Abstract
Background
Azithromycin is a second-generation macrolide antibiotic which can be used in the treatment of diseases caused by sensitive bacterial infections. This article aimed to investigate the safety of azithromycin in the treatment of infectious diseases in children by meta-analysis.
Methods
PubMed, Embase, and Cochrane were selected as the search database platforms. Randomized controlled trials (RCTs) published after 2010 were searched using the following keywords: “azithromycin”, “children”, “adverse event”, “intravenous event”, and “oral”. Included studies were all clinical trials of azithromycin in the treatment of pediatric diseases. After inclusion/exclusion criteria screening and bias risk assessment, data were extracted from the included studies. RevMan 5.3.5 software was used for statistical analysis to obtain both forest and funnel plots.
Results
A total of nine articles involving 3,597 pediatric patients were included in this study. The results of meta-analysis showed that the nine articles exhibited statistical heterogeneity (I2=70%; P=0.001), and thus, a random-effects model was used. The obtained statistic was [odds ratio (OR) =0.65; 95% confidence interval (CI): (0.43, 0.97)], the statistical effect value was Z=2.10, P=0.04, and the difference was statistically significant. After excluding one article, the remaining eight articles showed homogeneity (I2=12%; P=0.34). So, using fixed-effects model analysis, the statistic was [OR =0.79; 95% CI: (0.67, 0.94)], and the effect size value was Z=2.73, P=0.006.
Discussion
Compared with other antibiotics, the clinical safety of azithromycin is relatively good, but care should be taken when the dosage is high in treating some disease.
Keywords: Azithromycin, pediatric infectious diseases, safety, meta-analysis
Introduction
Azithromycin (molecular formula: C38H72N2O12; molecular weight: 749), a semisynthetic macrolide antibiotic, is a 15-ring compound derived from erythromycin (1). As a second-generation macrolide antibiotic, azithromycin can be used in the treatment of skin and soft tissue infections, respiratory tract infections, otitis media, sinusitis, tonsillitis, and other diseases caused by sensitive bacterial infections. Compared with the first generation, azithromycin has a better half-life in serum, better stability, faster penetration in tissues, and is widely used in clinical practice (2,3). Furthermore, it has strong anti-infective ability, can regulate immunity, has non-specific activity, also due to its broad antibacterial spectrum against Streptococcus pneumonia, Moraxella catarrhalis, and atypical pathogens, azithromycin has been used extensively for the treatment of pediatric infectious diseases and became one of the most commonly prescribed antibiotics in children (4). Gastrointestinal reactions (abdominal distension, abdominal pain, and diarrhea) are the most common adverse reactions associated with azithromycin. In addition, some studies have also reported allergic reactions (such as rash), elevated liver function, and neurological system adverse reactions (5,6). In order to understand the safety of azithromycin in the treatment of children with diseases, this study collected relevant reports from recent years using the Meta-analysis method, and performed quantitative analysis of the data to guide safe clinical application of this medication. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/tp-21-444).
Methods
Search strategy
PubMed, Embase, and Cochrane were selected as the search databases, and literature published after 2010 was searched. The search method was applied as follows: free combination of subject terms using the following keywords: “azithromycin”, “children”, “adverse event”, “intravenous”, and “oral”.
Literature inclusion and exclusion criteria
Inclusion criteria
(I) Articles specifying that the literature type is a randomized controlled trial (RCT); (II) the study subjects were sick children younger than 18 years old; (III) studies specifying that the intervention group was treated with azithromycin alone (oral, intravenous drip, sequential method), while the control group could be treated with placebo or other antibiotics; and (IV) the total number of study samples was greater than 20.
Exclusion criteria
(I) Single-group studies without a control group; observational studies without an intervention method, case studies, review studies, and evaluation studies; (II) articles in which the original text could not be obtained, and those in which the outcome indicator of adverse event incidence or data could not be extracted; and (III) studies in which intervention protocol was mixed with other factors.
Literature bias analysis
We read the full texts of the articles, and evaluated the literature in the following six aspects according to the Cochrane Handbook for Intervention Evaluation criteria (7): the generation of random sequence, classification concealment, blinding method, whether the outcome assessment was incomplete, selective reporting, and other biases. We described the risk of bias of the studies as “high risk”, “low risk”, and “unknown risk”.
Literature screening and data collection
Two researchers conducted the literature screening, risk of bias assessment, and data collection. If the opinions of the two researchers differed in the screening and data collection process, a third researcher intervened to discuss and assist in achieving a solution. The author’s information, publication year, ages of study subjects, route of administration, dose, and incidence of adverse reactions were collected and converted, and then collated into tables for recording.
Statistical analysis
The following statistical methods were utilized: (I) RevMan 5.3.5 software released by Cochrane Collaboration was used as the statistical tool. (II) The Mantel-Haenszel method was used to assess the binary variables for statistical analysis; the fixed-effect model was used for analysis (under the premise of no heterogeneity of the literature), and the odds ratio (OR) was used to report the adverse reaction rates of the two groups of patients. (III) P<0.05 was considered statistically significant. (IV) The analysis process was presented in the form of a forest plot. (V) I2 analysis and Q verification were used for literature heterogeneity; I2>50% or P<0.1 was used to indicate that the results were heterogeneous. (VI) Sensitivity analysis was performed via the exclusion method (one-by-one), and a funnel plot was used to indicate publication bias.
Results
Literature screening procedure
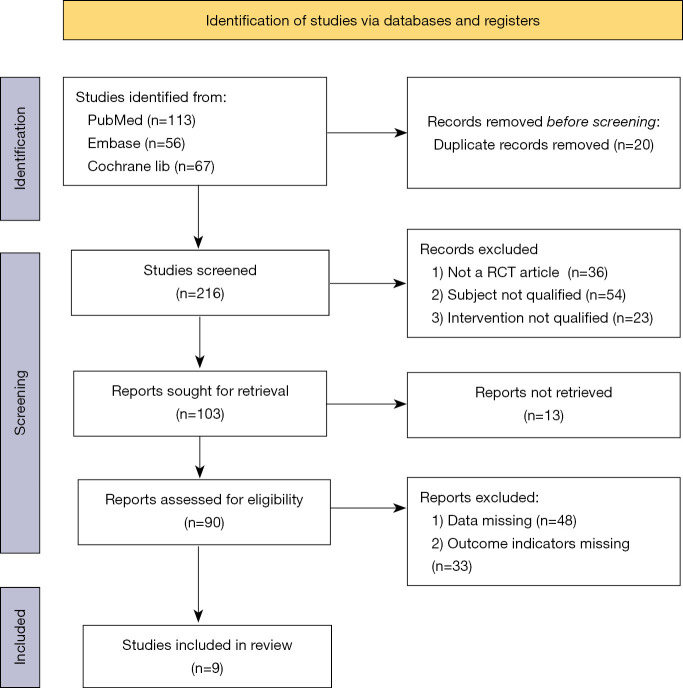
A total of 216 studies related to the adverse reactions of pediatric application of azithromycin (including 113 from PubMed, 56 from Embase, and 47 from Cochrane) were initially enrolled in this study. After reviewing the titles and abstracts of these studies, 53 articles were left after excluding repeated, non-RCT studies and those that did not meet the inclusion criteria. After reading the full texts and excluding articles without the indicator of “incidence of adverse reactions” as well as those for which the data could not be obtained, nine literatures were finally included for bias assessment. The study literature search and screening process is shown in Figure 1.
Figure 1.
Flowchart of literature search and screening.
Literature screening results
As shown in Table 1, a total of nine articles involving 3,597 pediatric patients were included in this study.
Table 1. Basic characteristics of the included studies.
| Author | Year | Condition treated | Number of cases (E/C) | Mean age (years) | Intervention group (azithromycin group) | Control group | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Route of administration | Dose and course of treatment | Adverse reactions | Therapeutic drug | Adverse reactions | ||||||
| Stokholm et al. (8) | 2016 | Asthma-like symptoms | 72/79 | 1–3 | Oral | 10 mg/kg per day for 3 days | 18 cases. The details were unknown | Placebo for 3 days | 24 cases. The details were unknown | |
| Li et al. (9) | 2019 | Tonsillitis in children | 83/79 | 5.6±2.3 | Oral | 10 mg/kg per day for 3 days | 2 cases: 1 diarrhea, 1 nausea | Amoxicillin 30 mg/kg per day for 10 days | 9 cases: 4 diarrhea, 2 rash, 3 nausea | |
| Goyal et al. (10) | 2018 | Bronchiectasis | 82/97 | 1–19 | Oral | 5 mg/kg per day for 21 days | 17 cases: 6 diarrhea, 2 rash, 9 nausea and vomiting | Amoxicillin-clavulanate 22.5 mg/kg, twice daily for 21 days | 23 cases: 15 diarrhea, 0 rash, 8 nausea and vomiting | |
| Sié et al. (11) | 2020 | Malaria | 230/220 | 1–4 | Oral | 20 mg/kg per day for 14 days | 35 cases: 0 abdominal pain, 18 diarrhea, 0 rash, 16 nausea and vomiting, 1 constipation | Placebo for 14 days | 21 cases: 2 abdominal pain, 13 diarrhea, 1 rash, 4 nausea and vomiting, 1 constipation | |
| Mandhane et al. (12) | 2017 | Acute asthma | 140/139 | 1–5 | Oral | 5 mg/kg per day for 5 days | 80 cases: 23 abdominal pain, 42 diarrhea, 26 rash, 22 nausea and vomiting, 8 headache | Placebo for 5 days | 97 cases: 34 abdominal pain, 44 diarrhea, 26 rash, 29 nausea and vomiting, 14 headache | |
| Oldenburg et al. (13) | 2018 | NA | 571/1142 | 1–5 months | Oral | 20 mg/kg for 14 days | 169 cases. The details were unknown | Placebo for 14 days | 391 cases. The details were unknown | |
| Mitjà et al. (14) | 2012 | Yaws | 110/113 | 0.5–15 | Oral | 30 mg/kg | 10 cases | Intramuscular injection of 50,000 units per kg benzathine benzylpenicillin | 8 cases | |
| Saiman et al. (15) | 2010 | CF patients with Chronic Pseudomonas aeruginosa infection | 131/129 | 6–18 | Oral | 250 mg 3 days per week for 6 months | 94 cases: 17 abdominal pain, 25 diarrhea, 22 nausea and vomiting, 30 headache | Placebo for 6 months | 127 cases: 20 abdominal pain, 36 diarrhea, 31 nausea and vomiting, 40 headache | |
| Wang et al. (16) | 2018 | Cholera | 91/89 | 1–5 | Intravenous injection | 30 mL/kg per day | 113 cases: 81 vomiting, 30 abdominal pain, and 2 abdominal distension | Ciprofloxacin 20 mg/kg | 115 cases: 78 vomiting, 32 abdominal pain, 5 abdominal distension | |
E, experiment; C, control; NA, not available; CF, cystic fibrosis.
Risk analysis of literature bias
Two articles (11,16) did not report on random generation sequence. Also, two articles (9,12) were not clear about the allocation concealment, which meant that there may be performance bias. Furthermore, two articles (11,13) were not clear about the optional reporting, which means that there may be reporting bias. The detailed risk of bias assessment of the nine articles included in this study is shown in Table 2.
Table 2. Risk of bias assessment based on the Cochrane systematic review criteria for randomized interventions.
| Study | Generation of random sequence | Allocation concealment | Blind method | Inadequate outcome assessment | Optional reporting | Other bias |
|---|---|---|---|---|---|---|
| Stokholm et al. (8) | Low | Low | Low | Low | Low | Low |
| Li et al. (9) | Low | Unclear | Low | Low | Low | Low |
| Goyal et al. (10) | Low | Low | Low | Low | Low | Low |
| Sié et al. (11) | Unclear | Low | Low | Low | Unclear | Low |
| Mandhane et al. (12) | Low | Low | Low | Low | Low | Low |
| Oldenburg et al. (13) | Low | Unclear | Low | Low | Unclear | Low |
| Mitjà et al. (14) | Low | Low | Low | Low | Low | Low |
| Saiman et al. (15) | Low | Low | Low | Low | Low | Low |
| Wang et al. (16) | Unclear | Low | Low | Low | Low | Low |
Meta-analysis results
Statistical analysis of adverse reactions to azithromycin
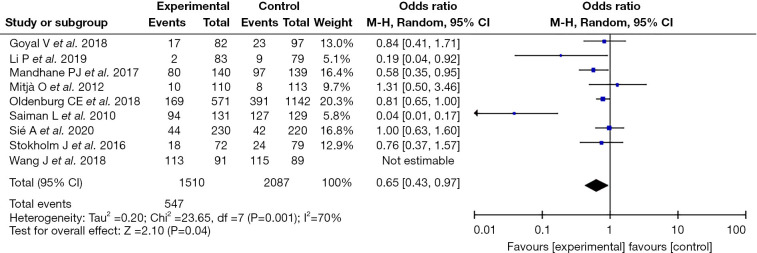
The common adverse reactions following the application of azithromycin included abdominal pain, diarrhea, nausea and vomiting, headache, rash, etc. According to the meta-analysis results, the nine included articles showed statistical heterogeneity (I2=70%; P=0.001), so the random effect model was used. The obtained statistic was [OR =0.65; 95% confidence interval (CI): (0.43, 0.97)], the statistical effect value was Z=2.10, P=0.04, and the difference as statistically significant. The adverse reactions in the intervention group using azithromycin were lower than those in the control group. Since all nine articles were low-risk studies, it was judged that their influence on the results was small (as shown in Figure 2).
Figure 2.
Forest plot of the incidence of adverse reactions following the application of azithromycin in the treatment of pediatric diseases.
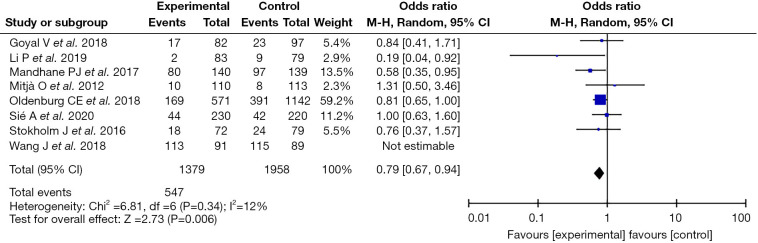
Heterogeneity investigation and sensitivity analyses
Given that the nine included articles exhibited heterogeneity, the case-by-case exclusion method was adopted. Following exclusion of one article (15), the remaining eight articles showed homogeneity (I2=12%; P=0.34). Using fixed effect model analysis, the statistic was (OR =0.79; 95% CI: 0.67−0.94) and effect size was Z=2.73; P=0.006, indicating that the remaining eight articles had good stability (Figure 3).
Figure 3.
Forest plot of sensitivity analysis.
Analysis of publication bias
The funnel plot showed that the eight studies were basically evenly distributed on both sides of the funnel, suggesting that the possibility of publication bias was small (Figure 4).
Figure 4.
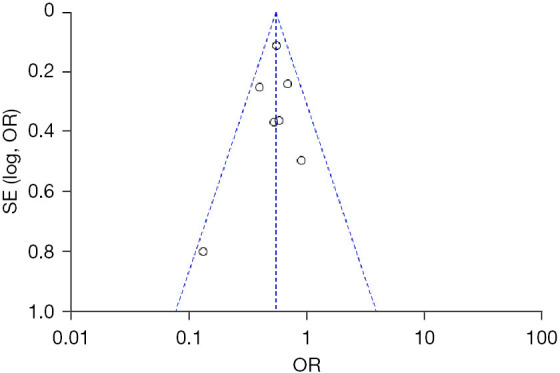
Funnel plot of the incidence of adverse reactions following the application of azithromycin in the treatment of pediatric diseases.
Discussion
As a second-generation macrolide antibiotic, azithromycin is widely used in the treatment of various infectious diseases, and its pediatric clinical application rate is high (17). Azithromycin is effective for Streptococcus pneumoniae and Haemophilus influenzae causing lower respiratory tract infections, and also has a control effect on Chlamydia pneumoniae and mycoplasma (18). In addition, its non-specific and immune effects have also led to its application in the adjuvant anti-infection treatment, which can regulate inflammatory cytokines and reduce inflammatory cell infiltration (19). However, there are no clear guidelines for its safe use in clinical practice at present.
Therefore, this paper summarized and analyzed recent articles on the adverse reactions related to the use of azithromycin in mainstream databases, which included a total of nine studies published after 2010. All of these articles were clearly described as randomized controlled studies. Using the random effects model, the results showed that compared with the control group, the OR of adverse reaction rate in the intervention group, which used azithromycin monotherapy, was [OR =0.65; 95% CI: (0.43, 0.97); P=0.04], indicating that the difference in the adverse reaction rate between the two groups was statistically significant. In other words, the safety of azithromycin monotherapy was good. In a meta-analysis by Smith et al. (20) on the use of azithromycin as a treatment in neonates, it was reported that the use of azithromycin was associated with fewer neurological and gastrointestinal adverse reactions, and significantly reduced the risk of bronchopulmonary dysplasia in neonates. The majority of the subjects included in the present study were children >1 year of age, and the findings were consistent with the previous reports. Another similar meta-analysis performed by Ruuskanen (21) in year 2004 found that for the patients treated with oral azithromycin, on average 9% of patients have treatment-related adverse events, which are most frequently gastrointestinal complaints, but symptoms were mild to tolerate. Our study had the similar outcome with the one mentioned, but included articles all published after 2010, the results were more reliable.
In the present study, the nine included articles exhibited heterogeneity (I2=70%; P=0.001), and the case-by-case elimination method was used for sensitivity analysis. After excluding one of the studies (15), the remaining eight literatures showed homogeneity (I2=12%; P=0.34), and the fixed utility model was used to obtain an effect value of Z=2.73, P=0.006. This result was only slightly different from that of the random effect model of the nine studies combined, which suggested good stability. The reason for the heterogeneity of the one excluded study (15) may lie in its specificity. In the treatment of chronic Pseudomonas aeruginosa infection in patients with fibrosis, the treatment cycle is up to 6 months, resulting in numerous adverse reactions. Thus, strictly controlling the dose, improving medication compliance, closely monitoring the adverse reactions, and timely intervention are needed.
In a study (22), the author pointed out that the medium-dosage group (10–30 mg/kg/day) had more adverse events than that of low-dosage group, which implying more dosage would bring more adverse events. In the study (21), single dose 30 mg/kg and 3-day 20-mg/kg/day regimens are well-tolerated, although these new dosages are associated with more adverse effects. Combined with our findings, in case of treating children with special diseases as fibrosis, trachoma, cholera when the dosage is high, we need to be careful of the adverse reactions, especially the cardiac reactions (prolonged QT or irregular heart beat).
In this study, the publication bias was evaluated after eliminating the one study (15). The funnel plot showed that both sides were basically symmetrical, indicating that the publication bias was small and the results had high reliability. However, this study still had shortcomings that should be noted. Among the nine included articles, some studies did not describe the generation method of random sequence, did not mention allocation concealment, and did not strictly implement the double-blind method, which may have resulted in bias in the results. In addition, a number of articles included in this study focused on abdominal pain, diarrhea, rash, nausea, vomiting, constipation, and other adverse reactions. The absence of statistics regarding neurological complications, allergy, etc., may affect the comprehensiveness of the results. This study only combined the statistics of all adverse reactions, but did not perform statistical analysis of single adverse reactions, which affects the depth of the study. Therefore, in-depth investigations on the safety of azithromycin in the treatment of pediatric diseases are required in future studies.
Conclusions
In this meta-analysis of the application of azithromycin in the treatment of infectious diseases in children, a total of nine studies involving 3,597 patients were included. The results showed that the clinical safety of azithromycin was relatively better than that of other antibiotics, but care should be taken when the dosage is high in treating some disease.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-444
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-444). Both authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Zhou P, Wang X, Zhang X, et al. Recommendations on off-label use of intravenous azithromycin in children. Int J Clin Pract 2021;75:e14010. 10.1111/ijcp.14010 [DOI] [PubMed] [Google Scholar]
- 2.Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med 2016;375:1231-41. 10.1056/NEJMoa1602044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keenan JD, Bailey RL, West SK, et al. Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med 2018;378:1583-92. 10.1056/NEJMoa1715474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshikoya KA, Wharton GT, Avant D, et al. Serious adverse events associated with off-label use of azithromycin or fentanyl in children in intensive care units: a retrospective chart review. Paediatr Drugs 2019;21:47-58. 10.1007/s40272-018-0318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Li ZY, Zhang J, et al. Xiyanping plus azithromycin chemotherapy in pediatric patients with mycoplasma pneumoniae pneumonia: a systematic review and meta-analysis of efficacy and safety. Evid Based Complement Alternat Med 2019;2019:2346583. 10.1155/2019/2346583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu JL, Huang L, Shao MY, et al. Efficacy and safety of azithromycin combined with glucocorticoid on refractory Mycoplasma pneumoniae pneumonia in children: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20121. 10.1097/MD.0000000000020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://www.cochrane.org/
- 8.Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2016;4:19-26. 10.1016/S2213-2600(15)00500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Jiang G, Shen X. Evaluation of 3-day azithromycin or 5-day cefaclor in comparison with 10-day amoxicillin for treatment of tonsillitis in children. Can J Physiol Pharmacol 2019;97:939-44. 10.1139/cjpp-2019-0087 [DOI] [PubMed] [Google Scholar]
- 10.Goyal V, Grimwood K, Byrnes CA, et al. Amoxicillin-clavulanate versus azithromycin for respiratory exacerbations in children with bronchiectasis (BEST-2): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet 2018;392:1197-206. 10.1016/S0140-6736(18)31723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sié A, Dah C, Bountogo M, et al. Adverse events and clinic visits following a single dose of oral azithromycin among preschool children: a randomized placebo-controlled trial. Am J Trop Med Hyg 2020. [Epub ahead of print]. doi: . 10.4269/ajtmh.20-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial. PLoS One 2017;12:e0182411. 10.1371/journal.pone.0182411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldenburg CE, Arzika AM, Maliki R, et al. Safety of azithromycin in infants under six months of age in Niger: a community randomized trial. PLoS Negl Trop Dis 2018;12:e0006950. 10.1371/journal.pntd.0006950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitjà O, Hays R, Ipai A, et al. Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial. Lancet 2012;379:342-7. 10.1016/S0140-6736(11)61624-3 [DOI] [PubMed] [Google Scholar]
- 15.Saiman L, Anstead M, Mayer-Hamblett N, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010;303:1707-15. 10.1001/jama.2010.563 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Yang C. Clinical effect of sequential therapy with azithromycin in children mycoplasma pneumoniae pneumonia. Pak J Pharm Sci 2018;31:1649-52. [PubMed] [Google Scholar]
- 17.Mori F, Pecorari L, Pantano S, et al. Azithromycin anaphylaxis in children. Int J Immunopathol Pharmacol 2014;27:121-6. 10.1177/039463201402700116 [DOI] [PubMed] [Google Scholar]
- 18.Shi H, Lan L, Lv X, et al. Effect of pidotimod combined with azithromycin on children with mycoplasma pneumonia and the expression levels of IL-10 and G-CSF in serum. Exp Ther Med 2019;18:1800-6. 10.3892/etm.2019.7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:710-6. 10.1016/j.bbmt.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C, Egunsola O, Choonara I, et al. Use and safety of azithromycin in neonates: a systematic review. BMJ Open 2015;5:e008194. 10.1136/bmjopen-2015-008194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruuskanen O. Safety and tolerability of azithromycin in pediatric infectious diseases: 2003 update. Pediatr Infect Dis J 2004;23:S135-9. 10.1097/01.inf.0000112528.75956.41 [DOI] [PubMed] [Google Scholar]
- 22.Zeng L, Xu P, Choonara I, et al. Safety of azithromycin in pediatrics: a systematic review and meta-analysis. Eur J Clin Pharmacol 2020;76:1709-21. 10.1007/s00228-020-02956-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as