Abstract
Background
Diabetes mellitus (DM) has detrimental effects on the function of microvascular beds, resulting in blood–brain barrier (BBB) dysfunction. The objective of the study was to investigate whether DM affects the brain physiology through composition of cerebrospinal fluid (CSF) and compare gas tension and electrolyte levels in CSF between the diabetic and nondiabetic populations.
Methods
Patients aged between 20 and 70 years scheduled for elective orthopedic or urologic surgery requiring spinal anesthesia were enrolled. They were assigned to either of the two groups (control or type 2 DM). Gas tension and electrolytes in the CSF and whole blood samples were measured in both groups.
Results
All 49 enrolled patients (24 in the control and 25 in the DM group) completed the study. The concentrations of Na+ and Mg2+ in the blood were significantly lower in the DM group than those in the control. The levels of pCO2 and in the CSF were lower in the DM group than in the control group. In addition, there was a marked increase in the glucose level in both the blood and CSF in the DM group.
Conclusion
The results show that there were some homeostatic changes in blood and CSF in patients with DM.
Keywords: cerebrospinal fluid, electrolyte, acid-base, diabetes mellitus
1. Introduction
Cerebrospinal fluid (CSF) fills the spaces between the brain and spinal cord, flowing through the brain ventricles and subarachnoid space. It is a major component of the extracellular fluid in the central nervous system (CNS). Due to its special location and interaction with the brain parenchyma, CSF has many important roles in brain function. An analysis of CSF composition may assist clinicians identify patients with CNS disorders [1,2].
Diabetes mellitus (DM) is a major public health issue worldwide. It is a metabolic disorder that results from defects in insulin production (type 1, insulin-dependent) and insulin resistance (type 2, insulin-independent). DM has detrimental effects on the function of vascular beds, resulting in various peripheral and CNS complications. Recent evidence from clinical and experimental studies suggested that prolonged hyperglycemic status induced tissue damage and elicited a progressive injury of the brain through several different pathogenetic mechanisms [3,4]. It has been reported that hyperglycemia and abnormal glucose metabolism lead to the generation of reactive oxygen species and elicit inflammatory responses, resulting in endothelial injury of the cerebral microvasculature [5,6]. Altered glycemic conditions in diabetic patients may contribute to the early breakdown of the blood–brain barrier (BBB) [7]. The BBB is a highly specialized endothelial structure in the brain that helps maintain the brain homeostasis by separating the circulating blood components from neurons. Recent studies have shown that patients with diabetes have related changes in BBB integrity. In animal models, hyperglycemia significantly exacerbates BBB dysfunction and edema formation after focal ischemia-reperfusion injury in the brain [8]. MRI images of the brain in DM patients also showed increased BBB permeability when compared to controls [9].
CSF is mainly produced by the choroid plexus via active transport instead of passive ultrafiltration [10]. It regulates the distribution of substances or metabolic waste products in different areas of the brain. Although the respective compositions in blood and CSF are quite similar, there does exist a small difference between them. Breakdown of the BBB alters enzymatic activity and transport of molecules between the blood and brain, leading to neuronal dysfunction and loss, as seen in many CNS disorders such as Alzheimer’s disease [11] and traumatic brain injury [12]. DM is characterized by neurovascular dysfunction involving an inflammatory cascade and BBB breakdown. Clinical manifestations in patients with DM include cognitive, learning, memory deficits, and structural brain abnormalities [13,14]. To our knowledge, there is limited information about ion homeostasis in the CNS, which may be crucial in the pathophysiology of brain dysfunction in DM patients. Therefore, the objective of the present study was to investigate how DM affects the brain physiology through CSF composition. In addition, the gas tension and electrolyte parameters in CSF between the DM and non-DM populations were compared.
2. Materials and methods
2.1. Patients
Forty-nine patients with American Society of Anesthesiologists physical status I or II scheduled for elective urologic or orthopedic surgery and receiving spinal anesthesia were enrolled in this controlled study. Of the 49 patients, while 25 had a history of type 2 DM and were taking oral hypoglycemic agents, the other 24 (control group) had no history of type 2 DM. All patients were between 20 and 70 years. Patients with contraindications for spinal anesthesia, severe cardiovascular dysfunction, renal dysfunction, type 1 DM, any history of neuropathy or neurodegenerative diseases, CNS infection, sepsis, or known allergies to any medications used in this study were excluded.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration and has been approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taoyuan, Taiwan (IRB: 1602240003).
2.2. Study protocol
According to DM status, the patients were assigned to the two groups (control or type 2 DM). All fasted for at least 8 h before sampling blood and CSF. In the operating room, standard monitoring, including noninvasive arterial blood pressure, electrocardiography, and pulse oximetry, was applied. The patient was positioned in the lateral decubitus position. Spinal anesthesia was performed at the L3–4 or L4–5 interspace with a 25-gauge Quincke-type needle through the midline. After discarding an initial volume of approximately 1 mL, another 1 mL of CSF was sampled, following which 15 mg of 0.5% isobaric bupivacaine was administered by an experienced anesthesiologist. After the regional anesthesia was complete, the patient was restored in the supine position. A whole blood sample (2.5 mL) was also collected through arterial puncture from the femoral or dorsalis pedis artery before the operation. CSF and whole blood samples were collected using commercial blood gas syringes with dry heparin preparation. Next, blood gas and electrolyte analyses of CSF and whole blood samples were performed using a StatProfile® Critical Care Xpress Analyzer (Nova Biomedical, Waltham, MA, USA).
2.3. Statistical analysis
All data analyses were performed using SPSS version 17.0 (SPSS Statistics for Windows; SPSS Inc., Chicago, IL). Data were assessed for normality using the Kolmogorov-Smirnov test. Categorical data were reported as numbers and percentages and analyzed using the chi-square test with the Yates correction. Continuous data were expressed as the mean ± standard deviation. While the parametric data were analyzed using an independent t-test, the Mann–Whitney U-test was employed for nonparametric data. Multivariate logistic regression was applied with adjustment for confounding factors, including age, sex, weight, and height. Differences were considered statistically significant at p < 0.05.
3. Results
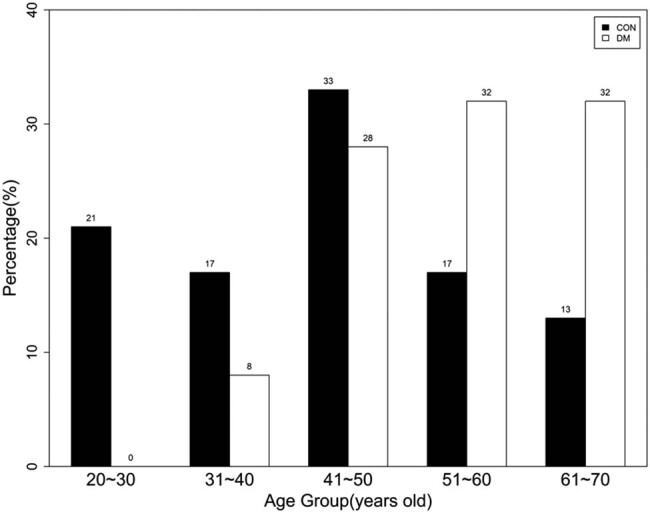
All 49 enrolled patients (24 in the control and 25 in the DM group) scheduled for elective urologic or orthopedic surgery and receiving spinal anesthesia completed the study. CSF and whole blood samples were collected from all the patients. The percentiles for the age categories are shown in Figure 1. In the control group, more samples were collected from patients aged 41 to 50 years than from the other age groups. In the DM group, most samples were collected from those between 41 and 70 years, and over 50% were above 51 years.
Figure 1.
Percentiles in different age categories in control and DM groups.
3.1. Patient characteristics in the control and DM groups
The characteristics of patients included in the two groups are depicted in Table 1. As seen from the table, the age in the DM group was higher than that in the control group (54.7 ± 10.0 vs 44.7 ± 14.0 years, p = 0.006). As compared to the control group, there were more males in the DM group (p = 0.001). In the DM group, weight and height of patients were higher as compared with those in the control group (71.4 ± 13.6 vs 61.7 ± 10.9 kg, p = 0.008; 168.5 ± 8.2 vs 163.0 ± 6.2 cm, p = 0.011, respectively). There was no significant difference in the preoperative blood pressure, heart rate, and oxyhemoglobin saturation between the two groups.
Table 1.
Patient characteristics
| Control group (n = 24) | DM group (n = 25) | p value | |
|---|---|---|---|
| Age (years) | 44.7 ± 14.0 | 54.7 ± 10.0 | 0.006* |
| M/F | 9(37.5)/15(62.5) | 21(84.0)/4(16.0) | 0.001* |
| Weight (kg) | 61.7 ± 10.9 | 71.4 ± 13.6 | 0.008* |
| Height (cm) | 163.0 ± 6.2 | 168.5 ± 8.2 | 0.011* |
| BMI (kg/m2) | 23.2 ± 3.6 | 25.2 ± 4.8 | 0.107 |
| Preoperative | |||
| SBP (mmHg) | 119.3 ± 19.1 | 123.3 ± 23.2 | 0.349 |
| DBP (mmHg) | 71.1 ± 11.1 | 71.4 ± 12.7 | 0.504 |
| HR (min) | 70.5 ± 10.4 | 68.5 ± 10.2 | 0.911 |
| SpO2 (%) | 99.46 ± 0.59 | 99.52 ± 0.59 | 0.983 |
M = male; F = female; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; SpO2: oxyhemoglobin saturation by pulse oximetry.
Data are reported as mean ± standard deviation or number (%).
*p < 0.05 when compared with the control group.
3.2. Comparison of gas tension, electrolyte variables, and glucose in blood between the control and DM groups
The results of the gas tension analysis, electrolyte variables, and blood glucose levels in the two groups are presented in Table 2. There was no significant difference in gas tension between the two groups. As compared to the control group, the concentration of Na+ and Mg2+ in blood was significantly lower in the DM group (133.71 ± 3.49 vs 136.74 ± 2.98 mEq/L, p = 0.005; 0.45 ± 0.10 vs 0.56 ± 0.06 mEq/L, p = 0.045). The glucose level was markedly increased in the DM group compared with that in the control group (171.60 ± 62.56 vs 123.33 ± 44.08 mg/dL, p = 0.041). Additionally, no significant differences in the levels of K+, Cl−, Ca2+, and lactate were observed between the two groups.
Table 2.
Comparison of gas tension, electrolyte variables, and glucose in blood between control and DM groups
| Control group (n = 24) | DM group (n = 25) | Adjusted p value | |
|---|---|---|---|
| pH | 7.40 ± 0.03 (7.39, 7.42) | 7.42 ± 0.04 (7.41, 7.44) | 0.556 |
| pCO2 (mmHg) | 35.44 ± 4.93 (33.36, 37.52) | 33.81 ± 5.61 (31.49, 36.412) | 0.984 |
| pO2 (mmHg) | 131.13 ± 67.12 (102.8, 159.53) | 110.54 ± 77.71 (78.46, 142.61) | 0.317 |
| (mEq/L) | 22.24 ± 2.06 (21.37, 23.11) | 22.14 ± 3.19 (20.82, 23.46) | 0.680 |
| BE (mEq/L) | −1.44 ± 1.46 (−2.06, −0.82) | −1.26 ± 2.86 (−2.44, −0.08) | 0.637 |
| BUN (mg/dL) | 11.92 ± 3.87 (10.28, 13.55) | 20.08 ± 16.42 (13.30, 26.86) | 0.325 |
| Na+ (mEq/L) | 136.74 ± 2.98 (135.51, 138.04) | 133.71 ± 3.49 (132.31, 135.24) | 0.005* |
| K+ (mEq/L) | 3.68 ± 0.33 (3.54, 3.82) | 3.92 ± 0.30 (3.79, 4.05) | 0.055 |
| Cl− (mEq/L) | 109.43 ± 2.35 (108.41, 110.44) | 108.62 ± 4.33 (106.81, 111.44) | 0.218 |
| Ca2+ (mEq/L) | 1.15 ± 0.05 (1.13, 1.17) | 1.17 ± 0.07 (1.14, 1.20) | 0.133 |
| Mg2+ (mEq/L) | 0.56 ± 0.06 (0.51, 0.62) | 0.45 ± 0.10 (0.40, 0.50) | 0.045* |
| Glucose (mg/dL) | 123.33 ± 44.08 (104.71, 141.94) | 171.60 ± 62.56 (145.81, 197.44) | 0.041* |
| Lac (mmol/L) | 1.16 ± 0.52 (0.86, 1.46) | 1.02 ± 0.48 (0.78, 1.26) | 0.479 |
BE: base excess; Lac: lactate.
Data are reported as mean ± standard deviation (95% CI).
p value is adjusted for age, gender, weight, and height.
*p < 0.05 when compared with the control group.
3.3. Comparison of gas tension, electrolyte variables, and glucose in lumbar CSF between the control and DM groups
The values of gas tension analysis, electrolyte variables, and glucose in the lumbar CSF from the two groups are shown in Table 3. There was no significant difference in the pH of the CSF between the two groups, and it was slightly alkaline in both groups. The levels of pCO2 and were lower in the DM group as compared with the control group (31.41 ± 3.77 vs 34.61 ± 4.90 mmHg, p = 0.012; 21.24 ± 2.37 vs 23.48 ± 2.32 mEq/L, p = 0.023, respectively). In both groups, the higher pH of the CSF is attributed to its lower pCO2, reflecting a decreased bicarbonate buffering capacity. As regard the electrolyte values of CSF, there was no significant difference in the levels of Na+, K+, Cl−, Ca2+, Mg2+, and lactate between the two groups. As expected, the glucose level was significantly higher in the DM group than that in the control group (83.20 ± 23.75 vs 55.04 ± 5.90 mg/dL, p = 0.013).
Table 3.
Comparison of gas tension, electrolyte variables, and glucose in lumbar CSF between control and DM groups
| Control group (n = 24) | DM group (n = 25) | Adjusted p value | |
|---|---|---|---|
| pH | 7.43 ± 0.02 (7.42, 7.45) | 7.44 ± 0.03 (7.43, 7.44) | 0.415 |
| pCO2 (mmHg) | 34.61 ± 4.90 (32.54, 36.68) | 31.41 ± 3.77 (29.85, 32.97) | 0.012* |
| pO2 (mmHg) | 122.61 ± 23.46 (112.74, 132.52) | 126.35 ± 16.91 (119.39, 133.32) | 0.165 |
| (mEq/L) | 23.48 ± 2.32 (7.39, 7.42) | 21.24 ± 2.37 (7.39, 7.42) | 0.023* |
| BE (mEq/L) | 0.35 ± 1.60 (−0.32, 1.03) | −1.58 ± 1.95 (−2.41, −0.76) | 0.014* |
| BUN | 10.96 ± 2.40 (10.14, 12.13) | 16.64 ± 12.23 (11.59, 21.69) | 0.262 |
| Na+ (mEq/L) | 137.17 ± 1.81 (136.39, 137.49) | 136.20 ± 2.89 (135.09, 137.42) | 0.331 |
| K+ (mEq/L) | 2.75 ± 0.08 (2.71, 2.78) | 2.80 ± 0.13 (2.75, 2.86) | 0.485 |
| Cl− (mEq/L) | 121.79 ± 1.86 (121.03, 122.62) | 120.84 ± 2.27 (119.89, 121.82) | 0.379 |
| Ca2+ (mEq/L) | 0.98 ± 0.03 (0.97, 0.99) | 0.99 ± 0.04(0.98, 1.01) | 0.422 |
| Mg2+ (mEq/L) | 0.78 ± 0.03 (0.76, 0.79) | 0.76 ± 0.04 (0.74, 0.78) | 0.971 |
| Glucose (mg/dL) | 55.04 ± 5.90 (52.55, 57.53) | 83.20 ± 23.75 (73.39, 93.00) | 0.013* |
| Lac (mmol/L) | 1.12 ± 0.13 (1.05, 1.19) | 1.31 ± 0.27 (1.18, 1.43) | 0.598 |
BE: base excess; Lac: lactate.
Data are reported as mean ± standard deviation (95% CI).
p value is adjusted for age, gender, weight, and height.
*p < 0.05 when compared with the control group.
3.4. Comparison of age subgroups in DM patients
In the DM group, nine of 25 patients were between 31 and 50 years, and the age of the remaining 16 ranged from 51 to 70 years (36 and 64%, respectively). We compared the gas tension and biochemical variables in the blood and lumbar CSF in the two age subgroups (Table 4). It was seen that there were no significant differences between the two age subgroups.
Table 4.
Comparison of gas tension, electrolyte variables, and glucose in blood and lumbar CSF between age subgroups in DM patients
| Age subgroup | Blood | Lumbar CSF | ||||
|---|---|---|---|---|---|---|
| Age <51 years (n = 9) | Age ≥51 years (n = 16) | p value | Age <51 years (n = 9) | Age ≥51 years (n = 16) | p value | |
| pH | 7.42 ± 0.04 | 7.42 ± 0.04 | 0.597 | 7.43 ± 0.02 | 7.44 ± 0.02 | 0.849 |
| pCO2 (mmHg) | 34.03 ± 5.05 | 33.68 ± 6.06 | 0.884 | 31.32 ± 3.16 | 31.46 ± 4.18 | 0.931 |
| pO2 (mmHg) | 121.48 ± 63.18 | 126.88 ± 62.11 | 0.365 | 119.89 ± 12.96 | 129.98 ± 18.14 | 0.156 |
| (mEq/L) | 21.97 ± 2.54 | 22.24 ± 3.58 | 0.843 | 21.09 ± 1.60 | 21.32 ± 2.75 | 0.821 |
| BE (mEq/L) | −1.52 ± 2.32 | −1.11 ± 3.18 | 0.739 | −1.64 ± 1.22 | −1.55 ± 2.33 | 0.909 |
| BUN (mg/dL) | 21.89 ± 16.43 | 19.06 ± 16.86 | 0.689 | 18.78 ± 13.30 | 15.44 ± 11.87 | 0.524 |
| Na+ (mEq/L) | 134.10 ± 1.63 | 133.49 ± 4.23 | 0.615 | 135.94 ± 3.70 | 136.33 ± 2.52 | 0.761 |
| K+ (mEq/L) | 3.97 ± 0.34 | 3.89 ± 0.29 | 0.534 | 2.81 ± 0.10 | 2.80 ± 0.14 | 0.758 |
| Cl− (mEq/L) | 108.30 ± 3.41 | 108.81 ± 4.86 | 0.785 | 120.59 ± 2.78 | 120.99 ± 2.03 | 0.683 |
| Ca2+ (mEq/L) | 1.18 ± 0.11 | 1.17 ± 0.05 | 0.656 | 0.99 ± 0.05 | 1.00 ± 0.03 | 0.497 |
| Mg2+ (mEq/L) | 0.44 ± 0.09 | 0.49 ± 0.11 | 0.382 | 0.76 ± 0.05 | 0.77 ± 0.04 | 0.775 |
| Glucose (mg/dL) | 172.11 ± 68.77 | 155.56 ± 54.57 | 0.514 | 85.11 ± 26.68 | 82.13 ± 22.79 | 0.770 |
| Lac (mmol/L) | 1.03 ± 0.57 | 1.01 ± 0.41 | 0.926 | 1.29 ± 0.30 | 1.32 ± 0.24 | 0.807 |
BE: base excess; Lac: lactate.
Data are reported as mean ± standard deviation.
3.5. Relation between Na+ CSF/blood ratio and blood glucose levels
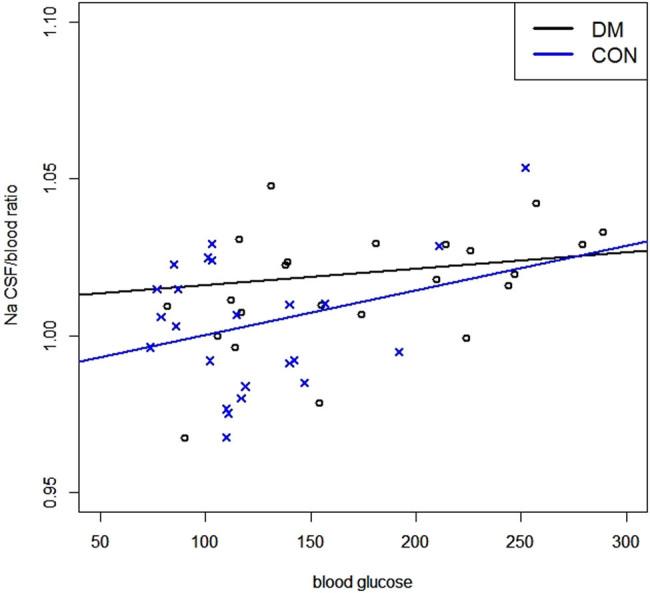
A linear correlation between the Na+ CSF/blood ratio and blood glucose is depicted in Figure 2. The ratio increased with blood glucose in both control and DM groups (r = 0.299 and 0.113, respectively; p < 0.05).
Figure 2.
The linear correlation between the values of Na+ CSF/blood ratio and blood glucose in control and DM groups.
4. Discussion
In this study, CSF samples for profile analysis of patients requiring spinal anesthesia were collected. We analyzed the gas tension and electrolyte parameters of blood and CSF in DM and non-DM adult patients, and some differences between the two groups were seen. It was seen that the concentration of Na+ and Mg2+ was lower in the blood of the DM group than that in the control group. In addition, there was a marked increase in the glucose level in both the blood and CSF in the DM group.
Diabetes is one of the largest epidemics worldwide. Globally, the number of people with type 2 DM has doubled in the past 20 years. With respect to age, a recent meta-analysis study found that the prevalence rate of type 2 diabetes was seven times higher in the 55–74 age group than that in the 20–34 group for Chinese adults, and similar prevalence rates were observed in both women and men [15]. In our prospective controlled study, the patients in the DM group were older than those in the control group (p = 0.006); approximately 60% of the patients were above 51 years, which is in line with the age distribution pattern in a previous meta-analysis study. Moreover, there were more males than females in the DM group. This may be because more patients scheduled for the urologic surgery and enrolled in the study were males. Therefore, for comparison, we adjusted the data for age and sex. Furthermore, in another previous study, the values of serum Na+ and K+ revealed a significant positive correlation with the age of patients [16]. However, in our study no significant difference in study variables was observed between the two age subgroups. This difference might be due to the renal dysfunction status; almost 50% patients had renal dysfunction in the previous study and those enrolled in our study had normal renal function.
Maintaining the pH of the CSF in a narrow range near 7.4 is important for homeostasis because the neurons in the CNS are highly susceptible to small changes in acid-base balance [17]. Many conditions, including respiratory acidosis/alkalosis or metabolic events, may lead to variations in CSF. In patients with both type 1 and type 2 diabetes, the incidence of lactic acidosis is reported to be 3% [18]. Diabetic ketoacidosis is also a severe complication observed in the diabetic population. The variations in pH of the CSF in response to changes in blood pH are related to the function of the BBB. The BBB is relatively freely permeable to CO2, but not to hydrogen or ions. Rapid compensation occurs through the central chemoreceptors in the medulla oblongata, which influence respiratory activity by changing the pCO2 levels. The central canal of the spinal cord also has lining neurons that are sensitive to both acidic and alkaline pH, which intrinsically regulates the pH of the CSF [19]. In our study, no significant difference (p = 0.415) in the CSF pH was observed between the two groups, although both were slightly alkaline. The higher pH in CSF contributes to its lower pCO2 and compensates for the decreased buffering capacity of the bicarbonate.
CSF is mainly produced by the choroid plexus. The respective distributions of main ions between blood and CSF are similar as seen in our study. We also observed that the respective concentrations of Na+ were very close in the blood and CSF in the control group, but not in the DM group. Likewise, the concentrations of K+ and Ca2+ were higher in the blood, whereas the concentrations of Cl− and Mg2+ were lower than those in the CSF.
The secretion of CSF across the choroid plexus occurs through active unidirectional flux via transporters and ion channels and not by the passive ultrafiltration of the plasma [10]. This may have been the reason for the concentrations of ions in the CSF being virtually constant, despite variations in the concentration of ions in the plasma. Furthermore, the major components responsible for transporting monovalent ions across the luminal membrane of the choroid plexus are Na+/K+ ATPase, Na+/K+/2Cl− cotransporter, and channels for the selective secretion of K+ and . The net effect of the interaction of these components is the unidirectional influx of Na+, Cl−, and from the choroid plexus epithelium into the brain ventricle, which forms an osmotic gradient that drives water into the CSF. Na+/K+ ATPase plays a major role in pumping sodium ions into the CSF and is important for the production of CSF. It also creates electrochemical gradients to drive Cl−, and cross the luminal membrane to the CSF [1,20]. In our study, the concentration of Na+ in the blood was significantly lower in the DM group (p = 0.005). Patients with DM usually have abnormal serum sodium concentrations. This dilutional hyponatremia is caused by an increased serum osmolality due to a high glucose level, which results in shifting of water from cells to extracellular fluid [21]. In the brain, Na+/K+ ATPase plays a vital role in maintaining the homeostasis of Na+ ions in the CSF. In an animal model of induced DM, the expression of Na+/K+ ATPase in the brain was shown to be associated with age and sex. In older animals, there seemed to be a significant loss of enzyme activity from diabetic insults. In addition, the activity of Na+/K+ ATPase was significantly lower in the brain of male diabetic animals than in the female group [22]. In our controlled study, though there were more male patients with higher age in DM group, the difference in the concentration of Na+ in the CSF between the two groups was not observed. Further studies are needed to investigate the activities of Na+/K+ ATPase in patients with DM compared to the control. We also found that patients with higher blood glucose levels had higher Na+ CSF/blood ratios (Figure 2). This may be attributed to dilutional hyponatremia caused by high blood glucose levels.
Increasing evidence suggests that Ca2+ and Mg2+ play key roles in the CNS. Ca2+ ions control neurotransmitter release, modulate the activity of ion channels, as well as regulate neuronal plasticity and cell death [23,24]. Ca2+ and Mg2+ in the CSF and serum may also modulate seizure activity [25]. A higher incidence of hypomagnesemia ranging from 13.5 to 47.7% has been reported in patients with type 2 DM [26]. In our study, a lower concentration of Mg2+ in the blood was observed in the DM group than in the control group. The contributory factors may be reduced oral intake and gastrointestinal absorption of magnesium due to diabetic autonomic neuropathies and enhanced renal loss. In the CNS, mechanisms influencing the concentration of CSF Mg2+ are not fully understood. A previous study showed that prolonged induced hypermagnesemia only leads to a marginal increase in CSF Mg2+ in the human brain [27]. Another study revealed that intravenous magnesium infusion did not increase CSF Mg2+ concentration in patients with intracranial hypertension [28]. The regulation of CSF Mg2+ may be controlled by active ion transport through a specific channel, and Mg2+ is maintained at a higher level in the CSF than in the blood. In our study, the concentrations of CSF Mg2+ were similar in the DM and control groups, despite the significant difference (p = 0.045) in the blood Mg2+ concentration between the two groups.
Glucose is the primary metabolic energy source for the mammalian brain. In the CNS, glucose crosses the BBB into the extracellular space of the brain through the glucose transport (GLUT) family, mainly GLUT-1 and GLUT-3. GLUT1 plays an important role in brain glucose uptake and is largely distributed in the BBB and astrocytes [29]. Previous studies undertaken in hyperglycemic animal models have shown that chronic elevated blood glucose downregulates GLUT-1 and GLUT-3 expression in the BBB of the brain [30]. However, another study undertaken in diabetic rats revealed no significant change in the expression of glucose transporters in the BBB [31]. These conflicting results may be due to the different animal models or analytical methods employed. In human adults, the general range of CSF glucose concentration is usually directly proportional to the plasma values and is approximately 50–60% of the plasma levels [32]. In our study, there were no significant differences in the CSF/blood glucose ratio between the control and the DM groups (0.40–0.62 vs 0.44–0.64). A low CSF/plasma glucose ratio (usually <0.4) may be indicative of a CNS infection; either bacterial, or fungal. On the other hand, an elevated CSF glucose concentration usually has no specific pathologic significance and may be merely related to high blood sugar levels [33], as observed in patients with DM in our study.
5. Conclusion
Diabetes-induced alterations in blood glucose levels may cause physiological changes. Our results showed that there are some homeostatic disorders of ions, acid-base, and sugar in the blood and CSF of patients with DM. A detailed analysis of BBB function, expression, and activity of glycated proteins that could induce endothelial damage or other solutes such as amino acids, inflammatory mediators, or metabolic products in CSF in DM patients is further indicated.
Footnotes
Funding information: This work was also supported in part by grants from the Ministry of Science and Technology (MOST 105-2314-B-182A-137-MY3) and Chang Gung Memorial Hospital (CMRPG3F1011-3, CMRPG3F1013) to Fu-Chao Liu, and Chang Gung Memorial Hospital (CORPG3G0601) to Chia-Chih Liao.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Reference
- [1].Speake T, Whitwell C, Kajita H, Majid A, Brown PD. Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech. 2001;52:49–59. [DOI] [PubMed]; Speake T, Whitwell C, Kajita H, Majid A, Brown PD. Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech. 2001;52:49–59. doi: 10.1002/1097-0029(20010101)52:1<49::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [2].Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13:913–22. [DOI] [PubMed]; Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S. et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13:913–22. doi: 10.1111/j.1468-1331.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- [3].Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. [DOI] [PubMed]; Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- [4].Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, Kjartansson O, et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009;32:1608–13. [DOI] [PMC free article] [PubMed]; Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, Kjartansson O. et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009;32:1608–13. doi: 10.2337/dc08-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–64. [DOI] [PubMed]; Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–64. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- [6].Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–83. [DOI] [PubMed]; Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–83. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- [7].Taguchi A. Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:859–64. [DOI] [PubMed]; Taguchi A. Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:859–64. doi: 10.3233/JAD-2009-0975. [DOI] [PubMed] [Google Scholar]
- [8].Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood–brain barrier dysfunction. Stroke. 2007;38:1044–9. [DOI] [PMC free article] [PubMed]; Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood–brain barrier dysfunction. Stroke. 2007;38:1044–9. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–6. [DOI] [PMC free article] [PubMed]; Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–6. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. [DOI] [PMC free article] [PubMed]; Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–8. [DOI] [PubMed]; Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [12].Saw MM, Chamberlain J, Barr M, Morgan MP, Burnett JR, Ho KM. Differential disruption of blood–brain barrier in severe traumatic brain injury. Neurocrit Care. 2014;20:209–16. [DOI] [PubMed]; Saw MM, Chamberlain J, Barr M, Morgan MP, Burnett JR, Ho KM. Differential disruption of blood–brain barrier in severe traumatic brain injury. Neurocrit Care. 2014;20:209–16. doi: 10.1007/s12028-013-9933-z. [DOI] [PubMed] [Google Scholar]
- [13].Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. [DOI] [PubMed]; Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H. et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- [14].Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Foster JK, et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241–8. [DOI] [PubMed]; Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Foster JK. et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241–8. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- [15].Yang L, Shao J, Bian Y, Wu H, Shi L, Zeng L, et al. Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): a meta-analysis. J Diabetes Investig. 2016;7:845–52. [DOI] [PMC free article] [PubMed]; Yang L, Shao J, Bian Y, Wu H, Shi L, Zeng L. et al. Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): a meta-analysis. J Diabetes Investig. 2016;7:845–52. doi: 10.1111/jdi.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Engwa G, Engwa, Nwalo N, Attama T-J, Abonyi C, Akaniro-Ejim N, et al. Influence of type 2 diabetes on serum electrolytes and renal function indices in patients. J Clin Diagnostic Res. 2018;12:BC13–6.; Engwa G, Engwa, Nwalo N, Attama T-J, Abonyi C, Akaniro-Ejim N. et al. Influence of type 2 diabetes on serum electrolytes and renal function indices in patients. J Clin Diagnostic Res. 2018;12:BC13–6. [Google Scholar]
- [17].Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. [DOI] [PubMed]; Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- [18].Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf). 2011;74:191–6. [DOI] [PubMed]; Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf) 2011;74:191–6. doi: 10.1111/j.1365-2265.2010.03891.x. [DOI] [PubMed] [Google Scholar]
- [19].Jalalvand E, Robertson B, Tostivint H, Wallen P, Grillner S. The spinal cord has an intrinsic system for the control of pH. Curr Biol. 2016;26:1346–51. [DOI] [PubMed]; Jalalvand E, Robertson B, Tostivint H, Wallen P, Grillner S. The spinal cord has an intrinsic system for the control of pH. Curr Biol. 2016;26:1346–51. doi: 10.1016/j.cub.2016.03.048. [DOI] [PubMed] [Google Scholar]
- [20].Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56:1695–716. [DOI] [PubMed]; Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56:1695–716. doi: 10.1016/j.addr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [21].Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126:256–63. [DOI] [PubMed]; Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126:256–63. doi: 10.1016/j.amjmed.2012.06.037. [DOI] [PubMed] [Google Scholar]
- [22].Kalocayova B, Mezesova L, Bartekova M, Vlkovicova J, Jendruchova V, Vrbjar N. Effect of duration of diabetes mellitus type 1 on properties of Na, K-ATPase in cerebral cortex. Mol Cell Biochem. 2015;405:41–52. [DOI] [PubMed]; Kalocayova B, Mezesova L, Bartekova M, Vlkovicova J, Jendruchova V, Vrbjar N. Effect of duration of diabetes mellitus type 1 on properties of Na, K-ATPase in cerebral cortex. Mol Cell Biochem. 2015;405:41–52. doi: 10.1007/s11010-015-2394-2. [DOI] [PubMed] [Google Scholar]
- [23].Lohmann C, Wong RO. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium. 2005;37:403–9. [DOI] [PubMed]; Lohmann C, Wong RO. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium. 2005;37:403–9. doi: 10.1016/j.ceca.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [24].Bano D, Nicotera P. Ca2+signals and neuronal death in brain ischemia. Stroke. 2007;38:674–6. [DOI] [PubMed]; Bano D, Nicotera P. Ca2+signals and neuronal death in brain ischemia. Stroke. 2007;38:674–6. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- [25].Apostol A, Apostol R, Ali E, Choi A, Ehsuni N, Hu B, et al. Cerebral spinal fluid and serum ionized magnesium and calcium levels in preeclamptic women during administration of magnesium sulfate. Fertil Steril. 2010;94:276–82. [DOI] [PubMed]; Apostol A, Apostol R, Ali E, Choi A, Ehsuni N, Hu B. et al. Cerebral spinal fluid and serum ionized magnesium and calcium levels in preeclamptic women during administration of magnesium sulfate. Fertil Steril. 2010;94:276–82. doi: 10.1016/j.fertnstert.2009.02.024. [DOI] [PubMed] [Google Scholar]
- [26].Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–73. [DOI] [PubMed]; Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–73. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- [27].McKee JA, Brewer RP, Macy GE, Phillips-Bute B, Campbell KA, Borel CO, et al. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: a study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med. 2005;33:661–6. [DOI] [PubMed]; McKee JA, Brewer RP, Macy GE, Phillips-Bute B, Campbell KA, Borel CO. et al. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: a study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med. 2005;33:661–6. doi: 10.1097/01.ccm.0000156293.35868.b2. [DOI] [PubMed] [Google Scholar]
- [28].Brewer RP, Parra A, Borel CO, Hopkins MB, Reynolds JD. Intravenous magnesium sulfate does not increase ventricular CSF ionized magnesium concentration of patients with intracranial hypertension. Clin Neuropharmacol. 2001;24:341–5. [DOI] [PubMed]; Brewer RP, Parra A, Borel CO, Hopkins MB, Reynolds JD. Intravenous magnesium sulfate does not increase ventricular CSF ionized magnesium concentration of patients with intracranial hypertension. Clin Neuropharmacol. 2001;24:341–5. doi: 10.1097/00002826-200111000-00005. [DOI] [PubMed] [Google Scholar]
- [29].Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. N Physiol Sci. 2001;16:71–6. [DOI] [PubMed]; Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. N Physiol Sci. 2001;16:71–6. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- [30].Hou WK, Xian YX, Zhang L, Lai H, Hou XG, Xu YX, et al. Influence of blood glucose on the expression of glucose trans-porter proteins 1 and 3 in the brain of diabetic rats. Chin Med J (Engl). 2007;120:1704–9. [PubMed]; Hou WK, Xian YX, Zhang L, Lai H, Hou XG, Xu YX. et al. Influence of blood glucose on the expression of glucose trans-porter proteins 1 and 3 in the brain of diabetic rats. Chin Med J (Engl) 2007;120:1704–9. [PubMed] [Google Scholar]
- [31].Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49:1016–21. [DOI] [PubMed]; Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49:1016–21. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- [32].Jerrard DA, Hanna JR, Schindelheim GL. Cerebrospinal fluid. J Emerg Med. 2001;21:171–8. [DOI] [PubMed]; Jerrard DA, Hanna JR, Schindelheim GL. Cerebrospinal fluid. J Emerg Med. 2001;21:171–8. doi: 10.1016/s0736-4679(01)00360-2. [DOI] [PubMed] [Google Scholar]
- [33].Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68:1103–8. [PubMed]; Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68:1103–8. [PubMed] [Google Scholar]


