Abstract
Objective:
To determine the incidence and worsening of lumbar spine structure and low back pain (LBP) and whether they are predicted by demographic characteristics or clinical characteristics or appendicular joint osteoarthritis (OA).
Methods:
Paired baseline (2003-2004) and follow-up (2006-2010) lumbar spine radiographs from the Johnston County Osteoarthritis Project were graded for osteophytes (OST), disc space narrowing (DSN), spondylolisthesis, and presence of facet joint OA (FOA). Spine OA was defined as at least mild OST and mild DSN at the same level for any level of the lumbar spine. LBP, comorbidities, and back injury were self-reported. Weibull models were used to estimate hazard ratios and 95% confidence intervals (CI) of spine phenotypes accounting for potential predictors including demographics, clinical characteristics, comorbidities, obesity, and appendicular OA.
Results:
Obesity was a consistent and strong predictor of incidence of DSN (HR=1.80, 95%CI 1.09–2.98), spine OA (HR=1.56, 95%CI 1.01–2.41), FOA (HR=4.99, 95%CI 1.46–17.10), spondylolisthesis (HR=1.87, 95%CI 1.02–3.43), and LBP (HR=1.75, 95%CI 1.19–2.56), and worsening of DSN (HR=1.51, 95%CI 1.09–2.09) and LBP (HR=1.51, 95%CI 1.12–2.06). Knee OA was a predictor of incident FOA (HR=4.18, 95%CI 1.44–12.2). Spine OA (HR=1.80, 95%CI 1.24–2.63) and OST (HR=1.85, 95%CI 1.02–3.36) were predictors of incidence of LBP. Hip OA (HR=1.39, 95%CI 1.04–1.85) and OST (HR=1.58, 95%CI 1.00–2.49) were predictors of LBP worsening.
Conclusion:
Among the multiple predictors of spine phenotypes, obesity was a common predictor for both incidence and worsening of lumbar spine degeneration and LBP.
Chronic low back pain (LBP) impacts over 31 million Americans at any given moment1 and has increased threefold in prevalence over a 10-year period.2 The traditional “gateway” to interventions for LBP is diagnostic clinical imaging.3 This is especially true within the primary care setting, where although plain film radiographs are not generally recommended by guidelines,4 they are nonetheless frequently performed for examining whether lumbar spine structure is linked to LBP.5,6 Improved understanding of the relationship between lumbar spine imaging and LBP is critically important,7-13 as discordance between spine degeneration and LBP may lead to additional tests and referrals, some of which may have questionable benefit.14
Disc space narrowing (DSN) from degeneration of the intervertebral disc, vertebral osteophytes (OST) formation, facet joint osteoarthritis (FOA), and spondylolisthesis are potential sources of nociceptive pain in the lower back. Cross-sectional studies have linked lumbar spine degeneration with demographic and clinical characteristics.7-9,15,16 However, longitudinal community-based studies are sparse, with most including only women, a considerable length of follow-up (approximately 9 years), and limited LBP examination.10,12,13 Muraki et al.,17 using data from a Japanese cohort, identified that sex was a significant predictor of incidence of lumbar spine degeneration; more severe spine degeneration was also a significant predictor of LBP. However, differences between Japanese and US lifestyles may result in different predictors of incidence and worsening of lumbar spine degeneration and LBP.
To our knowledge, no other community-based study within the US has examined the incidence, worsening, and the longitudinal relationship between demographic and clinical characteristics and appendicular joint OA as predictors of radiographic lumbar spine degeneration and LBP within the same cohort. Therefore, our objective was to: 1) describe the incidence and worsening of lumbar spine vertebral OST, DSN, FOA, spondylolisthesis, and LBP, and 2) determine demographic or clinical characteristics and appendicular joint OA predictors of incidence and worsening of lumbar spine degeneration and LBP. We hypothesized that there would be 1) multiple factors predictive of degeneration, 2) multiple factors predictive of LBP, and 3) few (if any) factors predictive of both degeneration and LBP. These hypotheses are driven by previous research which suggests that factors predictive of structural changes are not the same factors as those predictive of LBP.17-19 However, we intentionally posit a general hypothesis as we believe several factors are likely to be predictive of these outcomes.
Patients and Methods
Participants
Details of the sampling strategy and recruitment methods used for the Johnston County Osteoarthritis Project (JoCo OA) are described elsewhere.7,20 This ongoing longitudinal study of OA includes African American (nearly one-third of the cohort) and white participants living in a largely rural county in North Carolina. Civilian, noninstitutionalized residents aged ≥45 years from six townships in Johnston County were enrolled between 1991 and 1998 (n=3187, Original Cohort), and additional residents were enrolled in 2003–2004 (n=1015, Enrichment Cohort). Since the Enrichment Cohort aimed to supplement the sample for African Americans and younger participants, participants enrolled during 2003–2004 tended to be younger (mean age 59.3 vs. 65.8 years) and more likely to be African American (40% vs. 28%) than the Original Cohort participants at first follow-up (1999–2003); the two groups did not differ according to sex.21 Participants in JoCo OA completed follow-up clinical and interview data collection approximately every 5 years, with 1,695 participants seen during the 2006–2010 clinic visit (time point T2). All participants in JoCo OA have provided informed consent for participation, and JoCo OA has been continuously approved by the institutional review boards of the University of North Carolina and the Centers for Disease Control and Prevention in Atlanta, Georgia.
Radiographic Spine Structure
Lumbar spine radiographs were included in the JoCo OA study for participants at the T2 2006–2010 clinic visit (n=1685). There were 819 returning participants at the T3 time point (2013–2015) who completed lumbar spine radiographs. By protocol, women of reproductive age (<50 years) were excluded from having lumbar spine radiographs. Lumbar spine radiographs were performed with the participant lying on his/her left side, a common position for clinical radiographs, with the central beam centered at the lumbar spine. The Burnett Atlas22 was used to grade lumbar spine radiographic features of FOA, DSN, and OST. FOA was graded as absent or present at each lumbar level, while DSN and OST were graded in a semi-quantitative fashion (0=none, 1=mild, 2=moderate, and 3=severe). Spine OA was defined as the presence of at least mild OST and mild DSN at the same vertebral level,23,24 which is similar to studies that define spine degeneration with the Kellgren-Lawrence (K-L) atlas.13,17 Spondylolisthesis was graded based on the translation of the vertebrae relative to the diameter of the affected intervertebral disc space, with 0=no listhesis, 1=≤25%, 2=26%−50%, 3=51%−75%, 4=76%−100%, and 5=>100% translation. All lateral lumbar spine radiographs were graded at each lumbar level by an experienced single bone and joint radiologist (JBR) with an intra-rater reliability of kappa=0.73 for FOA, 0.89 for DSN, and 0.90 for OST.25
LBP
LBP was ascertained at the clinical interview by asking participants to answer “yes” or “no” to the question “On most days do you have pain, aching or stiffness in your lower back?” Those participants who reported “yes” were also asked to quantify the severity of their symptoms as “mild,” “moderate,” or “severe.”
Demographic and Clinical Characteristics
Demographic data were collected by clinical interview and examination, including age, sex, and race (African American/white). Clinical characteristics included self-reports of diabetes, high blood pressure, back injury, and history of cigarette smoking, as well as body mass index (BMI) at the time of interview (calculated from height measured without shoes and weight measured with a balance beam scale).
Appendicular Joint OA
The protocol for conducting appendicular joint OA radiographs has been described in detail elsewhere.26,27 All knee, hip, and hand radiographs were read for K-L28 score by the same radiologist (JBR). Inter-rater and intra-rater reliability have been reported previously with a kappa of 0.86 and 0.89, respectively, for both the hip and knee.26 Hip and knee OA for these analyses was defined as a K-L score of 2-4 in at least one extremity. Hand OA was defined as K-L grade of 2-4 in at least one distal interphalangeal of one extremity with at least two other interphalangeal joints or carpometacarpal joints affected (K-L grade 2-4) across both hands.27
Statistical Analysis
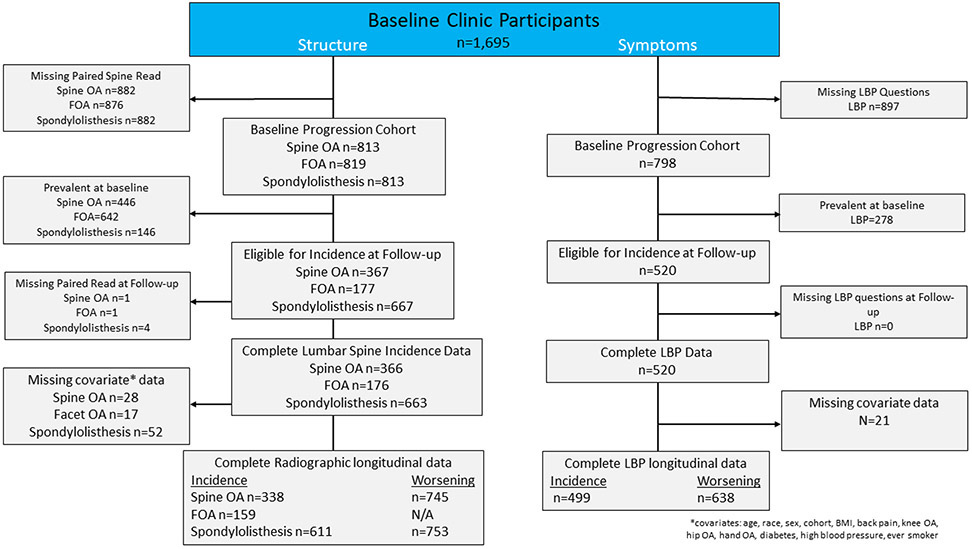
Descriptive statistics were generated for the total sample. Incidence was defined as the absence of a specific radiographic feature at all levels of the lumbar spine at baseline and the presence of that feature at any level of the lumbar spine at follow-up. Worsening was defined as ≥1-unit increase in severity from baseline to follow-up for OST, DSN, and spondylolisthesis. Incidence was measured for all radiographic features, whereas worsening was not measured for FOA since this was measured on a dichotomous scale. LBP was considered incident if the participant reported “no” LBP at baseline but “yes” at follow-up. LBP was considered worsening if there was a ≥ 1-unit increase in severity from baseline status to follow-up. Those with baseline severe LBP were excluded as they were unable to have a 1-unit increase in symptoms. Since the prevalence varied for each spine feature at baseline, the incidence and worsening sample sizes varied accordingly (Figure 1).
Figure 1.
Flow Diagram.
Our outcomes were interval-censored because the exact time of occurrence was not observed, but rather only whether the event occurred between time points. In addition, follow-up intervals were of varying length for JoCo OA participants (on average, 5.5 years). Due to these features of our data, we selected Weibull models to estimate hazard ratios accounting for potential predictors with corresponding 95% confidence intervals (CI). All models reported are multivariable and included demographic variables (age, race, and sex), clinical characteristics (diabetes, high blood pressure, smoking status, and BMI) and appendicular joint OA (knee, hip, and hand OA) predictors simultaneously. When FOA was the outcome, we also included spine OA; likewise, when spine OA, DSN, or OST was the outcome, we included FOA. We explored several pairwise interaction terms between each BMI interval and diabetes and demographic (sex, race, and age) and clinical characteristics (BMI, smoking, and diabetes), but we did not identify any significant interactions. Similar to other studies, we conducted post-hoc stratified analyses of the relationships between upper (L1-3) and lower (L4-5) lumbar levels for spine OA and FOA. Those results are provided in Supplemental Table 1. In addition, we compared differences in outcomes and predictors between participants in this study and those who were lost to follow-up (Supplemental Table 2). Finally, we conducted a simple sensitivity analysis to determine if our definition of spine OA would be influenced by the severity of OST and DSN. All analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC), and alpha was set at 0.05.
Results
Figure 1 illustrates the selection of participants in JoCo OA for both structure and symptoms for these analyses. For our structure outcomes, approximately 50% did not have lumbar spine radiographic data due to being lost to follow-up or failure to return for the clinic follow-up visit. A large proportion had baseline prevalent lumbar spine structural abnormality (54.9% with spine OA and 78.4% with FOA) or LBP (34.8%). Some were missing or refused lumbar spine radiographs at follow-up for lumbar spine structure (n=1 for FOA and n=1 for spine OA and n=6 spondylolisthesis), and some had missing covariate data for lumbar spine structure (n=28 for spine OA and spondylolisthesis, and n=17 for FOA). For our symptom outcome of LBP, approximately 50% had missing LBP data due to being lost to follow-up or failure to return for the clinic follow-up visit. After missing covariate data (n=21), 499 participants were eligible for incidence analysis and 638 were eligible for analysis of worsening.
Table 1 describes the baseline demographic and clinical characteristics as well as appendicular joint OA and lumbar spine degenerative factors. Just over two-thirds (67.9%) were women, and 31.8% were African American. OST and FOA were common at baseline: 84.3% and 78.4%, respectively; DSN and spondylolisthesis occurred less frequently: 26.1% and 17.8%, respectively. A majority (54.7%) of the participants were obese, a small percentage (1.6%) reported a history of back injury, and approximately 35% reported the presence of LBP. The proportions of knee (39.6%), hip (35.5%), and hand OA (32.0%) were similar among participants.
Table 1.
Characteristics of participants at baseline with paired lumbar spine radiographs (N=819)
| n/N | % (95% CI) | |
|---|---|---|
| Radiographic Spine Outcomes | ||
| OST | 691/819 | 84.3 (81.9-86.9) |
| Grade 0 vs 1-3 | ||
| Grade 0 | 128/819 | 15.6 (13.2-18.3) |
| Grade 1 | 511/819 | 62.4 (59.0-65.70 |
| Grade 2 | 154/819 | 18.8 (16.2-21.7) |
| Grade 3 | 26/819 | 3.2 (2.1-4.6) |
| DSN | 579/819 | 26.1 (23.0-29.3) |
| Grade 0 vs 1-3 | ||
| Grade 0 | 240/819 | 29.3 (26.2-32.6) |
| Grade 1 | 364/819 | 44.4 (41.0-47.9) |
| Grade 2 | 214/819 | 26.1 (23.2-9.3) |
| Grade 3 | 1/819 | 0.12 (0.0-0.7) |
| Spondylolisthesis | 146/813 | 17.8 (15.3-20.6) |
| Grade 0 | 673/813 | 82.2 (79.4-84.7) |
| Grade 1 | 139/813 | 17.0 (14.5-19.7) |
| Grade 2 | 5/813 | 0.61 (0.2-1.4) |
| Grade 3 | 2/813 | 0.24 (0.03-0.9) |
| Grade 4 | 0/813 | N/A |
| Grade 5 | 0/813 | N/A |
| Spine OA present | 446/813 | 54.9 (51.4-58.3) |
| FOA present | 642/819 | 78.4 (75.6-81.2) |
| Demographic Predictors | ||
| Age | ||
| <55 | 34/819 | 4.2 (2.9-5.8) |
| 55<65 | 371/819 | 45.3 (41.9-48.9) |
| 65+ | 414/819 | 50.5 (47.1-54.0) |
| Sex - female | 556/819 | 67.9 (65.0-71.0) |
| Race - African American | 260/819 | 31.8 (28.6-35.1) |
| Clinical Characteristic Predictors | ||
| Obesity (BMI≥30) | 448/819 | 54.7 (51.3-8.1) |
| Diabetes | 160/795 | 20.1 (17.3-23.0) |
| High blood pressure | 499/796 | 62.7 (59.3-66.1) |
| Smoking | 410/812 | 50.5 (47.0-54.0) |
| Back injury | 13/811 | 1.6 (1.0-2.0) |
| Back pain | 278/798 | 34.8 (31.5-38.1) |
| None | 520/798 | 65.2 (61.0-69.1) |
| Mild | 96/798 | 12.0 (9.5-15.1) |
| Moderate/severe | 182/798 | 22.8 (19.5-26.6) |
| Appendicular Joint OA Predictors | ||
| Knee | 318/803 | 39.6 (36.2-43.0) |
| Hip | 287/809 | 35.5 (32.2-38.8) |
| Hand | 260/813 | 32.0 (28.8-35.3) |
BMI=body mass index; DSN=disc space narrowing; FOA=facet joint osteoarthritis; N/A=not applicable; OA=osteoarthritis; OST=osteophytes.
The number of participants at risk for incident lumbar spine degeneration is described in Supplemental Table 3. A large proportion of participants developed OST at any level of the lumbar spine (59.0%). The incidences of DSN and spine OA were similar at 38.1% and 31.4%, respectively. The incidences of any FOA and spondylolisthesis were 10.2% and 9.1%, respectively. Approximately 34% of participants had a worsening of OST, 24% a worsening of DSN, and 8% a worsening of spondylolisthesis.
Table 2 describes the demographic predictors for incidence of lumbar spine degeneration in multivariable models including demographic, clinical characteristics, and OA variables as potential predictors simultaneously. Women were more likely to develop spondylolisthesis (HR=2.16, 95% CI 1.07–4.34). Obesity was a risk factor for incidence of DSN (HR=1.80, 95% CI 1.09–2.98), FOA (HR=4.99, 95% CI 1.46–17.10), spine OA (HR=1.56, 95% CI 1.01-2.41) and spondylolisthesis (HR=1.87, 95% CI 1.02–3.43). The presence of diabetes was a protective factor for the incidence of DSN (HR=0.54, 95% CI 0.30–0.97). Smokers were 39% less likely to develop DSN (HR=0.61, 95% CI 0.38–0.98) and 40% less likely to develop spine OA (HR=0.60, 95% CI 0.39–0.91). Knee OA was a risk factor for the incidence of FOA (HR=4.18, 95% CI 1.44–12.2). However, hip and hand OA were not risk factors for radiographic changes in the lumbar spine. In our sensitivity analysis of spine OA coding, we did not identify that the severity of OST or DSN changed the results.
Table 2.
Baseline demographics, clinical characteristics, and appendicular joint OA as predictors of incident lumbar spine degeneration in Weibull models
| OST | DSN | Spine OA | FOA | Spondylolisthesis | |
|---|---|---|---|---|---|
| N=114 | N=220 | N=338 | N=159 | N=611 | |
| Demographics | |||||
| Women vs. men | 2.31 (0.88-6.04) | 1.33 (0.79-2.23) | 1.26 (0.78-2.04) | 0.50 (0.14-1.75) | 2.16 (1.07-4.34) |
| African American vs. white | 1.19 (0.60-2.37) | 0.69 (0.41-1.17) | 0.81 (0.52-1.28) | 0.96 (0.29-3.23) | 1.20 (0.66-2.18) |
| Age 55-65 y vs. age <55 y | 0.41 (0.11-1.58) | 1.05 (0.34-3.23) | 2.51 (0.75-8.42) | 0.69 (0.03-14.9) | 0.67 (0.19-2.41) |
| Age 65+ y vs. age <55 y | 0.38 (0.09-1.55) | 1.24 (0.37-4.10) | 2.17 (0.62-7.58) | 1.09 (0.05-26.1) | 0.96 (0.26-3.59) |
| Clinical Characteristics | |||||
| Obesity | 0.63 (0.31-1.27) | 1.80 (1.09-2.98) | 1.56 (1.01-2.41) | 4.99 (1.46-17.1) | 1.87 (1.02-3.43) |
| Diabetes | 0.51 (0.17-1.57) | 0.54 (0.30-0.97) | 0.71 (0.42-1.18) | 0.82 (0.20-3.35) | 0.69 (0.34-1.39) |
| High blood pressure | 0.80 (0.42-1.50) | 0.76 (0.48-1.21) | 0.81 (0.54-1.22) | 0.51 (0.16-1.56) | 1.26 (0.69-2.29) |
| Smoking | 0.87 (0.49-1.56) | 0.61 (0.38-0.98) | 0.60 (0.39-0.91) | 1.04 (0.35-3.11) | 0.79 (0.46-1.38) |
| Back injury | 0.57 (0.07-4.59) | 0.56 (0.07-4.43) | 0.72 (0.17-3.02) | NR | 1.82 (0.24-14.0) |
| Appendicular Joint OA | |||||
| Knee OA | 1.28 (0.62-2.65) | 0.92 (0.54-1.57) | 0.95 (0.62-1.47) | 4.18 (1.44-12.2) | 0.88 (0.50-1.53) |
| Hip OA | 0.61 (0.34-1.10) | 0.80 (0.48-1.34) | 0.91 (0.59-1.38) | 0.41 (0.13-1.27) | 1.28 (0.74-2.22) |
| Hand OA | 1.02 (0.56-1.84) | 1.16 (0.68-1.97) | 1.31 (0.84-2.02) | 0.58 (0.16-2.09) | 1.20 (0.66-2.19) |
DSN=disc space narrowing; FOA=facet joint osteoarthritis; OA=osteoarthritis; OST=osteophytes; NR= not reported due to small sample size.
Data shown are HR (95% CI). Weibull models include sex, race, age, body mass index, diabetes, high blood pressure, smoking, and back injury, as well as knee, hip, and hand OA predictors simultaneously. Additionally, DSN and spine OA models included FOA presence, and FOA models included spine OA presence.
Table 3 describes the predictors for worsening of lumbar spine degeneration in multivariable models including demographic, clinical characteristics, and OA variables simultaneously as potential predictors. Women had a 46% increased hazard of worsening of DSN (HR=1.46, 95% CI 1.02–2.08). Obesity was a risk factor for worsening of DSN (HR=1.51, 95% CI 1.09–2.09) but a protective factor for vertebral OST worsening (HR=0.75, 95% CI 0.57–0.99). The presence of diabetes was a risk factor for the worsening of vertebral OST (HR=1.38, 95% CI 1.00–1.92).
Table 3.
Baseline demographics, clinical characteristics, and appendicular joint OA as predictors of lumbar spine degeneration worsening in Weibull models
| OST N=730 |
DSN N=754 |
Increased Levels of Spine OA N=745 |
Spondylolisthesis N=753 |
|
|---|---|---|---|---|
| Demographics | ||||
| Women vs. men | 0.85 (0.63-1.13) | 1.46 (1.02-2.08) | 1.22 (0.92-1.62) | 1.93 (0.96-3.87) |
| African American vs. white | 1.04 (0.77-1.40) | 0.79 (0.55-1.13) | 0.91 (0.69-1.21) | 1.19 (0.66-2.13) |
| Age 55-65 y vs. age <55 y | 0.84 (0.41-1.74) | 1.40 (0.55-3.60) | 2.95 (1.07-8.15) | 0.67 (0.19-2.40) |
| Age 65+ y vs. age <55 y | 0.89 (0.43-1.88) | 1.29 (0.49-3.38) | 3.01 (1.08-8.42) | 0.90 (0.24-3.33) |
| Clinical Characteristics | ||||
| Obesity | 0.75 (0.57-0.99) | 1.51 (1.09-2.09) | 1.24 (0.96-1.62) | 1.77 (0.97-3.22) |
| Diabetes | 1.38 (1.00-1.92) | 0.85 (0.56-1.28) | 0.77 (0.56-1.08) | 0.81 (0.40-1.62) |
| High blood pressure | 0.88 (0.67-1.16) | 0.88 (0.64-1.20) | 1.13 (0.87-1.47) | 1.15 (0.64-2.04) |
| Smoking | 1.00 (0.76-1.30) | 1.08 (0.79-1.47) | 0.95 (0.74-1.23) | 0.77 (0.44-1.35) |
| Back injury | 0.58 (0.18-1.83) | 0.61 (0.15-2.49) | 0.59 (0.19-1.85) | 1.41 (0.19-10.6) |
| Appendicular Joint OA | ||||
| Knee OA | 0.97 (0.74-1.28) | 0.84 (0.61-1.16) | 1.00 (0.78-1.30) | 0.83 (0.48-1.45) |
| Hip OA | 0.91 (0.69-1.19) | 0.72 (0.52-1.00) | 0.77 (0.60-1.01) | 1.11 (0.64-1.91) |
| Hand OA | 0.98 (0.73-1.32) | 1.06 (0.75-1.48) | 1.10 (0.84-1.43) | 1.05 (0.58-1.90) |
DSN=disc space narrowing; OA=osteoarthritis; OST=osteophytes.
Data shown are HR (95% CI). Weibull models include sex, race, age, body mass index, diabetes, high blood pressure, smoking, and back injury, as well as knee, hip, and hand OA predictors simultaneously.
Table 4 describes the predictors for incidence and worsening of low back pain in multivariable models including demographic, clinical characteristics, and OA variables simultaneously as potential predictors. Obesity was a strong risk factor of both LBP incidence (HR=1.75, 95% CI 1.19–2.56) and worsening (HR=1.51, 95% CI 1.12–2.06). Having mild or moderate LBP at baseline was a predictor for LBP worsening. Participants with hip OA at baseline were 39% more likely to progress in LBP severity (HR=1.39, 95% CI 1.04–1.85). Baseline OST and spine OA were predictors of incident LBP (HR=1.85, 95% CI 1.02–3.36; HR=1.80, 95% CI 1.24–2.63, respectively). The presence of vertebral OST at baseline was also a predictor of worsening LBP (HR=1.58, 95% CI 1.00–2.49).
Table 4.
Baseline demographics, clinical characteristics, appendicular joint OA, and lumbar spine degeneration as predictors of incident and worsening low back pain in Weibull Models
| Low Back Pain Incidence (None to Mild or Greater) N=509 |
Low Back Pain Worsening (1-Unit Change From Baseline; Excludes Severe at Baseline) N=652 |
|
|---|---|---|
| Demographics | ||
| Women vs. men | 1.19 (0.79-1.79) | 1.35 (0.97-1.87) |
| African American vs. white | 0.66 (0.43-1.01) | 0.81 (0.58-1.12) |
| Age 55-65 y vs. age <55 y | 0.70 (0.26-1.85) | 0.68 (0.33-1.43) |
| Age 65+ y vs. age <55 y | 0.55 (0.20-1.50) | 0.66 (0.31-1.42) |
| Clinical Characteristics | ||
| Obesity | 1.75 (1.19-2.56) | 1.51 (1.12-2.06) |
| Diabetes | 0.68 (0.40-1.16) | 0.92 (0.64-1.32) |
| High blood pressure | 1.33 (0.91-1.95) | 1.30 (0.96-1.76) |
| Smoking | 0.69 (0.47-1.01) | 0.83 (0.62-1.11) |
| Low back injury | 1.85 (0.42-8.10) | 2.39 (0.69-8.27) |
| Mild low back pain to moderate and severe | NR | 5.35 (3.62-7.92) |
| Moderate low back pain to severe | NR | 2.26 (1.55-3.29) |
| Appendicular Joint OA | ||
| Knee | 1.40 (0.97-2.02) | 1.15 (0.86-1.53) |
| Hip | 1.08 (0.75-1.57) | 1.39 (1.04-1.85) |
| Hand | 1.19 (0.79-1.77) | 0.97 (0.71-1.33) |
| Spine Degeneration | ||
| OST | 1.85 (1.02-3.36) | 1.58 (1.00-2.49) |
| DSN | 1.52 (0.99-2.33) | 1.11 (0.80-1.53) |
| Spine OA | 1.80 (1.24-2.63) | 1.27 (0.95-1.70) |
| FOA | 1.06 (0.69-1.63) | 1.05 (0.73-1.51) |
| Spondylolisthesis | 1.14 (0.71-1.82) | 1.12 (0.78-1.62) |
BMI=body mass index; DSN=disc space narrowing; NR= not reported due to small sample size; OA=osteoarthritis; OST=osteophytes.
Data shown are HR (95% CI). Weibull models include sex, race, age, body mass index, diabetes, high blood pressure, smoking, and back injury, as well as knee, hip, and hand OA predictors simultaneously.
The results for the post-hoc stratified analysis of the incidence of upper and lower lumbar spine OA and FOA are described in Supplemental Table 1. Findings were similar to our primary analysis of the lumbar spine degeneration features. In addition, African-Americans were 59% less likely (HR=0.41, 95% CI 0.24–0.72) to have incident upper lumbar spine OA with symptoms and 53% less likely (HR=0.47, 95% CI 0.28–0.79) to have lower FOA. Those with self-reported high blood pressure were 59% more likely (HR=1.59, 95% CI 1.01–2.51) to have incident upper spine OA.
Discussion
We determined the incidence and worsening of radiographic lumbar spine features commonly used in clinical practice to help better delineate relationship between spine structure and LBP. Obesity was a strong predictor for nearly all lumbar spine features and LBP. OST and spine OA had prognostic value as demonstrated by their prediction of LBP incidence and worsening. These findings may have important implications for clinical practice, especially where diagnostic imaging is being used for better understanding the relationship between lumbar spine degeneration and LBP.
We identified that obesity was a predictor of both the incidence and worsening of LBP. Muraki and colleagues17 did not find obesity to be a significant predictor of incident LBP; however, their Japanese study population had a considerably lower average BMI compared to ours. Since our cohort had a large proportion of participants considered to be obese, these findings may not be generalizable to other community-based studies with a much lower proportion of obese participants. For radiographic features commonly examined during initial visits for LBP, we found that spine OA was a significant risk factor for incident LBP, similar to findings of others.17 The baseline presence of at least mild OST was a significant predictor of LBP incidence and worsening. Although OST is common among the population, the presence of OST among those with LBP may be an indicator of continued or worse mechanical LBP. We also identified hip OA as a significant predictor of LBP worsening. Some have supported a “hip-spine syndrome” whereby influences from the presence of hip symptoms or OA have resulted in LBP.29-34 To our knowledge, this is the first study to report a longitudinal relationship between baseline hip OA and LBP worsening.
Obesity was a strong and consistent predictor for the incidence of DSN, spine OA, FOA, and spondylolisthesis, and only for worsening of DSN. Muraki et al.17 found that BMI was a significant risk factor for lumbar spine degeneration. However, our study also includes lumbar spine FOA and spondylolisthesis, which were not included in their study. Our findings support cross-sectional studies from both our group and others that indicate an association between obesity and FOA.7,14,35 Due to the older age of our sample in JoCo OA, over 75% of participants already had FOA at baseline. As such, the small number of incident cases for FOA limits these findings. The finding that obesity was a protective factor for the worsening of OST is difficult to explain. One reason may be related to the large number of prevalent OST at baseline that limited the number at risk during follow-up. As such, this sample may be a more “resistant” group to OST development. Similar to our findings, a Framingham Study analysis36 determined a higher proportion of women developed spondylolisthesis.
We identified some interesting relationships between diabetes and smoking and the incidence and worsening of DSN. Smoking has been associated with intervertebral disc disease in twin studies,37 while other community-based studies17-19 have found no significant association with spine degeneration. Our study, however, identified that smokers were significantly less likely to have incident DSN. It has been suggested that inadequate statistical control of BMI may be one factor that leads to this inverse association; however, this was not the case in our study. The inverse association of smoking with DSN is concordant with the inverse association of smoking with knee OA found in many studies.38,39 Self-reported presence of diabetes was identified as a significant risk factor for worsening of OST. This may be related to impaired glucose tolerance, which has been linked to ankylosing hyperostosis in the spine.40 However, we also identified self-reported diabetes as inversely associated with incidence of DSN. We explored any potential differences between those participants self-reporting insulin use for diabetes treatment, based on the study by Shirinsky and Shirinsky41 . In this prior study, they found that medication-treated diabetes was protective for knee OA progression. However, we did not identify any significant differences between these two groups. It is possible that other anti-diabetic medications—such as metformin, which has both antioxidant and anti-senolytic effects—might help explain the inverse association of diabetes and lumbar spine degeneration. The relationship between diabetes and DSN does appear to be focused more in the upper lumbar spine than the lower lumbar spine. A biologic rationale is not known for the BMI-independent inverse association of diabetes and smoking with DSN, but may be related to potential underlying nicotine effects or cellular mechanisms.39 In addition, there may be other non-biological reasons that could explain these findings. We did not adjust our findings for multiple comparisons, and several of these findings are nearly statistically significant by our established threshold. In addition, while our models included several potential predictors simultaneously, we did not include all potential confounders that might influence our findings, and therefore we cannot rule out the potential for residual confounding.
Our study has several strengths but is not without limitations. Lateral lumbar spine radiographs could lead to non-differential misclassification of FOA status since lateral views may underestimate the occurrence of FOA. However, prevalence estimates of FOA based on lateral spine radiography7 are similar to those previously reported based on computed tomography scans.35 The JoCo OA protocol excluded women of childbearing age from having lumbar spine radiographs to prevent unnecessary radiation exposure; therefore, the results may not be generalizable to this subgroup. While the average length of follow-up of 5.5 years is ideal for examining degeneration, it limits the examination of incident and worsening of LBP since it may be quite variable over a long period. We measured the presence and severity of LBP but not how LBP interfered with daily activity. In addition, our question for LBP also includes pain, aching, and stiffness, which may overestimate the true incidence of LBP since stiffness may be present without pain. We did not include lower back-specific functional measures or account for previous LBP episodes, widespread pain, or psychosocial factors that are known to be associated with incident or progressive LBP.42 Our study had over 40% loss to follow-up of cohort participants, which may limit generalizability since those lost to follow-up were more likely to be older and to have a BMI less than 30, self-reported diabetes, high blood pressure, and appendicular joint OA (knee, hip and hand OA). However, the primary reason for loss to follow-up was participant death. The loss to follow-up we experienced over this time frame may influence the direction and strength of our estimates relative to the true population values. This may be the case for some estimates without clear statistical significance such as the relationship we identified between DSN and diabetes. In addition, there was a high prevalence of OST and FOA among participants based upon the average age of the cohort. As such, future studies should consider cohorts with younger participants to enhance generalizability.
In conclusion, our study is unique in that it provides estimates of lumbar spine incidence and worsening of radiographic features commonly used in clinical practice to examine spine health. The predictors identified in this study are commonly used in routine primary care for participants with LBP. The finding that obesity, existing lumbar spine degeneration, and appendicular hip OA are predictive of future LBP may assist in the ongoing efforts to decrease LBP in the community.
Supplementary Material
Significance and Innovations.
This longitudinal study of 819 participants over an average of 5.5 years found that obesity and appendicular joint osteoarthritis were significant predictors of the incidence and worsening of both lumbar spine degeneration and low back pain.
Since obesity is a modifiable risk factor, efforts to decrease it could lessen the development and worsening of lumbar spine degeneration and low back pain.
Acknowledgments
Funding/support: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R01AR071440 (Goode, Cleveland, George, Schwartz, Kraus, Jordan, Golightly), R01AR075399 (Goode, George, Kraus, DeFrate) and R01AR07800 (DeFrate and Goode). Dr Golightly was supported by R01AR067743. Dr Kraus is supported by the National Institute on Aging (NIA) P30-AG-028716. The Johnston County Osteoarthritis Project is supported in part by cooperative agreements S043, S1734, and S3486 from the Centers for Disease Control and Prevention (CDC)/Association of Schools of Public Health; the NIAMS Multipurpose Arthritis and Musculoskeletal Disease Center grant 5-P60-AR30701; the NIAMS Multidisciplinary Clinical Research Center grant 5 P60 AR49465-03; and NIAMS Core Centers for Clinical Research grant 1P30AR072580-01A1. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC, NIA, or NIAMS.
Footnotes
Conflict of interest disclosures: All authors disclose they have no financial or personal relationships with other people or organizations that could potentially and inappropriately bias their work and conclusions.
References
- 1.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvik JG, Comstock BA, James KT, Avins AL, Bresnahan BW, Deyo RA, et al. Lumbar Imaging With Reporting Of Epidemiology (LIRE)--Protocol for a pragmatic cluster randomized trial. Contemp Clin Trials. 2015;45(Pt B):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791–2803. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Jarvik JG, Chou R. Low back pain in primary care. BMJ. 2014;349:g4266. [DOI] [PubMed] [Google Scholar]
- 7.Goode AP, Marshall SW, Renner JB, Carey TS, Kraus VB, Irwin DE, et al. Lumbar spine radiographic features and demographic, clinical, and radiographic knee, hip, and hand osteoarthritis. Arthritis Care Res (Hoboken). 2012;64:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pye SR, Reid DM, Smith R, Adams JE, Nelson K, Silman AJ, et al. Radiographic features of lumbar disc degeneration and self-reported back pain. J Rheumatol. 2004;31:753–758. [PubMed] [Google Scholar]
- 9.de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976). 2010;35:531–536. [DOI] [PubMed] [Google Scholar]
- 10.Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44:571–585. [DOI] [PubMed] [Google Scholar]
- 11.Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraki S, Oka H, Akune T, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of radiographic lumbar spondylosis and its association with low back pain in elderly subjects of population-based cohorts: the ROAD study. Ann Rheum Dis. 2009;68:1401–1406. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23–44. [DOI] [PubMed] [Google Scholar]
- 15.Pye SR, Reid DM, Adams JE, Silman AJ, O'Neill TW. Influence of weight, body mass index and lifestyle factors on radiographic features of lumbar disc degeneration. Ann Rheum Dis. 2007;66:426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pye SR, Reid DM, Lunt M, Adams JE, Silman AJ, O'Neill TW. Lumbar disc degeneration: association between osteophytes, end-plate sclerosis and disc space narrowing. Ann Rheum Dis. 2007;66:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muraki S, Akune T, Oka H, Ishimoto Y, Nagata K, Yoshida M, et al. Incidence and risk factors for radiographic lumbar spondylosis and lower back pain in Japanese men and women: the ROAD study. Osteoarthritis Cartilage. 2012;20:712–718. [DOI] [PubMed] [Google Scholar]
- 18.Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum. 2003;48:3112–3117. [DOI] [PubMed] [Google Scholar]
- 19.Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. I. Clinical findings. Ann Rheum Dis. 1991;50:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 21.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB, et al. Associations of occupational tasks with knee and hip osteoarthritis: the Johnston County Osteoarthritis Project. J Rheumatol. 2010;37:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett SJ HD, Cooper C, Spector TD. A Radiographic Atlas of Osteoarthritis. London: Springer-Verlag; 1994. [Google Scholar]
- 23.Goode AP, Cleveland RJ, George SZ, Kraus VB, Schwartz TA, Gracely RH, et al. Different phenotypes of osteoarthritis in the lumbar spine reflected by demographic and clinical characteristics: the Johnston County Osteoarthritis Project. Arthritis Care Res (Hoboken). 2020;72:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson AE, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM. Differences in multijoint radiographic osteoarthritis phenotypes among African Americans and Caucasians: the Johnston County Osteoarthritis project. Arthritis Rheum. 2011;63:3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goode AP, Nelson AE, Kraus VB, Renner JB, Jordan JM. Biomarkers reflect differences in osteoarthritis phenotypes of the lumbar spine: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2017;25:1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–250. [DOI] [PubMed] [Google Scholar]
- 27.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, et al. The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage. 2007;15:120–127. [DOI] [PubMed] [Google Scholar]
- 28.Kellgren JH. The epidemiology of rheumatic diseases. Ann Rheum Dis. 1964;23:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks GE, Sions JM, Velasco TO. Hip symptoms, physical performance, and health status in older adults with chronic low back pain: a preliminary investigation. Arch Phys Med Rehabil. 2018;99:1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner DK, Fang M, Gentili A, Kochersberger G, Marcum ZA, Rossi MI, et al. Deconstructing chronic low back pain in the older adult--step by step evidence and expert-based recommendations for evaluation and treatment: part I: Hip osteoarthritis. Pain Med. 2015;16:886–897. [DOI] [PubMed] [Google Scholar]
- 31.Sembrano JN, Polly DW Jr. How often is low back pain not coming from the back? Spine (Phila Pa 1976). 2009;34:E27–32. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Galim P, Ben-Galim T, Rand N, Haim A, Hipp J, Dekel S, et al. Hip-spine syndrome: the effect of total hip replacement surgery on low back pain in severe osteoarthritis of the hip. Spine (Phila Pa 1976). 2007;32:2099–2102. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh PH, Chang Y, Chen DW, Lee MS, Shih HN, Ueng SW. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213–218. [DOI] [PubMed] [Google Scholar]
- 34.Chimenti PC, Drinkwater CJ, Li W, Lemay CA, Franklin PD, O'Keefe RJ. Factors associated with early improvement in low back pain after total hip arthroplasty: a multi-center prospective cohort analyses. J Arthroplasty. 2016;31:176–179. [DOI] [PubMed] [Google Scholar]
- 35.Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O'Donnell CJ, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976). 2008;33:2560–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kauppila LI, Eustace S, Kiel DP, Felson DT, Wright AM. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976). 1998;23:1868–1873. [DOI] [PubMed] [Google Scholar]
- 37.Battie MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976). 1991;16:1015–1021. [PubMed] [Google Scholar]
- 38.Kong L, Wang L, Meng F, Cao J, Shen Y. Association between smoking and risk of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2017;25:809–816. [DOI] [PubMed] [Google Scholar]
- 39.Felson DT, Zhang Y. Smoking and osteoarthritis: a review of the evidence and its implications. Osteoarthritis Cartilage. 2015;23:331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crisp AJ, Heathcote JG. Connective tissue abnormalities in diabetes mellitus. J R Coll Physicians Lond. 1984;18:132–141. [PMC free article] [PubMed] [Google Scholar]
- 41.Shirinsky IV, Shirinsky VS. Effects of medication-treated diabetes on incidence and progression of knee osteoarthritis: a longitudinal analysis of the Osteoarthritis Initiative data. Rheumatol Int. 2017;37:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg R, Chiarotto A, Enthoven WT, de Schepper E, Oei EHG, Koes BW, et al. Clinical and radiographic features of spinal osteoarthritis predict long-term persistence and severity of back pain in older adults. Ann Phys Rehabil Med. 2020:S1877-0657(20)30153-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.