Abstract
Introduction
The objective of the present study was to describe the experience of the Blood and Tissues Bank of Aragon with the Reveos® Automated Blood Processing System and Mirasol® Pathogen Reduction Technology (PRT) System, comparing retrospectively routine quality data obtained in two different observation periods.
Methods
Comparing quality data encompassing 6,525 blood components from the period 2007–2012, when the semi-automated buffy coat method was used in routine, with 6,553 quality data from the period 2014–2019, when the Reveos system and subsequently the Mirasol system were implemented in routine.
Results
Moving from buffy coat to Reveos led to decreased discard rates of whole blood units (1.2 to 0.1%), increased hemoglobin content (48.1 ± 7.6 to 55.4 ± 6.6 g/unit), and hematocrit (58.9 ± 6.5% to 60.0 ± 4.9%) in red blood cell concentrates. Platelet concentrates (PCs) in both periods had similar yields (3.5 ×10<sup>11</sup>). Whereas in the earlier period, PCs resulted from pooling 5 buffy coats, in the second period 25% of PCs were prepared from 4 interim platelet units. The mean level of factor VIII in plasma was significantly higher with Reveos (92.8 vs. 97.3 IU). Mirasol PRT treatment of PCs reduced expiry rates to 1.2% in 2019. One septic transmission was reported with a non-PRT treated PCs, but none with PRT-treated PCs.
Conclusion
Automation contributed to standardization, efficiency, and improvement of blood processing. Released resources enabled the effortless implementation of PRT. The combination of both technologies guaranteed the self-sufficiency and improvement of blood safety.
Keywords: Automation, Pathogen reduction technology, Blood processing, Safety
Introduction
According to the World Health Organization (WHO), more than 117 million whole blood (WB) units are annually collected worldwide [1]. These donations may be processed into labile blood components, such as red blood cell concentrates (RCCs), plasma, and platelet concentrates (PCs), or into plasma-fractionated products [2]. Labile blood components are standardized according to regulatory guidelines like those of the Council of Europe [3]. In Europe, the most prevalent system for blood processing in the last decades has been the semi-automated buffy coat system, alternative to the manual platelet-rich plasma production, which is the most used method in the world [4]. This process of production of blood components requires numerous repetitive and time-consuming steps [5, 6]. Automation of blood processing has been aimed at increasing the efficiency by eliminating steps such as weighing, balancing, centrifugation, expression, and sealing and eventually decreasing and/or eliminating errors and avoiding deviations associated with multiple manual steps [7]. Previous partially automated systems, such as OrbiSac and TACSI® (Terumo BCT, Lakewood, CO, USA), enabled the pooling and processing of buffy coats into a leukoreduced unit of PC [5, 6, 7, 8]. Atreus (Terumo BCT) was the first full-automated system, able to process 1 unit of WB at a time into 2 or 3 blood components [9, 10]. It was followed by the Reveos® Automated Blood Processing System (Terumo BCT), which can process up to 4 units of WB within 20 min into 2 (plasma and RCC; 2C protocol) or 3 main components (plasma, RCC, and interim platelet unit, IPU; 3C protocol) [11, 12]. In Reveos, blood components are extracted from the fractionated WB during centrifugation, contrarily to the BC method where components are extracted after centrifugation on separate component extractors. Both 2C and 3C protocols collect a Leukopak unit containing the main white blood cell (WBC) fraction, contaminated with red cells and plasma, which is equivalent to a platelet-poor BC fraction of the semi-automated system. The resulting RCC has to be further leukoreduced by gravity using the in-line filter of the Reveos kit, whereas consistent leukoreduction of plasma is reached through the simultaneous centrifugation/extraction step. The system provides a platelet yield indicator, which estimates the platelet count in the IPU [10]. Moreover, the safety of the blood components has always been a crucial issue in blood supply. Pathogen reduction technology (PRT) of WB, plasma, RCCs, and PCs has been developed to minimize the risk of viral, bacterial, and parasites transmission [13, 14]. Mirasol® PRT System (Terumo BCT) uses riboflavin and ultraviolet light to irreversibly induce damage in the nucleic acids of infectious agents [15] and white cells [16]. The Blood and Tissues Bank of Aragon (BTBA) is a public service which collects 43,000 WB units per year and supplies blood components to the region of Aragon, in Spain, with more than 1.3 million of inhabitants [17].
The BTBA validated the Reveos system in November 2012 and implemented it routinely in July 2013. In 2015, the BTBA started using the Mirasol PRT for improving the safety of platelet transfusions. Mirasol PRT is a simple and short process with an illumination step and no postillumination manipulation. To our knowledge, there are limited publications describing the impact of automation and PRT in blood processing. The objective of the present study was to describe the experience with both technologies in the BTBA to supply the demand of blood components.
Materials and Methods
Study Design
This retrospective, observational study was performed with available quality control data between 2007 and 2019 in the Department of Blood Processing at the BTBA. Between 2007 and 2012, WB was processed using the semi-automated buffy coat method, and from 2013 on with the automated Reveos system. Since in 2013 both the buffy coat and Reveos systems co-existed, this year was excluded from the analysis. Regarding Mirasol PRT, the experience comprised the years between 2015 and 2019. Sample size calculation for QC investigation evolved from 1% of produced components in 2007 to numbers proposed in Table 2 of Appendix 4 of the 19th Edition of the EDQM Guidelines to reach 95% confidence of >90% of the components meeting the standard [3].
Table 2.
Production of platelet concentrates in the BTBA between 2015 and 2019
| 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|
| Total PC units, n | 6,021 | 6,624 | 6,605 | 6,478 | 6,306 |
| Treated with Mirasol PRT, n (%) | 939 (15.6) | 1,459 (22.0) | 3,321 (50.3) | 3,870 (59.7) | 3,934 (62.4) |
| PCs expired, n (%) | 207 (3.4) | 163 (2.2) | 100 (1.5) | 215 (3.3) | 77 (1.2) |
| Number of times out of stock | 11 | 4 | 2 | 0 | 1 |
PCs, platelet concentrates.
WB Processing
WB units (450 mL ± 10%) were placed on 1,4-butane-diol cooling plates (CompoCool WB, Fresenius Kabi, Bad Homburg, Germany) for a minimum of 2 h and maximum of 24 h. They were processed using the buffy coat method from 2007 to 2012 according to routine SOP. It consisted of a quadruple top and bottom bag system (Leukotrap® WB In-line collection set, Pall Medical España, Madrid, Spain) with 63 mL citrate-phosphate-dextrose as anticoagulant and 1 satellite bag (100 mL SAGM) as additive solution for RCC. The WB unit was centrifuged for 20 min at 4,532 g and 20°C (acceleration 8, brake 5) in a Jouan KR4I centrifuge (Thermo Electron Corporation, Barcelona, Spain). After hard spin centrifugation, WB was fractionated into components using the BagPressPlus semi-automated blood component separator (Bioelettronica, Milan, Italy). PCs were prepared from 5 buffy coats, randomly selected from fresh and overnight production, plus 300 mL platelet additive solution or PAS (SSP+, MacoPharma, Mouveaux, France) by the OrbiSac device (Terumo BCT), with a pooling kit (OrbiSac System, Terumo BCT) which had an integrated leukoreduction filter. The buffy coat had an average of 55 mL and hematocrit of 45%.
From 2014 to 2019, WB (450 mL) was processed with Reveos by using 2C or 3C protocols according to the blood component demand; 3C and 2C differ in centrifugal speed, i.e., 2,600 and 2,900 rpm, respectively. The “fresh” protocol was used to process WB collected between 2 and 12 h before fractionation, whereas the “overnight” protocol was applied for WB collected between 12 and 24 h before separation. Fresh and overnight protocols differ in the centrifugation time, i.e., 4.5 and 7.0 min, respectively. Internal validations showed no major differences in the quality of RCC between 3C fresh and 3C overnight. The same Reveos WB collection and processing set, Reveos LR (Terumo BCT), was used for both protocols. The freshly separated IPUs, average volume 30 mL, were placed for a minimum of 2 h in a shaker (Helmer PF96i, Helmer Scientific, Noblesville, IN, USA; 60 cycles/min) at 22 ± 2°C after 1 h of resting at room temperature. The identification of the most adequate combination of pools for producing the required number of PCs with a defined yield range was performed with the software T-Pool-Select by the Reveos Management System (RMS, Terumo BCT). Four or 5 IPUs, randomly produced with the fresh or overnight protocol, were pooled using a Reveos Platelet Pooling Set (Terumo BCT) with integrated leukoreduction filter to form a transfusable adult-size platelet dose. PCs were leukoreduced by gravity using the integrated filter in the Reveos pooling set, with 200 mL of T-Platelet Additive Solution (T-PAS+, Terumo BCT). Standard PCs were stored on a flat-bed agitator (Helmer PF96i, 60 cycles/min) in a temperature-controlled room at 22 ± 2°C in both periods up to 5 days.
Quality Controls
Hematological parameters were analyzed with an automated hematology analyzer (Sysmex KX-21N, Sysmex Corporation, Kobe, Japan) until 2015, subsequently with CDRuby (Abbott, IL, USA), and in 2018 with Emerald (Abbott), either right after production or at expiry of the respective blood components (Table 1). Residual leukocytes in RCCs, PCs, and plasma units were determined right after production by using the LeukoSure Enumeration Kit (Beckman Coulter Spain, Barcelona, Spain) in a flow cytometry (Cytomics FC500, Beckman Coulter) from 2013 to 2017. In 2018 and 2019, they were analyzed by the device ADAM (MacoPharma, Tourcoing, France). Factor VIII in plasma was determined after a freezing and thawing cycle by ACLTOP 700 (Werfen, Barcelona, Spain) in plasma processed with 3C overnight protocol, for logistic reasons. pH was measured at day 6 and day 8 in the case of Reveos-derived PCs in non-PRT treated and PRT-treated PCs, respectively.
Table 1.
Comparison of the semi-automated buffy coat (2007–2012) and the automated Reveos system (2013–2019) for blood processing
| Semi-automated buffy coat | Automated Reveos | p | |
|---|---|---|---|
| LR-RCCs leukodepleted (at expiry)* | |||
| Number of tested units | 1,667 | 1,522 | |
| Volume, mL | 245.4±21.5 | 287.4±22.5 | <0.050 |
| Hemoglobin content, g/unit | 48.1±7.6 | 55.4±6.6 | <0.050 |
| Hematocrit, % | 58.9±6.5 | 60.0±4.9 | <0.050 |
| LR-RCCs leukodepleted (after production)* | |||
| Number of tested units | 2,814 | 3,002 | |
| WBCs >1 ×106, % | 123 (4.09) | 2 (0.07) | <0.050 |
| WBCs 1 ×106 | 0.20±2.00 | 0.07±0.10 | <0.050 |
| Plasma (measured after production)* | |||
| Number of tested units | 701 | 701 | |
| Volume, mL | 280.6±21.4 | 264.2±21.7 | <0.050 |
| Number of tested units | 721 | 720 | |
| Platelets, 109/L | 9.6±7.1 | 19.0±13.0 | <0.050 |
| Platelets >50 ×109/L | 1 (0.1) | 33 (4.5) | <0.050 |
| Erythrocytes >6 ×109/L | 50 (6.9) | 41 (5.7) | 0.386 |
| Erythrocytes, 109/L | 1.3±8.6 | 0.72±1.2 | 0.138 |
| WBCs 1 ×109 | 0.02±0.032 | 0.00035±0.0006 | <0.050 |
| Plasma (after freezing/thawing)* | |||
| Number of tested units | 222 | 230 | |
| Factor VIII, IU | 92.5±27.5 | 99.4±29.8 | <0.050 |
| PCs (from WB and untreated after production)* | |||
| Number of tested samples | 661 | 692 | |
| Volume, mL | 336.0±47.5 | 311.4±73.7 | <0.050 |
| Platelet yield, ×1011/pool of 5 or 4 | 3.5±0.8 | 3.5±0.7 | >0.050 |
| WBCs, ×106 | 0.09±0.40 | 0.30±2.00 | <0.050 |
| WBCs >1 ×106 | 5 (0.8) | 12 (1.7) | 0.143 |
| PCs (from WB and untreated at expiry)* | 440 | 387 | |
| pH | 7.0±0.3 | 7.2±0.2 | <0.050 |
| pH >6.4 | 431 (98.0) | 387 (100.0) | <0.050 |
Values are means ± SD or numbers (percentages). LR-RCCs, leukoreduced red blood cell concentrates; SD, standard deviation; WBCs, white blood cells; IU, international unit; PC, platelet concentrates.
Quality control data are exclusively related to whole blood-derived platelet concentrates with no pathogen reduction technology treatment in both periods. They fulfilled the requirements established in guidelines by the Council of Europe.
Bacterial Contamination
Samples for bacterial culture (10 mL) were taken on the 43th day in the case of RCCs and on the 6th day in non-PRT-treated PCs, and on the 8th day in PRT-treated PCs, respectively. All cultures for aerobic and anaerobic bacteria were incubated up to 7 days following the BactAlert method (BioMerieux, Craponne, France).
Mirasol PRT
Platelets were treated with the Mirasol PRT system in accordance with the manufacturer's instructions. In short, 200–330 mL PCs were transferred together with a 35 mL solution of 500 µM riboflavin into a Mirasol illumination bag (Terumo BCT). Once this suspension was well mixed, the unit was weighed and then placed in the Mirasol illuminator device to be exposed to UV light centered at 313 nm. The light energy dose was calculated by the illuminator in accordance with the weight of the product. Mirasol-treated PCs have a shelf-life of 7 days.
Statistical Analyses and Other Calculations
Qualitative variables are expressed as absolute and relative frequencies (%), whereas quantitative variables are expressed as means ± standard deviations (SD). All statistical analyses were performed with Excel software (Microsoft). Comparisons between groups were carried out using the Mann-Whitney test for nonparametric variables and Fisher's exact test for percentages. Statistical significance was established when p ≤ 0.050. Expired rates of PCs were calculated in relation to the total amount of manufactured products with or without Mirasol treatment.
Results
A total of 6,525 blood components were controlled for quality between 2007 and 2012 with the semi-automated buffy coat method and 6,533 with the Reveos system between 2014 and 2019 (Table 1).
Red Blood Cell Concentrates
Comparative analysis of the two observation periods showed that the mean RCC volume (287.4 ± 22.5 mL), hemoglobin content (55.4 ± 6.6 g/unit), and hematocrit (60.0 ± 4.9%) were significantly higher with the Reveos than with the buffy coat protocol (245.4 ± 21.5 mL, 48.1 ± 7.6 g/unit, and 58.9 ± 6.5%; p < 0.050 in all; Table 1). The number of RCC units with <40 g Hb/unit decreased from 124 (4.40%) during the buffy coat observation period to 2 (0.06%) with Reveos. Hemolysis values ≥0.8% at the end of shelf life were observed in 4.9% of the tested units using the buffy coat method in contrast to 3.5% with Reveos. Four percent of the RCCs produced by the buffy coat method had WBC values greater than 1 ×106 versus 0.007% of RCCs produced with the Reveos method. The average of WBC content per unit was 2.4 ×105 and 0.7 ×105 for the buffy coat and Reveos periods, respectively.
Plasma
Volume of recovered plasma was significantly higher, i.e., 281 ± 21 mL with the buffy coat method than with the Reveos (264 ± 22 mL; p < 0.050), where 95% of the units were processed with the 3C protocol. Moreover, a difference of 33 mL was observed between 3C fresh and 2C overnight. The percentage of plasma units with >50 ×109 platelets/L was 0.1% in the buffy coat and 4.5% in the Reveos observation period (p < 0.050). Average residual platelet contents of 9.6 ± 7.1 ×109/L and 19.0 ± 13.0 ×109/L (p < 0.050) were observed for the buffy coat and Reveos periods, respectively. The percentage of plasma with red cell contamination above >6 ×109/L changed from 6.9% during the buffy coat period to 5.7% during the Reveos period (p = 0.38). In both production periods, WBC contamination levels were 100% under 1 ×109/L (not included in Table 1). The average of WBC counts was significantly higher with the buffy coat method in comparison to the Reveos method (0.02 vs. 0.00035; p < 0.050; Table 1), whereas 92% of the Reveos-derived plasma units had WBC levels <1 ×106. The mean level of factor VIII in plasma was significantly higher with the Reveos (97.3 IU) than with the buffy coat protocol (92.8 IU; p < 0.004).
WB-Derived PCs, non-PRT-Treated
The volume of PCs was significantly higher with buffy coat (336.0 ± 47.5 mL) than with Reveos (311.4 ± 73.7 mL; p < 0.050). Platelet content was similar in both periods (3.5 ± 0.8 ×1011 with buffy coat and 3.5 ± 0.7 ×1011 with Reveos), although all PCs produced by the buffy coat method resulted from pooling 5 BCs, while 25% of the PCs by Reveos were made from 4 IPUs. In the buffy coat group, 98% of units controlled at the 6th day had a pH >6.4, compared to 100% in the Reveos group. The average value of pH at the end of shelf life was 7.0 in the buffy coat period compared to 7.2 in the Reveos period (p < 0.050).
Equipment Footprint Reduction
Laboratory space was reduced by reducing the number of devices from 16, including 3 centrifuges, 8 expressors, 2 scales, and 3 OrbiSacs to 3 Reveos and 2 scales. Adding 3 Mirasol devices enabled the implementation of the PRT technology.
Labor Processing Hours Reduction
In the first observation period (2007–2012), pooling of PCs was performed until mid of the second working shift, at 7 p.m. With WB automation, routine manufacturing of PCs was finished by the end of the first working shift, at 3 p.m. The released working capacity led to the reduction of 1 full-time equivalent operator, independent of the additional PRT process and cryopreservation of PCs.
Bacterial Contamination
One case of septic transmission by a non-PRT-treated PC was reported to the BTBA in 2015, although no bacteria-positive culture results were observed during the two observed periods.
Discarded Units
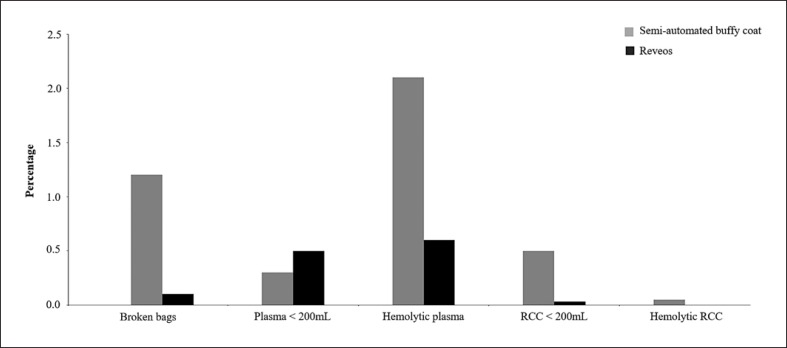
The percentage of discarded WB units decreased with the implementation of automation, including bag ruptures (1.2% with buffy coat vs. 0.1% with Reveos) and low-volume RCCs (<200 mL; 0.5% vs. 0.03%, respectively). A total of 3,259 of WB were discarded due to bag rupture during the buffy coat observation period, most likely due to the hard-shell nature of the in-line filter. There were 144 units discarded due to hemolysis after processing during the buffy coat observation period and none during the Reveos observation period. By contrast, the percentage of units with low-volume plasma (<200 mL) slightly increased with Reveos (0.5% vs. 0.3% with buffy coat), whereas the number of units discarded due to hemolytic plasma decreased (0.6% vs. 2.1% with buffy coat). No “pink” PC was observed in the Reveos period, in contrast to 0.1% during the buffy coat period (Fig. 1).
Fig. 1.
Comparison of discard rates and labor cost savings during the 2 observation periods: semi-automated buffy coat routine blood processing (2007–2012) and the automated Reveos system routine blood processing (2013–2019). FTE, full-time equivalent; RCC, red blood cell concentrate.
Experience with PRT
Twelve PCs can be PRT-treated and released within 34 min if the laboratory is set up with 3 Mirasol illumination devices and 2 lab technicians. Platelet loss is minimal at 1.6% (internal results not shown in this article). Between 2015 and 2019, the annual production of PCs increased from 6,021 to 6,306 units (Table 2). The percentage of PCs undergoing PRT treatment increased from 939 (15.6%) in 2015 to 3,934 (60.2%) in 2019. The total number of PCs treated with PRT was 13,523: 11,843 derived from Reveos production and 1,680 by apheresis. Expired PCs varied from 207 (3.4%) in 2015 to 77 (1.2%) in 2019 (p < 0.050). The number of PC stock disruption events decreased from 11 in 2015 to 0 in 2018 and 1 in 2019. The mean volume of PRT-treated PCs was 324 mL (range: 227–365 mL) and the mean yield was 3.5 ×1011 (range: 2.1–4.4 ×1011). At day 8, 100% of tested PRT-treated PCs showed pH values above 6.4. Control of sterility with bacterial culture of PRT-treated PCs at day 8 were negative in all cases. No adverse reactions, transmission of viruses, bacteria, or other pathogens were reported after transfusion of Mirasol-treated PCs. PRT-treated PCs were transfused to all kinds of patients, including 464 transfusions to patients under 18 years of age.
Discussion
WB has been processed manually or using semi-automated systems for decades [18, 19]. Although the cost of these methods is considered low, they require multiple devices, laboratory space, and several technicians spending time in many operational and maintenance tasks. For the semi-automated blood processing, individuals' expertise and attention during the processes have a relevant impact on the quality of the blood products [20]. Automation has become a cornerstone in the optimization of blood processing due to standardization and traceability, increasing the quality of blood components and minimizing procedural errors [5, 6, 7, 11, 21, 22]. In our study, the implementation of automation (Reveos System) led to a significant reduction in labor-processing hours, decreasing work complexity, eliminating strenuous repetitive tasks, and leading to increased staff satisfaction. In the hypothetical situation that all RCCs undergo Hb quality control check before release, and units with Hb content below the threshold of 40 g per unit would be eliminated due to nonconformity, one could estimate savings of approximately 5 EUR per released RCC with the introduction of the automated system. It finally culminated in the reduction of 1 full-time equivalent operator, while providing the capacity for the introduction of a new technology, i.e., PRT. Furthermore, automation released labor space, which positively contributed to the benefit/cost ratio of blood processing.
Our present study has also demonstrated that the performance with automation is stable and maintained over time. The clinical significance of increased hemoglobin content in red cell concentrates deserves further investigation. The application of T-Pool Select improved the standardization of PC yields, while maintaining flexibility for the combination of 4 or 5 IPUs, according to daily blood product demand. This tool made it possible that all PCs fulfilled quality standards, minimizing personal variations and waste of units. In our study, there were no statistical differences in platelet counts between the 2 processes; however, one should take into account that 25% of the Reveos-produced PCs were derived from pooling of 4 IPUs. It is worth noting that during the 2 observation periods, different methods for platelet counting have been applied: impedance in the earlier period; optical and impedance in the later period [23].
If recovered plasma residual cell content is compared, higher levels of platelets but lower white blood cell contamination levels are found with the Reveos process. Nevertheless, the quality of plasma met EU guidelines requirements for cellular residual content and can be considered leukoreduced based on the very low level of WBC contamination. Currently, the plasma fractionation industry demand increases by 7% per year in Europe, and in Spain the use of albumin and gamma-globulin has increased by 10% and 39% in 2013 and 2017, respectively [24]. Hence, being able to accommodate processing protocols to the actual product demand will be key in the future. Higher plasma recovery out of WB donation can be obtained with the semi-automated method using a different set of bags not designated for platelet production. On the other hand, recovered plasma can be increased by 33 mL when using the Reveos 2C overnight protocol with the single collection kit. Hence, disposable management is more complex and less flexible when using the semi-automated system. Moreover, the possibility of increasing the number of 2C runs with the Reveos system will decrease the monetary loss of lower plasma recovery using the 3C protocol. In fact, the percent of runs using the 2C protocol increased from 1.7% in 2017 to 6.2% in 2019; for 2020, the aim is to reach 10%. Lastly, higher recovery of factor VIII was observed with the fully automated system, which deserves further investigation.
Cid and Lozano [25] using a metanalysis methodology and Slichter et al. [26] with the PLADO clinical trial demonstrated the association between platelet doses and transfusion interval. Moreover, Kaufman et al. [27] in a posthoc analysis of the PLADO study established an association between high doses of PCs with increased transfusion-related adverse events, which might be related to the increased volume of plasma infused per transfusion. With the automated system, we can successfully obtain PCs with less plasma volume while maintaining the same platelet count. In this context, full automation of WB processing may be an important step in the development of customized blood components required to supply the needs of an aging population, not only of patients but also of blood donors.
According to our experience, the fully automated Reveos system may contribute to a balance between standardization and customization. Among the improvements observed with the introduction of automation with the Reveos system, we counted less WB discards, higher recovery of hemoglobin in RCCs, lower discard rates for hemolysis, and low RCC volume, as well as on average less IPUs per platelet adult dose. In summary, we observed an improvement in blood component quality and an increased productivity. The flexibility of the system for the selection of IPUs and upscale recovery of plasma lead to the maximization of the WB donor pool, supporting the increase in demand of PCs and plasma [28].
The adoption of Mirasol PRT technology became essential to guarantee transfusion safety [29, 30] in times of demographic and climate change. It did not compromise blood product quality [31] nor did it hamper the productivity. Mirasol PRT led to a slight increase in volume of the PC, but without any impact on platelet yield, nor on the number of IPUs included in the final PC. The pH remained above 6.4 at day 8 in 100% of components. The experience of BTBA with Mirasol PRT also confirmed that it is simple and easy to use. The treatment leads to minimal platelet loss. It was cost-effective since it allowed the extension of the shelf life in PCs from 5 to 7 days, reducing expiry rates [32, 33], it significantly decreased stock rupture as well as eliminating the need for gamma irradiation [16]. Moreover, if PRT is in place for a certain fraction of components as it is in our case, only a short lead time is required to scale up production to 100% of blood components in the case of infectious disease outbreaks [34]. Due to initial budget constraints, we gradually increased the number of PRT treatments with the aim of achieving 100% in the near future.
In conclusion, automation contributed to the standardization, efficiency, and improvement of blood processing. The released resources enabled the effortless implementation of PRT. The combination of both technologies clearly improved the self-sufficiency and contributed to higher blood safety in our blood center. The goal of this communication is to highlight the innovative operational considerations that lead to a more flexible product portfolio.
Statement of Ethics
Due to the nature of this research on the experience with 2 WB processing techniques through analysis of blood component quality control parameters, the ethical approval of an Ethics Committee was not required, as stated by the Ethics Committee for Research of the Community of Aragon (CEICA). Consent to participate was neither required.
Conflict of Interest Statement
A.I.P.A. has collaborated as guest speaker in Terumo BCT. M.C. is a Terumo BCT employee. The other authors declare no conflicts of interest.
Funding Sources
The authors received financial support from Terumo BCT for the publication of this article.
Author Contributions
A.I.P.A. and M.C. conceived and designed the study; A.I.P.A. executed data collection; A.I.P.A. and G.L. performed the statistical analysis; A.I.P.A. took the lead in writing the manuscript. All authors provided feedback and helped shape the research and analysis, critically reviewed and contributed to the final version of the manuscript.
Acknowledgements
The authors would like to thank the staff of the BTBA. They would also like to express their gratitude to Meisys for writing advice.
References
- 1.World Health Organization 10 facts on blood transfusion. 2015. [cited 06/05/2020]. Available from: https://www.who.int/news-room/facts-in-pictures/detail/blood-transfusion.
- 2.Pasqualetti D, Ghirardini A, Cristina Arista M, Vaglio S, Fakeri A, Waldman AA, et al. Blood component fractionation: manual versus automatic procedures. Transfus Apher Sci. 2004 Feb;30((1)):23–8. doi: 10.1016/j.transci.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 3.European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM) Guide to the preparation, use and quality assurance of blood Components. 19th ed. Strasbourg: Council of Europe; 2017. [Google Scholar]
- 4.Schrezenmeier H, Seifried E. Buffy-coat-derived pooled platelet concentrates and apheresis platelet concentrates: which product type should be preferred? Vox Sang. 2010 Jul;99((1)):1–15. doi: 10.1111/j.1423-0410.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 5.El Ekiaby M. Automation in blood processing. Voxs. 2017;12((1)):87–90. [Google Scholar]
- 6.Cid J, Magnano L, Lozano M. Automation of blood component preparation from whole blood collections. Vox Sang. 2014 Jul;107((1)):10–8. doi: 10.1111/vox.12131. [DOI] [PubMed] [Google Scholar]
- 7.Jurado M, Algora M, Garcia-Sanchez F, Vico S, Rodriguez E, Perez S. Automated processing of whole blood units: operational value and in vitro quality of final blood components. Blood Transfus. 2012 Jan;10((1)):63–71. doi: 10.2450/2011.0008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cid J. Automation in blood component preparation methods. In: Blajchman M, Cid J, Lozano M, editors. Blood component preparation: From benchtop to bedside. Bethesda, MD: AABB Press; 2011. pp. p. 271–86. [Google Scholar]
- 9.Thomas S, Beard M, Garwood M, Callaert M, Cardigan R. Platelet concentrates produced from whole blood using the Atreus processing system. Vox Sang. 2009 Aug;97((2)):93–101. doi: 10.1111/j.1423-0410.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 10.Cid J, Magnano L, Molina P, Diaz-Ricart M, Martínez N, Maymó RM, et al. Automated preparation of whole blood-derived platelets suspended in two different platelet additive solutions and stored for 7 days. Transfusion. 2014 Feb;54((2)):426–33. doi: 10.1111/trf.12283. [DOI] [PubMed] [Google Scholar]
- 11.Johnson L, Winter KM, Kwok M, Reid S, Marks DC. Evaluation of the quality of blood components prepared using the Reveos automated blood processing system. Vox Sang. 2013 Oct;105((3)):225–35. doi: 10.1111/vox.12051. [DOI] [PubMed] [Google Scholar]
- 12.Reveos® Automated blood processing system. Terumo BCT. [cited 06/05/2020]. Available from: https://www.terumobct.com/reveos. [Google Scholar]
- 13.Alter HJ. Pathogen reduction: a precautionary principle paradigm. Transfus Med Rev. 2008 Apr;22((2)):97–102. doi: 10.1016/j.tmrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terumo BCT Mirasol. [cited 06/05/2020]. Available from: https://www.terumobct.com/mirasol.
- 15.van der Meer PF, Bontekoe IJ, Daal BB, de Korte D. Riboflavin and UV light treatment of platelets: a protective effect of platelet additive solution? Transfusion. 2015 Aug;55((8)):1900–8. doi: 10.1111/trf.13033. [DOI] [PubMed] [Google Scholar]
- 16.Marschner S, Fast LD, Baldwin WM, 3rd, Slichter SJ, Goodrich RP. White blood cell inactivation after treatment with riboflavin and ultraviolet light. Transfusion. 2010 Nov;50((11)):2489–98. doi: 10.1111/j.1537-2995.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 17.de Aragón G. Cifras oficiales de población. [cited 06/05/2020]. Available from: https://www.aragon.es/en/-/cifras-oficiales-de-poblacion.
- 18.Hardwick J. Blood processing. ISBT Science Series. 2008;3((2)):148–76. [Google Scholar]
- 19.Basu D, Kulkarni R. Overview of blood components and their preparation. Indian J Anaesth. 2014 Sep-Oct;58((5)):529–37. doi: 10.4103/0019-5049.144647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strengers P. Key elements of a blood transfusion quality management system, the tools and objectives. ISBT Science Series. 2011;6((1)):21–5. [Google Scholar]
- 21.Sunheimer RL, Lifshitz MS, Threatte GA. Analysis: Clinical laboratory automation. In: McPherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 22nd ed. China: WB Saunders; 2011. pp. p. 64–72. [Google Scholar]
- 22.Malomgré W, Neumeister B. Recent and future trends in blood group typing. Anal Bioanal Chem. 2009 Mar;393((5)):1443–51. doi: 10.1007/s00216-008-2411-3. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer PF, Dijkstra-Tiekstra MJ, Mahon A, de Wildt-Eggen J. Counting platelets in platelet concentrates on hematology analyzers: a multicenter comparative study. Transfusion. 2009 Jan;49((1)):81–90. doi: 10.1111/j.1537-2995.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- 24.Spanish Department of Health Transfusion medicine. [cited 06/05/2020]. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/medicinaTransfusional/indicadores/indicadores.htm.
- 25.Cid J, Lozano M. Lower or higher doses for prophylactic platelet transfusions: results of a meta‐analysis of randomized controlled trials. Transfusion. 2007 Mar;47((3)):464–70. doi: 10.1111/j.1537-2995.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 26.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010 Feb 18;362((7)):600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman RM, Assmann SF, Triulzi DJ, Strauss RG, Ness P, Granger S, et al. Transfusion-related adverse events in the Platelet Dose study. Transfusion. 2015 Jan;55((1)):144–53. doi: 10.1111/trf.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escourt J. Why has demand for platelet components increased? A review. Transfus Med. 2014 Oct;24((5)):260–8. doi: 10.1111/tme.12155. [DOI] [PubMed] [Google Scholar]
- 29.Mundt JM, Rouse L, Van den Bossche J, Goodrich RP. Chemical and biological mechanisms of pathogen reduction technologies. Photochem Photobiol. 2014 Sep-Oct;90((5)):957–64. doi: 10.1111/php.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonemura S, Doane S, Keil S, Goodrich R, Pidcoke H, Cardoso M. Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology. Blood Transfus. 2017 Jul;15((4)):357–64. doi: 10.2450/2017.0320-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girona-Llobera E, Jimenez-Marco T, Galmes-Trueba A, Muncunill J, Serret C, Serra N, et al. Reducing the financial impact of pathogen inactivation technology for platelet components: our experience. Transfusion. 2014 Jan;54((1)):158–68. doi: 10.1111/trf.12232. [DOI] [PubMed] [Google Scholar]
- 32.Prioli KM, Karp JK, Lyons NM, Chrebtow V, Herman JH, Pizzi LT. Economic implications of pathogen reduced and bacterially tested platelet components: A US hospital budget impact model. Appl Health Econ Health Policy. 2018 Dec;16((6)):889–99. doi: 10.1007/s40258-018-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorria C, Labata G, Lezaun M, López FJ, Pérez Aliaga AI, Pérez Vaquero MÁ. Impact of implementing pathogen reduction technologies for platelets on reducing outdates. Vox Sang. 2020 Feb;115((2)):167–73. doi: 10.1111/vox.12860. [DOI] [PubMed] [Google Scholar]
- 34.Domanović D, Ushiro-Lumb I, Compernolle V, Brusin S, Funk M, Gallian P, et al. Pathogen reduction of blood components during outbreaks of infectious diseases in the European Union: an expert opinion from the European Centre for Disease Prevention and Control consultation meeting. Blood Transfus. 2019 Nov;17((6)):433–48. doi: 10.2450/2019.0288-19. [DOI] [PMC free article] [PubMed] [Google Scholar]