Abstract
Objective:
Classification criteria for calcium pyrophosphate deposition (CPPD) disease will facilitate clinical research on this common crystalline arthritis. We report on the first two phases of a four-phase process for developing CPPD classification criteria.
Methods:
CPPD classification criteria development is overseen by a 12-member Steering Committee. Item generation (Phase I) included a scoping literature review of five literature databases and contributions from a 35-member Combined Expert Committee and two Patient Research Partners. Item reduction and refinement (Phase II) involved a Combined Expert Committee meeting, discussions among Clinical, Imaging, and Laboratory Advisory Groups, and an item rating exercise to assess the influence of individual items toward classification. The Steering Committee reviewed the modal rating score for each item (range −3 [strongly pushes away from CPPD] to +3 [strongly pushes toward CPPD]) to determine items to retain for future phases of criteria development.
Results:
Item generation yielded 420 items (312 from the literature, 108 from experts/patients). The Advisory Groups eliminated items they agreed were unlikely to distinguish between CPPD and other forms of arthritis, yielding 127 items for the item rating exercise. Fifty-three items, most of which had a modal rating of +/− 2 or 3, were retained for future phases. As numerous imaging items were rated +3, the Steering Committee recommended focusing on imaging of the knee, wrist, and one additional affected joint for calcification suggestive of CPP crystal deposition.
Conclusion:
A data- and expert-driven process is underway to develop CPPD classification criteria. Candidate items comprise clinical, imaging, and laboratory features.
Keywords: classification criteria, CPPD, calcium pyrophosphate, pseudogout
INTRODUCTION
Calcium pyrophosphate deposition (CPPD) disease represents a symptomatic crystalline arthritis that affects an estimated 8–10 million adults in the United States.1,2 Chondrocalcinosis, a radiographic finding that has been used to estimate CPPD prevalence, is present in approximately 10% of adults in Italy and the United States aged 65 and older; CPPD accounts for a similar number of hospitalizations in France as does gout.3–5 CPPD was first recognized in the 1960s yet clinical research on this disease has lagged far behind other common arthritides. This crystalline arthritis presents with a host of manifestations including acute CPP crystal inflammatory arthritis, chronic CPP crystal inflammatory arthritis, and osteoarthritis with CPPD, and a patient may have more than one manifestation over time or simultaneously.6 Targeted treatments for CPP crystal deposition do not currently exist and many patients with CPPD suffer from inadequately treated joint pain, swelling, and stiffness.7 Advances in CPP-related arthritis, including treatment trials, have been hampered by lack of validated classification criteria, a framework that has facilitated clinical research and trials in other rheumatic diseases.8–11
The case-definition of CPPD in research studies has varied, which poses a major setback to advancing knowledge about CPPD and to developing therapeutics. Diagnostic criteria for CPPD proposed in the 1970s require evidence of chondrocalcinosis on plain radiographs and CPP crystals on synovial fluid crystal analysis, but have not been validated for use in clinical research and have several shortcomings.12,13 Plain radiography and synovial fluid crystal analysis each have limitations in their ability to detect CPP crystals, including low-to-moderate sensitivity of plain radiography, under-utilization of arthrocentesis, inter-observer differences in identifying CPP crystals via polarized light microscopy, and certain qualities of CPP crystals that pose challenges to identifying them via compensated polarized light microscopy.14–16 Sensitivity of synovial fluid CPP crystal identification has been variable in the literature, raising concerns that relying on this test to define CPPD for research could exclude a large percentage of subjects.17–19
Classification criteria for a disease allow investigators to identify relatively homogenous populations with that disease for inclusion in clinical research.20,21 The European League Against Rheumatism (EULAR) generated working terminology and diagnostic recommendations in 2011, but formal classification criteria for CPPD do not exist.2 The American College of Rheumatology (ACR) and EULAR are jointly sponsoring multi-phase development of CPPD classification criteria. The criteria system will aim to achieve high sensitivity while maximizing specificity for CPPD, ensuring that future study populations achieve a degree of homogeneity and represent a consensus definition of CPPD for research reflecting the most common manifestations of CPPD. Classification criteria for CPPD will need to encompass the spectrum of symptomatic manifestations of this arthritis, though future investigators may choose to limit their study population to patients with particular types of CPPD manifestations.
The CPPD classification criteria working group is utilizing established methodology that includes data-driven and expert-driven methods previously employed for developing classification criteria for gout, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, and other rheumatic diseases.8–11,22,23 This report describes the findings of Phases I and II -- item generation and item reduction -- for the CPPD classification criteria project.
METHODS
CPPD classification criteria are being developed through a four-phase process. The process began by generating a comprehensive list of all possible items to be considered for classification of the disease (Phase I – item generation), followed by reducing that list to a parsimonious number providing a comprehensive yet manageable set for collecting data from de-identified patient cases that form a derivation set (Phase II – item reduction), reported herein. In Phase III, the items will be structured into an evaluable framework and the relative importance of items will be compared and item weights assigned through a multi-criterion decision analysis exercise. A threshold score will then be determined for classifying a patient as CPPD. The classification criteria will be validated in Phase IV using an independent validation set of de-identified patient cases, and test performance characteristics will be determined (Figure 1).
Figure 1.
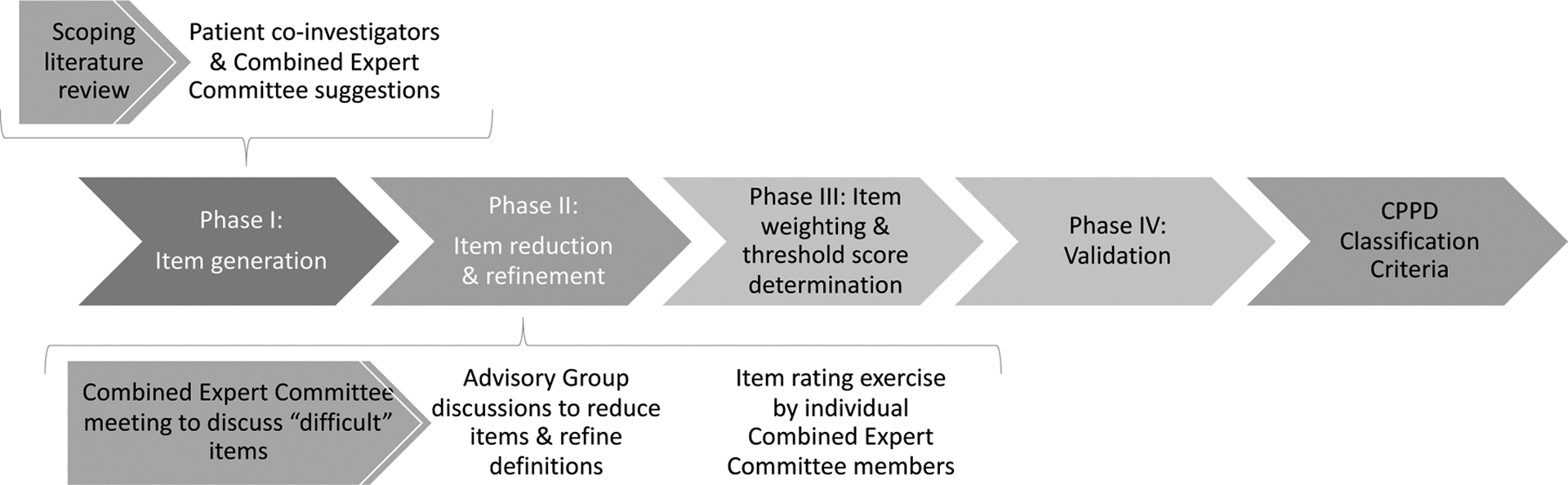
Overview of classification criteria development process, with a focus on sub-processes in Phases I and II.
Study oversight and management
Phases I and II were overseen by 4 co-PIs (AA, HC, WJT, RT) and 8 other members of the Steering Committee with expertise in CPPD research and/or classification criteria methodology (ND, AL, RN, TN, TP, FPR, AR, SKT).
Item generation
The purpose of item generation was to supply a comprehensive list of items to be considered as classification criteria. This should represent typical manifestations of CPPD and include items across a variety of categories including imaging, clinical, and laboratory features.
Scoping literature review to begin item generation
A scoping literature review was conducted within Web of Science, Embase, MEDLINE, CINAHL, and AMED databases through May 31, 2019 (see Supplement 1 for protocol). The search identified randomized controlled trials, cohort studies, cross-sectional studies, and case-control studies of adults with CPPD without language restriction. Studies with <20 participants were excluded to increase generalizability of features extracted from the literature. Terms included in the search included CPPD, CPDD, chondrocalcinosis, pseudogout, pyrophosphate, and calcium pyrophosphate. Literature search and data management were performed by an experienced systematic reviewer (BK). Four investigators divided the identified studies, screened titles and abstracts for eligibility, and performed full-text review on eligible studies (SKT, TP, AL, CG). A second investigator from among the four independently assessed 10% of titles, abstracts, and eligible studies to assess for agreement with the primary reviewer. Data extracted from full-text review included all features that were positively or negatively associated with CPPD. The list of identified features included demographics; signs and symptoms; imaging findings on plain radiograph, ultrasound, CT, and MRI; and laboratory findings in synovial fluid and peripheral blood. Risk factors for CPPD were also included in the list.
Expert and patient input for item generation
The Steering Committee invited 23 additional CPPD experts to form a 35-member Combined Expert Committee comprising 32 rheumatologists, two classification criteria methodologists, and one musculoskeletal radiologist with expertise in CPPD (Supplement 2). Combined Expert Committee members were asked to review the list of items generated from the literature and suggest additional items, including those that may not have been reported in the literature but influence clinical judgement. Two Patient Research Partners also reviewed the list of items from the literature and proposed additional items. The literature review list, expert suggestions, and patient suggestions were combined into one list with duplicates removed yielding a list of unique potential items to consider in developing the classification criteria.
Item reduction
The item reduction phase was guided by questions of specificity and generalizability. During each of three steps (Figure 1), experts were asked to consider the discriminating ability of items – i.e., whether they help distinguish CPPD vs. non-CPPD – a key feature of classification criteria. Experts were also asked to consider whether items might be highly specific for CPPD, but too rare to be implemented across a wide range of medical centers internationally.
The first step of this phase involved a four hour in-person meeting of the Combined Expert Committee immediately prior to the American College of Rheumatology meeting in Atlanta, in November 2019. Experts were presented with the list of items categorized according to clinical, imaging, and laboratory features and risk factors. The group held an open discussion as to whether each item could plausibly discriminate between CPPD and non-CPPD based on expert opinion. Consensus to eliminate an item was considered achieved if there were no objections to the proposed elimination.
The second step was led by three advisory groups working remotely in December 2019-January 2020. Clinical, Laboratory, and Imaging Advisory Groups each included 6–10 members from the Combined Expert Committee and were co-led by an expert in the topic and a junior faculty facilitator (Supplement 2). Each Advisory Group held 1–2 teleconferences to further reduce items following the same consensus-based discussion process that was used during the prior Combined Expert Committee meeting. Advisory Groups also refined item definitions to increase their precision. The goal was to consolidate the number of candidate items to approximately 150 to make the subsequent item rating exercise more feasible. Each group was encouraged to retain all items that, based on expert opinion, might distinguish between CPPD and non-CPPD.
The third step involved an item rating exercise in January 2020. Combined Expert Committee members were invited to use an online web-survey platform (www.surveymonkey.com) to rate each potential classification criterion on a 7-point scale indicating how much the presence of that item would push them toward or against classifying a patient as CPPD. Each item was rated from −3 to +3, with −3 signifying it pushed the expert strongly away from classifying the patient as CPPD, 0 indicating that did not influence them in classifying a patient as CPPD, and +3 meaning it pushed them strongly toward classifying the patient as CPPD. Experts were asked to select “not applicable” if they were unsure how to respond and were able to add comments as free text. The Steering Committee reviewed the distribution of ratings for each item and retained items with modal rating of −3, −2, +2, or +3, as these scores indicated a general consensus that the item is an important discriminator for or against CPPD. Items with a modal rating of −1, 0, or +1 were discussed in a Steering Committee teleconference before removal from the list of candidate items; those items that were considered to be potentially important discriminators were retained. Items retained at the end of Phase II (item reduction) will be included in subsequent phases of the classification criteria development.
RESULTS
Item generation
The scoping literature review yielded 67 manuscripts eligible for full-text review (Figure 2), from which 312 unique items were identified after removing duplicates and merging very similar items together. Twenty-five experts and two patients suggested 108 additional items yielding a total of 420 candidate items. These 420 items were grouped into three broad categories: clinical (n=264, 62.9%), imaging (n=90, 21.4%), and laboratory (n=66, 15.7%) The full list of candidate items is presented in Supplement 3.
Figure 2.
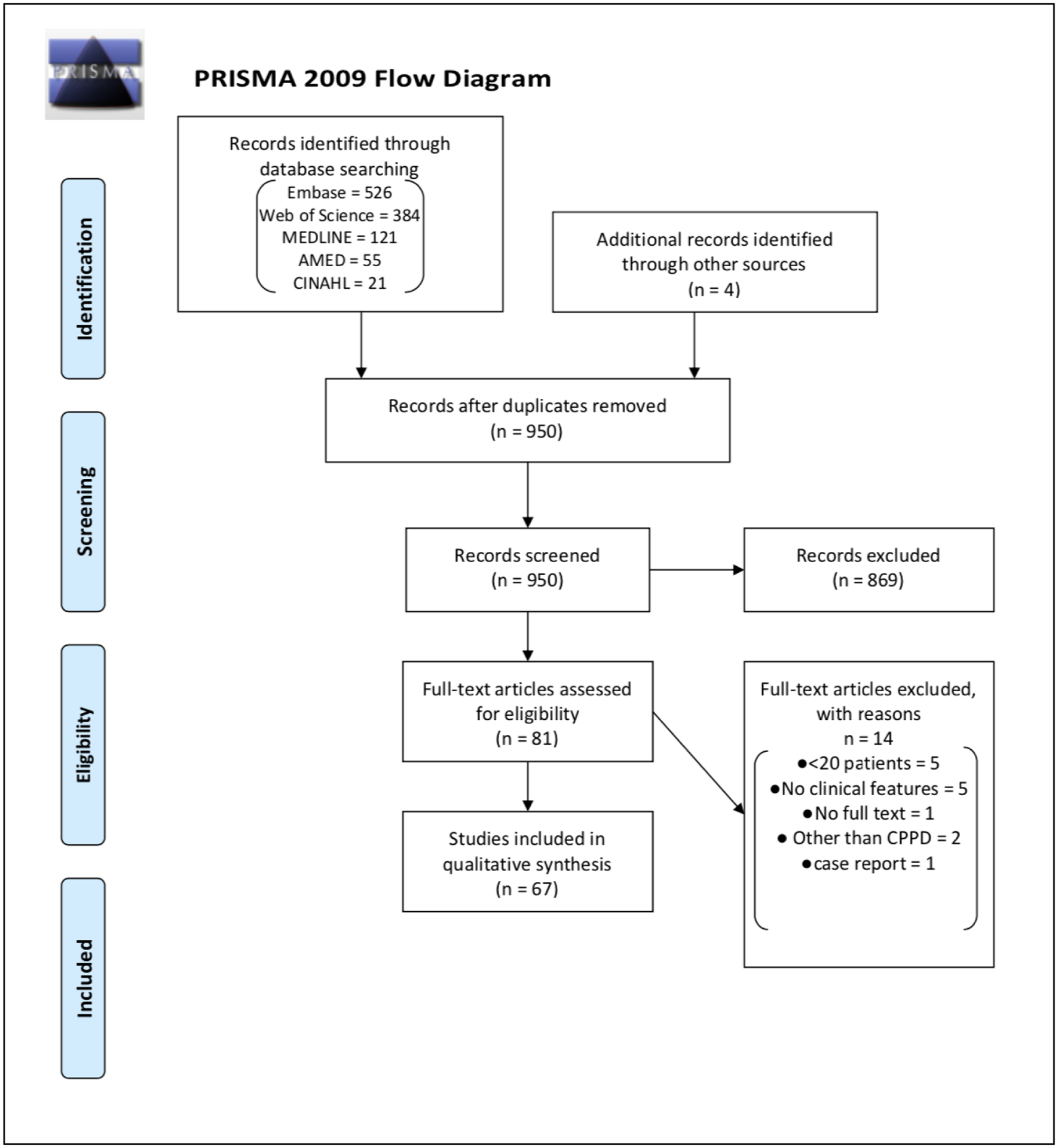
PRISMA flow diagram for scoping literature review for CPPD.
Item reduction at in-person meeting
Twenty-six Combined Expert Committee members attended the in-person meeting, during which 132 items were removed by consensus decision (Supplement 3). The group first discussed whether to exclude patients with other types of arthritis such as seropositive rheumatoid arthritis or sacroiliitis from being classified as CPPD. It was decided that because CPPD can co-exist with these conditions, other forms of arthritis should not be exclusion criteria for classification. Combinations of osteoarthritis in one joint plus chondrocalcinosis at another joint were removed from further consideration, as the group felt that complexity of these permutations would be impractical to operationalize as classification criteria. Other osteoarthritis items were retained for future discussion and refinement.
Age as a possible criterion was discussed at length. Some experts noted that age is to CPPD what serum urate is to gout, meaning that it is critical for disease pathogenesis. The group did not reach consensus on this issue, so it was decided to retain age at this step and re-consider it later. A number of features of acute CPP crystal arthritis were eliminated since they were not felt to distinguish between CPPD and other forms of inflammatory arthritis, e.g., fever, response to colchicine, and knee pain. Other clinical items were eliminated as they were complex constructs of items that had been included individually, e.g., pseudo-OA with synovitis, or were characteristic of many types of arthritis, e.g., disability with ambulation. Co-morbidities not thought to be pathogenically related to CPPD were eliminated, such as diabetes, end-stage renal disease, and hypertension. It was discussed whether certain medications might be pathogenic factors and these were retained, including bisphosphonates and diuretics.
Macroscopic appearance of synovial fluid and white blood cell count were considered non-specific for CPPD and all such features were eliminated. Absence of CPP crystals was discussed in depth. In the ACR/EULAR 2015 Gout Classification Criteria, absence of monosodium urate crystals from an aspirated joint carried negative weight.8 The group felt that absence of CPP crystals in synovial fluid may have a different impact on CPPD classification given that CPP crystals can be difficult to identify via compensated polarized light microscopy and there may be a high false negative rate. Experts favored creating a new item capturing repeatedly negative synovial fluid crystal analysis, distinct from an item characterized by a single negative synovial fluid crystal analysis. Monosodium urate crystals were retained as a potential criterion for two reasons: gout and CPPD can co-occur, CPP crystals can be found in gouty tophi, and there is a possibility that monosodium urate crystals could carry negative weight toward classifying a patient as CPPD.24,25
Many of the peripheral blood tests identified in the literature search were recognized to be non-specific for CPPD (e.g., high white blood count, high ESR), and these were eliminated. Autoimmune serologies were eliminated with the exception of rheumatoid factor and anti-citrullinated protein antibody (ACPA), as these may warrant consideration in future phases, for example, in potentially being assigned a negative weight toward CPPD classification. Genetic polymorphisms and mutations were considered highly specific, but too uncommon to be relevant for a classification criteria system and were eliminated. Metabolic abnormalities that are risk factors for CPPD were retained for future steps, such as high serum ferritin, hypomagnesemia, and hypophosphatasia. Laboratory assays that have been reported in research literature but are not routinely available for clinical use, e.g. high serum MMP3 and tissue-type plasminogen activator levels, were considered impractical for classification criteria and were omitted.
Candidate items in the imaging category were nearly uniformly retained during this discussion. The group felt that decisions about including imaging evidence of CPP crystal deposition at specific joints or combinations of joints were best left to future steps.
Advisory Group Meetings to further reduce and refine items
Each of the three Advisory Groups reached consensus on eliminating items that were not considered important discriminators between CPPD and non-CPPD. Supplement 3 presents the items that were retained and the updated definitions resulting from this step. Periarticular soft tissue swelling, asymmetric joint involvement, and hip pain are among the clinical items that were removed. Presence of CPP crystals specifically within white blood cells was removed, as laboratory experts reached consensus that extracellular and intracellular CPP crystals should carry equal importance in CPPD classification. Ultrasound findings characteristic of gout, such as double-contour sign and “snowstorm” appearance, were eliminated as potential items weighing against CPPD classification. The Advisory Group discussions yielded 61 clinical items, 57 imaging items, and 9 laboratory items (127 items total) for further consideration.
Item rating exercise
Twenty-nine Combined Expert Committee members (82.9%) completed the item rating exercise, which included 127 items. Eighty-three items (65.4%) had a modal rating of 3, 2, −2, or −3 and were retained for future phases based on this rating alone (Tables 1 and 2). Nearly all of the imaging items, synovial fluid CPP crystals, age >80 at symptom onset, crowned dens syndrome, history of familial CPPD, Gitelman syndrome, hemochromatosis, hyperparathyroidism, and hypomagnesemia were most strongly and consistently rated as influencing consideration of classifying as CPPD, with modal rating of +3 and standard deviation less than one. Four items were rated as strongly indicating the case was unlikely to be CPPD: palpable subcutaneous tophi, monosodium urate crystals (and no CPP crystals) in synovial fluid, positive synovial fluid culture, and high-titer anti-citrullinated peptide antibody (>3 times upper limit of normal).
Table 1.
CPPD Combined Expert Committee rating of influence on the likelihood of CPPD (−3 to +3) for candidate clinical and laboratory items
| Candidate criteria | Modal rating score | Standard deviation |
|---|---|---|
| Age at symptom onset | ||
| 91 years or older | 3 | 0.81 |
| 81–90 | 3 | 0.84 |
| 71–80 | 2 | 0.82 |
| 61–70 | 1 | 0.82 |
| 51–60 | 0 | 1.03 |
| 50 years or younger | −2 | 1.11 |
| Acute inflammatory arthritis of the following joint | ||
| Crowned dens syndrome | 3 | 0.84 |
| Knee | 2 | 0.90 |
| Wrist | 2 | 0.77 |
| Diffuse inflammatory hand swelling involving periarticular structures | 1 | 1.24 |
| Ankle | 0 | 1.21 |
| Spine | 0 | 1.50 |
| Mid-foot | −1 | 1.06 |
| 1st MTP joint | −2 | 1.21 |
| Recurrence and pattern of joint involvement | ||
| One self-limiting episode of acute inflammatory arthritis that resolved without treatment | 2 | 0.83 |
| Recurrent attacks of acute inflammatory arthritis in a characteristic joint (e.g. wrist, knee) | 2 | 0.77 |
| Additive attacks of inflammatory polyarthritis (i.e. the episode starts in one joint, then another joint becomes involved, then another, etc.) | 1 | 1.32 |
| Asymmetric attacks of inflammatory polyarthritis | 1 | 1.12 |
| Inflammatory oligoarthritis with asymmetric joint involvement | 1 | 0.98 |
| Persistent inflammatory polyarthritis with intermittent flares | 1 | 1.12 |
| Recurrent attacks of acute inflammatory arthritis in joints other than those that are typically involved by CPPD | 0 | 1.21 |
| Persistent inflammatory polyarthritis without flares | −2 | 1.32 |
| Onset and treatment response | ||
| Rapid onset acute inflammatory arthritis (time to maximal pain <24 hours)* | 1 | 1.01 |
| Acute inflammatory arthritis that developed in the context of acute illness, surgery, or joint trauma | 1 | 0.97 |
| Inflammatory oligoarthritis with good response to colchicine | 1 | 1.23 |
| Inflammatory oligoarthritis with good response to colchicine | 1 | 1.14 |
| Inflammatory polyarthritis with good response to colchicine | 1 | 1.15 |
| Acute inflammatory arthritis that developed in the context of recent bisphosphonate use | 1 | 1.06 |
| Physical findings | ||
| Erythema of the involved joint(s) during acute inflammatory arthritis | 1 | 0.70 |
| Hemarthrosis without other explanation (e.g. without joint injury, anticoagulation or pigmented villonodular synovitis) | 1 | 1.23 |
| Palpable subcutaneous tophus | −3 | 1.00 |
| Psoriasis | −2 | 1.16 |
| Co-morbidities and family history | ||
| History of familial CPPD | 3 | 0.81 |
| Gitelman disease | 3 | 0.86 |
| Hereditary hemochromatosis | 3 | 0.87 |
| Wilson disease | 2 | 1.24 |
| Ochronosis | 2 | 1.33 |
| History of meniscectomy or arthroscopy | 0 | 1.26 |
| Acromegaly | 0 | 1.10 |
| Short bowel disease | 0 | 1.76 |
| Osteoarthritis location and features | ||
| Wrist | 2 | 0.90 |
| 2nd and 3rd MCPJs | 2 | 1.08 |
| 2nd or 3rd MCPJs*+ | 1 | 0.84 |
| Scapho-trapezium joint* | 1 | 0.69 |
| Scapho-trapezium joint without OA of the trapezio-metacarpal joint | 1 | 0.82 |
| Scapho-lunate advanced collapse (SLAC) wrist* | 1 | 0.83 |
| Elbow | 1 | 1.00 |
| Patello-femoral joint without OA of the tibio-femoral joint | 1 | 1.09 |
| Ankle, without history of trauma | 1 | 1.01 |
| More than one of the following: ankle, wrist, shoulder, elbow | 1 | 0.97 |
| OA at one or more joint with signs of joint inflammation on examination | 1 | 0.90 |
| Scapho-trapezium joint without OA of the 1st MCP joint | 0 | 1.14 |
| Lateral tibiofemoral joint | 0 | 0.78 |
| Patello-femoral and tibio-femoral joints | 0 | 0.89 |
| Shoulder | 0 | 1.20 |
| Scapho-trapezium joint without OA of the 1st MCP joint | 0 | 0.95 |
| Scapho-trapezium and trapezio-metacarpal joints | 0 | 1.00 |
| Trapezio-metacarpal joint | 0 | 0.61 |
| Synovial fluid findings | ||
| At least one joint aspirate demonstrating CPP crystals | 3 | 0.26 |
| CPP crystals absent 1 occasion* | −1 | 0.63 |
| CPP crystals absent on 2 or more occasions | −2 | 0.85 |
| Monosodium urate crystals present, no CPPD crystals, and no micro-organisms | −3 | 0.83 |
| Micro-organisms on culture, no monosodium urate crystals, no CPPD crystals | −3 | 0.99 |
| Laboratory findings | ||
| Hypomagnesemia | 3 | 0.83 |
| Hyperparathyroidism | 3 | 0.78 |
| Hypercalcemia | 2 | 0.92 |
| Low-titer positive RF (1–3 times upper limit of normal) | 0 | 0.68 |
| High-titer positive RF (>3 times upper limit of normal) | −2 | 0.96 |
| Low-titer positive ACPA (1–3 times upper limit of normal)* | 0 | 0.98 |
| High-titer positive ACPA (>3 times upper limit of normal) | −3 | 0.97 |
Retained despite mode rating score −1, 0, or +1 per Steering Committee discussion
OA of 2nd MCP joint and OA of 3rd MCP joint were split into two discrete items
Table 2.
CPPD Combined Expert Committee rating of influence on the likelihood of CPPD (−3 to +3) for candidate imaging items+
| Affected joint+ | Knee*+ | Wrist*+ | 2nd and/or 3rd MCP* | Hip* | Symphysis pubis* | C1/C2* | Shoulder* | |
|---|---|---|---|---|---|---|---|---|
| Plain radiograph: calcification in regions of fibro- or hyaline cartilage | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Plain radiograph: calcification of the synovial membrane/capsule/tendon | 1** | 1** | 2 | 2 | -- | -- | 3 | -- |
| Conventional CT: calcification in regions of fibro- or hyaline cartilage | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Conventional CT: calcification of the synovial membrane/capsule/tendon | 2 | 2 | 2 | 2 | 2 | -- | 3 | -- |
| Ultrasound: CPP crystal deposition in fibro- or hyaline cartilage | 3 | 3 | 3 | 3 | -- | -- | -- | 3 |
| Ultrasound: CPP crystal deposition in synovial membrane/capsule/tendons | 2 | 2 | 2 | 3 | -- | -- | -- | -- |
| Cartilage calcium aggregates in the affected joint on ultrashort echo time (UTE) or in 3D dual echo steady-state gradient-echo MRI sequences | 2 | 1 | 2 | 3 | 3 | 3 | 2 | 2 |
| Dual-energy CT: CPP crystal deposition in fibro- or hyaline cartilage | 3 | 3 | 3 | 3 | 3 | -- | 3 | 3 |
| Dual-energy CT: CPP crystal deposition in synovial membrane/capsule/tendon | 3 | 3 | 3 | 3 | 3 | -- | 3 | -- |
The mode rating score is presented for each imaging modality and location
Imaging data from one affected joint, the knee (regardless of symptoms), and the wrist (regardless of symptoms) were ultimately recommended as candidate criteria by the Steering Committee
Regardless of symptoms at this joint
Plain radiograph of the affected joint and plain radiograph of the knee with calcification of the synovial membrane/capsule/tendon were retained per Steering Committee discussion
Seven respondents entered free-text comments indicating that the experience of the person performing polarized light microscopy is quite important, particularly when CPP crystals are not observed in synovial fluid. Thirteen respondents selected “not applicable” for questions related to MRI findings; aside from MRI, “not applicable” was rarely chosen.
The Steering Committee discussed twenty-eight items (22.0%) with a modal rating of −1, 0 or +1. Eight of these were retained at this stage to facilitate data collection to evaluate the potential relevance of these items in a data-driven approach (Tables 1 and 2). The modal score was 0 for history of meniscectomy or arthroscopy, and several experts commented that meniscectomy is prone to favor local disease but not systemic CPPD.
Because the number of candidate imaging items remained large and included numerous joints at this stage, the Steering Committee proposed a parsimonious approach for the imaging items: given that CPPD most commonly affects the knee and wrist, it was agreed that imaging data regarding calcification suggestive of CPP crystal deposition should be routinely assessed for these joints. Including imaging data from one additional affected joint was felt to provide a reasonable balance between informativeness for potential classification as CPPD and workload for future steps of data collection. Imaging data from any additional joints were deemed to likely provide only minimal additional value for discriminating CPPD from non-CPPD.
Following the item rating exercise, the Steering Committee and Laboratory Advisory Group added an additional item – histopathologic evidence of CPP crystals – as it was recognized that surgical specimens from knee replacement or spinal surgery may reveal these crystals in patients without arthrocentesis or synovial fluid crystal analysis. As such, 53 items were retained for future phases of classification criteria development.
DISCUSSION
We present results from the initial phases of CPPD classification criteria development, which will culminate in the first validated system to identify patients with CPPD for clinical research studies. A scoping review identified >300 unique clinical, laboratory, and imaging items from publications with a variety of study designs and a range of definitions of CPPD. Input from CPPD experts, some of whom have more than five decades of experience treating this disease, and CPPD patients was critical for identifying more than 100 additional items that are observed in clinical practice but may not have been published in the relatively scant literature on CPPD.
The item reduction process achieved its goal of honing a manageable number of items for future data collection and testing. Expert opinion was key for the rating exercise. Candidate items for which the mode was +3 or −3 had a standard deviation of less than one, indicating consensus among individual raters and providing face validity for these items. For a handful of items with a modal rating of −1, 0 or +1, the Steering Committee took an inclusive approach to retain now and analyze these items in a future data-driven step.
While the item generation phase had produced a large number of clinical features, the item reduction phase revealed that many of these occur in a large number of conditions and as a result, do not discriminate between CPPD and non-CPPD, a key requirement for an item to be considered for inclusion in classification criteria. Periarticular soft tissue swelling and ultrasound double-contour sign are among the items that were removed during item reduction (Supplement 3). Factors that are pathogenic and central to the construct of disease, such as older age, hyperparathyroidism, and osteoarthritis of the knee, wrist, 2nd or 3rd MCPs were retained.
It is notable that 40 of 49 items rated as strongly influencing experts toward classifying a patient as having CPPD (rating +3) were imaging findings of the affected joint or other joints characteristic of CPPD regardless of symptoms in those joints (e.g., incidental findings in the shoulder on CT chest). Given the large number of imaging items that were highly rated as +3, the Steering Committee proposed including imaging of the knee and wrist, as these are commonly involved in CPPD and most clinically impactful, and one additional affected joint. Dual-energy CT was consistently rated +3 for all joint locations, consistent with the limited literature suggesting high specificity for CPPD. Ultrasound evidence of CPPD was also rated highly, reflecting the published reports of high specificity in fibro- or hyaline cartilage.26,27 Plain radiograph calcification in regions of fibro- or hyaline cartilage in all proposed joint locations was also rated as strongly influencing a decision towards classifying as CPPD, while calcification in synovial membrane, capsule, or tendon generally was not as strongly rated because this was potentially more likely due to another form of calcium crystal deposition, e.g. calcium hydroxyapatite. Specific MRI sequences received mixed ratings and notably one-third of respondents did not answer questions about MRI due to lack of experience with these sequences and lack of sufficient data in the published literature to guide decision-making. Ongoing work for imaging items includes refining definitions to include technical parameters and developing of a set of representative CPPD images for reference.
The next phase of classification criteria development will involve de-identified prospective data collection from real-life patients to form a derivation cohort of patients with a range of probabilities for being classified as having CPPD. Collecting data on the 53 items from hundreds of patients will enable evaluation of the distribution of each item across a range of patient scenarios ranging from “definite CPPD” to “not CPPD” and inform a subsequent multicriterion decision analytic exercise using 1000Minds software. Prior to the 1000Minds exercise, latent class analysis will be performed to identifying which items cluster together in order to further reduce the number of items and identify potential domains. An expert consensus workshop and 1000Minds exercise will further reduce items to a manageable framework of domains and establish weights for individual items within each domain and across domains. The current item reduction phase was successful in achieving a manageable number of candidate criteria for the data collection step and subsequent testing.
CONCLUSION
Classification criteria for CPPD will provide a validated definition of CPPD for use in clinical research, facilitating comparisons across studies and enrollment into clinical trials. An international CPPD Combined Expert Committee developed a comprehensive list of candidate items, informed by the literature and by expert opinion. This list was then refined and narrowed to items that experts considered most influential in pushing them toward or against classifying an individual patient as having CPPD. Phase III, item weighting and threshold determination, is underway. The final phase, Phase IV, will validate the CPPD classification criteria system.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
The CPPD classification criteria working group is utilizing established methodology that includes data-driven and expert-driven methods to develop validated CPPD classification criteria.
This report describes the findings of Phases I and II -- item generation and item reduction -- for the CPPD classification criteria project.
The 53 candidate items retained in Phase II are broadly categorized as clinical, imaging, and laboratory features of CPPD.
Acknowledgements:
We would like to thank our Patient Research Partners, Diane A. Sachs (United States) and Patricia Brackley (United Kingdom), for their contributions.
Funding:
The project was supported by joint funding from the American College of Rheumatology and European League Against Rheumatism. Dr. Tedeschi receives support from the National Institutes of Health (K23 AR075070, L30 AR070514). Dr. Terkeltaub was supported by the VA Research Service and NIH (AR060772, AR075990). Dr. Neogi was supported by NIH K24 AR070892. Dr. Perez-Ruiz receives support from Cruces Rheumatology Association. Dr. Yokose receives support from the Rheumatology Research Foundation Scientist Development Award and National Institutes of Health (T32 AR007258).
Footnotes
- Sara K. Tedeschi: Consulting fees from NGM Biopharmaceuticals (<10K)
- Augustin Latourte: Advisory fees from Novartis
- Nicola Dalbeth: Consulting fees from AstraZeneca, Dyve, Selecta, Horizon, Arthrosi, and Cello Health (all <10K). Speaker fees from Abbvie and Janssen (all <10K)
- Fabio Becce: Personal consultant fees from Horizon Therapeutics; research agreement for dual-energy CT with Siemens Healthineers
- John FitzGerald: Patent pending for lens-free microscope to detect crystals in synovial fluid
- Tim L. Jansen: Consulting fees/compensation for educational talks for Abbvie, Amgen, BMS, Grunenthal, Olatec, Sanofi Genzyme
- Minna J. Kohler: Consultant fees from Novartis, speaker fees from Lilly
- Geraldine M. McCarthy: Consultant for PK Med
- Jasvinder A. Singh: Consultant fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio health, Medscape, WebMD, Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, Practice Point communications, the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals and Charlotte’s Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker’s bureau of Simply Speaking.
- Robert Terkeltaub: Consulting fees for SOBI, Horizon Therapeutics and Astra-Zeneca (all <10K), and Selecta (>10K) and has received research support from Astra-Zeneca
- Abhishek Abhishek: Consulting fees from NGM Biopharmaceuticals (<10K)
The following authors have no disclosures to report: Tristan Pascart, Cattleya Godsave, Burak Kundakci, Raymond P. Naden, William J. Taylor, Tuhina Neogi, Fernando Perez-Ruiz, Ann Rosenthal, Eliseo Pascual, Mariano Andres, Thomas Bardin, Michael Doherty, Hang-Korng Ea, Georgios Filippou, Marwin Guitierrez, Annamaria Iagnocco, Frédéric Lioté, Mark Matza, Roberta Ramonda, Anthony Reginato, Pascal Richette, Francisca Sivera, Alexander So, Lisa K. Stamp, Janeth Yinh, Chio Yokose, Hyon Choi
REFERENCES
- 1.Abhishek A, Neogi T, Choi H, et al. Review: Unmet needs and the path forward in joint disease associated with calcium pyrophosphate crystal deposition. Arthritis Rheumatol 2018;70:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Doherty M, Bardin T, et al. European league against rheumatism recommendations for calcium pyrophosphate deposition. Part i: Terminology and diagnosis. Ann Rheum Dis 2011;70:563–70. [DOI] [PubMed] [Google Scholar]
- 3.Ramonda R, Musacchio E, Perissinotto E, et al. Prevalence of chondrocalcinosis in italian subjects from northeastern italy. The prova study. Clin Exp Rheumatol 2009;27:981–84. [PubMed] [Google Scholar]
- 4.Maravic M, Ea HK. Hospital burden of gout, pseudogout and other crystal arthropathies in france. Joint Bone Spine 2015;82:326–9. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Anderson JJ, Naimark A, et al. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: The framingham study. J Rheumatol 1989;16:1241–5. [PubMed] [Google Scholar]
- 6.Fuller A, Cai K, Diaz-Torne C, et al. Outcome domains reported by patients, caregivers, healthcare professionals and stakeholders for calcium pyrophosphate deposition (cppd): A content analysis based on semi-structured qualitative interviews from the omeract cppd working group. Semin Arthritis Rheum 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai K, Fuller A, Hensey O, et al. Outcome domains reported in calcium pyrophosphate deposition studies: A scoping review by the omeract cppd working group. Semin Arthritis Rheum 2020;50:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheumatol 2015;67:2557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 10.Aringer M, Costenbader K, Daikh D, et al. 2019 european league against rheumatism/american college of rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–9. [DOI] [PubMed] [Google Scholar]
- 11.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: An american college of rheumatology/european league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 12.McCarty DJ. Calcium pyrophosphate dihydrate crystal deposition disease: Nomenclature and diagnostic criteria. Ann Intern Med 1977;87:241–2. [DOI] [PubMed] [Google Scholar]
- 13.Ryan L, McCarty D. Calcium pyrophosphate crystal deposition disease; pseudogout; articular chondrocalcinosis. In: McCarty D, ed. Arthritis and allied conditions. 10th ed. Philadelphia: Lea & Febiger; 1985:1515–46. [Google Scholar]
- 14.Utsinger PD, Resnick D, Zvaifler NJ. Wrist arthropathy in calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum 1975;18:485–91. [DOI] [PubMed] [Google Scholar]
- 15.Tanikawa H, Ogawa R, Okuma K, et al. Detection of calcium pyrophosphate dihydrate crystals in knee meniscus by dual-energy computed tomography. J Orthop Surg Res 2018;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andres M, Vela P, Jovani V, et al. Most needle-shaped calcium pyrophosphate crystals lack birefringence. Rheumatology (Oxford) 2019. [DOI] [PubMed] [Google Scholar]
- 17.Berendsen D, Neogi T, Taylor WJ, et al. Crystal identification of synovial fluid aspiration by polarized light microscopy. An online test suggesting that our traditional rheumatologic competence needs renewed attention and training. Clin Rheumatol 2017;36:641–7. [DOI] [PubMed] [Google Scholar]
- 18.Pascual E, Tovar J, Ruiz MT. The ordinary light microscope: An appropriate tool for provisional detection and identification of crystals in synovial fluid. Ann Rheum Dis 1989;48:983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumbreras B, Pascual E, Frasquet J, et al. Analysis for crystals in synovial fluid: Training of the analysts results in high consistency. Ann Rheum Dis 2005;64:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken) 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh JA, Solomon DH, Dougados M, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum 2006;55:348–52. [DOI] [PubMed] [Google Scholar]
- 22.Fransen J, Johnson SR, van den Hoogen F, et al. Items for developing revised classification criteria in systemic sclerosis: Results of a consensus exercise. Arthritis Care Res (Hoboken) 2012;64:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmajuk G, Hoyer BF, Aringer M, et al. Multi-center delphi exercise reveals important key items for classifying systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2018;70:1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ea HK, Gauffenic A, Nguyen QD, et al. Calcium pyrophosphate crystal deposition in gouty tophi. Arthritis Rheumatol 2020. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition disease. N Engl J Med 2016;374:2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippou G, Adinolfi A, Iagnocco A, et al. Ultrasound in the diagnosis of calcium pyrophosphate dihydrate deposition disease. A systematic literature review and a meta-analysis. Osteoarthritis Cartilage 2016;24:973–81. [DOI] [PubMed] [Google Scholar]
- 27.Cipolletta E, Filippou G, Scire CA, et al. The diagnostic value of conventional radiography and musculoskeletal ultrasonography in calcium pyrophosphate deposition disease: A systematic literature review and meta-analysis. Osteoarthritis Cartilage 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


