Abstract
To study the effects of foliar application of putrescine (distilled water (0), 0.75, 1.5, and 2.25 mM) and water deficit stress (20%, 40%, 60%, and 80% available soil water depletion (ASWD)) on the physiological, biochemical, and molecular attributes of Salvia officinalis L., a factorial experiment was performed in a completely randomized design with three replications in the growth chamber. The results of Real-Time quantitative polymerase chain reaction (qRT-PCR) analysis showed that putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced cineole synthase (CS), sabinene synthase (SS), and bornyl diphosphate synthase (BPPS) relative expression. The highest concentration of 1,8-cineole, camphor, α-thujone, β-thujone, CS, SS, and BPPS were obtained in the irrigation regime of 80% ASWD with the application of 0.75 mM putrescine. There was high correlation between expression levels of the main monoterpenes synthase and the concentration of main monoterpenes. The observed correlation between the two enzyme activities of ascorbate peroxidase (APX) and catalase (CAT) strongly suggests they have coordinated action. On the other hand, the highest peroxidase (PO) and superoxide dismutase (SOD) concentrations were obtained with the application of 0.75 mM putrescine under the irrigation regime of 40% ASWD. Putrescine showed a significant increase in LAI and RWC under water deficit stress. There was an increasing trend in endogenous putrescine when putrescine concentration was increased in all irrigation regimes. Overall, the results suggest that putrescine may act directly as a stress-protecting compound and reduced H2O2 to moderate the capacity of the antioxidative system, maintain the membrane stability, and increase secondary metabolites under water deficit stress.
Subject terms: Agricultural genetics, Plant sciences, Plant genetics, Plant physiology, Plant stress responses, Secondary metabolism
Introduction
Water deficit stress is considered the most limiting environmental factor for the growth and yield of many plant species in arid and semi-arid regions. Water deficit stress affects plants at the morphological, physiological, biochemical, and molecular level1.One way to deal with water shortages is to cultivate herbs that have more tolerance. Generally, 49% of medicinal and aromatic plants whose native habitat is the Mediterranean region are notably tolerant and adaptable to dry conditions2. In addition to their economic value, these plants are proper crops for cultivation in dry areas3. There is a remarkable demand for sage (Salvia officinalis L.). and various related products all over the world. Accommodating this demand requires an increase in its cultivation area under water deficit conditions, particularly in arid and semi-arid areas. Sage was introduced in old Latin as a medicinal plant. The genus salvia is one of the best known genera due to monoterpenes as major compounds used in food and pharmaceutical industries4. Geranyl diphosphate (GPP) is the widely accepted common substrate for monoterpene biosynthesis. Three of the most important monoterpene synthases in sage are cineole synthase, sabinene synthase and bornyl diphosphate synthase. The cineole synthase (CS) produces in one step 1,8-cineole. The ( +)-sabinene synthase (SS) catalyzes the production of sabinene, which undergoes further rearrangements leading to the two major monoterpenes α- and β-thujone. The ( +)-bornyl diphosphate synthase (BPPS) produces bornyl diphosphate, which is subsequently hydrolyzed to borneol and then oxidized to camphor5. These compounds have potent antioxidative and anti-inflammatory effects6.
Zwenger and Basu7 reported that many factors, i.e. water deficit, nutrient, ozone, and mechanical stress, contribute to the up-adjustment of terpene synthesis genes in Arabidopsis. The essential oil yield and compounds in plants may change in water deficit stress. Plants generally produce higher levels of secondary metabolites under water deficit stress8. Increases in percentages of essential oils and main compounds have been reported in both Ocimum basilicum and Ocimum americanum under water deficit stress9. A study by Nowak et al.10 determined that the total content of monoterpenes in sage is significantly increased by moderate water deficit stress. The production of secondary metabolites, including glycyrrhizin, in liquorice plants and gene expression levels involved in their biosynthesis are strongly correlated to growth conditions11. It has been suggested that signal molecules may be employed directly or indirectly in the production of plant secondary metabolites12. The signal components involved in monoterpene biosynthesis might therefore serve as potential allelochemicals for the induction of monoterpenes. Researchers have found that the expression of monoterpene synthase increased with increasing concentrations of gibberellin but decreased when gibberellin biosynthesis was blocked with daminozide. Increasing concentrations of gibberellin also increased 1,8-cineole and camphor contents13.
Water deficit stress reduces plant growth by reducing cell turgor and relative water content (RWC), which in turn decreases cell elongation and development, consequently reducing leaf area14, and an imbalance between antioxidant defenses and the amount of reactive oxygen species (ROS) leads to oxidative stress under water deficit conditions15. Excessive accumulation of ROS can destabilize cell membranes and cause direct damage to DNA, pigments, proteins, lipids, and other essential cellular molecules, leading to cell death and loss of biomass16. Enhanced antioxidant enzyme activity helps in ROS scavenging and protecting against stress16. superoxide dismutase (SOD), peroxidase (PO), catalase (CAT), and Ascorbate peroxidase (APX) activity is responsible for quenching ROS. Indeed, they are usually activated when excessive ROS is generated to protect plants against oxidative damage under various types of stress17. As the first line of protection against ROS, SOD catalyzes the anion superoxide (O2−) to O2 and H2O2; then CAT catalyzes the decomposition of the H2O2 to water and molecular oxygen and PO works in the extra-cellular space for scavenging H2O2. PO expression was increased by the application of putrescine and PEG18. A survey of the literature indicated that salicylic acid (SA) can affect antioxidant enzyme activities and cause a moderate increase in the content of ROS such as hydrogen peroxide (H2O2)19, which acts as a second messenger in regulating plant defense responses.
Polyamines (PAs) are considered plant growth regulators with multiple functions and are implicated in many processes of plant growth and development, such as cell division and DNA replication. In plant cells, putrescine, spermidine and spermine are three major constituents of PAs20. Putrescine is produced from arginine or ornithine, while putrescine and S-adenosylmethionine are precursors for production of spermidine and spermine21. Overexpression of arginine decarboxylase and S-adenosylmethionine decarboxylase, the key genes for polyamine synthesis, resulted in elevated levels of putrescine, spermidine and spermine and increased water deficit stress tolerance in transgenic rice and tobacco plants22, while down regulation of S-adenosylmethionine decarboxylase gene declined spermidine and spermine levels and PAs and led to decreased water deficit stress tolerance in transgenic rice plants23.
A prevalent physiological response in various plants under abiotic stress is the accumulation of proline. Proline, as a component of stress signal transduction pathways, could be the nitrogen and carbon source needed for stress recovery and finally to help enhance stress tolerance24. Kotakis et al.25 announced that putrescine (as buffer and osmolite), inducing increase in proline content, which leads to maintenance of leaf water status under stress. Excessive expression of putrescine revealed both the up- and down-regulation of various stress-responsive, hormone and signaling-related genes, involved in the biosynthesis of auxin, ethylene, ABA, gibberellin and SA. Also, genes for auxin transport, genes coding for auxin-responsive proteins, ethylene and ABA-responsive transcriptional factors, and also jasmonate -induced proteins were also noticed, which confirm the dual role of putrescine and PAs in general: direct protection and participation in acclimation signaling pathways26. ROS derived from PAs oxidation is urgent in order to trigger stress response signaling. However, the size and rate of its accumulation determines cell fate, which means that ROS should not exceed specific thresholds; if so, it no longer signals the expression of stress genes but, instead, triggers programmed cell death27. Typically, when cellular PAs contents are up, their catabolism also augments, the levels of H2O2 increase, and various ROS as well as the anti-oxidative system (enzymatic and non-enzymatic) is also induced, hence their roles in preventing damage from stress are beneficial as well as deleterious. This is consistent with the notion that an augment in cellular PAs titers contributes to both sides of the ROS-anti-oxidative equation under stress28. Exogenous PAs help to improve the function and activation of antioxidant enzymes under water deficit stress.
In generally, secondary metabolites (such as isoprenoids, phenols or alkaloids) production can be directly enhanced by using deliberate water deficit stress. This enhancement can be reached by applying special irrigation regimes that are both simple and inexpensive, but this approach requires intense examination to optimize secondary metabolites (such as isoprenoids, phenols or alkaloids)8. In the current study, putrescine in different concentrations was employed as an elicitor to study the physiological, biochemical, and molecular changes in sage under water deficit stress. The main goal of this study was to determine the effects of water deficit stress and putrescine on the contents of three main monoterpenes in sage. It was hypothesized that the expression pattern of the genes involved in monoterpene biosynthesis pathways and their connection with increases in essential oil compounds under water deficit stress and putrescine may facilitate the production of economically valuable medicinal plants through genetic engineering techniques.
Materials and methods
Plant material, growth conditions, and sampling
The plants used are not wild and cuttings were prepared from one-year-old mother plants of Salvia officinalis L. in the medicinal farm of the Institute of Medicinal Plants & Natural Products Research, Iranian Academic Center for Education, Culture & Research (ACECR) (Karaj, Iran). A total of 48 sage plants were sown in 48 plastic pots with a diameter of 19 cm, height of 15.2 cm and volume of 3 L, containing a 1: 1: 1 mixture of (peatmoss: soil: sand). The physical and chemical properties of the field soil were assessed as follows: (Total N: 0.121%; T.N.V: 5.5%; organic carbon: 1.034%; field capacity (FC): 22.51%; permanent wilting point (PWP): 8.01%; E.C: 2.610 dSm−1, texture: Silt loam; pH: 7.51. Initial soil test values (mg kg−1) for P, K, Fe, Mn, Zn, Cu and B were 269, 68.8, 4.17, 8.91, 0.86, 1.09, and 1.77, respectively. Fertilization was not done. The growth conditions were 16/8 h (light/dark) and the light intensity was 300 μmol photons m−2 s−1 29,30. Also, heating, cooling, relative humidity and vapor pressure deficit of growth chamber were set with 65 Ḟ, 75 Ḟ, %70 and 0.55 kPa, respectively. A factorial experiment was performed in a completely randomized design (CRD) with three replications. Treatments included irrigation regimes and putrescine, as follows:
Irrigation after depletion of 20% available soil water (control).
Irrigation after depletion of 40% available soil water.
Irrigation after depletion of 60% available soil water.
Irrigation after depletion of 80% available soil water.
Four concentrations of putrescine (distilled water (0), 0.75, 1.5, and 2.25 mM) were also applied. Foliar application of putrescine was performed one week before applying irrigation regimes. To investigate proper treatments, plants must have similar masses of foliage before treatments are applied. The plant height, number of nodes, number of leaves per plant and leaf area were 24 cm, 11, 52 and 8 cm2, respectively. Therefore, no water stress or putrescine were applied in the first 14 days of the growth cycle. During this period, all pots were irrigated when 20% of the available soil water was depleted (ASWD). The pots in unstressed control were irrigated daily and distance between upper and lower limits of water uptake was 20% available soil water which was determined by TDR. After leaf emergence, foliar application was conducted with 15 mL of putrescine for all aboveground parts of each plant. The depth of the irrigation water was assigned based on the available soil water and calculated using the following equations:
| 1 |
| 2 |
| 3 |
where FC and PWP (%) are the volumetric soil water amounts, D (cm) is the soil layer depth, Id is the irrigation depth (cm), ρ is the fraction of ASW that can be depleted from the root zone (20%, 40%, 60%, and 80%), Ig is the coarse depth of irrigation (cm), and Ea is the irrigation efficiency (%) assumed at an average of 65%31. The applied irrigation water was measured (based on Eq. (3)) at each irrigation32. Irrigation treatments were implemented based on the maximum allowable depletion (MAD) from the percentage of ASWD. Each treatment was irrigated when the available soil water reached its threshold value31. The applied treatments were 20%, 40%, 60%, and 80% MAD of ASWD. A TDR probe (Time-Domain Reflectometry, Model TRIME-FM, Germany) was applied to measure soil water amount at a depth of 12.5 cm (root zone of sage). TDR probes were taken from the first experiment to the last experiment. Sampling was conducted from the sage leaves one week after applying the irrigation regimes. Also, samples from all treatments were collected on the same day in the morning. The expression rates were considerably higher in young leaves (first and second nodes) than in fully mature leaves (fifth and upper nodes)30. Accordingly, only first and second node leaves were used. These samples were cleaned with ethanol and paper tissues to remove any surface contamination, immediately frozen in liquid nitrogen, and stored at − 80 °C for use in measuring physiological attributes and RNA extraction.
RWC, LAI (leaf area index) and putrescine endogenous
The length of time for the discs to reach saturation varies with species and conditions. Studies have shown that water uptake by leaf discs is initially rapid for several hours, followed by a slower rate uptake that can persist as long as the floated discs remain healthy. This usually occurs within 3 to 6 h. Our tests have shown that water uptake by leaf discs is initially rapid for four hours, followed by a slower rate uptake that could continue as 6 h as the floated discs remain healthy. On the other hand, errors arising from growth was minimized by growth inhibitors or by floating the discs at 4 °C. The water content of plants was easily determined by weighing the material immediately after sampling, drying at 75 °C and reweighing 24 h later.
To calculate RWC, 20 leaf discs (around 1 cm in diameter) were punched, and their fresh weight (FW) was recorded. The same leaf discs were kept in petri dishes containing distilled water for six hours to record saturation weight (SW), and after that discs were oven dried at 75 °C for 24 h to record the dry weight (DW). Calculations were performed using the formula: RWC = (FW – DW)/ (SW − DW)33.
Leaf tissue was ground under liquid nitrogen using a mortar and pestle. Putrescine extraction followed by HPLC quantification were carried out in accordance with Lütz et al.34.
RNA preparation and cDNA synthesis
Total RNAs were extracted from the sage leaves using a RNeasy Plant Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. RNA quality and concentrations were determined using 1.0% agarose gel electrophoresis and analysis by a NanoDrop 2000/2000c Spectrophotometer (Wilmington, DE 19,810 U.S.A.). Moreover, cDNA was synthesized with a SuperScript III Reverse Transcriptase reagent kit (Thermo Fisher Scientific, USA).
Real-Time quantitative polymerase chain reaction (qRT-PCR)
qRT-PCR Primers for the assayed genes were designed according to the GenBank accession (Table 1). qRT-PCR was conducted with a Corbett Model (Rotor gene 6000). The qRT-PCR reaction for target gene transcript amplification was carried out in a final volume of 25 μL containing PCR buffer, 2.5 μL MgCl2, 0.5 μL dNTP, 2.5 μL of SYBR green I (Sigma-Aldrich), 0.1 μL of Taq, 1.25 μL of each forward and reverse primer, and 2 μL diluted cDNA. The PCR reaction conditions were denaturation at 95 °C for 5 min followed by 45 cycles at 95 °C for 20 s, primer annealing at 60 °C for 20 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 2 min. Melting curve analysis include 95 °C for 60 s, 55 °C for 90 s, and 60 °C for 1 s. A housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase C2 (GapC2), was used as the control to account for variations in cDNA template levels35,36. All reactions were done in triplicate.
Table 1.
Description of reference genes and the monoterpene synthase primer sequences for qRT-PCR.
| Gene | GeneBank accession number | Primer name | Primer sequence |
|---|---|---|---|
| 1 ,8 cineole synthase | AF051899 | CS (FW) | 5́-TTCAAGCACAATTTCAACAAGAG-3ʹ |
| CS (RV) | 5́-AGCGTACCATAAATATCAAAGAC-3ʹ | ||
| Bornyl diphosphate synthase | AF051900 | B-PP-S (FW) | 5́-TATTTCACAGCTCTTGGATTCAG-3ʹ |
| B-PP-S (RV) | 5́-TGTAACATTCCCTTCGTATCTTG-3ʹ | ||
| Sabinene synthase | AF051901 | SS (FW) | 5́-AGGTGGTGATGAAATTGATGAAG-3ʹ |
| SS (RV) | 5́-ATATTGAAGTTGAGTTTGGCGAG-3ʹ | ||
| Glyceraldehyde-3-phosphate dehydrogenase C2 | JN083806.1 | Gap C2 (FW) | 5́-CAGTGTATTGATGGATGGTATTC-3ʹ |
| Gap C2 (RV) | 5́-CCAAACTCACTTACTTCAAACAG-3ʹ |
Essential oil extraction
The shade-dried foliage of collected sage was extracted by hydro-distillation in a Clevenger device with double-distilled water. The obtained essential oil was separated from the aqueous layer using a 100 ml capacity separatory hopper (a piece of laboratory glassware used in liquid–liquid extractions). The collected surplus aqueous essential oil was dried over anhydrous sodium sulfate. After extraction, the essential oil was conserved in vials sealed at 4 °C until GC/MS evaluation.
Identification of essential oil composition (GC–MS analysis)
Gas chromatography/mass spectrometry (GC/MS) analysis was accomplished using a Thermoquest-Finnigan TRACE GC–MS instrument (Manchester, UK) provided with the same gas chromatography situation as mentioned for the GC analyses. The percentages of compounds were calculated by the area normalization method on GC–MS chromatogram, without considering response factors. Helium was utilized as the vector gas at a flow rate of 1.1 ml min−1 with a split ratio of 1:50, and ionization voltage was 70 eV. The constituents of the essential oil were analyzed based on the retention index (RI) of the series of n-terpenes (C5h8)n and the oil on a Ph-5 column under the same chromatographic conditions. Single constituents were identified based on comparisons of their mass spectra fragmentation designs with standard ones found in the literature in wiley libraries or with those of valid compounds37.
Glycine betaine (GB), proline and total reducing sugars (TRS)
GB was determined using the method of Grieve and Grattan38, in which 5 ml of toluene-water mixture (0.5% toluene) was added to 0.1 g dried ground material. Afterwards, tubes were shaken for 24 h at 25 °C, the extract was filtered, and the volume was made to equal 100 ml. Then, 1 ml of 2 N HCl solution was added to 1 ml of filtrate, and an aliquot of 0.5 ml from this solution was mixed with 0.1 ml of potassium tri-iodide solution. After placing the mixture in an ice bath for 90 min, 4 ml of 1,2 dichloroethane was added to it. The optical density was determined spectrophotometrically at 365 nm.
Proline was estimated using the method described by Bates et al.39.
To measure total reducing sugars, the method introduced by Dubois et al.40 was employed.
Antioxidant enzymes (SOD, PO, APX and CAT) and 2,2-diphenyl-1-picrylhydrazyl (DPPH)
Leaf samples (500 mg) were pulverized in Na–P buffer (pH 7.0) containing 1 mM EDTA and 1% soluble polyvinyl pyrrolidone (PVP) using a mortar and pestle. The leaf extract was then centrifuged at 12,000 g for 20 min at 4 °C. The enzyme extracts obtained were used to determine the activity levels of SOD, CAT, PO, and APX enzymes. Enzyme activity was expressed as enzyme unit (EU) mg−1 protein. Furthermore, the extraction buffer for ascorbate peroxidase enzyme contained 1 mM ascorbic acid.
The protein concentration was analyzed using the method reported by Bradford41.
To estimate SOD activity (EC 1.15.1.1), the Bayer and Fridovich42 method was used. Briefly, 3 ml reaction mixture (containing 100 mM phosphate buffer with pH 7.8), 13 mM methionine, 75 µM NBT, 0.1 mM EDTA, 2 µM riboflavin, and 100 µL enzyme extract was incubated under a light intensity of 3600 lx for 15 min, and the reaction was stopped by switching off the light. The absorbance was read at 560 nm.
PO activity (EC 1.11.1.7) was determined utilizing the method introduced by Herzog and Fahimi43. The reaction mixture contained 0.15 M sodium phosphate-citrate buffer (pH 4.4), 50% (w/v) gelatin, 0.6% H2O2, and 5 μl enzyme extract. Absorption levels were read at 465 nm (extinction coefficient 2.47 mM−1 cm−1) for 3 min.
To estimate APX activity (EC 1.11.1.11), the Nakano and Asada44 method was employed. The reaction mixture contained 50 mM Na–P buffer with pH 7.0, 0.5 mM ascorbic acid, 0.1 mM EDTA-Na2, 0.12 mM H2O2, and 20 μl of enzyme extract. Absorbance was read at 290 nm.
CAT activity (EC.1.11.1.6) was determined using the method of Aebi45. Enzyme extract (100 µl) was added to 900 µl reaction solution (containing 50 mM sodium phosphate buffer with pH 7.0 mM H2O2), and absorbance was read at 240 nm.
The free radical scavenging activity of DPPH was determined spectrophotometrically as described by Hung et al.46. In brief, 2 mL of different extract solutions (16–500 g/ml) were mixed with 2 mL of DPPH solution. The mixture was allowed to stand for 30 min to achieve complete reaction at room temperature. Finally, the absorbance of samples was determined at 515 nm.
H2O2 and lipid peroxidation (MDA)
To calculate leaf H2O247, 100 mg fresh tissue was extracted with 5 mL trichloro acetic acid (0.1%) and then centrifuged at 10,000 rpm for 10 min. Finally, supernatant was mixed with potassium phosphate buffer (pH 7.0) and potassium iodide (KI), and absorbance was read at 390 nm. Fresh plant was ground into a fine powder in liquid nitrogen and homogenized with 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was then centrifuged at 12,000 rpm. Aliquots of the supernatant were mixed with 0.5% 2-thiobarbituric acid (TBA) (prepared in 20% TCA). First, the mixture was heated to 95 °C and then cooled on ice. The mixture was further centrifuged at 12,000 rpm. The absorbance of the supernatant was read at 532 and 600 nm.
The MDA content was calculated using its absorption coefficient at 155 mM−1 cm−1, after the non-specific absorbance was reduced to 600 nm. Finally, MDA content was expressed as nmol g-1 of fresh weight48.
Statistical analysis
Amplification data was analyzed using Rotor-Gene 6000 Series software (version 1.7). The threshold cycle (Ct) values of the triplicate PCRs were averaged and the relative quantification of the transcript levels was determined using the comparative Ct method 49. The Ct value of the calibrator (the sample with the highest Ct value) was subtracted from every other sample to produce the ∆∆Ct value, and 2-∆∆Ct was taken as the relative expression level for each sample. The data was subjected to analysis of variance (ANOVA) using SAS 9.3 software (SAS Institute, Cary, NC, USA). Mean comparisons were evaluated using the LSD Test at 5% probability levels. Pearson’s correlation coefficients were determined using the CORR procedure.
Results
RWC, LAI and endogenous putrescine
Based on the results of analysis of variance, putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced RWC, LAI and endogenous putrescine (Table 2).
Table 2.
Analysis of variance (mean square) of relative water content (RWC), endogenous putrescine, cineole synthase (CS), sabinene synthase (SS), bornyl diphosphate synthase (BPPS), 1,8-cineole, α-thujone, β-thujone, camphor, glycine betaine (GB), proline, total reducing sugars (TRS), superoxide dismutase activity (SOD), peroxidase activity (PO), ascorbate peroxidase activity (APX), catalase activity (CAT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Hydrogen peroxide (H2O2), lipid peroxidation (MDA) and leaf area index (LAI) of Salvia officinalis influenced by irrigation regimes (I) and putrescine concentrations (P).
| Sources of variation | I | P | I × P | Error |
|---|---|---|---|---|
| DF | 3 | 3 | 9 | 32 |
| RWC | 21,259.03** | 67,051.47** | 23,922.83** | 83.19 |
| Endogenous P | 10,521.54** | 325,915.86** | 8066.24** | 184.94 |
| CS | 0.11** | 0.24** | 0.05** | 0.0005 |
| SS | 0.21** | 0.64** | 0.21** | 0.0007 |
| BPPS | 0.004** | 0.006** | 0.002** | 0.0001 |
| 1,8-cineole | 15.50** | 1066.01** | 5.06** | 0.88 |
| α-thujone | 88.49** | 435.27** | 19.70** | 0.36 |
| β-thujone | 150.71** | 1025.30** | 42.33** | 0.48 |
| camphor | 63.12** | 350.47** | 18.57** | 0.23 |
| GB | 0.21** | 0.64** | 0.21** | 0.0007 |
| Proline | 15.50** | 1066.01** | 5.06** | 0.88 |
| TRS | 0.004** | 0.006** | 0.002** | 0.0001 |
| SOD | 63.12** | 350.47** | 18.57** | 0.23 |
| PO | 88.49** | 435.27** | 19.70** | 0.35 |
| APX | 102.90** | 856.03** | 32.37** | 0.33 |
| CAT | 150.71** | 1025.31** | 42.33** | 0.48 |
| DPPH | 21,259.03** | 67,051.47** | 23,922.83** | 83.19 |
| H2O2 | 10,521.54** | 325,915.86** | 8066.24** | 184.94 |
| MDA | 0.11** | 0.24** | 0.05** | 0.0005 |
| LAI | 102.90** | 856.03** | 32.37** | 0.33 |
ns: non-significant.
*α ≤ 0.05.
**: α ≤ 0.01.
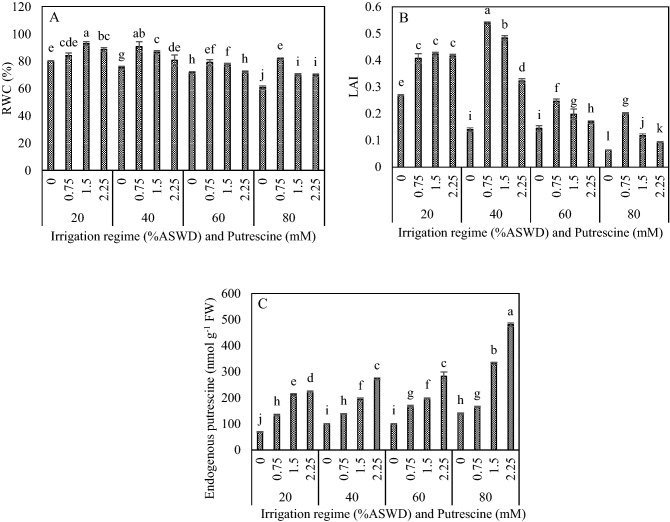
The current results showed a decreasing trend in RWC with increasing water deficit stress. The highest RWC was obtained with the application of 0.75 and 1.5 mM putrescine under the irrigation regimes of 80% and 20% ASWD, respectively (Fig. 1A). Generally, the highest RWC (92.93%) was observed with the application of 1.5 mM putrescine under the irrigation regime of 20% ASWD (Fig. 1A). LAI was decreased under water deficit stress. The highest LAI was observed with the application of 0.75 mM putrescine under the irrigation regimes of 40%, 60%, and 80% ASWD (Fig. 1B). Generally, the highest LAI (0.54) was shown with the application of 0.75 mM putrescine under the irrigation regime of 40% ASWD (Fig. 1B). The current results revealed an increasing trend in endogenous putrescine when putrescine concentration was increased under irrigation regimes of 20%, 40%, 60%, and 80% ASWD. Moreover, the highest concentration of endogenous putrescine was obtained with the application of 2.25 mM putrescine under irrigation regimes of 20%, 40%, 60%, and 80% ASWD (Fig. 1C). Generally, the highest concentration of endogenous putrescine (480.00 nmol g−1 FW) resulted from the application of 2.25 mM putrescine under the irrigation regime of 80% ASWD (Fig. 1C).
Figure 1.
Interaction between irrigation regime and putrescine on RWC (A), LAI (B) and Endogenous putrescine (C). The different letters show significantly different at the level of 0.01. The error bars represent standard error.
Gene Expression Assay
Expression analysis revealed that putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced the relative expression levels of CS, SS, and BPPS (Table 2).
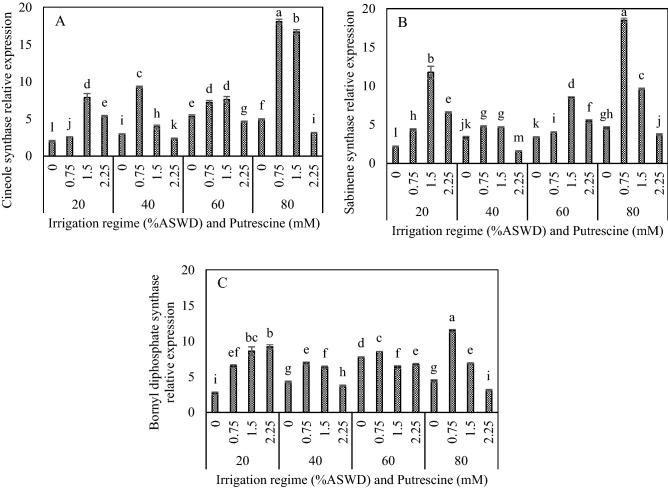
The CS, SS, and BPPS relative expression levels were increased under water deficit stress (Fig. 2A,B,C). The highest CS and SS relative expression levels were obtained with the application of 1.5 mM putrescine under the irrigation regime of 20% ASWD (Fig. 2A,B). The highest CS and BPPS relative expression level was achieved with the application of 0.75 mM putrescine under the irrigation regime of 40% ASWD (Fig. 2A,C). The highest SS and BPPS relative expression levels were obtained with the application of 1.5 and 0.75 mM putrescine under the irrigation regime of 60% ASWD, respectively (Fig. 2B,C). Generally, the highest relative expression levels of CS (18.06), SS (18.48), and BPPS (11.46) resulted from the application of 0.75 mM putrescine under the irrigation regime of 80% ASWD (Fig. 2A,B,C).
Figure 2.
Interaction between irrigation regime and putrescine on cineole synthase (A), sabinene synthase (B) and bornyl diphosphate synthase (C) relative expression. The different letters show significantly different at the level of 0.01. The error bars represent standard error.
Concentrations of 1, 8-cineole, α-thujone, β-thujone, and camphor
The results of GC/MS analyses revealed that the main compounds of the essential oils were α- thujone, β-thujone, camphor, and 1,8-cineole, respectively. These compounds accounted for more than 95% of the total monoterpenes amount. Therefore, the regulation of the biosynthesis of 1,8-cineole, camphor, α- thujone, and β-thujone was investigated in this study. The ANOVA results showed that putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced the concentrations of 1,8-cineole, α-thujone, β-thujone, and camphor (Table 2).
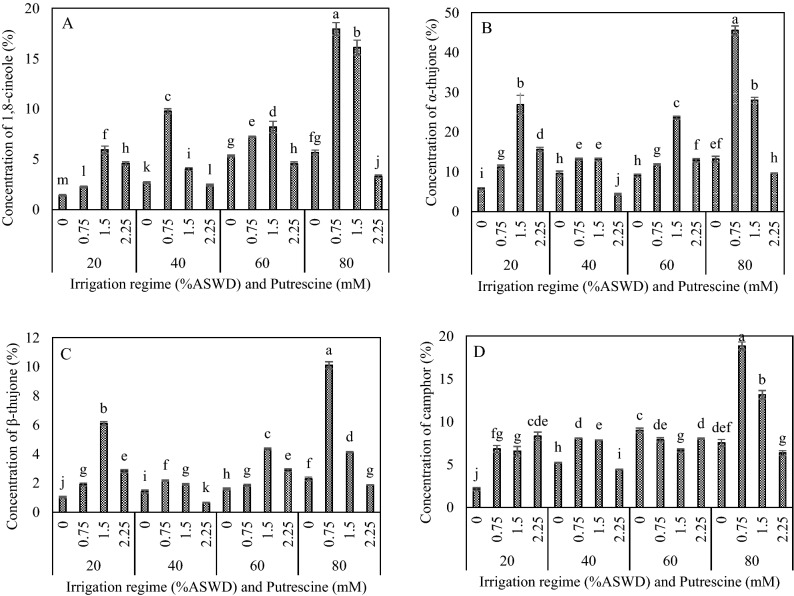
The concentrations of 1,8-cineole, α-thujone, β-thujone, and camphor were increased under water deficit stress (Fig. 3A,B,C,D). Also, the highest concentrations of 1,8-cineole, α-thujone, and β-thujone were obtained under irrigation regime of 80% compared to irrigation regime of 20% (Fig. 3A,B,C). The highest concentrations of 1,8-cineole, α-thujone, and β-thujone were obtained with the application of 1.5 mM putrescine under irrigation regimes of 20% and 60% ASWD (Fig. 3A,B,C). The highest concentration of camphor was observed with the application of 2.25 mM putrescine and distilled water under irrigation regimes of 20% and 60% ASWD, respectively (Fig. 3D). The highest concentrations of 1,8-cineole, β-thujone, and camphor were obtained with the application of 0.75 mM putrescine under irrigation regimes of 40% ASWD (Fig. 3A,C,D). Generally, the highest concentration of 1,8-cineole (17.95%), α-thujone (45.50%), β-thujone (10.10%), and camphor (18.83%) were observed in the irrigation regime of 80% ASWD with the application of 0.75 mM putrescine (Fig. 3A,B,C,D).
Figure 3.
Interaction between irrigation regime and putrescine on concentration of 1,8-cineole (A), α-thujone (B), β-thujone (C) and camphor (D). The different letters show significantly different at the level of 0.01. The error bars represent standard error. (Retention Index = 1031, 1103, 1115 and 1144, respectively).
Glycine betaine (GB), proline and total reducing sugars (TRS)
The analysis of variance results showed that putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced significantly GB, proline, and TRS (Table 2).
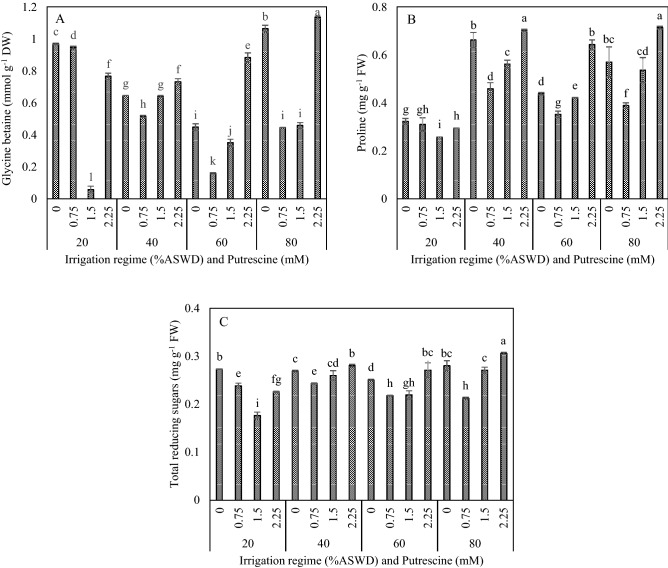
The highest GB and proline contents were obtained under irrigation regime of 80% compared to irrigation regime of 20% (Fig. 4A,B). The lowest GB, proline and TRS contents were obtained with the application of 1.5 and 0.75 mM putrescine under irrigation regimes of 20% and 40% ASWD, respectively (Fig. 4A,B,C). Moreover, the lowest GB and proline content were observed with the application of 0.75 mM putrescine under irrigation regime of 60% ASWD (Fig. 4A,B). Generally, the lowest GB (0.06 mmol g−1 DW), proline (0.26 mg g−1 FW), and TRS (0.18 mg g−1 FW) contents were obtained with the application of 1.5 mM putrescine under the irrigation regime of 20% ASWD (Fig. 4A,B,C).
Figure 4.
Interaction between irrigation regime and putrescine on glycine betaine (A), proline (B) and total reducing sugars (C). The different letters show significantly different at the level of 0.01. The error bars represent standard error.
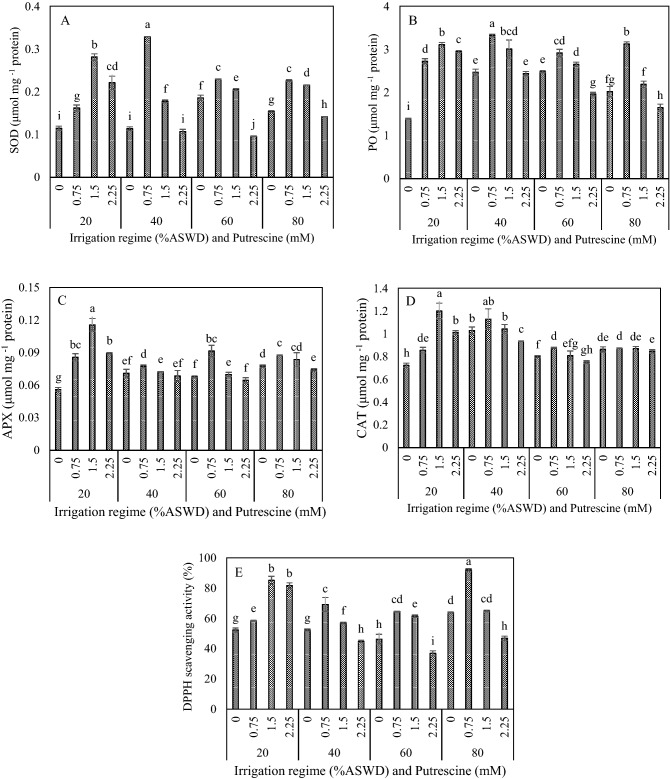
Antioxidant enzymes (SOD, PO, APX, and CAT) and DPPH
Based on the results of the analysis of variance, putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced antioxidant enzymes (SOD, PO, APX, and CAT) and DPPH levels (Table 2).
SOD, PO, APX, CAT, and DPPH values were increased under water deficit stress (Fig. 5A,B,C,D,E). The highest SOD, PO, and APX values were obtained with the application of 1.5, 0.75, and 0.75 mM putrescine under the irrigation regimes of 20%, 40%, and 60% ASWD, respectively (Fig. 5A,B,C). Moreover, the highest SOD and PO values were observed with the application of 0.75 mM putrescine under the irrigation regime of 80% ASWD (Fig. 5A,B). Furthermore, the highest CAT resulted from the application of 1.5 and 0.75 mM putrescine under the irrigation regimes of 20% and 60% ASWD, respectively (Fig. 5D). Generally, the highest SOD (0.33 μmol mg−1 protein) and PO (3.32 μmol mg−1 protein) values were obtained with the application of 0.75 mM putrescine under the irrigation regime of 40% ASWD (Fig. 5A,B), and the highest APX (0.11 μmol mg−1 protein) and CAT (1.20 μmol mg−1 protein) levels were obtained with the application of 1.5 mM putrescine under the irrigation regime of 20% ASWD (Fig. 5C,D). The highest DPPH was also obtained by applying 0.75 mM putrescine under the irrigation regimes of 40%, 60%, and 80% (Fig. 5E). Generally, the highest DPPH (91.89%) was observed with the application of 0.75 mM putrescine under the irrigation regime of 80% (Fig. 5E).
Figure 5.
Interaction between irrigation regime and putrescine on SOD (A), PO (B), APX (C), CAT (D) and DPPH (E). The different letters show significantly different at the level of 0.01. The error bars represent standard error.
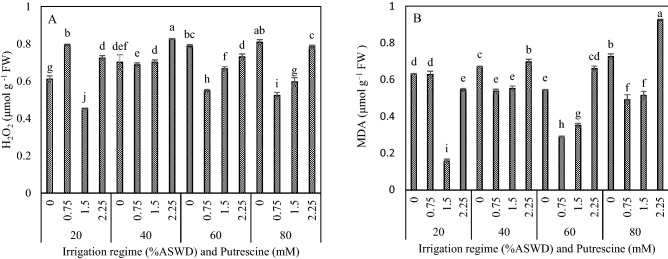
H2O2 and MDA
The analysis of variance results showed that putrescine concentration, irrigation regime, and the two-way interaction between irrigation regime and putrescine concentration significantly influenced significantly H2O2 and MDA (Table 2).
The values of H2O2 and MDA increased under water deficit stress (Fig. 6A,B). The lowest H2O2 and MDA were obtained with the application of 1.5 and 0.75 mM putrescine under irrigation regimes of 20% and 60% ASWD, respectively (Fig. 6A,B). Moreover, the lowest H2O2 was obtained with the application of 0.75 mM putrescine under irrigation regime of 80% (Fig. 6A). Generally, the lowest H2O2 (0.45 μmol g−1 FW) and MDA (0.16 μmol g–1 FW) content were obtained with the application of 1.5 mM putrescine under the irrigation regime of 20% ASWD (Fig. 6A,B).
Figure 6.
Interaction between irrigation regime and putrescine on H2O2 (A) and MDA (B). The different letters show significantly different at the level of 0.01. The error bars represent standard error.
Discussion
The current results showed a significant increase in compatible osmolytes (proline and GB) under the irrigation regime of 80% ASWD. Compatible osmolytes aid in the maintenance of turgor and stabilize macromolecular structures in response to stress50. The increase in sage proline in irrigation regimes of 40%, 60% and 80% indicates the important role of this osmolyte under water deficit stress. Indeed, the accumulation of proline and GB in stressed sage plants maintain membrane integrity, reduce oxidation of lipid membranes, stabilize ROS scavenging enzymes, and scavenge free radicals51. Generally, increases in compatible osmolytes stabilize redox potential and NAD(P) + /NAD(P)H ratio to prevent oxidative damage under stress conditions52.
In present study, LAI reduced under water deficit stress. On the other hand, this decrease was greater in irrigation regimes of 60% and 80% than 40% ASWD, which could be due to leaves with a smaller size and a higher falling rate. Exogenous application of 0.75 mM putrescine under irrigation regimes of 40%, 60% and 80% ASWD improved leaf area index. The highest LAI was obtained in irrigation regime of 40% ASWD under exogenous application of 0.75 mM putrescine. This improvement may be attributed to maintenance of LAI (assimilatory surface) due to high turgor and sustained photosynthetic ability of the crop by protecting the photosynthetic machinery from reactive oxygen species produced during drought stress and by increasing Rubp content under drought condition. Similarly, the positive effects of exogenously putrescine on LAI and tolerance to abiotic stresses in different plant species have been reported by others53.
In response to water deficit stress, RWC decreased in sage plants. Decreases in RWC subject the cell membranes to changes such as penetrability and decreased sustainability under water deficit stress54, that probably aims to create osmotic adjustment55. There was a negative correlation between proline and RWC (− 0.56; P < 0.05) (Table 3). Indeed, the decrease in RWC in irrigation regime of 80% AWSD were possibly due to the accumulation of proline and GB. Foliar application of putrescine alleviated the detrimental effects of water deficit stress and increased RWC considerably. These results are concordant with those of Farooq et al.53. The possible effect of foliar spray is related to the fact that while in direct contact with leaf surface, PAs improved the water status of epidermal cells and underlying cells53. It seems that the contribution of putrescine to osmotic adjustment can be considered as a mechanism to retain RWC for better growth and productivity. Two reasons for the responses of PAs under different adverse environmental conditions might be the ability to scavenge ROS and adjust osmosis53.
Table 3.
Pearson’s correlation coefficients among relative water content (RWC), endogenous putrescine, cineole synthase (CS), sabinene synthase (SS), bornyl diphosphate synthase (BPPS), 1,8-cineole, α-thujone, β-thujone, camphor, glycine betaine (GB), proline, total reducing sugars (TRS), superoxide dismutase activity (SOD), peroxidase activity (PO), ascorbate peroxidase activity (APX), catalase activity (CAT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Hydrogen peroxide (H2O2), lipid peroxidation (MDA) and leaf area index (LAI) of Salvia officinalis influenced by irrigation regimes and putrescine concentrations.
| RWC | Endogenous putrescine | CS | SS | BPPS | 1,8-Cineole | α-thujone | β-thujone | Camphor | GB | Prolin | TRS | SOD | PO | APX | CAT | DPPH | H2O2 | MDA | LAI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RWC | 1 | |||||||||||||||||||
| Endogenous putrescine | − 0.22 ns | 1 | ||||||||||||||||||
| CS | 0.04 ns | 0.10 ns | 1 | |||||||||||||||||
| SS | 0.22 ns | 0.07 ns | 0.83** | 1 | ||||||||||||||||
| BPPS | 0.41 ns | − 0.14 ns | 0.66** | 0.73** | 1 | |||||||||||||||
| 1,8-Cineole | − 0.06 ns | 0.14 ns | 0.98** | 0.74** | 0.58* | 1 | ||||||||||||||
| α-thujone | 0.15 ns | 0.08 ns | 0.88** | 0.99** | 0.71** | 0.81** | 1 | |||||||||||||
| β-thujone | 0.19 ns | 0.05 ns | 0.80** | 0.99** | 0.71** | 0.70** | 0.97** | 1 | ||||||||||||
| Camphor | − 0.05 ns | 0.11 ns | 0.88** | 0.80** | 0.75** | 0.85** | 0.83** | 0.78** | 1 | |||||||||||
| GB | − 0.42 ns | 0.20 ns | − 0.52* | − 0.47 ns | − 0.64** | − 0.48 ns | − 0.48 ns | − 0.45 ns | − 0.32 ns | 1 | ||||||||||
| Prolin | − 0.56* | 0.49 ns | − 0.22 ns | − 0.36 ns | − 0.56* | − 0.13 ns | − 0.32 | − 0.34 ns | − 0.12 ns | 0.43 ns | 1 | |||||||||
| TRS | − 0.64** | 0.35 ns | − 0.39 ns | − 0.60* | − 0.78** | − 0.28 ns | − 0.56* | − 0.59* | − 0.29 ns | 0.77** | 0.79** | 1 | ||||||||
| SOD | 0.54* | − 0.11 ns | 0.58* | 0.48 ns | 0.64** | 0.55* | 0.49 ns | 0.44 ns | 0.40 ns | − 0.66** | − 0.56* | − 0.69** | 1 | |||||||
| PO | 0.70** | − 0.28 ns | 0.37 ns | 0.43 ns | 0.74** | 0.31 ns | 0.42 ns | 0.40 ns | 0.39 ns | − 0.67** | − 0.42 ns | − 0.75** | 0.74** | 1 | ||||||
| APX | 0.45 ns | 0.09 ns | 0.40 ns | 0.56* | 0.63** | 0.32 ns | 0.52* | 0.52* | 0.34 ns | − 0.55* | − 0.52* | − 0.73** | 0.65** | 0.60* | 1 | |||||
| CAT | 0.65** | − 0.05 ns | 0.08 ns | 0.17 ns | 0.25 ns | 0.03 ns | 0.13 ns | 0.13 ns | − 0.01 ns | 0.42 ns | − 0.13 ns | − 0.42 ns | 0.59* | 0.69** | 0.60** | 1 | ||||
| DPPH | 0.49 ns | − 0.17 ns | 0.64** | 0.77** | 0.71** | 0.54* | 0.75** | 0.74** | 0.55* | − 0.49 ns | − 0.64** | − 0.74** | 0.74** | 0.64** | 0.76** | 0.50* | 1 | |||
| H2O2 | − 0.12 ns | 0.04 ns | − 0.70** | − 0.73** | − 0.69** | − 0.65** | − 0.74** | − 0.72** | − 0.58* | 0.71** | 0.43 ns | 0.68** | − 0.66** | − 0.48 ns | − 0.74** | − 0.31 ns | − 0.72** | 1 | ||
| MDA | − 0.52* | 0.31 ns | − 0.42 ns | − 0.49 ns | − 0.65** | − 0.36 ns | − 0.48 ns | − 0.46 ns | − 0.20 ns | 0.91** | 0.69** | 0.90** | − 0.66** | − 0.63** | − 0.63** | − 0.37 ns | − 0.58* | 0.74** | 1 | |
| LAI | 0.92** | − 0.23 ns | − 0.12 ns | − 0.02 ns | 0.25 ns | − 0.17 ns | − 0.07 ns | − 0.05 ns | − 0.16 ns | − 0.23 ns | − 0.44 ns | − 0.44 ns | 0.49 ns | 0.65** | 0.31 ns | 0.64** | 0.33 ns | 0.01 ns | − 0.35 ns | 1 |
ns non-significant.
*α ≤ 0.05.
**α ≤ 0.01.
In the present study, water deficit stress increased the production of H2O2 and MDA in plants, which is an indication of tissue damage, thus enhancing SOD, CAT, PO, APX and DPPH activities under water deficit conditions. The effectiveness of the antioxidant defense system function depends on the intensity of the water deficit stress56. The highest APX and DPPH concentrations for scavenging H2O2 and MDA protecting biomolecules were obtained in the 80% ASWD irrigation regime. This higher enzyme activity did not provide enough protection against ROS (H2O2) and MDA. Indeed, to improve the damage oxidative stress imposes on sage, foliar applications of putrescine (0.75 and 1.5 mM) are required. Similar results were reported by Tajti et al.57 achieved similar results on cadmium stress in wheat by increasing putrescine levels. Negative correlations between SOD, PO, and APX and MDA were observed (− 0.66, − 0.63, and − 0.63; P < 0.01, respectively), GB (− 0.66, − 0.67, and − 0.55; P < 0.05 and 0.01, respectively), and TRS (− 0.69, − 0.75, and − 0.73; P < 0.01, respectively) (Table 3). There were also negative correlations between SOD and APX and proline (− 0.56 and − 0.52; P < 0.05, respectively) (Table 3). Also, there was high correlations between H2O2 and MDA (0.74; P < 0.01) (Table 3). The lowest compatible osmolytes and H2O2 and MDA and highest antioxidant enzymes including CAT and APX were obtained with the application of 1.5 mM putrescine; this showed the role of putrescine in decreasing of lipid peroxidation, stabilizing the cell membranes, preventing degrading of cell membranes by free radicals like H2O2. This response indicates a good H2O2 scavenging ability in the application of putrescine. Indeed, putrescine inhibits NADPH oxidase enzymes in cell walls, which ultimately leads to less H2O2 production in putrescine-treated plants58.
The observed correlation between the two enzyme activities strongly suggests coordinated action between APX and CAT. Yiu et al.59 reported similar results. In the present study, with the application of 0.75 mM putrescine, SOD and PO played an important role in protecting H2O2 under the 40% ASWD irrigation regime. On the other hand, SOD (as the first line of defense against ROS) and APX have significant negative correlations with H2O2 (− 0.66, − 0.74; P < 0.01, respectively) (Table 3). Indeed, probably putrescine substantially improved the impacts of water deficit stress on the membrane stability index in sage plants by binding to the negatively-charged phospholipid head group60. It is well documented that PAs (e.g., putrescine) are able to induce adaptive changes to maintain plasma membrane integrity under water deficit stress. Moreover, the enzymatic antioxidant activity enhanced by putrescine seems to be the result of de novo synthesis and/or the activation of the enzymes, mediated by the transcription and/or translation of specific genes61, that potentially aids stressed plants to resist against oxidative stress induced by water deficit stress. There were also high correlations between LAI, SOD, PO and CAT and RWC (0.92, 0.54, 0.70 and 0.65; P < 0.01, respectively) (Table 3). Moreover, there were positive correlations between PO and CAT and LAI (0.65 and 0.64; P < 0.01) (Table 3). So, the highest RWC, LAI, PO, CAT and SOD were obtained with the application of 0.75 mM putrescine and the 40% ASWD irrigation regime. Also, the highest RWC and CAT were obtained with the application of 1.5 mM putrescine and the 20% ASWD irrigation regime.
Elevation of ambient CO2 concentration results in a massive enhancement of photosynthesis, quite often the rate is doubled or even tripled. The absolute demands for ATP and NAD(P)H by the plant cell as well as the demand for ATP relative to NAD(P)H vary in response to growth, development and stress conditions62. A dominant concept is that water deficit stress, similar some other stresses, favors electron flux to O2 based on the idea that the regeneration of NADP+ cannot keep pace with NADPH generation. However, any significantly increased in the NADPH: NADP+ ratio will have deep effects on the regulation of photosynthesis due to the vital coupling of electron transport to ATP synthesis63. An important point (In terms of the sustainability of alternative electron flow) is the mean turnover times of ATP and NADPH in the chloroplast stroma, which do not trespass a few seconds at moderate to high rates of steady-state photosynthesis64. In addition to any raise of the Mehler reaction, over reduce of the stroma will favor engagement of other ATP generating processes such as cyclic electron transport and chlororespiratory pathways. Classical energy dissipating mechanisms for eliminating the overflow of electrons comprise non-photochemical quenching, photorespiration, and the xanthophyll cycle65. As a result, photosynthetic control over plastoquinol oxidation at the cytochrome b6f. complex will limit overreduced of PSI and join to build electron pressure in PSII66. Increased photosynthetic control will not only promote energy dissipation in PSII but will also tend to increase the likelihood of singlet oxygen production. Furthermore, the synthesis of secondary metabolites and biosynthesis of highly reduced compounds like isoprene are significantly involved in the dissipation of excess photosynthetic energy67. The amount of energy decomposed by isoprene emission could account for up to 25% of the net photosynthesis energy store under stress conditions68. Indeed, synthesis and increased secondary metabolites could be considered as the machinery to minimize the reduction equivalents.
The current results showed that monoterpene concentration, monoterpene synthesis, and H2O2 were increased under water deficit stress conditions with the highest contents obtained with the 80% ASWD irrigation regime. Indeed, it is well known that ROS as signal components are involved in the activation of monoterpene biosynthetic enzymes, and to some extent, oxidative bursts could induce monoterpene biosynthesis69. The highest levels of expression of the main monoterpene synthase and concentrations of main monoterpenes were obtained with the application of 0.75 mM putrescine and the 80% ASWD irrigation regime. On the other hand, there were positive correlations between CS, BPPS, and SS and 1,8-cineol, camphor, α-thujone, and β-thujone (0.98, 0.75 and 0.99/0.99; P < 0.01, respectively) (Table 3). The increase in the secondary metabolite contents in sage are due to a “passive” shift of biosynthesis as a result of an over-reduced status and an “active” up-regulation of the enzymes involved in the corresponding biosynthesis30.
The biosynthesis of terpenes is composed of two distinct paths: methylerythritol 4-phosphate (MEP) and mevalonate (MVA), occurring in the plastid and cytoplasm of plants, respectively. The MEP pathway is for the synthesis of carotenoids, isoprene, mono- and diterpenes, plant hormones [abscisic acid (ABA), gibberellins (GA)], phytol, the side chain of chlorophyll, tocopherols, phylloquinone, plastoquinones, etc70. Monoterpenes biosynthesis is located in plastids71. PAs in plants are not only found in cytoplasm, but also in specified organelles like mitochondria, chloroplasts, and vacuoles72. Our results showed the ratio of (1,8-cineol and CS), (camphor and BPPS) and (α- thujone, β-thujone and SS) were obtained (3.16 and 3.65 times), (2.50 and 2.59 times), and (3.43, 4.41, and 4.04 times) with the application of 0.75 mM putrescine compared to the application of distilled water under the irrigation regime of 80% ASWD. So, in explaining this result, it should be said that putrescine enters the leaves by penetrating the cuticle or through the stomata before entering the plant cell, where they can be practical in metabolism and are further transported to other parts through plasmodesmata. Therefore, putrescine and monoterpenes were probably produced in the MEP pathway (plastid) and essential oil content affected by putrescine. Schmiderer et al.13 achieved similar results; they reported that the expression of monoterpene synthase and contents of 1,8-cineole and camphor were increased with the application of gibberellins. Methyl jasmonate and SA have also been applied as abiotic elicitors to induce secondary metabolite biosynthesis as terpene metabolism in Tanacetum parthenium73.
The highest amount of DPPH was observed with the application of 0.75 mM putrescine under the irrigation regime of 80% ASWD. High correlations were observed between DPPH and CS, BPPS, SS, 1,8-cineol, camphor, α- thujone, and β-thujone (0.64, 0.71, 0.77, 0.54, 0.55, 0.75 and 0.74; P < 0.05, 0.01, respectively) (Table 3). There were also high correlations between SOD and CS, BPPS, and 1,8-cineol (0.58, 0.64, and 0.55; P < 0.05, 0.01, respectively), between PO and BPPS (0.74; P < 0.01), and between APX and BPPS, SS, α-thujone, and β-thujone (0.63, 0,56, 0.52, and 0.52; P < 0.05, 0.01, respectively) (Table 3). High correlations were also observed between DPPH and SOD, PO, CAT, and APX (0.74, 0.64, 0.50, and 0.76, respectively) (Table 3).
According to the current results, the content of compatible osmolytes and concentration of antioxidant enzymes were increased and decreased, respectively, with the application of 2.25 mM putrescine. There is evidence that SA causes a rise in the quantity of ROS in the cell, suggesting the existence of a self-induced SA H2O2 cycle74. This is not surprising, as a close correlation was recently reported between the endogenous PAs and SA contents75, which may be responsible for the negative effects of greater concentrations of putrescine. Some reports on SA have been in agreement with the current results76. The treatment of 2.25 mM putrescine resulted in an inhibitory effect compared with the 0.75 mM putrescine treatment. This is why stress-induced H2O2 accumulation was lower in the 0.75 mM than the 2.25 mM putrescine-treated plants. Putrescine reduced the accumulation of total monoterpenes in concentrations of 2.25 mM which, to some extent, can be attributed to excessive oxidative burst-induced putrescine in high concentrations. Indeed, there was a decreasing trend in the concentration of monoterpenes and the expression of monoterpene synthases genes with increasing putrescine concentrations under the irrigation regime of 80% ASWD; however, further studies are needed to determine whether the application of putrescine in high concentrations at the cellular level decrease monoterpenes in sage under water deficit stress conditions.
Conclusion
The current results showed a foliar application of putrescine alleviated the detrimental effects of water deficit stress and considerably increased LAI and RWC. The lowest compatible osmolyte and H2O2 contents and the highest antioxidant enzymes including CAT and APX were obtained with the application of 1.5 mM putrescine, which showed the role of putrescine in decreasing of lipid peroxidation, stabilizing cell membranes and preventing the degradation of cell membranes by free radicals like H2O2. This response indicates a good H2O2 scavenging ability with the application of putrescine. In the present study, SOD and PO in the application of 0.75 mM putrescine played important roles in decreasing H2O2 under the irrigation regime of 40% ASWD. The highest levels of expression of the main monoterpenes synthase, concentrations of main monoterpenes, and DPPH were obtained with the application of 0.75 mM putrescine and the irrigation regime of 80% ASWD. PAs and monoterpenes were probably produced in the (MEP) pathway, and the essential oil content affected by putrescine. In general, the adequate putrescine concentration to trigger a best response is 0.75 mM. Indeed, putrescine could be a useful strategy to increase the main monoterpenes in sage plants. However, further researches are needed to determine the appropriate concentrations of exogenous putrescine under water deficit stress conditions in sage.
Statements on plant material
The plant material in this manuscript complies with relevant institutional, national and international guidelines and laws and can be obtained from various respective centers worldwide. The plants used are not wild and were purchased from Institute of Medicinal Plants & Natural Products Research, Iranian Academic Center for Education, Culture & Research (ACECR) (Karaj, Iran).
Author contributions
M.M.-C., performed all the experiments and data analysis and wrote the article; S.A.M.M.-S. and F.S., supervised the project and provided editorial input into the writing; S.R.-M., supported and verified the analysis of qRT-PCR; A.M.-B., advised compatible osmolytes and antioxidant enzymes and data analyses. All authors discussed the results and contributed to the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fathi A, Tari DB. Effect of drought stress and its mechanism in plants. Int. J. Health. Life Sci. 2016;10(1):1–6. doi: 10.3126/ijls.v10i1.14509. [DOI] [Google Scholar]
- 2.Gonzalez-Tejero MR, Casares-Porcel M, Sánchez-Rojas CP. Medicinal plants in the Mediterranean area: synthesis of the results of the project RUBIA. J. Ethnopharmacol. 2008;116(2):341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 3.Baghalian K, Abdoshah S, Khalighi-Sigaroodi F, Paknejad F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.) Plant. Physiol. Biochem. 2011;49:201–207. doi: 10.1016/j.plaphy.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi, S., Fernandez, L., Noon, J., Zapata, E. & Bhaskar, R. Exploring administrative records for race and Hispanic origin item non-response. Center for Administrative Records Research and Applications Working Paper. 2014–2016.
- 5.Grausgruber-Gröger S, Schmiderer C, Steinborn R, Novak J. Seasonal influence on gene expression of monoterpene synthases in Salvia officinalis (Lamiaceae) J. Plant. Physiol. 2012;169:353–359. doi: 10.1016/j.jplph.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Juergens U. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug. Res. 2014;64(12):638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- 7.Zwenger S, Basu Ch. Plant terpenoids: applications and future potentials. Biotechnol. Mol. Biol. Rev. 2007;3(1):1–7. [Google Scholar]
- 8.Selmar D, Kleinwachter M. Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant. Cell. Physiol. 2013;54(6):817–826. doi: 10.1093/pcp/pct054. [DOI] [PubMed] [Google Scholar]
- 9.Khalid KA. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.) Int. Agrophys. 2006;20(4):289–296. [Google Scholar]
- 10.Nowak M, Manderscheid R, Weigel HJ, Kleinwcachter M, Selmar D. Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J. Appl. Bot. Food. Qual. 2010;83:133–136. [Google Scholar]
- 11.Nasrollahi SM, Ghorbani GR, Khorvash M, Yang WZ. Effects of grain source and marginal change in lucerne hay particle size on feed sorting, eating behaviour, chewing activity, and milk production in mid-lactation Holstein dairy cows. J. Anim. Physiol. An. N. 2014;98:1110–1116. doi: 10.1111/jpn.12185. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Schmiderer C, Grausgruber-Groger S, Grassi P, Steinborn R, Novak J. Influence of gibberellin and daminozide on the expression of terpene synthases and on monoterpenes in common sage (Salvia officinalis) J. Plant Physiol. 2010;167:779–786. doi: 10.1016/j.jplph.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Alishah HM, Heidari R, Hassani A, Dizaji A. Effect of water stress on some morphological and biochemical characteristics of purple basil (Ocimum basilicum) Res. J. Biol. Sci. 2006;6(4):763–767. [Google Scholar]
- 15.DaCosta M, Huang B. Changes in antioxidant enzyme activities and lipid peroxidation for bent grass species in response to drought stress. J. Am. Soc. Hortic. Sci. 2007;132:319–326. doi: 10.21273/JASHS.132.3.319. [DOI] [Google Scholar]
- 16.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant. Cell. Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 17.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55(1):373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 18.Li ZY, Chen SY. Isolation and characterization of a salt-and drought-inducible gene for S- 25 adenosyl methionine decarboxylase from wheat (Triticum aestivum L.) J. Plant. Physiol. 2000;156(26):386–393. doi: 10.1016/S0176-1617(00)80078-4. [DOI] [Google Scholar]
- 19.Harfouche AL, Rugini E, Mencarelli F, Botondi R, Muleo R. Salicylic acid induces H2O2 production and endochitinase gene expression but not ethylene biosynthesis in Castanea sativa in vitro model system. J. Plant Physiol. 2008;165:734–744. doi: 10.1016/j.jplph.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Galston, A.W. & Kaur-Sawhney, R. Polyamines as endogenous growth regulators. In: Davies, P.J. (Ed.), Plant Hormones and their Role in Plant Growth and Development. Kluwer Academic Publisher, Dordrecht, Netherlands. 280–295 (1988).
- 21.Gupta K, Dey A, Gupta B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013;35:2015–2036. doi: 10.1007/s11738-013-1239-4. [DOI] [Google Scholar]
- 22.Wi SJ, Kim SJ, Kim WT, Park KY. Constitutive Sadenosylmethionine decarboxylase gene expression increases drought tolerance through inhibition of reactive oxygen species accumulation in Arabidopsis. Planta. 2014;30:30. doi: 10.1007/s00425-014-2027-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Chen J, Fang J, Guo Z, Lu S. Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2014;116:311–322. doi: 10.1007/s11240-013-0405-0. [DOI] [Google Scholar]
- 24.Sagor GHM, Berberich T, Takahashi Y, Niitsu M, Kusano T. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock- related genes. Transgenic. Res. 2013;22:595–605. doi: 10.1007/s11248-012-9666-3. [DOI] [PubMed] [Google Scholar]
- 25.Kotakis C, Theodoropoulou E, Tassis K, Oustamanolakis C, Ioannidis NE, Kotzabasis K. Putrescine, a fast-acting switch for tolerance against osmotic stress. J. Plant Physiol. 2014;171:48–51. doi: 10.1016/j.jplph.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Pál M, Szalai G, Janda T. Speculation: polyamines are important in abiotic stress signaling. Plant Sci. 2015;237:16–23. doi: 10.1016/j.plantsci.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell. 2008;20:1708–1724. doi: 10.1105/tpc.108.059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minocha R, Majumdar R, Minocha SC. Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 2014;5:175. doi: 10.3389/fpls.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caser M, Chitarra W, D’Angiolillo F, Perrone I, Demasi S, Lovisolo C, Pistelli L, Pistelli L, Scariot V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019;129:85–96. doi: 10.1016/j.indcrop.2018.11.068. [DOI] [Google Scholar]
- 30.Radwan A, Kleinwächter M, Selmar D. Impact of drought stress on specialised metabolism: Biosynthesis and the expression of monoterpene synthases in sage (Salvia officinalis) Phytochemistry. 2017;141:20–26. doi: 10.1016/j.phytochem.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Govahi M, Ghalavand A, Nadjafi F, Sorooshzadeh A. Comparing different soil fertility systems in Sage (Salvia officinalis) under water deficiency. Ind. Crop. Prod. 2015;74:20–27. doi: 10.1016/j.indcrop.2015.04.053. [DOI] [Google Scholar]
- 32.Bahreininejad B, Razmjoo J, Mirza M. Influence of water stress on morphophysiological and phytochemical traits in Thymus daenensis. Int. J. Plant Prod. 2013;7(1):151–166. [Google Scholar]
- 33.Turner NC. Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 1981;58:339–366. doi: 10.1007/BF02180062. [DOI] [Google Scholar]
- 34.Lütz C, Navakoudis E, Seidlitz HK, Kotzabasis K. Biochim. Biophys. Acta. 2005;1710:24–33. doi: 10.1016/j.bbabio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Mafra VK, Alves-Ferreira KS, Ribeiro-Alves M, Stuart M, Boava RM, Rodrigues LP, Machado CM. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE. 2012;7(2):e31263. doi: 10.1371/journal.pone.0031263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Li C, Zhang J, Chen F, Gong Y, Li Y, Su Y, Wei Y, Zhao Y. Selection of the reference gene for expression normalization in Papaver somniferum L. under abiotic stress and hormone treatment. Genes. 2020;11(2):124. doi: 10.3390/genes11020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, fourth ed. Allured Publishing Corporation, Carol Stream (2007).
- 38.Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant. Soil. 1983;70:303–307. doi: 10.1007/BF02374789. [DOI] [Google Scholar]
- 39.Bates LS, Waldren RP, Tears ID. Rapid determination of free proline for water stress studies. Plant. Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 40.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(248):254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Bayer WF, Fridovich JL. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 43.Herzog V, Fahimi HD. A new sensitive colorimetric assay for peroxidase using 3,3'- diaminobenzidineas hydrogen donor. Anal. Biochem. 1973;55:554–562. doi: 10.1016/0003-2697(73)90144-9. [DOI] [PubMed] [Google Scholar]
- 44.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific-peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 1981;22:867–880. [Google Scholar]
- 45.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie; 1983. pp. 673–684. [Google Scholar]
- 46.Huang D, Ou B, Prior RL. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 47.Velikova V, Yordanov I, Edereva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Protective role of exogenous polyamines. Plant. Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 48.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 49.Meireles-Filho ACA, Stark A. Comparative genomics of gene regulation-conservation and divergence of cis-regulatory infor-mation. Curr. Opin. Genet. Dev. 2009;19:565–570. doi: 10.1016/j.gde.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Misra N, Gupta AK. Effect of salt stress on proline metabolism in two high yielding 34 genotypes of green gram. Plant. Sci. 2005;169:331–339. doi: 10.1016/j.plantsci.2005.02.013. [DOI] [Google Scholar]
- 51.Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 52.Hare PD, Cress WA. Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102. doi: 10.1023/A:1005703923347. [DOI] [Google Scholar]
- 53.Farooq M, Wahid A, Lee D. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta. Physiol. Plant. 2009;31:937–945. doi: 10.1007/s11738-009-0307-2. [DOI] [Google Scholar]
- 54.Blokhina O, Virolainen E, Fagerstedt KV. Anti-oxidative damage and oxygen deprivation stress. Ann. Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer RF, Boyer JS. Osmoregulation solute distribution and growth in soybean seedlings having low water potentials. Planta. 1981;151:482–489. doi: 10.1007/BF00386543. [DOI] [PubMed] [Google Scholar]
- 56.Habibi G. Silicon supplementation improves drought tolerance in canola plants. Russ. J. Plant. Physiol. 2014;61(6):784–791. doi: 10.1134/S1021443714060077. [DOI] [Google Scholar]
- 57.Tajti JJ, Majláth T, Szalai IG, Pál M. Comparative study on the effects of putrescine and spermidine pre-treatment on cadmium stress in wheat. Ecotoxicol. Environ. Saf. 2018;148:546–554. doi: 10.1016/j.ecoenv.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 58.Shu Y, Zhou J, Lu K, Li K, Zhou Q. Response of the common cutworm Spodoptera litura to lead stress: changes in sex ratio, Pb accumulations, midgut cell ultrastructure. Chemosphere. 2015;139:441–451. doi: 10.1016/j.chemosphere.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 59.Yiu JC, Juang LD, Fang DYT, Liu CW, Wu SJ. Exogenous Putrescine reduces flooding induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci. Hortic. 2009;120:306–314. doi: 10.1016/j.scienta.2008.11.020. [DOI] [Google Scholar]
- 60.Takahashi T, Kakehi JI. Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009;105:1–6. doi: 10.1093/aob/mcp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajguz A. Effect of brassinosteroids on nucleic acid and protein content in cultured cell of Chlorella vulgaris. Plant. Physiol. Biochem. 2000;38:209–215. doi: 10.1016/S0981-9428(00)00733-6. [DOI] [Google Scholar]
- 62.Kramer DM, Evans JR. The importance of energy balancein improving photosynthetic productivity. Plant Physiol. 2011;155:70–78. doi: 10.1104/pp.110.166652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012;63:1637–1661. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- 64.Noctor G, Foyer CH. Homeostasis of adenylate status during photosynthesis in a fluctuating environment. J. Exp. Bot. 2000;51:347–356. doi: 10.1093/jexbot/51.suppl_1.347. [DOI] [PubMed] [Google Scholar]
- 65.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid. Redox. Signal. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 66.Joliot PJ, GN, Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA. 2011;108:13317–13322. doi: 10.1073/pnas.1110189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilhelm C, Selmar D. Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. J. Plant Physiol. 2011;168:79–87. doi: 10.1016/j.jplph.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Magel E, Mayrhofer S, Müller A, Zimmer I, Hampp R, Schnitzler JP. Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos. Environ. 2006;40:138–151. doi: 10.1016/j.atmosenv.2005.09.091. [DOI] [Google Scholar]
- 69.Zhao J, Matsunaga Y, Fujita K, Sakai K. Signal transduction and metabolic flux of β-thujaplicin and monoterpene biosynthesis in elicited Cupressus lusitanica cell cultures. Metab. Eng. 2006;8:14–29. doi: 10.1016/j.ymben.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Verma N, Shukla S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants. 2015;2:105–113. [Google Scholar]
- 71.Wise ML, Savage TJ, Katahira E, Croteau R. Monoterpene synthases from common sage (Salvia officinalis) cDNA isolation, characterization, and functional expression of (þ)-sabinene synthase, 1,8-cineole synthase, and (þ)-bornyl diphosphate synthase. J. Biol. Chem. 1998;273:14891–14899. doi: 10.1074/jbc.273.24.14891. [DOI] [PubMed] [Google Scholar]
- 72.Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 73.Majdi M, Abdollahi MR, Maroufi A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant. Cell. Rep. 2015;34:1909–1918. doi: 10.1007/s00299-015-1837-2. [DOI] [PubMed] [Google Scholar]
- 74.Pál M, Kovács V, Vida G, Szalai G, Janda T. Changes induced by powdery mildew in the salicylic acid and polyamine contents and the antioxidant enzyme activities of wheat lines. Eur. J. Plant Pathol. 2013;135:35–47. doi: 10.1007/s10658-012-0063-9. [DOI] [Google Scholar]
- 75.Szalai G, et al. Comparative analysis of polyamine metabolism in wheat and maize plants. Plant. Physiol. Biochem. 2017;112:239–250. doi: 10.1016/j.plaphy.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Hayat S, Fariduddin Q, Ali B, Ahmad A. Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron. Hung. 2005;53:433–437. doi: 10.1556/AAgr.53.2005.4.9. [DOI] [Google Scholar]