Abstract
Objective:
To determine if individuals newly diagnosed with opioid use disorder (OUD) who saw a primary care provider (PCP) prior to or on the date of diagnosis had higher rates of medication treatment for OUD (MOUD).
Methods:
Observational study using logistic regression with claims data from Medicaid and a large private insurer in North Carolina from January 2014 to July 2017.
Key Results:
Between 2014 and 2017, the prevalence of diagnosed OUD increased by 47% among Medicaid enrollees and by 76% among the privately insured. Over the same time period, the percent of people with an OUD who received MOUD fell among both groups, while PCP involvement in treatment increased. Of Medicaid enrollees receiving buprenorphine, the percent receiving buprenorphine from a PCP increased from 32% in 2014 to 39% in 2017. Approximately 82% of people newly diagnosed with OUD had a PCP visit in the 12 months before diagnosis in Medicaid and private insurance. Those with a prior PCP visit were not more likely to receive MOUD. Seeing a PCP at diagnosis was associated with a higher probability of receiving MOUD than seeing an emergency provider but a lower probability than seeing a behavioral health specialist or other provider type.
Conclusions:
People newly diagnosed with OUD had high rates of contact with PCPs prior to diagnosis, supporting the importance of PCPs in diagnosing OUD and connecting people to MOUD. Policies and programs to increase access to MOUD and improve PCPs’ ability to connect people to evidence-based treatment are needed.
Keywords: Primary care, opioid use disorder, medication assisted treatment
Introduction
Though the benefits of medication treatment for opioid use disorder (MOUD) are well established,1–4 research suggests few people with opioid use disorder (OUD) receive MOUD.5,6 An important contributor to low treatment rates is likely the shortage of providers. Evidence suggests nearly all states lack enough opioid treatment programs (OTPs) and buprenorphine-waivered providers to treat all people with OUD.7
In order to meet the demand for OUD treatment, there have been calls to mobilize primary care providers (PCPs) to offer MOUD.8,9 Models of office-based MOUD by PCPs have been developed and programs have been implemented to train PCPs in MOUD.10 Models of primary care treatment of OUD broadly involve pharmacotherapy with coordination or integration of services to address patients’ psychosocial needs.11–13 These models emphasize pharmacotherapy with buprenorphine over naltrexone, since evidence supporting the use of naltrexone in primary care is more limited.11
Despite efforts to increase primary care provision of buprenorphine, only 7.0% of primary care physicians were waivered to prescribe buprenorphine as of 2018.14,15 Studies have documented many barriers to prescribing buprenorphine among PCPs, including lack of psychosocial support services, time constrains, reimbursement concerns, and lack of confidence.16–18 A prior study in rural Pennsylvania of a primarily Medicaid expansion population found that the majority of Medicaid-enrolled adults with OUD had access to primary care, but that PCPs were seldom involved in diagnosing OUD.19
This study examined whether seeing a PCP prior to or at the time of OUD diagnosis was associated with a higher probability of receiving MOUD compared to seeing other providers. While not causal, this analysis provides insight into how well PCPs are connecting people with OUD to evidence-based treatment. We examined this question in both publicly and privately insured groups. We also described trends in MOUD in these publicly and privately insured populations.
Methods
Data
We accessed Medicaid claims and encounter data from North Carolina through the Carolina Cost and Quality Initiative for individuals with opioid-related diagnoses from January 2014 to July 2018.20 Approximately 18% of North Carolina’s population is covered by Medicaid, which was not expanded under the Affordable Care Act.21 North Carolina’s Medicaid program is currently a fee-for-service program, but has a capitated behavioral health (BH) carve-out wherein BH services are delivered by regional managed care organizations (MCOs). Our data included all claims from fee-for-service Medicaid and encounter data from the MCOs. We excluded individuals who were dually enrolled in Medicare in order to increase observability of pharmaceutical treatments in claims data. For analysis of privately insured individuals, we used claims data from a large private insurer in North Carolina for individuals with opioid-related diagnoses from January 2014 to July 2018. There are a number of dimensions on which those on Medicaid will differ from the privately insured, including expected lower income for Medicaid enrollees because of income thresholds, and a higher proportion female.22 For all analyses we restrict to individuals age 14 or older.
OUD Diagnosis and Treatment
We defined the population with OUD broadly as: (1) individuals with any claim containing an International Classification of Diseases (ICD) code for opioid abuse, dependence, or poisoning; or (2) individuals with any claims for methadone from an OTP or for a buprenorphine formulation for OUD treatment. We did not code individuals receiving naltrexone without an OUD diagnosis in the population with OUDsince naltrexone can also be used for the treatment of alcohol use disorder, but we did include naltrexone as an MOUD treatment for those with an OUD diagnosis. We did not include individuals whose only OUD diagnoses appeared in laboratory claims, since these may represent diagnoses of exclusion for individuals tested for OUD. We included individuals with at least one claim with an administrative diagnosis OUD or opioid poisoning, but note that 89% of our sample receive OUD diagnoses on multiple claims with different dates. Six percent of our sample received diagnosis of opioid poisoning without an OUD diagnosis. These individuals were retained in the sample in order to not inappropriately exclude those with poorer access to care.
When describing yearly trends (Table 1 and Figure 1), we counted all individuals with an OUD diagnosis within a year, as described above. For the remaining analyses, we included only newly observed OUD diagnoses, which we refer to as the index diagnosis. In order to ensure we were capturing new OUD diagnoses, we required that individuals be enrolled in Medicaid or private insurance for 10 out of 12 months before their index diagnosis.
Table 1.
OUD diagnosis and treatment in Medicaid and private insurance
| 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
|
| ||||
| Medicaid enrollment (Age>=14) | 1,390,796 | 1,501,573 | 1,570,787 | 1,653,106 |
| Number of enrollees with an OUD diagnosisa | 23,979 | 32,372 | 38,677 | 41,177 |
| Percent of enrollees with an OUD diagnosis | 1.7% | 2.2% | 2.5% | 2.5% |
| Number of enrollees with OUD who received MOUD | 10,764 | 12,982 | 14,644 | 16,996 |
| Percent of enrollees with an OUD diagnosis who received MOUDb | 45% | 40% | 38% | 41% |
| Buprenorphrine | 27% | 25% | 24% | 27% |
| Methadone | 19% | 16% | 15% | 16% |
| Naltrexone | 0.35% | 0.40% | 0.57% | 0.50% |
| Private enrollment (Age>=14) | 1,655,502 | 1,615,539 | 1,440,835 | 1,549,499 |
| Number of members with an OUD diagnosis | 8,980 | 11,519 | 12,519 | 14,713 |
| Percent of members with an OUD diagnosis | 0.54% | 0.71% | 0.87% | 0.95% |
| Number of enrollees with OUD who received MOUD | 3,772 | 4,492 | 4,632 | 5,738 |
| Percent of members with an OUD diagnosis who received MOUD | 42% | 39% | 37% | 39% |
| Buprenorphrine | 39% | 35% | 32% | 33% |
| Methadone | 1.6% | 2.5% | 2.5% | 2.8% |
| Naltrexone | 1.5% | 2.2% | 3.1% | 3.3% |
We defined the population with OUD broadly as: (1) individuals with any claim containing an International Classification of Diseases (ICD) code for opioid abuse, dependence, or poisoning involving opioids; or (2) individuals with any claims for methadone from an OTP or for a buprenorphine formulation for OUD treatment (see Appendix for codes).
We defined MOUD as the receipt of at least one buprenorphine or naltrexone prescription claim for formulations intended for use in OUD treatment, or as receipt of methadone dispensing services from an OTP.
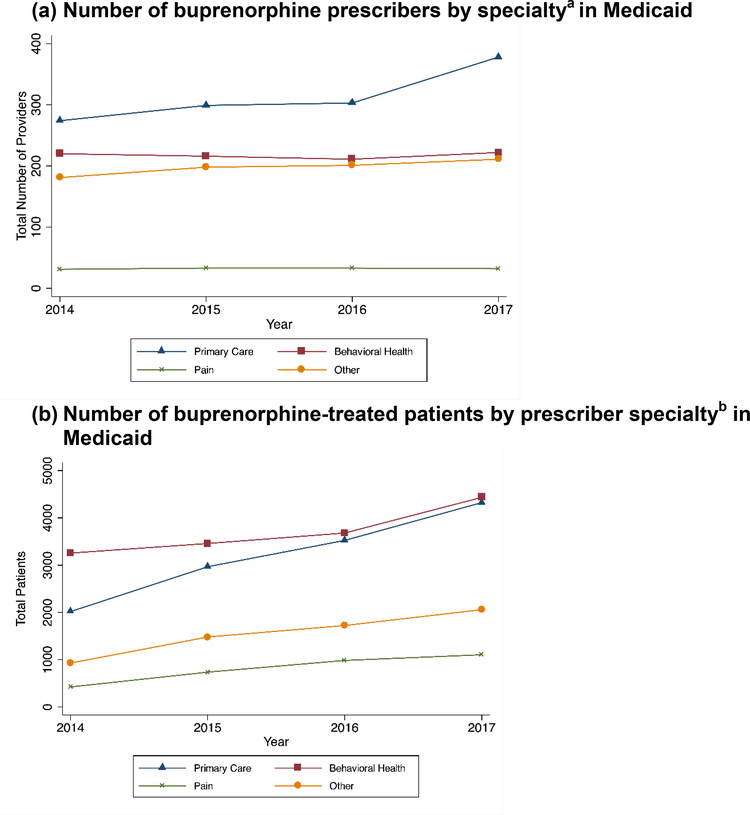
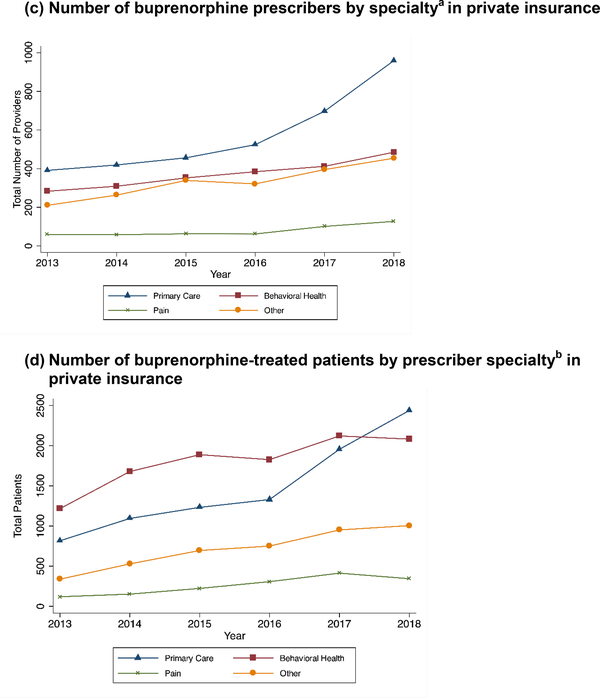
Figure 1. Patterns of buprenorphine prescriptions for OUD by specialty over time by payer.
a The number of prescribers reflects the yearly number of unique NPIs listed as prescribers of buprenorphine formulations for OUD. We grouped providers into types based on their primary taxonomy in NPPES.
b The number of unique Medicaid-enrolled individuals in a year who received at least one prescription for a buprenorphine formulation intended for OUD by the type of prescribing provider.
We defined MOUD initiation as the receipt of at least one buprenorphine or naltrexone prescription claim for formulations intended for use in OUD treatment, or as receipt of methadone dispensing services from an OTP (HCPCS code H0020, J1230, or S0109). We examine MOUD initiation within 2 and 12 months of the index diagnosis in order to examine both short-run and longer-run rates. We required individuals to be enrolled in Medicaid or private insurance for 10 out of 12 months after their index diagnosis in order to ensure observability of treatment claims.
Prior Service Use and Diagnosis
We examined whether patients saw a PCP or a BH provider in the 12 months prior to their index OUD diagnosis. We defined a visit with a PCP as a claim with an outpatient evaluation and management CPT code where the rendering provider met the definition of a PCP, as described below, without regard to prior affiliation between the PCP and patient. We defined receipt of BH services as any claims where the rendering provider met the definition of a BH provider also described below.
We also determined the provider type seen on the index diagnosis date as either PCP, BH provider, emergency provider, or other type of provider. If multiple providers were seen on the day of diagnosis, we assigned the provider based on the following hierarchy: PCP, BH provider, emergency provider, then other type of provider.
Provider Types
In order to identify provider type, we linked providers in the claims data with the National Plan and Provider Enumeration System (NPPES) data using national provider identifiers (NPI). We used the taxonomy code chosen by providers as their primary specialty to group them into provider types (see Appendix 1 for full description). There were few missing NPIs in claims data (0.4% in Medicaid and 3.9% in private insurance), and we were able to match nearly all NPIs to NPPES (99.3% match in Medicaid and 99.6% match in private insurance).
For Medicaid, we described annual trends in the types of providers prescribing buprenorphine and the number of patients receiving treatment from different provider types (Figure 1). We assigned patients receiving buprenorphine to their first buprenorphine prescriber in a year to calculate the number of patients treated by each provider type. We were unable to do this for the privately insured sample because the private insurance pharmaceutical claims did not identify prescribing providers.
Multivariate Analysis Methods
We used logistic regression models to examine the association between PCP engagement and MOUD initiation. Two variables described PCP engagement. The first was a categorical variable of whether an individual had a PCP visit, a BH visit but no PCP visit, or neither PCP nor BH visits in the 12 months prior to index diagnosis. The second was a categorical variable of the type of provider seen on the day of index diagnosis: a PCP, a BH provider, an emergency provider, or a different type of provider. We ran separate models of short and long-run treatment rates, where the dependent variable was either an indicator of MOUD initiation within 2 months of the index OUD diagnosis or within 12 months of index diagnosis.
The model controlled for demographic characteristics and comorbidities diagnosed in the year prior to the index OUD diagnosis using the medical conditions from the Elixhauser Index.23 The number of medical conditions were modeled as splines to provide an incremental different interpretation. We also included indicators of other behavioral health diagnoses observed in claims in the year prior to the index diagnosis because of the noted correspondence between OUD diagnosis and behavioral health conditions, and the potentially greater access to behavioral health providers in this population.24 Information on individuals’ race was not available in the private insurance data.
We report average marginal effects (AMEs) of each covariate on the rate of treatment within either 2 or 12 months of index OUD diagnosis along with 95% confidence intervals based on delta method standard errors. These AMEs reflect the difference in the predicted probability of initiating treatment at each time period between the covariate and the relevant referent group in percentage point units. This study was approved by the UNC-CH Institutional Review Board.
Results
Between 2014 and 2017, the prevalence of documented OUD increased by 47% among Medicaid enrollees and by 76% among the privately insured (Table 1). Over the same time period, while the number of people with an OUD diagnosis who initiated MOUD grew, the percent of people with a documented OUD who initiated MOUD in each year fell slightly from 45% to 41% in Medicaid and from 42% to 39% in private insurance. Buprenorphine was the most common treatment in Medicaid and private insurance. A sizeable proportion of Medicaid enrollees (15–19%) received methadone, whereas methadone was uncommon among the privately insured (<3% treatment rate each year).
PCPs represented the largest share of buprenorphine prescribers in both payers (Figures 1a&c), although the increase in primary care providers was much more dramatic in private insurance. BH prescribers treated more individuals with buprenorphine for OUD in Medicaid (Figure 1b), although primary care overtook the size of behavioral health in the last year of private insurance data (Figure 1d). The percent of all patients receiving buprenorphine who were treated by PCPs increased from 32% in 2014 to 39% in 2017.
Among individuals newly diagnosed with OUD, the vast majority, 82% in each insurance group, had seen a PCP 12 months prior to their index diagnoses (Table 2). Those with prior contact with a PCP were older, more likely to be female, and had a greater number of medical comorbidities than those who saw either a behavioral health provider but not a PCP or those who saw neither type of provider in the year prior to their index diagnosis. Not surprisingly, those who saw a PCP prior to their index OUD diagnosis were also more likely to have seen a PCP on the date of their index diagnosis. Approximately 21% of Medicaid enrollees and 15% of private insurance members initiated MOUD within 2 months of their index diagnoses. Rates of MOUD initiation within a year of index diagnosis were only moderately larger, with 25% of Medicaid enrollees and 19% of private insurance members receiving MOUD within a year of diagnosis.
Table 2:
Characteristics of people with new OUD diagnosis in Medicaid and private insurance
| Medicaid | ||||
| Service use in 12 months prior to index OUD diagnosis, By Provider Type Seen in Pre-Period |
||||
| PCP | BH, no PCP | Neither | p-value | |
|
| ||||
| N | 24,279 | 905 | 4507 | |
| Age, mean (SD) | 40.6 (12.8) | 33.0 (11.6) | 33.5 (11.6) | <0.001 |
| Female | 15282 (68.1%) | 570 (61.7%) | 2734 (66.4%) | <0.001 |
| Race | ||||
| White | 15054 (67.1%) | 560 (60.6%) | 3006 (73.0%) | <0.001 |
| Black | 5106 (22.8%) | 288 (31.2%) | 718 (17.4%) | |
| Othera | 2268 (10.1%) | 76 (8.2%) | 395 (9.6%) | |
| Number of Comorbid Medical Conditions, (prior to index diagnosis)b | 2.6 (2.5) | 0.9 (1.5) | 0.9 (1.7) | <0.001 |
| BH Comorbid Conditions (prior to index diagnosis) | ||||
| Psychosis | 3478 (15.5%) | 275 (29.8%) | 189 (4.6%) | <0.001 |
| Depression | 9221 (41.1%) | 359 (38.9%) | 430 (10.4%) | <0.001 |
| Anxiety | 2654 (11.8%) | 87 (9.4%) | 71 (1.7%) | <0.001 |
| Alcohol | 2310 (10.3%) | 216 (23.4%) | 182 (4.4%) | <0.001 |
| Provider Type seen on date of index diagnosis | ||||
| PC | 9825 (43.8%) | 170 (18.4%) | 1037 (25.2%) | <0.001 |
| BH, no PC | 5005 (22.3%) | 491 (53.1%) | 1515 (36.8%) | |
| Emerg, no PC or BH | 2338 (10.4%) | 108 (11.7%) | 530 (12.9%) | |
| Other | 5260 (23.5%) | 155 (16.8%) | 1037 (25.2%) | |
| Treatment at 2 months | 4415 (19.7%) | 196 (21.2%) | 1562 (37.9%) | <0.001 |
| Treatment at 1 year | 5396 (24.1%) | 244 (26.4%) | 1813 (44.0%) | <0.001 |
| Private insurance | ||||
| Prior Period (12 months) Service Use, By Provider | ||||
| PCP | BH, no PC | Neither | p-value | |
| N | 5719 | 201 | 998 | |
| Age, mean (SD) | 42.5 (13.4) | 31.1 (12.9) | 34.8 (12.4) | <0.001 |
| Female | 2696 (47.1%) | 63 (31.3%) | 282 (28.3%) | <0.001 |
| Physical Comorbid Conditions, (prior to index diagnosis date)b | 1.8 (2.1) | 0.4 (1.0) | 0.5 (1.2) | <0.001 |
| BH Comorbid Conditions (prior to index diagnosis date) | ||||
| Psychosis | 399 (7.0%) | 24 (11.9%) | 19 (1.9%) | <0.001 |
| Depression | 1903 (33.3%) | 76 (37.8%) | 64 (6.4%) | <0.001 |
| Anxiety | 583 (10.2%) | 38 (18.9%) | 20 (2.0%) | <0.001 |
| Alcohol | 516 (9.0%) | 40 (19.9%) | 46 (4.6%) | <0.001 |
| Provider Type Seen on Index Diagnosis date | ||||
| PC | 2138 (37.4%) | 32 (15.9%) | 187 (18.7%) | <0.001 |
| BH, no PC | 930 (16.3%) | 75 (37.3%) | 225 (22.5%) | |
| Emerg, no PC or BH | 667 (11.7%) | 20 (10.0%) | 116 (11.6%) | |
| Other | 1984 (34.7%) | 74 (36.8%) | 470 (47.1%) | |
| Treatment at 2 months | 744 (13.0%) | 36 (17.9%) | 250 (25.1%) | <0.001 |
| Treatment at 1 year | 973 (17.0%) | 47 (23.4%) | 321 (32.2%) | <0.001 |
Other race includes Asian/Pacific Islander, American Indian/Alaskan Native, Native Hawaiian/Other Islander, and more than one race.
Number of physical conditions from the Elixhauser Comorbidity Software that were documented in a claim prior to the index diagnosis.
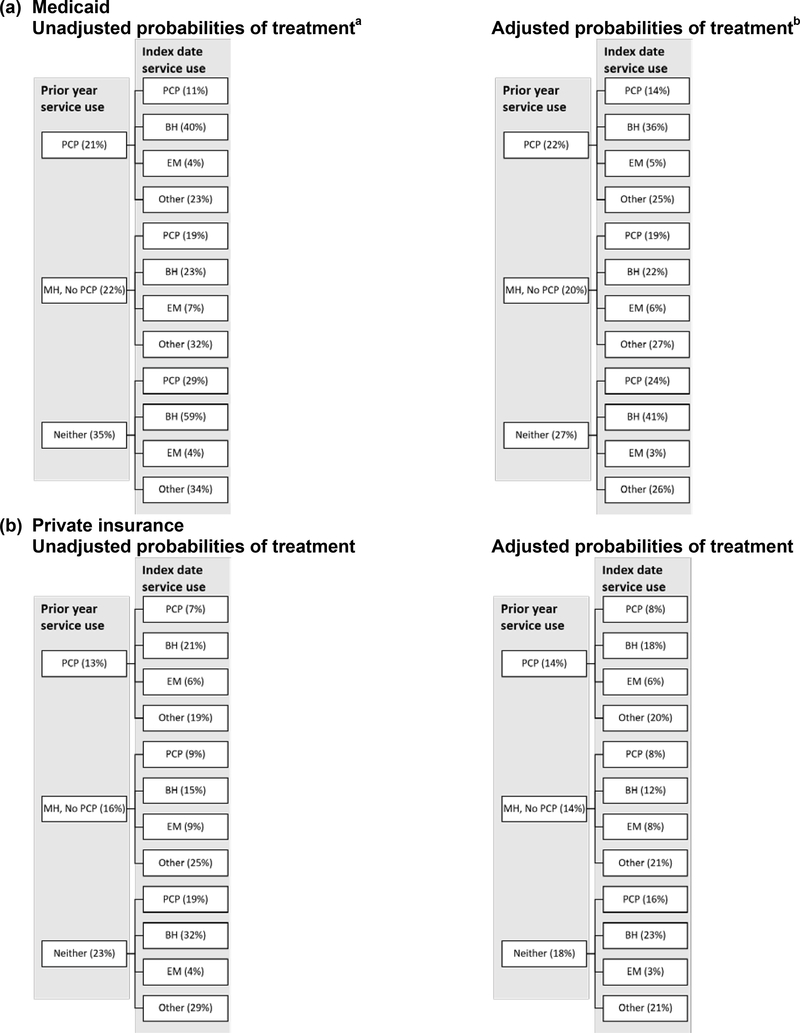
Figure 2 presents unadjusted and adjusted probabilities of MOUD initiation within 2 months of index OUD diagnosis among Medicaid enrollees and private insurance members. The unadjusted probabilities are the actual percent of individuals who initiated MOUD in each category. The adjusted figures are the predicted percent of people in each category who would initiate MOUD controlling for the variables in Table 2.
Figure 2: Probabilities of OUD treatment within two months of diagnosis by prior service use and on index OUD diagnosis date in Medicaid and private insurance.
a The unadjusted probabilities are the actual percent of individuals in each category that received MOUD.
b The adjusted probability are the predicted probabilities of MOUD controlling for variables in Table 2.
Treatment rates were generally low, but there was substantial variation in unadjusted treatment by types of providers accessed prior to and on the index diagnosis date. The differences in unadjusted probabilities of treatment by prior service use decreased after controlling for individual characteristics (Figure 2), indicating that many of these covariates, rather than providers’ treatment patterns, account for some of the differences in treatment rates. For Medicaid and private insurance, the adjusted probabilities of treatment were similar for those with a prior PCP or BH provider visits and strikingly highest for those without either type of visit. Those who saw BH or other types of providers on their index date had the highest probabilities of treatment for both Medicaid and private insurance, followed by PCP; emergency providers had the lowest MOUD initiation probabilities.
Consistent with the results shown in Figure 2, in multivariate logistic regression, we found no difference in the predicted probability of treatment for individuals who saw a PCP prior to their index diagnosis compared to those who saw only a BH provider (Table 3). By contrast, individuals who saw neither a PCP nor a BH provider were more likely to receive treatment than those who saw a PCP. These results were consistent for Medicaid and privately insured individuals. In Medicaid and private insurance, seeing a BH provider or other type of provider on the date of index diagnosis was associated with a higher probability of treatment compared to those seen by a PCP. Being seen by an emergency provider on the index date was associated with a lower probability of MOUD initiation in Medicaid at both time periods, but in private insurance only at 2 months.
Table 3.
Association of prior service use and provider type diagnosing with changes in the probability of treatment initation within two months and one year in Medicaid and private insurance
| Changes in the Probability of Treatment Initiation – Medicaid | Changes in the Probability of Treatment Initiation – Private insurance | |||
|---|---|---|---|---|
| Average Marginal Effects [95% confidence interval] | Average Marginal Effects [95% confidence interval] | |||
| 2 months post index | 1 year post index | 2 months post index | 1 year post index | |
| Prior Period (REF=PCP) | ||||
| BH, No PCP | −0.015 [−0.043, 0.013] | −0.008 [−0.038, 0.022] | −0.0069 [−0.052, 0.039] | −0.0073 [−0.059, 0.044] |
| Neither | 0.053 [0.039, 0.066] | 0.06 [0.045, 0.074] | 0.036 [0.012, 0.061] | 0.053 [0.026, 0.081] |
| Providers seen on Index Diagnosis Date (REF=PCP) | ||||
| BH | 0.20 [0.19, 0.21] | 0.21 [0.2, 0.23] | 0.093 [0.069, 0.118] | 0.11 [0.085, 0.14] |
| Emergency | −0.12 [−0.13, −0.11] | −0.090 [−0.104, −0.076] | −0.038 [−0.059, −0.017] | −0.022 [−0.049, 0.0043] |
| Other | 0.097 [0.085, 0.109] | 0.098 [0.085, 0.111] | 0.11 [0.086, 0.13] | 0.11 [0.085, 0.13] |
| Age | −0.0003 [−0.0007, 0.00011] | −0.00085 [−0.0013, −0.00042] | −0.00057 [−0.0011, −0.000001] | −0.0018 [−0.0025, −0.0011] |
| Female | 0.12 [0.11, 0.13] | 0.14 [0.13, 0.15] | −0.034 [−0.051, −0.017] | −0.045 [−0.063, −0.026] |
| White | 0.009 [0, 0.019] | 0.016 [0.006, 0.027] | ||
| County Size (REF=>200,000) | ||||
| <50,000 | 0.016 [0.002, 0.03] | 0.020 [0.005, 0.035] | 0.04 [0.014, 0.067] | 0.055 [0.025, 0.084] |
| 50,000–125,000 | 0.015 [0.003, 0.026] | 0.017 [0.004, 0.029] | 0.022 [0.002, 0.043] | 0.02 [−0.0027, 0.042] |
| 125,000–200,000 | 0.021 [0.009, 0.033] | 0.025 [0.012, 0.038] | 0.019 [−0.002, 0.04] | 0.0067 [−0.016, 0.03] |
| Psychosis | −0.046 [−0.061, −0.032] | −0.048 [−0.063, −0.033] | −0.0052 [−0.042, 0.031] | −0.0014 [−0.04, 0.038] |
| Depression | −0.024 [−0.034, −0.014] | −0.023 [−0.034, −0.013] | −0.0018 [−0.022, 0.018] | 0.012 [−0.01, 0.033] |
| Anxiety | −0.015 [−0.03, −0.0005] | −0.015 [−0.031, 0.001] | −0.017 [−0.046, 0.012] | −0.02 [−0.051, 0.012] |
| Alcohol | −0.040 [−0.058, −0.022] | −0.040 [−0.058, −0.022] | 0.048 [0.022, 0.075] | 0.045 [0.016, 0.075] |
| Phys. Comorbid Splinesa | ||||
| 0–1 | −0.032 [−0.043, −0.021] | −0.037 [−0.049, −0.025] | −0.033 [−0.053, −0.013] | −0.04 [−0.063, −0.018] |
| 1–2 | −0.033 [−0.041, −0.025] | −0.036 [−0.045, −0.028] | −0.025 [−0.042, −0.0076] | −0.022 [−0.04, −0.003] |
| 3–4 | −0.024 [−0.036, −0.012] | −0.024 [−0.036, −0.012] | −0.016 [−0.045, 0.012] | −0.027 [−0.058, 0.0034] |
| 5+ | −0.0067 [−0.016, 0.0026] | −0.0095 [−0.019, −0.00024] | 0.0034 [−0.018, 0.025] | 0.0017 [−0.023, 0.026] |
| Year (Ref=2014) | ||||
| 2015 | −0.057 [−0.07, −0.045] | −0.051 [−0.064, −0.038] | −0.022 [−0.044, 0.00033] | −0.01 [−0.035, 0.014] |
| 2016 | −0.072 [−0.085, −0.06] | −0.069 [−0.083, −0.056] | −0.036 [−0.058, −0.013] | −0.03 [−0.055, −0.005] |
| 2017 | −0.059 [−0.073, −0.044] | −0.061 [−0.076, −0.045] | −0.026 [−0.054, 0.001] | −0.025 [−0.055, 0.0048] |
| N | 27467 | 27467 | 6896 | 6896 |
Estimates represent differences between each category and the referent group in terms of the probability of treatment initiation. Bolded estimates are statistically significant at a p<0.05 level.
Number of physical conditions from the Elixhauser Comorbidity Software that were documented in a claim prior to the index diagnosis.
Females were more likely to receive treatment in Medicaid but less likely in private insurance, while whites were more likely to receive treatment in Medicaid. The number of comorbid conditions generally decreased the probability of treatment, especially among populations with few such conditions. Smaller counties were generally associated with higher probabilities of treatment. Behavioral health comorbidities were associated with lower probabilities of treatment in Medicaid but not private insurance. Having an alcohol use disorder was associated with a lower probability of MOUD initiation in Medicaid but a higher probability in private insurance. Finally, as observed in the unadjusted estimates, the treatment rate was lower in years after 2014 in the Medicaid population, even controlling for model covariates.
Discussion
This study found that the percent of Medicaid enrollees and private insurance members with a documented OUD diagnosis increased substantially between 2014 and 2017. These increases may result from new cases of OUD or from new diagnoses of previously undiagnosed OUD. This trend is consistent with the increase in opioid overdose deaths in NC during this time period, which increased rom 8.6 deaths per 100,000 population in 2014 to 18.3 deaths per 100,000 in 2017, more than doubling the death rate.25 ED visits per capita for opioid overdoses in NC similarly increased by 87% during this time period.
Several limitations are worthy of mention. Our estimates likely undercount the true prevalence of OUD since they do not capture people with OUD who are not diagnosed or whose providers do not document OUD in claims, but may also overcount people with OUD if the administrative diagnoses do not meet clinical criteria for OUD. The extent to which these or other sources of measurement error have changed over this time period are unknown. Our approach of counting individuals receiving buprenorphine formulations for OUD as being diagnosed with OUD may inflate the prevalence if some of these individuals received buprenorphine for off-label uses. However, Medicaid and the private insurer have policies against reimbursing off-label prescriptions of buprenorphine. While NC’s private insurance market has been noted to be concentrated,26 these results may not generalize to other private insurance companies or other states. Our estimates reflect only associations with treatment initiation and do not imply causation. There are likely a number of sources of unmeasured confounding, such as severity of illness, that are not observable in our data. The requirement of 10 months of enrollment prior to and following the index diagnosis may render the sample less generalizable to the full population of Medicaid enrollees with an OUD diagnosis.
The number of people initiating MOUD increased substantially in the study period. Overall, the state saw a 21% increase in opioid use disorder treatments per capita provided through the regional carve-outs during this time period,25 which is a slower growth rate than overdose deaths or ED visits for overdoses. Consistent with these statewide trends, we found that the treatment rate as a percent of those with administrative OUD diagnoses fell during this time. MOUD initiation rates were slightly higher among Medicaid enrollees compared to privately insured individuals. Rates of methadone treatment were low among the privately insured, possibly as a result of methadone not being a covered service.27
We found that the number of PCPs prescribing buprenorphine in Medicaid increased as did the number of people receiving buprenorphine from PCPs. This finding presents compelling evidence that PCP involvement in OUD care is increasing, though it remains concentrated in a relatively small number of providers. These increases may result from efforts to increase MOUD provider capacity in NC under the state’s Opioid Action Plan, which include buprenorphine waiver trainings and UNC ECHO for MAT.10,28,29 UNC ECHO for MAT is a multi-faceted intervention including (1) televideo-enabled ECHO sessions with case-based discussions and didactic presentations; (2) individual provider-to-provider consultations; and (3) practice coaching. Interviews with participants of UNC ECHO for MAT found the ECHO sessions were particularly beneficial though barriers to MOUD provision remained.29
We found most people who were newly diagnosed with OUD had a PCP visit in the 12 months prior to diagnosis, providing support for the importance of PCPs in diagnosing OUD and connecting patients to treatment. People diagnosed with OUD who had a seen a PCP in the year prior to diagnosis were as likely to receive treatment as those who only saw a BH provider. However, those who saw neither type of provider were the most likely to receive treatment. These individuals had fewer comorbid health needs, which may explain their lack of engagement with providers prior to their index OUD diagnoses. This group’s high treatment rate may be because they sought out healthcare providers with the specific goal of receiving OUD treatment.
Individuals seen by a BH provider on their index date were more likely to initiate MOUD compared to those diagnosed by a PCP. While our analyses were not causal, this result may suggest that BH providers are better equipped to provide or refer to MOUD. We cannot rule out the explanation, however, that those individuals most committed to MOUD treatment may be more likely to seek a diagnosis from behavioral health specialists. The difference in treatment rates between those seeing PCPs or BH specialists on the index date was larger in Medicaid than private insurance. One potential reason for this difference could be the BH carve-out in Medicaid as an additional barrier to PCPs connecting patients to MOUD. We found low treatment rates among those diagnosed by emergency providers, consistent with previous studies.30 Notably, we found that treatment rates within 2 months and 1 year of index diagnosis did not differ substantially, which may point to the importance of early intervention after diagnosis. Further research should examine whether this finding holds in other settings, patient decision making for OUD treatment, and what role PCP visits prior to OUD diagnosis play in the treatment trajectory.
Conclusion
In all, our findings show some progress in expanding MOUD and PCPs’ involvement in its provision. The high rate of PCP engagement among people diagnosed with OUD points to the value of involving PCPs in the diagnosis and treatment of OUD. Nevertheless, the continued shortages of treatment options for individuals with OUD and the low rates of treatment point to a need to identify new leverage points to address these gaps to address the Nation’s opioid overdose epidemic.
Supplementary Material
Acknowledgments
Funding:
This project was funded by the Agency for Health Care Research and Quality (R18HS25065-01) and the North Carolina Department of Health and Human Services (TI17014). The database infrastructure used for this project was supported in part by the Cecil G. Sheps Center for Health Services Research and the CER Strategic Initiative of UNC’s Clinical and Translational Science Award (UL1TR001111). Alex Gertner received support from the National Institute on Drug Abuse (F30DA044668). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH, AHRQ or NC DHHS.
Footnotes
Conflicts of interest: none
References
- 1.Comer SD, Sullivan MA, Yu E, et al. Injectable, Sustained-Release Naltrexone for the Treatment of Opioid Dependence. Arch Gen Psychiatry. 2006;63(2):210. doi: 10.1001/archpsyc.63.2.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. In: Minozzi S, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2011:4–6. doi: 10.1002/14651858.CD001333.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. In: Mattick RP, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2014. doi: 10.1002/14651858.CD002207.pub4 [DOI] [Google Scholar]
- 5.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population HHS Public Access. J Subst Abus Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon AJ, Lo-Ciganic W-H, Cochran G, et al. Patterns and Quality of Buprenorphine Opioid Agonist Treatment in a Large Medicaid Program. J Addict Med. 2015;9(6):470–477. doi: 10.1097/ADM.0000000000000164 [DOI] [PubMed] [Google Scholar]
- 7.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Heal. 2015;105(8):e1–e9. doi: 10.2105/ajph.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakeman SE, Barnett ML. Primary Care and the Opioid-Overdose Crisis — Buprenorphine Myths and Realities. N Engl J Med. 2018. doi: 10.1056/nejmp1802741 [DOI] [PubMed] [Google Scholar]
- 9.Saitz R, Daaleman TP. Now is the time to address substance use disorders in primary care. Ann Fam Med. 2017;15(4):306–308. doi: 10.1370/afm.2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20–24. doi: 10.1080/08897077.2015.1129388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou R, Korthuis PT, Weimer M, et al. Medication-Assisted Treatment Models of Care for Opioid Use Disorder in Primary Care Settings. Agency Healthc Res Qual. 2016;(Technical Brief 28). http://effectivehealthcare.ahrq.gov/ehc/products/636/2225/opioid-use-disorder-draft-report-160513.pdf. [PubMed] [Google Scholar]
- 12.Wilson CG, Fagan EB. Providing office-based treatment of opioid use disorder. Ann Fam Med. 2017;15(5):481. doi: 10.1370/afm.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan LE, Fiellin D a. Review Annals of Internal Medicine Narrative Review : Buprenorphine for Opioid-Dependent Patients in. Ann Intern Med. 2008;148(10):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblatt R a, Andrilla CHA, Catlin M, Larson EH. Geographic and Specialty Distribution of US Physicians Trained to Treat Opioid Use Disorder. Ann Fam Med. 2015;13(1):23–26. doi: 10.1370/afm.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove LR, Rao N, Spero J, Fraher E, Domino ME. Which providers obtain DATA 2000 Waivers for Opioid Use Disorder Treatment? Unpublished.
- 16.Andrilla CHA, Coulthard C, Larson EH. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med. 2017;15(4):359–362. doi: 10.1370/afm.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchinson E, Catlin M, Andrilla CHA, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med. 2014;12(2):128–133. doi: 10.1370/afm.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walley AY, Alperen JK, Cheng DM, et al. Office-based management of opioid dependence with buprenorphine: Clinical practices and barriers. J Gen Intern Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole ES, DiDomenico E, Cochran G, et al. The Role of Primary Care in Improving Access to Medication-Assisted Treatment for Rural Medicaid Enrollees with Opioid Use Disorder. J Gen Intern Med. 2019. doi: 10.1007/s11606-019-04943-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Carolina Cost and Quality Initiative; 2019. https://www.shepscenter.unc.edu/data/bcbsnc-claims-data-ccqi/.
- 21.The Henry J Kaiser Family Foundation. Health Insurance Coverage of the Total Population Based on the Census Bureau’s American Community Survey; 2019. https://www.kff.org/other/state-indicator/total-population/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D.
- 22.Distribution of Nonelderly Adults with Medicaid by Sex. Kaiser Family Foundation. https://www.kff.org/medicaid/state-indicator/medicaid-distribution-nonelderly-adults-by-sex/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Published 2019.
- 23.Healthcare Cost and Utilization Project. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Published 2017.
- 24.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78–82. doi: 10.1016/j.drugalcdep.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 25.Opioid Action Plan Data Dashboard. North Carolina Department of Health and Human Services. https://www.ncdhhs.gov/about/department-initiatives/opioid-epidemic/opioid-action-plan-data-dashboard.
- 26.Carey L Complex factors create lack of health insurance competition in rural areas. North Carolina Health News. https://www.northcarolinahealthnews.org/2019/08/14/complex-factors-create-lack-of-health-insurance-competition-in-rural-sreas/.
- 27.Reif S, Horgan CM, Hodgkin D, Matteucci A-MM, Creedon TB, Stewart MT. Access to Addiction Pharmacotherapy in Private Health Plans. J Subst Abuse Treat. 2016;66:23–29. doi: 10.1016/j.jsat.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansagra SM, Cohen MK. The Opioid Epidemic in NC. N C Med J. 2018;79(3):157–162. doi: 10.18043/ncm.79.3.157 [DOI] [PubMed] [Google Scholar]
- 29.Shea C, Gertner A, Green S. Barriers and Perceived Usefulness of an ECHO Intervention for Office-Based Buprenorphine Treatment for Opioid Use Disorder in North Carolina: A Qualitative Study. Unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyawala N, Landis R, Barry CL, Stein BD, Saloner B. Changes in Outpatient Services and Medication Use Following a Non-fatal Opioid Overdose in the West Virginia Medicaid Program. J Gen Intern Med. 2019. doi: 10.1007/s11606-018-4817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.