Abstract
The search for new compounds with activity against Paracoccidioides, etiologic agents of Paracoccidioidomycosis (PCM), is extremely necessary due to the current scenario of the available therapeutic arsenal. Treatment is restricted to three classes of antifungals with side effects. Curcumin is a polyphenol with antifungal effects that is extracted from Curcuma longa. The present work aimed to evaluate the activity of curcumin in different species of Paracoccidioides and to evaluate the potential molecular targets of curcumin using computational strategies. In addition, interactions with classic antifungals used in the treatment of PCM were evaluated. Curcumin inhibits the growth of Paracoccidioides spp. exerting a fungicidal effect. The combination of curcumin with amphotericin B, co-trimoxazole, and itraconazole showed a synergistic or additive interaction. Molecular targets as superoxide dismutase, catalase, and isocitrate lyase were proposed based on in silico approaches. Curcumin affects the fungal plasma membrane and increases the production of reactive oxygen species. Therefore, curcumin is a good alternative for the treatment of PCM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00548-6.
Keywords: Antifungal activity, Curcumin, Paracoccidioides spp.
Introduction
Paracoccidioidomycosis (PCM), a systemic mycosis caused by Paracoccidioides, has presented a serious public health problem. The majority of the patients is unable to work due to the sequelae that compromise their vital functions [1, 2]. The treatment of the disease is usually long and lasts from 1 to 2 years, depending on the degree of progression of the disease [3]. In addition, the limitations of antifungals have become increasingly evident in the clinic, such as toxicity, drug interactions, restricted routes of administration, and reduced bioavailability in target tissues [4], demonstrating the importance of antifungal drug research.
Nature is a rich source of new antifungal compounds and the therapeutic potential of plants is commonly used in popular medicine [5]. Due to the chemical diversity of plants, several researchers have concentrated efforts on the evaluation of the antifungal activity of extracts and essential oils from medicinal plants [6]. Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a polyphenolic compound described as the main phytochemical of Curcuma longa L. (Zingiberaceae family) rhizome, popularly known as turmeric and has been extensively investigated due to its benefit to human health. This compound presents anti-inflammatory, antimutagenic and anticancer activity [7]. Regarding its antimicrobial potential, antibacterial, antiviral, and antifungal activities have already been reported [8]. Studies have explored the toxicity of turmeric and curcumin and showed that the compound is relatively safe at an orally dose of 6 g/day for 4–7 weeks [9]. In addition, nephroprotective, hepatoprotective, and immunomodulatory effects have been associated with curcumin [10, 11]. Therefore, curcumin is an alternative to current antifungal therapies.
The identification of targets is an important step in drug development, either by mechanism analysis or identification of interactions that may cause undesirable effects [12]. The advance of bioinformatics has led to the development of several databases in order to organize the proteomic data and other tools that facilitate the sequential, structural, and functional analysis of proteins [13]. Platforms, such as PharmMapper, gather information available in databases [14] such as UniProt [15], BindingDB [16], PDTD [17], and DrugBank [18] in order to identify target compounds, thus increasing the reliability of predictions. PharmMapper is a free online server that predicts targets using a pharmacophore mapping approach [19] and identifies possible targets of compounds [20, 21].
Based on the potential of curcumin, this work aimed at the investigation of its antifungal activity against Paracoccidioides spp., the evaluation of curcumin interaction with the antifungals employed in the treatment of PCM, the investigation of targets through in silico approach, and the evaluation of its effect on the plasma membrane and induction of reactive oxygen species (ROS) in Paracoccidioides cells.
Materials and methods
Biological activity evaluation
Chemicals
Curcumin, amphotericin B (AmB), and itraconazole (ITZ) were purchased from Sigma-Aldrich. Co-trimoxazole (CMX) was purchased from Prati Donaduzzi®. All compounds were solubilized in dimethylsulfoxide (DMSO) at 5%.
Microorganisms and culture conditions
Paracoccidioides lutzii (Pb01), P. brasiliensis (Pb18), P. americana (Pb03), and P. restrepiensis (EPM83 and Pb6085) yeast cells were cultivated in liquid Fava-Netto medium (0.3% protease peptone, 1% peptone, 0.5% (w/v) meat extract, 0.5% (w/v) yeast extract, 4% glucose, 0.5% NaCl), pH 7.2 for 48 h at 37 °C under agitation at 120 rpm. Subsequently, the experiments were carried out using RPMI 1640 medium (Sigma-Aldrich).
Analysis of fungal susceptibility
The determination of the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) was performed using the microdilution technique as described by Silva et al. [22]. Serial dilutions of curcumin (125 to 0.48 μg/mL) were added to fungal suspension (final concentration of 1 × 105 cells/mL) and maintained at 37 °C under agitation for 48 h. Samples without curcumin were used as positive growth control. Subsequently, 20 μL of resazurin at 0.02% (a redox indicator) was added and samples were incubated for 24 h at 37 °C. The MIC value was visually determined based on the color change of resazurin (blue) into resorufin (pink). To determine the MFC, 20 μL of the corresponding MIC sample was cultivated in a solid Fava-Netto medium. The positive control was also plated. Plates were incubated at 37 °C for 7 days. The MFC was defined as the lowest concentration where fungal growth was not visualized.
Cytotoxicity
The cytotoxicity (compound concentration required to decrease total cell viability) was evaluated using BALB/c 3T3 clone A31 (ATCC® CCL-163™). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (Nutricell). 1 × 105 cells/mL were incubated at different concentrations of curcumin at 37 °C for 48 h, 5% CO2. Then, 20 μL of resazurin at 0.02% was added to each micro-well and incubated for 24 h. The cytotoxicity was visually determined based on the reduction of the dye and the color change of resazurin. The calculation of the selectivity index was defined as the ratio of the cytotoxic concentration to antifungal concentration.
Interaction of curcumin and antifungals
The interaction of antifungal agents (ITZ, AmB, and CMX) and curcumin was evaluated using the checkerboard method [23]. Dilutions of the antifungal and curcumin were disposed of in an orderly manner. The concentrations of curcumin decreased along the vertical direction and the concentrations of the antifungal decreased along the horizontal direction. Pb18 cells were added at the final concentration of 1 × 105 cells/mL. Samples were incubated at 37 °C and kept under agitation for 48 h. Resazurin solution (0.02%) was added to the samples and incubated for 24 h. Growth inhibition was determined by visual inspection. Fractional inhibitory concentration (FIC) was calculated using the formula: FICs = FICA + FICB, where FICA is the MIC of compound A combined with compound B divided by the MIC value of compound A; FICB is MIC of compound B combined with compound A divided by the MIC value of compound B. FICI values were interpreted as follows: synergic for FIC ≤ 0.5, additive for FIC > 0.5 to ≤ 1, indifferent effect for FIC > 1 to ≤ 4, antagonism for FIC > 4.
Evaluation of reactive oxygen species
The production of reactive oxygen species (ROS) was evaluated using dichlorodihydrofluorescein 2ʹ, 7ʹ-diacetate dye (DCFH-DC) (Sigma-Aldrich). 1 × 105 cells/mL of P. brasiliensis cells were incubated in the presence of 3.90 μg/mL of curcumin. Cells grown only in RPMI 1640 medium were used as control. Aliquots of 1 mL were collected after 3, 6, 9, 12, 24, 48, and 72 h. The cells were centrifuged at 2000 g for 5 min at 4 °C, resuspended in 1mL of PBS 1 × , and 25 μM of DCFH-DC was added. The mixture was kept in the dark for 30 min. The cells were washed twice with PBS 1 ×, resuspended in 1 mL of PBS 1x and observed under fluorescence microscopy (Zeiss Axiocam MRc – Scope A1) using λex equals 490 nm and λem equals 516 nm. The quantification of cells based on the fluorescence intensity was performed using the AxioVision V 4.8.2.0 (Carl Zeiss) apparatus where the values of pixels density and the area (in pixels2) of the cells were measured. We analyzed cells that showed fluorescence in the image and with well-defined borders. The fluorescence intensity was calculated by the ratio between the logarithmic values of the cell’s density and the area of control and treated samples of each time interval. The statistical difference between control and treated samples was assessed using Student’s T-test and p values ≤ 0.05 were significant.
Plasma membrane permeability assay
A total of 1 × 105 cells/mL of Pb18 was incubated in the presence of 3.90 μg/mL. Then, cells were centrifuged at 2000 g for 5 min at 4 °C, resuspended in PBS 1 × , and added 1.4 μM of propidium iodide (PI) (Sigma-Aldrich). The mixture was kept in the dark for 20 min. The cells were washed twice with PBS 1 ×, resuspended in 1mL of PBS 1x and observed under fluorescence microscopy (Zeiss Axiocam MRc – Scope A1) using λex equals 482 nm and λem equals 608 nm. The verification of changes in the membrane permeability was performed by measuring the proportion of fluorescent cells in relation to the total population of cells for both controls and treated samples in each period of time in triplicate. The statistical difference between control and treated samples was assessed using Student’s T-test and p values ≤ 0.05 were significant.
In silico analysis
Target prediction
To predict the potential target of curcumin, the MDL SDF format of the curcumin was obtained from the PubChem server (https://pubchem.ncbi.nlm.nih.gov) and submitted to the webserver PharmaMapper (http://www.lilab-ecust.cn/pharmmapper/) in order to perform reverse docking [19]. The parameters used in PharmMapper server were (i) conformation generation: generate conformers, yes; maximum generated conformations: 300 and (ii) pharmacophore mapping: pharmacophore models with pKd > 6.0 (v2017, 52,431); number of reserved matched targets (Max 1,000): 300. The first hundred targets suggested by the pharmacophore models in PharmMapper were selected and their sequences were obtained in the Uniprot server (http://www.uniprot.org/). The targets predicted were compared with the Pb18 (taxid: 502,780) proteins in the Blast server (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to select homologs proteins. Proteins with identity ≥ 40% were considered potential targets and subsequently classified functionally. Duplicate proteins were identified and considered only once.
Construction of protein–protein interaction network for potential targets
The STRING (Version 11.0) database (http://string-db.org/) was used to analyze the protein–protein interactions of the targets [24]. The sequences of the Pb18 proteins were submitted to the database and the string’s default parameters were used. (i) For the network edges, we selected the evidence option; (ii) active interaction sources were based on text mining, neighborhood, experiments, gene fusion, databases, co-occurrence, and co-expression; (iii) the minimum required interaction score was 0.0400; (iv) the max number of interactors for the 1st shell was none/query proteins only and for the 2nd shell was none; and (v) the network display mode was interactive SVG and no options were checked in the simplification display. STRING generates a score for each protein–protein interaction based on the supporting evidence, specificity, and reproducibility. Supporting evidence is classified into evidence channels that are represented by different edge colors. These channels are important to calculate the combined confidence score that is used to build the interaction networks. The evidence channels are experiment channel, database channel, text mining channel, co-expression channel, neighborhood channel, and co-occurrence channel [24].
Molecular docking of selected targets with curcumin
Homology modeling
The sequences of isocitrate lyase (PADG_04709), catalase (PADG_00225), superoxide dismutase (PADG_01755), and glucose-6-phosphate -1-dehydrogenase (PADG_04200) were retrieved from the NCBI server (https://www.ncbi.nlm.nih.gov/protein/) and the 3D structures were built using the SWISS-MODEL server [25]. Four main steps were used to build the homology model: (i) identification of structural template in Protein Data Bank (PDB) [26], (ii) alignment of protein sequence and template structures, (iii) model building; and (iv) analysis of the geometrical and stereochemical quality of the structures. Then, the best homology model was structurally optimized using the 3Drefine server [27], which refine the model in two stages. The first by optimizing the hydrogen network and the second by minimizing energy using composite physics and knowledge-based force fields [28]. The 3D structures were imported into the H + + server [29] and the protonation states of their residues were estimated at neutral pH 7.0. The MolProbity was used to analyze the quality of the generated models. In the MolProbity server, the quality of the dihedral angles of the backbone (ψ against φ) and angles of the side chain (χ) are indicated by Ramachandran and rotamers [30]. Other rating criteria are the clashscores, which is the amount of bad atom–atom overlaps per thousand atoms ≥ 0.4 [30] and MolProbity scores, a general statistic based on the values of clashscore, Ramachandran not favored, and bad side-chain rotamers [31].
Ligand preparation
The SMILES sequences of the curcumin, amitrole, epigallocatechin gallate, camphene thiosemicarbazide, and 3-nitropropionic acid were retrieved from the PubChem server [32] and carefully standardized according to the protocol proposed by Fourches and colleagues [33]. After structure standardization, up to 2000 conformations were generated for each drug using the OMEGA v.3.0.0.1 software [34]. The protonation states at neutral pH (7.4 ± 1.0) and AM1-BCC charges [35] were estimated using QUACPAC v.1.7.0.2 [36].
Molecular docking
The models of the isocitrate lyase, catalase, superoxide dismutase, and glucose-6-phosphate 1-dehydrogenase were subjected to the grid-generation protocol using a molecular probe available on OEDocking suite v.3.2.0 [37] for the detection of the binding pockets around the protein. Finally, molecular docking calculations were performed using the high-resolution protocol of the FRED program with the ChemGauss4 score function [38], both available on the OEDocking suite.
Results
Curcumin is fungicidal and presents a high index of the selectivity
The anti-Paracoccidioides activity of curcumin was verified in this work (Table 1). The results showed that curcumin inhibited the growth of Paracoccidioides spp. with MIC varying from 3.90 (10.59 μΜ) to 7.81 μg/mL (21.20 μΜ). Pb18 and Pb03 showed greater susceptibility to curcumin compared to other species. Curcumin showed a fungicidal effect at the same MIC concentration for all species. No cytotoxicity to mammalian cells was observed until 1000 μg/mL (2714.59 μΜ). Thus, the selectivity index showed different values between the species; for Pb18 and Pb03, the value was ≥ 255.7 while for Pb01, EPM83, and Pb60855 was ≥ 128.0.
Table 1.
Biological activity of curcumin against Paracoccidioides spp
| Isolates | MIC100 | MFC | CC100 | SI |
|---|---|---|---|---|
| Pb01 | 7.81 | 7.81 | > 1000 | > 128.0 |
| Pb03 | 3.90 | 3.90 | > 1000 | > 255.7 |
| Pb18 | 3.90 | 3.90 | > 1000 | > ≥ 255.7 |
| PbEPM83 | 7.81 | 7.81 | ≥ 1000 | > ≥ 128.0 |
| Pb60855 | 7.81 | 7.81 | > 1000 | > 128.0 |
MIC, minimal inhibitory concentration; MFC, minimum fungicidal concentration; CC, cytotoxic concentration; SI, selectivity index. Paracoccidioides lutzii (Pb01), Paracoccidioides brasiliensis (Pb18), Paracoccidioides americana (Pb03), Paracoccidioides restrepiensis (EPM83 and Pb60855). MIC and MIF values are expressed in μg/mL
Curcumin interacts positively with antifungals used in the treatment of PCM
The association of curcumin and antifungals was evaluated. The combination of curcumin and antifungals used in the treatment of PCM showed additive and synergistic effects (Table 2). When combined with AmB, the effect was synergistic with a FIC index of 0.31 and a reduction of the MIC values from 3.90 to 0.96 μg/mL for curcumin and from 3.90 to 0.24 μg/mL for AmB. In combination with ITZ, the effect was synergistic with a FIC index of 0.50 and a reduction of the MIC value from 3.90 to 0.96 μg/mL for curcumin and from 0.007 to 0.001 μg/mL for ITZ. The combination of curcumin with CMX resulted in an additive effect with a FIC index of 0.56 and a decrease in MIC values from 3.90 to 1.95 μg/mL for curcumin and from 1000 to 62.5 μg/mL for CMX, respectively.
Table 2.
Interaction of curcumin with amphotericin B, itraconazole, and co-trimoxazole
| CUR + AmB | CUR + ITZ | CUR + CMX | |||
|---|---|---|---|---|---|
| MICCUR | 3.90 | MICCUR | 3.90 | MICCUR | 3.90 |
| MICAmB | 3.90 | MICITZ | 0.007 | MICCMX | 1000 |
| MICCUR combined | 0.96 | MICCUR combined | 0.96 | MICCUR combined | 1.95 |
| MICAmB combined | 0.24 | MICITZ combined | 0.001 | MICCMX combined | 62.5 |
| FIC index | 0.31 | FIC index | 0.50 | FIC index | 0.56 |
| Interaction | Synergistic | Interaction | Synergistic | Interaction | Additive |
MIC, minimal inhibitory concentration; FIC, fractional inhibitory concentration; CUR, curcumin; AmB, amphotericin B; ITZ, itraconazole; CMX, co-trimoxazole. MIC values are expressed in μg/mL
Important enzymes were proposed as molecular targets of curcumin in Paracoccidioides brasiliensis
PharmMapper server was used to identify the targets of curcumin in the fungus. The top 100 targets suggested for curcumin were classified according to the adjustment scores between the corresponding pharmacophoric models. The targets showed significant identity with 49 proteins from Paracoccidioides brasiliensis (Supplementary Table S1).
Those proteins were classified according to their functional categories (Table 3) as lipid, fatty acid, and isoprenoid metabolism (1 protein), tricarboxylic acid pathway (1 protein), glyoxylate cycle (1 protein), signal transduction (1 protein), and cell cycle (1 protein); translation (2 proteins), nucleotide metabolism (2 proteins), and protein fate (2 proteins); C-compound and carbohydrate metabolism (3 proteins), transcription (3 proteins), and ribosome biogenesis (3 proteins); amino acid metabolism (4 proteins); defense and virulence (6 proteins); and uncharacterized protein (19 proteins).
Table 3.
Curcumin targets in Paracoccidioides brasiliensis predicted in silico
| PharmMapper | Blast/Uniprot | ||||
|---|---|---|---|---|---|
| Rank | PDB ID | Fit score | PADG | Protein | Per. Ident |
| Amino acid metabolism | |||||
| 7 | 2HZP | 2.459 | PADG_00349 | Kynureninase | 45.14% |
| 17 | 1D7C | 3.286 | PADG_02101 | Glutamine amidotransferase type-2 domain-containing protein | 48.15% |
| 81 | 3HBX | 2.71 | PADG_06319 | Glutamate decarboxylase | 56.53% |
| 81 | 3HBX | 2.71 | PADG_11803 | Glutamate decarboxylase | 46.04% |
| C-compound and carbohydrate metabolism | |||||
| 9 | 1EXV | 2.986 | PADG_02145 | Alpha-1,4 glucan phosphorylase | 50.12% |
| 22 | 1QKI | 2.118 | PADG_04200 | Glucose-6-phosphate 1-dehydrogenase | 49.19% |
| 85 | 1DOS | 2.671 | PADG_00668 | Fructose-bisphosphate aldolase | 53.98% |
| Lipid, fatty acid and isoprenoid metabolism | |||||
| 55 | 2PFF | 2.876 | PADG_00254 | Fatty acid synthase subunit alpha | 59.92% |
| Tricarboxylic acid pathway | |||||
| 25 | 3CDK | 2.984 | PADG_04939 | Succinyl-CoA:3-ketoacid-coenzyme A transferase | 51.97% |
| Glyoxylate cycle | |||||
| 30 | 1IGW | 2.959 | PADG_04709 | Isocitrate lyase | 42.02% |
| Defense and virulence | |||||
| 8 | 1ONF | 2.459 | PADG_02367 | Glycerol-3-phosphate dehydrogenase | 54.84% |
| 34 | 1MA1 | 2.941 | PADG_01755 | Superoxide dismutase | 44.04% |
| 64 | 1RSO | 2.817 | PADG_05941 | Phospholipase | 41.94% |
| 94 | 2A9E | 3.93 | PADG_00324 | Catalase | 54.79% |
| 94 | 2A9E | 3.93 | PADG_00225 | Catalase | 41.58% |
| 94 | 2A9E | 3.93 | PADG_04740 | Catalase | 40.66% |
| Cell cycle | |||||
| 39 | 1YF6 | 2.926 | PADG_06763 | Septum formation protein Maf | 50.00% |
| Transcription | |||||
| 19 | 2Z6E | 2.726 | PADG_01508 | U1 small nuclear ribonucleoprotein C | 45.65% |
| 21 | 2CV8 | 2.139 | PADG_12304 | tRNA-splicing endonuclease subunit Sen2 | 40.43% |
| 54 | 2Z7X | 2.878 | PADG_01576 | Glucose-repressible alcohol dehydrogenase transcriptional effector | 41.03% |
| Translation | |||||
| 29 | 2ZU6 | 2.963 | PADG_11711 | ATP-dependent RNA helicase eIF4A | 74.87% |
| 93 | 1P7O | 2.623 | PADG_03560 | Translation elongation factor Tu | 48.15% |
| Signal transduction | |||||
| 14 | 2G18 | 2.882 | PADG_06642 | STE/STE7 protein kinase | 42.31% |
| Nucleotide metabolism | |||||
| 15 | 1FCX | 2.875 | PADG_04803 | FAD-binding FR-type domain-containing protein | 42.86% |
| 72 | 1K44 | 2.787 | PADG_07524 | Nucleoside diphosphate kinase | 47.73% |
| Protein fate | |||||
| 28 | 1TG6 | 2.964 | PADG_04169 | ATP-dependent Clp protease proteolytic subunit | 59.04% |
| 31 | 2FXT | 2.958 | PADG_01853 | Mitochondrial import inner membrane translocase subunit TIM44 | 42.67% |
| Ribosome biogenesis | |||||
| 29 | 2ZU6 | 2.963 | PADG_05787 | ATP-dependent RNA helicase FAL1 | 69.97% |
| 29 | 2ZU6 | 2.963 | PADG_03283 | ATP-dependent RNA helicase DBP5 | 41.49% |
| 29 | 2ZU6 | 2.963 | PADG_05924 | ATP-dependent RNA helicase DHH1 | 40.92% |
| Uncharacterized protein | |||||
| 10 | 3CPR | 2.366 | PADG_12138 | Uncharacterized protein | 83.33% |
| 10 | 3CPR | 2.366 | PADG_03515 | Uncharacterized protein | 44.83% |
| 12 | 3GH8 | 2.907 | PADG_01104 | Uncharacterized protein | 40.74% |
| 16 | 2NR0 | 2.284 | PADG_07365 | Uncharacterized protein | 40.00% |
| 17 | 1D7C | 3.286 | PADG_03386 | Uncharacterized protein | 43.33% |
| 17 | 1D7C | 3.286 | PADG_03633 | Uncharacterized protein | 42.11% |
| 19 | 2Z6E | 2.726 | PADG_01772 | Uncharacterized protein | 41.67% |
| 33 | 2IF2 | 2.946 | PADG_07398 | Uncharacterized protein | 44.12% |
| 34 | 1MA1 | 2.941 | PADG_01263 | Uncharacterized protein | 41.67% |
| 61 | 2RMR | 2.834 | PADG_05214 | Uncharacterized protein | 52.38% |
| 61 | 2RMR | 2.834 | PADG_03864 | Uncharacterized protein | 47.83% |
| 61 | 2RMR | 2.834 | PADG_01852 | Uncharacterized protein | 46.88% |
| 61 | 2RMR | 2.834 | PADG_05741 | Uncharacterized protein | 40.00% |
| 64 | 1RSO | 2.817 | PADG_01098 | Uncharacterized protein | 45.24% |
| 66 | 2RFA | 2.808 | PADG_03495 | Uncharacterized protein | 62.50% |
| 66 | 2RFA | 2.808 | PADG_06137 | Uncharacterized protein | 46.51% |
| 83 | 2AA7 | 3.148 | PADG_00825 | Uncharacterized protein | 52.38% |
| 91 | 1FI6 | 2.639 | PADG_12437 | Uncharacterized protein | 40.85% |
| 94 | 2A9E | 3.93 | PADG_04926 | Uncharacterized protein | 48.00% |
Rank: statistically significant classification of the targets assessed by PharmMapper; PDB ID: identification number of the PDB templates used by PharmMapper to indentify targets; Fit Score: score corresponding to the analysis of the pharmacophore models generated with the investigated compound; PADG: identification number of P. brasiliensis proteins; Per. Ident: percentage of identity after Blast analysis of proteins suggested as targets of P. brasiliensis proteins by PharmMapper.
Interaction network
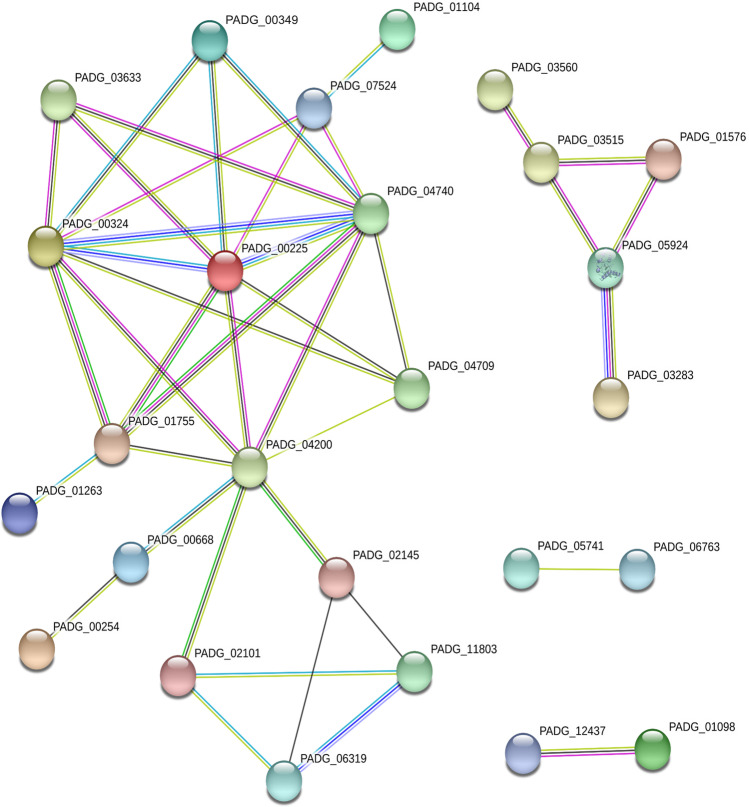
STRING database was used to identify interacting proteins with the 49 target proteins suggested for curcumin. The interaction network resulted in 49 nodes with 41 edges. Out of the 49 target proteins, 23 did not interact with any other protein. STRING identified the interaction of a group of 13 proteins, of which three were uncharacterized and the others belong to amino acid metabolism, nucleotide metabolism, defense and virulence, glyoxylate cycle, C-compound and carbohydrate metabolism, lipid metabolism, fatty acids, and isoprenoids. A smaller group of five proteins were identified interacting with each other and only was uncharacterized, two belong to ribosome biogenesis and two to translation. In addition, STRING identified two nodes with only two proteins in each and only one of them was classified functionally, a component of the cell cycle (Fig. 1).
Fig. 1.
Interaction network of Paracoccidioides brasiliensis target proteins. Network statistics: number of nodes 49; the number of edges 41; average node degree 1.67, average local clustering coefficient 0.382; expected number of edges 16; PPI enrichment p-value 1.7 e-07. Node color: colored nodes—query proteins and first shell of interactors; white nodes—second shell of interactors; node content: empty nodes—proteins of unknown 3D structure; filled nodes—some 3D structures are known or predicted. Edges: known interactions: turquoise is from curated databases and pink is experimentally determined; predicted interactions: green is gene neighborhood, red is gene fusion, and blue is gene co-occurrence; others: yellow is text mining, black is co-expression, and lilac is protein homology
Homology modeling and Molecular docking
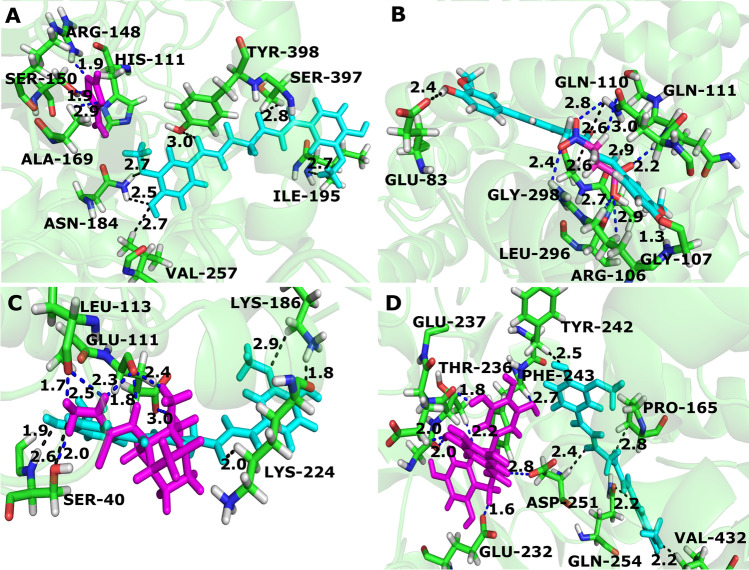
In silico approach was used to identify the amino acids involved in the interaction of curcumin and target proteins. In the molecular docking, we selected proteins related to virulence and infectious processes of the fungus, such as the superoxide dismutase [39], catalase [40], and isocitrate lyase [41, 42], in addition to glucose-6-phosphate dehydrogenase that is important to NADPH production [43]. All details of the homology modeling, such as templates, statistical results, and validation results, are listed in Supplementary Table S2. The values of the clashscore of the models ranged from 0 to 0.55 and the MolProbity scores from 0.79 to 1.13. These results showed that proteins models generated by homology modeling are acceptable. Analyzing the docking results, curcumin performed hydrogen bond with the residues ILE-195, SER-397, TYR-398, ASN-184, and VAL-257 of the catalase protein (Fig. 2A). The residues of superoxide dismutase that interact with curcumin via hydrogen bonds are SER-40, LYS-224, and LYS-186 (Fig. 2B). Most interactions of protein residues with curcumin occur between the oxygen atoms attached to the rings of the curcumin molecule and the oxygen atoms present in the middle of the structure. Glucose-6-phosphate dehydrogenase residues that interacted with curcumin are TYR-242, ASP-251, PRO-165, VAL-432, LEU-437, SER-440, and GLN-254 (Fig. 2C). Curcumin interacted with GLU-83, GLY-298, GLN-111, GLN-110, and GLY-107 residues of isocitrate lyase (Fig. 2D).
Fig. 2.
Interaction of Paracoccidioides brasiliensis proteins with curcumin. In green are the residues of each protein that interact with the curcumin represented in turquoise. A Catalase (PADG_00225); B superoxide dismutase (PADG_01755); C glucose-6-phosphate 1-dehydrogenase (PADG_04200); D isocitrate lyase (PADG_04709)
To compare whether the interaction mode of curcumin with proteins selected for docking is similar to its interaction with known inhibitors of these proteins, we selected amitrole (catalase inhibitor [44]), 3-nitropropionic acid (isocitrate lyase inhibitor [45]), epigallocatechin gallate (glucose-6-phosphate dehydrogenase inhibitor [46]), and camphene thiosemicarbazide (superoxide dismutase inhibitor [47]). The interaction mechanism of curcumin with catalase and glucose-6-phosphate dehydrogenase is different from the interaction of these proteins and their inhibitors amitrole and epigallocatechin gallate, respectively; they interact with different amino acid residues, despite being close residues (Fig. 3A and D). Curcumin has similar mechanisms of interaction with isocitrate lyase and superoxide dismutase and their inhibitors, 3-nitropropionic acid and camphene thiosemicarbazide, respectively. They interact with the residues GLN-111 and GLN-110 of isocitrate lyase and with SER-40 of superoxide dismutase (Fig. 3B and C).
Fig. 3.
Comparison of the mode of binding of curcumin with catalase, isocitrate lyase, superoxide dismutase, and glucose-6-phosphate 1-dehydrogenase inhibitors. A Despite interacting with nearby amino acid residues, curcumin and amitrole have different interaction mechanisms with catalase. B Compared to the interactions of 3-nitropropionic acid (blue dot lines), curcumin (black dot lines) showed a similar interaction pattern with isocitrate lyase. C The camphene thiosemicarbazide (blue dot lines) and curcumin (black dot lines) both interact with the residues SER-40 of superoxide dismutase. D Curcumin and epigallocatechin gallate interacted with different amino acid residues of glucose-6-phosphate 1-dehydrogenase
Curcumin is a ROS-inducing agent in Paracoccidioides brasiliensis
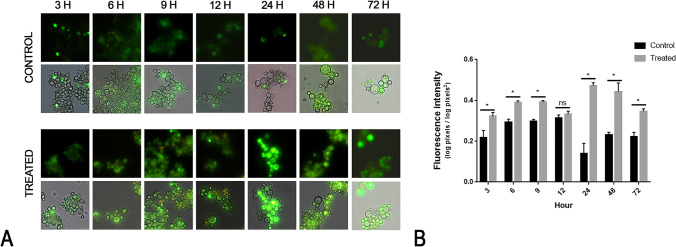
The target prediction assays identified enzymes related to oxidative stress. We assessed whether curcumin could act as a ROS-inducing agent in P. brasiliensis. In fact, fungal cells grown in the presence of curcumin at the MIC concentration had higher ROS production when compared to control after 3 h of exposure to the compound (Fig. 4).
Fig. 4.
Reactive oxygen species are produced in Paracoccidioides brasiliensis in the presence of curcumin. P. brasiliensis labeled with DCFH-DC showed that curcumin is a ROS-inducing agent in this pathogen. A The fluorescence photomicrographs show green-labeled cells indicating the ROS production after 3, 6, 9, 12, 24, 48, and 72 h with (treated) or without (control) curcumin at 10.59 μM. B Fluorescence intensity graph. All fluorescent cells from the control and treated samples were analyzed using the AxioVision V 4.8.2.0 software (Carl Zeiss). Density and area were measured to calculate the fluorescence intensity as described in the materials and methods section. The data were expressed as the mean fluorescence intensity of the twenty cells ± standard error. The asterisk (*) indicates a significant difference between the control and treated samples with a p value ≤ 0.05 and the ns symbol indicates a p value > 0.05
Curcumin affects the integrity of the plasma membrane
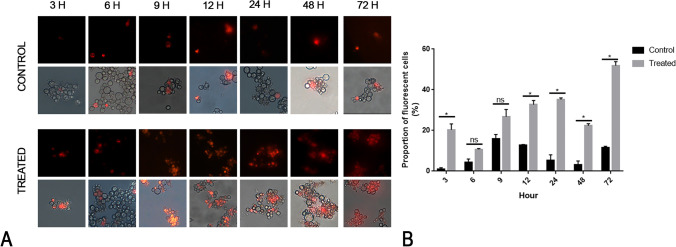
Curcumin exerts its antifungal activity through the disruption of the plasma membrane [48]. We used a fluorescence microscopy approach to evaluate the propidium iodide influx in cells cultured in the presence of curcumin. The effect of curcumin on the plasma membrane of P. brasiliensis was evaluated using the fluorescent dye PI, which marks cells with abnormal membrane permeability. The microscopy has shown that curcumin affects the membrane at MIC concentration. This effect is time dependent, with significantly more PI influx compared to the control after 12 h of exposure (Fig. 5).
Fig. 5.
Membrane permeability assay according to the time variable. A The panels show the fungus under a fluorescence microscopy evaluation after labeling with PI at different time points. B The verification of changes in membrane permeability was performed by measuring the proportion of fluorescent cells in relation to the total population of cells for both controls and treated samples in each period of time in triplicate. Data were expressed as mean of fluorescent cells related to non-fluorescent ± standard error. The asterisk (*) indicates a significant difference between the control and treated samples with a p value ≤ 0.05, and the ns symbol indicate a p value > 0.05
Discussion
Recently, a great effort has been devoted to the planning and development of new antifungals for the treatment of PCM in order to improve efficacy and safety [22, 49, 50]. Several natural products and their derivatives are active against several species of Paracoccidioides, as oenothein B from Eugenia uniflora [51], Copaiba oil [23], argentilactone from Hyptis ovalifolia [52], and a chalcone derivative [22]. Among this attractive universe of medicinal plants or derivatives, this work explores the potential of curcumin as an antifungal.
Among pathogenic fungi, the purified curcumin extract was able to inhibit 80% of clinical and environmental Cryptococcus gattii growth at concentrations up to 32 μg/mL. Curcumin showed a synergistic effect in combination with fluconazole [53]. The inhibitory effect of curcumin was also evaluated against Aspergillus spp., Candida spp., Cryptococcus neoformans, and Sporothrix schenckii, with inhibition values ranging from 2 to 256 μg/mL [54]. Besides antifungal activity, anti-inflammatory [55], antiplatelet, and anticoagulant properties of curcumin have been reported [56]. These effects may be relevant for the treatment of PCM, due to the characteristics of this mycosis, such as inflammatory responses, pulmonary fibrosis, and acute lung injury [2, 57].
Here, we point out the fungicidal effect of curcumin against Paracoccidioides. An effective immune response is able to prevent fungal replication. However, Paracoccidioides spp. can persist in latent form within granulomas, especially in a compromised immune system. Then, the infection can be reactivated, leading to the most severe form of mycosis [58]. Drugs with a fungicidal profile are promising as an alternative option for the treatment of immunosuppressed patients. Unfortunately, among the therapeutic arsenal for the treatment of PCM, AmB is the only drug reported to have fungicidal activity. It is indicated for the most severe cases of the disease due to its high toxicity and severe side effects [59, 60].
The therapeutic options for PCM are limited to three structurally distinct classes of antifungal compounds. These drugs have great cytotoxic effects [3]. Thus, the combined antifungal therapy has been considered as an alternative to monotherapy, increasing the spectrum of activity and fungicidal activity through the association of antifungals. In addition, it reduces the development of fungal resistance and toxicity [61]. Sharma et al. showed the synergistic anticandidal activity of polyphenol curcumin in combination with azoles and polyenes, leading to ROS production [62]. In Paracoccidioides spp., curcumin interacts synergistically with ITZ and AmB and additively with co-trimoxazole, expanding the antifungal potential of these drugs. This finding makes curcumin even more promising as an antifungal, reducing the concentration of AmB, co-trimoxazole, and ITZ requested for the PCM treatment and consequently reducing the side effects of such drugs.
Curcumin interacts with several molecular targets, which explains the wide range of therapeutic applications and efficacy as a chemotherapeutic agent [63]. Studies showed that the antifungal activity of curcumin in C. albicans may be related to cell apoptosis induction by ROS [64], impairs the extrusion of H+ by the plasma membrane-ATPase transporter [65], reduces the ergosterol synthesis [66], and promotes cell wall disruption [67].
The plasma membrane acts as a physical barrier between the inside and outside of the cell, controlling the entry and exit of compounds. Two antifungals used in the treatment of PCM target the plasma membrane, AmB binds to ergosterol inducing the formation of pores in the membrane, which increases its permeability [68, 69], and ITZ inhibits the enzyme 14-α-lanosterol demethylase, blocking ergosterol biosynthesis [70]. However, these antifungals are associated with high hepatoxicity and nephrotoxicity, in addition to several side effects [71, 72].
It has been shown that curcumin promotes changes in the integrity of the membrane, leading to the rupture of the lipid bilayer. We verified the effect of curcumin on the membrane of P. brasiliensis. In fact, the permeability of the membrane of P. brasiliensis is altered in the presence of curcumin in a time-dependent manner. Some studies have demonstrated the interaction of curcumin with the membrane of several organisms. Scanning microscopy and fluorescence of Escherichia coli and Pseudomonas aeruginosa cells showed membrane damage and altered permeability caused by curcumin [73]. Curcumin is able to reduce the membrane thickness, as it can bind to the lipid bilayers and affect membrane proteins [74]. In addition, curcumin impairs the plasma membrane stability in organisms closely related to P. brasiliensis, including the phytopathogen Fusarium graminearum [75] and the mammalian-related pathogens Aspergillus flavus [76] and Candida albicans [48].
It is a difficult task to deeply understand the cell response in the presence of a drug but bioinformatics tools, such as PharmMapper, are useful to identify possible targets of drug compounds [19]. The first 100 targets suggested by PharmMapper showed significant identity with proteins of Paracoccidioides spp. with homology greater than 40%. Studies suggest that homologs with an identity of 30% or more can have the same interaction mechanism [77] and that proteins with 40% identity tend to share the first 3 digits of the Enzyme Commission Numbers [78]. The use of this tool was valuable in driving experimental validation tests.
The oxidative stress induces changes in gene expression, which are necessary for virulence and pathogenicity of Paracoccidioides spp. These changes include modulation of the expression of superoxide dismutase [39], glutathione, thioredoxin, cytochrome c peroxidase [79], and catalases [40]. The success of Paracoccidioides spp. infection is related to the modulation of its antioxidant system in the hostile environment of hosts [80]. The in silico prediction of curcumin targets in P. brasiliensis identified enzymes related to the regulation of oxidative stress in the fungus, such as catalase, superoxide dismutase, and glutathione reductase. In addition, the increase in the production of ROS was confirmed by microscopy tests, leading to the understanding that the Paracoccidioides spp. antioxidant defense system may be inhibited in the presence of curcumin. Curcumin has also been associated with increased production of ROS in Aspergillus niger, A. flavus, Penicillium griseofulvum, P. chrysogenum, Fusarium oxysporum, Candida albicans, and Zygosaccharomyces bailii, interacting directly with the membrane of these fungi [81]. Studies showed that in Candida spp., the growth of mutants to oxidative stress response genes (ICaIpf7817 and ∆Cap1) was extensively impaired in the presence of curcumin [64].
Other compounds tested against Paracoccidioides, such as argentilactone and camphene thiosemicarbazide, showed similar effects to those caused by the presence of curcumin, increasing ROS production by inhibiting the enzymatic activity of superoxide dismutase. However, argentilactone presented a MIC value of 91 μM [52] and camphene thiosemicarbazide with a MIC value of 79 μM [82], a higher concentration when compared to curcumin (10.59-21.20 μM).
A metabolic pathway widely studied in several organisms is the glyoxylate cycle, which allows the synthesis of glucose and tricarboxylic acid cycle intermediates from acetyl CoA. Such a pathway is important for survival, response to oxidative stress [83], and virulence [41, 42]. The enzymes of the glyoxylate cycle, malate synthase, and isocitrate lyase, are promising antifungal targets, as they exist only in the pathogen and not in the human host. In Paracoccidioides spp., isocitrate lyase is important for the infectious process since its expression is induced during the transition from mycelium to yeast and in the phagocytosis process by murine macrophages [84, 85]. The in silico analysis performed in the present work suggests that isocitrate lyase is a possible curcumin target. Similar results were found for Acinetobacter baumannii, in which the expression of isocitrate lyase was inhibited by curcumin [86]. In addition, the interaction mode of curcumin and 3-nitropropionic acid with isocitrate lyase is similar. The 3-nitropropionic acid is a standard compound for the inhibition of isocitrate lyase, used as a positive control for the analysis of the inhibition potential of new drugs [87].
The STRING analyses showed an interaction between isocitrate lyase (PADG_04709), glucose-6-phosphate dehydrogenase (PADG_04200), catalases (PADG_00324, PADG_00225, and PADG_047740), and superoxide dismutase (PADG_01755). This suggests an additional role of isocitrate lyase and glucose-6-phosphate dehydrogenase in response to oxidative stress. Studies have shown that the glyoxylate cycle plays an important role in response to oxidative stress [42]. Since the main function of the tricarboxylic acid cycle is the production of NADH and FADH2, the activation of alternative pathways, such as glyoxylate, can disturb the potential redox of cells by decreasing the flow of electrons directed to respiration. In plants, when catalase is inactive, H2O2 is able to inhibit isocitrate lyase and degrade glyoxylate. Thus, catalase could be associated with isocitrate lyase providing greater protection against H2O2 [88]. The glyoxylate cycle is induced by Pseudomonas aeruginosa under conditions of oxidative stress induced by the limitation of carbon sources [83]. In addition, Paracoccidioides yeast cells increased expression of isocitrate lyase in response to oxidative stress [89] and carbon deprivation [90], indicating a role in regulatory processes of respiration and ROS response.
The role of glucose-6-phosphate dehydrogenase is related to the catalysis of a limiting stage of the oxidative pentose-phosphate pathway, providing NADPH for several biosynthetic reactions, such as the synthesis of fatty and nucleic acids and detoxification [43]. The homeostatic levels of NADPH contribute to the maintenance of important proteins belonging to the antioxidant system, thioredoxin, glutaredoxin, peroxiredoxin, and catalase. Glucose-6-phosphate dehydrogenase is required by Salmonella typhimurium and E. coli for virulence and resistance to ROS and protection against oxidative stress [91, 92]. In P. brasiliensis, genes coding for glucose-6-phosphate dehydrogenase are important for glutathione biosynthesis and NADPH regeneration [93], which corroborates its interaction with other proteins related to oxidative defense.
Conclusion
Seeking new antifungals for the treatment of PCM has led to the discovery that curcumin may be a viable alternative. Curcumin is an efficient inhibitor of P. brasiliensis growth with an attractive selectivity index. Such features are important for the development of a new drug. Along with in vitro tests, in silico analysis allowed us to suggest the mode of action of curcumin. The compound inhibits the activity of proteins, such as superoxide dismutase, catalase, isocitrate lyase, and glucose-6-phosphate dehydrogenase that are important defenses against oxidative stress. In addition, curcumin affects the plasma membrane of P. brasiliensis.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Each author has contributed significantly to this work. O.B.R., L.C.S., and M.A.B.C.J. performed the biological assays. A.A.O. performed the in silico assay. All authors contributed to the manuscript writing. All authors have given approval for the final version of the manuscript. O.B.R. and L.C.S. share the first authorship.
Funding
This work was performed at Universidade Federal de Goiás supported by MCTI/CNPq (Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico), FNDCT (Fundo Nacional de Desenvolvimento Científico e Tecnológico), FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/ Finance Code 001), FINEP (Financiadora de Estudos e Projetos), PRONEX (Programa de Apoio a Núcleos de Excelência), and INCT-IPH (Instituto Nacional de Ciência e Tecnologia de Estratégias de Interação Patógeno-Hospedeiro). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Olívia Basso Rocha and Lívia do Carmo Silva contributed equally to this work and share the first authorship.
References
- 1.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017;17:e367–e377. doi: 10.1016/S1473-3099(17)30306-7. [DOI] [PubMed] [Google Scholar]
- 2.Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F de, Kono ASG, Paniago AMM, Nathan A, Valle ACF do, Bagagli E, Benard G, Ferreira MS, Teixeira M de M, Silva-Vergara ML, Pereira RM, Cavalcante R de S, Hahn R, Durlacher RR, Khoury Z, Camargo ZP de, Moretti ML, Martinez R (2017) Brazilian guidelines for the clinical management of paracoccidioidomycosis. Revista da Sociedade Brasileira de Medicina Tropical 50:715–740. 10.1590/0037-8682-0230-2017 [DOI] [PubMed]
- 3.Shikanai-Yasuda MA. Paracoccidioidomycosis treatment. Rev Inst Med Trop Sao Paulo. 2015;57:31–37. doi: 10.1590/S0036-46652015000700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travassos LR, Taborda CP, Colombo AL. Treatment options for paracoccidioidomycosis and new strategies investigated. Expert Rev Anti Infect Ther. 2008;6:251–262. doi: 10.1586/14787210.6.2.251. [DOI] [PubMed] [Google Scholar]
- 5.Freitas e Silva KS, C. Silva L, Gonçales RA, Neves BJ, Soares CMA, Pereira M (2020) Setting new routes for antifungal drug discovery against pathogenic fungi. CPD 26:1509–1520. 10.2174/1381612826666200317125956 [DOI] [PubMed]
- 6.Negri M, Salci T, Shinobu-Mesquita C, Capoci I, Svidzinski T, Kioshima E. Early state research on antifungal natural products. Molecules. 2014;19:2925–2956. doi: 10.3390/molecules19032925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewlings S, Kalman D (2017) Curcumin: a review of its’ effects on human health. Foods 6:92. 10.3390/foods6100092 [DOI] [PMC free article] [PubMed]
- 8.Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:1–12. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018;32:985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 10.Osawa T. Nephroprotective and hepatoprotective effects of curcuminois. In: Aggarwal BB, Surh Y-J, Shishodia S, editors. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Boston, MA: Springer; 2007. pp. 407–423. [Google Scholar]
- 11.Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019;66:1–16. doi: 10.1016/j.jnutbio.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler S, Pries V, Hedberg C, Waldmann H. Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chem Int Ed. 2013;52:2744–2792. doi: 10.1002/anie.201208749. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Huang H, Wu CH. Protein bioinformatics databases and resources. Methods Mol Biol. 2017;1558:3–39. doi: 10.1007/978-1-4939-6783-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comess KM, McLoughlin SM, Oyer JA, Richardson PL, Stöckmann H, Vasudevan A, Warder SE. Emerging approaches for the identification of protein targets of small molecules - a practitioners’ perspective. J Med Chem. 2018;61:8504–8535. doi: 10.1021/acs.jmedchem.7b01921. [DOI] [PubMed] [Google Scholar]
- 15.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilson MK, Liu T, Baitaluk M, Nicola G, Hwang L, Chong J. BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016;44:D1045–1053. doi: 10.1093/nar/gkv1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Z, Li H, Zhang H, Liu X, Kang L, Luo X, Zhu W, Chen K, Wang X, Jiang H (2008) PDTD: a web-accessible protein database for drug target identification. BMC Bioinformatics 9:104. 10.1186/1471-2105-9-104 [DOI] [PMC free article] [PubMed]
- 18.DrugBank 5.0: a major update to the DrugBank database for 2018 - PubMed. https://pubmed.ncbi.nlm.nih.gov/29126136/. Accessed 22 Jul 2020 [DOI] [PMC free article] [PubMed]
- 19.Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:W356–W360. doi: 10.1093/nar/gkx374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu T, Ma C, Fan S, Deng N, Lian Y, Tan L, Du W, Zhang S, Liu S, Ren B, Li Z, Wang Q, Wang X, Cheng F (2018) Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid Based Complement Alternat Med 2018. 10.1155/2018/2582843 [DOI] [PMC free article] [PubMed]
- 21.Xu T, Ma C, Fan S, Deng N, Lian Y, Tan L, Du W, Zhang S, Liu S, Ren B, Li Z, Wang Q, Wang X, Cheng F (2018) Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid Based Complement Alternat Med 2018. 10.1155/2018/2582843 [DOI] [PMC free article] [PubMed]
- 22.Silva LC, Neves BJ, Gomes MN, Melo-Filho CC, Soares CM, Andrade CH, Pereira M. Computer-aided identification of novel anti-paracoccidioidomycosis compounds. Future Microbiol. 2018;13:1523–1535. doi: 10.2217/fmb-2018-0175. [DOI] [PubMed] [Google Scholar]
- 23.do Carmo Silva L, Miranda MACM, de Freitas JV, Ferreira SFA, de Oliveira Lima EC, de Oliveira CMA, Kato L, Terezan AP, Rodriguez AFR, Faria FSEDV, de Almeida Soares CM, Pereira M (2020) Antifungal activity of Copaíba resin oil in solution and nanoemulsion against Paracoccidioides spp. Braz J Microbiol 51:125–134. 10.1007/s42770-019-00201-3 [DOI] [PMC free article] [PubMed]
- 24.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, Von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, De Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose PW, Prlić A, Altunkaya A, Bi C, Bradley AR, Christie CH, Di Costanzo L, Duarte JM, Dutta S, Feng Z, Green RK, Goodsell DS, Hudson B, Kalro T, Lowe R, Peisach E, Randle C, Rose AS, Shao C, Tao YP, Valasatava Y, Voigt M, Westbrook JD, Woo J, Yang H, Young JY, Zardecki C, Berman HM, Burley SK. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017;45:D271–D281. doi: 10.1093/nar/gkw1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya D, Nowotny J, Cao R, Cheng J. 3Drefine: an interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016;44:W406–W409. doi: 10.1093/nar/gkw336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya D, Cheng J. 3Drefine: Consistent protein structure refinement by optimizing hydrogen bonding network and atomic-level energy minimization. Proteins: Structure. Function, and Bioinformatics. 2013;81:119–131. doi: 10.1002/prot.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anandakrishnan R, Aguilar B, Onufriev AV. H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012;40:W537–W541. doi: 10.1093/nar/gks375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, Jain S, Lewis SM, Arendall WB, Snoeyink J, Adams PD, Lovell SC, Richardson JS, Richardson DC. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 2018;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Bryant SH, Cheng T, Wang J, Gindulyte A, Shoemaker BA, Thiessen PA, He S, Zhang J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017;45:D955–D963. doi: 10.1093/nar/gkw1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fourches D, Muratov E, Tropsha A. Trust, but verify II: a practical guide to chemogenomics data curation. J Chem Inf Model. 2016;56:1243–1252. doi: 10.1021/acs.jcim.6b00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OMEGA v.3.0.0.1: OpenEye Scientific Software, Santa Fe, NM. http://www.eyesopen.com. Accessed 10 Dec 2020
- 35.Jakalian A, Jack DB, Bayly CI. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II Parameterization and validation. J Comput Chem. 2002;23:1623–1641. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 36.QUACPAC v.1.7.0.2: OpenEye Scientific Software, Santa Fe, NM. http://www.eyesopen.com. Accessed 10 Dec 2020
- 37.(2017) OEDocking v.3.2.0: OpenEye Scientific Software, Santa Fe, NM, USA. http://www.eyesopen.com. Accessed 10 Dec 2020
- 38.McGann M. FRED and HYBRID docking performance on standardized datasets. J Comput Aided Mol Des. 2012;26:897–906. doi: 10.1007/s10822-012-9584-8. [DOI] [PubMed] [Google Scholar]
- 39.Tamayo D, Muñoz JF, Lopez Á, Urán M, Herrera J, Borges CL, Restrepo Á, Soares CM, Taborda CP, Almeida AJ, McEwen JG, Hernández O (2016) Identification and analysis of the role of superoxide dismutases isoforms in the pathogenesis of Paracoccidioides spp. PLoS Negl Trop Dis 10:e0004481 . 10.1371/journal.pntd.0004481 [DOI] [PMC free article] [PubMed]
- 40.Tamayo D, Muñoz JF, Almeida AJ, Puerta JD, Restrepo Á, Cuomo CA, McEwen JG, Hernández O. Paracoccidioides spp. catalases and their role in antioxidant defense against host defense responses. Fungal Genet Biol. 2017;100:22–32. doi: 10.1016/j.fgb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn MF, Ramirez-Trujillo JA, Hernandez-Lucas I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009;155:3166–3175. doi: 10.1099/mic.0.030858-0. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001;412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 43.Luzzatto L, Battistuzzi G. Glucose-6-phosphate dehydrogenase. In: Harris H, Hirschhorn K, editors. Advances in human genetics 14. Boston, MA: Springer; 1985. pp. 217–329. [DOI] [PubMed] [Google Scholar]
- 44.Chagas RF, Bailão AM, Fernandes KF, Winters MS, Pereira M, de Soares CM, A Purification of Paracoccidioides brasiliensis catalase P: subsequent kinetic and stability studies. J Biochem. 2010;147:345–351. doi: 10.1093/jb/mvp182. [DOI] [PubMed] [Google Scholar]
- 45.Schloss JV, Cleland WW. Inhibition of isocitrate lyase by 3-nitropropionate, a reaction-intermediate analog. Biochemistry. 1982;21:4420–4427. doi: 10.1021/bi00261a035. [DOI] [PubMed] [Google Scholar]
- 46.Shin ES, Park J, Shin J-M, Cho D, Cho SY, Shin DW, Ham M, Kim JB, Lee TR. Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg Med Chem. 2008;16:3580–3586. doi: 10.1016/j.bmc.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 47.e Silva KS, da S Neto BR, Zambuzzi-Carvalho PF, de Oliveira CM, Pires LB, Kato L, Bailão AM, Parente-Rocha JA, Hernández O, Ochoa JG, de A Soares CM, Pereira M (2018) Response of Paracoccidioides lutzii to the antifungal camphene thiosemicarbazide determined by proteomic analysis. Future Microbiol 13:1473–1496. 10.2217/fmb-2018-0176 [DOI] [PubMed]
- 48.Lee W, Lee DG. An antifungal mechanism of curcumin lies in membrane-targeted action within candida albicans. IUBMB Life. 2014;66:780–785. doi: 10.1002/iub.1326. [DOI] [PubMed] [Google Scholar]
- 49.da Silva LS, Barbosa UR, Silva L do C, Soares CM, Pereira M, da Silva RA (2019) Identification of a new antifungal compound against isocitrate lyase of Paracoccidioides brasiliensis. Future Microbiol 14:1589–1606. 10.2217/fmb-2019-0166 [DOI] [PubMed]
- 50.Bueno PSA, Rodrigues FAV, Santos JL, Canduri F, Biavatti DC, Pimentel AL, Bagatin MC, Kioshima ÉS, de Freitas Gauze G, Seixas FAV (2019) New inhibitors of homoserine dehydrogenase from Paracoccidioides brasiliensis presenting antifungal activity. J Mol Model 25:325. 10.1007/s00894-019-4221-2 [DOI] [PubMed]
- 51.Santos GD, Ferri PH, Santos SC, Bao SN, Soares CMA, Pereira M. Oenothein B inhibits the expression of PbFKS1 transcript and induces morphological changes in Paracoccidioides brasiliensis. Med Mycol. 2007;45:609–618. doi: 10.1080/13693780701502108. [DOI] [PubMed] [Google Scholar]
- 52.Prado RS do, Alves RJ, Oliveira CMA de, Kato L, Silva RA da, Quintino GO, do Desterro Cunha S, de Almeida Soares CM, Pereira M (2014) Inhibition of Paracoccidioides lutzii Pb01 isocitrate lyase by the natural compound argentilactone and its semi-synthetic derivatives. PLoS ONE 9:e94832. 10.1371/journal.pone.0094832 [DOI] [PMC free article] [PubMed]
- 53.Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice - Silva - 2016 - Journal of Applied Microbiology - Wiley Online Library. https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/jam.12966. Accessed 13 Jul 2020 [DOI] [PubMed]
- 54.Martins CVB, da Silva DL, Neres ATM, Magalhaes TFF, Watanabe GA, Modolo LV, Sabino AA, de Fatima A, de Resende MA. Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother. 2008;63:337–339. doi: 10.1093/jac/dkn488. [DOI] [PubMed] [Google Scholar]
- 55.dos Santos PDF, Francisco CRL, Coqueiro A, Leimann FV, Pinela J, Calhelha RC, Porto Ineu R, Ferreira ICFR, Bona E, Gonçalves OH. The nanoencapsulation of curcuminoids extracted from Curcuma longa L. and an evaluation of their cytotoxic, enzymatic, antioxidant and anti-inflammatory activities. Food Funct. 2019;10:573–582. doi: 10.1039/C8FO02431F. [DOI] [PubMed] [Google Scholar]
- 56.Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A. Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol. 2018;233:4497–4511. doi: 10.1002/jcp.26249. [DOI] [PubMed] [Google Scholar]
- 57.Borges-Walmsley MI, Chen D, Shu X, Walmsley AR. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002;10:80–87. doi: 10.1016/S0966-842X(01)02292-2. [DOI] [PubMed] [Google Scholar]
- 58.Benard G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia. 2008;165:209–221. doi: 10.1007/s11046-007-9065-0. [DOI] [PubMed] [Google Scholar]
- 59.Harmsen S, McLaren AC, Pauken C, McLemore R (2011) Amphotericin B is cytotoxic at locally delivered concentrations. Clinical Orthopaed Related Res 469:3016–3021. 10.1007/s11999-011-1890-2 [DOI] [PMC free article] [PubMed]
- 60.Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26:223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer M, Robbins N, Wright GD. Combinatorial strategies for combating invasive fungal infections. Virulence. 2017;8:169–185. doi: 10.1080/21505594.2016.1196300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma M, Manoharlal R, Negi AS, Prasad R (2010) Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis: curcumin is synergistic to antifungals in Candida. FEMS Yeast Research no-no . 10.1111/j.1567-1364.2010.00637.x [DOI] [PubMed]
- 63.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases: Curcumin: from kitchen to clinic. Br J Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma M, Manoharlal R, Puri N, Prasad R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci Rep. 2010;30:391–404. doi: 10.1042/BSR20090151. [DOI] [PubMed] [Google Scholar]
- 65.Dao TT, Sehgal P, Tung TT, Møller JV, Nielsen J, Palmgren M, Christensen SB, Fuglsang AT (2016) Demethoxycurcumin is a potent inhibitor of P-type ATPases from diverse kingdoms of life. PLoS ONE 11:e0163260 . 10.1371/journal.pone.0163260 [DOI] [PMC free article] [PubMed]
- 66.Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA. Curcumin as a promising anticandidal of clinical interest. Can J Microbiol. 2011;57:204–210. doi: 10.1139/W10-117. [DOI] [PubMed] [Google Scholar]
- 67.Kumar A, Dhamgaye S, Maurya IK, Singh A, Sharma M, Prasad R. Curcumin targets cell wall integrity via calcineurin-mediated signaling in Candida albicans. Antimicrob Agents Chemother. 2014;58:167–175. doi: 10.1128/AAC.01385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 69.Baginski M, Czub J, Sternal K. Interaction of amphotericin B and its selected derivatives with membranes: molecular modeling studies. Chem Rec. 2006;6:320–332. doi: 10.1002/tcr.20096. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida Y, Aoyama Y. Sterol 14 alpha-demethylase and its inhibition: structural considerations on interaction of azole antifungal agents with lanosterol 14 alpha-demethylase (P-450(14DM)) of yeast. Biochem Soc Trans. 1991;19:778–782. doi: 10.1042/bst0190778. [DOI] [PubMed] [Google Scholar]
- 71.Catalán M, Montejo JC. Antifúngicos sistémicos. Farmacodinamia y farmacocinética. Rev Iberoam Micol. 2006;23:39–49. doi: 10.1016/S1130-1406(06)70012-2. [DOI] [PubMed] [Google Scholar]
- 72.Mishra J, Dey A, Singh N, Somvanshi R, Singh S. Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res. 2013;137:767–776. [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K (2015) Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 10:e0121313. 10.1371/journal.pone.0121313 [DOI] [PMC free article] [PubMed]
- 74.Hung W-C, Chen F-Y, Lee C-C, Sun Y, Lee M-T, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–4338. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C, Long L, Zhang F, Chen Q, Chen C, Yu X, Liu Q, Bao J, Long Z (2018) Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 13:e0194284. 10.1371/journal.pone.0194284 [DOI] [PMC free article] [PubMed]
- 76.Ferreira FD, Mossini SAG, Ferreira FMD, Arrotéia CC, da Costa CL, Nakamura CV, Machinski Junior M. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci World J. 2013;2013:1–6. doi: 10.1155/2013/343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aloy P, Ceulemans H, Stark A, Russell RB. The relationship between sequence and interaction divergence in proteins. J Mol Biol. 2003;332:989–998. doi: 10.1016/j.jmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Tian W, Skolnick J. How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol. 2003;333:863–882. doi: 10.1016/j.jmb.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 79.Parente-Rocha JA, Parente AFA, Baeza LC, Bonfim SMRC, Hernandez O, McEwen JG, Bailão AM, Taborda CP, Borges CL, Soares CM de A (2015) Macrophage interaction with Paracoccidioides brasiliensis yeast cells modulates fungal metabolism and generates a response to oxidative stress. PLoS ONE 10:e0137619. 10.1371/journal.pone.0137619 [DOI] [PMC free article] [PubMed]
- 80.Campos EG, Jesuino RSA, Dantas A da S, Brígido M de M, Felipe MSS (2005) Oxidative stress response in Paracoccidioides brasiliensis. Genet Mol Res 4:409–429 [PubMed]
- 81.Al-Asmari F, Mereddy R, Sultanbawa Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J Photochem Photobiol, B. 2017;173:301–306. doi: 10.1016/j.jphotobiol.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 82.do Carmo Silva L, Tamayo Ossa DP, Castro SV da C, Bringel Pires L, Alves de Oliveira CM, Conceição da Silva C, Coelho NP, Bailão AM, Parente-Rocha JA, Soares CM de A, Ruiz OH, Ochoa JGM, Pereira M (2015) Transcriptome profile of the response of Paracoccidioides spp. to a camphene thiosemicarbazide derivative. PLOS ONE 10:e0130703. 10.1371/journal.pone.0130703 [DOI] [PMC free article] [PubMed]
- 83.Ahn S, Jung J, Jang I-A, Madsen EL, Park W. Role of glyoxylate shunt in oxidative stress response. J Biol Chem. 2016;291:11928–11938. doi: 10.1074/jbc.M115.708149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costa M, Borges CL, Bailao AM, Meirelles GV, Mendonca YA, Dantas SFIM, de Faria FP, Felipe MSS, Molinari-Madlum EEWI, Mendes-Giannini MJS, Fiuza RB, Martins WS, Pereira M, Soares CMA. Transcriptome profiling of Paracoccidioides brasiliensis yeast-phase cells recovered from infected mice brings new insights into fungal response upon host interaction. Microbiology. 2007;153:4194–4207. doi: 10.1099/mic.0.2007/009332-0. [DOI] [PubMed] [Google Scholar]
- 85.Bastos KP, Bailão AM, Borges CL, Faria FP, Felipe MSS, Silva MG, Martins WS, Fiúza RB, Pereira M, Soares CMA (2007) The transcriptome analysis of early morphogenesis in Paracoccidioides brasiliensis mycelium reveals novel and induced genes potentially associated to the dimorphic process. BMC Microbiol 7:29. 10.1186/1471-2180-7-29 [DOI] [PMC free article] [PubMed]
- 86.Kaur A, Sharma P, Capalash N (2018) Curcumin alleviates persistence of Acinetobacter baumannii against colistin. Sci Rep 8:11029. 10.1038/s41598-018-29291-z [DOI] [PMC free article] [PubMed]
- 87.(2012) Synthesis and in vitro antimycobacterial and isocitrate lyase inhibition properties of novel 2-methoxy-2′-hydroxybenzanilides, their thioxo analogues and benzoxazoles. European J Med Chem 56:108–119 . 10.1016/j.ejmech.2012.08.016 [DOI] [PubMed]
- 88.Yanik T, Donaldson RP. A protective association between catalase and isocitrate lyase in peroxisomes. Arch Biochem Biophys. 2005;435:243–252. doi: 10.1016/j.abb.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 89.de Arruda GD, Bailão AM, Vieira Rezende TC, Borges CL, de Oliveira MAP, Parente JA, de Almeida Soares CM. Response to oxidative stress in Paracoccidioides yeast cells as determined by proteomic analysis. Microbes Infect. 2013;15:347–364. doi: 10.1016/j.micinf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Lima P de S, Casaletti L, Bailão AM, Vasconcelos ATR de, Fernandes G da R, Soares CM de A (2014) Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Neglected Tropical Diseases 8:e2855. 10.1371/journal.pntd.000285 [DOI] [PMC free article] [PubMed]
- 91.Sandoval JM, Arenas FA, Vásquez CC (2011) Glucose-6-phosphate dehydrogenase protects Escherichia coli from tellurite-mediated oxidative stress. PLoS ONE 6:e25573. 10.1371/journal.pone.0025573 [DOI] [PMC free article] [PubMed]
- 92.Lundberg BE, Wolf RE, Dinauer MC, Xu Y, Fang FC. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect Immun. 1999;67:436–438. doi: 10.1128/IAI.67.1.436-438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dantas AS, Andrade RV, de Carvalho MJ, Felipe MSS, Campos ÉG. Oxidative stress response in Paracoccidioides brasiliensis: assessing catalase and cytochrome c peroxidase. Mycol Res. 2008;112:747–756. doi: 10.1016/j.mycres.2007.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.