the neurosecretory protein VGF in nociceptive processing. J Neurosci. 2009;29(42):13377–13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schechter I, Berger A.. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27(2):157–162. [DOI] [PubMed] [Google Scholar]
- 55. Fujihara H, Sasaki K, Mishiro-Sato E, et al. Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology. 2012;153(3):1377–1386. [DOI] [PubMed] [Google Scholar]
- 56. Yamaguchi H, Sasaki K, Satomi Y, et al. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J Biol Chem. 2007;282(36):26354–26360. [DOI] [PubMed] [Google Scholar]
- 57. Namkoong C, Toshinai K, Waise TMZ, et al. NERP-2 regulates gastric acid secretion and gastric emptying via the orexin pathway. Biochem Biophys Res Commun. 2017;485(2):409–413. [DOI] [PubMed] [Google Scholar]
- 58. Toshinai K, Yamaguchi H, Kageyama H, et al. Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am J Physiol Endocrinol Metab. 2010;299(3):394–401. [CVOCROSSCVO] [DOI] [PubMed] [Google Scholar]
- 59. Dalbøge LS, Jacobsen JM, Mehrotra S, et al. Evaluation of VGF peptides as potential anti-obesity candidates in pre-clinical animal models. Peptides. 2021;136:170444. [DOI] [PubMed] [Google Scholar]
- 60. D’Amato F, Noli B, Angioni L, et al. VGF peptide profiles in type 2 diabetic patients’ plasma and in obese mice. PLoS One. 2015;10(11):e0142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brancia C, Noli B, Boido M, et al. TLQP peptides in amyotrophic lateral sclerosis: Possible blood biomarkers with a neuroprotective role. Neuroscience. 2018;380:152–163. [DOI] [PubMed] [Google Scholar]
- 62. Cho K, Jang YJ, Lee SJ, et al. TLQP-21 mediated activation of microglial BV2 cells promotes clearance of extracellular fibril amyloid-β. Biochem Biophys Res Commun. 2020;524(3):764–771. [DOI] [PubMed] [Google Scholar]
- 63. Petrocchi-Passeri P, Cero C, Cutarelli A, et al. The VGF-derived peptide TLQP-62 modulates insulin secretion and glucose homeostasis. J Mol Endocrinol. 2015;54(3):227–239. [DOI] [PubMed] [Google Scholar]
- 64. Behnke J, Cheedalla A, Bhatt V, et al. Neuropeptide VGF promotes maturation of hippocampal dendrites that is reduced by single nucleotide polymorphisms. Int J Mol Sci. 2017;18(3):612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thakker-Varia S, Jean YY, Parikh P, et al. The neuropeptide VGF is reduced in human bipolar postmortem brain and contributes to some of the behavioral and molecular effects of lithium. J Neurosci. 2010;30(28):9368–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang C, Lin WJ, Sadahiro M, et al. VGF function in depression and antidepressant efficacy. Mol Psychiatry. 2018;23(7):1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Foglesong GD, Huang W, Liu X, et al. Role of hypothalamic VGF in energy balance and metabolic adaption to environmental enrichment in mice. Endocrinology. 2016;157(3):983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin WJ, Jiang C, Sadahiro M, et al. VGF and its C-terminal peptide TLQP-62 regulate memory formation in hippocampus via a BDNF-TrkB-dependent mechanism. J Neurosci. 2015;35(28):10343–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Succu S, Mascia MS, Melis T, et al. Pro-VGF-derived peptides induce penile erection in male rats: Involvement of paraventricular nitric oxide. Neuropharmacology. 2005;49(7):1017–1025. [DOI] [PubMed] [Google Scholar]
- 70. Noda Y, Motoyama S, Nakamura S, Shimazawa M, Hara H.. Neuropeptide VGF-derived peptide LQEQ-19 has neuroprotective effects in an in vitro model of amyotrophic lateral sclerosis. Neurochem Res. 2019;44(4):897–904. [DOI] [PubMed] [Google Scholar]
- 71. Soliman N, Okuse K, Rice ASC.. VGF: A biomarker and potential target for the treatment of neuropathic pain? Pain Reports. 2019;4(5):e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akhter MS, Arif J.. The pathways involved in TLQP-62 mediated biological functions. J Adv Biotechnol. 2018;7(1):953–966. [Google Scholar]
- 73. Thakker-Varia S, Behnke J, Doobin D, et al. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res. 2014;12(3):762–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang C, Lin WJ, Salton SR.. Role of a VGF/BDNF/TrkB autoregulatory feedback loop in rapid-acting antidepressant efficacy. J Mol Neurosci. 2019;68(3):504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rantamäki T, Vesa L, Antila H, et al. Antidepressant drugs transactivate trkb neurotrophin receptors in the adult rodent brain independently of bdnf and monoamine transporter blockade. PLoS One. 2011;6(6):e20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fred SM, Laukkanen L, Brunello CA, et al. Pharmacologically diverse antidepressants facilitate TRKB receptor activation by disrupting its interaction with the endocytic adaptor complex AP-2. J Biol Chem. 2019;294(48):18150–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen T, Wu Y, Wang Y, et al. Brain-derived neurotrophic factor increases synaptic protein levels via the MAPK/Erk signaling pathway and Nrf2/Trx axis following the transplantation of neural stem cells in a rat model of traumatic brain injury. Neurochem Res. 2017;42(11):3073–3083. [DOI] [PubMed] [Google Scholar]
- 78. Mirza FJ, Zahid S.. The role of synapsins in neurological disorders. Neurosci Bull. 2018;34(2):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hannedouche S, Beck V, Leighton-Davies J, et al. Identification of the C3a receptor (C3AR1) as the target of the VGF-derived peptide TLQP-21 in rodent cells. J Biol Chem. 2013;288(38):27434–27443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cero C, Vostrikov VV, Verardi R, et al. The TLQP-21 peptide activates the G-protein-coupled receptor C3aR1 via a folding-upon-binding mechanism. Structure. 2014;22(12):1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Molteni L, Rizzi L, Bresciani E, et al. Pharmacological and biochemical characterization of TLQP-21 activation of a binding site on CHO cells. Front Pharmacol. 2017;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Molteni L, Rizzi L, Bresciani E, et al. STIM proteins and Orai Ca2+ channels are involved in the intracellular pathways activated by TLQP-21 in RAW264.7 macrophages. Front Pharmacol. 2018;9:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen YC, Pristerá A, Ayub M, et al. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J Biol Chem. 2013;288(48):34638–34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Akhter S, Chakraborty S, Moutinho D, et al. The human VGF-derived bioactive peptide TLQP-21 binds heat shock 71 kDa protein 8 (HSPA8) on the surface of SH-SY5Y cells. PLoS One. 2017;12(9):e0185176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang JTJ, Leweke FM, Oxley D, et al. Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis. PLoS Med. 2006;3(11):e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huang JT, Leweke FM, Tsang TM, et al. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS One. 2007;2(8):e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiang H, Chen S, Lu N, et al. Reduced serum VGF levels were reversed by antidepressant treatment in depressed patients. World J Biol Psychiatry. 2017;18(8):586–591. [DOI] [PubMed] [Google Scholar]
- 88. Li X, Ge H, Zhou D, et al. Reduced serum VGF levels are linked with suicide risk in Chinese Han patients with major depressive disorder. BMC Psychiatry. 2020;20(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hölttä M, Minthon L, Hansson O, et al. An integrated workflow for multiplex CSF proteomics and peptidomics-identification of candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. J Proteome Res. 2015;14(2):654–663. [DOI] [PubMed] [Google Scholar]
- 90. Rüetschi U, Zetterberg H, Podust VN, et al. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196(2):273–281. [DOI] [PubMed] [Google Scholar]
- 91. van der Ende EL, Meeter LH, Stingl C, et al. Novel CSF biomarkers in genetic frontotemporal dementia identified by proteomics. Ann Clin Transl Neurol. 2019;6(4):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rotunno MS, Lane M, Zhang W, et al. Cerebrospinal fluid proteomics implicates the granin family in Parkinson’s disease. Sci Rep. 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hendrickson RC, Lee AYH, Song Q, et al. High resolution discovery proteomics reveals candidate disease progression markers of Alzheimer’s disease in human cerebrospinal fluid. PLoS One. 2015;10(8):e0135365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Llano DA, Bundela S, Mudar RA, Devanarayan V; for the Alzheimer’s Disease Neuroimaging Initiative (ADNI). A multivariate predictive modeling approach reveals a novel CSF peptide signature for both Alzheimer’s disease state classification and for predicting future disease progression. PLoS One. 2017;12(8):e0182098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Llano DA, Devanarayan P, Devanarayan V; Alzheimer’s Disease Neuroimaging Initiative (ADNI). VGF in cerebrospinal fluid combined with conventional biomarkers enhances prediction of conversion from MCI to AD. Alzheimer Dis Assoc Disord. 2019;33(4):307–314. [DOI] [PubMed] [Google Scholar]
- 96. Duits FH, Brinkmalm G, Teunissen CE, et al. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao Z, Lange DJ, Ho L, et al. Vgf is a novel biomarker associated with muscle weakness in amyotrophic lateral sclerosis (ALS), with a potential role in disease pathogenesis. Int J Med Sci. 2008;5(2):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Park SA, Jung JM, Park JS, et al. SWATH-MS analysis of cerebrospinal fluid to generate a robust battery of biomarkers for Alzheimer’s disease. Sci Rep. 2020;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sathe G, Na CH, Renuse S, et al. Quantitative proteomic profiling of cerebrospinal fluid to identify candidate biomarkers for Alzheimer’s disease. Proteomics Clin Appl. 2019;13(4):1800105–1800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Steenoven I, Noli B, Cocco C, et al. VGF peptides in cerebrospinal fluid of patients with dementia with Lewy bodies. Int J Mol Sci. 2019;20(19):4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Van Steenoven I, Koel-Simmelink MJA, Vergouw LJM, et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: proteomic approach. Mol Neurodegener. 2020;15(1):1–15. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pasinetti GM, Ungar LH, Lange DJ, et al. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66(8):1218–1222. [DOI] [PubMed] [Google Scholar]
- 103. Cocco C, Corda G, Lisci C, et al. VGF peptides as novel biomarkers in Parkinson’s disease. Cell Tissue Res. 2020;379(1):93–107. [DOI] [PubMed] [Google Scholar]
- 104. Remnestål J, Öijerstedt L, Ullgren A, et al. Altered levels of CSF proteins in patients with FTD, presymptomatic mutation carriers and non-carriers. Transl Neurodegener. 2020;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Noli B, Sanna F, Brancia C, et al. Profiles of VGF peptides in the rat brain and their modulations after phencyclidine treatment. Front Cell Neurosci. 2017;11(158):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen S, Jiang H, Hou Z, et al. Higher serum VGF protein levels discriminate bipolar depression from major depressive disorder. J Neurosci Res. 2019;97(5):597–606. [DOI] [PubMed] [Google Scholar]
- 107. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460. [Google Scholar]
- 108. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K.. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82(12):4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Brion JP, Couck AM, Passareiro E, Flament-Durand J.. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J Submicrosc Cytol. 1985;17(1):89–96. [PubMed] [Google Scholar]
- 111. Wang X, Allen M, Li S, et al. Deciphering cellular transcriptional alterations in Alzheimer’s disease brains. Mol Neurodegener. 2020;15(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tasaki S, Gaiteri C, Mostafavi S, De Jager PL, Bennett DA.. The molecular and neuropathological consequences of genetic risk for Alzheimer’s dementia. Front Neurosci. 2018;12(699):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bai B, Wang X, Li Y, et al. Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron. 2020;105(6):975–991.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wingo AP, Dammer EB, Breen MS, et al. Large-scale proteomic analysis of human brain identifies proteins associated with cognitive trajectory in advanced age. Nat Commun. 2019;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carrette O, Demalte I, Scherl A, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics. 2003;3(8):1486–1494. [DOI] [PubMed] [Google Scholar]
- 116. Khoonsari PE, Shevchenko G, Herman S, et al. Improved differential diagnosis of Alzheimer’s disease by integrating ELISA and mass spectrometry-based cerebrospinal fluid biomarkers. J Alzheimers Dis. 2019;67(2):639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pedrero-Prieto CM, García-Carpintero S, Frontiñán-Rubio J, et al. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin Proteomics. 2020;17(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Prim. 2017;3(17071):1–18. [DOI] [PubMed] [Google Scholar]
- 119. Noda Y, Tanaka M, Nakamura S, et al. Identification of VGF nerve growth factor inducible-producing cells in human spinal cords and expression change in patients with amyotrophic lateral sclerosis. Int J Med Sci. 2020;17(4):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jordan K, Murphy J, Singh A, Mitchell CS.. Astrocyte-mediated neuromodulatory regulation in preclinical ALS: A metadata analysis. Front Cell Neurosci. 2018;12(491):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Walker Z, Possin KL, Boeve BF, Aarsland D.. Lewy body dementias. Lancet. 2015;386(10004):1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Outeiro TF, Koss DJ, Erskine D, et al. Dementia with Lewy bodies: An update and outlook. Mol Neurodegener. 2019;14(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rajkumar AP, Bidkhori G, Shoaie S, et al. Postmortem cortical transcriptomics of Lewy body dementia reveal mitochondrial dysfunction and lack of neuroinflammation. Am J Geriatr Psychiatry. 2020;28(1):75–86. [DOI] [PubMed] [Google Scholar]
- 124. Olney NT, Spina S, Miller BL.. Frontotemporal dementia. Neurol Clin. 2017;35(2):339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ross CA, Tabrizi SJ.. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 126. MacDonald ME, Ambrose CM, Duyao MP, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. [DOI] [PubMed] [Google Scholar]
- 127. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M.. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:17013–17021. [DOI] [PubMed] [Google Scholar]
- 129. Henderson-Smith A, Corneveaux JJ, De Both M, et al. Next-generation profiling to identify the molecular etiology of Parkinson dementia. Neurol Genet. 2016;2(3):e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:1–20. [DOI] [PubMed] [Google Scholar]
- 131. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vieta E, Berk M, Schulze TG, et al. Bipolar disorders. Nat Rev Dis Prim. 2018;4:18008–18016. [DOI] [PubMed] [Google Scholar]
- 133. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: A systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cattaneo A, Sesta A, Calabrese F, Nielsen G, Riva MA, Gennarelli M.. The expression of VGF is reduced in leukocytes of depressed patients and it is restored by effective antidepressant treatment. Neuropsychopharmacology. 2010;35(7):1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mizoguchi T, Hara H, Shimazawa M.. VGF has roles in the pathogenesis of major depressive disorder and schizophrenia: Evidence from transgenic mouse models. Cell Mol Neurobiol. 2019;39(6):721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bozdagi O, Rich E, Tronel S, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28(39):9857–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mizoguchi T, Minakuchi H, Tanaka M, Tsuruma K, Shimazawa M, Hara H.. Sensorimotor gating deficits and effects of antipsychotics on the hyperactivity in VGF-overexpressing mice. Pharmacol Reports. 2018;70(3):476–480. [DOI] [PubMed] [Google Scholar]
- 138. Malberg JE, Eisch AJ, Nestler EJ, Duman RS.. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Prim. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 140. Busse S, Bernstein HG, Busse M, et al. Reduced density of hypothalamic VGF-immunoreactive neurons in schizophrenia: A potential link to impaired growth factor signaling and energy homeostasis. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):365–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed.