ABSTRACT
Background
Asthma has become one of the major public health challenges, and recent studies show promising clinical benefits of dietary interventions, such as the Dietary Approaches to Stop Hypertension (DASH) diet.
Objective
The objective of this study was to examine whether changes in diet quality are associated with changes in inflammatory markers important in asthma pathophysiology.
Methods
In this exploratory study in patients with poorly controlled asthma participating in a randomized controlled trial of a DASH intervention study, changes in concentrations of a broad panel of serum proteins (51-plex Luminex assay, Affymetrix) were determined, and their relation to diet quality (DASH score) assessed by combining data of both intervention and usual-care control groups. Second, the relation between the serum proteins, other biomarkers of inflammation and nutrition, and Asthma Control Questionnaire (ACQ) was assessed.
Results
During the first 3 mo, diet quality (DASH scores) were inversely associated (P < 0.05, false discovery rate P < 0.09) with serum concentrations of a large number serum proteins, reflecting not only general proinflammatory markers such as IL-1β, transforming growth factor α (TGF-α), and IL-6 (r = −0.31 to −0.39) but also a number of proteins associated with asthmatic conditions, specifically several T-helper (Th) 2 (Th2; r = −0.29 to −0.34) and Th17 (r = −0.4) associated cytokines and growth factors. Monokine induced by gamma/chemokine (C-X-C motif) ligand 9 (CXCL9) (MIG/CXCL9), a T-cell attractant induced by IFN-γ previously linked to asthma exacerbations, appeared to be the marker most consistently associated with DASH diet quality for the entire 6-mo study period (r = −0.40 and −0.30 for 0–3 and 3–6 mo, respectively, and standardized coefficient loadings −0.13 in the partial least squares analyses). Decreases in 19 serum protein concentrations were also correlated with improved asthma control during the 6-mo study period.
Conclusions
Our data in adult patients with poorly controlled asthma suggest that dietary changes, like the introduction of DASH, may have beneficial effects on reducing inflammatory status. This trial was registered at http://www.clinicaltrials.gov as NCT01725945.
Keywords: DASH diet, asthma, blood proteins, cytokines, inflammatory markers
Introduction
Asthma has become one of the major public health challenges, currently affecting ∼20 million US adults or 7.7% of the population (1). Uncontrolled asthma strongly impairs quality of life for patients. Adverse changes in diet composition and quality have been suggested to have contributed to the increased asthma prevalence as well as poor asthma control (2, 3). Thus, with the aim of providing dietary advice to help control asthma attacks, potential benefits of dietary changes have been investigated, with particular attention to components in healthy diets, like fruits, vegetables, fish, or nutrients (4, 5). The evidence, however, is inconsistent, and mainly consists of epidemiological association studies, without mechanistic information strengthening causal plausibility. With regard to the impact of whole-diet approaches, meta-analyses do not show any association with asthma prevalence in adults, albeit individual studies report associations between dietary patterns and risk of uncontrolled asthma, measures of lung function, and/or frequency of asthma attacks (6).
The Dietary Approaches to Stop Hypertension (DASH) diet (7) was recommended in the 2015 US Dietary Guidelines as an overall dietary pattern to reduce blood pressure (8). In a recent pilot randomized intervention trial (the DASH for Asthma Trial), a behavioral intervention promoting the DASH diet in adult patients with uncontrolled asthma showed promising clinical benefits for improved asthma control and functional status while maintaining patients’ weight as adjunct therapy to usual care (9). However, less is known about the immunological mechanisms. C-reactive protein (CRP) has been assessed as a marker of inflammation, and a recent meta-analysis of 6 non-asthma randomized trials with the DASH diet concluded that circulating CRP concentrations were lowered when compared with unhealthy or habitual diets (10). Few studies, however, have reported on other inflammatory markers including IL-6, TNF-α, and IL-2, and found decreased IL-6 or no effects associated with the introduction of the DASH diet (11). While these observations were performed in patient groups with metabolic syndrome, obesity, or type 2 diabetes, to our knowledge, no studies have investigated the effects on inflammatory markers in asthmatic patients or used a multiplex approach for investigation of proteins.
The main objective of the present secondary analysis of biomarkers from the original pilot trial was to determine whether changes in diet quality, assessed as DASH score, were associated with inflammatory markers important in asthma pathophysiology by combining data of both intervention and usual-care control participants. We investigated whether this association was detectable at 2 follow-up time points: 3 and 6 mo. We assessed a broad panel of cytokines, chemokines, and growth factors in the peripheral blood of patients with poorly controlled asthma. In addition, the relation between the changes in nutritional biomarkers (as possible objective indications of altered diet) and the serum markers of inflammation during the first 3 mo was investigated. As a secondary objective, the relation between changes in inflammatory markers and asthma control was assessed.
Methods
Study participants and intervention
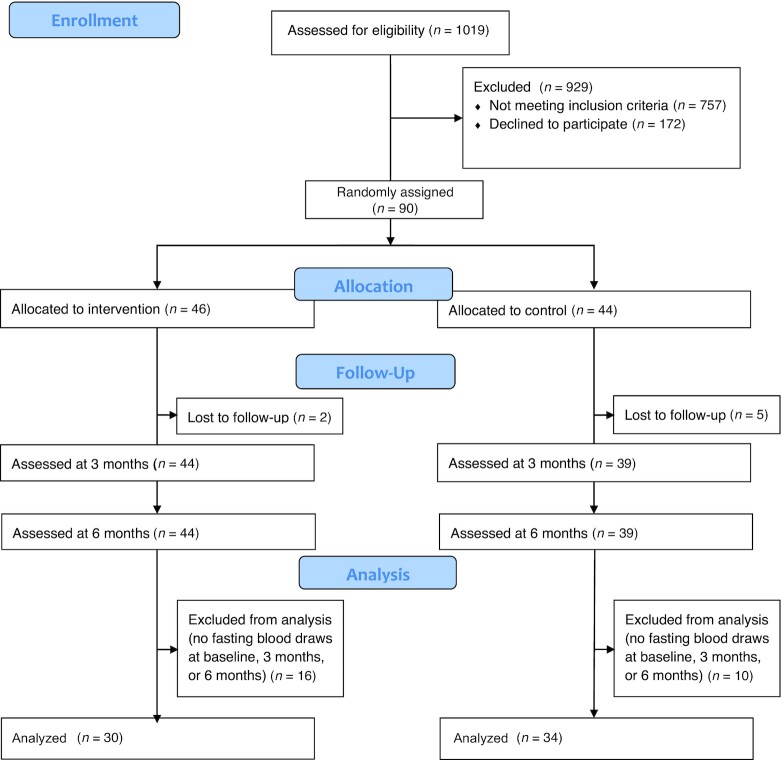
The DASH for Asthma Trial was conducted among 90 adults with uncontrolled asthma confirmed through a multistage screening process [i.e., electronic asthma registry queries, completion of the Asthma Control Test (12) pre- and post-bronchodilator spirometry, and if necessary, medical chart review by an asthma specialist]. Participants were randomly assigned to usual care (n = 44) or usual care with a behavioral intervention promoting the DASH diet (n = 46) for 6 mo (Figure 1). The trial protocol as well as the main findings of the intervention study were previously published (9, 13). In short, participants recruited in 2013 from primary care patients at Kaiser in San Francisco and Hayward medical centers were aged 20–70 y old, had a BMI (kg/m2) between 18.9 and 39.9, and confirmed uncontrolled persistent asthma through a multistage screening process. All participants initially had a diet of low quality (score of ≤6 out of 9 total on the DASH concordance index summing 9 nutrient targets, including total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium) (14). The 6-mo intervention curriculum included an intensive phase (3 individual and 8 group sessions) over the first 3 mo and a maintenance phase (monthly or more frequent phone consultations depending on participant needs, preferences, and availability) for the last 3 mo. The primary intervention goal over the entire 6 mo was to achieve and maintain daily recommendations for 1) fruit and vegetable servings (usually 7–8 for women or 8–10 for men), 2) low-fat or fat-free dairy food servings (usually 2–3), 3) total fat grams (27% of estimated caloric needs), and 4) sodium intake (<2300 mg/d). All but the sodium goal were calorie based for weight maintenance. Exercise was not a component of the intervention. For details, see the Supplemental Methods and Ma et al. (13). This study reports results on the association between changes in diet quality and changes in inflammatory markers important in asthma pathophysiology by combining data of both intervention and usual-care control groups.
FIGURE 1.
Patient flow from enrollment and follow-up to data analysis.
Ethics
The procedures followed were in accordance with the ethical standards of the responsible institutional or regional committee on human experimentation or in accordance with the Helsinki Declaration of 1975 as revised in 1983. The study was approved by an Institutional Review Board of the Kaiser Foundation Research Institute in Northern California and the study Data and Safety Monitoring Board and registered at ClinicalTrials.gov (NCT01725945). All participants provided written informed consent.
Assessment of asthma control, DASH score, and blood sample markers
All measures reported in this analysis were assessed by blinded outcome assessors at 0 (baseline), 3, and 6 mo. The primary clinical outcome was asthma control, assessed by the Asthma Control Questionnaire (ACQ) score (15), whereas overall diet quality was assessed as the DASH score (14) based on multiple-pass 24-h dietary recalls. Data from multiple-pass 24-h diet recalls (16) using the window-based Nutrition Data System for Research (version 2012; University of Minnesota) were used to compute the DASH concordance index based on the 9 nutrient targets (0- to 9-point DASH score) as a measure of overall diet quality (14). The majority of the participants completed fasting blood draws at the onsite clinical laboratory at all or some of the clinical visits at 0 (baseline), 3, and 6 mo (n = 64, 58, and 60, respectively). In all available samples, a panel of 51 cytokines, chemokines, and growth factors (Table 1) in serum were measured by Luminex technology. Plasma concentrations of CRP (by ELISA), folate, and vitamin B-12 (by RIA), carotene (by HPLC), and vitamin D (by LC-MS/MS) were measured. For further methodological details, see the Supplemental Methods.
TABLE 1.
The 51 serum proteins measured by Luminex technology, alphabetically listed
| Marker/abbreviation | Name/synonym |
|---|---|
| CD40L | Soluble CD40 ligand, CD154 |
| Eotaxin | Eotaxin 1, CCL11 |
| ENA-78 | Epithelial-derived neutrophil-activating peptide 78, CXCL5 |
| FASL | Soluble Fas ligand |
| FGF-2 | Fibroblast growth factor 2 |
| G-CSF | Granulocyte-colony stimulating factor, CSF-3 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor, CSF-2 |
| GRO-α | Growth-regulated protein alpha, CXCL1 |
| HGF | Hepatocyte growth factor |
| ICAM-1 | Soluble intercellular adhesion molecule-1 |
| IFN-α | Inteferon alpha, Type 1 interferon |
| IFN-β | Interferon beta |
| IFN-γ | Interferon gamma |
| IL-1α | Interleukin 1α |
| IL-1β | Interleukin 1β |
| IL-1RA | Interleukin 1 receptor antagonist |
| IL-2 | Interleukin 2 |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-7 | Interleukin 7, pre–B-cell factor |
| IL-8 | Interleukin 8, CXCL8 |
| IL-10 | Interleukin 10 |
| IL-12 p40 | Interleukin 12 p40 monomer, subunit of IL-12 and IL-23 |
| IL-12 p70 | Interleukin 12 (bioactive), dimer consisting of p40 and p35 monomers |
| IL-13 | Interleukin 13 |
| IL-15 | Interleukin 15 |
| IL-17A | Interleukin 17A, CTLA-8 |
| IL-17F | Interleukin 17F |
| IP-10 | Interferon γ–induced protein 10, CXCL10, functionally related to CXCL9 |
| Leptin | Leptin |
| LIF | Leukemia inhibitory factor |
| M-CSF | Macrophage colony-stimulating factor, CSF-1, MIG-IM |
| MCP-1 | Monocyte chemoattractant protein 1, CCL2 |
| MCP-3 | Monocyte chemoattractant protein 3, CCL7 |
| MIG/CXCL9 | Monokine induced by γ interferon (MIG), CXCL9 |
| MIP-1α | Macrophage inflammatory protein 1α, CCL3 |
| MIP-1β | Macrophage inflammatory protein 1β, CCL4 |
| NGF | Nerve growth factor |
| PAI-1 | Plasminogen activator inhibitor 1, serpin |
| PDGF-BB | Platelet-derived growth factor-BB (dimer of 2 B subunits) |
| RANTES | Regulated on activation, normal T-cell expressed and secreted, CCL5 |
| Resistin | Adipose tissue–specific secretory factor (ADSF), XCP1 |
| SCF | Stem cell factor, c-kit ligand |
| TGF-α | Transforming growth factor α |
| TGF-β | Transforming growth factor β |
| TNF-α | Tumor necrosis factor α |
| TNF-β | Tumor necrosis factor β |
| TRAIL | TNF-related apoptosis-inducing ligand, CD253 |
| VCAM-1 | Soluble vascular cell adhesion molecule 1, CD106 |
| VEGF-A | Vascular endothelial growth factor A |
Statistical analyses
Data from all participants in both the intervention and control groups with blood samples available at baseline and at either 3- or 6-mo follow-up were included. Pearson correlation coefficients were generated to measure the linear relations between individual markers and outcomes. Although multiple comparison adjustments were not planned as the original trial was a pilot study designed to use the CI approach for data interpretation (17), we provided both original P values and false discovery rate (FDR)–controlled P values as references (an FDR-corrected P < 0.1 threshold is also reported given the exploratory nature) (18). By combining data from both intervention and control groups, participants’ weight remained stable from baseline to 3 and 6 mo [mean (95% CI) weight change from baseline: −0.44 (−0.88, +0.00) kg, P = 0.051, at 3 mo and −0.70 (−1.66, 0.26) kg, P = 0.15, at 6 mo]. Therefore, we did not correct for weight in the Pearson correlation analyses. As the number of independent variables was significantly larger than the number of data points, partial least squares (PLS) was used to further identify linear combinations of predictors (factors) to predict outcomes. To investigate the association of baseline asthma endotypes with changes in serum proteins and DASH score, we divided the participants into 2 groups with better or worse asthma control by setting a cutoff at below or above an ACQ of 2.1 (sample mean at baseline) and compared the changes in serum proteins and DASH scores from 0 to 3 mo, 0 to 6 mo, and 3 to 6 mo between the 2 groups of baseline asthma control. All analyses were conducted using SAS, version 9.2 (SAS Institute, Inc.).
Results
Participant characteristics
The participants were middle-aged, 67% female, and racially/ethnically and socioeconomically diverse (Table 2). They had poor asthma control at baseline (mean ± SD ACQ score: 2.1 ± 0.8), and most participants were atopic as determined by allergen-specific IgE >0.35 kU/L for any of 6 allergens tested, and reported asthma onset after age of 12 y. The DASH scores at baseline were low (mean DASH score: 2.2; range: 0–9) (Table 2), indicating poor diet quality.
TABLE 2.
Characteristics among study participants, collected at baseline1
| Characteristic | Overall (n = 64) |
|---|---|
| Age, mean ± SD, y | 52.3 ± 12.5 |
| Female | 67.2 |
| Race/ethnicity | |
| Non-Hispanic White | 48.4 |
| Non-Hispanic Black | 12.5 |
| Asian/Pacific Islander | 26.6 |
| Hispanic | 12.5 |
| Current/ex-smoker | 23.4 |
| BMI, mean ± SD, kg/m2 | 27.7 ± 4.7 |
| Education | |
| High school/GED | 6.3 |
| Some college | 35.9 |
| College or above | 57.8 |
| Employment status | |
| Working full time | 57.8 |
| Working part time | 10.9 |
| Unemployed/retired/disabled | 31.3 |
| Annual family income (n = 62)2 | |
| <$35,000 | 9.7 |
| $35,000 to <$55,000 | 14.5 |
| $55,000 to <$75,000 | 21.0 |
| $75,000 to <$100,000 | 19.3 |
| $100,000 to <$125,000 | 14.5 |
| ≥$125,000 | 21.0 |
| Asthma-onset age | |
| <12 y old | 28.1 |
| ≥12 y old | 71.9 |
| Atopy3 | 76.6 |
| DASH score, mean ± SD4 | 2.2 ± 1.3 |
| ACQ score, mean ± SD | 2.1 ± 0.8 |
Values are % unless otherwise indicated. ACQ, Asthma Control Questionnaire (range: 0–6, with higher scores indicating worse asthma control); DASH, Dietary Approaches to Stop Hypertension (range: 0–9, with higher scores indicating better DASH concordance); GED, General Educational Development.
The denominator was less than the total number (64) of the participants due to missing data.
Atopy was defined as any tested aeroallergen having a value ⩾0.35 kU/L based on ImmunoCAP-specific IgE testing. The specific allergens tested were dust mite (Dermatophagoides pteronyssinus), animal dander (cat and dog), grasses (ragweed and timothy grass), and tree (juniper cedar).
DASH scores were calculated based on combining 9 nutrient targets (i.e., total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium). The intermediate target of each nutrient was halfway between the DASH target and population mean (based on the NHANES 2007–2008, the latest data available at the inception of this study). For a nutrient, participants reaching the DASH target were assigned 1 point, those reaching the intermediate target were assigned a half-point, and those not meeting the intermediate target were given 0 points. The DASH score was the sum of points for all 9 nutrients.
Serum proteins in relation to the DASH score
At baseline, no significant correlations between serum concentrations of any of the proteins and the DASH score were observed (Supplemental Table 1).
However, from 0 to 3 mo, changes in protein concentrations of 18 of the 47 serum proteins were significantly correlated with changes in DASH scores (Table 3), all inversely correlated (FDR-corrected P < 0.1 for 15 of these). The regression plots in Figure 2 show the inverse associations between decrease in selected cytokines and improvements in the DASH scores.
TABLE 3.
Serum protein concentration changes over the periods of 0–3 mo, 0–6 mo, and 3–6 mo1
| 0 to 3 mo (n = 47) | 0 to 6 mo (n = 47) | 3 to 6 mo (n = 49) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum protein | Baseline (n = 64), pg/mL | Concentration change, pg/mL | r | FDR P value | Concentration change, pg/mL | r | FDR P value | Concentration change, pg/mL | r | FDR P value |
| Th2-related | ||||||||||
| IL-4 | 15.2 ± 5.2 | −2.1 ± 4.7 | −0.29 | 0.132 | −1.1 ± 5.1 | −0.10 | 0.91 | 0.7 ± 4.6 | 0.35 | 0.092 |
| IL-5 | 163.1 ± 70.4 | −39.5 ± 62.7 | −0.31 | 0.092 | −15.8 ± 60.9 | −0.14 | 0.85 | 17.7 ± 56.0 | 0.27 | 0.19 |
| IL-13 | 14.4 ± 5.7 | 6.7 ± 7.6 | −0.14 | 0.51 | 9.3 ± 8.6 | 0.06 | 0.99 | 2.2 ± 10.2 | 0.22 | 0.24 |
| Eotaxin | 29.3 ± 9.8 | −13.4 ± 8.4 | −0.34 | 0.092 | −10.0 ± 8.8 | −0.26 | 0.85 | 2.7 ± 6.1 | 0.27 | 0.19 |
| Th1-related | ||||||||||
| IL-12P40 | 15.9 ± 28.7 | −4.0 ± 11.2 | −0.01 | 0.97 | 2.4 ± 12.9 | 0.00 | 1.00 | 5.4 ± 10.8 | 0.40 | 0.092 |
| IL-12P70 | 113.6 ± 53.4 | −24.1 ± 46.7 | −0.31 | 0.092 | −4.8 ± 49.0 | −0.15 | 0.85 | 16.4 ± 46.3 | 0.27 | 0.19 |
| IFN-γ | 2.1 ± 0.9 | −0.3 ± 0.8 | −0.39 | 0.092 | 0.1 ± 0.8 | −0.11 | 0.91 | 0.4 ± 0.9 | 0.16 | 0.38 |
| IP-10 | 55.5 ± 17.2 | −5.5 ± 14.3 | −0.24 | 0.20 | −3.6 ± 15.8 | −0.08 | 0.94 | 1.1 ± 14.2 | 0.43 | 0.092 |
| MIG/CXCL9 | 26.6 ± 34 | −21.2 ± 30.7 | −0.40 | 0.092 | −12.1 ± 25.5 | −0.30 | 0.632 | 5.0 ± 11.3 | 0.16 | 0.36 |
| Th17-related | ||||||||||
| IL-17F | 4.6 ± 21.3 | −0.5 ± 0.9 | −0.13 | 0.55 | −0.3 ± 0.7 | −0.06 | 0.99 | 0.0 ± 0.5 | 0.22 | 0.23 |
| IL-17 | 20.6 ± 20.2 | −1.7 ± 12.3 | −0.40 | 0.092 | 2.2 ± 10.9 | 0.05 | 0.99 | 5.6 ± 15.0 | −0.12 | 0.49 |
| Regulatory | ||||||||||
| IL-10 | 26.3 ± 10.4 | −0.2 ± 9.2 | −0.33 | 0.092 | 5.1 ± 11.0 | 0.04 | 0.99 | 5.2 ± 11.3 | 0.21 | 0.24 |
| Inflammatory | ||||||||||
| IL-1α | 22.5 ± 9.2 | 8.5 ± 11.4 | −0.08 | 0.71 | 8.6 ± 11.3 | 0.13 | 0.85 | −1.1 ± 13.0 | 0.32 | 0.162 |
| IL-1β | 5.3 ± 2.1 | −1.6 ± 2.0 | −0.38 | 0.092 | −0.6 ± 2.3 | −0.17 | 0.85 | 1.0 ± 1.9 | 0.25 | 0.20 |
| IL-2 | 25.3 ± 10.3 | 3.2 ± 11.4 | −0.26 | 0.16 | 7.7 ± 11.8 | 0.01 | 1.00 | 4.3 ± 12.8 | 0.26 | 0.19 |
| IL-6 | 39.4 ± 29.2 | −0.1 ± 28.2 | −0.39 | 0.092 | 10.0 ± 28.9 | −0.03 | 0.99 | 10.3 ± 35.2 | 0.26 | 0.19 |
| IL-8 | 3 ± 2.1 | −0.7 ± 2.6 | 0.04 | 0.89 | −1.0 ± 1.5 | 0.05 | 0.99 | −0.4 ± 2.6 | 0.08 | 0.65 |
| IL-15 | 24.5 ± 10.2 | 3.4 ± 9.4 | −0.24 | 0.20 | 6.2 ± 8.9 | 0.00 | 1.00 | 2.0 ± 10.1 | 0.27 | 0.19 |
| IFN-β | 121.9 ± 909 | −2.4 ± 2.7 | −0.24 | 0.20 | −0.6 ± 3.1 | −0.09 | 0.91 | 1.1 ± 2.6 | 0.18 | 0.32 |
| TGF-α | 16.2 ± 16.1 | −1.9 ± 5.8 | −0.31 | 0.092 | −0.6 ± 6.1 | −0.06 | 0.99 | 1.0 ± 6.2 | 0.24 | 0.22 |
| TGF-β | 17.9 ± 8.4 | −10.3 ± 7.4 | −0.24 | 0.20 | −8.1 ± 5.8 | −0.17 | 0.85 | 1.4 ± 3.7 | 0.22 | 0.23 |
| TNF-α | 4.5 ± 2 | 0.3 ± 2.5 | −0.14 | 0.51 | 1.3 ± 2.5 | 0.15 | 0.85 | 1.1 ± 3.5 | 0.25 | 0.20 |
| MIP-1α | 13.4 ± 25.8 | −3.0 ± 6.3 | −0.24 | 0.20 | −1.3 ± 5.1 | −0.18 | 0.85 | 0.7 ± 5.7 | 0.20 | 0.28 |
| MIP-1β | 74.2 ± 51.6 | −19.7 ± 41.1 | −0.27 | 0.16 | −13.3 ± 38.5 | −0.23 | 0.85 | 1.2 ± 36.3 | 0.35 | 0.092 |
| MCP-1 | 13 ± 4.3 | −2.0 ± 3.7 | −0.18 | 0.37 | −0.2 ± 4.1 | −0.10 | 0.91 | 1.6 ± 4.1 | 0.32 | 0.192 |
| MCP-3 | 18.9 ± 7 | −4.4 ± 6.8 | −0.12 | 0.55 | −2.0 ± 6.8 | 0.09 | 0.91 | 1.8 ± 6.3 | 0.24 | 0.21 |
| RANTES | 590.8 ± 149.2 | 62.2 ± 159 | 0.13 | 0.54 | 40.4 ± 209 | 0.22 | 0.85 | −32.3 ± 203 | 0.17 | 0.36 |
| Resistin | 601.6 ± 335.2 | −121 ± 204 | 0.11 | 0.56 | −133 ± 256 | −0.18 | 0.85 | −85.2 ± 252 | 0.08 | 0.65 |
| CD40L | 17,020.7 ± 33,497.6 | −17E3 ± 35E3 | −0.04 | 0.86 | −11E3 ± 28E3 | 0.00 | 1.00 | 570.6 ± 1643 | −0.02 | 0.90 |
| Adhesion | ||||||||||
| ICAM-1 | 8335.8 ± 7210.8 | −3630 ± 4502 | −0.15 | 0.50 | −3037 ± 4211 | −0.19 | 0.85 | −34.0 ± 3388 | 0.22 | 0.23 |
| VCAM-1 | 3967.5 ± 2207.3 | −883 ± 1845 | 0.19 | 0.37 | −591 ± 1520 | −0.04 | 0.99 | 97.1 ± 704 | −0.13 | 0.47 |
| Inflammation-associated growth factors | ||||||||||
| IL-7 | 272.3 ± 129.6 | −94.2 ± 107 | −0.30 | 0.122 | −59.2 ± 108 | −0.15 | 0.85 | 30.8 ± 82.6 | 0.29 | 0.192 |
| VEGF | 116.1 ± 68 | −60.3 ± 62.8 | −0.34 | 0.092 | −33.2 ± 48.7 | −0.24 | 0.85 | 23.6 ± 36.9 | 0.08 | 0.64 |
| G-CSF | 11.7 ± 5.8 | −2.8 ± 8.2 | −0.31 | 0.092 | 3.9 ± 8.0 | −0.03 | 0.99 | 6.7 ± 9.7 | 0.06 | 0.72 |
| GM-CSF | 35 ± 13.1 | 6.3 ± 13.4 | −0.27 | 0.16 | 12.0 ± 14.4 | 0.01 | 1.00 | 4.9 ± 15.4 | 0.27 | 0.19 |
| NGF | 72.7 ± 23.5 | −21.6 ± 25.5 | −0.32 | 0.092 | −8.2 ± 22.8 | −0.10 | 0.91 | 11.5 ± 20.7 | 0.17 | 0.36 |
| Other growth factors | ||||||||||
| LIF | 15.2 ± 7.2 | −4.4 ± 6.8 | −0.32 | 0.092 | −1.4 ± 7.2 | −0.14 | 0.85 | 2.9 ± 6.2 | 0.30 | 0.192 |
| SCF | 27.9 ± 10.5 | −0.8 ± 9.1 | −0.29 | 0.132 | 2.3 ± 11.0 | −0.01 | 1.00 | 3.2 ± 11.1 | 0.27 | 0.19 |
| FGFβ | 54.1 ± 22.8 | −4.6 ± 16.7 | −0.35 | 0.092 | −3.9 ± 16.3 | 0.04 | 0.99 | 1.8 ± 17.1 | 0.14 | 0.43 |
| HGF | 24.8 ± 7.5 | −3.3 ± 5.7 | −0.02 | 0.94 | −3.5 ± 6.8 | 0.02 | 1.00 | −0.9 ± 7.1 | 0.25 | 0.20 |
| Other | ||||||||||
| Leptin | 2208.6 ± 3381.7 | −615 ± 1471 | −0.07 | 0.76 | −626 ± 1515 | −0.05 | 0.99 | −36.7 ± 398 | 0.13 | 0.47 |
| PAI-1 | 349.8 ± 43.2 | 40.0 ± 53.1 | 0.08 | 0.71 | 7.1 ± 44.2 | 0.41 | 0.192 | −30.8 ± 53.4 | −0.10 | 0.60 |
| FASL | 7.9 ± 7.9 | 3.2 ± 6.2 | 0.12 | 0.56 | 4.7 ± 8.3 | 0.30 | 0.632 | 1.4 ± 6.3 | −0.09 | 0.61 |
| ENA-78 | 59.4 ± 68.5 | −6.2 ± 22.9 | −0.01 | 0.97 | −4.7 ± 28.6 | 0.20 | 0.85 | 0.8 ± 24.4 | 0.22 | 0.23 |
| PDGFBB | 1187.8 ± 726.2 | −565 ± 507 | −0.17 | 0.41 | −534 ± 495 | −0.13 | 0.85 | 5.2 ± 244 | 0.07 | 0.66 |
| TRAIL | 40.3 ± 32 | −0.9 ± 22.9 | 0.05 | 0.85 | 0.1 ± 15.7 | 0.17 | 0.85 | −0.4 ± 13.7 | 0.25 | 0.20 |
| IL-1RA | 105.1 ± 27.4 | 7.7 ± 24.4 | 0.02 | 0.94 | 5.2 ± 29.7 | 0.12 | 0.90 | −5.7 ± 29.0 | 0.39 | 0.092 |
For each of the serum proteins, means ± SDs of serum concentrations at baseline and the changes in serum concentrations are shown, as well as Pearson correlation coefficients (r) and the FDR-corrected P values for correlations between changes in the serum protein concentrations and changes in the DASH score. DASH, Dietary Approaches to Stop Hypertension; ENA-78, epithelial-derived neutrophil-activating peptide 78; FASL, soluble Fas ligand; FDR, false discovery rate; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulatory factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; ICAM-1, soluble intercellular adhesion molecule 1; IP-10, interferon γ–induced protein 10; LIF, leukemia inhibitory factor; MCP, macrophage colony-stimulating factor; MIG/CXCL9, monokine induced by γ interferon; MIP, macrophage inflammatory protein; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor 1; PDGFBB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; SCF, stem cell factor; TGF, transforming growth factor; Th, T-helper; VCAM-1, soluble vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Indicates correlation coefficients with original P values of <0.05.
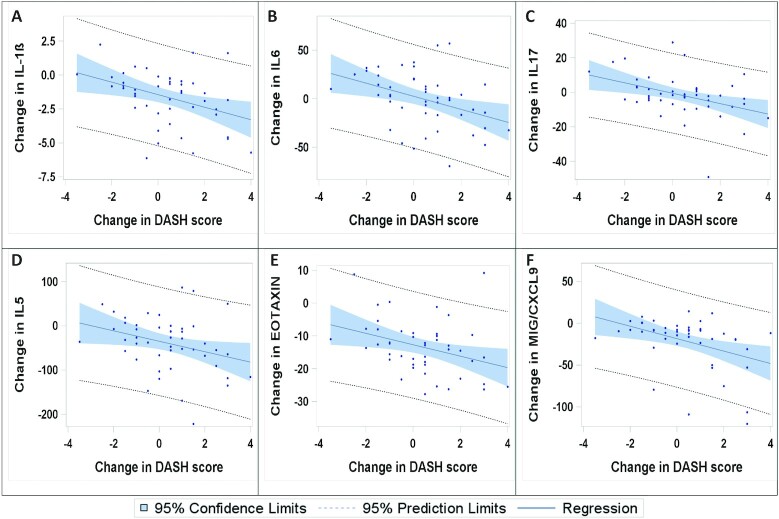
FIGURE 2.
Regression plots for changes in DASH score and changes in selected serum protein concentrations from baseline (0 mo) to 3 mo (n = 47): IL- 1β (A), IL-6 (B), IL-17 (C), IL-5 (D), eotaxin (E), and MIG/CXCL9 (F). These proteins were selected as representative examples of the cytokines with strong correlation with changes in DASH scores, and MIG and IL-17 were selected due to their strong effects in the multifactorial analyses. DASH, Dietary Approaches to Stop Hypertension; MIG/CXCL9, monokine induced by γ interferon.
The proteins that decreased with an improved DASH score were cytokines reflecting T-helper (Th) (Th2) (IL-4, IL-5, and eotaxin), Th17 (IL-17), Th1 [IL12p70, IFN-γ, monokine induced by γ interferon (MIG/CXCL9)], regulatory/Th2 (IL-10), and classical proinflammatory [IL-1β, transforming growth factor α (TGF-α), IL-6] responses, in addition to inflammation-associated growth factors [IL-7; vascular endothelial growth factor (VEGF); granulocyte colony stimulatory factor (G-CSF); nerve growth factor (NGF)] and other more general growth factors [leukemia inhibitory factor (LIF), fibroblast growth factor (FGF) β, and stem cell factor (SCF)]. In terms of Pearson correlation coefficient values, the strongest associations were observed for MIG/CXCL9, IL-1β, IL-6, IL-10, IL-17, IFN-γ, eotaxin, FGFβ, and VEGF.
However, only one of these factors, MIG/CXCL9, was still significantly correlated when assessing the changes in serum protein concentrations and DASH scores from 0 to 6 mo. In addition, plasminogen activator inhibitor 1 (PAI-1) and soluble Fas ligand (FASL) showed a significant positive correlation with DASH score changes from 0 to 6 mo, but neither of these were significant for the 0–3- or 3–6-mo periods separately.
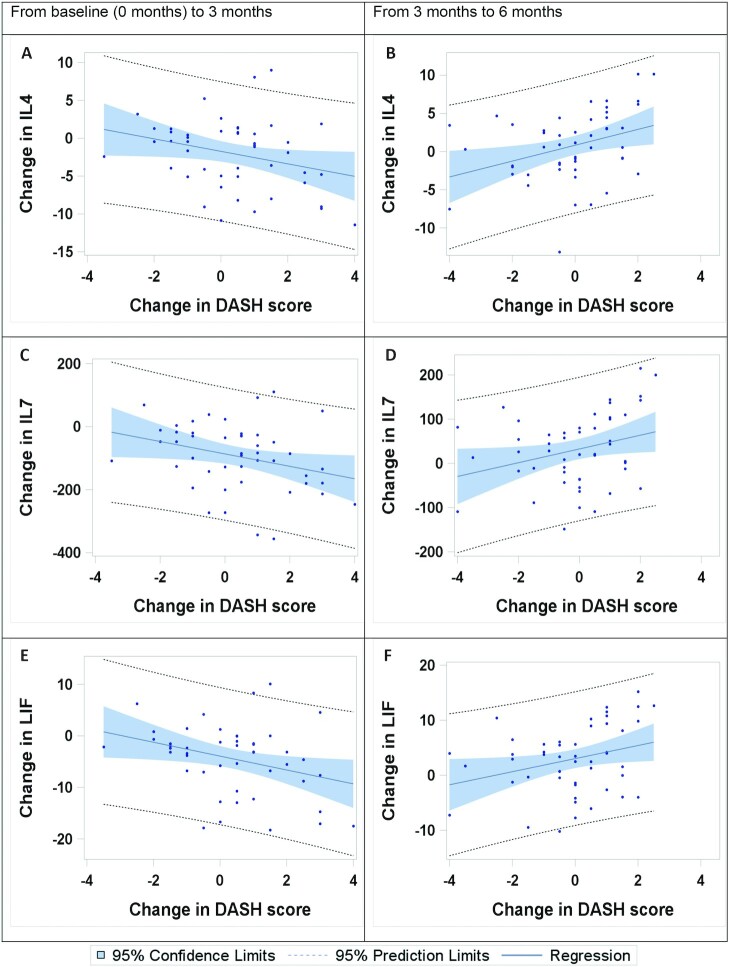
From 3 to 6 mo, significant correlations between changes in serum protein concentrations and changes in DASH score were observed for 9 proteins (5 of these had P < 0.1 after FDR correction), but these were all positively associated with changes in the DASH score (Table 3). Three of these proteins (IL-4, IL-7, and LIF; Figure 3) are the same proteins that were inversely correlated with DASH score between 0 and 3 mo. The other serum proteins that showed significant positive correlation with DASH score changes between 3 and 6 mo were interferon γ–induced protein 10 (IP-10), IL-12p40, Macrophage inflammatory protein 1β (MIP1β), Monocyte chemoattractant protein-1 (MCP-1), IL-1α, and IL-1RΑ (Table 3).
FIGURE 3.
Regression plots for changes in DASH score and changes in serum proteins from baseline (0 mo) to 3 mo (n = 47) and from 3 to 6 mo (n = 49). Plots are shown for the 3 markers demonstrating a significant (P < 0.05) correlation with the DASH scores for both time periods. Regression plots for changes in DASH score and IL-4 from baseline to 3 mo (A) and from 3 to 6 mo (B); for changes in DASH score and IL-7 from baseline to 3 mo (C) and from 3 to 6 mo (D); and for changes in DASH score and LIF from baseline to 3 mo (E) and from 3 to 6 mo (F). DASH, Dietary Approaches to Stop Hypertension; LIF, leukemia inhibitory factor.
Patients with asthma may have heterogeneous underlying endotypes that may affect the inflammatory profile of patients and raises the question of whether subgroups of patients respond differently (or do not respond) to the dietary intervention. To investigate this, we divided the participants into 2 groups with better or worse asthma control by setting a cutoff at below or above an ACQ of 2.1 (sample mean at baseline). Results are reported in Supplemental Table 2. Interestingly, we found a pattern that, from 3 to 6 mo, participants with worse asthma control at baseline (ACQ ≥2.1) seemed to have stronger improvement in the serum biomarkers (i.e., decrease in proteins) than those with better asthma control (ACQ <2.1). There was no influence on change in DASH score. We also examined the partial correlation between changes in serum proteins and changes in DASH score after controlling for baseline ACQ score and the results are reported in Supplemental Table 3.
Because the majority of, and the strongest, associations were seen with changes from baseline to 3 mo, all further investigations were focused on this initial time period.
Multifactorial analyses of the serum proteins in relation to the DASH score
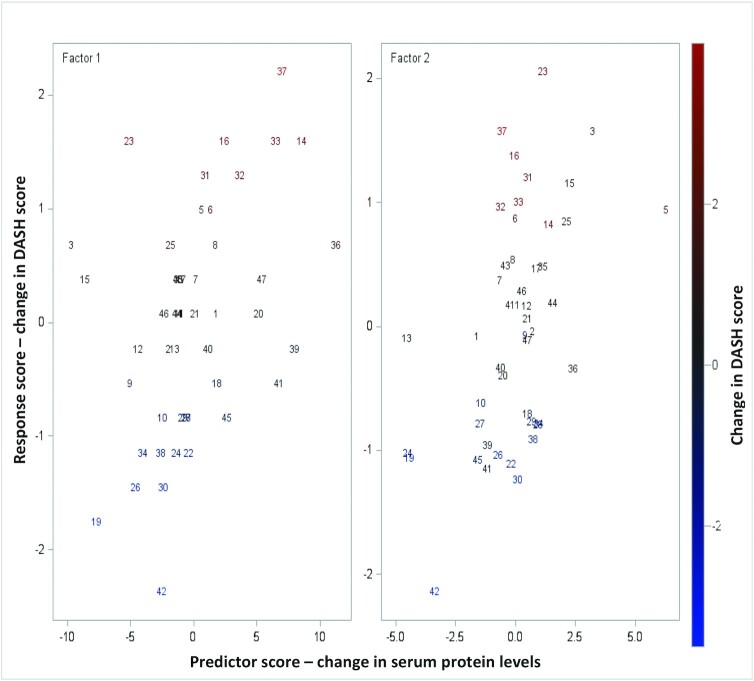
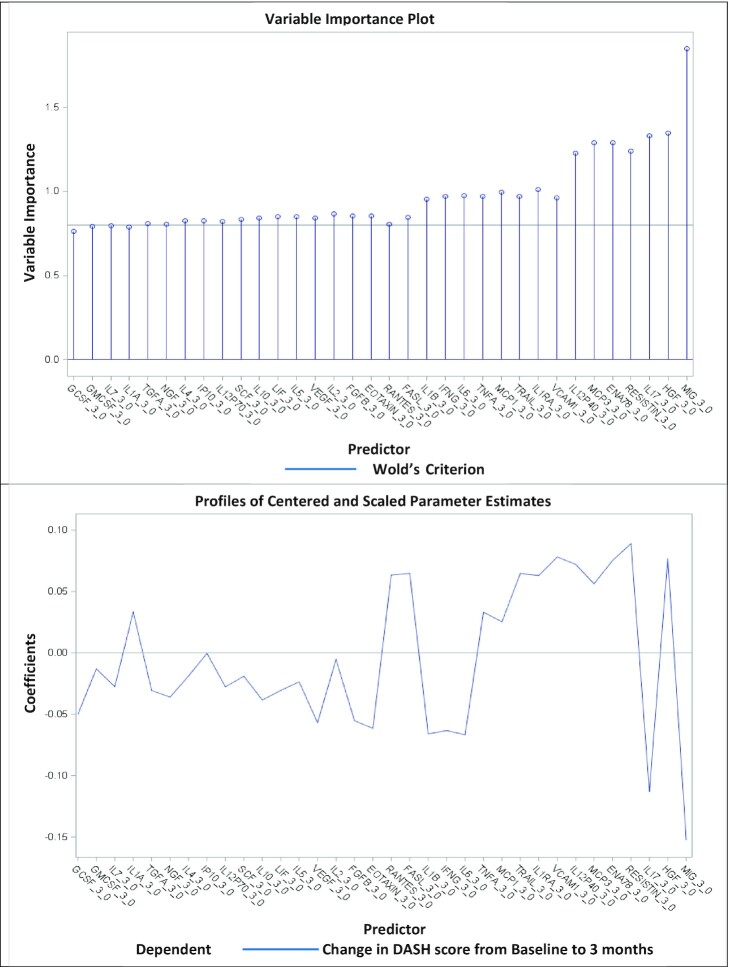
By use of multifactorial PLS analyses including all 47 serum proteins, 2 factors were extracted explaining 35.7% of the variation in the dependent variable (i.e., change in the DASH score from 0 to 3 mo), whereas 57.2% of the predictor variation (changes in serum proteins) was explained. Figure 4 indicates that the changes in serum proteins in both factor 1 and factor 2 were correlated with the changes in DASH scores from baseline to 3 mo. Based on the variable importance plot and the regression coefficient profile (Figure 5), which represents the contribution of each predictor in the prediction of the dependent variable (i.e., changes in the DASH score from 0 to 3 mo), the 2 factors were mainly driven by the changes in MIG(CXCL9) and IL-17 from baseline to 3 mo. Changes in both these serum proteins were negatively correlated with the concurrent change in DASH score (standardized coefficient loadings: −0.13 and −0.10, respectively).
FIGURE 4.
Changes in DASH scores predicted by changes in serum proteins from baseline to 3 mo in the PLS analyses. Two factors were extracted in the PLS analyses, explaining 35.7% of the variation in the dependent variable (i.e., change in the DASH score from 0 to 3 mo) and 57.2% of the variation in the predictors (changes in serum proteins) (n = 47). The graphs show correlation between the response scores (change in DASH score from 0 to 3 mo) and the predictor scores (changes in serum proteins from 0 to 3 mo) for the 2 extracted factors. Each number represents a participant. The color bar indicates changes in DASH score from negative (blue) to positive (red). DASH, Dietary Approaches to Stop Hypertension; PLS, partial least squares.
FIGURE 5.
Variance importance plot (top) and regression parameter profile (bottom). This graph shows the variance importance plot (top) and regression parameter profile (bottom) after removing predictors with the VIP statistic of Wold of <0.8. The VIP statistic of Wold in the variance importance plot illustrates the contribution of each predictor in fitting the PLS model for both predictors and response, the most important serum proteins contributing to the PLS model. The regression parameter profile shows absolute coefficients for the centered and scaled parameter estimates. DASH, Dietary Approaches to Stop Hypertension; VIP, Variable Importance for Projection.
Serum CRP concentrations in relation to DASH score and serum proteins
As expected, serum CRP concentrations were low (all <10 mg/L; all but 7 measurements <1 mg/L), and were not correlated with changes in the DASH score from 0 to 3 mo (Table 4). For the initial time period, changes in serum CRP were significantly correlated with changes in 6 of the serum proteins epithelial-derived neutrophil-activating peptide 78 (ENA-78), IFN-β, IL-12p40, IL-1RA, hepatocyte growth factor (HGF), and resistin (resistin also P < 0.05 after FDR correction; Table 4).
TABLE 4.
Pearson correlation coefficients (r) and FDR-corrected P values for changes in serum protein concentrations, DASH score, and asthma control parameter (ACQ) score, and changes in the dietary biomarkers and CRP from baseline to 3 mo1
| Folate | Carotene | Vitamin B-12 | Vitamin D | CRP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum protein | r | FDR P value | r | FDR P value | r | FDR P value | r | FDR P value | r | FDR P value |
| Th2-related | ||||||||||
| IL-4 | 0.24 | 0.67 | −0.27 | 0.36 | −0.20 | 0.74 | −0.04 | 0.96 | 0.07 | 0.89 |
| IL-5 | 0.29 | 0.672 | −0.22 | 0.40 | −0.06 | 0.90 | 0.09 | 0.96 | 0.00 | 0.99 |
| IL-13 | 0.05 | 0.84 | −0.26 | 0.37 | −0.17 | 0.74 | −0.03 | 0.96 | 0.22 | 0.70 |
| Eotaxin | 0.22 | 0.68 | −0.09 | 0.73 | −0.09 | 0.90 | 0.00 | 1.00 | −0.17 | 0.70 |
| Th1-related | ||||||||||
| IL-12P40 | 0.11 | 0.74 | −0.09 | 0.73 | −0.17 | 0.74 | −0.10 | 0.96 | 0.33 | 0.292 |
| IL-12P70 | 0.21 | 0.68 | −0.30 | 0.36 | −0.15 | 0.83 | 0.06 | 0.96 | 0.00 | 0.99 |
| IFN-γ | 0.19 | 0.68 | −0.18 | 0.44 | −0.06 | 0.90 | 0.10 | 0.96 | −0.08 | 0.88 |
| IP-10 | 0.26 | 0.67 | −0.31 | 0.362 | −0.05 | 0.90 | −0.12 | 0.96 | 0.19 | 0.70 |
| MIG/CXCL9 | 0.09 | 0.82 | 0.05 | 0.89 | −0.20 | 0.74 | 0.25 | 0.82 | −0.25 | 0.61 |
| Th17-related | ||||||||||
| IL-17F | −0.16 | 0.68 | 0.11 | 0.69 | −0.03 | 0.98 | 0.26 | 0.82 | 0.17 | 0.70 |
| IL-17 | 0.03 | 0.91 | −0.20 | 0.40 | 0.33 | 0.492 | 0.24 | 0.82 | 0.05 | 0.92 |
| Regulatory | ||||||||||
| IL-10 | 0.19 | 0.68 | −0.21 | 0.40 | −0.18 | 0.74 | −0.04 | 0.96 | 0.11 | 0.79 |
| Inflammatory | ||||||||||
| IL-1α | 0.07 | 0.84 | −0.25 | 0.38 | −0.18 | 0.74 | −0.03 | 0.96 | 0.27 | 0.49 |
| IL-1β | 0.24 | 0.67 | −0.17 | 0.46 | 0.00 | 1.00 | −0.04 | 0.96 | −0.03 | 0.92 |
| IL-2 | 0.16 | 0.68 | −0.23 | 0.40 | −0.13 | 0.87 | 0.01 | 0.96 | 0.15 | 0.70 |
| IL-6 | 0.16 | 0.68 | −0.28 | 0.36 | 0.01 | 0.99 | 0.14 | 0.96 | 0.09 | 0.87 |
| IL-8 | −0.18 | 0.68 | 0.02 | 0.94 | −0.06 | 0.90 | −0.01 | 0.96 | 0.04 | 0.92 |
| IL-15 | 0.15 | 0.68 | −0.29 | 0.36 | −0.12 | 0.87 | 0.10 | 0.96 | 0.14 | 0.71 |
| IFN-β | 0.02 | 0.94 | −0.21 | 0.40 | 0.08 | 0.90 | 0.22 | 0.82 | 0.31 | 0.292 |
| TGF-α | 0.12 | 0.71 | −0.28 | 0.36 | −0.08 | 0.90 | 0.04 | 0.96 | 0.15 | 0.70 |
| TGF-β | 0.06 | 0.84 | −0.13 | 0.61 | 0.08 | 0.90 | 0.11 | 0.96 | 0.03 | 0.92 |
| TNF-α | 0.11 | 0.71 | −0.22 | 0.40 | −0.31 | 0.492 | −0.05 | 0.96 | 0.11 | 0.79 |
| MIP-1α | 0.06 | 0.84 | −0.03 | 0.93 | 0.11 | 0.89 | 0.23 | 0.82 | 0.16 | 0.70 |
| MIP-1β | 0.13 | 0.68 | −0.17 | 0.46 | 0.05 | 0.92 | 0.13 | 0.96 | −0.16 | 0.70 |
| MCP-1 | 0.28 | 0.67 | −0.27 | 0.36 | −0.17 | 0.74 | −0.10 | 0.96 | 0.07 | 0.89 |
| MCP-3 | 0.13 | 0.68 | −0.29 | 0.36 | −0.21 | 0.74 | 0.01 | 0.96 | 0.18 | 0.70 |
| RANTES | 0.13 | 0.68 | −0.08 | 0.75 | 0.02 | 0.98 | −0.07 | 0.96 | 0.03 | 0.92 |
| Resistin | 0.01 | 0.94 | 0.11 | 0.68 | −0.36 | 0.492 | −0.26 | 0.82 | 0.50 | <0.052 |
| CD40L | 0.03 | 0.94 | 0.21 | 0.40 | −0.14 | 0.84 | −0.06 | 0.96 | 0.03 | 0.92 |
| Adhesion | ||||||||||
| ICAM-1 | 0.08 | 0.83 | 0.00 | 0.99 | 0.05 | 0.90 | 0.03 | 0.96 | 0.06 | 0.89 |
| VCAM-1 | 0.14 | 0.68 | 0.09 | 0.73 | −0.29 | 0.492 | −0.18 | 0.96 | 0.07 | 0.89 |
| Inflammation-associated growth factors | ||||||||||
| IL-7 | 0.26 | 0.67 | −0.27 | 0.36 | −0.14 | 0.84 | 0.03 | 0.96 | −0.10 | 0.79 |
| VEGF | 0.18 | 0.68 | −0.18 | 0.44 | 0.02 | 0.98 | 0.15 | 0.96 | −0.15 | 0.70 |
| G-CSF | 0.11 | 0.71 | −0.22 | 0.40 | 0.02 | 0.98 | 0.22 | 0.82 | 0.23 | 0.65 |
| GM-CSF | 0.14 | 0.68 | −0.22 | 0.40 | −0.25 | 0.63 | 0.01 | 0.96 | 0.10 | 0.79 |
| NGF | 0.14 | 0.68 | −0.27 | 0.36 | −0.02 | 0.98 | 0.07 | 0.96 | 0.14 | 0.71 |
| Other growth factors | ||||||||||
| LIF | 0.24 | 0.67 | −0.21 | 0.40 | −0.11 | 0.87 | −0.03 | 0.96 | 0.04 | 0.92 |
| SCF | 0.17 | 0.68 | −0.18 | 0.44 | −0.09 | 0.90 | −0.01 | 0.96 | 0.10 | 0.79 |
| FGFβ | 0.15 | 0.68 | −0.30 | 0.362 | 0.07 | 0.90 | 0.09 | 0.96 | 0.01 | 0.97 |
| HGF | 0.08 | 0.83 | −0.14 | 0.57 | −0.25 | 0.63 | −0.23 | 0.82 | 0.42 | 0.102 |
| Other | ||||||||||
| Leptin | −0.04 | 0.88 | 0.09 | 0.73 | −0.33 | 0.492 | 0.01 | 0.96 | −0.06 | 0.89 |
| PAI-1 | 0.25 | 0.67 | 0.02 | 0.94 | −0.06 | 0.90 | −0.08 | 0.96 | 0.15 | 0.70 |
| FASL | 0.07 | 0.84 | −0.18 | 0.44 | 0.03 | 0.98 | 0.10 | 0.96 | 0.06 | 0.89 |
| ENA-78 | 0.01 | 0.94 | 0.00 | 0.99 | 0.00 | 1.00 | 0.10 | 0.96 | 0.30 | 0.332 |
| PDGFBB | 0.16 | 0.68 | −0.11 | 0.68 | −0.12 | 0.87 | 0.09 | 0.96 | −0.11 | 0.79 |
| TRAIL | 0.08 | 0.83 | −0.03 | 0.94 | −0.11 | 0.87 | 0.10 | 0.96 | 0.17 | 0.70 |
| IL-1RA | −0.02 | 0.94 | −0.06 | 0.84 | −0.22 | 0.74 | −0.03 | 0.96 | 0.35 | 0.292 |
| DASH score | −0.21 | 0.68 | 0.03 | 0.94 | −0.18 | 0.74 | −0.13 | 0.96 | 0.20 | 0.70 |
| ACQ | 0.06 | 0.84 | −0.19 | 0.44 | −0.21 | 0.74 | −0.24 | 0.82 | 0.15 | 0.70 |
ACQ, Asthma Control Questionnaire; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; ENA-78, epithelial-derived neutrophil-activating peptide 78; FASL, soluble Fas ligand; FDR, false discovery rate; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulatory factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; ICAM-1, soluble intercellular adhesion molecule 1; IP-10, interferon γ–induced protein 10; LIF, leukemia inhibitory factor; MCP, macrophage colony-stimulating factor; MIG/CXCL9, monokine induced by γ interferon; MIP, macrophage inflammatory protein; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor 1; PDGFBB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; SCF, stem cell factor; TGF, transforming growth factor; Th, T-helper; VCAM-1, soluble vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Indicates correlation coefficients with original P values of <0.05.
Nutritional biomarkers in relation to serum proteins, DASH score, and ACQ
In correlation analyses (Table 4), few or no serum protein changes were significantly correlated with changes in the folate and vitamin D3 concentrations, respectively. Changes in the vitamin B-12 concentrations, however, were significantly correlated with changes in 5 proteins: leptin, TNF-α, resistin, and vascular cell adhesion molecule 1 (VCAM-1) negatively and IL-17 positively correlated. Changes in carotene were significantly inversely correlated with changes in IP-10 and FGFβ, and 12 of the serum proteins had a negative correlation coefficient with P ≤ 0.1. The majority of these proteins (7 of 12) were the same as those that were inversely associated with DASH score and ACQ.
Neither changes in the DASH score nor the asthma control–related parameter ACQ correlated with the nutritional biomarker changes from 0 to 3 mo (Table 4), suggesting that the nutritional biomarkers were not the best to reflect the dietary changes relevant for the effects on asthma control. Taken together, in the present group of patients, changes in the nutritional serum biomarkers, with the exception of carotene, showed limited associations with serum proteins and did not reflect dietary changes or asthma control.
Serum proteins in relation to asthma control (ACQ)
When investigating the relation between the changes in serum proteins and the asthma control score parameters from 0 to 3 mo, only platelet-derived growth factor-BB (PDGFBB) changes were significantly correlated (positive Pearson correlation coefficient 0.36, P = 0.014, FDR corrected P = 0.52; Supplemental Table 4) with the asthma control outcome ACQ.
However, for the whole study time period (i.e., from 0 to 6 mo), 19 of the serum proteins were positively correlated with the change in the asthma control outcome ACQ (i.e., decreased serum protein concentrations were correlated with improved asthma control) (Table 5). These were the serum proteins SCF, MPC3, FASL, IL-1α, IL-1RA, IL-2, IL-5, IL-6, IL-12p40, IL-12p70, IL-13, IL-15, IP-10, TGF-α, PDGFBB, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, FGFβ. After correcting for multiple comparisons, IL-13 remained significant, but also MCP3, IL-1α, IL-1RA, IL-2, IL-5, IL-6, IL-12p40, IL-12p70, IL-15, IP-10, TGF-α, PDGFBB, and GM-CSF had P values <0.1. Seven of these (SCF, IL-5, IL-6, IL-12p70, TGF-α, IFN-γ, FGFβ) were the ones significantly inversely correlated with dietary changes (DASH score) for the early period (0–3 mo). Of the serum proteins being associated with ACQ, IL-12p40 and IL-1RA were also associated with changes in CRP for the 0–3-mo period, and IP-10 and FGFβ were significantly inversely correlated with changes in carotene concentrations (see above).
TABLE 5.
Pearson correlations coefficients (r) and FDR-corrected P values for correlations between changes in serum protein concentrations and changes in the asthma control parameter (ACQ) from baseline to 6 mo1
| Serum protein | r | FDR P value |
|---|---|---|
| Th2-related | ||
| IL-4 | 0.27 | 0.12 |
| IL-5 | 0.31 | 0.102 |
| IL-13 | 0.48 | <0.052 |
| Eotaxin | 0.08 | 0.62 |
| Th1-related | ||
| IL-12P40 | 0.34 | 0.082 |
| IL-12P70 | 0.30 | 0.102 |
| IFN-γ | 0.32 | 0.102 |
| IP-10 | 0.34 | 0.082 |
| MIG/CXCL9 | 0.20 | 0.26 |
| Th17-related | ||
| IL-17F | 0.14 | 0.43 |
| IL-17 | 0.10 | 0.57 |
| Regulatory | ||
| IL-10 | 0.29 | 0.11 |
| Inflammatory | ||
| IL-1α | 0.40 | 0.072 |
| IL-1β | 0.19 | 0.30 |
| IL-2 | 0.37 | 0.072 |
| IL-6 | 0.36 | 0.072 |
| IL-8 | 0.10 | 0.58 |
| IL-15 | 0.39 | 0.072 |
| IFN-β | 0.07 | 0.67 |
| TGF-α | 0.36 | 0.072 |
| TGF-β | 0.12 | 0.49 |
| TNF-α | 0.32 | 0.102 |
| MIP-1α | 0.25 | 0.17 |
| MIP-1β | 0.21 | 0.26 |
| MCP-1 | 0.28 | 0.11 |
| MCP-3 | 0.34 | 0.082 |
| RANTES | 0.24 | 0.19 |
| Resistin | 0.18 | 0.30 |
| CD40L | −0.06 | 0.68 |
| Adhesion | ||
| ICAM-1 | −0.05 | 0.74 |
| VCAM-1 | 0.09 | 0.61 |
| Inflammation-associated growth factors | ||
| IL-7 | 0.29 | 0.11 |
| VEGF | 0.18 | 0.30 |
| G-CSF | 0.16 | 0.38 |
| GM-CSF | 0.40 | 0.072 |
| NGF | 0.22 | 0.22 |
| Other growth factors | ||
| LIF | 0.22 | 0.22 |
| SCF | 0.29 | 0.102 |
| FGFβ | 0.31 | 0.102 |
| HGF | 0.23 | 0.19 |
| Other | ||
| Leptin | 0.12 | 0.49 |
| PAI-1 | 0.25 | 0.17 |
| FASL | 0.29 | 0.112 |
| ENA-78 | 0.23 | 0.20 |
| PDGFBB | 0.34 | 0.082 |
| TRAIL | 0.17 | 0.34 |
| IL-1RA | 0.33 | 0.082 |
ACQ, Asthma Control Questionnaire; DASH, Dietary Approaches to Stop Hypertension; ENA-78, epithelial-derived neutrophil-activating peptide 78; FASL, soluble Fas ligand; FDR, false discovery rate; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulatory factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; ICAM-1, soluble intercellular adhesion molecule 1; IP-10, interferon γ–induced protein 10; LIF, leukemia inhibitory factor; MCP, macrophage colony-stimulating factor; MIG/CXCL9, monokine induced by γ interferon; MIP, macrophage inflammatory protein; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor 1; PDGFBB, platelet-derived growth factor-BB; RANTES, regulated on activation, normal T-cell expressed and secreted; SCF, stem cell factor; TGF, transforming growth factor; Th, T-helper; VCAM-1, soluble vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Indicates correlation coefficients with original P values of <0.05.
Discussion
This article presents a secondary analysis of biomarkers from the original pilot trial of a DASH-promoting behavioral intervention in adult patients with poorly controlled asthma. The data support and extend existing epidemiological data by demonstrating associations between reduced serum concentrations of a number of asthma-associated serum proteins and improved diet scores, as well as with improved asthma control. The results inform future interventional studies of health benefits and underlying mechanisms of the DASH diet for patients with asthma.
DASH scores in relation to serum proteins
Interestingly, the proteins showing negative associations with an improved DASH score at 3 mo included Th2-related cytokines IL-4, IL-5, and eotaxin; the inflammatory markers IL-17, IL12p70, IFN-γ, and MIG/CXCL9; as well as the classical proinflammatory cytokines IL-1β and IL-6, which all may play a role in asthma development and exaggeration (19–21). Others included the regulatory/Th2 cytokine IL-10 and inflammation-associated growth factors such as IL-7 (a lymphoid growth factor), VEGF (also involved in macrophage migration), TGF-α (a transforming growth factor for epithelial cells), and G-CSF. Also, NGF [increased during inflammation, produced by mast cells as well as by T-cell clones inducing T-cell maturation during infections (22)] and the more general growth factors LIF, FGFβ, and SCF, were all inversely associated with improved diet. MIG/CXCL9, a T-cell attractant induced by IFN-γ, was the only marker significantly associated with increased DASH score at 3 mo that persisted at 6 mo, as well as being 1 of 2 proteins most strongly driving the associations in the multifactorial PLS analyses. It is worth noticing that MIG/CXCL9 has previously been suggested to be a sensitive marker for the amount of bioactive IFN-γ present (23) as well as been suggested as a useful inflammatory marker of asthma exacerbation (24), as discussed below.
Our results extend several previous studies reporting effects of the DASH diet on single inflammatory markers, such as CRP (10, 11), in various non-asthma patient groups. Furthermore, adherence to a DASH-like diet in female adults (25), and components of the DASH diet, like low-fat dairy products, legumes, vegetables, B-carotene, and vitamin C, have also been reported to be associated with decreased concentrations of the inflammatory markers IL-6 and TNF-α (26–28). The novelty in our study is the demonstration of consistent findings for a high number of parameters, reflecting not only the general proinflammatory markers TNF-α and IL-6 but also a number of proteins related to the asthmatic state, like Th2- and Th17-associated cytokines. Together with other studies, our data in adult patients with poorly controlled asthma suggest that dietary changes, like the introduction of DASH, may have beneficial effects on reducing the inflammatory status in various diseases with chronic inflammation. Interestingly, our results also suggest that the improvement in proinflammatory markers during the later phase depended on asthma control degree, and/or indirectly the asthma endotype, at baseline. How different endotypes of asthma patients may benefit from dietary interventions should therefore be taken into consideration in future studies.
The PLS analyses identified MIG/CXCL9 and IL-17 as the main drivers of the factors describing the changes from 0 to 3 mo, which negatively correlated with the concurrent change in DASH score. The degree of variation explained by the PLS factors, however, suggests that, although we see reductions in inflammatory markers associated with improved DASH score, the variation in these serum proteins is also influenced by factors other than the DASH score. It could also imply that there are only some constituents of the dietary components in the DASH score that are responsible for the change in the serum inflammatory markers.
Different associations at 3 and 6 mo
Of the 18 proteins inversely associated with the DASH score between 0 and 3 mo, only 1 chemokine, MIG/CXCL9, remained significantly inversely correlated when looking at changes in DASH score and serum proteins over 0–6 mo (P = 0.042). We speculate that the transient associations seen for the other proteins may be explained by a “counteracting/adaption effect” during the maintenance period (3–6 mo). The general lack of clear, lasting associations over the 6 mo seemed to be explained by a considerable number of the proteins demonstrating inverse associations with the DASH score changes during 0–3 mo but positive associations from 3 to 6 mo (statistically significant or trends). Alternatively, the positive associations for the period from 3 to 6 mo may be explained by a continued decrease in cytokines due to the initial dietary changes in the cytokines while the dietary changes flattened out/were reversed between 3 and 6 mo. Indeed, the major changes in the DASH score appeared to occur during the 0–3-mo period, suggesting that the changes in DASH score flattened out from 3 to 6 mo (9). This is typical of behavioral interventions in that, in general, most of the effect occurs during the intensive treatment phase, peaking around 3–6 mo, followed by plateauing or regressing over time. Last, an explanation could also be that the cytokine changes diminish over time due to an “adaption” to the diet. Our results illustrate the importance of considering intervention intensity and time intervals. Previous studies reporting no effects of DASH, vitamin, or antioxidant interventions on serum IL-2, IL- 6, and TNF-α concentrations in children (11, 28) and adults with asthma (29) may support the limited associations with serum proteins we observed over the 6-mo trial period.
Potential mechanism from improved dietary quality to improved asthma control
Neither causality nor clinical impact of the observed changes in inflammatory markers can be concluded from our study. However, we observed apparent diet-related decreases in numerous serum factors that have a plausible link to asthma severity, exacerbation, and/or lung function (19), including IL-17, MIG/CXCL9, TNF-α, IL-6, and eotaxin (20, 21, 24, 30–33). Although we did not observe a full overlap in markers affected, the majority of changes in cytokines were seen as inverse correlations with diet quality and positive correlations with asthma control. This consistency and the direction in the associations suggest that the effect of dietary improvements on increased asthma control [as reported in Ma et al. (9)] may be reflected in decreased concentrations of inflammatory serum markers. In addition, DASH score reflects overall dietary quality, with a higher score indicating a healthier diet. A previous study reported that a diet containing yogurt, fruit, vegetables, and fish in early life was associated with increased butyrate production by the gut microbiota and reduced allergy outcomes in childhood (34). Another study found that obesity and the microbiome play an important role for the immune system and asthma outcomes (35). What we observed in this study may be microbiome-induced changes on consuming a more “healthy diet.”
Strengths and limitations
As serum proteins tend to show high interindividual variations, the assessment of changes in the serum markers over time is a considerable strength of the present study. The broad assessment of markers reflecting a more comprehensive measure of immune system function adds strength to our conclusions; earlier studies only assessed 1 or a narrow selection of inflammatory markers. Limitations of this study are the low power due to a modest overall sample size given the pilot study design, blood samples missing, and a relatively modest overall change in the DASH score values over time, making significant changes harder to identify. Also, other lifestyle factors such as physical activity probably interact with dietary effects [recently reviewed in (36)] and integrated studies of such factors may be needed for more targeted interventions on the individual level.
Conclusions
In asthmatics, this study demonstrates inverse associations between DASH scores and reduced general proinflammatory biomarkers such as cytokines and growth factors, which have been associated with asthma. Our observations provide support and expand existing epidemiological data suggesting that introduction of the DASH diet may have beneficial effects on inflammatory status. The results provide suggestive mechanistic data for the clinical indications of beneficial health outcomes with the inclusion of the DASH diet. These data may assist with guidelines for the management of asthma.
Data Sharing
To comply with the study informed consent form, we would share deidentified data and associated data dictionary only under a formal data sharing and use an agreement that provides for a commitment to the following: 1) using the data only for research purposes and not to identify any individual participant; 2) securing the data using appropriate computer technology, which needs to be specified, 3) destroying or returning the data after analyses are completed; 4) accepting reporting responsibilities; 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties; and 6) proper acknowledgement of the data resource. Data sharing requests shall be submitted to the Institutional Review Board for the University of Illinois at Chicago whose contact information is as follows: telephone: 1-(312) 996-1711; e-mail: uicirb@uic.edu.
Supplementary Material
Acknowledgments
The authors thank Dr. Holden Macker and Dr. Yael Rosenberg at the Human Immune Monitoring Center (HIMC) at Stanford University for performing the Luminex assays. Vanitha Sampath is highly acknowledged for critically reviewing the manuscript. We also thank the DASH participants and their families who made this study possible.
The authors’ responsibilities were as follows—JM, CAC, PS, and KCN: designed the research project (project conception, development of overall research plan, and study oversight); PS, NL, UCN, and KH: conducted research (hands-on conduct of the experiments and data collection); LX and UCN: analyzed data or performed statistical analysis; UCN, LX, and JM: wrote the manuscript (only authors who made a major contribution); UCN and JM: had primary responsibility for final content; UCN, LX, KCN, KH, CAC, and JM: performed data interpretations; and all authors: critically revised the manuscript and read and approved the final manuscript. KCN reports grants from National Institute of Allergy and Infectious Diseases (NIAID), Food Allergy Research and Education (FARE), End Allergies Together (EAT), Allergenis, and Ukko Pharma; was a grant awardee at NIAID, National Institute of Environmental Health Sciences (NIEHS), National Heart, Lung, and Blood Institute (NHLBI), and the Environmental Protection Agency (EPA); is involved in clinical trials with Regeneron, Genentech, Aimmune Therapeutics, DBV Technologies, AnaptysBio, Adare Pharmaceuticals, and Stallergenes-Greer; received a research sponsorship by Novartis, Sanofi, Astellas, and Nestlé; Data and Safety Monitoring Board member at Novartis and NHLBI; cofounded Before Brands, Alladapt, ForTra, and Iggenix; Chief Intellectual Office at FARE, Director of the World Allergy Organization (WAO) Center of Excellence at Stanford; received personal fees from Regeneron, Astrazeneca, ImmuneWorks, and Cour Pharmaceuticals; Consultant and Advisory Board Member at European Academy of Allergy and Clinical Immunology (EAACI) Research and Outreach Committee, Ukko, Before Brands, Alladapt, IgGenix, Probio, Vedanta, Centecor, Seed, Novartis, NHBLI, EPA, National Scientific Committee of Immune Tolerance Network (ITN) and NIH programs; has US patents for basophil testing, multifood immunotherapy and prevention, monoclonal antibody from plasmoblasts, and a device for diagnostics. JM is a paid scientific consultant for Health Mentor, Inc. (San Jose, CA). The other authors report no conflicts of interest.
Notes
The study was supported by grant R34 HL108753 from the National Heart, Lung and Blood Institute, Sean N. Parker Center for Allergy and Asthma Research at Stanford University and The Norwegian Institute of Public Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Supplemental Methods and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
UCN and LX contributed equally to this work.
Abbreviations used: ACQ, Asthma Control Questionnaire; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; ENA78, epithelial cell-derived neutrophil-activating peptide-78; FASL, soluble Fas ligand; FDR, false discovery rate; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulatory factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO, growth-regulated protein alpha; HGF, hepatocyte growth factor; IP-10, interferon γ–induced protein 10; LIF, leukemia inhibitory factor; MCP, monocyte chemoattractant protein; M-CSF, macrophage colony-stimulating factor; MIG/CXCL9, monokine induced by gamma/chemokine (C-X-C motif) ligand 9 (CXCL9); MIP, macrophage inflammatory protein; NGF, nerve growth factor; PAI, plasminogen activator inhibitor; PDGFBB, platelet-derived growth factor-BB; PLS, partial least squares; RANTES, regulated on activation, normal T-cell expressed and secreted; SCF, stem cell factor; TGF, transforming growth factor; Th, T-helper; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
Contributor Information
Unni C Nygaard, Sean N Parker Center for Allergy and Asthma Research, Division of Pulmonary and Critical Care Medicine and Division of Allergy, Immunology and Rheumatology, Stanford University, Stanford, CA, USA; Department for Environmental Health, Norwegian Institute of Public Health, Oslo, Norway.
Lan Xiao, Department of Medicine, Stanford University, Palo Alto, CA, USA.
Kari C Nadeau, Sean N Parker Center for Allergy and Asthma Research, Division of Pulmonary and Critical Care Medicine and Division of Allergy, Immunology and Rheumatology, Stanford University, Stanford, CA, USA.
Kinjal M Hew, Sean N Parker Center for Allergy and Asthma Research, Division of Pulmonary and Critical Care Medicine and Division of Allergy, Immunology and Rheumatology, Stanford University, Stanford, CA, USA.
Nan Lv, Institute of Health Research and Policy, University of Illinois at Chicago, Chicago, IL, USA.
Carlos A Camargo, Jr, Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Peg Strub, Department of Allergy, Asthma and Immunology, Kaiser Permanente San Francisco, San Francisco, CA, USA.
Jun Ma, Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
References
- 1. National Center for Health Statistics. Asthma.[Internet]. [Accessed 2019 Feb 13]. Available from: https://www.cdc.gov/nchs/fastats/asthma.htm. [Google Scholar]
- 2. Global Initiative for Asthma (GINA).. Global strategy for asthma management and prevention. 2018; [Internet]. Available from: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. [Google Scholar]
- 3. Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009;64:610–9. [DOI] [PubMed] [Google Scholar]
- 4. Barros R, Moreira A, Fonseca J, Delgado L, Castel-Branco MG, Haahtela T, Lopes C, Moreira P. Dietary intake of alpha-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br J Nutr. 2011;106:441–50. [DOI] [PubMed] [Google Scholar]
- 5. Iikura M, Yi S, Ichimura Y, Hori A, Izumi S, Sugiyama H, Kudo K, Mizoue T, Kobayashi N. Effect of lifestyle on asthma control in Japanese patients: importance of periodical exercise and raw vegetable diet. PLoS One. 2013;8:e68290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, Champagne CM, Hoben KP. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S19–27. [DOI] [PubMed] [Google Scholar]
- 8. USDA; US Department of Health and Human Services.. 2015–2020 Dietary guidelines for Americans. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 9. Ma J, Strub P, Lv N, Xiao L, Camargo CA Jr, Buist AS, Lavori PW, Wilson SR, Nadeau KC, Rosas LG. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur Respir J. 2016;47:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of Dietary Approaches to Stop Hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37:542–50. [DOI] [PubMed] [Google Scholar]
- 11. Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross-over clinical trial. Ann Nutr Metab. 2014;64:20–7. [DOI] [PubMed] [Google Scholar]
- 12. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- 13. Ma J, Strub P, Lavori PW, Buist AS, Camargo CA Jr, Nadeau KC, Wilson SR, Xiao L. DASH for asthma: a pilot study of the DASH diet in not-well-controlled adult asthma. Contemp Clin Trials. 2013;35:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–14. [DOI] [PubMed] [Google Scholar]
- 15. Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. [DOI] [PubMed] [Google Scholar]
- 16. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. [DOI] [PubMed] [Google Scholar]
- 17. Althouse AD. Adjust for multiple comparisons? It's not that simple. Ann Thorac Surg. 2016;101:1644–5. [DOI] [PubMed] [Google Scholar]
- 18. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statist. 2001;29:23. [Google Scholar]
- 19. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl Ret al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. [DOI] [PubMed] [Google Scholar]
- 20. Jiang XG, Yang XD, Lv Z, Zhuang PH. Elevated serum levels of TNF-alpha, IL-8, and ECP can be involved in the development and progression of bronchial asthma. J Asthma. 2018;55:111–8. [DOI] [PubMed] [Google Scholar]
- 21. Lv H, Lu B, Qian XJ, Huang JA, Qiu TF. Serum IL-17 & eotaxin levels in asthmatic patients with allergic rhinitis. Pak J Med Sci. 2016;32:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D'Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–14. [DOI] [PubMed] [Google Scholar]
- 23. Berthoud TK, Dunachie SJ, Todryk S, Hill AV, Fletcher HA. MIG (CXCL9) is a more sensitive measure than IFN-gamma of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J Immunol Methods. 2009;340:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai ST, Hung CH, Hua YM, Hsu SH, Jong YJ, Suen JL. T-helper 1-related chemokines in the exacerbation of childhood asthma. Pediatr Int. 2008;50:99–102. [DOI] [PubMed] [Google Scholar]
- 25. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 26. Esmaillzadeh A, Azadbakht L. Dairy consumption and circulating levels of inflammatory markers among Iranian women. Public Health Nutr. 2010;13:1395–402. [DOI] [PubMed] [Google Scholar]
- 27. Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aeberli I, Molinari L, Spinas G, Lehmann R, l'Allemand D, Zimmermann MB. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006;84:748–55. [DOI] [PubMed] [Google Scholar]
- 29. Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–43. [DOI] [PubMed] [Google Scholar]
- 30. Bazan-Socha S, Mastalerz L, Cybulska A, Zareba L, Kremers R, Zabczyk M, Pulka G, Iwaniec T, Hemker C, Undas A. Prothrombotic state in asthma is related to increased levels of inflammatory cytokines, IL-6 and TNFalpha, in peripheral blood. Inflammation. 2017;40:1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SCet al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao P, Gibson PG, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJet al. Anti-inflammatory deficiencies in neutrophilic asthma: reduced galectin-3 and IL-1RA/IL-1beta. Respir Res. 2015;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie M, Mustovich AT, Jiang Y, Trudeau JB, Ray A, Ray P, Hu H, Holguin F, Freeman B, Wenzel SE. IL-27 and type 2 immunity in asthmatic patients: association with severity, CXCL9, and signal transducer and activator of transcription signaling. J Allergy Clin Immunol. 2015;135:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, Schiavi E, Barcik W, Rodriguez-Perez N, Wawrzyniak Met al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74:799–809. [DOI] [PubMed] [Google Scholar]
- 35. Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, Van Horn S, Sokolowska M, Altunbulakli C, Eljaszewicz Aet al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun. 2019;10:5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nyenhuis SM, Dixon AE, Ma J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: what is the evidence?. J Allergy Clin Immunol In Practice. 2018;6:751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.