Summary
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are intracellular Ca2+ channels that link extracellular stimuli to Ca2+ signals. Ca2+ release from intracellular stores is “quantal”: low IP3 concentrations rapidly release a fraction of the stores. Ca2+ release then slows or terminates without compromising responses to further IP3 additions. The mechanisms are unresolved. Here, we synthesize a high-affinity partial agonist of IP3Rs and use it to demonstrate that quantal responses do not require heterogenous Ca2+ stores. IP3Rs respond incrementally to IP3 and close after the initial response to low IP3 concentrations. Comparing functional responses with IP3 binding shows that only a tiny fraction of a cell’s IP3Rs mediate incremental Ca2+ release; inactivation does not therefore affect most IP3Rs. We conclude, and test by simulations, that Ca2+ signals evoked by IP3 pulses arise from rapid activation and then inactivation of very few IP3Rs. This allows IP3Rs to behave as increment detectors mediating graded Ca2+ release.
Keywords: Ca2+ signaling, endoplasmic reticulum, IP3 receptor, partial agonist, quantal Ca2+ release, receptor inactivation, intracellular Ca2+ stores, IP3 receptor antagonist
Graphical abstract

Highlights
-
•
IP3 evokes quantal Ca2+ release from the endoplasmic reticulum
-
•
IP3 receptors rapidly activate and then inactivate
-
•
Submaximal responses to IP3 require activation of very few IP3 receptors
-
•
Rapid activation and inactivation of very few IP3Rs allow incremental responses to IP3
Rossi et al. define mechanisms for IP3-evoked quantal Ca2+ release that reconcile response termination with undiminished sensitivity to further IP3 additions. They show that responses require very few IP3 receptors that rapidly open and then inactivate, allowing graded responses despite the ability of IP3 receptors to propagate regenerative Ca2+ signals.
Introduction
Inositol 1,4,5-trisphosphate (IP3) links receptors in the plasma membrane to Ca2+ release from the endoplasmic reticulum (ER) through IP3 receptors (IP3Rs), which are intracellular Ca2+ channels (Berridge, 2016). This redistribution of Ca2+ from the ER generates cytosolic Ca2+ signals, transfers Ca2+ to other organelles, and stimulates store-operated Ca2+ entry. IP3-evoked Ca2+ signals initiate at small clusters of IP3Rs, where IP3 binding primes IP3Rs to open in response to Ca2+ released by their neighbors (Smith and Parker, 2009; Thillaiappan et al., 2017, 2021). Similar Ca2+-induced Ca2+ release (CICR) occurs with ryanodine receptors (RyRs), the other major family of ER Ca2+ channels (Ríos, 2018). However, CICR is potentially explosive and might prevent cells from generating graded responses. Dispersed clusters of channels constrain regenerative propagation of Ca2+ signals between them (Ríos, 2018), and inhibition of IP3Rs (and RyRs) by increased cytosolic free Ca2+ concentration ([Ca2+]c) may contribute to terminating Ca2+ release, but it may not be the only mechanism (Wiltgen et al., 2014).
IP3-evoked Ca2+ release is “quantal”: submaximal IP3 concentrations rapidly release only a fraction of the Ca2+ stores before release terminates (Muallem et al., 1989; Taylor and Potter, 1990). This pattern of response occurs without compromising responses to further incremental increases in IP3 concentration (Meyer and Stryer, 1990), indicating that it is not mediated by a conventional form of desensitization. Quantal Ca2+ release by IP3Rs has been reported by many laboratories (reviewed in Bootman [1994] and Yamashita [2006]), and it is also a feature of RyRs (Cheek et al., 1994; Wang et al., 2004). Quantal responses have been most thoroughly examined in permeabilized cells, but they have also been reported in intact cells responding to stimuli that evoke IP3 formation (Bootman et al., 1992; Muallem et al., 1989). Hence, quantal Ca2+ release is an essential feature of both major families of ER Ca2+ channels.
The mechanism of quantal Ca2+ release is unknown, but it is not due to IP3 metabolism or compensatory Ca2+ re-uptake, nor does it require ATP (Meyer and Stryer, 1990; Taylor and Potter, 1990). Two categories of mechanism have been proposed. One suggests that submaximal concentrations of IP3 completely empty the stores that are most sensitive to IP3, and higher IP3 concentrations then recruit the less sensitive stores (Ferris et al., 1992; Hirose and Iino, 1994; Muallem et al., 1989; Oldershaw et al., 1991; Parker and Ivorra, 1990) (Figure 1A). This model requires both extremely cooperative responses to IP3 and compartmentalized Ca2+ stores with heterogenous sensitivities to IP3. The second model suggests that IP3R activity attenuates before Ca2+ stores are fully depleted (Figure 1B) (Irvine, 1990; Missiaen et al., 1992; Parys et al., 1993; Tregear et al., 1991). The mechanisms that might curtail IP3R-mediated Ca2+ release include IP3-mediated inactivation (Hajnóczky and Thomas, 1994), inhibition by increased [Ca2+]c, loss of the electrochemical Ca2+ gradient for Ca2+ release as Ca2+ leaves the ER without compensatory charge movements (Yamashita et al., 2006), and regulation of IP3Rs by luminal Ca2+ (Figure 1C). The latter mechanism proposes that opening of an IP3R requires binding of IP3 and of Ca2+ at both the cytosolic and luminal sides of the IP3R (Irvine, 1990; Marchant and Taylor, 1997). Regulation by luminal Ca2+ predicts that as ER [Ca2+] falls, the sensitivity of IP3Rs to cytosolic IP3 and Ca2+ declines, and Ca2+ release then terminates with Ca2+ trapped in the ER (Figure 1C). In the 30 years since quantal Ca2+ release was first identified (Muallem et al., 1989; Taylor and Potter, 1990), numerous studies have confirmed the phenomenon and provided evidence that supports or challenges each mechanism (Yamashita, 2006). Single-channel recordings from IP3Rs (Ionescu et al., 2006; Taufiq-Ur-Rahman et al., 2009) and high-resolution optical methods (Callamaras and Parker, 2000) have also failed to identify the mechanisms underlying incremental responses to IP3.
Figure 1.
Quantal Ca2+ release by IP3Rs
(A–C) Proposed mechanisms for quantal Ca2+ release: all-or-nothing emptying of stores with different IP3 sensitivities (A), or mechanisms that terminate Ca2+ release before stores have fully emptied (B). The latter could be mediated by luminal Ca2+ regulating IP3Rs, such that as the luminal [Ca2+] falls after IP3R activation, Ca2+ dissociates from the luminal site (Lu), and IP3Rs close trapping Ca2+ within the ER (C) (Irvine, 1990).
(D and E) Ca2+ content of the ER of permeabilized Mag-fluo 4-loaded DT40-IP3R1 cells after addition of ATP (1.5 mM) followed by CPA (10 μM) to inhibit SERCAs, and then the indicated concentrations (μM) of IP3 (D) or ionomycin (E). Results (typical of three to four experiments, each with two replicates) show Ca2+ content (%) relative to steady-state Ca2+ content; (D) is reproduced from Rossi and Taylor (2020).
(F) Responses to IP3 and ionomycin plotted after subtraction of the basal Ca2+ leak.
(G and H) Effects of submaximal (100 nM) (G) or maximal (10 μM) (H) concentrations of IP3 added directly or by incremental additions to permeabilized DT40-IP3R1 cells. Results show mean ± SD of three replicates.
(I and J) Summary results (individual values, mean ± SEM, n = 3, each with three replicates) show the Ca2+ released from the stores of permeabilized DT40-IP3R1 cells (determined at 150 s) for the three incremental additions (cumulative IP3 concentrations shown in μM). The final Ca2+ release was not significantly different between any of the incremental additions (one-way repeated ANOVA with Bonferroni’s multiple comparisons test).
(K) Ca2+ content of the ER of intact wild-type HEK expressing G-CEPIA1er after addition of CPA (5 μM) to inhibit SERCAs, and then the indicated concentrations of carbachol (CCh, μM). Results (typical of seven experiments, each with three replicates) show Ca2+ content (%) relative to the Ca2+ content determined before adding CPA.
(L) Responses to the indicated concentrations of carbachol in intact wild-type HEK expressing G-CEPIA1er after subtraction of the basal Ca2+ leak.
(M) Summary results (mean ± SEM, n = 7, each with three replicates) show the Ca2+ released from the stores of intact wild-type HEK expressing G-CEPIA1er determined at 400 and 700 s for the indicated concentrations of carbachol. The Ca2+ release at 400 and 700 s was not significantly different at any of the carbachol concentrations (one-way repeated ANOVA with Bonferroni’s multiple comparisons test).
(N) Incremental responses from intact wild-type HEK cells to the indicated concentrations of carbachol added after CPA (5 μM) and recorded using G-CEPIA1er (cumulative carbachol concentrations are shown in μM).
(O) Summary results from intact wild-type HEK cells expressing G-CEPIA1er (individual values, mean ± SEM, n = 3, each with 8–12 replicates) show Ca2+ released from the stores (determined at 150 s) for the incremental additions (cumulative carbachol concentrations shown in μM). ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant (for indicated comparisons; one-way repeated ANOVA with Bonferroni’s multiple comparisons test).
(P) Incremental responses to IP3 compared with the graded rates of Ca2+ release expected if increasing concentrations of IP3 uniformly increased the permeability of the ER to Ca2+.
See also Figures S1–S4.
Here, we synthesize a high-affinity, partial agonist of IP3R with exceptionally low efficacy, and use it to establish that quantal responses are not mediated by all-or-nothing emptying of stores with heterogenous sensitivities to IP3 (Figure 1A). We show that all three subtypes of IP3Rs mediate incremental responses to IP3, and that the responses arise from a tiny fraction of a cell’s IP3Rs opening in response to IP3 and then inactivating. This mechanism resolves long-standing confusion and establishes how cells respond to incremental changes in IP3 concentration.
Results and discussion
Quantal Ca2+ release by all IP3R subtypes
To measure IP3-evoked Ca2+ release without the opposing activity of ER Ca2+ pumps (sarcoplasmic/ER Ca2+-ATPases [SERCAs]), the ER of permeabilized DT40 cells expressing only IP3R1 (DT40-IP3R1 cells) was loaded with Mag-fluo 4, a low-affinity Ca2+ indicator (Rossi and Taylor, 2020). ATP was added to fuel Ca2+ uptake before addition of cyclopiazonic acid (CPA) to inhibit SERCAs; IP3 was then added to stimulate Ca2+ release (Figure 1D). A maximally effective IP3 concentration released ∼70% of the ER Ca2+ content. Submaximal IP3 concentrations rapidly released a smaller fraction of the stores, after which there was no further effect of IP3 on the rate of Ca2+ release or its effect was much reduced (Figure 1D). These quantal responses to IP3 are clearest after correction for the basal Ca2+ leak evident after SERCA inhibition (Figure 1F). The responses to IP3 are very different to those evoked by the Ca2+ ionophore, ionomycin, which stimulated monophasic Ca2+ loss from the entire ER at rates that increased with ionomycin concentration (Figures 1E, 1F, S1A, and S1B). Similar quantal responses from type 1 IP3R (IP3R1) were observed with IP3 in the presence of the K+ ionophore, valinomycin, to dissipate any ER membrane potential; with Cs+ replacing K+ to inhibit K+ channels; with mitochondria inhibited; with IP3 added in larger volumes to avoid possible artifacts arising from bolus additions; and with inositol 2,4,5-trisphosphate ((2,4,5)IP3), a non-metabolized analog of IP3 (Hill et al., 1988) (Figures S1C–S1J).
Although responses to low IP3 concentrations rapidly attenuated (Figures 1D, 1F, and S1B), subsequent addition of more IP3 evoked further rapid Ca2+ release. Furthermore, the fraction of the Ca2+ stores released by maximal or submaximal IP3 concentrations was the same whether IP3 was delivered immediately at its final concentration or as incremental additions (Figures 1G–1J). Similar “incremental” responses (Beecroft and Taylor, 1997; Ferris et al., 1992; Meyer and Stryer, 1990) were obtained in the presence of the fast Ca2+ buffer, BAPTA, at a concentration (10 mM) sufficient to rapidly buffer even very local increases in [Ca2+]c (Vais et al., 2012). This indicates that inhibition of IP3Rs by increases in [Ca2+]c are not required for incremental responses (Figures S2A–S2F). That conclusion is consistent with two additional lines of evidence. First, IP3Rs from Capsaspora owczarzaki are not regulated by cytosolic Ca2+, but they do mediate quantal Ca2+ release (Alzayady et al., 2015). Second, in ER depleted of Ca2+, retrograde movement of Mn2+ through open IP3Rs can be used to report IP3R opening. Under these conditions, responses to IP3 have been reported to be quantal, but only after fragmentation of the ER (Hajnóczky et al., 1994; Renard-Rooney et al., 1993). Responses were also incremental in permeabilized DT40 cells expressing only IP3R2 or IP3R3 (Figures S2G–S2J), and in wild-type human embryonic kidney (HEK) cells, which express all three IP3R subtypes (Mataragka and Taylor, 2018) (Figure S3). In each case, the total amount of Ca2+ released by a submaximal concentration of IP3 was the same whether IP3 was presented as a single addition or as incremental additions. Incremental responses to IP3 were also observed using a low-affinity genetically encoded Ca2+ indicator targeted to the ER lumen (G-CEPIA1er) (Suzuki et al., 2014) (Figures S4A–S4H).
In single-cell analyses of permeabilized HEK cells stably expressing G-CEPIA1er, all cells responded to a maximal IP3 concentration with a rapid decrease in ER luminal [Ca2+]. Submaximal IP3 concentrations rapidly released a fraction of the IP3-sensitive stores, and the response then terminated without preventing subsequent responses to a maximal IP3 concentration (Figures S4I–S4N). Quantal responses observed in populations of permeabilized cells are not, therefore, due to all-or-nothing responses from different cells with heterogeneous IP3 sensitivities. In permeabilized hepatocytes too, quantal responses to IP3 were observed in single cells (Renard-Rooney et al., 1993). We also measured changes in G-CEPIA1er fluorescence within small subcellular regions and, although the spatial resolution was limited, the results suggest that different regions within a HEK cell have similar sensitivity to IP3 (Figures S4K–S4N).
It has been reported that quantal responses to IP3 are observed in permeabilized hepatocytes only when permeabilization is accompanied by fragmentation of the ER (Renard-Rooney et al., 1993). Most studies do not address whether the ER remains continuous after permeabilization. In our analyses of permeabilized HEK cells, there is some fragmentation of the ER, whether reported by G-CEPIA1er or compartmentalized Mag-fluo 4, and attempts to avoid it by adjusting conditions failed to achieve ATP-dependent Ca2+ uptake without some ER fragmentation (Figure S4O). However, several lines of evidence establish that quantal responses are not an artifact arising from ER fragmentation. First, others have observed quantal responses from stimuli that evoke IP3 formation in intact cells (Bootman et al., 1992; Muallem et al., 1989). By measuring quantal responses directly using a Ca2+ indicator within the ER lumen, we confirmed that stimulation of the endogenous muscarinic acetylcholine receptors of HEK cells with carbachol to evoke IP3 formation caused quantal Ca2+ release from intracellular stores (Figures 1K–1M). Successive additions of carbachol evoked incremental responses (Figures 1N and 1O). Second, fragmentation of the ER might create the heterogenous Ca2+ stores required for the all-or-nothing model of quantal Ca2+ release (Figure 1A), but it is difficult to envisage how it might create the conditions required for a model where IP3Rs close before the stores have fully emptied (Figure 1B). Our subsequent experiments demonstrate that all-or-nothing emptying of discrete Ca2+ stores cannot explain quantal responses (see Figures 2 and 3), and we provide direct evidence that IP3Rs inactivate before the stores have emptied (see Figures 5, 6, and 7).
Figure 2.
A 2-O-modified analog of IP3 (2) is a high-affinity, partial agonist
(A) Synthetic scheme for 2 and structure of IP3.
(B) Equilibrium-competition binding to purified full-length IP3R1 using [3H]IP3 (1.5 nM) and the indicated concentrations of IP3 or 2 in Tris-EDTA medium (TEM) at 4°C (mean ± SEM, n = 7, many error bars are smaller than the symbols).
(C) Effects of IP3 or 2 on Ca2+ release (determined after 20 s) from permeabilized DT40-IP3R1 cells (mean ± SEM, n = 3, each with three replicates).
(D) Patch-clamp recordings, typical of at least five recordings, from excised nuclear patches of DT40-IP3R1 cells, at a holding potential of +40 mV with K+ as the charge carrier, and ligands within the pipette (cytosolic surface; IP3, 10 μM; 2, 10 μM, except where shown otherwise). C, closed state.
(E) Summary results (mean ± SEM, n = 5–7) show channel activity (NPo) for the indicated stimuli (enlarged in the inset). ∗∗∗∗p < 0.0001, ANOVA with Bonferroni’s test, relative to IP3 alone.
(F) Permeabilized DT40-IP3R1 cells stimulated (30 s) with 2 (1 μM, which evoked 31% ± 7% Ca2+ release) were then stimulated with the indicated concentrations of IP3. Ca2+ release evoked by IP3 (determined after a further 20 s) is shown as a percentage of the store content after the first stimulus (mean ± SEM, n ≥ 4, each with three replicates).
(G) Equilibrium-competition binding to cerebellar membranes using [3H]IP3 (7.5 nM) and the indicated concentrations of IP3 or 2 in CLM at 20°C (mean ± SEM, n = 3–4 with duplicate determinations). pKD values were 6.10 ± 0.06 (mean ± SEM, n = 4, KD = 794 nM) for IP3 and 6.26 ± 0.01 (n = 3, KD = 549 nM) for 2. Hill coefficients (h) were 3.2 ± 1.3 and 2.0 ± 0.7 for IP3 and 2.
(H) IP3 binds between the α and β domains of the IBC, but the SD is required for IP3 to evoke channel opening.
(I and J) Equilibrium-competition binding of IP3 and 2 to the IBC or NT using [3H]IP3 (0.3 or 0.75 nM for IBC and NT, respectively). Mean ± SEM, n = 4.
(K) IP3R moves between an unknown numbers of closed states (C) to an open state (O) after IP3 binding. Rate(s) of movement through the closed to the open state occur more slowly with 2 bound to the IP3R.
Tables S1–S3 summarize the properties of IP3 and 2.
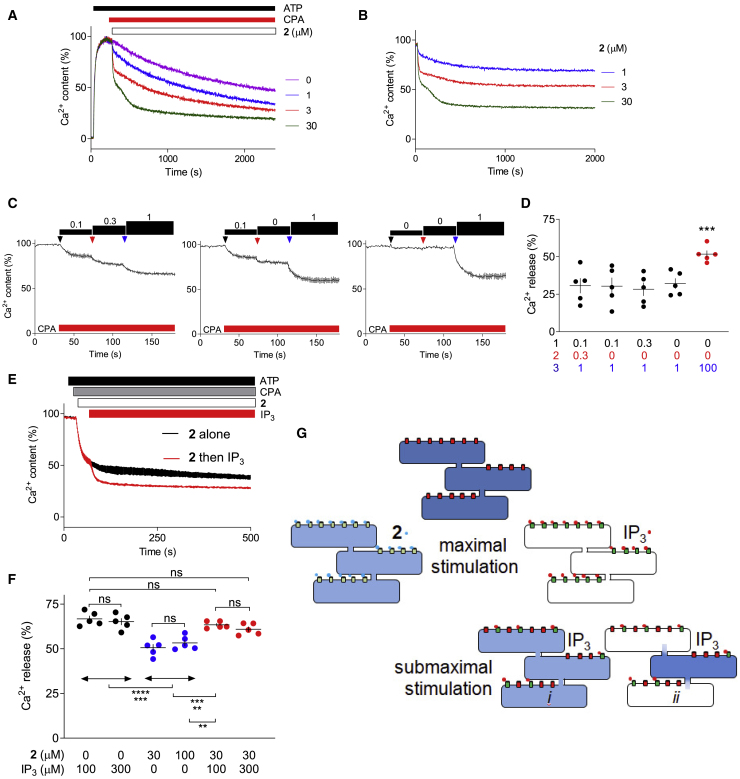
Figure 3.
Quantal responses are not mediated by heterogenous stores
(A) Ca2+ content of the ER of populations of permeabilized DT40-IP3R1 cells after addition of ATP, CPA (10 μM), and then the indicated concentrations of 2. Results are typical of three experiments, each with duplicate determinations.
(B) Responses to 2 shown after subtraction of the basal Ca2+ leak.
(C) Effects of a submaximal concentration of 2 (1 μM) added directly or by incremental additions. Results show mean ± SD of three replicates.
(D) Summary results (individual values, mean ± SEM, n = 5, each with three replicates) show the final Ca2+ content of the stores of permeabilized DT40-IP3R1 cells (determined at 150 s). A maximal concentration of 2 (30 μM) released 52% ± 3% of the Ca2+ stores (red symbols). ∗∗∗p < 0.001, one-way repeated ANOVA with Bonferroni’s test relative to all other values.
(E) Ca2+ release from wild-type HEK cells evoked by 2 (30 μM) alone or followed by a supra-maximal concentration of IP3 (100 μM). Mean of duplicate determinations.
(F) Summary results (individual values, mean ± SEM, n = 5) show Ca2+ release (determined at 500 s) evoked by the indicated concentrations of 2 followed by IP3 in wild-type HEK cells. ∗∗p < 0.01, ∗∗∗p < 0.001,∗∗∗∗p < 0.0001; ns, not significant (for indicated comparisons, ANOVA with Bonferroni’s test).
(G) Maximal IP3 concentrations fully empty the IP3-sensitive Ca2+ stores, but it was unclear whether quantal responses to submaximal IP3 concentrations arise from incomplete emptying of the entire ER (i) or complete emptying of a fraction of the ER (ii). Results with a maximally effective concentration of 2, which partially activates all IP3Rs leaving Ca2+ trapped within the ER, indicate that quantal responses are not due to all-or-nothing emptying of heterogenous stores (ii).
Figure 5.
IP3Rs close after rapid Ca2+ release evoked by low IP3 concentrations
(A) If IP3Rs remain active after an incremental Ca2+ release; stores should re-accumulate Ca2+ after addition of an IP3R antagonist.
(B–G) The ER of permeabilized HEK-IP3R1 cells was loaded with Ca2+ by addition of ATP (t = 0); IP3 (0–30 μM) and heparin (10 mg/mL) were then added as indicated. Each trace (mean of duplicate determinations) shows results with a different IP3 concentration. The code in (B) applies to all panels.
(H and I) Summary results (mean ± SEM, n = 6, each with two determinations) show the extent to which stores have refilled (relative to cells without IP3) 250 s after IP3 addition (H), as well as the initial rate of refilling from the slope of the curve immediately after heparin addition (I). In (H), 0% refilling is defined as the store content in control conditions measured 250 s after addition of a maximal IP3 concentration. In these experiments, the EC50 for IP3-evoked Ca2+ release was 166 nM (pEC50 = 6.78 ± 0.08) and maximal Ca2+ release was 86% ± 2% (mean ± SEM, n = 6).
(J and K) Summary results (mean ± SEM, n = 5–7, each with duplicate determinations) from similar analyses using 50 μM dequalinium (J; Figures S7A–S7F) or 125 μM 2-APB (K; Figures S7G–S7L) to block IP3Rs. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, one-way repeated ANOVA with Bonferroni’s test for the indicated comparisons (H–K).
(L) IP3 was incubated with permeabilized HEK-IP3R1 cells (pre-incubated) or without cells (control) for 260 s under conditions identical to those used to show IP3R inactivation. The supernatant was then bio-assayed. Summary results (mean ± SEM, n = 4, each with three replicates) show no significant difference between the potency of IP3 after incubation under control conditions or with permeabilized cells. The results establish that refilling of stores during sustained incubation with low concentrations of IP3 is not due to its metabolism. Table S4 provides a summary and detailed methods.
See also Figures S6 and S7 and Table S4.
Figure 6.
IP3Rs of HEK-IP3R1 cells close after incremental responses to low concentrations of a stable analog of IP3
(A–F) The experiments are similar to those shown in Figure 5, but using HEK-IP3R1 cells, (2,4,5)IP3 at the indicated concentrations, and dequalinium (DQ, 50 μM). Permeabilized cells were loaded with Ca2+ by addition of ATP before adding the indicated concentrations of (2,4,5)IP3 (without inhibiting SERCAs) and then the IP3R antagonist, dequalinium. Traces show means of two replicates.
(G and H) Summary results (mean ± SEM, n = 4, each with two determinations) show the extent to which stores refill (relative to cells without (2,4,5)IP3) 250 s after (2,4,5)IP3 addition (G), and the initial rate of refilling from the slope of the curve immediately after DQ addition (H). ∗p < 0.05, ∗∗∗∗p < 0.0001, one-way ANOVA with Bonferroni’s test.
(I) Stores rapidly refill after stimulation with the lowest IP3 concentrations, as the very few active IP3Rs inactivate. Higher concentrations of IP3 activate many more IP3Rs than needed to empty the stores, hence even substantial inactivation leaves enough active IP3Rs to keep the stores empty.
See also Figures S6 and S7.
Figure 7.
Incremental responses are mediated by very few IP3Rs that rapidly inactivate
(A) IP3 must bind to all four IP3-binding sites of a tetrameric IP3R for the channel to open (Alzayady et al., 2016). Since IP3 binds independently to each site and with the same KD, we compute the fractional occupancy of all IP3-binding sites (α) at any ligand concentration ([L]) from: . The fraction of tetrameric IP3R in which all four sites are occupied is then α4. The plot shows the relationship between normalized ligand concentration ([L]/KD) and the fraction of IP3Rs in which all four IP3-bindings sites are occupied (α4) compared to the fractional occupancy at a single site (α).
(B) Equilibrium competition binding with [3H]IP3 in TEM was used to define the KD and from that the maximal number of binding sites (Bmax) in cerebellar membranes, which were then used to calibrate western blots to estimate the number of IP3Rs in a HEK-IP3R1 cell. Loadings are for serial 2-fold dilutions from 3.2 to 0.1 μL/lane (cerebellum) and from 12.8 to 0.8 μL/lane (HEK-IP3R1 cells). The HEK-IP3R1 cell lysate contained 10,000 cells/μL. Bmax values are mean ± SEM, n = 6.
(C) Predicted relationship for HEK-IP3R1 cells between IP3 concentration, number of IP3Rs (% of total) with all four IP3-binding sites occupied (calculated from the KD for IP3 determined under conditions used for functional assays, 794 nM), and IP3-evoked Ca2+ release (% of IP3-sensitive stores; EC50 = 186 nM, pEC50 = 6.73 ± 0.04; n = 17).
(D) Relationships between IP3 concentrations around the EC50 value, Ca2+ release, and the number of tetra-liganded IP3Rs/cell.
(E) We assume that IP3Rs in which four subunits have bound IP3 move between closed (C4), open (O4), and inactivated (I4) states with forward rate constants k1 > k2 >> k3.
(F–H) Simulations show numbers of open (F) and inactivated IP3Rs/well (G), and the ER Ca2+ content (H) for HEK-IP3R1 cells after sequential additions to give the indicated final IP3 concentrations (nM) or a single addition of the final IP3 concentration. Each addition of IP3 evokes a rapid, transient surge in the number of open IP3Rs, which then rapidly inactivate, leading to transient Ca2+ release. Note the tiny fraction of open and inactivated IP3Rs (right axes in F and G).
(I–K) Simulations for HEK-IP3R1 cells with SERCAs active show ER Ca2+ content after addition of the indicated IP3 concentrations and then heparin (similar to analyses in Figures 5 and 6). Addition of heparin was simulated by making krel = 0.
(L–N) In the simulations of cell populations (F–H), stepwise increases in IP3 concentration caused the predicted number of tetra-liganded IP3Rs (IP3R4) to increase by 4.41-fold (for 60 to 90 nM) and then by 2.76-fold (90 to 120 nM). Here, we simulate responses from a single wild-type HEK cell expressing 9,101 IP3Rs, with the first IP3 addition triggering formation of 5 IP3R4s/cell (143 nM), 10 IP3R4s/cell (177 nM), or 20 IP3R4s/cell (219 nM). We then deliver two further IP3 additions calculated to cause the numbers of IP3R4s to increase by 4.41-fold and then by 2.76-fold to mimic the stimuli used in simulations of larger numbers of cells (F–G). Simulations show the effects of adding the indicated concentrations of IP3 on the number of open (L) and inactivated IP3Rs (M), and the Ca2+ content of the ER (N). Colored code indicates concentrations of IP3 (nM) added at each step.
(O–T) We predict the number of open IP3Rs during incremental responses of permeabilized DT40-IP3R1 cells by subtracting the ER Ca2+ leak after inhibition of SERCA (Figure 1D, purple trace) from the results obtained with incremental additions of IP3 (Figures 1G–1H), and then estimate the time-dependent loss of ER Ca2+ due to opening of IP3Rs (see Analysis in STAR Methods). For each sequence of IP3 additions, results are shown for each of three independent analyses (blue, red, and black). The results show that after each IP3 addition, IP3Rs abruptly open and then close (inactivate), and the time course of the number of open IP3Rs matches predictions of the simple activation-inactivation scheme (F–H), confirming that our scheme captures essential features of IP3Rs responding incrementally to IP3.
The results so far confirm that incremental responses to IP3 occur within single cells, they are not a consequence of cell permeabilization, and they are not mediated by increases in [Ca2+]c, by IP3 metabolism, or by ineffective movement of counter-ions, nor do mitochondria contribute. Incremental responses to IP3 (Figure 1P) are a feature of all IP3R subtypes, and genetically encoded IP3R heterogeneity is not required for quantal Ca2+ release. The present results do not, however, distinguish between the proposed mechanisms (Figures 1A–1C).
Characterization of a high-affinity, weak partial agonist of IP3Rs
Because partial agonists activate receptors less effectively than do full agonists (Rossi et al., 2009), we predicted that if a weak partial agonist occupied all IP3Rs and evoked quantal Ca2+ release, the quantal mechanism could not be due to heterogenous IP3R sensitivity. We previously developed 2-O-modified IP3 analogs that are partial agonists (Rossi et al., 2009), but none has sufficiently low efficacy for our present needs. Reasoning that enlarging the 2-O-substituent might further reduce efficacy, we synthesized additional analogs and found Cbz3-spermine-IP3 (compound 2) to be an exceptionally weak partial agonist (Figure 2A). Compound 2 bound to IP3R1 with about 2-fold greater affinity than IP3 (Figure 2B), but it was significantly less potent than IP3 in stimulating Ca2+ release from permeabilized DT40-IP3R1 cells (Figures 2C; Table S1) or wild-type HEK cells (Table S2). The difference between binding and functional analyses is captured by comparing the ligand concentrations required to release 50% of the IP3-sensitive Ca2+ stores (ECI50) and to occupy 50% of IP3-binding sites (KD, equilibrium dissociation constant); the ratio (ECI50/KD) is 41-fold greater for 2 than for IP3 (Table S1). The weakest known partial agonist with an affinity comparable to IP3 (compound 4 in Rossi et al., 2009) has an ECI50/KD ratio only 5.7-fold greater than that of IP3.
In patch-clamp recordings from the outer nuclear envelope, which is continuous with the ER, the increase in channel open probability (NPo) of IP3R1 was the same for 10 and 100 μM of 2, confirming that both concentrations were maximally effective (Figures 2D and 2E). However, the maximal NPo for 2 was 40-fold less than for IP3; the single-channel conductance (γK) was the same for both agonists (Figures 2D and 2E; Table S1). Because partial agonists bind to the same site as full agonists, but less effectively activate the receptor, they behave as competitive antagonists of full agonists. This behavior is evident in patch-clamp and Ca2+-release assays, where 2 reduced the response to IP3 (Figures 2D–2F). These observations, which are expected for a partial agonist, are important for subsequent experiments because they demonstrate that IP3 and 2 compete for occupancy of the same IP3Rs, but 2 causes less effective activation.
The KD of 2 determined from dose-ratio analysis (KD = 426 nM) (Table S3) was similar to the half-maximally effective concentration of 2 in Ca2+-release assays (EC50 = 479 nM, Table S1), as expected for a weak partial agonist. The KD for 2 derived from functional assays (∼450 nM) is much greater than that determined by radioligand binding (5.2 nM) because they use different assay conditions (Ding et al., 2010). However, in binding analyses performed under conditions that mimic functional assays, the KD for 2 was 549 nM, which is comparable to its EC50 for Ca2+ release, and 1.5-fold lower than the KD for IP3 determined under these conditions (794 nM) (Figure 2G).
We reported previously that the reduced open probability (Po) of IP3Rs activated by partial agonists was due to an increase in mean channel closed time (τc), with no effect on mean open time (τo) (Rossi et al., 2009). Similar behavior appears to underlie the reduced efficacy of 2 because the modest (∼2-fold) decrease in τo (relative to IP3) is insufficient to explain the 40-fold decrease in NPo (Table S1). With such low Po, it is impracticable to determine τc directly for 2.
IP3 binds to the IP3-binding core of the IP3R (IBC, residues 224–604) (Bosanac et al., 2002), but communication with the channel requires the suppressor domain (SD, residues 1–223) (Rossi et al., 2009; Yoshikawa et al., 1999) (Figure 2H). The difference in ligand affinities (ΔΔG = ΔGIBC − ΔGNT, where ΔG = −RTlnKD) for the IBC and N-terminal (NT, residues 1–604) reports the binding energy diverted into changing the NT conformation (Rossi et al., 2009). Analyses of IP3 and 2 binding to the IBC and NT confirm that IP3 diverts more energy into changing the NT conformation than does 2 (Figures 2I and 2J; Table S1).
We conclude that 2 is a high-affinity, partial agonist of IP3R. It is the weakest known partial agonist with high affinity for IP3R. The basis of its low efficacy is similar to that of related partial agonists (Rossi et al., 2009): it perturbs communication between the IBC and SD, causing the channel to dwell longer in a closed state and so open infrequently (Figure 2K). Our results suggest strategies to develop high-affinity antagonists of IP3R and they provide the tool needed to assess whether all-or-nothing emptying of Ca2+ stores with heterogenous IP3R sensitivities underlies quantal responses (Figure 1A).
Quantal responses are not mediated by heterogenous stores
Compound 2 stimulated quantal Ca2+ release, and the responses to sequential additions were incremental (Figures 3A–3D). However, the quantal response evoked by a maximally effective concentration of 2 (Figures 3A and 3D) was smaller than the maximal response to IP3 (Figure 2C). Hence, even when all IP3Rs are occupied by 2, the IP3-sensitive Ca2+ stores are not fully depleted. However, the Ca2+ stores remain sensitive to a subsequent IP3 addition, albeit at a high concentration of IP3 because it must now surmount the competitive antagonism of 2 (Figures 3E and 3F). Since 2 evokes quantal Ca2+ release when it occupies all IP3Rs, the quantal phenomenon cannot be due to all-or-nothing emptying of stores with heterogenous sensitivities. The response to a maximal concentration of 2 in Figure 3A is biphasic, and we also observed some biphasic responses to IP3 (see Figures S5L and S5M), but such biphasic responses were not consistently observed and were not further analyzed.
When cells are maximally stimulated with 2, IP3 can bind to IP3R only after 2 dissociates. Rapid IP3-evoked Ca2+ release after maximal stimulation with 2 (Figures 3E and 3F) therefore demonstrates that quantal Ca2+ release is accompanied by rapid dissociation and re-association of agonists: IP3 is not “locked” onto activated IP3Rs. We conclude that during quantal responses, there is incomplete emptying of the entire ER, and IP3 continues to associate with and dissociate from IP3Rs (Figures 1B and 3G).
Quantal Ca2+ release is not a property of the ER
Since quantal Ca2+ release occurs with IP3Rs (Figure 1) (Yamashita, 2006) and RyRs (Cheek et al., 1994; Wang et al., 2004), it might reflect properties of the ER rather than its channels. Increases in [Ca2+]c can, for example, restructure the ER (Subramanian and Meyer, 1997), suggesting that loss of Ca2+ from the ER might cause its further fragmentation and deny active Ca2+ channels access to the remaining Ca2+. Transient receptor potential mucolipin 1 (TRPML1) channels are usually expressed in the membranes of lysosomes (Shen et al., 2012), but when overexpressed TRPML1 channels also release Ca2+ from the ER and some TRPML1 populates the reticular ER in which IP3Rs are expressed (Figures 4A and S5A–S5F). In permeabilized HEK cells expressing CFP-TRPML1, but not in mock-transfected cells, ML-SA1 (a selective TRPML1 agonist) (Shen et al., 2012) stimulated a concentration-dependent Ca2+ release from the ER (Figures 4A and S5A). In contrast to the quantal responses evoked by IP3 and 2, ML-SA1 caused a concentration-dependent increase in the rate of Ca2+ release from the entire ML-SA1-sensitive ER (Figures 4B and S5B). These results establish that quantal Ca2+ release is not a property of the ER or an inexorable consequence of cell permeabilization with the associated ER fragmentation (Figure S4O), but it is instead a feature of IP3Rs and RyRs. That conclusion is consistent with evidence that purified IP3Rs mediate quantal Ca2+ release (Ferris et al., 1992).
Figure 4.
Neither ER reorganization nor luminal Ca2+ mediates quantal Ca2+ release
(A) Effects of the indicated concentrations of ML-SA1 on the ER Ca2+ content of permeabilized HEK cells expressing CFP-TRPML1.
(B) Semi-logarithmic plots show mono-exponential loss of ER Ca2+ after treatment with ML-SA1. Results (A and B) are typical of four experiments, each with duplicate determinations. Summary results are in Figure S5B.
(C and D) Permeabilized DT40-IP3R1 cells were loaded to steady state with Ca2+ before addition of thapsigargin (TG, 1 μM) to inhibit SERCAs. At intervals thereafter (0–35 min), IP3 (C) or 2 (D) was added. Results are typical of 29–36 experiments.
(E and F) Summary results (each point from a single concentration-effect relationship) show the relationships between ER Ca2+ content at the time of addition of IP3 or 2 (%) and pEC50 value (−logEC50) (E) and maximal Ca2+ release (F). Lines (least-squares linear regression) have slopes that are not significantly different from 0.
See also Figure S5.
Neither luminal Ca2+ nor erlin 2 determines quantal responses
An appealing hypothesis is that luminal Ca2+ is required for IP3R gating, such that as the ER loses Ca2+ the IP3R channel closes trapping Ca2+ within the ER (Irvine, 1990) (Figure 1C). Previous analyses of the effects of luminal Ca2+ provide conflicting results. There is evidence that a very substantial loss of ER Ca2+ reduces IP3R sensitivity (Barrero et al., 1997; Marshall and Taylor, 1994) and that overloading stores with Ca2+ promotes Ca2+ release (Missiaen et al., 1992), while other reports suggest that luminal Ca2+ reduces IP3R sensitivity (Vais et al., 2020) or has no effect (Beecroft and Taylor, 1997; Shuttleworth, 1992).
We anticipated that if three ligands are required to open IP3Rs (IP3, cytosolic Ca2+, and luminal Ca2+, Figure 1C), channel opening by IP3 might require less luminal Ca2+ than the weak partial agonist 2. We used thapsigargin to irreversibly inhibit SERCA and slowly drain the ER of Ca2+, and then evaluated responses to IP3 and 2 at different ER Ca2+ contents (Figures 4C and 4D). The fraction of the remaining Ca2+ stores released by IP3 or 2 and the EC50 for each ligand were unaffected as stores lost their Ca2+. We conclude that there is no significant effect of ER Ca2+ content on the sensitivity or maximal response to IP3 or 2 (Figures 4E and 4F), even when the Ca2+ content is reduced to levels well below those observed after quantal responses to IP3 or 2. Luminal Ca2+ does not, therefore, affect the number of open IP3Rs. We conclude, and consistent with some previous reports (Beecroft and Taylor, 1997; Combettes et al., 1992; Hajnóczky and Thomas, 1994; Oldershaw et al., 1991; Shuttleworth, 1992), that regulation of IP3Rs by luminal Ca2+ does not underlie quantal responses.
Activation of IP3Rs initiates a sequence that can lead to their proteasomal degradation. An early step in this sequence is recognition of active IP3Rs by an ER-membrane protein, erlin 2 (ER lipid raft-associated protein 2) (Wojcikiewicz, 2018). We considered whether erlin 2 might associate with active IP3Rs, terminate their activity, and thereby contribute to quantal Ca2+ release. However, substantial depletion of erlin 2 using small interfering RNA (siRNA) had no effect on quantal responses to IP3 (Figures S5G–S5M). We conclude that neither erlin 2 nor changes in ER luminal [Ca2+] contribute to incremental Ca2+ release by IP3.
IP3 evokes Ca2+ release and then IP3R inactivation
Previous analyses of IP3R inactivation provide conflicting results, perhaps indicating a need for unidentified labile accessory factors (Bezprozvanny and Ehrlich, 1994; Hajnóczky and Thomas, 1994; Mak and Foskett, 1997; Marchant and Taylor, 1998; Oldershaw et al., 1992; Taufiq-Ur-Rahman et al., 2009; Stehno-Bittel et al., 1995). To examine any possible contribution of IP3R inactivation to quantal Ca2+ release, we therefore examined IP3R inactivation under conditions that exactly replicate those we used to show incremental responses to IP3.
To assess the activity of IP3Rs during the sustained phase of incremental responses, we examined IP3-evoked Ca2+ release without inhibiting SERCAs and then added an IP3R antagonist during the response. The rationale is that if IP3Rs remain active, an antagonist should reverse any ongoing activity and allow stores to refill (Figure 5A). We used three unrelated antagonists since each has limitations (Figure S6). Heparin is a competitive antagonist (Richardson and Taylor, 1993) and 2-aminoethoxydiphenyl borate (2-APB) is a non-competitive inhibitor with some selectivity for IP3R1 (Saleem et al., 2014). We show that dequalinium, which blocks K+ channels, is also an IP3R antagonist (Figures S6A–S6G). At the concentrations used, the antagonists completely (heparin and dequalinium) or partially (2-APB) blocked IP3-evoked Ca2+ release from permeabilized HEK-IP3R1 cells (Figures S6H–S6J).
During sustained stimulation of permeabilized HEK-IP3R1 cells with a maximal concentration of IP3, the stores remained empty, but they refilled after adding heparin (Figure 5B). The incomplete refilling is probably due to partial inhibition of SERCAs by heparin (Figures S6K–S6M). The results demonstrate that during maximal stimulation, enough IP3Rs remain open to counteract opposing SERCA activity. Since an IP3R can conduct ∼500,000 Ca2+/s (Vais et al., 2010) while a SERCA transports fewer than 40 Ca2+/s (Lytton et al., 1992), it may require very little residual IP3R activity to overwhelm SERCAs and keep the ER depleted of Ca2+. After stimulation with the lowest IP3 concentrations (30–100 nM), the stores rapidly refilled and the rate was unaffected by heparin (Figures 5E–5I). This indicates that after the initial Ca2+ release evoked by low IP3 concentrations, there were no detectable open IP3Rs. The pattern of response to intermediate concentrations of IP3 fell between these extremes: there was residual sustained IP3R activity with the higher IP3 concentrations, and IP3R inactivation occurred with the lower concentrations, but more slowly than for the lowest IP3 concentration (Figures 5C, 5D, 5H, and 5I). Similar results were obtained with different antagonists (Figures 5J, 5K, and S7), with heparin in wild-type HEK cells (Figures S7M–S7T), and with a metabolically stable analog of IP3, (2,4,5)IP3 (Figures 6A-6H). We also used a bioassay of IP3 to confirm that refilling of Ca2+ stores during prolonged incubation with low concentrations of IP3 was not due to IP3 degradation (Figure 5L; Table S4).
Previous studies provide conflicting evidence for IP3-evoked IP3R inactivation. Single-channel recordings of IP3Rs support (Mak and Foskett, 1997; Stehno-Bittel et al., 1995) or challenge inactivation (Bezprozvanny and Ehrlich, 1994; Taufiq-Ur-Rahman et al., 2009), and while some analyses of permeabilized cells show IP3-evoked inactivation (Hajnóczky and Thomas, 1994; Marchant and Taylor, 1998), others suggest that responses are sustained (Oldershaw et al., 1992). Our results, from analyses where incremental responses and inactivation were assessed under identical conditions, show that incremental responses to low IP3 concentrations are accompanied by rapid inactivation of IP3Rs (Figure 6I).
Incremental responses involve activation of very few IP3Rs
If quantal responses to low IP3 concentrations are terminated by closure of IP3Rs, how can stores retain undiminished responsiveness to further IP3 additions? Comparison of the relationship between the occupancy of IP3Rs by IP3 with functional responses provides a possible answer to this conundrum.
Analyses of [3H]IP3 binding establish the fraction of IP3-binding sites to which each concentration of IP3 has bound (α) without revealing how the binding is distributed between IP3Rs or their different states. However, Hill coefficients for IP3 binding to IP3R1 are close to one (0.95 ± 0.08 in Figure 2B) (Hannaert-Merah et al., 1994; Iwai et al., 2005; Rossi et al., 2009; Supattapone et al., 1988), indicating that each IP3R subunit binds IP3 independently (Hannaert-Merah et al., 1994) and that differences in the affinities of different IP3R states are either small or masked by the predominance of a single state. We therefore assume that IP3 binding is independently distributed across all available IP3-binding sites. Since IP3R opening requires binding of IP3 to all four IP3R subunits (Alzayady et al., 2016), we need to predict the relationship between IP3 concentration and the fraction of IP3R with all four sites occupied (α4, Figure 7A). We begin with analyses of HEK-IP3R1 cells.
Our measurements of IP3-evoked Ca2+ release lack the temporal resolution needed to determine the initial rates of Ca2+ release that would directly report the number of open IP3Rs (Marchant and Taylor, 1998). However, the assays do establish the IP3 sensitivity of quantal responses, because (with SERCAs inhibited) the Ca2+ release determined after 20 s for each IP3 concentration reports the cumulative response to IP3 throughout that interval. When addressing mechanisms of quantal Ca2+ release, it is, therefore, appropriate to compare the sensitivity to IP3 of quantal Ca2+ release (the fraction of Ca2+ stores released) to estimates of how many IP3Rs are likely to be tetra-liganded by that concentration of IP3. We used IP3R1 from cerebellum (2.75 × 108 IP3Rs/μL of sample, determined from [3H]IP3 binding) to calibrate western blots, and so determine the number of IP3Rs in HEK-IP3R1 cells (49,146 ± 15,001 IP3Rs/cell) (Figure 7B). Comparing the affinity of IP3R1 for IP3 under conditions that replicate functional assays (Figure 2G) with the number of IP3Rs in HEK-IP3R1 cells, we predict that the Ca2+ release evoked by a half-maximally effective concentration of IP3 is associated with opening of only 0.13% of a cell’s IP3Rs, or about 60 IP3Rs/cell (Figures 7C and 7D). This situation, where only a tiny fraction of available receptors elicits a response, is analogous to the “spare receptors” found in many signaling pathways initiated by plasma membrane receptors. Here, all receptors are competent to respond, but there are many more receptors than required to elicit a maximal response (Stephenson, 1956). Our argument does not require an additional level of IP3R regulation, although that may also occur (Thillaiappan et al., 2017; Thillaiappan et al., 2021); it requires only that cells express more IP3Rs than are needed to deplete the ER of Ca2+. In analogy with spare receptors elsewhere, we expect this feature to increase the sensitivity of Ca2+ release to IP3.
An open IP3R is predicted to mediate movement of about 500,000 Ca2+/s out of the ER (Vais et al., 2010). We estimate the ER Ca2+ content of a single permeabilized HEK cell to be about 9 × 10−16 mol (see Simulations in STAR Methods). This suggests that sustained opening of a single IP3R for the 20 s taken for incremental responses to plateau would be sufficient to cause loss of almost 2% of the ER Ca2+ content of a cell. The key point is that very few of the many IP3Rs in a cell are active during incremental responses to IP3 and they are sufficient to account for the observed responses (Figure 7D).
A mechanism for incremental responses to IP3
We propose that after IP3 addition, very few of the many IP3Rs in a cell rapidly bind IP3 to each of their four subunits; many more IP3Rs will be partially occupied (Figures 7A, 7C, and 7D). The fully occupied IP3Rs open and rapidly release Ca2+; they then inactivate and more slowly recover (Figure 7E). After the initial Ca2+ release, when activation of IP3Rs is effectively synchronized by IP3 addition, additional IP3Rs will open as they bind four IP3 molecules and then proceed to inactivate, but their openings will be asynchronous. Hence, addition of IP3 drives a few IP3Rs through a near-synchronous sequence of opening and inactivation, after which very small numbers of IP3Rs proceed through the sequence asynchronously. The next step in IP3 concentration then drives another pulse of near-synchronous IP3R openings.
Two features of this scheme contribute to understanding incremental responses. First, incremental responses are mediated by only a tiny fraction of the IP3Rs in a cell (<1%, Figures 7C and 7D). Inactivation therefore negligibly impacts the number of IP3Rs available for subsequent activation, ensuring that despite some inactivation of IP3Rs, responses to subsequent IP3 additions are unperturbed. Second, because IP3Rs move inexorably from an open to an inactivated state, there will always be more open IP3Rs immediately after an IP3 addition (when openings are synchronized) than later (when IP3Rs progress to inactivated states) (Figure 7E). A surge of Ca2+ release after each IP3 addition is followed by a period of much reduced activity, thereby explaining the quantal pattern of Ca2+ release.
There is evidence, albeit with conflicting observations, that rapid activation of IP3Rs by IP3 is followed by slower inactivation (half-time [t½] of about 15–30 s) (Hajnóczky and Thomas, 1994; Mak et al., 2000, 2001; Stehno-Bittel et al., 1995) and even slower recovery (t½ of 30 s to several minutes) (Hajnóczky and Thomas, 1994; Ionescu et al., 2006). This mechanism would allow incremental responses only if inactivation requires most (probably all four) IP3-binding sites to be occupied; otherwise, IP3Rs would inactivate before they opened. An alternative mechanism is “feed-through” inhibition of IP3Rs by Ca2+ as it passes through an open channel (Dawson et al., 2003), but even these very local Ca2+ signals would probably be intercepted by 10 mM BAPTA (Vais et al., 2012), which did not prevent quantal responses (Figures S2A–S2F). We suggest that IP3R inactivation is directly linked to activation rather than via feedback effects from increased [Ca2+]c.
A simple activation-inactivation scheme predicts incremental responses to IP3
We developed an empirical scheme with stochastic simulations to assess whether it captured key features of the experimental observations. A simplified scheme is needed to reduce computation times for the stochastic simulations of the many IP3Rs required to address the consequences of a few IP3Rs opening together and then losing synchrony as they inactivate. The scheme envisages that an IP3R opens rapidly (t½ of ∼2.5 s) after it has bound four molecules of IP3, before slowly inactivating (t½ of ∼5 s) and then very slowly recovering from the inactivated to the closed state (t½ of ∼250 s) (Figure 7E). The detailed mechanisms of IP3-evoked IP3R inactivation are unresolved and may require interaction with additional unidentified molecules. Hence, our empirical scheme based on the activation-inactivation sequence shown in Figure 7E is not a closed cycle (see Simulations in STAR Methods).
Mathematical simulations using our simplified scheme (Figure 7E) and with the numbers of IP3Rs determined in functional analyses of HEK-IP3R1 cells in 96-well plates (4 × 105 cells/well, ∼2 × 1010 IP3Rs/well) (Figure 7B) capture the key experimental observations. Each addition of a submaximal IP3 concentration evokes a surge of IP3R openings followed by stable inactivation of a small fraction of the IP3R population. The ER Ca2+ content responds incrementally to successive IP3 additions. Finally, with SERCAs active, stores rapidly refill in the presence of low IP3 concentrations, but remain depleted with higher concentrations (Figures 7F–7K).
Most of our analyses used populations of cells in 96-well plates, but incremental responses to IP3 are also evident in single wild-type HEK cells (Figures S4J–S4N). We therefore measured the number of IP3Rs in wild-type HEK cells (9,101 ± 934 IP3Rs/cell, mean ± SD, n = 3) and applied our scheme to a single cell. The simulations confirm that here too, the scheme predicts behavior consistent with experimental observations. Incremental additions of IP3 evoke graded loss of ER Ca2+, which is associated with transient opening of very small numbers of IP3Rs and sustained inactivation of a few IP3Rs (Figures 7L–7N).
We also compared the activation-inactivation scheme with experimental data from DT40-IP3R1 cells responding incrementally to IP3 (Figures 1G–1J). Using the experimental data, we identified the component of ER Ca2+ release attributable to open IP3Rs by subtracting the basal IP3-independent Ca2+ leak (see Analysis in STAR Methods). By dividing this IP3R-dependent Ca2+ flux by the time-matched ER Ca2+ content, we estimated the time course of the number of open IP3Rs (in arbitrary numbers) during sequential additions of IP3 (Figures 7O–7T). The analysis establishes that IP3Rs must close within about 10 s of each IP3 addition. Furthermore, the close correspondence of the time course of IP3R openings in the experimental analysis with the predictions of our simple, albeit incomplete, activation-inactivation scheme (Figure 7E) confirms that the scheme captures all essential features of the experimental observations.
Conclusions
Quantal Ca2+ release is an unusual feature of IP3Rs, first described in 1989 (Muallem et al., 1989), but never adequately explained (Yamashita, 2006). We confirmed that in both intact and permeabilized cells and under conditions where SERCAs cannot counteract IP3R activity, submaximal concentrations of IP3 rapidly release only a fraction of the ER Ca2+ stores without compromising responses to further incremental IP3 additions. These features are properties of all three IP3R subtypes; they are evident in single cells; they do not arise from ineffective charge compensation during electrogenic Ca2+ efflux, IP3 metabolism, increases in [Ca2+]c, or mitochondrial activity; and they are a property of IP3Rs rather than the ER.
We developed a synthetic high-affinity agonist of IP3Rs with very low efficacy, 2, to distinguish between the mechanisms proposed to explain quantal Ca2+ release (Figures 1A and 1B); 2 has the lowest efficacy reported for any high-affinity agonist of IP3R. We used 2 to show that quantal responses do not arise from all-or-nothing emptying of heterogenous Ca2+ stores. We do not exclude the possibility that IP3Rs within a cell may differ in their IP3 sensitivity, but it is clear that any such heterogeneity is not required for quantal responses (Figure 3). Instead, after the initial rapid Ca2+ release evoked by low concentrations of IP3, IP3Rs close with Ca2+ still trapped in the ER. Neither erlin 2, an increase in [Ca2+]c, nor a decrease in ER luminal [Ca2+] contributes to this IP3R inactivation. Instead, inactivation is probably an inexorable consequence of IP3R activation (Hajnóczky and Thomas, 1994), although the detailed mechanisms are unresolved.
Comparison of receptor occupancy and functional responses reveals that only a tiny fraction of a cell’s IP3Rs contribute to incremental responses. These analyses, supported by simulations (Figures 7E–7T), reconcile incremental responses with IP3R inactivation. A surge of coordinated opening of IP3Rs occurs with each step in IP3 concentration. This is followed by IP3R inactivation. Thereafter, there will never be the same balance in favor of the open state until the next step in IP3 concentration triggers another coordinated sequence of IP3R opening and then inactivation (Figures 7F and 7L). Pulsatile delivery of IP3 to intact cells may be provided by changes in extracellular stimulus intensity or by cycles of IP3 production and degradation driven by feedback regulation of phospholipase C in the sustained presence of an extracellular stimulus (Bartlett et al., 2015; Dupont et al., 2011; Meyer and Stryer, 1988; Nash et al., 2001).
Our results suggest a mechanism for incremental responses to IP3, a phenomenon that has remained unresolved for more than 30 years. Unrestrained IP3Rs might evoke explosive responses through CICR. The mechanism we propose allows cells to evoke rapid Ca2+ signals through IP3Rs that are graded with stimulus intensity (Figure 1P).
Limitations of the study
Two essential features underpin our explanation for incremental responses to IP3: rapid inactivation of IP3Rs after their opening, and physiological responses mediated by a tiny fraction of available IP3Rs. We do not yet understand the molecular mechanisms underlying IP3R inactivation, and notably whether accessory proteins are required. Understanding these mechanisms would allow us to refine our simplified scheme, which presently includes an irreversible step that forbids transitions from the closed (C4) to inactivated (I4) state of IP3Rs. Hence, our simplified scheme is not a closed cycle, suggesting that it is coupled to an additional mechanism, perhaps reflecting the need for additional proteins to mediate inactivation. High-resolution optical microscopy reveals that low concentrations of IP3 evoke small, highly localized Ca2+ signals (“Ca2+ puffs”) in intact cells neighbors (Smith and Parker, 2009; Thillaiappan et al., 2021). It is not yet clear how incremental responses to IP3 relate to Ca2+ puffs.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-IP3R Ab (AbC, recognizes all vertebrate IP3R subtypes, rabbit, monoclonal) (1:1000) | Cell Signaling Technology (Danvers, MA, USA) | Cat#8568S |
| Clone # D53A5 | ||
| RRID: AB_10890699 | ||
| Anti-β-actin Ab (mouse monoclonal) (1:30000) | Cell Signaling Technology | Cat#3700S |
| Clone # 8H10D10 | ||
| RRID: AB_2242334 | ||
| Anti-erlin 2 Ab (rabbit, monoclonal) (1:1000) | Abcam (Cambridge, UK) | Cat#ab129207 |
| Clone # EPR8088 | ||
| RRID:AB_11143745 | ||
| Anti-mouse secondary Ab (mouse IgG κ-binding protein conjugated to horseradish peroxidise, HRP) (1:5000) | Santa Cruz (Dallas, TX, USA) | Cat#sc-516102 |
| RRID: AB_2687626 | ||
| Anti-rabbit secondary Ab (goat monoclonal, conjugated to HRP) (1:5000) |
SeraCare (Milford, MA, USA) |
Cat#5220-0336 |
| RRID: AB_2857917 | ||
| Chemicals, peptides, and recombinant proteins | ||
| 2-aminoethoxy diphenyl borate (2-APB) | BioVision (Milpitas, CA, USA) | Cat#1798-100 |
| ATP | Sigma-Aldrich (St. Louis, MO, USA) | Cat#A6419 |
| Bafilomycin A1 | Fluorochem (Hadfield, UK). | Cat#M01404 |
| BAPTA | Phion (Dorset, UK) | Cat#61061782 |
| Bis(4-nitrophenyl) carbonate | Acros Organics via ThermoFisher (Waltham, MA, USA) | Cat#171600250 |
| Cbz3-spermine-IP3 (2) | Synthesis and properties described in this paper | Figure 2A |
| Chelex-100 resin Na+ form | Sigma-Aldrich | Cat#C7901 |
| Cyclopiazonic acid (CPA) | Tocris (Bristol, UK) | Cat#1235 |
| Dequalinium chloride hydrate | Sigma-Aldrich | Cat#D3768 |
| d-myo-inositol 1,4,5-trisphosphate (IP3) | Enzo (Exeter, UK) | Cat#ALX-307-007-M005 |
| d-myo-[3H]inositol 1,4,5-trisphosphate ([3H]IP3) (19.3-21 Ci/mmol) | PerkinElmer (Beaconsfield, UK) | Cat#NET911005UC |
| d-myo-inositol 2,4,5-trisphosphate ((2,4,5)IP3) | Cayman Chemical (Ann Arbor, MI, USA) | Cat#10007779 |
| Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 with GlutaMAX (DMEM/F-12 GlutaMAX) | ThermoFisher | Cat#10565018 |
| Foetal bovine serum (FBS) | Sigma | Cat#F7524 |
| G418 | Formedium (Norfolk, UK) | Cat#G4181 |
| Heparin, sodium salt | Calbiochem via Merck (Watford, UK) | Cat#375095 |
| Ionomycin, Ca2+ salt | Apollo Scientific (Stockport, UK) | Cat#BII0123 |
| LiChroprep RP-18 (25-40 μm) | Merck | Cat#109303 |
| Mag-fluo 4 AM | ThermoFisher | Cat#M14206 |
| ML-SA1 | Merck | Cat#648493 |
| Poly-l-lysine (1% solution) | Sigma | Cat#P8920 |
| Q-Sepharose Fast Flow resin | GE Healthcare (Chicago, IL, USA) | Cat#GE17-0510-01 |
| Saponin | Sigma-Aldrich | Cat#S4521 |
| Thapsigargin | Bio-Techne (Middlesex, UK) | Cat#1138/1 |
| Triethylamine | Sigma-Aldrich | Cat#90340 |
| Critical commercial assays | ||
| 8-well glass-bottom μ-slides | Ibidi (Munich, Germany) | Cat#80827 |
| 35-mm glass bottom dishes (#1 glass) | Cellvis (Mountain View, CA 94039) | Cat#D35-14-1-N |
| ECL-Prime/Select western blotting reagents | Amersham via GE Healthcare | Cat#RPN2236/RPN2235 |
| Half-area 96-well black-walled plates | Greiner, Bio-One (Stonehouse, UK) | Cat#675090 |
| Neon transfection system kit | ThermoFisher | Cat#MPK10096 |
| iBlot gel-transfer stacks PVDF | ThermoFisher | Cat#IB401001 |
| Protease inhibitor cocktail (Roche) | Merck | Cat#11836153001 |
| NuPAGE, SDS-PAGE gels (3-8% Tris-acetate or 4-12% Bis-Tris) | ThermoFisher | Cat#EA0375BOX or NP0321BOX |
| TransIT-LT1 transfection reagent | Geneflow (Lichfield, UK) | Cat#MIR 2305 |
| Experimental models: Cell lines | ||
| Gallus gallus DT40-IP3R1 (DT40-IP3R2; DT40-IP3R3) cells | Tovey et al., 2006 | N/A |
| Homo sapiens HEK-3KO cells | Kerafast (Boston, MA, USA) (Alzayady et al., 2016) | Cat#EUR030 |
| Homo sapiens HEK-G-CEPIAer cells | This paper | N/A |
| Homo sapiens wild-type HEK293 cells | Dr D Yule, University of Rochester, NY, USA | N/A |
| Oligonucleotides | ||
| siRNA targeting sequence of Erlin 2 (siRNA 1) ATCTACTTTGACAGAATTGAA | QIAGEN (Manchester, UK) | Cat#SI04952689 |
| siRNA targeting sequence of Erlin 2 (siRNA 2) AAGATAGAAGAGGGACATATT | QIAGEN | Cat#SI04952696 |
| Non-silencing (NS) siRNA | QIAGEN | Cat#1027280 |
| Recombinant DNA | ||
| CFP-TRPML1 plasmid | Addgene (Venkatachalam et al., 2006) | Cat#18827 |
| G-CEPIA1er plasmid | Addgene (Suzuki et al., 2016) | Cat#58215 |
| IP3R1 in pcDNA3.1(-) /Myc-His B plasmid | Dohle et al., 2019 | N/A |
| EGFP-IP3R1 in pcDNA3.2/DEST | Pantazaka and Taylor, 2011 | N/A |
| Residues 1-604 of rat IP3R1 (NT) in pTrcHisA plasmid | Rossi et al., 2009 | N/A |
| Residues 224-604 of rat IP3R1 (IBC) in pTrcHisA plasmid | Rossi et al., 2009 | N/A |
| Software and algorithms | ||
| Fiji | Schindelin et al., 2012 | |
| MetaMorph. Version 7.10.1.161 | Molecular Devices (San Jose, CA, USA) | N/A |
| PRISM. Version 8.4.2 | GraphPad Software (La Jolla, CA, USA) | N/A |
| QuB | Qin, 2004 | N/A |
| SoftMax Pro. Version 5.4 | Molecular Devices | N/A |
| Codes for simulations | This paper | Available from: https://github.com/genedupont/Quantal |
Resource availability
Lead contact
Requests for resources and reagents should be directed to the Lead Contact, Colin W. Taylor (cwt1000@cam.ac.uk).
Materials availability
All plasmids used are commercially available, except EGFP-IP3R1 which is available from the Lead Contact. The cell lines generated in this study are available from the Lead Contact. Compound 2 is available from Barry V. L. Potter (barry.potter@pharm.ox.ac.uk).
Experimental model and subject details
Cells
DT40 cells are an avian B cell line, wherein high rates of DNA recombination have been widely exploited for analyses of cell signaling and B cell function (Winding and Berchtold, 2001). DT40 cells were the first cells in which all three IP3R subtypes were genetically disrupted to provide a null-background for IP3R expression (Sugawara et al., 1997). We used DT40 cells without native IP3Rs to generate cell lines stably expressing single subtypes of mammalian IP3R (DT40-IP3R1-3) (Tovey et al., 2006).
Human embryonic kidney 293 (HEK293 cells) are hypotriploid cells derived from embryonic kidney and immortalized by transformation with adenovirus (Shaw et al., 2002). We used wild-type HEK cells (which express all three IP3R subtypes) (Mataragka and Taylor, 2018), and a stable cell line in which CRISPR/Cas9 was used to prevent expression of all native IP3Rs (HEK-3KO) (Alzayady et al., 2016). HEK-3KO cells were transfected with mammalian IP3R1 to generate a monoclonal HEK-IP3R1 cell line, and wild-type HEK cells were used to generate HEK-G-CEPIA1er cells stably expressing the ER-targeted genetically-encoded Ca2+ indicator, G-CEPIA1er.
The identity of the parental HEK293 cell line used to generate HEK-3KO cells was confirmed by genotyping (Alzayady et al., 2016), and we confirmed the absence of all IP3Rs by western blotting (Mataragka and Taylor, 2018). None of the other cell lines was authenticated. All cells were periodically (every 2 months) confirmed to be free of mycoplasma.
Method details
Synthesis of 2
We originally synthesized 2 (2-O-(1,5,10-tris(benzyloxycarbonyl)-15-oxo-1,5,10,14,16-pentaazaoctadecan-18-yl)-1D-myo-inositol 1,4,5-trisphosphate, Figure 2A) as an intermediate in the synthesis of a novel polyamine-IP3 conjugate, but 2 proved to be most useful for our present purposes. Bis(4-nitrophenyl) carbonate was recrystallized from CH2Cl2/hexane. d-2-O-(2-aminoethyl)-IP3 was synthesized as previously reported (Riley et al., 2004) and used as the triethylammonium salt. N1,N5,N10-tri(benzyloxycarbonyl)spermine was synthesized according to (Blagbrough and Geall, 1998). Triethylammonium bicarbonate (TEAB) buffer was made by bubbling CO2 through a chilled aqueous solution of triethylamine. Anion-exchange chromatography was carried out on Q-Sepharose Fast Flow resin (GE Healthcare) using a Pharmacia Biotech Gradifrac system and P-1 pump, eluting at 5 mL/min with a linear gradient (0 to 100%) of 2.0 M aqueous TEAB buffer, collecting 10-mL fractions. Phosphate-containing fractions were identified using a modification of the Briggs phosphate test (Lampe et al., 1994). Reverse-phase ion-pair chromatography was carried out on LiChroprep RP-18 (25-40 μm, Merck) using a BioLogic LP system (BioRad), eluting at 5 mL/min with a linear gradient (0 to 70%) of CH3CN in 0.05 M TEAB buffer, collecting 7-mL fractions. Compound 2 was accurately quantified using the Ames phosphate assay (Ames and Dubin, 1960). All water used was MilliQ quality.
To a solution of bis(4-nitrophenyl) carbonate (200 mg, 0.66 mmol) in dry CH2Cl2 (5 mL) under N2 was added a solution of N1,N5,N10-tri(benzyloxycarbonyl)spermine (400 mg, 0.66 mmol) in dry CH2Cl2, dropwise over 10 min. The solution was stirred for 1 hr, after which TLC (CH2Cl2/MeOH 10:1 v/v) showed that all the amine (streak, Rf 0.06) had been consumed, with appearance of a product at Rf 0.72 (yellow on heating and stains with phosphomolybdic acid), together with 4-nitrophenol (Rf 0.5) and a trace of unreacted bis(4-nitrophenyl) carbonate (Rf 0.86). CH2Cl2 (20 mL) was added, and the solution was washed with saturated aqueous NaHCO3 (3 × 20 mL). The pale yellow organic layer was dried (MgSO4) and concentrated to give a yellow oil, which was purified by flash chromatography (CH2Cl2, then CH2Cl2:MeOH, 50:1 v/v) to give 4-nitrophenyl N-alkylcarbamate (436 mg) as a colorless oil; TLC Rf 0.24 (CH2Cl2/EtOAc, 5:1). A solution of this alkylcarbamate (46 mg, 60 μmol) in CD3OD (0.75 mL) was added to solid 2-O-(2-aminoethyl)-IP3 (triethylammonium salt, 21 mg, 28 μmol) under N2 in a 5-mL round-bottom flask, followed by dry triethylamine (40 μL). A deep yellow color (4-nitrophenol) appeared within seconds. The clear yellow solution was stirred at room temperature for 2 hr, then transferred to an NMR tube. The 31P NMR spectrum of this solution showed three major peaks (1.01, 3.17 and 4.18 ppm) corresponding to 2, together with other, smaller peaks. The solution was left for 16 hr in the NMR tube, after which a second 31P NMR spectrum showed only the three product peaks. The reaction was therefore judged to be complete. The contents of the NMR tube were transferred to a 100-mL round-bottom flask and the NMR tube was rinsed with MeOH (1 mL). To the combined washings was added triethylammonium bicarbonate (TEAB) buffer (0.05 M, pH 7.6, 75 mL), and the resulting cloudy yellow solution was left for 16 hr at room temperature to hydrolyse any unreacted N-alkylcarbamate. The solution was then loaded onto a column of Q-Sepharose Fast Flow resin (120 mm × 16 mm, bicarbonate form). The column was washed well with water (200 mL) and then eluted with a gradient of TEAB (2.0 M, 0 to 100%). At approx. 40% 2.0 M TEAB, the column eluent took on an intense yellow color (4-nitrophenol) and this buffer concentration was held constant until the eluent became colorless. When the concentration of TEAB was then allowed to increase, the target compound eluted above 90% 2.0 M TEAB. Fractions containing the target compound were identified using the Briggs phosphate assay, combined and concentrated under reduced pressure to give a colorless glassy residue (40 mg). 1H NMR spectroscopy of the product at this stage showed that it contained significant amounts of alkylammonium contaminants, resulting from the high concentration of TEAB buffer needed to elute it from the column. It was therefore purified further using reverse-phase ion-pair chromatography: the 40 mg of material was taken up in 0.05 M TEAB (5 mL) and loaded onto a column of LiChroprep RP-18 resin (100 mm × 16 mm). The column was washed well with 0.05 M TEAB, and then eluted with a gradient of CH3CN in 0.05 M TEAB (0 to 70% CH3CN). The target eluted at 33%–40% CH3CN, as detected by UV absorption at 254 nm. Fractions containing the target compound were combined and concentrated under reduced pressure. MeOH was repeatedly added and evaporated until a colorless glass remained (∼35 mg). Finally, this material was dissolved in water (5 mL) and passed through a column of Chelex-100 resin (Na+ form, 70 mm deep in a Pasteur pipette) to remove triethylammonium ions. The column was washed with water (5 mL) and the combined eluents were lyophilised to give 2 as a white, fluffy solid (28 mg, 19.7 μmol, 70% as determined by total phosphate assay); 1H NMR (Na+ salt in D2O, 400 MHz) δ 1.16–1.48 (4H, broad m, 2 × spermine CH2), 1.49–1.80 (4H, broad m, 2 × spermine CH2), 2.90–3.32 (12H, broad m, 6 × spermine CH2), 3.39 (2H, broad s, NCH2CH2O), 3.80 (1H, broad d, J 9.9 Hz, inositol H-3), 3.87–4.13 (5H, m, inositol H-1, H-5. H-6 and NCH2CH2O), 4.15 (1H, broad s, inositol H-2), 4.33 (1H, q, J 9.1 Hz, inositol H-4), 4.92–5.14 (6H, m, 3 × OCH2Ph), 7.14–7.45 (15H, m, Ph); 13C NMR (Na+ salt in D2O, 126 MHz) δ 24.42, 29.96, 27.35 and 28.02 (spermine CH2), 37.40 and 38.01 (spermine CH2), 40.20 (NCH2CH2O), 44.04, 45.01 and 46.40 (spermine CH2), 66.51 and 67.20 (3 × OCH2Ph), 71.38 (inositol C-3), 71.91 (inositol C-6), 72.72 (NCH2CH2O), 74.59 (inositol C-1), 76.46 (inositol C-4), 78.31 (inositol C-5), 79.69 (inositol C-2), 127.53–128.58 (CH of Ph), 136.27 and 136.60 (ipso-C of Ph), 157.21, 157.37 and 157.82 (3 × urethane C = O), 160.56 (urea C = O); 31P NMR (triethylammonium salt in CD3OD, 162 MHz, 1H-decoupled) δ 0.81 (1 P), 1.55 (1 P) and 2.50 (1 P); HRMS (m/z) [M–H]– calcd for C43H62N5O22P3, 1092.3027; found 1092.3017.
Cell culture and transfections
Methods used to generate and culture DT40 cells without native IP3Rs and then transfected to stably express a single subtype of mammalian IP3R (DT40-IP3R1-3) were described previously (Tovey et al., 2006). HEK293 cells were cultured in DMEM/F-12 GlutaMAX medium with 10% FBS at 37°C in 95% air and 5% CO2. Cells were passaged or used for experiments when they reached confluence. Plasmids were transfected into HEK cells using TransIT-LT1 reagent. The transfection efficiency of HEK cells transiently expressing CFP-TRPML1 or EGFP-IP3R1 was ∼60%, assessed by CFP or EGFP fluorescence. For double transfections (CFP-TRPML1 and EGFP-IP3R1), the transfection efficiency was ∼15%. To generate HEK cells expressing only IP3R1 (HEK-IP3R1 cells), HEK-3KO cells were transfected with the gene encoding rat IP3R1 (lacking the S1 splice site) (Rossi et al., 2009) cloned into pcDNA3.1(-)/Myc-His B plasmid (Dohle et al., 2019). To produce HEK cells stably expressing G-CEPIA1er (HEK-G-CEPIA1er cells), wild-type HEK cells were transfected with G-CEPIA1er plasmid (Suzuki et al., 2014). To generate stable cell lines (HEK-IP3R1 and HEK-G-CEPIA1er cells), cells were passaged 48 hr after transfection in medium with G418 (1 mg/mL). Selection was maintained for 2 weeks with medium changes every 3 days. Monoclonal HEK-IP3R1 cell lines were selected by plating cells (∼1 cell/well) into 96-well plates in medium containing G418 (1 mg/mL). After 4 days, wells with only one cell were identified, cells were then grown to confluence, and cell lines were expanded and their expression of IP3R1 was confirmed by western blot using an antibody specific for IP3R1 (Rossi et al., 2009). To obtain polyclonal HEK-G-CEPIA1er cells, cells with the highest G-CEPIA1er expression (∼1%) were isolated using fluorescence-activated cell sorting (FACS). siRNA transfections of wild-type HEK cells used a Neon transfection system with a final siRNA concentration of 150 nM. Cells were incubated for 48 hr before use. The siRNAs used are listed in the Key Resources table.
Ca2+ release by IP3Rs
Incubation of cells with the acetoxymethylester (AM) of Mag-fluo 4 traps low-affinity forms of the indicator ( = 1.15 mM measured in situ) within the ER. Fluorescence, which is approximately linearly related to ER free [Ca2+] (since [Ca2+]ER < ), can then be used, without further calibration, to reliably report free [Ca2+] within the ER lumen (Rossi and Taylor, 2020). The ER of DT40 or HEK293 cells was loaded with indicator by incubating cells with Mag-fluo 4 AM (20 μM, 60 min, 22°C) in HEPES-buffered saline (HBS: 135 mM NaCl, 5.9 mM KCl, 11.6 mM HEPES, 1.5 mM CaCl2, 11.5 mM glucose, 1.2 mM MgCl2, pH 7.3) as described (Rossi et al., 2009). After washing and permeabilization with saponin (10 μg/mL, 37°C, 2-3 min) in Ca2+-free cytosol-like medium (Ca2+-free CLM), cells were centrifuged (650 × g, 2 min) and resuspended in Mg2+-free CLM supplemented with CaCl2 to give a final free [Ca2+] of 220 nM after addition of 1.5 mM MgATP. Ca2+-free CLM comprised: 20 mM NaCl, 140 mM KCl, 1 mM EGTA, 20 mM PIPES, 2 mM MgCl2, pH 7.0. Cells (4 × 105 cells/well) were attached to poly-L-lysine-coated 96-well black-walled plates (Greiner Bio-One, Stonehouse, UK), and fluorescence (excitation and emission at 485 nm and 520 nm, respectively) was recorded at intervals of 1.44 s using a FlexStation III plate-reader (Molecular Devices, Sunnyvale, CA, USA). MgATP (1.5 mM) was added to initiate Ca2+ uptake, and when the ER had loaded to steady state with Ca2+ (∼150 s), ligands were added. Responses to the ligands show ER Ca2+ contents as either time courses or, in summary results, as the Ca2+ content recorded 20 s after addition of the stimulus. The distribution of samples across 96-well plates was varied between experiments to avoid any systematic ‘position-related’ artifacts. HEK-G-CEPIA1er cells were permeabilized and assayed as described for Mag-fluo 4-loaded cells.
Fluorescence microscopy
Fluorescence microscopy imaging was performed using an inverted Olympus IX83 microscope equipped with either a 60 × oil-immersion objective (numerical aperture, NA 1.45) or a 100 × oil-immersion total internal reflection fluorescence (TIRF) objective (NA 1.49), a multi-line laser bank (excitation light 425 nm and 488 nm) and an iLas2 targeted laser illumination system (Cairn, Faversham, Kent, UK). Excitation light was transmitted through either a quad dichroic beam splitter (TRF89902-QUAD) or a dichroic mirror (for 425 nm; ZT442rdc-UF2) (Chroma). Emitted light was passed through appropriate filters (Cairn Optospin; peak/bandwidth: 480/40 and 525/50) and detected using an iXon Ultra 897 electron-multiplied charge-coupled device (EMCCD) camera (512 × 512 pixels, Andor). Spinning-disc confocal microscopy used a spinning disc with a 70-μm pinhole (X-Light, Crest Optics). TIRFM used the iLas2 illumination system and the penetration depth was 90-140 nm. For analyses of the distribution of CFP-TRPML1 and EGFP-IP3R1 (Figure S5F), we confirmed that there was no bleed-through between channels. Image capture and processing used MetaMorph (Molecular Devices) and Fiji (Schindelin et al., 2012). All images were corrected for background (MetaMorph) by subtracting fluorescence detected from a region without cells.
Single-cell analyses of Ca2+ release
HEK-G-CEPIA1er cells were permeabilized with saponin, and resuspended in Mg2+-free CLM supplemented with CaCl2 to give a final free [Ca2+] of 220 nM after addition of 1.5 mM MgATP. Cells were attached (4 × 105 cells/well) to a poly-L-lysine-coated 8-well glass bottomed μ-slide. MgATP (1.5 mM) was added to initiate Ca2+ uptake, and when the ER had loaded to steady state with Ca2+ (∼700 s), ligands were added.
Cells were imaged (2 s/frame) using spinning-disk confocal microscopy with a 60 × oil-immersion objective. Images were collected using MetaMorph, corrected for background fluorescence and analyzed using the Time Series Analyzer plugin (Fiji). Fluorescence intensity values from regions of interest (ROI) that delineated individual cells or ROI within individual cells are reported in arbitrary fluorescence units.
[3H]IP3 binding
IP3R1, which accounts for ∼99% of IP3Rs in cerebellum (Wojcikiewicz, 1995), was purified from cerebella of adult Wistar rats using heparin-affinity chromatography (Rossi et al., 2009). The purified protein (10 μg/mL) was stored at −80°C in 500 mM NaCl, 50 mM Tris, 10% glycerol, 1 mM 2-mercaptoethanol, 1 mM benzamidine, 1 mM EGTA, 50 mM Tris, 1% CHAPS, pH 8.0. N-terminal fragments of IP3R1 (IBC, residues 224-604; NT, residues 1-604) were expressed as N-terminally tagged His6-fusion proteins in E. coli and cleaved from the tag with thrombin (Rossi et al., 2009). Most equilibrium-competition binding assays (4°C, 5 min) were performed in Tris-EDTA medium (TEM, 500 μL) comprising 50 mM Tris, 1 mM EDTA, pH 8.3 with [3H]IP3 (21 Ci/mmol, 0.3-1.5 nM), bacterial lysate (7 μg protein) or purified IP3R1 (2 μg protein), and competing ligands (Rossi et al., 2009). Bound and free ligand were separated by centrifugation (4°C, 5 min, 14,000 × g). Non-specific binding, determined by addition of 10 μM IP3, was less than 10% of total binding.
For analyses of [3H]IP3 binding under conditions that mimicked those used for functional assays (Figure 2G), equilibrium-competition binding assays (22°C, 5-10 min) were performed in Mg2+-free CLM supplemented with CaCl2 to give a final free [Ca2+] of 220 nM, [3H]IP3 (19.3 Ci/mmol, 7.5 nM), membranes from cerebella of adult Wistar rats (∼100 μg protein) and competing ligands. Bound and free ligand were separated by centrifugation (22°C, 5 min, 14,000 × g). Non-specific binding, determined by addition of 100 μM IP3, was less than 10% of total binding.
Nuclear patch-clamp recording from IP3Rs
The methods used for patch-clamp recording from patches excised from the outer nuclear envelope of DT40-IP3R1 cells, with K+ as charge-carrier and using QuB (Qin, 2004) for analysis of currents, were as described previously (Taufiq-Ur-Rahman et al., 2009; Rossi et al., 2009). It typically took 30-45 s to establish a recording, and Po was then stable throughout recordings that typically lasted up to 8-10 mins.
Western blotting
Cells were harvested in radio-immunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% NP-40 or Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) with protease inhibitor cocktail. The cell suspension was passed through a 25-gauge needle, incubated for 30 min at 4°C with rotation, sonicated (3 × 10 s pulses on ice), and then cleared by centrifugation (14,000 × g, 60 min, 4°C). Proteins were separated using 3%–8% or 4%–12% SDS-PAGE gels (NuPAGE), and transferred to PVDF membranes using an iBlot system. Antibodies are listed in the Key Resources table. Proteins were visualized using ECL Prime/Select western blotting detection reagents and the GeneGnome imaging system (SynGene, Cambridge, UK) and chemiluminescence was quantified using Fiji (Schindelin et al., 2012). For all quantitative analyses, we used bands for which we confirmed, by serial dilution of samples, a linear relationship between protein loading and band intensity.
Quantification and statistical analyses
Simulations
Simulations first consider the number of open IP3Rs after sequential additions of IP3. The scheme assumes that IP3Rs to which four IP3 molecules have bound move between three stable states, closed (C4), open (O4) and inactivated (I4), each with similar affinity for IP3 (Figure 7E). Transitions between receptors states are described by a triangular kinetic scheme (Tveito and Lines, 2010), with k1 = 0.4 s-1, k2 = 0.2 s-1 and k3 = 0.004 s-1, but the scheme includes an irreversible step in a way that is reminiscent of a published model for adaptation in cell signaling (Ferrell, 2016). The transitions between these states (rate constants, ki, 0.004 - 0.4 s-1, and 100-times slower for k-i) are much slower than the rates of IP3 association (∼3.5 × 107 M-1.s-1; kon ∼3.5 s-1 with 100 nM IP3) (Hannaert-Merah et al., 1994) or dissociation (∼28 s-1, estimated from the KD in Figure 2G). Our scheme therefore assumes that for each IP3 concentration, there is a fixed number of IP3Rs with all four IP3-binding sites occupied, determined by the (794 nM) and the number of IP3Rs/cell (49,146 in HEK-IP3R1 cells) (Figure 7B). IP3 addition is simulated by an immediate increase in C4. Simulations are performed stochastically, with each IP3R simulated independently. The probability of transition i is equal to ki. Δt. At each time step (12.5 ms), the algorithm generates a number X drawn uniformly at random on the [0,1] interval. If X < ki. Δt, the IP3R changes state; otherwise it remains in the same state. The behavior of the IP3R population is obtained by summing the number of IP3Rs in each state at every time step. The output provides the temporal evolution of the number of open IP3Rs. The same scheme and parameters were used to simulate the behavior of IP3Rs within a single wild-type HEK cell, for which we determined the expression level to be 9101 ± 934 IP3Rs/cell.
To simulate the change in ER free [Ca2+] (CER) over time, we consider the following equation:
| (1) |
where O4 is the number of open IP3Rs determined from the stochastic simulations, kleak is the rate constant for the IP3-independent Ca2+ leak from the ER (1.8 × 10−4 s-1, see Analysis section), krel is the rate constant for Ca2+ flux through an open IP3R (0.0008 s-1). We estimated krel by assuming that the free [Ca2+] within the ER was 500 μM, the buffer capacity was 5 (hence, total ER [Ca2+] = 2.5 mM; see Analysis section), and an open IP3R conducts 5 × 105 Ca2+ s-1 (Vais et al., 2010). C, the cytosolic free [Ca2+], is fixed at 220 nM by the EGTA included in CLM. At the beginning of each simulation, CER is set to 500 μM (see Rossi and Taylor, 2020) and then computed at each time step for the corresponding O4. The fraction of releasable ER Ca2+ was set to 80%.
To simulate experiments where the ER can refill through SERCA, we used:
| (2) |
with = 100 s.
Codes for the simulations are available from https://github.com/genedupont/Quantal.
Analysis
Rates of Ca2+ release evoked by IP3, ionomycin, carbachol or 2, corrected for the basal Ca2+ leak (Jleak), were calculated by fitting a mono-exponential function to the ER Ca2+ content determined from the time of adding CPA alone. The derivative of this relationship at each time provides Jleak. From the relationship between Jleak and ER Ca2+ content (fitted to a second-degree polynomial or exponential function), Jleak was estimated at each time and subtracted from the observed Ca2+ release to identify the Ca2+ release evoked by IP3, ionomycin, carbachol or 2 (Figures 1F, 1L, 3B, S3D, and S4B).
For each IP3 or ionomycin concentration, the time evolution of ER free [Ca2+] ([Ca2+]ER) was then numerically reconstructed by integration over time of the IP3- or ionomycin-evoked Ca2+ flux. Jleak values from [Ca2+]ER of 500 μM were also used to estimate the rate constant of Ca2+ leak from the ER (kleak = 1.8x10−4 s-1) used in the simulations. To obtain the evolution of open receptors (Figures 7R–7T), the leak-independent Ca2+ flux was subtracted from the rate of Ca2+ release before division of each instantaneous rate by the corresponding Ca2+ gradient. Following Equation (1), this gives the time-evolution of the number of open receptors in arbitrary units.
To estimate the total ER Ca2+ content of a single HEK cell, we assume a luminal free [Ca2+] of ∼500 μM (Rossi and Taylor, 2020), a buffer ratio of ∼5 (Prins and Michalak, 2011) (suggesting a total luminal [Ca2+] of ∼2.5 mM); a cell volume of 1.1 × 1012 L (http://bionumbers.hms.harvard.edu/search.aspx) of which ∼10% is occupied by the nucleus and ∼35% of the remaining cytoplasm is occupied by ER (∼3.64 × 10−13 L) (Valm et al., 2017). Hence, we estimate the total ER Ca2+ content within a single HEK cell to be ∼9 × 10−16 mol.
For equilibrium binding and concentration-effect relationships, results from each experiment were fitted to Hill equations (PRISM) from which pIC50 (-log of half-maximal inhibitory concentration) or pEC50 (-log of half-maximally effective concentration) values were determined. was calculated from: = - [3H-IP3] . In functional assays, dose ratios (DR) were used to calculate antagonist affinities (Arunlakshana and Schild, 1959): = [antagonist]/(DR-1). Statistical analyses of IP3 sensitivity used pEC50 and pKD values.
Statistical analyses
Experiments were performed without prior power calculations, blinding or systematic randomization. The distribution of treatments across wells in multi-well plates was varied between experiments to avoid any systematic bias arising from place-related effects. Statistical significance was assessed using Student’s t test or one-way repeated ANOVA followed by Bonferroni’s multiple comparisons test, with ∗p < 0.05 considered significant (PRISM). The numbers of independent experiments and replicates for each biological experiment are indicated in figure legends. Details of all statistical tests and exact pvalues are provided in Supplemental materials and methods.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BB/P005330/1 to C.W.T.), the Medical Research Council (MR/T028378/1 to C.W.T. and A.M. Rossi), Fondation Philippe Wiener-Maurice Anspach (to G.D. and C.W.T.), Senior Investigator Awards from Wellcome to C.W.T (101844) and B.V.L.P (101010), and a Royal Society Fellowship to T.R. (UF110479). A.M. Rossi is a fellow of Queens’ College, Cambridge. G.D. is a Research Director at the Belgian FRS-FNRS. We thank Abdelhamid Yousef (Cambridge) for contributing ideas relating to erlin 2.
Author contributions
A.M. Rossi performed all biological experiments, except the single-channel recordings. T.R. performed single-channel recordings. A.M. Riley and B.V.L.P. developed and synthesized compound 2. G.D. implemented the mathematical models. C.W.T. and A.M. Rossi designed the study, analyzed and interpreted results, and, with input from the other authors, wrote the manuscript. All authors reviewed the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: November 2, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109932.
Contributor Information
Ana M. Rossi, Email: amr50@cam.ac.uk.
Colin W. Taylor, Email: cwt1000@cam.ac.uk.
Supplemental information
Data and code availability
No large datasets were generated by this study. The computer code used for the simulations is available at: https://github.com/genedupont/Quantal. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Alzayady K.J., Sebé-Pedrós A., Chandrasekhar R., Wang L., Ruiz-Trillo I., Yule D.I. Tracing the evolutionary history of inositol, 1,4,5-trisphosphate receptor: Insights from analyses of Capsaspora owczarzaki Ca2+ release channel orthologs. Mol. Biol. Evol. 2015;32:2236–2253. doi: 10.1093/molbev/msv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayady K.J., Wang L., Chandrasekhar R., Wagner L.E., 2nd, Van Petegem F., Yule D.I. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 2016;9:ra35. doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B.N., Dubin D.T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J. Biol. Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- Arunlakshana O., Schild H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero M.J., Montero M., Alvarez J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. A comparative study. J. Biol. Chem. 1997;272:27694–27699. doi: 10.1074/jbc.272.44.27694. [DOI] [PubMed] [Google Scholar]
- Bartlett P.J., Metzger W., Gaspers L.D., Thomas A.P. Differential regulation of multiple steps in inositol 1,4,5-trisphosphate signaling by protein kinase C shapes hormone-stimulated Ca2+ oscillations. J. Biol. Chem. 2015;290:18519–18533. doi: 10.1074/jbc.M115.657767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecroft M.D., Taylor C.W. Incremental Ca2+ mobilization by inositol trisphosphate receptors is unlikely to be mediated by their desensitization or regulation by luminal or cytosolic Ca2+ Biochem. J. 1997;326:215–220. doi: 10.1042/bj3260215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: Conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagbrough I.S., Geall A.J. Practical synthesis of unsymmetrical polyamine amides. Tetrahedron Lett. 1998;39:439–442. [Google Scholar]
- Bootman M. Intracellular calcium. Questions about quantal Ca2+ release. Curr. Biol. 1994;4:169–172. doi: 10.1016/s0960-9822(94)00041-2. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J., Taylor C.W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J. Physiol. 1992;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac I., Alattia J.-R., Mal T.K., Chan J., Talarico S., Tong F.K., Tong K.I., Yoshikawa F., Furuichi T., Iwai M., et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- Callamaras N., Parker I. Phasic characteristic of elementary Ca2+ release sites underlies quantal responses to IP3. EMBO J. 2000;19:3608–3617. doi: 10.1093/emboj/19.14.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T.R., Berridge M.J., Moreton R.B., Stauderman K.A., Murawsky M.M., Bootman M.D. Quantal Ca2+ mobilization by ryanodine receptors is due to all-or-none release from functionally discrete intracellular stores. Biochem. J. 1994;301:879–883. doi: 10.1042/bj3010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Claret M., Champeil P. Do submaximal InsP3 concentrations only induce the partial discharge of permeabilized hepatocyte calcium pools because of the concomitant reduction of intraluminal Ca2+ concentration? FEBS Lett. 1992;301:287–290. doi: 10.1016/0014-5793(92)80258-i. [DOI] [PubMed] [Google Scholar]
- Dawson A.P., Lea E.J., Irvine R.F. Kinetic model of the inositol trisphosphate receptor that shows both steady-state and quantal patterns of Ca2+ release from intracellular stores. Biochem. J. 2003;370:621–629. doi: 10.1042/BJ20021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Rossi A.M., Riley A.M., Rahman T., Potter B.V.L., Taylor C.W. Binding of inositol 1,4,5-trisphosphate (IP3) and adenophostin A to the N-terminal region of the IP3 receptor: Thermodynamic analysis using fluorescence polarization with a novel IP3 receptor ligand. Mol. Pharmacol. 2010;77:995–1004. doi: 10.1124/mol.109.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle W., Su X., Mills S.J., Rossi A.M., Taylor C.W., Potter B.V.L. A synthetic cyclitol-nucleoside conjugate polyphosphate is a highly potent second messenger mimic. Chem. Sci. (Camb.) 2019;10:5382–5390. doi: 10.1039/c9sc00445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Combettes L., Bird G.S., Putney J.W. Calcium oscillations. Cold Spring Harb. Perspect. Biol. 2011;3:a004226. doi: 10.1101/cshperspect.a004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J.E., Jr. Perfect and near-perfect adaptation in cell signaling. Cell Syst. 2016;2:62–67. doi: 10.1016/j.cels.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Ferris C.D., Cameron A.M., Huganir R.L., Snyder S.H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992;356:350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Thomas A.P. The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature. 1994;370:474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Lin C., Thomas A.P. Luminal communication between intracellular calcium stores modulated by GTP and the cytoskeleton. J. Biol. Chem. 1994;269:10280–10287. [PubMed] [Google Scholar]
- Hannaert-Merah Z., Coquil J.-F., Combettes L., Claret M., Mauger J.-P., Champeil P. Rapid kinetics of myo-inositol trisphosphate binding and dissociation in cerebellar microsomes. J. Biol. Chem. 1994;269:29642–29649. [PubMed] [Google Scholar]
- Hill T.D., Dean N.M., Boynton A.L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988;242:1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Hirose K., Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- Ionescu L., Cheung K.H., Vais H., Mak D.O., White C., Foskett J.K. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal [corrected] Ca2+ release. J. Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F. “Quantal” Ca2+ release and the control of Ca2+ entry by inositol phosphates—A possible mechanism. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J. Biol. Chem. 2005;280:10305–10317. doi: 10.1074/jbc.M413824200. [DOI] [PubMed] [Google Scholar]
- Lampe D., Liu C., Potter B.V.L. Synthesis of selective non-Ca2+-mobilizing inhibitors of D-myo-inositol 1,4,5-trisphosphate 5-phosphatase. J. Med. Chem. 1994;37:907–912. doi: 10.1021/jm00033a007. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S.E., Shull G.E., MacLennan D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- Mak D.-O., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O., McBride S., Raghuram V., Yue Y., Joseph S.K., Foskett J.K. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 2000;115:241–256. doi: 10.1085/jgp.115.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O., McBride S., Foskett J.K. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 insp3 receptors. J. Gen. Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J.S., Taylor C.W. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 1997;7:510–518. doi: 10.1016/s0960-9822(06)00222-3. [DOI] [PubMed] [Google Scholar]
- Marchant J.S., Taylor C.W. Rapid activation and partial inactivation of inositol trisphosphate receptors by inositol trisphosphate. Biochemistry. 1998;37:11524–11533. doi: 10.1021/bi980808k. [DOI] [PubMed] [Google Scholar]
- Marshall I.C.B., Taylor C.W. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem. J. 1994;301:591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataragka S., Taylor C.W. All three IP3 receptor subtypes generate Ca2+ puffs, the universal building blocks of IP3-evoked Ca2+ signals. J. Cell Sci. 2018;131:jcs220848. doi: 10.1242/jcs.220848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. USA. 1988;85:5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA. 1990;87:3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992;357:599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S.J., Beeker T.G. Hormone-evoked calcium release from intracellular stores is a quantal process. J. Biol. Chem. 1989;264:205–212. [PubMed] [Google Scholar]
- Nash M.S., Young K.W., Challiss R.A.J., Nahorski S.R. Intracellular signalling. Receptor-specific messenger oscillations. Nature. 2001;413:381–382. doi: 10.1038/35096643. [DOI] [PubMed] [Google Scholar]
- Oldershaw K.A., Nunn D.L., Taylor C.W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem. J. 1991;278:705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw K.A., Richardson A., Taylor C.W. Prolonged exposure to inositol 1,4,5-trisphosphate does not cause intrinsic desensitization of the intracellular Ca2+-mobilizing receptor. J. Biol. Chem. 1992;267:16312–16316. [PubMed] [Google Scholar]
- Pantazaka E., Taylor C.W. Differential distribution, clustering, and lateral diffusion of subtypes of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2011;286:23378–23387. doi: 10.1074/jbc.M111.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990;250:977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Parys J.B., Missiaen L., De Smedt H., Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J. Biol. Chem. 1993;268:25206–25212. [PubMed] [Google Scholar]
- Prins D., Michalak M. Organellar calcium buffers. Cold Spring Harb. Perspect. Biol. 2011;3:004069. doi: 10.1101/cshperspect.a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys. J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard-Rooney D.C., Hajnóczky G., Seitz M.B., Schneider T.G., Thomas A.P. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J. Biol. Chem. 1993;268:23601–23610. [PubMed] [Google Scholar]
- Richardson A., Taylor C.W. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J. Biol. Chem. 1993;268:11528–11533. [PubMed] [Google Scholar]
- Riley A.M., Dozol H., Spiess B., Potter B.V.L. 2-O-(2-aminoethyl)-myo-inositol 1,4,5-trisphosphate as a novel ligand for conjugation: physicochemical properties and synthesis of a new Ins(1,4,5)P(3) affinity matrix. Biochem. Biophys. Res. Commun. 2004;318:444–452. doi: 10.1016/j.bbrc.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Ríos E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018;150:521–537. doi: 10.1085/jgp.201711959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A.M., Taylor C.W. Reliable measurement of free Ca2+ concentrations in the ER lumen using Mag-Fluo-4. Cell Calcium. 2020;87:102188. doi: 10.1016/j.ceca.2020.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A.M., Riley A.M., Tovey S.C., Rahman T., Dellis O., Taylor E.J.A., Veresov V.G., Potter B.V.L., Taylor C.W. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 2009;5:631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem H., Tovey S.C., Molinski T.F., Taylor C.W. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharmacol. 2014;171:3298–3312. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Morse S., Ararat M., Graham F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Shen D., Wang X., Li X., Zhang X., Yao Z., Dibble S., Dong X.P., Yu T., Lieberman A.P., Showalter H.D., Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T.J. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+] J. Biol. Chem. 1992;267:3573–3576. [PubMed] [Google Scholar]
- Smith I.F., Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L., Lückhoff A., Clapham D.E. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Stephenson R.P. A modification of receptor theory. Br. J. Pharmacol. Chemother. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K., Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–971. doi: 10.1016/s0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki M., Takata M., Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P.F., Baraban J.M., Snyder S.H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J. Biol. Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- Suzuki J., Kanemaru K., Ishii K., Ohkura M., Okubo Y., Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Kanemaru K., Iino M. Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys. J. 2016;111:1119–1131. doi: 10.1016/j.bpj.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taufiq-Ur-Rahman, Skupin A., Falcke M., Taylor C.W. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Potter B.V.L. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem. J. 1990;266:189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan N.B., Chavda A.P., Tovey S.C., Prole D.L., Taylor C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017;8:1505. doi: 10.1038/s41467-017-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan N.B., Smith H.A., Atakpa-Adaji P., Taylor C.W. KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals. Nat. Commun. 2021;12:4514. doi: 10.1038/s41467-021-24739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey S.C., Sun Y., Taylor C.W. Rapid functional assays of intracellular Ca2+ channels. Nat. Protoc. 2006;1:259–263. doi: 10.1038/nprot.2006.40. [DOI] [PubMed] [Google Scholar]
- Tregear R.T., Dawson A.P., Irvine R.F. Quantal release of Ca2+ from intracellular stores by InsP3: tests of the concept of control of Ca2+ release by intraluminal Ca2+ Proc. Biol. Sci. 1991;243:263–268. doi: 10.1098/rspb.1991.0040. [DOI] [PubMed] [Google Scholar]
- Tveito A., Lines G.T. SpringerOpen; 2010. Computing Characterizations of Drugs for Ion Channels and Receptors Using Markov Models. [Google Scholar]
- Vais H., Foskett J.K., Mak D.O. Unitary Ca2+ current through recombinant type 3 InsP3 receptor channels under physiological ionic conditions. J. Gen. Physiol. 2010;136:687–700. doi: 10.1085/jgp.201010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H., Foskett J.K., Ullah G., Pearson J.E., Mak D.O. Permeant calcium ion feed-through regulation of single inositol 1,4,5-trisphosphate receptor channel gating. J. Gen. Physiol. 2012;140:697–716. doi: 10.1085/jgp.201210804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H., Wang M., Mallilankaraman K., Payne R., McKennan C., Lock J.T., Spruce L.A., Fiest C., Chan M.Y., Parker I., et al. ER-luminal [Ca2+] regulation of InsP3 receptor gating mediated by an ER-luminal peripheral Ca2+-binding protein. eLife. 2020;9:e53531. doi: 10.7554/eLife.53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm A.M., Cohen S., Legant W.R., Melunis J., Hershberg U., Wait E., Cohen A.R., Davidson M.W., Betzig E., Lippincott-Schwartz J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546:162–167. doi: 10.1038/nature22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Hofmann T., Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 2006;281:17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Q., Stern M.D., Ríos E., Cheng H. The quantal nature of Ca2+ sparks and in situ operation of the ryanodine receptor array in cardiac cells. Proc. Natl. Acad. Sci. USA. 2004;101:3979–3984. doi: 10.1073/pnas.0306157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen S.M., Dickinson G.D., Swaminathan D., Parker I. Termination of calcium puffs and coupled closings of inositol trisphosphate receptor channels. Cell Calcium. 2014;56:157–168. doi: 10.1016/j.ceca.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winding P., Berchtold M.W. The chicken B cell line DT40: A novel tool for gene disruption experiments. J. Immunol. Methods. 2001;249:1–16. doi: 10.1016/s0022-1759(00)00333-1. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H. The making and breaking of inositol 1,4,5-trisphosphate receptor tetramers. Messenger (Los Angel.) 2018;6:45–49. doi: 10.1166/msr.2018.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. “Quantal” Ca(2+) release reassessed—A clue to oscillation and synchronization. FEBS Lett. 2006;580:4979–4983. doi: 10.1016/j.febslet.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Sugioka M., Ogawa Y. Voltage- and Ca2+-activated potassium channels in Ca2+ store control Ca2+ release. FEBS J. 2006;273:3585–3597. doi: 10.1111/j.1742-4658.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa F., Iwasaki H., Michikawa T., Furuichi T., Mikoshiba K. Cooperative formation of the ligand-binding site of the inositol 1,4, 5-trisphosphate receptor by two separable domains. J. Biol. Chem. 1999;274:328–334. doi: 10.1074/jbc.274.1.328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets were generated by this study. The computer code used for the simulations is available at: https://github.com/genedupont/Quantal. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.