Abstract
Physical exercise has acute and chronic effects on inflammatory balance, metabolic regulation, and redox status. Exercise-induced adaptations are mediated by enhanced 70-kDa heat shock protein (HSP70) levels and an improved heat shock response (HSR). Therefore, exercise could be useful against disease conditions [obesity, diabetes mellitus (DM), and exposure to atmospheric pollutants] marked by an impaired HSR. However, exercise performed by obese or diabetic subjects under pollution conditions might also be dangerous at certain intensities. Intensity correlates with an increase in HSP70 levels during physical exercise until a critical point at which the effort becomes harmful and impairs the HSR. Establishing a unique biomarker able to indicate the exercise intensity on metabolism and cellular fatigue is essential to ensure adequate and safe exercise recommendations for individuals with obesity or DM who require exercise to improve their metabolic status and live in polluted regions. In this review, we examined the available evidence supporting our hypothesis that HSP70 could serve as a biomarker for determining the optimal exercise intensity for subjects with obesity or diabetes when exposed to air pollution and establishing the fine threshold between anti-inflammatory and pro-inflammatory exercise effects.
Keywords: Fine particulate matter, Exercise, Diabetes, Heat shock response, Fatigue
Introduction
The ability of an organism to respond adequately and quickly to environmental or internal challenges is essential to survival, as stressful situations can either result in damage or adaptations by an organism (Pedersen 2017). High-intensity challenges overload the response mechanism, whereas moderate-intensity challenges can modulate physiological characteristics by triggering the internal recalibration of many systems, allowing an organism to survive in an aggressive external environment or adjust to internal dysfunctions (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013; Hochachka and Somero 2002).
This evolutionary perspective informs the sensitive, complex, and fascinating effect of physical exercise on several aspects of cellular physiology, including the inflammatory balance, metabolic regulation, and redox status. Exercise induces chronic adaptations by introducing acute challenges during exertion (Ropelle et al. 2010). The adaptations generated by engaging in exercise are associated with anti-inflammatory and antioxidant effects that are better able to respond to other external or internal challenges, such as environmental pollution (Marmett et al. 2020) and diabetes (Pedersen 2006; Pedersen 2017), respectively.
Both environmental pollution and diabetes are common modern-day conditions that represent physiological and biochemical challenges and must be carefully addressed. Fine particulate matter (PM2.5) is among the most abundant and harmful air pollutants. Due to their small size (0.1–2.5 μm), PM2.5 particles are inhaled into a deeper part of the respiratory tract, able to overcome the alveolar–capillary barrier, triggering immune, metabolic, and cardiovascular effects (Furuyama et al. 2009). The global prevalence of diabetes mellitus (DM) has also increased, primarily due to physical inactivity and diet-induced obesity. Diet-induced obesity combined with exposure to PM2.5 can result in synergistic effects on the development of type 2 DM (DM2) (Costa-Beber et al. 2021a; Costa Beber et al. 2020; Goettems-Fiorin et al. 2016). Physical exercise under these conditions can be useful but must be carefully prescribed due to dual effects. For example, individual variations and exaggerated glycemic responses to a session of acute exercise can be dangerous in patients with DM2 (Wormgoor et al. 2018), indicating that obesity and DM are critical health conditions that should be considered when recommending exercise under conditions of PM2.5 exposure (Kostrycki et al. 2019).
Metabolic impairments, inflammation, and oxidative stress can occur in individuals with DM2 who exercise under conditions of exposure to air pollution. Metabolism, inflammation, and redox state are interrelated, making it possible that a single biomarker able to serve as an indicator of the physiological interactions among these three states can be identified to monitor the strength of these interactions. Such a biomarker could be used when determining the appropriate level of exercise intensity to ensure that exercise provides sufficient stress to induce physiological adaptations without exacerbating inflammatory conditions. In this review, we propose that both extracellular and intracellular levels of 70-kDa heat shock protein (HSP70) can serve as biomarkers for evaluating the immune, redox, and inflammatory status.
HSP expression is a well-known cellular and molecular event that has often been used as a biomarker in many studies, starting as early as the 1960s (Brocchieri et al. 2008). First discovered in Drosophila sp., HSPs have been found to be expressed in response to a wide range of internal and environmental challenges (Brocchieri et al. 2008; Ritossa 1962; Ritossa 1996). Both oxidative and metabolic stress (e.g., hyperglycemia and hypoglycemia) induce intracellular HSP70 (iHSP70) expression (Hall and Martinus 2013; Ludwig et al. 2014). The primary physiological trigger for the activation of heat shock transcription factor (HSF1) and iHSP70 expression is hyperthermia (Miragem and Homem de Bittencourt Jr 2017; Newsholme and de Bittencourt Jr 2014), such as during physical exercise (Morton et al. 2009) or inflammation-induced fever. An acute inflammatory stimulus leads to the nuclear factor kappa beta (NFκB)-dependent activation of cyclooxygenase 2 (COX-2), which drives prostaglandin E2 (PGE2) synthesis and fever (Newsholme and de Bittencourt Jr 2014). Fever, in turn, forms a negative feedback loop and induces iHSP70 expression, which inhibits NFκB activation through canonical pathways to control the initial inflammatory stimulus. iHSP70 associates with the NFκB–inhibitor of NFκB (IκB) complex and inhibits IκB kinase (IKK) activity to prevent the translocation of NFκB to the nucleus (Chen et al. 2005; Costa-Beber et al. 2020; Kim et al. 2005) (Figure 1A). In the nucleus, iHSP70 mediates the degradation of the NFκB p65 subunit, inhibiting inflammatory signaling (Tanaka et al. 2014).
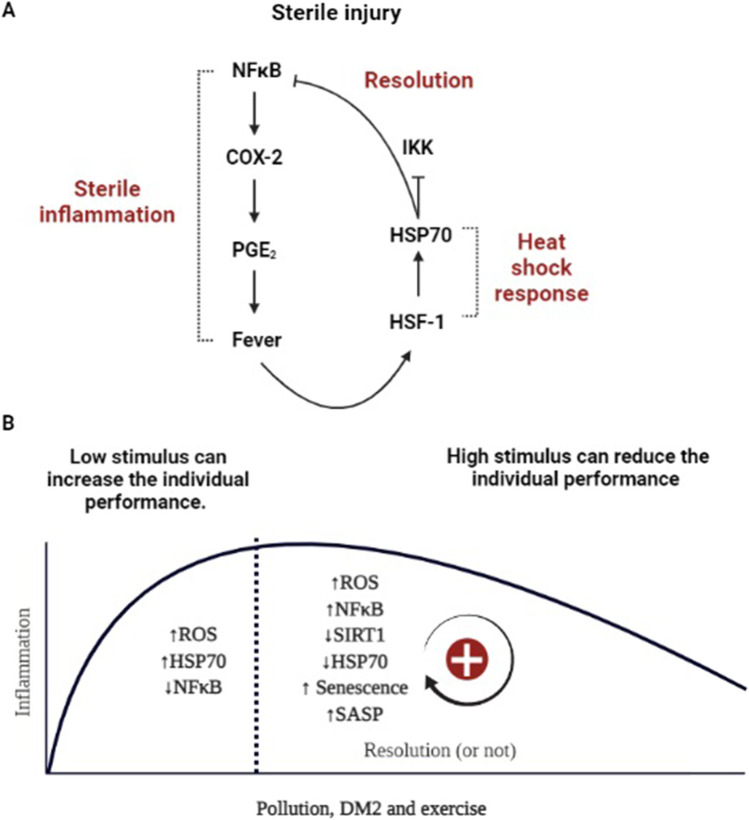
Fig. 1.
A The induction of HSP70 during inflammation and its role in counteracting the initial stimulus. An acute inflammatory stimulus leads to nuclear factor kappa B (NFκB)-dependent cyclooxygenase 2 (COX-2) activation, which drives prostaglandin E2 (PGE2) synthesis and fever. Fever induces iHSP70 expression, which associates with the NFκB–inhibitor of NFκB (IκB) complex and inhibits IκB kinase (IKK) activity, preventing the translocation of NFκB to the nucleus. B The effects of exercise on HSP70 levels in individuals with diabetes who are exposed to pollution: the hormesis theory. According to the hormesis theory, before becoming deleterious, a stimulus can lead to a preconditioning effect due to physiological adaptations, making the organism stronger and better able to address future harmful stress. In this scenario, a low-level stimulus can increase individual performance within a limited zone, which is typically characterized by increased HSP70 levels. A high-level stimulus (i.e., not within the normal limits) can reduce individual performance, which is typically characterized by decreased HSP70 levels. Thus, each condition (pollution, DM, and exercise) represent metabolic, oxidative, and inflammatory challenges that can induce HSP70 until a certain limit, when the organism is no longer able to induce a proper heat shock response (HSR) to counteract the initial harmful inflammatory stimulus. Created with BioRender.com
Among the known HSP families, the 70-kDa protein family plays an intriguing role in cell homeostasis that varies depending on whether the protein is expressed inside or outside of the cell. These proteins exert chaperone, cytoprotective, and anti-inflammatory effects inside the cell (iHSP70), whereas HSPs released in the extracellular fluid (eHSP70) mediate inflammatory events (De Maio 2011; Kampinga and Craig 2010; Pockley and Multhoff 2008). High levels of eHSP70 are released during exercise, depending on the exercise intensity (Heck et al. 2017), and high levels of eHSP70 have also been reported during conditions of both hypoglycemia (Ludwig et al. 2014) and hyperglycemia (Krause et al. 2015b). eHSP70 binds to Toll-like receptors 2 and 4 (TLR2 and TLR4, respectively) on the surface of antigen-presenting cells, inducing the production of interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α) in an NFκB-dependent manner (Ao et al. 2009; Asea 2003; Asea 2005; Asea et al. 2002; Vabulas et al. 2002). Excessive eHSP72 levels have been reported in association with several metabolic (Alemi et al. 2019; Krause et al. 2015b; Rodrigues-Krause et al. 2012b), pulmonary (Ogawa et al. 2008), and cardiovascular conditions (Krepuska et al. 2011; Szeberin 2012; Wright et al. 2000; Yadav et al. 2013; Zhang et al. 2010). However, during exercise, eHSP70 plays dual roles by simultaneously activating a chronic low-grade inflammatory state in addition to activating the immune system and mediating important signaling pathways, including those associated with fatigue (Heck et al. 2011).
HSP70 expression and release represent the cell’s ability to respond to stressful conditions, serving as a vital indicator of cellular adaptive mechanism, which can have effects on aging and longevity (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013; Dattilo et al. 2015). In healthy cells, inflammatory (Heck et al. 2017; Miragem and Homem de Bittencourt Jr 2017; Newsholme and de Bittencourt Jr 2014), metabolic (Ludwig et al. 2014), and thermal stimuli (Nava and Zuhl 2020; Ritossa 1962; Ritossa 1996) induce the translocation of HSF-1 from the cytoplasm to the nucleus, where it undergoes posttranslational modifications, such as trimerization, and binds to the heat shock element (HSE) in the HSP promoter region (Torok et al. 2014). This process allows for cells to mount an adequate heat shock response (HSR) through the increased expression of HSP70 (Torok et al. 2014), counteracting the initial inflammatory stimulus (Newsholme and de Bittencourt Jr 2014). According to the ‘hormesis’ theory, each type of insult is followed by a biphasic dose-response curve. A weak stimulus can stimulate the organism, increase performance and the survival rate, while a high dose stimulus can inhibit the defense system and decrease performance (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013; Dattilo et al. 2015). Therefore, a weak stimulus can induce the HSR, resulting in preconditioning through physiological adaptations, whereas a stronger stimulus impairs the HSR by reducing iHSP70 expression, and reduces survival (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013; Dattilo et al. 2015). The lack of a proper HSR can trigger a senescence-associated secretory phenotype (SASP), which propagates the low-grade and chronic inflammation associated with chronic diseases (Miragem and Homem de Bittencourt Jr 2017; Newsholme and de Bittencourt Jr 2014). Metabolic impairment can disrupt the cellular response to redox and other metabolic conditions, reducing cell survival (Costa-Beber et al. 2021a; Costa-Beber et al. 2021b; Costa-Beber et al. 2020; Costa Beber et al. 2020; Goettems-Fiorin et al. 2019).
HSP70 has been documented to serve as a biomarker and mediator of immune and (anti)inflammatory pathways in obesity and DM and during exposure to pollutants and exercise, leading us to propose that eHSP70 and iHSP70 might also serve as potential biomarkers for the immune and inflammatory response to various exercise intensities performed by patients with DM when exposed to air pollution. Here, we review available evidence to support the use of HSP70 as a biomarker of exercise intensity and the subsequent occurrence of tissue and systemic damage or protection under varying conditions to provide guidance for recommendations and limitations on exercising when exposed to air pollution in patients with DM.
HSP70 in obesity and diabetes
Even before clinical diagnosis, the early stages of DM development are marked by metabolic, inflammatory, and redox impairments. The immune-inflammation that initiates type 1 DM (DM1) and the chronic low-grade inflammation that marks DM2 can be prevented or monitored prior to developing into a severe condition.
In both DM1 and DM2, inflammatory cells (monocytes, macrophages, and T helper lymphocytes) are stimulated to express high levels of the inducible form of nitric oxide synthase (iNOS, encoded by NOS2) in response to NFκB activation (Enos et al. 2013). The activation of NFκB generates an influx of L-arginine in pancreatic islet β-cells through the iNOS pathway (Rojas et al. 2018), resulting in increased nitric oxide (NO) production, which acts as a toxic free radical in islet β-cells due to a limited antioxidant defense (Munhoz et al. 2016). During hyperglycemia or hypoglycemia, patients with DM are more susceptible to the development of a pro-oxidant and pro-inflammatory status (Eik et al. 2016; Ludwig et al. 2014).
In patients with DM, a pro-inflammatory status is associated with changes in HSP72 levels (Ludwig et al. 2014). Decreased iHSP70 levels might be key factors in both DM1 and DM2 development (Hooper 2003; Hooper and Hooper 2005; Hooper and Hooper 2009; Rogers et al. 2016). Patients with obesity present with reduced iHSP72 levels in muscle and hepatic cells (Rogers et al. 2016), although one study reported an increase in hepatic iHSP70 levels associated with diet-related steatosis (Zhang et al. 2018). In adipose tissue, the iHSP72 levels are increased in patients with obesity compared to the baseline (Tiss et al. 2014). However, among patients with obesity, a significant attenuation of adipose iHSP72 levels was reported in patients with DM compared with that in patients without DM (Tiss et al. 2014), indicating an impaired HSR in patients with DM.
As previously noted, inflammation and oxidative stress can induce dual HSR signaling pathways. An acute stimulus triggers the HSR, whereas chronic stimuli can silence the HSR, reducing iHSP70 levels (Adachi et al. 2009). The lack of a proper HSR is associated with deficiencies in the processes that control the resolution of inflammation. Briefly, low iHSP70 levels compromise the cellular ability to inhibit NFκB translocation, which is necessary to reduce inflammation [see Costa-Beber et al. 2020 and Mulyani et al. 2020 for review] and trigger the development of SASP (Newsholme and de Bittencourt Jr 2014).
Pancreatic β-cells are especially susceptible to inflammatory and oxidative stress due to the poor expression of catalase and glutathione peroxidase (Lenzen et al. 1996). Thus, iHSP70 induction can protect against NO and superoxide-induced necrosis (Burkart et al. 2000) and prevent the activation of NADPH oxidase (NOX), which is the main source of reactive oxygen species (ROS) (Krause et al. 2015a). During hyperglycemia, the chaperone properties of iHSP70 are necessary to assist with the proper folding of proteins in response to the upregulation of protein synthesis. The lack of iHSP70 in pancreatic cells can result in the induction of the unfolded protein response (UPR) and endoplasmic reticulum (ER) stress (Huang et al. 2014). Therefore, low pancreatic iHSP70 levels are associated with an increased vulnerability to stress, an exacerbated oxidative milieu, and impaired insulin secretion, culminating in cell death (Krause et al. 2015a). Inflammation can also lead to insulin resistance by inducing an increase in c-Jun N-terminal kinase (JNK) phosphorylation (Mulyani et al. 2020; Newsholme and de Bittencourt Jr 2014) and the inhibition of phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) signaling and glucose transporter 4 (GLUT-4) translocation (Mulyani et al. 2020; Newsholme and de Bittencourt Jr 2014). However, iHSP70 overexpression prevents JNK phosphorylation in the skeletal muscle and IKK phosphorylation in the liver (Chung et al. 2008). The prevention of IKK phosphorylation allows iHSP70 to increase the stability of the NFκB–IκB-α complex, preventing its translocation to the nucleus (Costa-Beber et al. 2020). Therefore, iHSP70 overexpression prevents the development of diet-induced insulin resistance (Chung et al. 2008).
Conversely, eHSP70 mediates a different response. Although iHSP70 plays a cytoprotective role, eHSP70 mediates pro-inflammatory pathways and acts in the immune signaling pathway. High circulating levels of eHSP70 can induce the release of inflammatory cytokines through two different signalings, the NFκB translocation to the nucleus and activating protein 1 (AP-1) activation (Gonzalez-Ramos et al. 2013). eHSP70 binds to TLRs to enhance NOX expression (Krause et al. 2015a; Zhang et al. 2016), activate the JNK pathway (Costa-Beber et al. 2020; Zhu and Mohan 2010), and impair insulin release.
Previous studies have documented increased levels of eHSP70 in patients with DM2 and obesity than in patients with DM2 without obesity (Rodrigues-Krause et al. 2012a), which is correlated with insulin resistance (Alemi et al. 2019). Circulating levels of eHSP70 have been associated with several DM-related complications (Alemi et al. 2019; Krause et al. 2014a; Nakhjavani et al. 2010; Rodrigues-Krause et al. 2012b; Xie et al. 2016) and the time-course of the disease (Xie et al. 2016). eHSP70 levels increase with disease duration, such that patients recently diagnosed with diabetes typically present with lower levels of eHSP70 than patients with 5 years of diagnosed disease (Nakhjavani et al. 2010). In agreement with these findings, some studies have reported increased levels of anti-HSP72 antibodies in animal models of high-fat-diet-induced obesity, DM2, and atherosclerosis (Ghayour-Mobarhan et al. 2007; Leng et al. 2013). Under such conditions, an increase in anti-HSP72 antibodies could represent an immune response to the increase in eHSP72 levels, which agrees with the findings of other studies (see Table 1 for review).
Table 1.
Summary of the studies evaluating the impact of the HFD-induced obesity, or DM2, on the tissue and plasma HSP70 concentration and level of anti-HSP70 antibodies
| Reference | Design | Protein of interest | Outcome |
|---|---|---|---|
| (Xie et al. 2016) |
Sprague Dawley male rats HFD for 12 weeks to induce obesity and atherosclerosis |
eHSP70 | ↑ |
| (Zhang et al. 2018) |
C57BL/6 mice HFD for 16 weeks |
iHSP70 hepatic expression | ↑ |
| HepG2 cells (hepatocytes) | mRNA iHSP70 expression | ↑ | |
| (Leng et al. 2013) |
Sprague Dawley rats HFD |
Anti-HSP72 antibodies | ↑ |
| (Ghayour-Mobarhan et al. 2007) |
White rabbits HFD for 13 weeks |
Anti-HSP70 antibodies | ↑ |
| (Krause et al. 2014) | 50 elderly (63.4 ± 4.4 years old) with BMI = 25.5 ± 2.7 kg/m2. | Association between eHSP72 and insulin resistance | ↑ |
| (Rodrigues-Krause et al. 2012a) | Non-diabetic obese, DM2 obese, DM2 non-obese (54 ± 9 years old) | eHSP70 due to DM2 | ↑ |
| iHSP70 expression in the vast lateral of obese subjects | ↓ | ||
| (Nakhjavani et al. 2010) | Patients with DM2 (49.17 ± 1.58 years old) | eHSP70 | ↑ |
| (Alemi et al. 2019) | Patients with DM2 (54.5 years old) | eHSP70 | ↑ |
| Association between eHSP72 and insulin resistance | ↑ |
BMI, body mass index; DM2, type 2 diabetes mellitus; eHSP70, extracellular 70-kDa-heat shock protein; iHSP70, intracellular 70-kDa heat shock protein; HFD, high fat diet. Possible outcomes: Up arrows indicate an increase in the protein of interest while down arrows indicate a reduction in that context
Although eHSP70 levels are increased in patients with obesity and DM at rest, the eHSP70 levels decrease in response to a challenging condition, such as physical exercise (Kostrycki et al. 2019), which is of particular importance considering that approximately 2 billion people are overweight and one third of them are obese (Seidell and Halberstadt 2015). Although this metabolic dysfunction represents a powerful challenge to the HSR on its own, exposure to air pollution also affects the HSR.
Diabetes and atmospheric pollution
In both high- and low-income countries, air pollution represents an aggressive, modern, urban threat to human health (Molina and Molina 2004). Epidemiologic evidence shows that as little as a 1 μg/m3 increase in the PM2.5 level above the annual average (12.5 10 μg/m3) enhances total cholesterol, triglyceride, and low-density lipoprotein levels in humans (McGuinn et al. 2019). Moreover, a 5 μg/m3 increase in PM2.5 levels is associated with a 6% increase in IL-6 levels (Hajat et al. 2015), whereas a 10 μg/m3 increase in PM2.5 levels increases blood pressure (Xie et al. 2018).
The lungs are the first organs that contact PM2.5 and suffer both direct and indirect adverse effects. Direct effects occur due to contact between particles and pneumocytes, whereas indirect effects occur through the secretory phenotype of alveolar macrophages during the immune reaction (Martin et al. 2019; Niemann et al. 2017). Alveolar macrophages entrap these particles through phagocytosis, resulting in an immunological response marked by an increase in ROS and inflammatory cytokines production (Dubowsky et al. 2006; Ghio et al. 2012). Due to their small size (0.1–.5 μm), PM2.5 can directly cross the alveolar–capillary barrier by transcytosis or be transported by monocytes via blood circulation (Furuyama et al. 2009). The immunological response of alveolar macrophages is thought to mediate PM2.5-related systemic effects (Jiang et al. 2018; Martin et al. 2019).
PM2.5 overwhelms the antioxidant response of endothelial cells that form the pulmonary barrier, resulting in an oxidative status (Klein et al. 2017). Once they reach the blood circulation, PM2.5 can induce systemic oxidative stress and inflammation (Costa Beber et al. 2020; Goettems-Fiorin et al. 2016). IL-6 is among the inflammatory factors most responsible for this outcome, and both PM10 and PM2.5 have been shown to increase IL-6 production by alveolar macrophages (Marchini et al. 2016), resulting in enhanced IL-6 levels in the bronchoalveolar fluid and the subsequent translocation of IL-6 into the systemic circulation (Kido et al. 2011; Marchini et al. 2016). The knockout or biological neutralization of IL-6 (Kido et al. 2011; Marchini et al. 2016) and TNF-α has been shown to prevent many of the adverse outcomes associated with PM exposure (Marchini et al. 2016). Together, IL-6 and TNF-α lead to lung (Jiang et al. 2018; Rhoden et al. 2008; Rhoden et al. 2004; Rhoden et al. 2005) and vascular damage (Haberzettl et al. 2016b), which propagates systemic oxidative stress and increases the risks of developing DM and cardiovascular diseases (Jiang et al. 2018).
PM2.5 represents a novel additional risk factor for DM2 (Alderete et al. 2017; Costa-Beber et al. 2021a; Costa Beber et al. 2020; Haberzettl et al. 2016a; Haberzettl et al. 2016b; Xu et al. 2011). Chronic exposure to air pollution can exacerbate diet-induced glucose intolerance, oxidative stress, and systemic insulin resistance (Costa Beber et al. 2020; Gasparotto et al. 2018; Goettems-Fiorin et al. 2016; Haberzettl et al. 2016a; Haberzettl et al. 2016b; Xu et al. 2010); hepatic steatosis (Gasparotto et al. 2018); and adipose tissue inflammation (Xu et al. 2010). Thus, air pollution can affect both DM1 and DM2 through an inflammatory process initiated by redox imbalance (Brook et al. 2010).
HSPs are naturally sensitive to redox alterations triggered by chemical attacks on the cells and are extensively used as biomarkers of environmental pollution exposure (Esposito et al. 2018; Kim et al. 2014; La Porte 2005; Mitra et al. 2018; Mukhopadhyay et al. 2003; Ravaschiere et al. 2017; Somasundaram et al. 2019). Several traffic-related air pollutants have been shown to affect HSP70 expression. Pollutant particle size represents an important factor in determining iHSP70 levels, as 3 days of exposure to PM10 and PM2.5 was shown to increase pulmonary and cardiac iHSP70 levels (Farina et al. 2013a; Sancini et al. 2014), whereas the same exposure to PM1 decreases pulmonary iHSP70 levels (Farina et al. 2013b).
The strong correlations observed between PM exposure, oxidative stress, and inflammation, and changes in the HSR reinforce the hypothesis that HSP70 may be an important biomarker of homeostatic equilibrium during environmental challenges. Only a few available studies have examined the effects of pollution on the HSR in diet-induced DM. Particulate pollutant exposure can disrupt the HSR in pancreatic tissue, marked by an increased eHSP70/pancreas iHSP70 ratio in obese animals (Goettems-Fiorin et al. 2016), linking obesity with insulin resistance. Furthermore, chronic exposure to residual oil fly ash (ROFA), which comprises the inorganic portion of PM2.5, induces a modest increase in iHSP70 levels in the cardiac tissue, whereas the combination with other challenging condition, such as ovariectomy (used to surgically induce hypoestrogenism in animal models), reduced iHSP70 levels similar to control (Costa-Beber et al. 2021b). Similarly, ROFA exposure reduced the iHSP70 levels, which tended to increase in a high-fat diet plus ovariectomy condition (Costa-Beber et al. 2021a).
The HSR varies across metabolic tissues in obese animals exposed to pollution. Although the pancreas shows decreased iHSP70 levels following pollution exposure in obese animals, increased iHSP70 levels were observed in the white adipose tissue of the epididymis and the liver (Goettems-Fiorin et al. 2016). In addition, chronic exposure to ROFA enhances the susceptibility to ovariectomy-induced increases in hepatic iHSP70 levels (Goettems-Fiorin et al. 2019). By contrast, evidence regarding the impacts of pollution on eHSP70 levels has not been conclusive, with studies reporting that eHSP70 levels increase (Baldissera et al. 2018; Gasparotto et al. 2013; Watterson et al. 2009), remain unaltered (Costa-Beber et al. 2021a; Costa-Beber et al. 2021b; Gasparotto et al. 2018; Goettems-Fiorin et al. 2019; Goettems-Fiorin et al. 2016), or decrease in response to pollution exposure (see Table 2 for review).
Table 2.
Summary of the studies evaluating the impact of the exposure to atmospheric pollutants on the HSP70 tissue expression and plasmatic concentration
| Reference | Design | Protein of interest | Outcome |
|---|---|---|---|
| (Baldissera et al. 2018) |
Wistar rats Acute exposure (3 days) to 750 μg/100 μL of ROFA |
eHSP70 | ↑ |
| Expression of iHSP70 in the lymph node | = | ||
| Expression of iHSP70 in the thymus | = | ||
| Expression of iHSP70 in the spleen | = | ||
| eHSP70/iHSP70 ratio in the lymph node | = | ||
| eHSP70/iHSP70 ratio in the thymus | ↑ | ||
| eHSP70/iHSP70 ratio in the spleen | = | ||
| (Sancini et al. 2014) |
BALB/c male mice Intratracheal instillation of 100 mg of PM2.5 three times, every three days |
Pulmonary parenchyma iHSP70 expression | ↑ |
| Cardiac iHSP70 expression | ↑ | ||
| (Gasparotto et al. 2013) |
RAW 264.7 macrophages Incubation with nanoparticles and ultrafine particles of coal fly ashes 1 mg/mL for 24 h |
iHSP70 expression | ↑ |
| (Watterson et al. 2009) |
Human bronchial epithelial cells (BEAS-2B) PM2.5 and PM10 12.5 and 25 μg/mL 24 h |
iHSP70 expression | ↑ |
| (Farina et al. 2013b) |
BALB/c mice Intratracheal instillation of 100 μg/animal of PM1 Three times, every three days |
Pulmonary iHSP70 expression | ↓ |
| (Farina et al. 2013a) |
BALB/c mice Intratracheal instillation of 100 μg/animal of PM10 Three times, every three days |
Pulmonary iHSP70 expression | ↑ |
| Cardiac iHSP70 expression | ↑ | ||
| (Gasparotto et al. 2018) |
Wistar rats HFD consumption (60%) for 24 weeks At the 21ª week, they also started to receive coal dust, in a chamber (10 mg/m3, 3 h/day) for 4 weeks. |
eHSP70 | = |
| (Goettems-Fiorin et al. 2016) |
B6.129SF2/J male mice HFD consumption (58.3%) for 24 weeks At the 13ª week, they also started to receive an intranasal instillation of 5 μg/10 μL of PM2.5 for 12 weeks |
eHSP70 | = |
| Adipose tissue iHSP70 expression | ↑ | ||
| Hepatic iHSP70 expression | ↑ | ||
| Gastrocnemius iHSP70 expression | = | ||
| Pancreatic iHSP70 expression | ↓ | ||
| eHSP70/iHSP70 ratio in the adipose tissue | = | ||
| eHSP70/iHSP70 ratio in the liver | = | ||
| eHSP70/iHSP70 ratio in the gastrocnemius | = | ||
| eHSP70/iHSP70 ratio in the pancreas | ↑ | ||
| (Costa-Beber et al. 2021a) |
Wistar female rats HFD consumption (58.3%) for 24 weeks ROFA exposure (250 μg/50 μL), once a day, 5 days/week, for 24 weeks OVX at the 12th week |
iHSP70 in the cardiac tissue | ↓* |
| eHSP70 | = | ||
| eHSP70/iHSP70 ratio in the cardiac tissue | = | ||
| (Costa-Beber et al. 2021b) |
Wistar female rats ROFA exposure (250 μg/50 μL), once a day, 5 days/week, for 24 weeks OVX at the 12th week |
iHSP70 in the cardiac tissue | ↓* |
| eHSP70 | = | ||
| eHSP70/iHSP70 ratio in the cardiac tissue | = | ||
| (Goettems-Fiorin et al. 2019) |
Wistar female rats ROFA exposure (250 μg/50 μL), once a day, 5 days/week, for 24 weeks OVX at the 12th week |
iHSP70 in the hepatic tissue | ↑ |
| eHSP70 | = | ||
| eHSP70/iHSP70 ratio in the hepatic tissue | = |
eHSP70, extracellular 70-kDa heat shock protein; HFD, high fat diet; iHSP70, intracellular 70-kDa heat shock protein; PM1, fine particulate matter (0.1 to 1 μm); PM2.5, fine particulate matter (0.1 to 2.5 μm); PM10, coarse particulate matter (2.5 to 10 μm); ROFA, residual oil fly ash. Possible outcomes: Up arrows indicate an increase in the protein of interest; down arrows indicate a reduction [* reduction back to basal levels]; equal signals ‘=’ indicate the absence of significant changes in that context
Although the precise effects on the HSR due to the combination of obesity with pollution exposure have not yet been established, both risk factors have been shown to exert synergistic effects on the metabolic, oxidative, and inflammatory environments (Costa-Beber et al. 2021a; Costa Beber et al. 2020; Goettems-Fiorin et al. 2016). Approximately 9 out of 10 people in the world breathe air that exceeds the recommended WHO guidelines (WHO 2021) for pollution exposure, which requires the evaluation of potential treatments that are capable of mitigating the effects of pollution exposure. One of the most powerful non-pharmacological treatments for obesity and insulin resistance-related conditions is physical exercise; however, whether exercise can alleviate the effects of pollution exposure under obesity conditions and how much exercise is optimal remains unknown.
Why exercise? The role of the HSP70 in exercise-induced benefits in diabetes
For patients with well-controlled DM who are treated with insulin or insulin secretagogues, moderate or intense physical exercise is associated with the risk of hypoglycemic events (Younk et al. 2011), particularly among individuals with DM who do not regularly engage in exercise. Exercise can ameliorate metabolic conditions by improving the cellular anti-inflammatory and antioxidant status. Exercise promotes the upregulation of antioxidant enzymes and increases serum and tissue anti-inflammatory cytokines, promoting better conditions glycemic metabolic control in individuals with DM (Nunes et al. 2008; Pedersen 2006; Pedersen 2013; Pedersen and Febbraio 2008; Pedersen et al. 2003).
Glutamine serves as a critical factor involved in antioxidant and anti-inflammatory defenses. Patients with both DM1 and DM2 present with low serum glutamine levels due to increased inflammatory metabolism (glutamine consumption by immune cells) (Liu et al. 2019). Therefore, glutamine released by active muscles during physical exercise (Raizel et al. 2016) can improve the antioxidant, immune, and metabolic status by serving as a substrate for the synthesis of glutathione, which is the major antioxidant found in pancreatic β-cells (Cruzat et al. 2018). In addition, glutamine concentrations modulate iHSP70 levels by inducing HSF-1 O-glycosylation or phosphorylation (Petry et al. 2015; Petry et al. 2014; Petry et al. 2019; Raizel et al. 2016). Thus, physical exercise can modulate HSP70 expression levels by altering glutamine levels (Hung et al. 2008).
A three-week treadmill protocol [30–60 min/day, at the intensity of 1.6 km/h, 5 days/week] is sufficient to increase HSP70 levels in cardiac and skeletal muscle (Hung et al. 2008). This exercise-induced iHSP70 level may be responsible for attenuating DM1-related outcomes and improving survival following lipopolysaccharide (LPS)-induced endotoxemia (Hung et al. 2008). The role played by HSP70 in reducing sepsis-related mortality was also reported by our group (Sulzbacher et al. 2020). However, HSP70 is sensitive to stress, and longer periods of exercise-related training may demand a progressive protocol, as adaptation may reduce the response to exercise, resulting in a decrease in the expression levels of HSP70. For example, in a streptozotocin-induced DM1 model, a 5-week ladder-climbing protocol reduced iHSP70 levels in the muscle, whereas iHSP70, IL-6, and TNF-α levels were increased in the adipose tissue (Molanouri Shamsi et al. 2016). According to the authors, this increase in adipose iHSP70 levels may be related to the promotion of lipolysis by exercise-generated acute stress. By contrast, the observed reduction of iHSP70 in muscle may be due to the interruption of diabetes-related inflammatory stress and muscle atrophy by exercise (Molanouri Shamsi et al. 2016).
DM1 and DM2 show distinct physiopathologies, and the optimal exercise regimens may differ between these two diseases. In a genetic animal model of obesity, treadmill training [60 min/day, at the intensity of 20 m/min, 5 days/week, 10 weeks] increased iHSP72 levels in the gastrocnemius muscle and liver (Tsuzuki et al. 2017). A 3-month protocol combining aerobic exercise with resistance training in people with obesity reduced the expression levels of various HSPs to levels observed in lean subjects, with a parallel decrease in the endogenous levels of IL-6 and TNF-α (Tiss et al. 2014). However, physical training alleviated cellular stress in adults with obesity, regardless of DM diagnosis, attenuating the inflammatory response (Khadir et al. 2018). Consistent with this finding, the same exercise protocol and duration increased HSP70 mRNA levels in the adipose tissue of people with obesity and DM, which were reduced at baseline compared with people with obesity but without DM (Khadir et al. 2018).
These data suggest that exercise-induced effects are mediated by the modulation of the HSR. In addition to the protective role displayed by iHSP70, the expression of iHSP70 under stressful conditions mediates other counter-regulatory pathways. A study by Tsuzuki et al. (2017) showed that the attenuation of exercise-induced iHSP72 expression, mediated by decreasing the room temperature, also attenuated improvements in lipid metabolism and whole-body insulin resistance. Additionally, the set of studies reviewed here emphasizes the need to perform time-course monitoring of both iHSP70 and eHSP70 levels during an exercise protocol to determine the most effective exercise intensity to obtain metabolic improvements among individuals with DM. This time-course study would also be valuable for determining the level of cellular stress experienced by individuals with obesity and DM, which would contribute to evaluating the benefits of training or supplementation programs (Antunes-Neto et al. 2006).
The benefits of physical training may also differ according to individual biology, which is influenced by each individual’s metabolic condition, the presence or absence of obesity or DM, and the quality of the air to which they are exposed. Air pollution is a relevant external challenge, and the use of different exercise protocols may serve as a potential means for preventing or improving the harmful effects associated with pollution exposure. Monitoring HSP70 levels could also provide important information regarding cellular stress in response to exercise intensity among populations with the combined risk factors of DM and exposure to poor-quality air.
The paradox of exercising under polluted conditions
Studies involving exercise as a non-pharmacological strategy for the prevention or treatment of PM2.5-induced insulin resistance or obesity-associated comorbidities differ in the exercise modality, intensity, and duration and the timing of exercise relative to pollution exposure, which likely contributes to the variability of the reported results. This variability has resulted in controversy regarding whether exercise under polluted conditions is advisable and whether exercise under polluted conditions prevents, treats, or exacerbates insulin resistance.
There is a recommendation to avoid exercise in streets or highways due to the toxic effects caused by air pollution (Heck et al. 2015b; Sharman et al. 2004; Sharman et al. 2002). Several studies that have examined exercise under polluted conditions have reported a decrease in pulmonary function (i.e., forced vital capacity and forced expiratory volume) (Rundell et al. 2008), highlighting the risks associated with exercising in inadequate air conditions (Sharman 2005; Sharman et al. 2004). During exercise, even at low intensities, the rates of both respiration and oral ventilation increase, which enhances PM2.5, inhalation five-fold and enhances PM0.1 inhalation two-fold (Daigle et al. 2003; Slezakova et al. 2020), potentiating the deleterious effects of air pollution (Bennett et al. 1985).
Previous studies have shown that particle inhalation or exposure to urban air pollution during exercise can increase pulmonary and cardiac oxidative stress (Heck et al. 2015a, 2015b). Similarly, an increase in lung inflammation after training sessions was detected in young athletes after exercising under conditions with high PM levels (Ferdinands et al. 2008), in addition to enhanced respiratory airway constriction and reduced respiratory function (Rundell et al. 2008). Corroborating these studies, other research showed that cycling in an urban environment, resulting in PM exposure, induces an increase in systemic inflammation markers (Jacobs et al. 2010), which can be observed as changes to routine hematological lab results, such as increasing white blood cells and platelets number (Kargarfard et al. 2015). Therefore, the strict consensus has been to avoid exercising under polluted conditions.
However, recent studies have shown contradictory results regarding whether exercise under polluted conditions is advisable. Using the same protocol of 2-h exposure in high or low traffic-related air pollution, intermittent moderate physical activity [15 min of cycling on a stationary bicycle alternating with 15 min of rest, four times, at the intensity of 50–70% of the maximum heart rate] was shown to prevent an increase in systolic blood pressure caused by exposure to traffic-related PM10 and PM>10 (Kubesch et al. 2015) and attenuated the PM-induced impairment of pulmonary capacity (Matt et al. 2016). However, a study evaluating only one-time outdoor running under conditions of low and high PM2.5 exposure did not find any effects on pulmonary function (Wagner and Brandley 2020). Another study reported that no pulmonary or autonomic effects were experienced by young individuals practicing vigorous activities [30 min, high-intensity cycling at the intensity of 77% of VO2peak] under polluted conditions (Giles et al. 2018). Together, this evidence suggests that exercising under polluted conditions may not contribute to harmful effects and may even prevent the damage induced by pollution under non-exercising conditions.
Studies in animal models have presented interesting evidence that a moderate level of physical exercise can prevent the damage induced by pollution. A treadmill pre-training protocol performed at moderate intensity prevented pulmonary dysfunction and the progression of lesions (local bleeding, pus exudation, inflammatory cell infiltration of lung tissues, and bronchial mucosal exfoliation) induced by one bout of PM2.5 exposure [chambers with 269.31 ± 30.79 μg/m3 and 509.84 ± 36.74 μg/m3 of PM2.5 in medium and high concentration, respectively] (Qin et al. 2020). However, in this study, only healthy animals were used, and exposure to air pollution occurred only once (Qin et al. 2020). In a follow-up study, these authors extrapolated their results to an already susceptible population by showing that the same exercise pre-training protocol was able to ameliorate lung injury induced by subchronic exposure to PM2.5 in aging rats (Qin et al. 2021). These protective effects were attributed to antioxidant and anti-inflammatory adaptations induced by exercise, characterized by increased catalase activity and glutathione levels and decreased TNF-α and IL-1β levels in the bronchoalveolar fluid (Qin et al. 2020). These findings may explain why 2 h of intermittent exercise performed by healthy volunteers under real-world, polluted conditions have been reported to result in the immediate repression of the negative effects that are typically induced by PM exposure on pulmonary function, including peak expiratory flow and forced vital capacity (Matt et al. 2016).
The benefits of exercise extrapolate to improved respiratory capacity and improved vascular and systemic conditions. A physical exercise protocol prevented endothelial dysfunction in animals subsequently exposed to PM2.5 (Feng et al. 2019). In addition, physical training increased high-density lipoprotein levels and function by improving cholesterol efflux capacity and reducing the oxidation index (Feng et al. 2019). The antioxidant response in the gastrocnemius muscle was also improved by exercising under polluted conditions (ROFA exposure) (Marmett et al. 2018) (see Table 3 for review).
Table 3.
Summary of the studies evaluating the impact of exercising in polluted conditions
| Reference | Design | Major outcomes |
|---|---|---|
| (Qin et al. 2020) |
Wistar rats Moderate treadmill-training (5 days/week, for 8 weeks) Cycle: 5 min, 50 to 55% of VO2 max + 3 min, 65 to 70% of VO2 max + 4 min, 80 to 90% of VO2 max + 5 min, 50 to 55% of VO2 max, 7 times. At the end of the exercising protocol, the animals were acutely exposed to different PM2.5 levels. Low: 149.16 ± 30.88 μg/m3 Medium: 269.31 ± 30.79 μg/m3 High: 509.84 ± 36.74 μg/m3 |
Compared with the corresponding different concentration of sedentary animals exposed to PM2.5, the exercise reduced MDA, TNF-α and IL-1β in the lungs, and increased CAT and GSH. |
| (Qin et al. 2021) |
Aging rats Moderate aerobic training (30 min, 7 days/week for 8 weeks) Cycle: warm-up (3 min), cooldown (2 min) with 45–55% of VO2 max and two sessions of moderate aerobic exercise (15 min, 65–75% VO2 max) with 5 min rest. At the end of the exercising protocol, the animals were exposed to PM2.5 4 h/day, 7days/week for two weeks. |
Aerobic exercise alleviated PM2.5-induced airway obstruction, deterioration of pulmonary function and inflammatory responses in aging rats. It increased the expression of iHSP70, reduced the eHSP70 levels and the H-Index and suppressed the NF-kB activation. |
| (Slezakova et al. 2020) | Local calculation of the UFP levels | Highly intense activities (i.e., running) led to twice-higher UFP exposure. Finally, UFP inhalation doses estimated for walking (commuting to work and/or schools) were 1.6–7.5 times lower than when conducting sport activities. |
| (Marmett et al. 2020) |
Healthy men experiencing a physical training program (> 150 min/week), indoor or outdoor, for at least six months. Personal monitoring exposure to the air pollutants O3 and NO2. |
Despite the greatest O3 exposure, exercise counteracts the negative effects of air pollution on cardiorespiratory function and lipid accumulation. |
| (Wagner and Clark 2018) | Two 3200 m outdoors running trials: one with low ambient PM2.5 (0.6 to 14.7 μg/m3), and the other during high PM2.5 (19.1 to 42.5 μg/m3). | No differences in the post-exercise forced vital capacity and in the post-exercise forced expiratory volume. |
| (Feng et al. 2019) |
Wistar rats Treadmill training with moderate-intensity intervals in week 1 to 6, 5 days/week. Cycle: Low intensity running (17 m/min, 10 min) interspersed by moderate intensity running (21 m/min, 10 min) for 50 min/day. At the seventh week, the animals received three repeated intranasal instillations of PM2.5 (3 mg/kg) on every other day. |
The exercise training prevented the endothelium-dependent vasorelaxation dysfunction and nitric oxide bioavailability reduction from subsequent PM2.5 instillation. It also improved the HDL function, by increasing its levels, enhancing the cholesterol efflux capacity and reducing the oxidization index. |
| (Kostrycki et al. 2019) |
B6129F2/J male mice Consumption of HFD (58.6%) for 16 weeks. Then, mice were acutely exposed to 50 μg of PM2.5 by intranasal instillation and submitted to moderate (6% of workload) - or high-intensity swimming exercise (8% of workload). |
HFD mice submitted to moderate- or high-intensity exercise and exposed to PM2.5 had a worse swim performance and showed lower levels of eHSP70 and H-index. PM2.5 exposure modified the glycemic response to exercise and modified hematological responses in HFD mice. |
| (Marmett et al. 2018) |
Wistar rats Daily exposure to 50 μg of ROFA. Moderate aerobic training (70%), 5 days/week. Daily supplementation of chromium picolinate (1 mg/kg/day) by oral gavage. All the interventions occurred during 90 days. |
There was no effect of the combination of exercise and pollutant on the oxidative markers in the pulmonary and cardiac tissues. The exercise increased the SOD activity and reduced MDA levels in the gastrocnemius muscle. |
| (Giles et al. 2018) | Males performed 30-min trials of low-intensity or high-intensity cycling (30 and 60% of power at VO2peak). For each subject, each trial was performed once breathing filtered air and once breathing PM2.5 (300μg/m3, six trials in total). |
Throat and chest symptoms were significantly greater immediately following PM2.5 exposure. PM2.5 exposure did not modify any effects of exercise intensity on heart rate variability or norepinephrine. |
| (Mai et al. 2017) |
B6129F2/J male mice Daily exposure to 5 μg of PM2.5 by intranasal instillation. Moderate (4% of workload) - or high-intensity swimming exercise (8% of workload) 5 days/week, along 12 weeks. |
The association of moderate- or high-intensity exercise and PM2.5 exposure increased the heart lipid peroxidation. High-intensity exercise under polluted conditions decreased eHSP70 and H-index compared to sedentary animals. |
| (Matt et al. 2016) | Healthy adults completed four 2-h exposure scenarios that included either rest or intermittent exercise in high- and low-traffic environments. |
An increase in exposure to one unit (1 μg/m3) of PMcoarse was associated with a decrease in forced expiratory volume and forced vital capacity, respectively. On the other hand, for an otherwise equivalent exposure an increase of physical activity by one unit (1% heart rate max) was found to reduce the immediate negative effects of the PM upon peak expiratory flow and the several hours delayed negative effects of PM upon forced vital capacity. |
| (Kubesch et al. 2015) | Healthy participants in four different exposure scenarios: 2 h exposure in high or low-traffic related air pollutant (TRAP) environment, each at rest and combined with intermittent moderate physical activity consisting of 15 min intervals alternating rest and cycling on a stationary bicycle. |
Exposure to high TRAP was associated with higher diastolic blood pressure post-exposure, irrespective of exercise status. Intermittent physical activity was associated with lower systolic blood pressure post-exposure. Physical activity lowered systolic blood pressure more after exposure to low-TRAP site compared with the high-TRAP site. There was an interaction between PA and both PM10 and PMcoarse, increasing systolic blood pressure. |
| (Kargarfard et al. 2015) |
Trained and complete training cessation/detrained athletes (21 to 27 years old) All participants performed a run test on two separate days in either polluted air (37.4 carbon monoxide part per million) or clean air (2.5 carbon monoxide part per million) condition. |
Exercising under polluted conditions increased the mean corpuscular hemoglobin, mean red blood cell volume, white blood cells and platelets in trained and detrained participants. |
| (Heck et al. 2015b) |
Wistar rats Exposure to urban air particles, in chamber, during 60 min 20–60 min of swimming exercise |
The exposure to urban ambient particles during 60 min of swimming exercise increased the lipid peroxidation and reduced the CAT activity in the lungs. |
| (Heck et al. 2014) |
Wistar rats Exposure to ROFA (500 μg/100 μL) before a 20-min session of swimming exercise |
The ROFA exposure increased the lipid peroxidation in the lungs and heart of animals undergoing exercise. The acute exposure to ROFA did not alter the alterations in the hemodynamic variables. |
| (Rundell et al. 2008). | Healthy participants undergoing two 30-min exercise bouts 4–5 days apart, inhaling low (7382 ± 1727 particles/cm3) or high (252290 ± 77529 particles/cm3) PM levels |
Normal resting lung function did not change after low PM exercise. After high PM exercise, forced expiratory volume and forced expiratory flow fell significantly. MDA increased 40% after low PM exercise and increased 208% after high PM exercise. Thus, high PM inhalation during exercise caused a reduced alveolar contribution to nitric oxide. |
| (Ferdinands et al. 2008) |
Adolescents athletes Daily outdoor long-distance running, for 10 days |
There was no acute effect of air pollution exposure during exercise on breath pH. |
| (Bennett et al. 1985) |
Healthy participants Bicycle ergometer |
We found that deposition fraction was unaffected. Exercise increased the mouth deposition, and the central-to-peripheral distribution of deposited aerosol. |
CAT, catalase; eHSP70, extracellular 70-kDa heat shock protein; GSH, reduced glutathione; HFD, high fat diet; iHSP70, intracellular 70-kDa heat shock protein; IL-1β, interleukin-1β; MDA, malondialdehyde; PM2.5, fine particulate matter (0.1 to 2.5 μm); PM10, coarse particulate matter (2.5 to 10 μm); ROFA, residual oil fly ash; TNF-α, tumor necrosis factor; TRAP, traffic-related air pollutant; UFP, ultrafine particulate matter
Despite differences in methodologies, the results of multiple studies have suggested that exercising before an acute or a subchronic exposure to PM2.5 was able to prevent pollutant-induced cardiovascular and metabolic injuries (Feng et al. 2019; Qin et al. 2021; Qin et al. 2020). These studies also suggest that intermittent moderate exercise performed under polluted conditions was also associated with beneficial effects (Kubesch et al. 2015; Matt et al. 2016). Recently, some researchers have concluded that healthy individuals derive more benefits from exercise outdoors, even in contact with air pollution, than remaining sedentary under pollution-free conditions (Marmett et al. 2020). However, whether exercise has the same benefits in individuals with DM who are exposed to air pollution remains unknown. The extra challenge posed by DM requires a better biomarker of exercise intensity and inflammatory status. We propose the use of HSP70 for this purpose.
How much exercise is sufficient, and how can HSP70 help in prescribing exercise intensity?
Regular exercise is known to serve as a prophylaxis or treatment for many chronic diseases (Ashor et al. 2015; Chang et al. 2015; Eijsvogels and Thompson 2015; Pedersen 2006; Pedersen 2017; Pedersen et al. 2003; Rinella 2015; Schmitz et al. 2015; Simon 2015; Wedell-Neergaard et al. 2018). The benefits of exercise for health include improved antioxidant and anti-inflammatory capacities that improve metabolic control (Ropelle et al. 2010). These effects occur at the molecular level, including the modulation of exercise-induced iHSP70 levels (Locke and Noble 2002; Milne et al. 2012; Mulyani et al. 2020; Noble et al. 2008), and are related to both oxidative and inflammatory signaling.
Exercise has been described as a ‘real polypill,’ a ‘drug’ with multiple applications (Fiuza-Luces et al. 2013), that can be compared against pharmacological treatments for many diseases. Similar to other drugs, exercise requires adequate doses, and varying levels of effort may be necessary for each individual. Even short sessions of moderate exercise can lead to transient tissue damage due to increased ROS production during exercise metabolism, which overloads an antioxidant defense system that is sufficient for rest conditions, resulting in oxidative stress. During physical exertion, increased oxygen consumption is directly proportional to ROS production (Banerjee et al. 2003). However, exercise induces a subsequent antioxidant and anti-inflammatory counter-regulatory response that benefits the organism by increasing its baseline capacity to cope with stressful conditions.
These benefits are delayed when untrained individuals are subjected to an acute session of intense or extenuating exercise. Acute exercise in untrained individuals can result in oxidative stress (Antunes-Neto et al. 2006; Liu et al. 2000; Pittaluga et al. 2006), tissue damage (Antunes-Neto et al. 2006), immunity depression (Murase et al. 2016; Nieman et al. 1994), and inflammation (Silveira et al. 2007; van Helvoort et al. 2005), which are all marked by an increase in salivary eHSP70 levels (Murase et al. 2016). Untrained individuals are more susceptible to extenuating exercise-induced oxidative impairments (Fisher-Wellman and Bloomer 2009; Pittaluga et al. 2006), because untrained individuals only have sufficient antioxidant capacity to address rest levels of oxidation whereas trained athletes maintain an oxidative capacity capable of addressing the oxidative load experienced during exercise.
Intense exercise sessions trigger antioxidant and anti-inflammatory counter-regulatory responses (Antunes-Neto et al. 2006; Cuthbert et al. 2019; Peake et al. 2005), resulting in adaptations throughout the course of an ongoing exercise program (training protocol), with positive metabolic, antioxidant (Liu et al. 2000), and anti-inflammatory effects (Antunes-Neto et al. 2006; Hinkley et al. 2017; Pittaluga et al. 2006). Physical effort has been shown to increase iHSP70 levels in leukocytes (Periard et al. 2015), cardiac, skeletal muscle (Milne and Noble 2002), and mesenteric lymphocytes (Heck et al. 2017), and the release of eHSP70 by macrophages (Scholer et al. 2016) appears to occur in an exercise intensity–dependent manner (Heck et al. 2017; Milne and Noble 2002; Periard et al. 2015; Scholer et al. 2016). Consistent with these findings, a pilot study showed that iHSP70 levels in the vastus lateralis were induced by high-intensity training in well-trained rowers but not by endurance training performed at low intensity (Liu et al. 2004).
A previous study evaluated plasma eHSP70 levels in athletes participating in various running protocols, with different durations and similar intensities showed that the marathon (260 ± 39 min) resulted in a 2.5-fold increase in eHSP72 levels compared with the long run (120 min) (Fehrenbach et al. 2005). When duration and intensity varied, a significantly greater increase in eHSP72 was found in athletes who performed exercise at an intensity resulting in 80% maximal oxygen consumption (VO2 max) compared with lower intensities (Fehrenbach et al. 2005). Therefore, different running durations and intensities increased eHSP72 levels to differing degrees in athletes (Fehrenbach et al. 2005).
These studies indicate that a threshold intensity must be achieved to induce iHSP70, with the largest HSP70 induction occurring when subjects exercise near or above their lactate threshold (Liu et al. 2004; Milne and Noble 2002; Pilis et al. 1993). Therefore, HSP70 levels could serve as a useful biomarker of physical exhaustion, which has been reported in a study of untrained individuals (Periard et al. 2012; Periard et al. 2015). For example, in humans, cycling to exhaustion at intensities of both 60% and 75% VO2 max resulted in enhanced monocytic iHSP70 and eHSP70 levels (Periard et al. 2012; Periard et al. 2015).
These findings highlight that HSP70 expression and export are sensitive to fatigue and transmit this signaling to the central nervous system (Dos Santos et al. 2020; Heck et al. 2011). HSP70 is also a potential chaperone whose expression is stimulated by physiological hyperthermia, in addition to mechanical, hormonal, metabolic, oxidative, energetic, and inflammatory stressors, all of which characterize physical effort (Silver et al. 2012). Therefore, the injury and recovery process following the exercise is associated with HSP70 expression and signaling. Recently, a study showed that eHSP70 released during exercise induces delayed-onset muscle soreness by activating the microglial TLR-4/IL-6 and TNF-α pathway in the spinal cord (Dos Santos et al. 2020). Consistent with this finding, the overexpression of iHSP70 improves physical performance, reduces serum lactate and muscle damage, and enhances the superoxide dismutase activity following exhaustive exercise (Liu et al. 2013). Thus, HSP70 serves as a powerful biomarker and an important mediator of physical fatigue and recovery.
However, in animals, surpassing a certain level of physical effort can result in exhaustion that renders them unable to properly develop an HSR, resulting in lower iHSP70 and higher eHSP70 levels (Heck et al. 2017), limiting the modulation of innate monocyte and macrophage functions (Scholer et al. 2016) and culminating in an inflammatory milieu (see Table 4 for review). Whether individual responses to acute intense exercise are similar for chronic diseases, such as obesity and DM2, that are characterized by impaired HSR remains to be carefully assessed. Under conditions of chronic disease and exercise, a threshold may exist beyond which homeostasis can no longer be maintained, characterized by asthma or cardiovascular events (Rundell et al. 2008; Thompson et al. 2007) and mediated by extreme inflammatory and oxidative stimuli. Individual exercise recommendations may be particularly relevant for patients who present with pre-existing pathology or injury conditions (Brody 2012).
Table 4.
Summary of the studies evaluating the impact of different modalities and intensities of exercising in healthy, DM1 or DM2
| Reference | Design | Major outcomes |
|---|---|---|
| Healthy animal models | ||
| (Milne and Noble 2002) | Sprague-Dawley rats remained sedentary or underwent one of seven exercise intensities, for which treadmill running speed (15, 18, 21, 24, 27, 30, and 33 m/min) on a 2% incline during 60 min. |
Cardiac HSP70 was significantly elevated only when animals were exercised at 24 m/min and beyond. Similarly, HSP70 was elevated in vastus red portion at running speeds above 24 m/min but did not increase in vastus white portion until 27 m/min. In contrast, HSP70 content was initially elevated in the soleus but subsequently declined at the highest running speeds. Exercise-induced elevation of HSP70 is intensity dependent. |
| (Antunes-Neto et al. 2006) | Wistar rats remained sedentary or ran in treadmill for 60 min, in a progressive protocol, with velocities varying between 10 and 30 m/min, considered an extenuous exercise. |
There was a correspondence between erythrocyte CAT and GR activities and leukocyte HSP70 levels, principally 3 h after the acute exercise. The HSP70 concentration remained elevated 4 h after exhaustion. The increase in levels of TBARS occurred principally after two hours of exercise. Thus, tissue damage occurred before the expression of any antioxidant system markers. |
| (Melling et al. 2007) | Male Sprague-Dawley rats remained sedentary or aerobically trained (8 wk, low intensity), and were further submitted to an acute exercise (60 min at 30 m/min) |
Trained animals (independent of acute exercise) increased the myocardial HSP70 content and protein kinase A activity, without differences between the exercised groups. However, acute exercise (independent of previous training) increased the HSP70 mRNA and a significant increase in HSF1-HSE DNA binding compared with control animals. Acute-trained animals presented higher ERK1/2 phosphorylation. |
| (Silver et al. 2012) |
HSP70 mRNA was characterized via in situ hybridization experiments analyzing fast-muscle, white vastus: 1, 3, 10, and 24 h after a single bout of intense treadmill running (1 h, 30 m/min, 6% grade), or after a full-body heating for 15 min at 40.0 °C (HS-40 °C) or 42.0°C (HS-42 °C) core body temperature. |
HSP70 mRNA signal was significantly greater 1 h post-exercise and continued to rise until 3 h post-exercise. |
| (Akin et al. 2017) | Female Sprague-Dawley ran 60 min a day at 30 m/min for 4 consecutive days. The exercise was performed at 25 °C (WE group) or 4 °C (CE). | Exercise increased body temperature only in the WE group. Adrenal Hsp72 and Hsp25 levels were significantly higher in the WE group compare to the other groups. |
| (Dos Santos et al. 2020) | Mice ran 40 min to a progressive speed, starting at 5 m/min, 10 m/min, 15 m/min and 17 m/min. | The Hsp70 released during exercise-induced delayed-onset muscle soreness (DOMS) activates the microglial TLR4/IL-6/TNF-α pathway in the spinal cord. |
| Obese and/or diabetic animal models | ||
| (Molanouri Shamsi et al. 2016) |
Streptozotocin-induced type 1 diabetic or healthy rats, trained or sedentary. Trained animals performed 5-week ladder climbing resistance training, with a 48 h-interval between the sessions. |
In diabetic rats, resistance training decreased inflammatory cytokines and HSP70 protein levels in fast skeletal muscle, increased adipose tissue inflammatory cytokines and HSP70, and preserved fast-twitch flexor hallucis longus (FHL) muscle mass. |
| (Tsuzuki et al. 2017) |
Otsuka Long-Evans Tokushima Fatty rats remained sedentary (Sed), trained in a thermal-neutral environment (NTr: 25 °C), or trained in a cold environment (CTr: 4 °C). Training was conducted 5 days/week for 10 weeks. |
Cold environment attenuated the exercise-induced increase of HSP72 levels in the gastrocnemius muscle and liver. It also blunted the improvement in whole-body insulin resistance and lipid metabolism in type 2 diabetic rats induced by physical training. |
| Clinical trial | ||
| (Shastry et al. 2002) | Trained (TR) and untrained (UT) subjects, ran on a treadmill for 1 h at 70% VO2. | Baseline HSP70 levels in TR subjects were lower than in UT subjects, while HSP90 levels were similar in TR and UT subjects. Exercise at an intensity that is within normal limits for a moderately trained individual did not induce HSP70 production in leucocytes 15 and 24 h after the session. |
| (Liu et al. 2004) | Well-trained rowers strength underwent a training program consisted of 3 weeks high-intensity training (HIT) and 3 weeks low intensity endurance training (ET), followed by 1 week of recovery each (R1 and R2, respectively). | HSP70 increased significantly at the end of HIT, decreased at the end of R1, and remained unchanged throughout ET and R2. HSP70 mRNA increased significantly after HIT and decreased gradually afterwards. |
| (Fehrenbach et al. 2005) | Athletes performed a continuous treadmill run of 60 min (CR) with an intensity of 75% VO2 max, a long treadmill run of 120 min (LR) with an intensity of 60% VO2 max, an extensive interval training (IT) program of 35 min, with 88% VO2 max), or a competitive marathon run (MA) within 260 ± 39 min, with an intensity of 65% VO2. |
The concentration of sHSP72 in the athletes’ plasma was increased immediately (0 h) after exercise. The highest levels of sHSP72 were detected directly after the MA. If both runs were performed with similar intensity, the running time as twice as long in MA resulted in a more than 2.5-fold increase in sHSP72 levels compared with LR. Further, the exercise duration was kept constant while the exercise intensity was varied from 80% to 60% VO2 max. The higher intensity resulted in a significantly higher post-exercise leukocytosis. Likewise, a significantly greater increase of sHSP72 was found in the serum of the athletes who performed the exercise at an intensity of 80% VO2 max. Running exercise of different duration and intensity significantly increased the level of soluble HSP72 (sHSP72) in the plasma/serum of the athletes. |
| (Peake et al. 2005) |
Subjects submitted to (1) level treadmill running at 60% VO2 max (moderate-intensity trial) for 60 min; (2) level treadmill running at 85% VO2 max (high-intensity trial) for 60 min; (3) downhill treadmill running (10% gradient) at 60% VO2 max (downhill running trial) for 45 min. |
The plasma concentrations of IL-1ra, IL-12p40, MCP-1 and HSP70 increased significantly after all three trials. |
| (Pittaluga et al. 2006) |
Young subjects with different training levels (professional athletes and non-agonists (NA)). Subjects were submitted to cycle ergometer exhaustive test, with different intensities. |
Agonistic training led to an oxidative insult (high baseline values of oxidized glutathione (GSSG), micronuclei and hemolysis). On the contrary, NA with the lowest level of training frequency showed a well-balanced profile at rest, but they were more susceptible to exercise-induced variations (GSSG/GSH and diene increased values). In vitro heat shock of lymphocytes showed increased HSP70 baseline expression, especially in NA-Int (indicating ability to counteract stress). |
| (Hom et al. 2012) |
Healthy males underwent 11 days of heat acclimation. Subjects walked for 90 min (50 ± 8% VO2max) on a treadmill (3.5 mph, 5% grade), in an environmental chamber (33 °C, 30–50% relative humidity). On days 1, 4, 7, 10, and 11, peripheral blood mononuclear cells were isolated from pre and post-exercise blood samples. |
Heat acclimation was achieved in the absence of significant changes in intracellular HSP70 and percent of lymphocytes during acclimation. Furthermore, there was no increased cellular heat tolerance during secondary ex vivo heat shock of the lymphocytes acquired from subjects during acclimation. |
| (Periard et al. 2012) | Male subjects cycled to exhaustion at 60 and 75 % VO2 in hot conditions (40 °C, 50 % RH). |
A significant increase and correlation was observed between eHSP72 and eHSP27 concentrations at exhaustion. Rectal temperature and VO2 max were significant predictor variables of eHSP72 expression. The exercise-induced eHSP72 release may be duration, intensity and exhaustion dependent. |
| (Periard et al. 2015) | Male subjects cycled to exhaustion at 60 and 75 % VO2 in hot conditions (40 °C, 50 % RH). | iHSP72 concentration increased similarly at exhaustion relative to pre-exercise and then increased further at 24 h. Exercise to exhaustion at high (75 %) and moderate (60 % of VO2 max) intensities in the heat induces a similar increase in iHSP72 concentration in monocytes. |
| (Tuttle et al. 2015) | Physically active but not heat-acclimated participants exercised at lactate threshold in either temperate (20 °C, 50% relative humidity; RH) or hot (30 °C, 50% RH) environmental conditions. Within each condition, participants completed a flat running (temperate flat or hot flat) and a downhill running (temperate downhill or hot downhill), separated by at least 7 days. |
Downhill running and exercise in hot conditions induced the largest stimuli for leukocyte Hsp72 and Hsp90 mRNA, which increased immediately after exercise. Rectal temperature increased significantly during downhill running compared with flat running. Significant correlations were observed between Hsp72 mRNA and peak exercising rectal temperature. Exercising rectal temperature appeared to be the major physiological stimuli with increased metabolic strain during hot conditions and activation of the innate immune response potentially also contributing. |
| (Murase et al. 2016) | Healthy sedentary young males performed 59 min of cycling exercise at 75 % VO2 max. Samples were collected before (Pre), immediately after (Post), and at 1, 2, 3, and 4 h after completion of the exercise. | Exercise stress elevated salivary eHSP70 levels, with a peak in its secretion 4 h post-exercise. However, it partially downregulated oral immune function. |
| (Cuthbert et al. 2019) | Recreationally trained males cycled for 1 hour at 60% VO2 peak in 7 °C, 20 °C, and 33 °C with biopsies taken pre and 3 hours post-exercise. | HSP70 mRNA was higher 3 h post-exercise when compared to pre-exercise but was not significantly different between temperatures. Skeletal muscle mRNA of cold shock proteins decrease, while HSP70 mRNA increases in response to a low to moderate intensity aerobic exercise bout. |
| Clinical trials with obese and/or diabetic humans | ||
| (Tiss et al. 2014) |
Lean and obese humans, both non-diabetic, undergoing 3-month moderate physical exercise. The exercise regimen involved a combination of moderate-intensity aerobic exercise and resistance training using a treadmill or stationary bicycle. Each exercise session included 10-min warm-up and cooldown steps at 50–60% max HR and 40 min of the prescribed exercise program at 65–80% max HR. |
Obese subjects had increased expression of HSP60, HSC70, HSP72, HSP90 and GRP94 and lower expression of DNAJB3/HSP40 in the SAT and PBMC. Higher levels of HSP72 and GRP94 proteins correlated positively with the indices of obesity (body mass index and percent body fat). This expression pattern was concomitant with increased inflammatory response in the adipose tissue as monitored by increased levels of IL-6, TNF-α and RANTES. Physical exercise reduced the expression of various HSPs in obese to normal levels observed in lean subjects with a parallel decrease in the endogenous levels of IL-6, TNF-α, and RANTES. |
| (Khadir et al. 2018) |
Obese human with and without diabetes undergoing 3-month, 3x/week, physical exercise or remaining sedentary. The exercise protocol involved a combination of moderate-intensity aerobic exercise and resistance training using a treadmill or stationary bicycle. Each exercise session included 10-min warm-up and cooldown steps at 50–60% max HR and 40 min of the prescribed exercise program at 65–80% max HR. |
Compared with obese adults without diabetes, HSP60 mRNA and protein levels were decreased in subcutaneous adipose tissue (SAT) in diabetic obese together with increased inflammatory marker expression and glycemic levels but lower VO2 Max. More interestingly, a 3-month physical exercise decreased endogenous levels of IL-6 and TNF-α. It also reduced the expression of HSP60 in SAT along with decreased HSP72 expression in the diabetes group. Interestingly, the downregulation of HSP60 in adults with diabetes was concomitant with the increased expression of the tissue inflammatory cytokines produced by macrophage upon TLR or Th1 activation, IL-6 and TNF-a. HSP60 levels in blood serum were lower in the diabetes groups. Indeed, HSP60 levels were increased by exercise in the diabetes group together with an increase in HSP72 levels, whereas clear decreases in IL-6 and TNF-a levels were noted in this group. However, an opposite pattern was observed in the non-diabetes group for HSP60 and HSP72, in addition to a decrease in inflammatory marker levels. |
| (Brinkmann et al. 2019) | Overweight/ obese men with T2DM performed endurance training (3 times per week for 3 months, moderate intensity). The analysis were done at T1 (6 weeks pre-training), T2 (1 week pre-training) and T3 (3 to 4 days post-training). | HSP70 was upregulated in the muscle vastus lateralis following endurance training. There was a fiber type-specific distribution of HSP70 with increased protein contents in type I fibers. A significant change in the fiber type distribution with an increase in type I fibers and a decrease in type II fibers was observed post-training. |
How does HSP70 reflect the complexity of exercise performed under polluted conditions in people with obesity or diabetes?
HSP70 could potentially contribute to identifying the thin threshold between the beneficial and damaging effects of exercise performed under polluted conditions in individuals with obesity or DM. We propose that the evidence regarding HSP70 expression under these circumstances should be interpreted through the lens of the hormesis theory. According to the hormesis theory, before becoming deleterious, a stimulus can lead to a preconditioning effect, mediated by physiological adaptations that strengthen an organism’s ability to address future harmful stress (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013; Dattilo et al. 2015). A low-level stimulus can increase individual performance within a limited zone (Calabrese et al. 2015), whereas a high-level stimulus can lead to the inhibition and reduction of individual performance (Calabrese et al. 2015). Thus, each condition (pollution, DM2, and exercise) exerts different metabolic, oxidative, and inflammatory challenges that can induce HSR until a specific threshold is met, at which point the effects become deleterious (Figure 1B).
DM and PM2.5 impair survival by decreasing the ability to exert a proper HSR under conditions of continued exposure. Obesity and DM2 affect an organism through similar mechanisms, starting with an increase in HSP70 levels (Miragem and Homem de Bittencourt Jr 2017) to prevent the propagation of low-grade inflammation induced by metabolic disruption (Newsholme and de Bittencourt Jr 2014). Enhanced HSP70 levels indicate the stimulation phase of the hormesis curve. Otherwise, as chronic low-grade inflammation continues, HSP70 expression decreases and becomes inhibited (Bittencourt et al. 2020; Miragem and Homem de Bittencourt Jr 2017; Newsholme and de Bittencourt Jr 2014). The lack of a proper HSR induces SASP and the propagation of the inflammatory milieu, which links obesity and insulin resistance (Bittencourt et al. 2020; Miragem and Homem de Bittencourt Jr 2017; Newsholme and de Bittencourt Jr 2014). Thus, reduced HSP70 levels represent the inhibition phase of the hormesis curve.
Similarly, a large set of environmental pollutants were reviewed for their ability to induce a hormetic response, although the effects of PM2.5 in the hormetic curve were not elucidated yet (Iavicoli et al. 2018). However, the studies reviewed here suggest that the intensity of PM2.5 exposure and its association with other risk factors might reduce HSP70 levels (Costa-Beber et al. 2021a; Costa-Beber et al. 2021b; Costa Beber et al. 2020). HSP70 transcription is regulated by HSF-1, which can be modulated by sirtuin 1 (SIRT-1) and is closely related to cell survival and longevity (Calabrese et al. 2010; Cornelius et al. 2013; Dattilo et al. 2015). Therefore, the decrease in SIRT-1 expression reported during PM2.5 exposure (Jin et al. 2016; Ribeiro Junior et al. 2019; Tanwar et al. 2017), combined with the observed reduction in the HSR in obesity, DM2 (Bittencourt et al. 2020; de Lemos Muller et al. 2018; Krause et al. 2015a; Krause et al. 2014a), and acute respiratory distress syndrome (Durand et al. 2000) might result in worse prognosis. Evidence from our lab also demonstrated that these outcomes vary in tissue- and pollutant-specific manners (Baldissera et al. 2018; Costa-Beber et al. 2021a; Costa-Beber et al. 2021b; Costa Beber et al. 2020; Goettems-Fiorin et al. 2019; Goettems-Fiorin et al. 2016; Kostrycki et al. 2019; Mai et al. 2017), and recent studies have demonstrated the normalization of these inflammatory conditions mediated by exercise (Khadir et al. 2018; Tiss et al. 2014; Tsuzuki et al. 2017). Accordingly, a recent study supported our hypothesis, showing that aerobic exercise prevented PM2.5-induced lung injury in aging rats by enhancing pulmonary iHSP70 expression and reducing circulating eHSP70 levels (Qin et al. 2021).
When performing moderate-intensity physical training, which has been suggested to be the safest intensity performed under polluted conditions (Mai et al. 2017; Qin et al. 2021; Qin et al. 2020), HSP70 levels can serve to verify that a safe intensity is being practiced (Qin et al. 2021). Although HSP70 serves as a fatigue signal (Heck et al. 2011; Liu et al. 2013), it also correlates with exercise intensity (Milne and Noble 2002) and is necessary for the induction of exercise-related benefits (Heck et al. 2017; Tsuzuki et al. 2017) (see item 6 for review). The dual role of HSP70 in the intracellular and extracellular processes indicates that monitoring both may be valuable for determining the specific threshold between benefits and damage induced by exercise. We suggest that HSP70 levels can be used to monitor the proper intensity of exercise appropriate for individual requirements to ensure that exercise is being performed at an intensity that does not further exacerbate the effects of pollution by inducing an appropriate HSR to address stressful conditions (Qin et al. 2021). Thus, monitoring HSP70 can help professionals manipulate exercise intensity and maintain an individual within the first phase of hormesis.
Recently, our lab and coworkers proposed the Heck Index (H-Index) as a potential biomarker for the immune–inflammatory status in exercise (Heck et al. 2017). The H-Index represents the eHSP70 to HSP70 ratio, which is independent of the method used for measurement (for details, see Heck et al. (2017) and should present a baseline value near 1 (1:1 eHSP70/HSP70 ratio) when measured in plasma or any tissue (Costa-Beber et al. 2020). Independently, metabolic diseases, exposure to high levels of pollution, and cardiovascular conditions are sufficient factors to increase the H-Index to values considered risky (Bruxel et al. 2019; Costa-Beber et al. 2020; Goettems-Fiorin et al. 2016; Heck et al. 2017; Qin et al. 2021). Moreover, the H-Index can be exacerbated if exercise intensity exceeds an individual’s capacity to cope with the physical effort (Heck et al. 2017), and the H-Index can be reduced to basal levels when an appropriate exercise intensity reduces inflammation triggered by pollution (Qin et al. 2021). However, a decrease in the H-Index below basal levels can serve as an indicator of a repressed HSR, as observed in animals exposed to PM2.5 while undergoing intense physical training (Mai et al. 2017).
Therefore, we suggest that monitoring the HSR via HSP70 levels may be valuable when determining an appropriate level of exercise (Heck et al. 2017; Kruger et al. 2019; Scholer et al. 2016) and determining the transition point between various phases (induction and inhibition) of hormesis (Calabrese et al. 2015; Calabrese et al. 2010; Cornelius et al. 2013). We propose that HSP70 can serve as a biomarker for establishing the fine threshold between anti-inflammatory and pro-inflammatory exercise levels.
Is it possible to measure the HSP70, H-Index, and heat shock response in such conditions? How to evolve to a unique biomarker of these conditions?
The circadian cycle affects HSP70 levels, which can be influenced by diurnal variations in adrenocorticotropic hormone (ACTH) levels, which are known to mediate the induction of adrenal HSP72 (Blake et al. 1991). A previous study demonstrated that basal levels of iHSP70 vary over a 24-h period in monocytes isolated from healthy male subjects (Sandstrom et al. 2009). Disruptions in the circadian cycle (e.g., light interference) serve as a powerful stressor that alters HSP70 mRNA expression and protein levels in the brain and hepatic tissues (Ashkenazi and Haim 2012; Moore et al. 2021). Therefore, the circadian rhythm of each species must be acknowledged and respected when performing experiments (nocturnal or daytime habit).
The adrenal gland plays a vital role in mediating the fight-or-flight response by releasing catecholamines and corticosteroids, and the hypothalamus–pituitary–adrenocortical axis is involved in the physiological effects of physical exercise (Tahara et al. 2017). Based on the levels of HSP70 and IL-6, combined with leukocyte parameters, training during the mornings has been recommended to avoid unnecessary homeostatic perturbations (Boukelia et al. 2018). It was already demonstrated that exercise induces decreased HSP70 levels and leukocytosis at 1800 h (Boukelia et al. 2018).Therefore, daily fluctuations in ACTH and cortisol levels may not necessarily be relevant confounding factors in the exercise-induced increase in HSP70 levels, as long as the nature of each species is appropriately considered (nocturnal or daytime habit). For our proposed use of HSP70 as a biomarker, exercising and sample collection should preferentially occur during the morning or prior to 1800 h to avoid biases due to diurnal variations.
After an exercise stimulus, the time courses of HSP70 mRNA and protein expression differ (Khassaf et al. 2001; Liu et al. 2004). The HSP70 mRNA signal increases significantly immediately after (Tuttle et al. 2015) and 1- to 3-h post-exercise (Cuthbert et al. 2019; Silver et al. 2012). An acute session of exercise increases HSP70 mRNA levels in both trained and sedentary animals, which can be verified 30 min after the physical challenge (Melling et al. 2007). By contrast, an increase in myocardial iHSP70 contents can be observed after this time only in trained animals (Melling et al. 2007).
Although HSP70 mRNA and protein expression occur on different time scales, HSP70 release has been consistently reported at the same time points. Increased circulating eHSP70 circulating has been reported 4-h post-exercise (Murase et al. 2016), immediately (0 h) after exercise (Fehrenbach et al. 2005), and at the point of exhaustion (i e., during exercise) (Periard et al. 2012). In peripheral blood mononuclear cells (PBMCs), following a stimulus, eHSP70 release peaks after 2 h of culture but continues to increase at a reduced rate for up to 24 h (Hunter-Lavin et al. 2004). Therefore, the patterns of mRNA and protein levels must be established individually because they do not necessarily change in tandem.
Both iHSP70 and eHSP770 can be assessed from the same total blood sample. PBMCs can be separated from plasma using the Histopaque technique, allowing for the separate measurement of iHSP70 and eHSP70 contents (de Lemos Muller et al. 2018; Madden et al. 2010). With the appropriate equipment, muscle micro-biopsies can also be obtained to measure iHSP70 levels (Brinkmann et al. 2019; Cuthbert et al. 2019; Hinkley et al. 2017; Khadir et al. 2018; Krause et al. 2014b; Liu et al. 2004; Martin-Ventura et al. 2007; Rodrigues-Krause et al. 2012b); however, this is an invasive technique and should not be considered part of routine analysis, whereas blood collection is minimally invasive and often part of routine clinical assessments. HSP70 measurements could be analyzed separately or used to calculate the H-Index.
Total blood samples (or previously isolated PBMCs) can be used to measure HSR. Two samples from the same patient are collected, and one is submitted to a thermal challenge at 42 °C (HSR sample) whereas the other is retained in a thermoneutral zone at 36 °C (control sample) for 2 h, followed by a rest and response period, during which both samples are maintained at 36 °C for 6 h (de Lemos Muller et al. 2018; Heck et al. 2017; Krause et al. 2020) (see Figure 2). Cells from a healthy patient should be capable of HSP70 expression, developing a proper HSR following exposure to thermal stress. We recommend challenging donors’ samples using thermal stress to properly evaluate the HSR, rather than evaluating the HSR in response to exercise, as moderate-intensity exercise was recommended as the optimal intensity for the prevention of pollution-related effects (Mai et al. 2017; Qin et al. 2021; Qin et al. 2020), but HSP70 is only released by lymphocytes during high-intensity exercise (Heck et al. 2017). HSP70 expression can be verified using highly sensitive enzyme-linked immunosorbent assays or western blotting. The choice of the method depends on the expertise and reagent availability; the measurement method is not a limiting factor because the H-Index requires normalization by a control (as previously described) and does not depend on the measurement method.
Fig. 2.
Schematic representation of intracellular and extracellular HSP70 measurement in human blood. Peripheral blood mononuclear cells (PBMCs) can be separated from plasma using the Histopaque technique to measure intracellular (iHSP70) and extracellular (eHSP70) contents, respectively (First approach). PBMCs can also be used to assess the heat shock response (HSR) by submitting two samples from the same donor to either a thermal challenge at 42 °C (HSR sample) or a thermoneutral zone at 36 °C (control sample) for 2 h, followed by a ‘rest and response period’ at 36 °C for 6 h (second approach). Following centrifugation, the supernatant can be used to assess eHSP70 levels, whereas the PBMC pellet can be used to assess iHSP70 levels. These measurements can be analyzed separately or used to calculate the H-Index. Cells from a healthy patient should be able to express HSP70 and, therefore, mount a proper HSR following thermal stress. Created with BioRender.com
To date, no cutoff values for iHSP70 or eHSP70 have been established to guide the interpretation of results. Until such cutoffs are established, we suggest the collection of blood samples from each subject before and after exercise. As described in Section 6, intense exercise may increase iHSP70 levels, whereas less strenuous exercise may not (Heck et al. 2017). In addition, the H-Index can be used, as previous studies from our research lab indicated that low-level physical training is typically associated with an H-Index value < 1.00, an acute moderate-intensity session presents an H-Index value of 1.00–2.00, and an acute high-intensity to strenuous exercise results in an H-Index value of > 5.00 (Heck et al. 2017). In addition, the ability of the donor’s PBMCs to mount a proper HSR, indicated by an H-Index > 2.00, should be evaluated by using the thermal stress assay, whereas the presence of chronic inflammatory disease is typically associated with an H-Index value of > 5.00 at the baseline (thermoneutral zone) (Heck et al. 2017).
Our proposition has some limitations concerning to the requirement of an equipped lab, which could be overcome by partnerships. As far as the studies advance, we look forward developing the possibility of technologically evolving towards an accurate and rapid assessment of HSP levels, as if using a glucometer, for example. Until then, more studies are necessary to properly establish the reference values of HSP70 for each condition.
Conclusion
Obesity and DM are conditions that should be addressed with a physical exercise regimen. The biometric and metabolic benefits associated with physical exercise are mediated by iHSP70 and eHSP70 signaling. However, eHSP70 is also a marker of physical fatigue and a mediator of chronic inflammation. Thus, the balance between iHSP70 and eHSP7 should be maintained and can be used to determine the appropriate exercise intensity to achieve maximal potential benefits. We reviewed the available evidence to support our hypothesis that HSP70 can be used as a biomarker for determining optimal exercise intensity among individuals with obesity or DM when exposed to air pollution, which could contribute to establishing the fine threshold between the anti- and pro-inflammatory effects mediated by exercise.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AP-1
activator protein 1
- COX-2
cyclooxygenase 2
- DM
diabetes mellitus
- DM1
type I diabetes mellitus
- DM2
type II diabetes mellitus
- eHSP70
extracellular 70-kDa heat shock proteins
- ER
endoplasmic reticulum
- GLUT-4
glucose transporter 4
- HFD
high-fat diet
- H-Index
Heck Index
- HSE
heat shock element
- HSF-1
heat shock factor 1
- HSP70
70-kDa heat shock proteins
- HSR
heat shock response
- iHSP70
intracellular 70-kDa heat shock proteins
- IKK
IκB kinase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- iNOS
inducible oxide nitric synthase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- NFκB
nuclear factor kappa beta
- NO
nitric oxide
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- PBMC
peripheral blood mononuclear cells
- PGE2
prostaglandin E2
- Pi3k
phosphoinositide 3-kinases-protein kinase B
- PM1
ultrafine particulate matter (≤ 1 μm)
- PM2.5
fine particulate matter (0.1–2.5 μm)
- PM10
gross particulate matter (2.5–10 μm)
- ROFA
residual oil fly ash
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SIRT-1
sirtuin 1
- TLR-2
Toll-like receptor 2
- TLR-4
Toll-like receptor 4
- TNF-α
tumor necrosis factor-α
- UPR
unfolded protein response
- WHO
World Health Organization
Author contribution
TGH elaborated the first version of the review, and LCCB extended the literature search, redefined the main questions to be addressed, and elaborated the last version of the manuscript. PBGF and MSL helped in the discussion and approved the final version. Finally, TGH and LCCB had the same contribution to the manuscript and share first authorship.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adachi M, Liu Y, Fujii K, Calderwood SK, Nakai A, Imai K, Shinomura Y. Oxidative stress impairs the heat stress response and delays unfolded protein recovery. PLoS One. 2009;4:e7719. doi: 10.1371/journal.pone.0007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin S, Naito H, Ogura Y, Ichinoseki-Sekine N, Kurosaka M, Kakigi R, Demirel HA. Short-term treadmill exercise in a cold environment does not induce adrenal Hsp72 and Hsp25 expression. J Physiol Sci. 2017;67:407–413. doi: 10.1007/s12576-016-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, et al. Longitudinal associations between ambient air pollution with insulin sensitivity, beta-cell function, and adiposity in Los Angeles Latino Children. Diabetes. 2017;66:1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi H, et al. Association of extracellular heat shock protein 70 and insulin resistance in type 2 diabetes; independent of obesity and C-reactive protein. Cell Stress Chaperones. 2019;24:69–75. doi: 10.1007/s12192-018-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Neto JM, Toyama MH, Carneiro EM, Boschero AC, Pereira-da-Silva L, Macedo DV (2006) Circulating leukocyte heat shock protein 70 (HSP70) and oxidative stress markers in rats after a bout of exhaustive exercise. Stress 9:107-115 doi:10.1080/10253890600772211 [DOI] [PubMed]
- Ao L, Zou N, Cleveland JC, Jr., Fullerton DA, Meng X (2009) Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. Am J Physiol Heart Circ Physiol 297:H21-28 [DOI] [PMC free article] [PubMed]
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Ashkenazi L, Haim A. Light interference as a possible stressor altering HSP70 and its gene expression levels in brain and hepatic tissues of golden spiny mice. J Exp Biol. 2012;215:4034–4040. doi: 10.1242/jeb.073429. [DOI] [PubMed] [Google Scholar]
- Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015;45:279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- Baldissera FG, et al. Subacute exposure to residual oil fly ash (ROFA) increases eHSP70 content and extracellular-to-intracellular HSP70 ratio: a relation with oxidative stress markers. Cell Stress Chaperones. 2018;23:1185–1192. doi: 10.1007/s12192-018-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/A:1026032404105. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Messina MS, Smaldone GC. Effect of exercise on deposition and subsequent retention of inhaled particles. J Appl Physiol (Bethesda, Md : 1985) 1985;59:1046–1054. doi: 10.1152/jappl.1985.59.4.1046. [DOI] [PubMed] [Google Scholar]
- Bittencourt A, Schroeder HT, Porto RR, de Lemos Muller CH, Krause M, Homem de Bittencourt PI., Jr Heat shock response to exercise in pancreatic islets of obese mice. Biochimie. 2020;168:28–40. doi: 10.1016/j.biochi.2019.10.015. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Udelsman R, Feulner GJ, Norton DD, Holbrook NJ. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci U S A. 1991;88:9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukelia B, Gomes EC, Florida-James GD. Diurnal variation in physiological and immune responses to endurance sport in highly trained runners in a hot and humid environment. Oxidative Med Cell Longev. 2018;2018:3402143. doi: 10.1155/2018/3402143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann C, Kuckertz A, Schiffer T, Bloch W, Predel HG, Brixius K. Endurance training alters YKL40, PERM1, and HSP70 skeletal muscle protein contents in men with type 2 diabetes mellitus. Endocr Res. 2019;44:1–8. doi: 10.1080/07435800.2018.1474920. [DOI] [PubMed] [Google Scholar]
- Brocchieri L, Conway de Macario E, Macario AJ. hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LT. Effective therapeutic exercise prescription: the right exercise at the right dose. J Hand Ther. 2012;25:220–231. doi: 10.1016/j.jht.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Brook RD, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Bruxel MA, et al. Chronic whole-body heat treatment relieves atherosclerotic lesions, cardiovascular and metabolic abnormalities, and enhances survival time restoring the anti-inflammatory and anti-senescent heat shock response in mice. Biochimie. 2019;156:33–46. doi: 10.1016/j.biochi.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Burkart V, et al. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;275:19521–19528. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Dhawan G, Kapoor R, Iavicoli I, Calabrese V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16:693–707. doi: 10.1007/s10522-015-9601-0. [DOI] [PubMed] [Google Scholar]
- Chang YK, Chu CH, Wang CC, Wang YC, Song TF, Tsai CL, Etnier JL (2015) Dose-response relation between exercise duration and cognition. Med Sci Sports Exerc 47:159-165 doi:10.1249/MSS.0000000000000383 [DOI] [PubMed]
- Chen HW, Kuo HT, Wang SJ, Lu TS, Yang RC. In vivo heat shock protein assembles with septic liver NF-kappaB/I-kappaB complex regulating NF-kappaB activity. Shock. 2005;24:232–238. doi: 10.1097/01.shk.0000174020.87439.f2. [DOI] [PubMed] [Google Scholar]
- Chung J, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a "chi". Immunity & ageing : I & A. 2013;10:15. doi: 10.1186/1742-4933-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Beber LC, et al. The association of subchronic exposure to low concentration of PM2.5 and high-fat diet potentiates glucose intolerance development, by impairing adipose tissue antioxidant defense and eHSP72 levels. Environ Sci Pollut Res Int. 2020;27:32006–32016. doi: 10.1007/s11356-020-09581-8. [DOI] [PubMed] [Google Scholar]
- Costa-Beber LC, Hirsch GE, Heck TG, Ludwig MS (2020) Chaperone duality: the role of extracellular and intracellular HSP70 as a biomarker of endothelial dysfunction in the development of atherosclerosis. Arch Physiol Biochem1-8 doi:10.1080/13813455.2020.1745850 [DOI] [PubMed]
- Costa-Beber LC, et al. Ovariectomy enhances female rats' susceptibility to metabolic, oxidative, and heat shock response effects induced by a high-fat diet and fine particulate matter. Exp Gerontol. 2021;145:111215. doi: 10.1016/j.exger.2020.111215. [DOI] [PubMed] [Google Scholar]
- Costa-Beber LC, Goettems-Fiorin PB, Dos Santos JB, Friske PT, Heck TG, Hirsch GE, Ludwig MS (2021b) Ovariectomy reduces the cardiac cytoprotection in rats exposed to particulate air pollutant. Environ Sci Pollut Res Int. 10.1007/s11356-021-12350-w [DOI] [PubMed]
- Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10:1564. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert RL, Shute RJ, Slivka DR. Skeletal muscle cold shock and heat shock protein mRNA response to aerobic exercise in different environmental temperatures. Temperature. 2019;6:77–84. doi: 10.1080/23328940.2018.1555414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdorster G, Utell MJ, Frampton MW. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- Dattilo S, et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immunity & ageing : I & A. 2015;12:20. doi: 10.1186/s12979-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos Muller CH, et al. Heat-induced extracellular HSP72 release is blunted in elderly diabetic people compared with healthy middle-aged and older adults, but it is partially restored by resistance training. Exp Gerontol. 2018;111:180–187. doi: 10.1016/j.exger.2018.07.014. [DOI] [PubMed] [Google Scholar]
- De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated Ferruccio Ritossa Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos RS, Veras FP, Ferreira DW, Sant'Anna MB, Lollo PCB, Cunha TM, Galdino G. Involvement of the Hsp70/TLR4/IL-6 and TNF-alpha pathways in delayed-onset muscle soreness. J Neurochem. 2020;155:29–44. doi: 10.1111/jnc.15006. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand P, Bachelet M, Brunet F, Richard MJ, Dhainaut JF, Dall'Ava J, Polla BS. Inducibility of the 70 kD heat shock protein in peripheral blood monocytes is decreased in human acute respiratory distress syndrome and recovers over time. Am J Respir Crit Care Med. 2000;161:286–292. doi: 10.1164/ajrccm.161.1.9812150. [DOI] [PubMed] [Google Scholar]
- Eijsvogels TM, Thompson PD. Exercise is medicine: at any dose? JAMA. 2015;314:1915–1916. doi: 10.1001/jama.2015.10858. [DOI] [PubMed] [Google Scholar]
- Eik WF, Marcon SS, Krupek T, Previdelli IT, Pereira OC, Silva MA, Bazotte RB. Blood levels of pro-inflammatory and anti-inflammatory cytokines during an oral glucose tolerance test in patients with symptoms suggesting reactive hypoglycemia. Br J Med Biolog Res = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2016;49:e5195. doi: 10.1590/1414-431X20165195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–163. doi: 10.1194/jlr.M030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, et al. In-field and in-vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: ultrastructural damage, oxidative stress and HSP70 induction. PLoS One. 2018;13:e0195717. doi: 10.1371/journal.pone.0195717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina F, Sancini G, Battaglia C, Tinaglia V, Mantecca P, Camatini M, Palestini P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS One. 2013;8:e56636. doi: 10.1371/journal.pone.0056636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina F, Sancini G, Longhin E, Mantecca P, Camatini M, Palestini P. Milan PM1 induces adverse effects on mice lungs and cardiovascular system. Biomed Res Int. 2013;2013:583513. doi: 10.1155/2013/583513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- Feng B, et al. Exercise training prevented endothelium dysfunction from particulate matter instillation in Wistar rats. Sci Total Environ. 2019;694:133674. doi: 10.1016/j.scitotenv.2019.133674. [DOI] [PubMed] [Google Scholar]
- Ferdinands JM, Crawford CA, Greenwald R, Van Sickle D, Hunter E, Teague WG. Breath acidification in adolescent runners exposed to atmospheric pollution: a prospective, repeated measures observational study. Environ Health. 2008;7:10. doi: 10.1186/1476-069X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- Furuyama A, Kanno S, Kobayashi T, Hirano S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch Toxicol. 2009;83:429–437. doi: 10.1007/s00204-008-0371-1. [DOI] [PubMed] [Google Scholar]
- Gasparotto J, et al. Coal and tire burning mixtures containing ultrafine and nanoparticulate materials induce oxidative stress and inflammatory activation in macrophages. Sci Total Environ. 2013;463-464:743–753. doi: 10.1016/j.scitotenv.2013.06.086. [DOI] [PubMed] [Google Scholar]
- Gasparotto J, et al. Obese rats are more vulnerable to inflammation, genotoxicity and oxidative stress induced by coal dust inhalation than non-obese rats. Ecotoxicol Environ Saf. 2018;165:44–51. doi: 10.1016/j.ecoenv.2018.08.097. [DOI] [PubMed] [Google Scholar]
- Ghayour-Mobarhan M, Lamb DJ, Tavallaie S, Ferns GAA. Relationship between plasma cholesterol, von Willebrand factor concentrations, extent of atherosclerosis and antibody titres to heat shock proteins-60, -65 and -70 in cholesterol-fed rabbits. Int J Exp Pathol. 2007;88:249–255. doi: 10.1111/j.1365-2613.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Giles LV, Carlsten C, Koehle MS. The pulmonary and autonomic effects of high-intensity and low-intensity exercise in diesel exhaust. Environ Health. 2018;17:87. doi: 10.1186/s12940-018-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettems-Fiorin PB, et al. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem. 2016;72:643–656. doi: 10.1007/s13105-016-0503-7. [DOI] [PubMed] [Google Scholar]
- Goettems-Fiorin PB et al (2019) Ovariectomy predisposes female rats to fine particulate matter exposure's effects by altering metabolic, oxidative, pro-inflammatory, and heat-shock protein levels. Environ Sci Pollut Res Int. 10.1007/s11356-019-05383-9 [DOI] [PubMed]
- Gonzalez-Ramos M, et al. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-beta1 up-regulation. Int J Biochem Cell Biol. 2013;45:232–242. doi: 10.1016/j.biocel.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Haberzettl P, McCracken JP, Bhatnagar A, Conklin DJ. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol. 2016;310:H1423–H1438. doi: 10.1152/ajpheart.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P, O'Toole TE, Bhatnagar A, Conklin DJ. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect. 2016;124:1830–1839. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L, Martinus RD. Hyperglycaemia and oxidative stress upregulate HSP60 & HSP70 expression in HeLa cells. SpringerPlus. 2013;2:431. doi: 10.1186/2193-1801-2-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck TG, Schöler CM, de Bittencourt PIH. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29(3):215–226. doi: 10.1002/cbf.1739. [DOI] [PubMed] [Google Scholar]
- Heck GH, Nunes RB, Petry MR, Maslinkiewicz A, Saldiva PHN, Dal Lago P, Rhoden CR. Residual Oil Fly Ash (ROFA) inhalation promotes lung and heart oxidative stress without hemodynamic effects inexercising rats. Journal of Exercise Physiology (online) 2014;17:78–89. [Google Scholar]
- Heck TG, Nunes RB, Petry MR, Maslinkiewicz A, Saldiva PHN, Lago PD, Rhoden CR. Residual Oil Fly Ash (ROFA) Inhalation promotes lung and heart oxidative stress without hemodynamic effects in exercising rats. J Exercise Physiol Online. 2015;17:11. [Google Scholar]
- Heck TG, Petry MR, Maslinkiewicz A, Goettems-Fiorin PB, Ludwig MS, Saldiva PHN, Dal Ago P, Rhoden CR (2015b) Effects of ambient particles inhalation on lung oxidative stress parameters in exercising rats. J Exercise Physiol Online 17:11
- Heck TG et al. (2017) Acute exercise boosts cell proliferation and the heat shock response in lymphocytes: correlation with cytokine production and extracellular-to-intracellular HSP70 ratio Cell Stress. Chaperones 22:271-291 doi:10.1007/s12192-017-0771-3 [DOI] [PMC free article] [PubMed]
- Hinkley JM, Konopka AR, Suer MK, Harber MP. Short-term intense exercise training reduces stress markers and alters the transcriptional response to exercise in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2017;312:R426–R433. doi: 10.1152/ajpregu.00356.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. 1 edition edn. Oxford: Oxford University Press; 2002. [Google Scholar]
- Hom LL, Lee EC, Apicella JM, Wallace SD, Emmanuel H, Klau JF, Poh PY, Marzano S, Armstrong LE, Casa DJ, Maresh CM. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones. 2012;17:29–39. doi: 10.1007/s12192-011-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL. Diabetes, nitric oxide, and heat shock proteins. Diabetes Care. 2003;26:951–952. doi: 10.2337/diacare.26.3.951. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. 2005;7:204–208. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Xie H, Liu H. Endoplasmic reticulum stress, diabetes mellitus, and tissue injury. Curr Protein Pept Sci. 2014;15:812–818. doi: 10.2174/1389203715666140930125426. [DOI] [PubMed] [Google Scholar]
- Hung CH, Chen YW, Shao DZ, Chang CN, Tsai YY, Cheng JT. Exercise pretraining attenuates endotoxin-induced hemodynamic alteration in type I diabetic rats. Appl Physiol Nutr Metab = Physiologie appliquee, nutrition et metabolisme. 2008;33:976–983. doi: 10.1139/H08-081. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Leso V, Fontana L, Calabrese EJ. Nanoparticle exposure and hormetic dose-responses: an update. Int J Mol Sci. 2018;19:805. doi: 10.3390/ijms19030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, et al. Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution: an intervention study. Environ Health. 2010;9:64. doi: 10.1186/1476-069X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, et al. The severity of lung injury and metabolic disorders induced by ambient PM2.5 exposure is associated with cumulative dose. Inhal Toxicol. 2018;30:239–246. doi: 10.1080/08958378.2018.1508258. [DOI] [PubMed] [Google Scholar]
- Jin X, Su R, Li R, Song L, Chen M, Cheng L, Li Z. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere. 2016;144:459–466. doi: 10.1016/j.chemosphere.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity Nature reviews. Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargarfard M, et al. Effects of polluted air on cardiovascular and hematological parameters after progressive maximal aerobic exercise. Lung. 2015;193:275–281. doi: 10.1007/s00408-014-9679-1. [DOI] [PubMed] [Google Scholar]
- Khadir A, Kavalakatt S, Cherian P, Warsame S, Abubaker JA, Dehbi M, Tiss A. Physical Exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human. Diabet Obese Front Endocrinol. 2018;9:16. doi: 10.3389/fendo.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Kido T, et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am J Respir Cell Mol Biol. 2011;44:197–204. doi: 10.1165/rcmb.2009-0427OC. [DOI] [PubMed] [Google Scholar]
- Kim I, Shin HM, Baek W. Heat-shock response is associated with decreased production of interleukin-6 in murine aortic vascular smooth muscle cells. Naunyn Schmiedeberg's Arch Pharmacol. 2005;371:27–33. doi: 10.1007/s00210-004-1007-5. [DOI] [PubMed] [Google Scholar]
- Kim BM, Rhee JS, Jeong CB, Seo JS, Park GS, Lee YM, Lee JS. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp Biochem Physiol Toxicol Pharmacol : CBP. 2014;166:65–74. doi: 10.1016/j.cbpc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Klein SG, et al. Endothelial responses of the alveolar barrier in vitro in a dose-controlled exposure to diesel exhaust particulate matter. Part Fibre Toxicol. 2017;14:7. doi: 10.1186/s12989-017-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrycki IM, et al. Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate. Matter J Diabetes Res. 2019;2019:4858740. doi: 10.1155/2019/4858740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci. 2014;126:739–752. doi: 10.1042/CS20130678. [DOI] [PubMed] [Google Scholar]
- Krause M, et al. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol. 2014;114:251–260. doi: 10.1007/s00421-013-2769-6. [DOI] [PubMed] [Google Scholar]
- Krause M, Bock PM, Takahashi HK, Homem De Bittencourt PI, Jr, Newsholme P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci. 2015;128:789–803. doi: 10.1042/CS20140695. [DOI] [PubMed] [Google Scholar]
- Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI., Jr The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm. 2015;2015:249205. doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Gerchman F, Friedman R. Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: is heat shock response determinant for the disease complications? Diabetol Metab Syndr. 2020;12:63. doi: 10.1186/s13098-020-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepuska M, et al. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16:257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K, Reichel T, Zeilinger C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J Appl Physiol. 2019;126:916–927. doi: 10.1152/japplphysiol.01052.2018. [DOI] [PubMed] [Google Scholar]
- Kubesch N, et al. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur J Prev Cardiol. 2015;22:548–557. doi: 10.1177/2047487314555602. [DOI] [PubMed] [Google Scholar]
- La Porte PF. Mytilus trossulus hsp70 as a biomarker for arsenic exposure in the marine environment: laboratory and real-world results. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2005;10:417–428. doi: 10.1080/13547500500264371. [DOI] [PubMed] [Google Scholar]
- Leng X, et al. Evidence of a role for both anti-Hsp70 antibody and endothelial surface membrane Hsp70 in atherosclerosis. Cell Stress Chaperones. 2013;18:483–493. doi: 10.1007/s12192-013-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol. 2000;89:21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lormes W, Wang L, Reissnecker S, Steinacker JM. Different skeletal muscle HSP70 responses to high-intensity strength training and low-intensity endurance training. Eur J Appl Physiol. 2004;91:330–335. doi: 10.1007/s00421-003-0976-2. [DOI] [PubMed] [Google Scholar]
- Liu CC, Lin CH, Lin CY, Lee CC, Lin MT, Wen HC. Transgenic overexpression of heat shock protein 72 in mouse muscle protects against exhaustive exercise-induced skeletal muscle damage. J Formosan Med Assoc = Taiwan yi zhi. 2013;112:24–30. doi: 10.1016/j.jfma.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: case-cohort study within the PREDIMED trial. Nutr Metab Cardiovasc Diseases : NMCD. 2019;29:1040–1049. doi: 10.1016/j.numecd.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Noble EG. Exercise and stress response: the role of stress proteins. New York: CRC press; 2002. [Google Scholar]
- Ludwig MS, Minguetti-Camara VC, Heck TG, Scomazzon SP, Nunes PR, Bazotte RB, Homem de Bittencourt PI., Jr Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNF-alpha. Mol Cell Biochem. 2014;397:97–107. doi: 10.1007/s11010-014-2176-2. [DOI] [PubMed] [Google Scholar]
- Madden J, Coward JC, Shearman CP, Grimble RF, Calder PC. Hsp70 expression in monocytes from patients with peripheral arterial disease and healthy controls: monocyte Hsp70 in PAD. Cell Biol Toxicol. 2010;26:215–223. doi: 10.1007/s10565-009-9134-x. [DOI] [PubMed] [Google Scholar]
- Mai AS, et al. Exercise training under exposure to low levels of fine particulate matter: effects on heart oxidative stress and extra-to-intracellular HSP70 ratio. Oxidative Med Cell Longev. 2017;2017:9067875. doi: 10.1155/2017/9067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini T, et al. Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages. Basic Res Cardiol. 2016;111:44. doi: 10.1007/s00395-016-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmett B, Nunes RB, de Souza KS, Lago PD, Rhoden CR. Aerobic training reduces oxidative stress in skeletal muscle of rats exposed to air pollution and supplemented with chromium picolinate. Redox Rep : communications in free radical research. 2018;23:146–152. doi: 10.1080/13510002.2018.1475993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmett B, Carvalho RB, Dorneles GP, Nunes RB, Rhoden CR. Should I stay or should I go: can air pollution reduce the health benefits of physical exercise? Med Hypotheses. 2020;144:109993. doi: 10.1016/j.mehy.2020.109993. [DOI] [PubMed] [Google Scholar]
- Martin PJ, et al. Cellular response and extracellular vesicles characterization of human macrophages exposed to fine atmospheric particulate matter. Environ Pollut. 2019;254:112933. doi: 10.1016/j.envpol.2019.07.101. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Matt F, Cole-Hunter T, Donaire-Gonzalez D, Kubesch N, Martinez D, Carrasco-Turigas G, Nieuwenhuijsen M. Acute respiratory response to traffic-related air pollution during physical activity performance. Environ Int. 2016;97:45–55. doi: 10.1016/j.envint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, et al. Association of long-term PM2.5 exposure with traditional and novel lipid measures related to cardiovascular disease risk. Environ Int. 2019;122:193–200. doi: 10.1016/j.envint.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melling CW, Thorp DB, Milne KJ, Krause MP, Noble EG. Exercise-mediated regulation of Hsp70 expression following aerobic exercise training. Am J Physiol Heart Circ Physiol. 2007;293:H3692–H3698. doi: 10.1152/ajpheart.00827.2007. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;1985(93):561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Wolff S, Noble EG. Myocardial accumulation and localization of the inducible 70-kDa heat shock protein, Hsp70, following exercise. J Appl Physiol (Bethesda, Md : 1985) 2012;113:853–860. doi: 10.1152/japplphysiol.00131.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragem AA, Homem de Bittencourt PI., Jr Nitric oxide-heat shock protein axis in menopausal hot flushes: neglected metabolic issues of chronic inflammatory diseases associated with deranged heat shock response. Hum Reprod Update. 2017;23:600–628. doi: 10.1093/humupd/dmx020. [DOI] [PubMed] [Google Scholar]
- Mitra T, et al. Expression patterns of heat shock protein genes in Rita rita from natural riverine habitat as biomarker response against environmental pollution. Chemosphere. 2018;211:535–546. doi: 10.1016/j.chemosphere.2018.07.093. [DOI] [PubMed] [Google Scholar]
- Molanouri Shamsi M, Mahdavi M, Quinn LS, Gharakhanlou R, Isanegad A. Effect of resistance exercise training on expression of Hsp70 and inflammatory cytokines in skeletal muscle and adipose tissue of STZ-induced diabetic rats. Cell Stress Chaperones. 2016;21:783–791. doi: 10.1007/s12192-016-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina MJ, Molina LT. Megacities and atmospheric pollution. J Air Waste Manage Assoc. 2004;54:644–680. doi: 10.1080/10473289.2004.10470936. [DOI] [PubMed] [Google Scholar]
- Moore NS, Mans RA, McCauley MK, Allgood CS, Barksdale KA. Critical effects on Akt signaling in adult zebrafish brain following alterations in light exposure. Cells. 2021;10:637. doi: 10.3390/cells10030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39:643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2003;17:249–254. doi: 10.1002/jbt.10086. [DOI] [PubMed] [Google Scholar]
- Mulyani WRW, et al. Chaperone-based therapeutic target innovation: heat shock protein 70 (HSP70) for type 2 diabetes mellitus. Diabetes, Metab Syndrome Obes : targets and therapy. 2020;13:559–568. doi: 10.2147/DMSO.S232133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz AC, Riva P, Simoes D, Curi R, Carpinelli AR. Control of insulin secretion by production of reactive oxygen species: study performed in pancreatic islets from fed and 48-hour fasted Wistar rats. PLoS One. 2016;11:e0158166. doi: 10.1371/journal.pone.0158166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase Y, Shimizu K, Tanimura Y, Hanaoka Y, Watanabe K, Kono I, Miyakawa S. Salivary extracellular heat shock protein 70 (eHSP70) levels increase after 59 min of intense exercise and correlate with resting salivary secretory immunoglobulin A (SIgA) levels at rest. Cell Stress Chaperones. 2016;21:261–269. doi: 10.1007/s12192-015-0656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava R, Zuhl MN. Heat acclimation-induced intracellular HSP70 in humans: a meta-analysis. Cell Stress Chaperones. 2020;25:35–45. doi: 10.1007/s12192-019-01059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, de Bittencourt PI., Jr The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr Opin Clin Nutr Metab Care. 2014;17:295–305. doi: 10.1097/MCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Nieman DC, et al. Effect of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med. 1994;15:199–206. doi: 10.1055/s-2007-1021047. [DOI] [PubMed] [Google Scholar]
- Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: Part 3 of a 3-Part Series. J Am Coll Cardiol. 2017;70:230–251. doi: 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33:1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Nunes RB, Tonetto M, Machado N, Chazan M, Heck TG, Veiga AB, Dall Ago P. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol (Bethesda, Md : 1985) 2008;104:1641–1647. doi: 10.1152/japplphysiol.00062.2008. [DOI] [PubMed] [Google Scholar]
- Ogawa F, Shimizu K, Hara T, Muroi E, Hasegawa M, Takehara K, Sato S. Serum levels of heat shock protein 70, a biomarker of cellular stress, are elevated in patients with systemic sclerosis: association with fibrosis and vascular damage. Clin Exp Rheumatol. 2008;26:659–662. [PubMed] [Google Scholar]
- Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol. 2005;95:514–521. doi: 10.1007/s00421-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Investig. 2017;47:600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- Periard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17:375–383. doi: 10.1007/s12192-011-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periard JD, Ruell PA, Thompson MW, Caillaud C. Moderate- and high-intensity exhaustive exercise in the heat induce a similar increase in monocyte Hsp72. Cell Stress Chaperones. 2015;20:1037–1042. doi: 10.1007/s12192-015-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry ER, Cruzat VF, Heck TG, Leite JS, Homem de Bittencourt PI, Jr, Tirapegui J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: involvement of heat shock protein pathways. Life Sci. 2014;94:130–136. doi: 10.1016/j.lfs.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Petry ER, Cruzat VF, Heck TG, Homem de Bittencourt PI, Jr, Tirapegui J. L-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exercise Metab. 2015;25:188–197. doi: 10.1123/ijsnem.2014-0131. [DOI] [PubMed] [Google Scholar]
- Petry ER et al (2019) Oral glutamine supplementation attenuates inflammation and oxidative stress-mediated skeletal muscle protein content degradation in immobilized rats: role of 70kDa heat shock protein Free radical. biology & medicine 145:87-102. 10.1016/j.freeradbiomed.2019.08.033 [DOI] [PubMed]
- Pilis W, Zarzeczny R, Langfort J, Kaciuba-Uscilko H, Nazar K, Wojtyna J. Anaerobic threshold in rats. Comp Biochem Physiol Comp Physiol. 1993;106:285–289. doi: 10.1016/0300-9629(93)90513-4. [DOI] [PubMed] [Google Scholar]
- Pittaluga M et al. (2006) Cellular and biochemical parameters of exercise-induced oxidative stress: relationship with training levels. Free Radic Res 40:607-614 [DOI] [PubMed]
- Pockley AG, Multhoff G. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found Symp. 2008;291:86–95. doi: 10.1002/9780470754030.ch7. [DOI] [PubMed] [Google Scholar]
- Qin F, Xu MX, Wang ZW, Han ZN, Dong YN, Zhao JX. Effect of aerobic exercise and different levels of fine particulate matter (PM2.5) on pulmonary response in Wistar rats. Life Sci. 2020;254:117355. doi: 10.1016/j.lfs.2020.117355. [DOI] [PubMed] [Google Scholar]
- Qin F, Cui S, Dong Y, Xu M, Wang Z, Qu C, Zhao J. Aerobic exercise ameliorates particulate matter-induced lung injury in aging rats. Environ Pollut. 2021;280:116889. doi: 10.1016/j.envpol.2021.116889. [DOI] [PubMed] [Google Scholar]
- Raizel R, Leite JS, Hypolito TM, Coqueiro AY, Newsholme P, Cruzat VF, Tirapegui J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br J Nutr. 2016;116:470–479. doi: 10.1017/S0007114516001999. [DOI] [PubMed] [Google Scholar]
- Ravaschiere A, Cutler C, Edleson K, Halem Z, Magun H, Meckler F, Cox R. Quantification of heat shock protein 70 and acetylcholinesterase over a time course suggests environmental adaptation in a foundational molluscan species. Ecotoxicol Environ Saf. 2017;142:222–229. doi: 10.1016/j.ecoenv.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Lawrence J, Godleski JJ, Gonzalez-Flecha B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci : an official journal of the Society of Toxicology. 2004;79:296–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, Gonzalez-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic. Stimul Biochim et Biophysica Acta. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Ghelfi E, Gonzalez-Flecha B. Pulmonary inflammation by ambient air particles is mediated by superoxide anion. Inhal Toxicol. 2008;20:11–15. doi: 10.1080/08958370701758379. [DOI] [PubMed] [Google Scholar]
- Ribeiro Junior G, et al. Diesel exhaust exposure intensifies inflammatory and structural changes associated with lung aging in mice. Ecotoxicol Environ Saf. 2019;170:314–323. doi: 10.1016/j.ecoenv.2018.11.139. [DOI] [PubMed] [Google Scholar]
- Rinella ME. The "dose" of exercise and its effects beyond weight loss. Hepatology. 2015;61:1115–1117. doi: 10.1002/hep.27669. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:DOTHSR>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Krause J et al. (2012a) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
- Rodrigues-Krause J, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RS, et al. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes. 2016;65:3341–3351. doi: 10.2337/db16-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas J, et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res. 2018;2018:9601801. doi: 10.1155/2018/9601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rundell KW, Slee JB, Caviston R, Hollenbach AM. Decreased lung function after inhalation of ultrafine and fine particulate matter during exercise is related to decreased total nitrate in exhaled breath condensate. Inhal Toxicol. 2008;20:1–9. doi: 10.1080/08958370701758593. [DOI] [PubMed] [Google Scholar]
- Sancini G, et al. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5) PLoS One. 2014;9:e109685. doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom ME, Madden LA, Taylor L, Siegler JC, Lovell RJ, Midgley A, McNaughton L. Variation in basal heat shock protein 70 is correlated to core temperature in human subjects. Amino Acids. 2009;37:279–284. doi: 10.1007/s00726-008-0144-4. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, et al. Dose-response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2015;154:309–318. doi: 10.1007/s10549-015-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer CM, Marques CV, da Silva GS, Heck TG, de Oliveira Junior LP, Homem de Bittencourt PI., Jr Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol Cell Biochem. 2016;421:111–125. doi: 10.1007/s11010-016-2791-1. [DOI] [PubMed] [Google Scholar]
- Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2):7–12. doi: 10.1159/000375143. [DOI] [PubMed] [Google Scholar]
- Sharman JE. Clinicians prescribing exercise: is air pollution a hazard? Med J Aust. 2005;182:606–607. doi: 10.5694/j.1326-5377.2005.tb06843.x. [DOI] [PubMed] [Google Scholar]
- Sharman JE, Coombes JS, Geraghty DP, Fraser DI. Exposure to automotive pollution increases plasma susceptibility to oxidation. Arch Environ Health. 2002;57:536–540. doi: 10.1080/00039890209602085. [DOI] [PubMed] [Google Scholar]
- Sharman JE, Cockcroft JR, Coombes JS. Cardiovascular implications of exposure to traffic air pollution during exercise. QJM : monthly journal of the Association of Physicians. 2004;97:637–643. doi: 10.1093/qjmed/hch104. [DOI] [PubMed] [Google Scholar]
- Shastry S, Toft DO, Joyner MJ. HSP70 and HSP90 expression in leucocytes after exercise in moderately trained humans. Acta Physiol Scand. 2002;175:139–146. doi: 10.1046/j.1365-201X.2002.00979.x. [DOI] [PubMed] [Google Scholar]
- Silveira EM, et al. Acute exercise stimulates macrophage function: possible role of NF-kappaB pathways. Cell Biochem Funct. 2007;25:63–73. doi: 10.1002/cbf.1365. [DOI] [PubMed] [Google Scholar]
- Silver JT, Kowalchuk H, Noble EG. hsp70 mRNA temporal localization in rat skeletal myofibers and blood vessels post-exercise. Cell Stress Chaperones. 2012;17:109–120. doi: 10.1007/s12192-011-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HB. Exercise and health: dose and response, considering both ends of the curve. Am J Med. 2015;128:1171–1177. doi: 10.1016/j.amjmed.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Slezakova K, Pereira MC, Morais S. Ultrafine particles: levels in ambient air during outdoor sport activities. Environ Pollut. 2020;258:113648. doi: 10.1016/j.envpol.2019.113648. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Abraham JS, Maurya S, Toteja R, Gupta R, Makhija S. Expression and molecular characterization of stress-responsive genes (hsp70 and Mn-sod) and evaluation of antioxidant enzymes (CAT and GPx) in heavy metal exposed freshwater ciliate. Tetmemena sp. Mol Biol Rep. 2019;46:4921–4931. doi: 10.1007/s11033-019-04942-0. [DOI] [PubMed] [Google Scholar]
- Sulzbacher MM, et al. A single dose of eHSP72 attenuates sepsis severity in mice. Sci Rep. 2020;10:9198. doi: 10.1038/s41598-020-66011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberin Z. Association of fetuin-A and heat shock protein 70 with arterial calcification in patients with peripheral vascular disease. Interv Med Appl Sci. 2012;4:50–55. [Google Scholar]
- Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci : JPS. 2017;67:1–10. doi: 10.1007/s12576-016-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shibazaki A, Ono R, Kaisho T. HSP70 mediates degradation of the p65 subunit of nuclear factor kappaB to inhibit inflammatory signaling. Sci Signal. 2014;7:ra119. doi: 10.1126/scisignal.2005533. [DOI] [PubMed] [Google Scholar]
- Tanwar V, et al. In utero particulate matter exposure produces heart failure, electrical remodeling, and epigenetic changes at adulthood. J Am Heart Assoc. 2017;6:e005796. doi: 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- Tiss A, et al. Immunohistochemical profiling of the heat shock response in obese non-diabetic subjects revealed impaired expression of heat shock proteins in the adipose tissue. Lipids Health Dis. 2014;13:106. doi: 10.1186/1476-511X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok Z, et al. Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications. Biochim Biophys Acta. 2014;1838:1594–1618. doi: 10.1016/j.bbamem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Kobayashi H, Yoshihara T, Kakigi R, Ichinoseki-Sekine N, Naito H. Attenuation of exercise-induced heat shock protein 72 expression blunts improvements in whole-body insulin resistance in rats with type 2 diabetes. Cell Stress Chaperones. 2017;22:263–269. doi: 10.1007/s12192-017-0767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle JA, Castle PC, Metcalfe AJ, Midgley AW, Taylor L, Lewis MP. Downhill running and exercise in hot environments increase leukocyte Hsp72 (HSPA1A) and Hsp90alpha (HSPC1) gene transcripts. J Appl Physiol. 2015;118:996–1005. doi: 10.1152/japplphysiol.00387.2014. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- van Helvoort HA, van de Pol MH, Heijdra YF, Dekhuijzen PN. Systemic inflammatory response to exhaustive exercise in patients with chronic obstructive pulmonary disease. Respir Med. 2005;99:1555–1567. doi: 10.1016/j.rmed.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Wagner DR, Clark NW. Effects of ambient particulate matter on aerobic exercise performance. J Exerc Sci Fit. 2018;16:12–15. doi: 10.1016/j.jesf.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DR, Brandley DC. Exercise in thermal inversions: PM2.5 air pollution effects on pulmonary function and aerobic performance. Wilderness Environ Med. 2020;31:16–22. doi: 10.1016/j.wem.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Watterson TL, Hamilton B, Martin R, Coulombe RA., Jr Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol Sci. 2009;112:111–122. doi: 10.1093/toxsci/kfp186. [DOI] [PubMed] [Google Scholar]
- Wedell-Neergaard AS, Eriksen L, Gronbaek M, Pedersen BK, Krogh-Madsen R, Tolstrup J. Low fitness is associated with abdominal adiposity and low-grade inflammation independent of BMI. PLoS One. 2018;13:e0190645. doi: 10.1371/journal.pone.0190645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021) Ambient air pollution. World Health Organization. https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/ambient-air-pollution. Accessed September 09, 2021 2021
- Wormgoor SG, Dalleck LC, Zinn C, Harris NK. Acute blood glucose, cardiovascular and exaggerated responses to HIIT and moderate-intensity continuous training in men with type 2 diabetes mellitus. J Sports Med Phys Fit. 2018;58:1116–1126. doi: 10.23736/S0022-4707.17.07639-3. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessel. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Xie F, Zhan R, Yan LC, Gong JB, Zhao Y, Ma J, Qian LJ. Diet-induced elevation of circulating HSP70 may trigger cell adhesion and promote the development of atherosclerosis in rats. Cell Stress Chaperones. 2016;21:907–914. doi: 10.1007/s12192-016-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, et al. Long-term effects of ambient particulate matter (with an aerodynamic diameter </=2.5 mum) on hypertension and blood pressure and attributable risk among reproductive-age adults in China. J Am Heart Assoc. 2018;7:e008553. doi: 10.1161/JAHA.118.008553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Kumar V, Jha V. Heat shock proteins 60 and 70 specific proinflammatory and cytotoxic response of CD4+CD28null cells in chronic kidney disease. Mediat Inflamm. 2013;2013:384807. doi: 10.1155/2013/384807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younk LM, Mikeladze M, Tate D, Davis SN. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev Endocrinol Metab. 2011;6:93–108. doi: 10.1586/eem.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15:675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shan P, Srivastava A, Jiang G, Zhang X, Lee PJ. An endothelial Hsp70-TLR4 axis limits Nox3 expression and protects against oxidant injury in lungs. Antioxid Redox Signal. 2016;24:991–1012. doi: 10.1089/ars.2015.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan N, Peng Y. Heat shock protein 70 promotes lipogenesis in HepG2 cells. Lipids Health Dis. 2018;17:73. doi: 10.1186/s12944-018-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mohan C. Toll-like receptor signaling pathways—therapeutic opportunities. Mediat Inflamm. 2010;2010:781235. doi: 10.1155/2010/781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.