Abstract
The most common nosocomial fungal infections are caused by several species of Candida, of which Candida glabrata is the second most frequently isolated species from bloodstream infections. C. glabrata displays relatively high minimal inhibitory concentration values (MIC) to the antifungal fluconazole and is associated with high mortality rates. To decrease mortality rates, the appropriate treatment must be administered promptly. C. glabrata contains in its genome several non-identical copies of species-specific sequences. We designed three pairs of C. glabrata-specific primers for endpoint PCR amplification that align to these species-specific sequences and amplify the different copies in the genome. Using these primers, we developed a fast, sensitive, inexpensive, and highly specific PCR-based method to positively detect C. glabrata DNA in a concentration-dependent manner from mixes of purified genomic DNA of several Candida species, as well as from hemocultures and urine clinical samples. This tool can be used for positive identification of C. glabrata in the clinic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00584-2.
Keywords: Molecular detection, Candida glabrata, Species-specific sequences, PCR amplification, Responsible Editor: Sandro Rogerio de Almeida
Introduction
The most common causal agents of nosocomial invasive fungal infections are several species of the genus Candida [1–3], of which bloodstream infections (candidemias) are associated with very high mortality rates (30–40%) [1, 4]. Only four Candida species account for 90% of all candidemias: Candida albicans, Candida glabrata, Candida parapsilosis, and Candida tropicalis [5–8]. Despite new antifungals, mortality rates of candidemia have not decreased substantially. To provide an effective treatment for candidemia, an early identification of the species causing the infection is crucial since each species displays different susceptibility to antifungals [9]. In addition, a delay in antifungal therapy correlates with an increase in mortality rate [4, 10, 11]. To diagnose candidemia, blood cultures are still the gold standard used in the clinic, but the sensitivity is only ~ 50% and the species are not identified [12, 13]. Furthermore, blood cultures have relatively long turnaround times (it takes from 1 to 5 days to detect the genus and species) (reviewed in [14, 15]). Non-culture methods such as those that detect different antigens from Candida directly in blood samples or serum are much faster than blood cultures but have relatively low sensitivity. However, molecular methods are fast and show higher sensitivity and specificity [14]. The most commonly used molecular methods are various PCR-based amplification of different fragments of the rDNA array of Candida spp. These are mostly in-house methods with varying degrees of specificity and usually require further steps to identify the Candida species, such as sequencing or restriction enzyme analysis of the amplified fragments [9, 14, 16].
In this work, we describe a fast, low-cost, and highly accurate endpoint PCR method, for positive identification of C. glabrata in blood cultures and urine samples. The method amplifies C. glabrata-specific sequences present in ~ 10 copies per cell. This method can detect 0.5 ng of C. glabrata genomic DNA with high specificity. Its performance in the clinical setting will be evaluated after which it could be used in the clinic.
Materials and methods
Strains, oligonucleotides, and plasmids
Table 1 describes reference yeast strains (Candida spp., Saccharomyces spp., and Kluyveromyces lactis) and Candida glabrata clinical isolates. We used 86 C. glabrata clinical isolates to test the specificity of our method.
Table 1.
Strains and Candida sp. clinical isolates used in this study
| Reference strains | Relevant genotype | Source or reference |
|---|---|---|
| CBS138 | C. glabrata clinical isolate from feces (ATCC2001) | http://www.candidagenome.org/ |
| BG14 | C. glabrata. Vaginal clinical isolate BG2. Same as BG2 but ura3∆::Tn903 G418R | [31] |
| ATCC MYA2876 | Candida albicans SC5314 | Dr. L. Riego’s Lab. collection |
| ATCC 750 | Candida tropicalis | Lab. collection |
| NCYC 3133 | Candida bracarensis | [32] |
| CD36 | Candida dublinensis | Dr. H. Mora’s Lab. collection |
| Candida guilliermondii | Laboratory collection | |
| Candida krusei | Laboratory collection | |
| CLIB 209 | Kluyveromyces lactis | Dr. L. Riego’s Lab. collection |
| ATCC 204508 | Saccharomyces cerevisiae (S288c) | Dr. L. Riego’s Lab. collection |
| NC4c2829 | Saccharomyces castellii | Dr. L. Riego’s Lab. collection |
| CLI 251 | Saccharomyces bayanus uvarum | Dr. L. Riego’s Lab. collection |
| IFO1815 | Saccharomyces mikatae | Dr. L. Riego’s Lab. collection |
|
Clinical isolates (86 total) |
Description | Origin |
|
MC1 to MC7 (7 isolates) |
Group 1: Clinical isolates from urinea | Hospital Central, SLP, Méxicob |
|
MC8 to MC11 (3 isolates) |
Group 2: Clinical isolates from vagina or urine | Clinic León, GTO, México |
|
MC12 to MC20 (9 isolates) |
Group 3: Clinical isolates from blood cultures | INCMNSZ, CDMX, Méxicoc |
|
MC21 to MC23 (3 isolates) |
Group 4: Clinical isolates from urine or blood cultures | Hospital Central, SLP, México |
|
MC25 to MC74 (48 isolates) |
Group 5: Clinical isolates from blood cultures or urine | INCMNSZ, CDMX, México |
|
MC77 to MC84 (8 isolates) |
Clinical isolates | Hospital Central, SLP, México |
|
MC85 to MC92 (8 isolates) |
Clinical isolates from blood cultures | INCMNSZ, CDMX, México |
aIsolated from urinary tract
bHospital Central Ignacio Morones Prieto, San Luis Potosí, SLP, México
cInstituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, México
C. glabrata-specific oligonucleotides are listed in Table 2 and the position in the genome where they align is shown in Fig. 1 and Supplementary Fig. 1. The C. glabrata-specific oligonucleotides are included in patents MX352246 B and EP2410052 B1.
Table 2.
Sequence of the C. glabrata-specific oligonucleotides
| Primer No | Name | Sequence (5′-3′) |
|---|---|---|
| 242 | Cg1 Fw | CCAAAGTTCTAGTATCTTATTCTGTATAAT |
| 243 | Cg3 Rv | ATACCACTCATATTCGTGCCAC |
| 244 | Cg3 Fw | GTGGAGTAGTTGGTACTTGTGTCC |
| 245 | Cg1 Rv & Cg2 Rv | GTGGAGTAGTTAGTTCTTGTGTCCAG |
| 246 | Cg2 Fw | CGCTCATACAGGCACAGAAG |
| Sequence of universal primers to amplify the rDNA array | ||
| Primer No | Name | Sequence (5′-3′) |
| 1404 | ITS-1 | TCCGTAGGTGAACCTGCGG |
| 1405 | ITS-4 | TCCTCCGCTTATTGATATGC |
Fig. 1.
Chromosomal localization of NE copies. Schematic representation of the chromosomal localization of 5 copies of the NE associated with subtelomeric genes encoding cell wall proteins and the position of the C. glabrata-specific primer pairs Cg1, Cg2, and Cg3. Line 1: right telomere and subtelomeric region of chromosome E (Chr E-R) and its subtelomeric region where the EPA1, EPA2, and EPA3 gene cluster is localized. The telomere (T) is indicated. Primer pairs (small arrows) Cg1 (primers #242 and #245) and Cg2 (primers #246 and #245) share the reverse primer and they align just downstream of the functional NE associated with EPA1 generating 139-bp and 324-bp fragments respectively. Line 2: alignment of Cg1 and Cg2 primer pairs on the right subtelomere of chromosome H (Chr H-R) where AWP13 is located. Cg1 and Cg2 amplification products of this region generate 141-bp and 364-bp fragments respectively. Line 3: left telomere of chromosome K (Chr K-L), Cg1 and Cg2 align between EPA22 and AWP2 with amplification products of 137 and 348 bp. Lines 4 and 5: left and right subtelomeric regions of chromosome C respectively (Chr C-L Chr E-R). Cg3 pair (primers #244 and #243) aligns downstream of both EPA6 and EPA7 with 602-bp and 665-bp expected amplification products as indicated
Plasmids constructed are described in Supplementary Table S1.
GenBank accession numbers for the sequenced PCR (polymerase chain reaction) products obtained from strain BG14, with primer pairs Cg1, Cg2, and Cg3, are included in Supplementary Table S1.
Media and growth conditions
Escherichia coli strain DH10 was used to transform plasmids by electroporation. Cells were grown at 30 °C in LB (Luria–Bertani) medium containing 10 g/L peptone, 5 g/L yeast extract, and 5 g/L NaCl. Transformants were selected on LB-agar plates (LB medium with 1.5% agar) supplemented with 50 µg/mL carbenicillin. Yeast was grown at 30 °C in rich media YPD (yeast peptone dextrose-rich media) containing 10 g/L yeast extract, 20 g/L peptone, and 2%. 2% agar was added for solid media [17].
Genomic DNA extraction from cultured yeast strains and clinical isolates
We used 86 clinical isolates MC1–MC92 (Table 1, not numbered consecutively) and DNA was extracted from cultures of each isolate grown at 30 °C in YPD for 24 h as described in [17] using a FastPrep-24 equipment (MP Biomedicals) to lyse the cells during 1 s at 4 m/seg. Genomic DNA was resuspended in 200 µL of TE (Tris–EDTA: 10 mM Tris pH 8.0; 0.1 mM EDTA pH8.0) supplemented with 0.5 µL of RNAse cocktail (Ambion®; ThermoFisher).
PCR protocol to amplify C. glabrata-specific DNA sequences using primer pairs Cg1, Cg2, and Cg3
All the reactions were performed using Taq DNA polymerase (Biotechmol, Amplificasa EA-500). The reactions were prepared in a total volume of 30 µL and the reaction performed for 30 cycles using 100 ng/µL of genomic DNA. Three-microliter samples of each reaction were loaded onto 2% agarose gels. Amplification reactions for each primer pair were optimized and the reaction conditions are shown in Table 3.
Table 3.
Amplification and reaction conditions for the three C. glabrata-specific primer pairs and the ITS regions of the rDNA array
| Primer pair namea | Primer No | Annealing temperature | Final primer concentration in PCR reaction | Final dNTP concentration in PCR reaction | Expected sizes of ampliconsb (chromosome) |
|---|---|---|---|---|---|
| Cg1 | 242 | 59 °C | 500 nM | 30 µM |
137 bp (chr. K) 139 bp (chr. E) 141 bp (chr. H) |
| 245 | |||||
| Cg2 | 245 | 67.1 °C | 200 nM | 30 µM |
324 bp (chr. E) 348 bp (chr. K) 364 bp (chr. H) |
| 246 | |||||
| Cg3 | 243 | 63.4 °C | 500 nM | 30 µM |
602 bp (chr. C left) 667 bp (chr. C right)c |
| 244 | |||||
| rDNA | ITS1 | 60 °C | 500 nM | 500 µM |
878 bp (C. glabrata)d 520 bp (C. parapsilosis) 524 bp (C. tropicalis) 535 bp (C. albicans) |
| ITS4 |
aEach pair of primers consists of a forward and reverse primer which anneal to several positions in the C. glabrata genome and generate several amplicons
bThe size of the amplicons is calculated in silico with the sequence of the strain CBS138
cThe amplicon of the NE adjacent to EPA7 corresponds to the sequence from strain BG14 (GenBank Acc. No. AY646926.1)
dThe amplicon expected for C. glabrata is 878 bp, while the amplicons for C. parapsiolisis, C. tropicalis, and C. albicans are very similar (520 bp, 524, and 535 bp respectively)[25]
Cloning of PCR fragments into cloning vector pMB11
To sequence individually all the DNA fragments amplified with the three species-specific primer pairs, we cloned several of these PCR products. For this, we performed PCR reactions with the three primer pairs (Table 2) using high-fidelity Taq polymerase enzyme iProof (Bio-Rad) and the products were purified using Qiaquick (Qiagen) PCR purification columns. Fragments were cloned in the vector pMB11 which is optimized to clone PCR products [18]. Five clones from each primer pair were sequenced at LANBAMA-IPICYT, our in-house sequencing facility (http://lanbama.ipicyt.edu.mx), and the results are described in Supplementary Table S1.
In addition, to verify that some of the positively identified C. glabrata isolates by our PCR method were indeed C. glabrata, we amplified from various clinical isolates (MC2, MC16, MC25, MC27, MC29, MC39, MC56, MC65, and MC68) and cloned and sequenced several different genes (SIR3, HDF1, SIR2, SIR4, MTLa1, MTLalpha1). All sequences were compared using BLAST (NCBI) to the publicly available genomic sequence of reference strain CBS138 (http://www.candidagenome.org/). All genes were determined to belong to C. glabrata (data not shown).
Experiments to determine the minimum amount of DNA detected
To determine the minimum amount of DNA detected with each primer pair, a solution of purified genomic DNA from our standard laboratory strain BG14 at 1000 ng/µL was serially diluted tenfold in sterile Tris 10 mM pH 8.0. PCR reactions were set up as described above and in Table 3 containing 100, 10, 1, 0.5, 0.1, 0.05, and 0.002 ng each, of genomic DNA.
Experiments to determine the number of cells detected by endpoint PCR and the specificity of the oligonucleotide pairs
To determine the minimum number of cells detected by each set of primers, C. glabrata cells from a stationary phase culture were counted with a hemocytometer and adjusted to obtain a suspension of 107 cells/mL in 10 mM Tris pH 8.0. These suspensions were serially diluted tenfold in 10 mM Tris pH 8.0. Genomic DNA was extracted as described above (Genomic DNA extraction from cultured yeast strains) from each suspension corresponding to 107, 106, 105, or 104 C. glabrata cells. The DNA was resuspended 50 µL of Tris 10 mM pH8. Two microliters of each genomic DNA was used for PCR reactions with each set of primers in a final volume of 30 µL, and to visualize the PCR fragments, 10 µL of the reaction was loaded in agarose gels. The cell suspensions were also serially diluted in sterile whole blood and genomic DNA was extracted as above after the red blood cells were removed as described below (DNA extraction from blood cultures).
To determine the specificity of the oligonucleotide pairs, we performed experiments using mixtures of genomic DNA from several yeast species. Thirty-microliter PCR reactions were set up, each containing a mix of 100 ng of each genomic DNA from C. albicans, C. tropicalis, C. parapsilosis, C. bracarensis, and S. cerevisiae (500 ng total) with decreasing concentrations of genomic DNA from C. glabrata (100, 10, 1, or 0 ng) added. The PCR reactions were performed with each set of primers.
rDNA amplification
We selected 5 clinical isolates (MC15, MC18, MC77, MC79, and MC89) that were negative for PCR with the C. glabrata-specific primer pairs, to amplify the internal transcribed spacer (ITS) from the rDNA array and sequence the PCR product. Genomic DNA was extracted and 50-µL PCR reactions using universal primers ITS1 and ITS4 (Table 2) were set up [19] with the high-fidelity DNA Polymerase iProof (Bio-Rad). We extracted genomic DNA from isolates MC15, MC18, MC77, MC79, and MC89 to set up endpoint PCR reactions using the conditions outlined in Table 3.
DNA extraction from blood cultures
We tested 24 yeast-positive blood cultures (diagnosed in the hospital using Vitek-2®) shown in Table 4 (cultures AN342–AN463, which are non-consecutive numbers, Table 4). Total DNA was prepared directly from each blood culture as described previously [17]. Briefly, to remove red blood cells from the cultures, 500 µL of Candida sp.-positive blood cultures was mixed with 500 µL of Buffer GB (10 mM Tris–HCl pH 8.0, 1% Triton X-100, 11% sucrose) [20] and vortexed. The mix was centrifuged for 3 min at 14,000 rpm and the pellet resuspended in 500 µL of Buffer GB. This was repeated 4 times and the final pellet resuspended in 50 µL of TE. The genomic DNA was extracted as described [17].
Table 4.
Identification of C. glabrata from DNA extracted from blood cultures using species-specific oligonucleotide pairs Cg1, Cg2, and Cg3 and comparison with reference method API® ID 32C
| No | Strain (from blood culture) | Identified by | ||
|---|---|---|---|---|
| Hospital(Vitek-2®) | Biochemical(API® ID 32C) | PCR from blood culture(Cg1, Cg2 and Cg3)b | ||
| 1 | AN342c | C. lusitaniae | C. pelliculosa | NDd, e |
| 2 | AN349c | C. glabrata | C. albicans | ND |
| 3 | AN358c | C. albicans | C. parapsilosis | ND |
| 4 | AN428 | C. parapsilosis | C. parapsilosis | ND |
| 5 | AN430 | C. albicans | C. albicans | ND |
| 6 | AN431 | C. tropicalis | C. tropicalis | ND |
| 7 | AN435 | C. tropicalis | C. tropicalis | ND |
| 8 | AN436 | C. glabrata | C. glabrata | C. glabrata |
| 9 | AN440 | C. glabrata | C. glabrata | C. glabrata |
| 10 | AN442 | C. tropicalis | C. tropicalis | ND |
| 11 | AN445 | C. tropicalis | C. tropicalis | ND |
| 12 | AN446 | C. tropicalis | C. tropicalis | ND |
| 13 | AN447 | C. dubliniensis | C. dubliniensis | ND |
| 14 | AN448 | C. dubliniensis | C. dubliniensis | ND |
| 15 | AN452 | C. glabrata | C. glabrata | C. glabrata |
| 16 | AN453 | C. glabrata | C. glabrata | C. glabrata |
| 17 | AN454 | C. glabrata | C. glabrata | C. glabrata |
| 18 | AN455 | C. glabrata | C. glabrata | C. glabrata |
| 19 | AN456 | C. albicans | C. albicans | ND |
| 20 | AN457 | C. albicans | C. albicans | ND |
| 21 | AN459 | C. glabrata | C. glabrata | C. glabrata |
| 22 | AN460 | C. albicans | C. albicans | ND |
| 23 | AN462 | C. parapsilosis | C. parapsilosis | ND |
| 24 | AN463 | C. parapsilosis | C. parapsilosis | ND |
aStrains isolated from the indicated blood cultures were used for detection with API® ID 32C
bPCR was performed as described in “Materials and methods” using the 3 C. glabrata-specific oligonucleotide pairs Cg1, Cg2, and Cg3. The results were identical with all three primers
cFor strains AN342, AN349 and AN358 the result of the diagnostic test made at the hospital is different from the result obtained with the diagnostic made using the method API® ID 32C. The species-specific PCR is correct since it did not amplify any product with C. glabrata-specific primers (true negative C. glabrata samples)
dND means no PCR product detected with the three pairs of species-specific oligonucleotides
eAll the cases where no PCR product was detected (ND) correspond to true negative C. glabrata isolates as judged by the method API® ID 32C and in several cases by rDNA sequencing. In these cases, the isolates were other non-C. glabrata, Candida spp. (C. pelliculosa, C. albicans, C. tropicalis, C. parapsilosis, and C. dubliniensis)
DNA extraction from whole blood
The procedure for genomic DNA extraction from blood cultures was also used to purify DNA from C. glabrata cells resuspended in sterile whole blood. C. glabrata cells were counted in a haemocytometer and 107 cells were resuspended in 1 mL of whole blood. The cell suspensions were serially diluted in sterile whole blood and genomic DNA was extracted as described above (Supplementary Fig. S2).
Identification of Candida spp. using the biochemical standard test API® ID 32C
We isolated the Candida spp. from the 24 blood cultures listed in Table 4 by plating 20 µL of each blood culture on rich media (YPD) to obtain single colonies. With these purified isolates from each blood culture, we performed the identification of the Candida spp. by API® ID 32C (BioMerieux Inc., France) following the manufacturer’s instructions. Strips were read and scored after 48 h and the profile reading was made using APIWeb™ software.
DNA extraction from urine samples
We obtained 19 urine samples from patients hospitalized with suspected urinary tract infections but were not diagnosed. Five hundred microliters µL of fresh urine were centrifuged and the pellet was resuspended in 1 mL of 10 mM Tris–HCl pH 8.0 with 0.5% Triton. The samples were centrifuged for 5 min at 13,000 rpm and the pellet washed in 1 mL of 10 mM Tris–HCl pH 8.0. The pellet was resuspended in 20 µL of 10 mM Tris–HCl pH 8.0, incubated 15 min at 94 °C, and centrifuged for 5 min at 13,000 rpm. The supernatant was transferred to a clean tube and stored at − 20 °C until use for PCR reactions.
Ethics statement
The clinical isolates and hemocultures were obtained from Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INMNSZ) and the protocol to obtain and diagnose the isolates was previously approved by the local Committee for Ethics in Research from the INMNSZ (Ref-1912) and by the local committees from IPICYT: CBMA-IPICYT (REF-02) and CSH&MA-IPICYT (REF-15).
Results
Accurate and expedite diagnosis of the Candida species causing candidemia in patients is critical in the clinic since (a) there is a clear correlation between the time of treatment and associated mortality [10] and (b) species differ in their susceptibility to antifungals [21].
The Candida glabrata genome contains several copies of C. glabrata species-specific sequences
C. glabrata adheres to epithelial cells in vitro, which is mediated by the adhesin Epa1 [22]. Expression of EPA1 is negatively regulated by a cis-acting regulatory element (NE) localized downstream from EPA1 [18], which is only found in the genome of C. glabrata but not in other microbial species. The CBS138 strain (ATCC2001) contains 10 non-identical copies of the NE, some associated with EPA genes, others to additional cell wall protein-encoding (CWP) genes (see Fig. 1), and the rest to other non-cell wall protein genes (http://www.candidagenome.org/).
Primers design for C. glabrata-specific DNA amplification
We considered that the NE sequences present in the genome could be used as a barcode to specifically detect C. glabrata by PCR amplification. We selected five copies of the NE and put them into two groups: the first group contains the NE associated with EPA1, EPA22, and AWP13 (Fig. 1 lines 1, 2, and 3); and the second group includes the NEs associated with EPA6 and EPA7 (Fig. 1 lines 4 and 5) (see Supplementary Fig. S1A and B).
We designed two sets of primers to detect the first group of NE copies: two forward primers (242 and 246) and one common reverse primer (245) (Fig. 1 lines 1, 2, and 3 and Table 2). Using primer pair 242 and 245 (which we called pair Cg1), we expected to amplify three fragments and with primer pair 246 and 245 (pair Cg2) another three fragments (Table 3). For the second group of NE copies, we designed one set of primers called Cg3 consisting of primers 244 (Fw) and 243 (Rv) (Table 2), with which we expected to amplify two fragments as shown in Table 3 and Fig. 1 lines 4 and 5 and Supplementary Fig. S1A and B.
A fragment of the NE can be used to specifically detect the presence of C. glabrata DNA
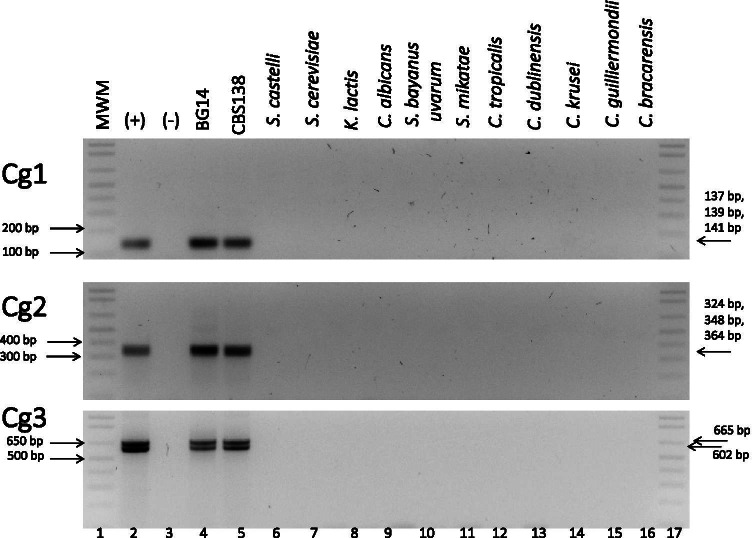
We first tested whether we could specifically detect C. glabrata but not other related species. We tested the three primer pairs using DNA extracted from two C. glabrata control strains: CBS138 (http://www.candidagenome.org/) and BG14 (Table 1). In addition, we extracted DNA from 5 other species of the closely related Saccharomyces clade (S. mikatae, S. bayanus, S. cerevisiae, S. castelli, Kluyveromyces lactis) as well as 7 other species from the Candida clade (C. albicans, C. dubliniensis, C. tropicalis, C. parapsilosis, C. guilliermondii, C. krusei, and C. bracarensis). The results show that we can only amplify the expected specific fragments from C. glabrata DNA but not from DNA extracted from any other of the related species used (Fig. 2). We obtained the expected band of ~ 140 bp, with Cg1, comprised of three fragments that cannot be resolved by agarose gel electrophoresis. In the case of Cg2 primer pair, we detected one band that contains three different fragments. With primer pair Cg3, we could distinguish the two expected bands (see Table 3 for expected fragment sizes with each oligo pair). To confirm that we indeed amplified the expected PCR products, we cloned each of these bands (from strain BG14) and sequenced individual clones from each primer set. The results show that the expected amplification products from chromosomes C, E, H, and K were obtained: 4 clones corresponding to the NE-EPA1 on chromosome E, 2 clones of the NE-AWP13 on chromosome H with primer pair Cg1; two clones of the NE-EPA22 on chromosome K with Cg2 and one clone each of the NE-EPA6 and NE-EPA7 with Cg3 (Supplementary Table S1). We also obtained clones with other NEs such as the NE-YPS3 on chromosome E (with Cg1) and the NE-PFY1 on chromosome I using Cg2 (Supplementary Table S1). These results indicate that the NE from C. glabrata can be specifically amplified and used as barcodes to identify C. glabrata.
Fig. 2.
Primer pairs Cg1, Cg2, and Cg3 specifically detect only C. glabrata. Genomic DNA from the indicated species from the Saccharomyces clade was used to amplify C. glabrata-specific sequences using each of the pairs Cg1, Cg2, and Cg3 as labeled in each gel. Lane 1: MWM relevant MWM bands are indicated on the left side with an arrow; lane 2: ( +) positive control (genomic DNA from strain BG14 previously tested for PCR reactions). Expected sizes of the bands obtained with each pair of primers is indicated with an arrow on the right side of the image; lane 3: (-) negative control: reaction mixture without DNA. Lanes 4 to 16: PCR reactions using genomic DNA from: parental BG14 strain, standard CBS138, Saccharomyces castellii (NC4c2829); Saccharomyces cerevisiae (S288c; ATCC 204,508); Kluyveromyces lactis (CLIB 209); Candida albicans (SC5314, ATCC MYA2876); Saccharomyces bayanus uvarum (CLI 251); Saccharomyces mikatae (IFO1815); Candida tropicalis (ATCC 750); Candida dublinensis (CD36); Candida krusei; Candida guilliermondii; Candida bracarensis (NCYC 3133) respectively; lane 17: MWM
The Cg primer sets amplify small amounts of cognate DNA in mixes of DNA from related species
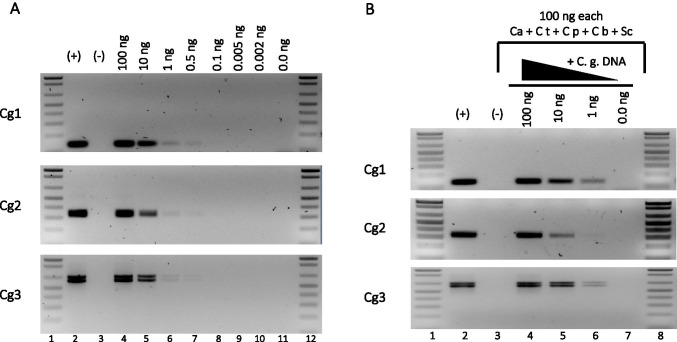
To determine the smallest amount of DNA that can be amplified with the C. glabrata-specific primers, we used serial dilutions of genomic DNA (from strain BG14). We can detect from 1 to 0.5 ng of C. glabrata genomic DNA under these conditions with the three primer pairs (Fig. 3A). We then asked whether we could amplify the specific C. glabrata fragments using mixes of excess genomic DNA (100 ng each) from 5 other related species from the Saccharomycotina group with decreasing concentrations of C. glabrata genomic DNA (see “Materials and methods”). We observed that the specific C. glabrata NE fragments are amplified from these complex mixes of DNA with all three primer pairs (Fig. 3B). This amplification depends on the presence of the cognate (C. glabrata) DNA, and the amount of PCR product is proportional to the amount of C. glabrata DNA added to the mix. We also determined that pairs Cg1 and Cg2 can detect a minimum of 3,300 cells while Cg3 can detect a minimum of 33,000 cells (data not shown). In addition, when we extracted DNA from C. glabrata cells suspended in sterile whole blood (see “Materials and methods”), we detected the same number of cells as when the DNA extraction was performed using cells resuspended in sterile water (3,300 cells with Cg1 and Cg2 and 33,000 with Cg30). These results indicate that DNA extraction from whole blood can be used for PCR identification of C. glabrata (Fig. S2 A and data not shown).
Fig. 3.
C. glabrata-specific primer pairs Cg1, Cg2, and Cg3 detect small amounts of genomic DNA. (A) Amplification products obtained by endpoint PCR with primer pairs Cg1, Cg2, and Cg3 with decreasing amounts of genomic DNA as indicated (lanes 4 to 11). Lanes 1 and 12: molecular weight markers (MWM). Lanes 2 ( +) and 3 (-): positive and negative controls as described for Fig. 2B Amplification products with C. glabrata-specific primer pairs Cg1, Cg2, and Cg3 using as template mixes of 100 ng each of purified genomic DNA from C. albicans (Ca), C. tropicalis (Ct), C. parapsilosis (Cp), C. bracarensis (Cb), and S. cerevisiae (Sc), in addition to decreasing amounts of C. glabrata DNA. Lanes 1 and 8: MWM; lanes 2 ( +) and 3 (-): positive and negative controls as described for Fig. 2. Lanes 4 to 7: mixes of 100 ng each Ca, Ct, Cp, Cb, Sc genomic DNA plus 100 ng (lane 4), 10 ng (lane 5), 1 ng (lane 6), or 0 ng (lane 7) of C. glabrata genomic DNA
Cg1, Cg2, and Cg3 primer pairs can correctly identify C. glabrata from a collection of 86 clinical isolates
We used a collection of 86 C. glabrata clinical isolates from three hospitals in Mexico. Each hospital had isolated and identified the C. glabrata isolates with VITEK-2®. We tested whether we could amplify the expected PCR products with our primers. In Fig. 4, we show an example of the amplicons obtained with the genomic DNAs from the first 16 clinical isolates (the rest of the clinical isolates are shown in Supplementary Figs. S3, S4, and S5). All of the 16 isolates shown in Fig. 4 except MC15 show the specific bands expected with all the primers. MC15 was negative with the three primer pairs, suggesting that it could be a species other than C. glabrata.
Fig. 4.
Primer pairs Cg1, Cg2, and Cg3 correctly identify C. glabrata clinical isolates. Genomic DNA from the indicated clinical isolates was used as template DNA for PCR reactions with Cg1, Cg2, and Cg3 C. glabrata-specific primer pairs. Lanes 1 and 20: molecular weight markers (MWM) and position of relevant sized bands are indicated with arrows on the left. Lanes 2 ( +) and 3 (-): positive and negative controls as described for Fig. 2. Lanes 4 through 19: genomic DNA from clinical isolates: MC1, MC2, MC3, MC4, MC5, MC6, MC7, MC8, MC10, MC11, MC12, MC13, MC14, MC15, MC16, and MC17 as indicated. The size and position (arrow) of the expected bands obtained with each pair of primers is indicated on the right side. With primer pair Cg3 over 80% of the isolates present only the 602-bp amplicon (associated with EPA6) but not the 665-bp fragment (associated with EPA7). The positive control (lane 2) shows both amplicons obtained using genomic DNA from BG14. Note that MC15 did not produce amplification bands with any of the primer pairs since it is not C. glabrata but C. albicans
With primer pair Cg3, most of the isolates display the smallest fragment (602 pb associated with EPA6); however, not all isolates show the 665-bp fragment associated with EPA7 (Fig. 4 and Fig. S5), in contrast to BG14 and the CBS138 reference strains which contain both fragments.
Of the 86 isolates tested, only 5 were negative for all three primer pairs (MC15, MC18, MC77, MC79, and MC89) (see Fig. 4 and Figs. S3, S4, and S5). The genomic DNA from these isolates was used to amplify a fragment of the rDNA locus with universal primers ITS1 and ITS4 [23, 24]. We obtained a 535-bp band from the 5 samples (Fig. S6). The size of this amplicon suggests that these isolates are not C. glabrata since the expected size for this organism is 878 bp [25]. To corroborate that the PCR-negative clones are not C. glabrata, we sequenced the amplicon obtained with primers ITS1 and ITS4. The sequence showed that the PCR-negative clinical isolates are C. albicans (data not shown). Therefore, we can detect 100% of the C. glabrata isolates, with no false positive results, and thus with 100% specificity.
The C. glabrata-specific primer pairs can detect C. glabrata from yeast-positive blood cultures and urine samples
We next used Cg1, Cg2, and Cg3 primer pairs to detect C. glabrata in samples of DNA extracted from blood cultures that tested positive for yeast at the hospital using BACTEC™ equipment as well as urine specimens (that had not been cultured) from hospitalized patients. We selected 24 yeast-positive blood cultures and extracted DNA directly from the culture (“Materials and methods”). We found that 7 of 24 blood cultures were positive for the three sets of C. glabrata primers (AN436, AN440, AN552, AN453, AN454, AN455, and AN459; Table 4). We also obtained the Candida sp. strain from each of the blood cultures (corresponding isolates) and we performed the API® ID 32 C test to identify the 24 isolates. The 7 blood culture samples identified as C. glabrata by our PCR method were also diagnosed as C. glabrata by API® ID 32C (as well as with Vitek-2® procedure at the hospital; Table 4). This indicates that our method correctly identifies C. glabrata from blood cultures and therefore gives no false positive results when compared with API® ID 32C. All the other PCR-negative isolates from the blood cultures were identified as a different Candida species by API® ID 32C (Table 4).
It is noteworthy that three of the 24 isolates from the blood cultures did not coincide between the result obtained with Vitek-2® and API® ID 32C. AN342, AN349, and AN358 were identified as C. pelliculosa, C. albicans, and C. parapsilosis respectively with API® ID 32C but identified as C. lusitaniae, C. glabrata, and C. albicans respectively with Vitek-2®. Importantly, these isolates were negative for C. glabrata with our method, confirming that we obtain no false positive results (Table 4).
We also tested our three C. glabrata primer pairs using DNA extracted from 18 urine samples from patients from Hospital Central Ignacio Morones Prieto in San Luis Potosí. These samples were not cultured or tested previously for the presence of yeast. Four samples (SL17, SL31, SL101, and SL202) were positive with all three primer pairs (Fig. S7 and data not shown).
Discussion
Candidemia usually occurs in patients with severely compromised immune systems [26]. While C. albicans is still the most frequently encountered species in candidemia in the intensive care units worldwide, there has been a shift towards non-albicans species. C. glabrata is now the second most common species in northern Europe, Canada, the USA, and Mexico [5, 8, 27, 28]. Since Candida spp. differ in the susceptibility to antifungals, it is extremely important to have an accurate identification of the species causing the candidemia to provide the appropriate treatment promptly. When the appropriate antifungal is administered as early as possible after the first positive blood culture, the outcome of the patient is better [11, 29]. Available diagnostic methods in the clinical setting are not sensitive enough, or they have long turnaround times or they have not been standardized or FDA approved. The gold standard to diagnose candidemia is still blood culture, although this method takes 3–6 days to identify the species with low sensitivity (~ 50%) [13–15, 29]. Non-culture methods are much faster and some are routinely used. For example, serological methods, which determine the presence of common Candida sp. antigens (mannan antigen or B-d-glucan) or anti-Candida antibodies (anti-mannan Ab). Although their negative predictive value is very good, these methods frequently give false positive results [14]. Molecular methods based on PCR amplification of the conserved rDNA array in Candida spp. are widely used because they are fast and easy to use. Amplification of these regions has the advantage that the rDNA array is present in multiple copies in the genome so it is easily amplified, but the amplicon size obtained from three of the four most common Candida spp. is very similar (C. albicans, C. parapsilosis, and C. tropicalis), so additional steps such as nested PCR, digestion, or sequencing of the amplicon are needed to identify the species. Multiplex PCR can also be used as an efficient method to detect several species when qPCR is used to analyze the melting curves to differentiate between species, or when the PCR products are sequenced. Other promising molecular methods exist using FISH to target specific areas in the genome or MALDI-TOF MS (proteomic technology) but these methods require expensive specialized equipment, although once the first investment has been done, the cost per sample can be less expensive. In general, PCR tests have ± 95% sensitivity and ± 92% specificity and have the potential to be used for diagnosis but need to be standardized and/or FDA approved [4, 14] (Global Action Fund for Fungal Infections GAFFI: https://www.gaffi.org/). Ideally, diagnosis of candidemia to the species level should be made directly from blood samples, without having to use blood cultures (which increase the turnaround time and can interfere with later amplification steps). But the disadvantage is that non-culture methods start with very few Candida cells in the blood of patients (below the detection limit of many methods). However, one FDA-approved method based on magnetic resonance can detect only 1–3 CFU/mL of blood with a turnaround time of 4–6 h (T2MR) [30] and another PCR-based method can detect as little as 3 CFU/mL of blood but has not been validated yet in the clinic (MycoReal Candida PCR) [15].
Here, we developed a highly specific method, which can be used for positive identification of C. glabrata directly from whole blood or urine samples with high specificity, detecting C. glabrata-specific sequences (Supplementary Fig. S2 and S7). We compared our low-cost and easy to use method with API® ID 32C with 96 additional purified genomic DNA extracts from clinical isolates (Supplementary Table S2). We found no false positive or false negative samples with a 100% sensitivity and specificity compared to API® ID 32C performed on the same additional isolates.
The most accurate identification method is MALDI-TOF MS or DNA sequencing, and in this regard, we have sequenced several amplified genes from isolates identified as C. glabrata with our three primer pairs. Analysis of these sequences confirmed that they indeed correspond to C. glabrata. In addition, sequencing of the amplified ITS region from the rDNA array from several isolates that were negative for PCR with the C. glabrata-specific primers showed that all of them were Candida albicans (Table 4 and data not shown).
We can also identify C. glabrata from positive blood cultures and urine samples, or directly from sterile whole blood spiked with C. glabrata cells. However, the sensitivity of the method is not sufficiently high to detect C. glabrata directly from clinical specimens without previous culture to amplify the number of C. glabrata cells. Using endpoint PCR, we can detect 1–0.5 ng of purified DNA (Fig. 3A) or ~ 3,300 or ~ 33,000 C. glabrata cells depending on the primers used (Supplementary Fig. S2 and S7).
The sensitivity of the method can be expected to increase if qPCR is used. We have started optimizing qPCR conditions so that a lower number of cells can be detected. Another alternative is to briefly incubate blood cultures for 12 to 24 h so that more C. glabrata cells per mL of blood culture are obtained. This would decrease the total blood culture incubation period used now by 24–48 h and it is expected that once there are enough cells of C. glabrata in the blood culture, the detection time will be an additional ~ 4 h for the PCR amplification.
The set of C. glabrata-specific oligonucleotide pairs Cg1, Cg2, and Cg3 is a promising tool for which we will next determine its performance in the clinic. In addition, we are currently designing species-specific primers for the other three most common Candida spp.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Lina Riego, Dr. Héctor Mora, and Dr. Celia Pais for providing reference strains and Dr. Areli Martínez, Dr. José Sifuentes, Dr. Pedro Torres from Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán: INCMNSZ, and Dr. Javier Araujo from Hospital Central Ignacio Morones Prieto for providing Candida sp. clinical isolates and blood cultures.
We thank Mayra Cuéllar-Cruz for technical support in the initial experiments.
The authors also thank Verónica Zárate and LANBAMA-IPICYT for sample sequencing.
Author contribution
C. H. H.; G. H. H.; and B. E. G. G. were involved in the optimization of PCR conditions. O. H. C.; C. H. H.; G. H. H.; M. S. H. B.; and B. E. G. G. performed PCR identification of Candida isolates, hemocultures, and urine samples and API® ID 32C validation experiments. G. G. E. planned and executed experiments. O. H. C. and C. H.-H. determined minimal amount of DNA detected. N. I. G. G. and D. B. P. wrote and followed up the patent applications. A. D. L. P. and I. C. were involved in conceptualization, design of the experiments, and design of the species-specific oligonucleotide pairs. I. C. wrote the manuscript and all authors were involved in reviewing and editing of the manuscript.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) Grants No. CB 2005–01-48304 and No. CB 2014–239629 to I.C. and Fondo Sectorial de Salud-CONACyT to A. D. L. P. No. 2005–13927.
O.H.-C.; C.H.-H.; and M.S.H.-B. were supported by CONACyT fellowship nos. 611321, 279,071, and 932,042 respectively. B.E.G.-G. was supported by a postdoctoral fellowship from CONACyT No. 205158 and G.H.-H. received a CEN BES fellowship by Secretaría de Educación Pública and a fellowship from IPICYT.
Data availability
All plasmids and yeast strains are available upon request.
The oligonucleotide pairs Cg1, Cg2, and Cg3 described in this work were designed by A. D. L. P. and have been included in patents MX352246 B and EP2410052 B1.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Oscar Hernández-Carreón, Email: oscar.hecar@gmail.com.
Cesia Hernández-Howell, Email: cesiahdez@hotmail.com.
Grecia Hernández-Hernández, Email: grecia.hernandez@ipicyt.edu.mx.
M. Selene Herrera-Basurto, Email: seleneherrerabasurto@gmail.com.
Blanca E. González-Gómez, Email: blancaeglezgomez@gmail.com
Guadalupe Gutiérrez-Escobedo, Email: maria.gutierrez@ipicyt.edu.mx.
Norma I. García-Calderón, Email: ngarcia@patentech.mx
Daniel Barrón-Pastor, Email: 4dbarron@gmail.com.
Alejandro De Las Peñas, Email: cano@ipicyt.edu.mx.
Irene Castaño, Email: icastano@ipicyt.edu.mx.
References
- 1.Chang A, Neofytos D, Horn D. Candidemia in the 21st century. Future Microbiol. 2008;3(4):463–472. doi: 10.2217/17460913.3.4.463. [DOI] [PubMed] [Google Scholar]
- 2.Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis and treatment. Med Mycol. 2007;45(4):321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36(1):1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 4.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis Nat Rev Dis Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 6.Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson M, Rautemaa R. How the host fights against Candida infections. Front Biosci (Landmark Ed) 2009;14:4363–4375. doi: 10.2741/3533. [DOI] [PubMed] [Google Scholar]
- 8.Toda M, Williams SR, Berkow EL, Farley MM, Harrison LH, Bonner L, et al. Population-based active surveillance for culture-confirmed candidemia - four sites, United States, 2012–2016. MMWR Surveill Summ. 2019;68(8):1–15. doi: 10.15585/mmwr.ss6808a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 10.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 11.Puig-Asensio M, Peman J, Zaragoza R, Garnacho-Montero J, Martin-Mazuelos E, Cuenca-Estrella M, et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med. 2014;42(6):1423–1432. doi: 10.1097/CCM.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 12.Clancy CJ, Nguyen MH 2018 Diagnosing invasive Candidiasis. J Clin Microbiol;56(5). 10.1128/JCM.01909-17 [DOI] [PMC free article] [PubMed]
- 13.Phoompoung P, Chayakulkeeree M. Recent progress in the diagnosis of pathogenic Candida species in blood culture. Mycopathologia. 2016;181(5–6):363–369. doi: 10.1007/s11046-016-0003-x. [DOI] [PubMed] [Google Scholar]
- 14.Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27(3):490–526. doi: 10.1128/CMR.00091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs S, Lass-Florl C, Posch W 2019 Diagnostic performance of a novel multiplex PCR assay for Candidemia among ICU patients. J Fungi (Basel);5(3). 10.3390/jof5030086 [DOI] [PMC free article] [PubMed]
- 16.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 17.Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: Wiley; 2001. [Google Scholar]
- 18.Gallegos-Garcia V, Pan SJ, Juarez-Cepeda J, Ramirez-Zavaleta CY, Martin-del-Campo MB, Martinez-Jimenez V, et al. A novel downstream regulatory element cooperates with the silencing machinery to repress EPA1 expression in Candida glabrata. Genetics. 2012;190(4):1285–1297. doi: 10.1534/genetics.111.138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 20.Riera MA, Rojas ME, Zapata PD. Protocolo de extracción de DNA por salting-out para pequeños volúmenes de sangre. Rev Cienc Tecnol. 2010;12(14):4–7. [Google Scholar]
- 21.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis. 2012;73(1):45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Cormack BP, Ghori N, Falkow S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science. 1999;285(5427):578–582. doi: 10.1126/science.285.5427.578. [DOI] [PubMed] [Google Scholar]
- 23.White PL, Shetty A, Barnes RA. Detection of seven Candida species using the Light-Cycler system. J Med Microbiol. 2003;52(Pt 3):229–238. doi: 10.1099/jmm.0.05049-0. [DOI] [PubMed] [Google Scholar]
- 24.White TJ, Bruns, T, Lee, S, Taylor J 1990 Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. San Diego, USA: Academic Press, Inc; p. 315-22
- 25.Fujita SI, Senda Y, Nakaguchi S, Hashimoto T. Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains. J Clin Microbiol. 2001;39(10):3617–3622. doi: 10.1128/JCM.39.10.3617-3622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Editorial. Correction: Stop neglecting fungi. Nat Microbiol. 2017;2:17123. 10.1038/nmicrobiol.2017.123 [DOI] [PubMed]
- 27.Corzo-Leon DE, Alvarado-Matute T, Colombo AL, Cornejo-Juarez P, Cortes J, Echevarria JI, et al. Surveillance of Candida spp bloodstream infections: epidemiological trends and risk factors of death in two Mexican tertiary care hospitals. PLoS ONE. 2014;9(5):e97325. doi: 10.1371/journal.pone.0097325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74(4):323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez J, Erstad BL, Petty W, Nix DE. Time to positive culture and identification for Candida blood stream infections. Diagn Microbiol Infect Dis. 2009;64(4):402–407. doi: 10.1016/j.diagmicrobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller MA, Wolk DM, Lowery TJ. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol. 2016;11(1):103–117. doi: 10.2217/fmb.15.111. [DOI] [PubMed] [Google Scholar]
- 31.Cormack BP, Falkow S. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics. 1999;151(3):979–987. doi: 10.1093/genetics/151.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correia A, Sampaio P, James S, Pais C. Candida bracarensis sp. nov., a novel anamorphic yeast species phenotypically similar to Candida glabrata. Int J Syst Evol Microbiol. 2006;56(Pt 1):313–7. doi: 10.1099/ijs.0.64076-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All plasmids and yeast strains are available upon request.
The oligonucleotide pairs Cg1, Cg2, and Cg3 described in this work were designed by A. D. L. P. and have been included in patents MX352246 B and EP2410052 B1.