Abstract
Fruit juices have shown promising results as new probiotic carriers. This study aimed to evaluate acerola, jelly palm, and passion fruit juices as substrates for fermentation using Lactiplantibacillus plantarum CCMA 0743 and Lacticaseibacillus paracasei LBC-81 in single and mixed cultures. First, the juices were evaluated as substrate and selected based on bacterial growth performance during fermentation. Afterward, the impact of fermentation on sugars, organic acids, and bioactive compounds was also appraised. Phytochemical modification of three different juices fermented by lactic acid bacteria at 37 °C/24 h was evaluated. After 18 h of fermentation, passion fruit juice showed higher cell viable counts of single and mixed L. plantarum CCMA 0743 culture, above 9.00 Log CFU/mL, and pH between 4.07 and 4.10. Sugars consumption and organic acid production were influenced by juice composition and culture used. The mixed culture reduced the total sugars in the passion fruit juice by approximately 53.0% (8.51 g/L). Lactic acid was the main product of the sugars fermentation, with higher concentrations detected in passion fruit juice (8.39–11.23 g/L). Bioactive compounds were analyzed on the selected substrate. The fermentative process reduced antioxidant activity and carotenoid content. However, single L. plantarum CCMA 0743 culture increased the yellow flavonoid content of passion fruit juice by approximately 3.0 µg/mL. L. plantarum CCMA 0743 showed high and suitable cell, viable counts, to claimed probiotic products, increasing bioactive compounds in passion fruit juice. Therefore, this strain and passion fruit substrate showed attractive potential to produce alternative and functional fermented fruit beverages.
Keywords: Chemical composition, Fermented beverages, Functional food, Lactobacillus, Principal component analysis, Probiotic
Introduction
Fermented beverages have become known for their functional attributes in many regions of the world. The popularity of these fermented foods increased because of their nutritional quality and remarkable health benefits associated with probiotic microorganisms [1]. Traditionally, fermented milk has been considered the best carrier for probiotic bacteria. However, an increasing number of people allergic to milk proteins, lactose intolerant, or who have adopted a strictly vegetarian diet cannot consume dairy products [2]. For this reason, nondairy matrices, including fruit juices, have become an alternative to innovate and develop new probiotic fermented beverages [3, 4].
Nowadays, fruit juices have been reported as appropriate substrates for lactobacilli cultures. Potential beneficial effects of fruit juices added with probiotics are reported in the literature [4] and the effects of the fruit bioactive compounds on human wellness [5]. Many studies have reported that commercial and non-commercial LAB species may show high viability in vegetable fermentation [6–8]. On the other hand, some researchers have observed viable cell counts reduction after fruit juice fermentation [9, 10]. Although juices contain essential nutrients (minerals, vitamins, dietary fibers, antioxidants), some parameters such as acidity, antinutrients presence, processing, and others can limit LAB survival in juices [11]. Thereby, strain and substrate choices become essential criteria for fermentation success since microbial metabolism depends entirely on the interaction of these intrinsic and extrinsic parameters. Fermented foods are also significantly influenced by the single or mixed fermentation of LAB or fruit‐based substrate [12, 13]. Hashemi and Jafarpour [6] noted that viable cell levels were higher in mixed cultures than in single cultures in bergamot juice fermentation. Furthermore, mixed fermentation by LAB increased bioactive compounds of cherry silverberry fruit [14].
The fermentation modifies the composition of the food by microbial metabolism and can improve several health benefits [15]. For example, lactic acid bacteria (LAB), especially Lactiplantibacillus plantarum (former Lactobacillus plantarum) species, have been related to increasing bioactive compounds, such as anthocyanin, phenolic, flavonoid, and antioxidant activity, of mulberry [16], Momordica charantia [17], and orange juices [18]. This species is generally used in the fermentation of plant-based matrices due to its more adaptable metabolism to these substrates [6, 19]. Nonetheless, growth potential and bioconversion capacity are strain-specific characteristics [20], and exposure to environmental stresses results in changed gene expression in LAB and induces shifts in the production of metabolites [21]. Therefore, changes in the nutritional and phytochemical compositions depend highly on the matrix, the LAB strain, and the fermentation conditions [1].
Tropical fruits have increased recognition of their nutritional and functional values. Researches have demonstrated that passion fruit, acerola, and jelly palm fruits show interesting nutritional properties as relevant sources of bioactive compounds [22–24]. Among these exciting properties, acerola displays a considerable amount of vitamin C [23], jelly palm contains high antioxidant capacities [25], and passion fruit has great total phenolic content [26].
The indigenous L. plantarum CCMA 0743 strain has demonstrated proper growth over the fermentation of nondairy beverages [27]. Additionally, this strain showed interesting in vitro probiotic properties, including reducing pathogen colonization in human epithelial cells [28]. Because the fermentation process is dependent on the substrate and the starter culture and considering the consumers’ demand for attractive and alternative probiotic beverages, this work aimed to evaluate acerola, jelly palm, and passion fruit juices as substrates for fermentation using potential probiotic Lactiplantibacillus plantarum CCMA 0743 and commercial probiotic Lacticaseibacillus paracasei subsp. paracasei LBC-81 in single and mixed cultures. First, the juices were evaluated as substrate and selected based on bacterial growth performance during fermentation. Afterward, the effect of the fermentation of sugars and the production of organic acids and bioactive compounds were also evaluated.
Materials and methods
Fruit juices preparation
The passion fruit (Passiflora edulis), acerola (Malpighia emarginata), and jelly palm (Butia capitata) pulps were obtained from Cooperativa Grande Sertão (Montes Claros, Minas Gerais, Brazil). The juices were prepared by diluting frozen pulp in potable water (1:5) as recommended by the manufacturer. After adjusting the pH to 5.6 with a 3 M NaOH sterile solution, the juices were pasteurized at 80 °C for 5 min and then cooled at 37 °C.
Microorganisms and inoculum preparation
The strains L. plantarum CCMA 0743 (from the Culture Collection of Agricultural Microbiology) and L. paracasei subsp. paracasei LBC-81 (Danisco-DuPont, USA) were used in this study. The L. plantarum CCMA 0743 strain was used due to its probiotic potential evaluated previously [28]. Inoculums were prepared by transferring the culture stored at − 80 °C of L. plantarum CCMA 0743 and L. paracasei LBC-81 to MRS broth. Strains were twice subcultured statically at 37 °C to obtain an active cell population culture of 8.0 Log CFU/mL and used as starter cultures in the juice fermentations.
Fermentations
The bacterial cultures were washed with sterile phosphate buffer saline (PBS) and re-suspended in sterile deionized water. Fermentations were performed in Erlenmeyer flasks (250 mL) containing 150 mL of prepared juices inoculated with 1% (v/v) (~ 6.0 Log CFU/mL) of L. plantarum CCMA 0743, L. paracasei LBC-81, and L. plantarum CCMA 0743 + L. paracasei LBC-81 (1:1). The samples were statically fermented for 24 h at 37 °C. Finally, viable cells and pH were evaluated at every 6 h of fermentation. The fruit juice and the fermentation time, which showed lower pH and higher viable cell counts, were selected for further analysis. The fermentations were performed with 3 independent repetitions.
LAB analysis and pH determination
LAB analysis, growth media, and incubation conditions were performed according to Szutowska et al. [29], with some modifications. Serial dilutions of fermented juices were performed using sterile peptone water (0.1% w/v), and aliquots of appropriate dilutions were plated in duplicate by the spread plate method. Total viable cell counts of the LAB strains were enumerated on MRS (Kasvi, Italy) agar at 37 °C for 48 h under aerobic conditions. Results were expressed as Log CFU/mL.
The juices pH was determined by direct measurement in a Digimed DM-22 potentiometer.
Sugars and organic acids determination
Organic acids (lactic, citric, malic, succinic, acetic, and butyric acid) and sugars (glucose, fructose, and sucrose) analyses of juices at time 0 and after 18 h fermentation were performed as described by Freire et al. [7], with modifications. A Shimadzu Liquid Chromatography System (Shimadzu Corp., Japan), equipped with a dual detection system consisting of a UV–Vis detector (SPD 10Ai) (for acids) and a refractive index detector (RID 10Ai) (for sugars), was used. Organic acids were determined using a Shimadzu ion exclusion column, Shim-pack SCR-101H (300 mm × 7.9 mm i.d., 10 μm) at an operating temperature of 50 °C, using an aqueous solution of perchloric acid as mobile phase. Sugars were analyzed using a Shimadzu ion exclusion column, Shim-pack SCR-101C (300 mm × 7.9 mm i.d., 10 μm) at an operating temperature of 80 °C, using water as mobile phase. The acids and sugars were identified by comparison with retention times of authentic standards. The quantification was performed using calibration curves constructed with standard compounds. All samples were analyzed in triplicate.
Total phenolic and antioxidant activity determination
The extracts were obtained according to the method described by De Souza et al. [30], with slight modifications. Briefly, 5 mL of the samples were added in centrifuge tubes and extracted sequentially with 10 mL of methanol/water (50:50, v/v) at room temperature (25 °C) for 1 h. The tubes were centrifuged at 5,240 × g at room temperature for 10 min, and the supernatants were recovered. Then, 10 mL of acetone/water (70:30, v/v) was added to the pellet at room temperature. The samples were extracted for 1 h and centrifuged again under the same conditions as before. The methanol and acetone extracts were used for the determination of antioxidant activity and phenolic content as follows.
Total phenolic content determination
Total phenolic content was determined by the Fast Blue (FB) method described by Medina [31], with some modifications. Sample (2 mL) was homogenized by vortex with 0.2 mL of 0.1% (w/v) Fast Blue BB reagent and 0.2 mL 5% (w/v) NaOH for 1 min. The reaction was completed at room temperature for 90 min, and the absorbance was measured spectrophotometrically (UV–VIS SP-2000UV spectrophotometer) at 420 nm. A calibration curve was prepared using a gallic acid solution (20–200 µg/mL). Results were expressed as µg of gallic acid equivalent per mL of sample (µg GAE/mL).
Phosphomolybdenum complex method (PCM)
Antioxidant activity was determined by the PCM according to the modified methodology described by Prieto et al. [32]. An aliquot of 0.1 mL of the sample solution was placed in tubes and mixed with 3 mL of reagent solution (1.8 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were capped and incubated in a water bath at 95 °C for 90 min. Then, the samples were cooled down to room temperature, and the absorbance of the green phosphomolybdenum complex was measured at 695 nm. A mixture containing methanol 50% and acetone 70% (1:1) was used as a blank. The quantification was based on a standard curve of ascorbic acid (1.95 to 500 µg), and the results were expressed in mg ascorbic acid equivalents (AAEs) per mL of sample.
ABTS assay
The antioxidant activity was determined using the ABTS (2,20-azinobis-3-ethylbenzothiazoline-6-sulfonate) assay according to the method of Re et al. [33] with minor modifications. The ABTS radical cation (ABTS+) was generated by the reaction of 5 mL of aqueous ABTS solution (7 mM) with 88 µL of 140 mM (2.45 mM final concentration) potassium persulfate. The mixture was kept in the dark for 16 h before use and then diluted with ethanol to obtain an absorbance of 0.7 ± 0.05 units at 734 nm using a spectrophotometer. The juice extracts (30 µL) or a reference substance (Trolox) was allowed to react with 3 mL of the resulting blue-green ABTS radical solution in the dark. The decrease in absorbance at 734 nm was measured after 6 min. The quantification was based on a standard curve of Trolox (0.1 to 2 mM), and the results were expressed as micromoles of Trolox equivalents (TEs) per milliliter of the sample (µmol of TE/mL).
Total carotenoids content determination
The extraction of total carotenoids was carried out according to Carbonell-Capella et al. [34], with slight modifications. Sample (2 mL) was homogenized with 5 mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v/v) and centrifuged at 4,520 × g for 5 min at 4 °C. The top layer of hexane containing the color was recovered and transferred to a 25 mL volumetric flask. The volume of recovered was then adjusted to 25 mL with hexane. The total carotenoid determination was carried out on an aliquot of the hexane extract by measuring the absorbance at 450 nm. The extinction coefficient used was of β-carotene, E1% = 2505.
Anthocyanins and yellow flavonoids determination
Anthocyanins and yellow flavonoids determination was carried out as described by Francis [35], with modifications. Briefly, 2.5 mL of each juice sample was suspended in 20 mL of extraction solution (ethanol 95%: 1.5 N HCl — 85:15 v/v). Samples were homogenized for 1 min and then transferred to a 50 mL volumetric flask. The volume was complete to 50 mL with the same extraction solution and incubated for 16 h under refrigeration (7 °C) in the dark. After this period, the extracts were filtered, and the absorbances at 535 nm (anthocyanins) and 374 nm (yellow flavonoids) were measured. The anthocyanins and yellow flavonoids content was calculated using Eq. 1 and absorption coefficients of 98.2 and 76.6 (mol/cm), respectively.
| 1 |
where ABS is the absorbance reading of the sample and is the absorption coefficient.
Determination of ascorbic acid content
The ascorbic acid content was determined using the iodine titration method modified from Suntornsuk et al. [36]. One milliliter of juice was transferred into a 125 mL conical flask and diluted with 4 mL distilled water. Then, 5 mL of 2 N sulfuric acid was added, mixed with 2 mL of 1% starch as an indicator. The solution was directly titrated with a 0.001 N standardized iodine solution. A blank titration was performed before the titration of each sample. Each milliliter of 0.001 N iodine was equivalent to 88.06 µg of ascorbic acid.
Statistical analysis
All the treatments and assays were carried out thrice, and the results were presented as mean ± standard error. The analysis of variance (ANOVA) was performed to compare all variables, and the Tukey test was used to calculate significant differences at p < 0.05. Principal component analysis (PCA) was applied to the data set through multivariate exploratory techniques. It was chosen to include just enough k components to explain at least 80% of the total variance. The analyses were performed using Statistica software version 10.0 (Statsoft, USA).
Results
LAB growth and acidification
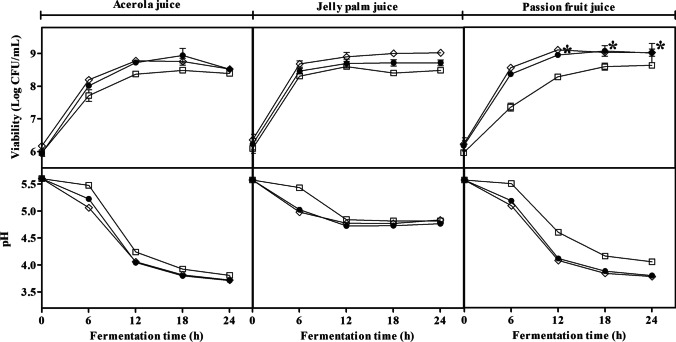
In general, L. plantarum CCMA 0743 and L. paracasei LBC-81 strains, in the single and mixed assays, showed high total viable cell counts during fermentation in the three evaluated fruit juices (Fig. 1). However, single and mixed cultures containing the potentially probiotic strain L. plantarum CCMA 0743 showed faster growth, as demonstrated by increasing around 2 Log CFU/mL at 6 h of fermentation. At the same time, the commercial probiotic strain in single culture reached the desired counts above 8 Log CFU/mL only in the jelly palm juice. After 12 h of the fermentative process in all evaluated juices, no significant increase in cell counts was observed for all cultures. The highest viable cell counts (8.95–9.11 Log CFU/mL) were observed from 12 h of fermentation in the passion fruit juice inoculated by single L. plantarum CCMA 0743 and mixed cultures.
Fig. 1.
Variations in growth and pH during 24 h of fermentation at 37 °C of acerola, jelly palm, and passion fruit juices fermented by LAB strains. Asterisks indicate greater viable cell counts, regardless of the substrate used and fermentation time. Bars represent the standard error of triplicate measurements of three independent assays. L. plantarum CCMA 0743 monoculture (◊); L. paracasei LBC-81 monoculture (□); L. plantarum CCMA 0743 and L. paracasei LBC-81 binary inoculation (●). The standard errors ranged from 0.01 to 0.29 and from 0.01 to 0.04, respectively, for viability and pH
A decrease in pH was observed in all juices and assays (Fig. 1). The most significant reductions occurred between 6 and 12 h of fermentation. By comparing the juices, the pH reduction in the jelly palm juice fermentation reached values close to 4.9, while in the other two evaluated juices, the pH values were below 4.3 for all strains. Between the strains, the single and mixed L. plantarum CCMA 0743 strain showed a higher pH decrease (p < 0.05) than the L. paracasei LBC-81 strain in all evaluated juices.
According to the obtained results (Fig. 1), the fermentation time of 18 h was able to maintain an adequate viable cell concentration for the probiotic purpose (above 9 Log CFU/mL), and the pH was reduced to a value around 4.0, which is considered enough to avoid spoilage and to ensure microbiological stability. After 18 h of fermentation, the viable cell counts were not significantly improved (p > 0.05). Thus, this fermentation time was considered for chemical evaluations.
Sugars and organic acids metabolism
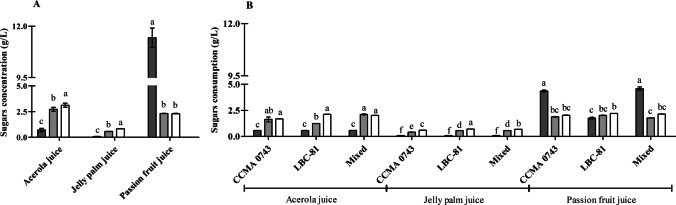
The sugar composition of the fruit juices is shown in Fig. 2A. The passion fruit and jelly palm juices contained, respectively, the highest (16.13 g/L) and the lowest (1.49 g/L) amount of fermentable sugars. Acerola juice showed 6.48 g/L of total sugars.
Fig. 2.
Sugars content (g/L) of unfermented juices (A) and sugars consumption (g/L) in fermented juices (B). Bars indicate standard error of sugar content of unfermented juices and sugars consumption in juices fermented with single and mixed cultures of L. plantarum CCMA 0743 and L. paracasei LBC-81 after 18 h of fermentation. The same lowercase letters are not significantly different between treatments within the same study juice at p > 0.05. sucrose (■); glucose (■); fructose (□)
Figure 2B shows sugar metabolism by the strains in the three evaluated juices. As observed, in acerola and jelly palm juices, the strains exhibited higher glucose and fructose consumption than sucrose hydrolysis. Although there was lower sucrose hydrolysis than monosaccharides consumption in jelly palm juice, this sugar was not detected after 18 h of fermentation. In contrast, in the passion fruit juice, the strain L. plantarum CCMA 0743 in single and mixed showed higher (p < 0.05) sucrose hydrolysis than the monosaccharides consumption (Fig. 2B). In this juice, when fermented by a single L. plantarum CCMA 0743 or by mixed cultures, there was a reduction of approximately 39.0% (4.5 g/L) of the total sucrose content.
In contrast, glucose and fructose total consumptions in passion fruit juices were approximately 4.0 g/L (86.0% of the total monosaccharides). Regarding passion fruit juice, it seems that the sucrose hydrolysis rate was faster than the rate of monosaccharides consumption. However, the initial concentration of this sugar was higher than the monosaccharides (Fig. 2A). Thus, sugar metabolism is affected by the levels of these sugars in the fermentation medium.
The organic acids in the three different juice fermentations were evaluated before and after 18 h of fermentation and are shown in Table 1. Lactic acid was the most abundant organic acid produced after fermentation and was detected in all fermented juices, ranging from 2.36 to 11.23 g/L. In acerola juice, the lower lactic acid content (5.26 g/L) was produced by strain L. paracasei LBC-81 in a single culture, while this strain was responsible for the higher production of this organic acid in jelly palm juice (3.36 g/L). Citric acid decreased significantly (p < 0.05) after fermentation in jelly palm juice for all evaluated strains, which may be related to the low consumption of fermentable sugars in this juice. On the other hand, malic acid was not detected after fermentation in all juices. Succinic acid decreased in all fermentations, except in acerola juice fermented by L. paracasei LBC-81. According to the obtained results, it seems that succinic acid catabolism was higher for the L. plantarum CCMA 0743 strain in single or mixed cultivation than for single L. paracasei LBC-81. In the single culture, acetic acid was produced in jelly palm juice fermentation and acerola juice fermented by L. paracasei LBC-81, with concentrations ranging from 0.23 to 1.12 g/L. Butyric acid was not detected in the acerola juice at any evaluated time of the fermentation process. However, this organic acid was consumed in passion fruit juice, while in jelly palm juice, this acid was produced by the three evaluated starter cultures.
Table 1.
Changes in organic acid of the fermented juices by lactic acid bacteria cultures
| Juice | Fermentation time | Strain | Organic acid (g/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Lactic acid | Citric acid | Malic acid | Succinic acid | Acetic acid | Butyric acid | |||
| Acerola | Before fermentation (0 h) | NDc | 0.17 ± 0.01a | 0.66 ± 0.03a | 0.46 ± 0.03a | NDb | NDa | |
| After fermentation (18 h) | 0743 | 7.49 ± 0.19a | 0.15 ± 0.03a | NDb | 0.03 ± 0.01b | NDb | NDa | |
| 81 | 5.26 ± 0.46b | NDa | NDb | 0.37 ± 0.03a | 0.23 ± 0.03a | NDa | ||
| Mix | 7.76 ± 0.13a | 0.13 ± 0.01a | NDb | 0.03 ± 0.01b | NDb | NDa | ||
| Jelly palm | Before fermentation (0 h) | NDc | 2.99 ± 0.11a | 2.16 ± 0.03a | 6.30 ± 0.02a | NDd | NDb | |
| After fermentation (18 h) | 0743 | 2.72 ± 0.17b | 1.26 ± 0.17b | NDb | 0.78 ± 0.01c | 0.35 ± 0.01c | 0.27 ± 0.01a | |
| 81 | 3.36 ± 0.03a | 0.71 ± 0.01bc | NDb | 3.97 ± 0.02b | 1.12 ± 0.01a | 0.26 ± 0.01a | ||
| Mix | 2.36 ± 0.02b | 0.57 ± 0.04c | NDb | 0.85 ± 0.03c | 0.94 ± 0.01b | 0.27 ± 0.01a | ||
| Passion fruit | Before fermentation (0 h) | 0.06 ± 0.02b | 33.60 ± 0.88a | 0.60 ± 0.02a | 0.27 ± 0.01a | NDa | 0.45 ± 0.06a | |
| After fermentation (18 h) | 0743 | 9.67 ± 1.32a | 27.73 ± 3.88a | NDb | NDb | NDa | NDb | |
| 81 | 8.39 ± 0.26a | 32.98 ± 0.52a | NDb | NDb | NDa | NDb | ||
| Mix | 11.23 ± 0.06a | 30.99 ± 0.16a | NDb | NDb | NDa | NDb | ||
Means ± standard error in the same column for each juice, followed by different lowercase letters, indicate statistically significant differences at p ≤ 0.05, according to Tukey test (n = 3). 0743, L. plantarum CCMA 0743 monoculture; 81, L. paracasei LBC-81 monoculture; Mix, L. plantarum CCMA 0743 and L. paracasei LBC-81 binary inoculation
ND, not detected
Based on the high viable cell counts, pH reduction to value around 4.0, sugars consumption, and organic acid production, the passion fruit juice fermented by L. plantarum CCMA 0743 strain in single and mixed cultures was selected for further analysis.
Bioactive compounds
Antioxidant activity and bioactive compounds were evaluated and are shown in Table 2. As observed, passion fruit juice’s antioxidant activity and carotenoid levels were reduced (p < 0.05) after fermentation. The highest antioxidant activity decrease (around 40%) was obtained by the mixed culture evaluated by ABTS and PCM methods (Table 2). A reduction (p < 0.05) of about 30% in carotenoid concentrations (3.7 to 2.6 µg/mL) was also observed for single and mixed cultures. On the other hand, the passion fruit juice fermented by L. plantarum CCMA 0743 strain increased yellow flavonoid content (74.15 µg/mL versus 71.26 µg/mL) demonstrates that LAB activity can enhance this phytochemical concentration in passion fruit juice. There was no difference (p > 0.05) in the evaluated unfermented and fermented juices’ total phenolic and anthocyanin contents. Furthermore, even after the pasteurization and fermentation processes, there was no significant modification (p > 0.05) in the ascorbic acid levels, which showed 67.51 µg/mL and 74.85 µg/mL for single and mixed cultures, respectively.
Table 2.
Antioxidant activity (ABTS and PCM), total phenolic, anthocyanin, yellow flavonoid, carotenoid, and ascorbic acid contents of unfermented and fermented passion fruit juices
| Treatment | Antioxidant activity | Total phenolic (µg of GAE/mL) | Anthocyanin (µg/mL) | Yellow flavonoid (µg/mL) | Carotenoid (µg/mL) | Ascorbic acid (µg/mL) | |
|---|---|---|---|---|---|---|---|
| ABTS (µmol of TE/mL) | PCM (mg of AAE/mL) | ||||||
| Unfermented juice (Control) | 8.99 ± 0.68a | 25.78 ± 0.84a | 662.91 ± 24.70a | 1.98 ± 0.24a | 71.26 ± 0.95b | 3.74 ± 0.02a | 86.59 ± 3.88a |
| 0743 | 6.20 ± 0.67ab | 19.76 ± 0.24b | 720.48 ± 16.25a | 1.25 ± 0.32a | 74.15 ± 0.54a | 2.61 ± 0.10b | 67.51 ± 1.47a |
| Mix | 5.10 ± 0.61b | 15.67 ± 0.18c | 620.89 ± 35.66a | 1.25 ± 0.38a | 71.61 ± 0.14ab | 2.63 ± 0.10b | 74.85 ± 6.73a |
Means ± standard error in the same column followed by different lowercase letters indicate statistically significant differences at p ≤ 0.05, according to the Tukey test (n = 3)
ABTS, 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonate; PCM, phosphomolybdenum complex method; TE, trolox equivalent; AAE, ascorbic acid equivalent; GAE, gallic acid equivalent. 0743, L. plantarum CCMA 0743 monoculture; Mix, L. plantarum CCMA 0743 and L. paracasei LBC-81 binary inoculation
Principal component analysis
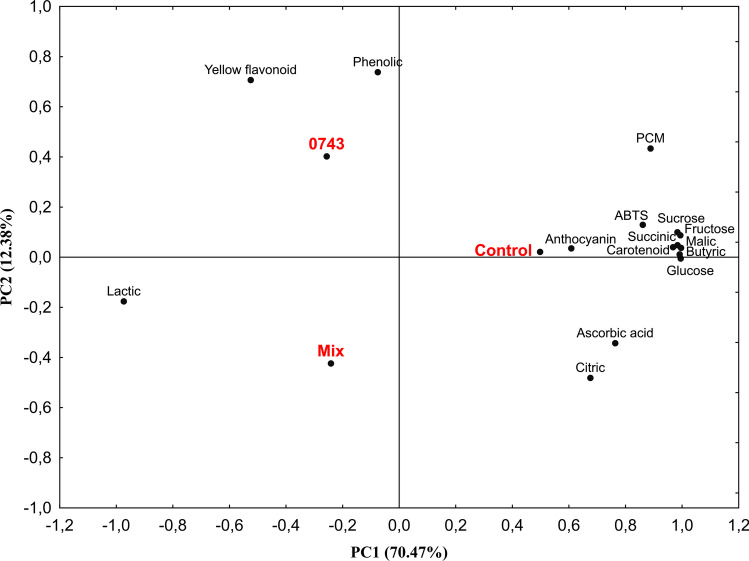
The PCA was obtained using data from unfermented and fermented passion fruit juices’ sugars, organic acids, and bioactive compounds data. The first three components had eigenvalues greater than 1, indicating that they should be interpreted (Kaiser criterion). The first principal component (PC1) explained 70.47% of the variability contained in the original variables, whereas the second (PC2) and third (PC3) principal components explained 12.38 and 7.87%, respectively, counting 90.72% of the total variability (Fig. 3). However, as the total variability of PC1 and PC2 was greater than 80%, PC3 was disregarded.
Fig. 3.
Principal component analysis of sugars, organic acids, and bioactive compounds of unfermented and fermented passion fruit juices. Control, unfermented juice; 0743, passion fruit juice fermented by L. plantarum CCMA 0743; Mix, passion fruit juice fermented by L. plantarum CCMA 0743 and L. paracasei LBC-81; ABTS, 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonate; PCM, phosphomolybdenum complex method
Hence, attributes that showed absolute loading values (> 0.700) of each feature were considered significant. In PC1, carotenoid, sugars, succinic, butyric, and malic acids, followed by ascorbic acid’s antioxidant activity, ascorbic acid, were positively correlated with unfermented treatment, while lactic acid was negatively affected correlated. This result can be observed in Fig. 3, where fermented juices were on the negative side, and unfermented juice was on the positive side of PC1. On the other hand, the juice fermented by L. plantarum CCMA 0743 was positively correlated with yellow flavonoid and phenolic compounds in the positive side of PC2 and the negative side of PC1.
Discussion
In recent years, the use of fruit juices as substrates for Lactobacillus and emended genera growth has been investigated [6, 13, 37]. The present study obtained high viable cell counts (above 8 Log CFU/mL), effective doses usually indicated for probiotic purposes [38], for the three evaluated functional cultures in the different fruit juices. However, there were differences in fermentation time to reach desired viable cells in the three evaluated fruit juices since they have different profiles of fermentable sugars (Fig. 2A). In addition to the chemical composition of juices, the nutrients availability, the presence and generation of inhibitory compounds, and the strains’ ability to adapt to the stress condition imposed by the fruit juice may be related to the obtained variable fermentation parameters [13]. As a result of fermentation, pH reduction by organic acid production occurs due to the microbial metabolism of carbohydrates [39]. Thereby, pH value can be used as an indicator of the fermentation progress. The most significant decrease in pH values during fermentation was registered between 6 and 12 h, reaching pH values close to 4.0 in acerola and passion fruit juices. The drop in the pH may be mainly related to lactic acid production, which reaches a concentration above 5.0 g/L after 18 h of fermentation. This pH result is similar to those previously reported by Costa et al. [39] evaluating pineapple juice fermented by Lactobacillus casei NRRL B-442. In the present study, the highest viable cell counts and minimal pH values were achieved at 18 h of fermentation in passion fruit juice inoculated with single and mixed L. plantarum CCMA 0743 culture. In addition to fermentative capacity in the juices, this strain has demonstrated probiotic properties [28], which may be an important option for food industry applications.
The carbohydrate metabolism of heterofermentative LAB occurs via the phosphoketolase pathway. However, most heterofermentative LAB grows poorly with glucose as the only carbon source. To increase the ATP yield of the pathway, heterofermentative LAB use as a strategy for the phosphorolytic cleavage of disaccharides and alternative electron acceptors to convert acetyl-phosphate into acetate instead of ethanol [40], which can be explained by the more significant sucrose hydrolysis observed in the present study. The three evaluated starter cultures showed the ability to metabolize sucrose, glucose, and fructose. However, the availability of carbohydrates in the different substrates strongly influenced carbon source utilization by the microorganism, as reported by Ricci et al. [19]. In parallel, the greater capacity to reduce sucrose levels by the L. plantarum CCMA 0743 strain in passion fruit juice (Fig. 1) makes it more advantageous than the commercial culture L. paracasei LBC-81 since the low-calorie food product market is in continuous increase. Moreover, this reduction is quite interesting from a nutritional perspective since the excessive consumption of this disaccharide is related to some metabolic diseases [41].
Lactic acid bacteria metabolize carbon sources via fermentation, leading to the formation or degradation of organic acids [21]. Among the various LAB, L. plantarum and L. paracasei species are facultative heterofermentative lactobacilli groups [42], producing lactic acid and others as final products. Nevertheless, lactic acid is the primary fermentation final product reported in other studies [8, 19]. Malic acid can be converted into lactic acid by malolactic fermentation and is associated with strain adaptation to the acid environment [19]. L. paracasei and L. plantarum species may utilize citric acid to form acetic acid and lactic acid under the catalysis of citrate lyase [21]. The increase in acetic acid and citric acid in acerola juice inoculated with L. paracasei LBC-81 (Table 1) may be related to the action of citrate lyase. Normally, lactobacilli metabolize succinate from citrate via the reductive tricarboxylic acid (TCA) pathway. Contrarily, succinic acid was almost totally consumed in most samples (Table 1). Our results corroborate with Lee et al. [43], and the data likely indicated that acidic conditions are inhibitory to one or more enzyme(s) that would lead to succinate production through the oxidative TCA cycle [44]. Conversion from lactate to butyrate is associated with low energy supply or when bacteria use some prebiotic energy sources [45]. However, butyric acid can be used as a precursor to aromatic compounds [46]. In the present study, strains produced and consumed butyric acid depending on the substrate, as observed, respectively, in jelly palm and passion fruit juices (Table 1). These findings suggest that fruit substrate composition influences microbial metabolism. Other studies using different substrates and single or mixed LAB strains as starter cultures also described variable organic acid profiles during fermentation [6, 14]. The higher availability of fermentable sugars associated with some organic acids makes the passion fruit juice an interesting substrate for LAB fermentation.
Bioactive compounds are phytochemicals secondary metabolites synthesized by plants with functional properties and are responsible for fruits’ color, flavor, and odor [15]. Therefore, the balanced consumption of fruit juices offers properties that promote good health and reduce the risk of disease [5]. LAB is recognized by its ability to produce hydrolytic enzymes, increasing phytochemical concentration [16]. However, antioxidant activity and carotenoid content decreased after passion fruit juice fermentation (Table 2). This reduction can probably be attributed to the pasteurization process (a process not carried out in unfermented juice) since heat treatment is related to the degradation of antioxidant compounds [47].
On the other hand, the production of approximately 3.00 µg/mL of yellow flavonoids by a single L. plantarum CCMA 0743 culture was observed. It is known that flavonoids possess several important biological activities, such as antioxidant properties, anti-inflammatory, antiulcer, antiviral, anti-cancer, anti-diabetic, and cytotoxic effects [48]. There are several studies of bioactive compounds in LAB-fermented fruit juices, with no predictable trend of change. According to Ogrodowczyk and Drabińska [1], the variations are associated with the analysis of different fermentation media, cultures, process conditions, and assays used to measure bioactive compounds.
Conclusions
In single or mixed cultures, the L. plantarum CCMA 0743 and L. paracasei LBC-81 strains could grow in high concentrations (above 8.0 Log CFU/mL) on all substrates evaluated; however, the population was obtained at different times of fermentation. Furthermore, the different fermentation showed different profiles of sugars and acid metabolisms. The passion fruit showed high sugars source among the juices, and consequently, higher viable cell counts and low pH value after 18 h of fermentation. Moreover, fermentation using L. plantarum CCMA 0743 as the starter culture impacted the flavonoid content and consumed exceptional levels of sugars in passion fruit juice. Therefore, L. plantarum CCMA 0743 was suitable to produce fermented passion fruit beverages. Hence, applying single or mixed LAB strains in passion fruit juice results from a beverage with proper bioactive and chemical properties, and it can be used as an alternative to dairy products. However, it is still necessary to assess the impacts of fermentation on the product’s shelf life and changes in volatile aromatic compounds and sensory profiles.
Acknowledgements
The authors thank the following Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brasil (CNPQ), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author contribution
Hugo Calixto Fonseca: conceptualization, methodology, investigation, formal analysis, data curation, writing — original draft preparation. Dirceu de Souza Melo and Cíntia Lacerda Ramos: writing — review and editing, visualization, supervision. Disney Ribeiro Dias and Rosane Freitas Schwan: supervision, writing — reviewing and editing, project administration, funding acquisition.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Consent to participate
The corresponding author states that all authors agreed to participate in this manuscript on behalf of all authors.
Consent for publication
On behalf of all authors, the corresponding author states that all authors agreed to publish this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ogrodowczyk A, Drabińska N. Crossroad of tradition and innovation — the application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products—a review. Polish J Food Nutr Sci. 2021;71:107–134. [Google Scholar]
- 2.Valero-Cases E, Cerdá-Bernad D, Pastor JJ, Frutos MJ. Nondairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients. 2020 doi: 10.3390/nu12061666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh AJ, Hill C, Ross RP, Cotter PD. Fermented beverages with health-promoting potential: past and future perspectives. Trends Food Sci Technol. 2014;38:113–124. [Google Scholar]
- 4.Pimentel TC, da Costa WKA, Barão CE, Rosset M, Magnani M. Vegan probiotic products: a modern tendency or the newest challenge in functional foods. Food Res Int. 2021 doi: 10.1016/j.foodres.2020.110033. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj RL, Nandal U, Pal A, Jain S. Bioactive compounds and medicinal properties of fruit juices. Fruits. 2014;69:391–412. [Google Scholar]
- 6.Hashemi SMB, Jafarpour D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: chemical composition, antioxidant activity and sensorial properties. Lwt. 2020;131:109803. [Google Scholar]
- 7.Ricci A, Cirlini M, Levante A, Dall’Asta C, Galaverna G, Lazzi C. Volatile profile of elderberry juice: effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res Int. 2018;105:412–422. doi: 10.1016/j.foodres.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Cirlini M, Ricci A, Galaverna G, Lazzi C. Application of lactic acid fermentation to elderberry juice: changes in acidic and glucidic fractions. Lwt. 2020;118:108779. [Google Scholar]
- 9.Kaprasob R, Kerdchoechuen O, Laohakunjit N, Sarkar D, Shetty K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017;59:141–149. [Google Scholar]
- 10.Roberts D, Reyes V, Bonilla F, Dzandu B, Liu C, Chouljenko A, Sathivel S. Viability of Lactobacillus plantarum NCIMB 8826 in fermented apple juice under simulated gastric and intestinal conditions. Lwt. 2018;97:144–150. [Google Scholar]
- 11.Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. [Google Scholar]
- 12.Zhao MN, Zhang F, Zhang L, Liu BJ, Meng XH. Mixed fermentation of jujube juice (Ziziphus jujuba Mill.) with L. rhamnosus GG and L. plantarum-1: effects on the quality and stability. Int J Food Sci Technol. 2019;54:2624–2631. [Google Scholar]
- 13.Espirito-Santo AP, Carlin F, Renard CMGC. Apple, grape or orange juice: which one offers the best substrate for lactobacilli growth?—a screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res Int. 2015;78:352–360. doi: 10.1016/j.foodres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Lizardo RCM, Cho HD, Won YS, Il SK. Fermentation with mono- and mixed cultures of Lactobacillus plantarum and L. casei enhances the phytochemical content and biological activities of cherry silverberry (Elaeagnus multiflora Thunb.) fruit. J Sci Food Agric. 2020;100:3687–3696. doi: 10.1002/jsfa.10404. [DOI] [PubMed] [Google Scholar]
- 15.Septembre-Malaterre A, Remize F, Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res Int. 2018;104:86–99. doi: 10.1016/j.foodres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Kwaw E, Ma Y, Tchabo W, Apaliya MT, Wu M, Sackey AS, Xiao L, Tahir HE. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Wen J-J, Hu J-L, Nie Q-X, Chen H-H, Nie S-P, Xiong T, Xie M-Y. Momordica charantia juice with Lactobacillus plantarum fermentation: chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019;29:62–72. [Google Scholar]
- 18.Multari S, Carafa I, Barp L, Caruso M, Licciardello C, Larcher R, Tuohy K, Martens S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrussinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. Lwt. 2020;125:109205. [Google Scholar]
- 19.Ricci A, Cirlini M, Maoloni A, Del Rio D, Calani L, Bernini V, Galaverna G, Neviani E, Lazzi C. Use of dairy and plant-derived lactobacilli as starters for cherry juice fermentation. Nutrients. 2019;11:1–14. doi: 10.3390/nu11020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Li T, Qi J, Jiang T, Xu H, Lei H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. Lwt. 2020;122:109064. [Google Scholar]
- 21.Papadimitriou K, Alegría Á, Bron PA, et al. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev. 2016;80:837–890. doi: 10.1128/MMBR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva LMR, De Figueiredo EAT, Ricardo NMPS, Vieira IGP, De Figueiredo RW, Brasil IM, Gomes CL. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Rufino M do SM, Alves RE, de Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. [Google Scholar]
- 24.Magalhaes HM, Brandao TM, Stracieri J, de Jesus HF, Mendes DST, Pasqual M. Evaluating chemical composition of Butia capitata pulp among various populations and locations using multivariate analysis. African J Biotechnol. 2017;16:1902–1910. [Google Scholar]
- 25.Pereira MC, Steffens RS, Jablonski A, Hertz PF, Rios A de O, Vizzotto M, Flôres SH. Characterization, bioactive compounds and antioxidant potential of three Brazilian fruits. J Food Compos Anal. 2013;29:19–24. [Google Scholar]
- 26.Ramaiya SD, Bujang JS, Zakaria MH, King WS, Shaffiq Sahrir MA. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J Sci Food Agric. 2013;93:1198–1205. doi: 10.1002/jsfa.5876. [DOI] [PubMed] [Google Scholar]
- 27.Freire AL, Ramos CL, da Costa Souza PN, Cardoso MGB, Schwan RF. Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int J Food Microbiol. 2017;248:39–46. doi: 10.1016/j.ijfoodmicro.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca HC, Melo DS, Ramos CL, Dias DR, Schwan RF (2020) Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob Proteins. 10.1007/s12602-020-09659-2 [DOI] [PubMed]
- 29.Szutowska J, Rybicka I, Pawlak-Lemańska K, Gwiazdowska D. Spontaneously fermented curly kale juice: microbiological quality, nutritional composition, antioxidant, and antimicrobial properties. J Food Sci. 2020;85:1248–1255. doi: 10.1111/1750-3841.15080. [DOI] [PubMed] [Google Scholar]
- 30.De Souza VR, Pereira PAP, Queiroz F, Borges SV, Carneiro JDDS. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012;134:381–386. [Google Scholar]
- 31.Medina MB. Determination of the total phenolics in juices and superfruits by a novel chemical method. J Funct Foods. 2011;3:79–87. [Google Scholar]
- 32.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 33.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 34.Carbonell-Capella JM, Buniowska M, Esteve MJ, Frígola A. Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chem. 2015;184:122–130. doi: 10.1016/j.foodchem.2015.03.095. [DOI] [PubMed] [Google Scholar]
- 35.Francis FJ. Analysis of anthocyanins. Anthocyanins as food color. 1982 doi: 10.1016/b978-0-12-472550-8.50011-1. [DOI] [Google Scholar]
- 36.Suntornsuk L, Gritsanapun W, Nilkamhank S, Paochom A. Quantitation of vitamin C content in herbal juice using direct titration. J Pharm Biomed Anal. 2002;28:849–855. doi: 10.1016/s0731-7085(01)00661-6. [DOI] [PubMed] [Google Scholar]
- 37.Hashemi SMB, Mousavi Khaneghah A, Barba FJ, Nemati Z, Sohrabi Shokofti S, Alizadeh F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: chemical composition, antioxidant and antibacterial activities. J Funct Foods. 2017;38:409–414. [Google Scholar]
- 38.Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME, Tremblay A, Ouwehand AC. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front Microbiol. 2020;11:1–9. doi: 10.3389/fmicb.2020.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa MGM, Fonteles TV, De Jesus ALT, Rodrigues S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: process optimisation and product stability. Food Chem. 2013;139:261–266. doi: 10.1016/j.foodchem.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 40.Gänzle MG. Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci. 2015;2:106–117. [Google Scholar]
- 41.Qi X, Tester RF. Lactose, maltose, and sucrose in health and disease. Mol Nutr Food Res. 2020;64:1–9. doi: 10.1002/mnfr.201901082. [DOI] [PubMed] [Google Scholar]
- 42.Kõll P, Mändar R, Marcotte H, Leibur E, Mikelsaar M, Hammarström L. Characterization of oral lactobacilli as potential probiotics for oral health. Oral Microbiol Immunol. 2008;23:139–147. doi: 10.1111/j.1399-302X.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee PR, Boo CX, Liu SQ. Fermentation of coconut water by probiotic strains Lactobacillus acidophilus L10 and Lactobacillus casei L26. Ann Microbiol. 2013;63:1441–1450. [Google Scholar]
- 44.Dudley EG, Steele JL. Succinate production and citrate catabolism by Cheddar cheese nonstarter lactobacilli. J Appl Microbiol. 2005;98:14–23. doi: 10.1111/j.1365-2672.2004.02440.x. [DOI] [PubMed] [Google Scholar]
- 45.Ningegowda MA, Gurudutt PS. In vitro fermentation of prebiotics by Lactobacillus plantarum CFR 2194: selectivity, viability and effect of metabolites on β-glucuronidase activity. World J Microbiol Biotechnol. 2012;28:901–908. doi: 10.1007/s11274-011-0887-z. [DOI] [PubMed] [Google Scholar]
- 46.Chen C, Lu Y, Yu H, Chen Z, Tian H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019;27:30–36. [Google Scholar]
- 47.Schvab M del C, Ferreyra MM, Davies CV, Stefani A, Cayetano MC, Gerard LM, Gonzalez RF. Effects of orange winemaking variables on antioxidant activity and bioactive compounds. Food Sci Technol. 2015;35:407–413. [Google Scholar]
- 48.Karak P. Biological activities of flavonoids: an overview. Int J Pharm Sci Res. 2019;10:1567–1574. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.