Abstract
Objectives
To summarise the current evidence regarding interventions for accurate and timely cancer diagnosis among symptomatic individuals.
Design
A scoping review following the Joanna Briggs Institute’s methodological framework for the conduct of scoping reviews and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews checklist.
Data sources
MEDLINE (Ovid), CINAHL (EBSCOhost) and PsycINFO (Ovid) bibliographic databases, and websites of relevant organisations. Published and unpublished literature (grey literature) of any study type in the English language were searched for from January 2017 to January 2021.
Eligibility and criteria
Study participants were individuals of any age presenting at clinics with symptoms indicative of cancer. Interventions included practice guidelines, care pathways or other initiatives focused on achieving predefined benchmarks or targets for wait times, streamlined or rapid cancer diagnostic services, multidisciplinary teams and patient navigation strategies. Outcomes included accuracy and timeliness of cancer diagnosis.
Data extraction and synthesis
We summarised findings graphically and descriptively.
Results
From 21 298 retrieved citations, 88 unique published articles and 16 unique unpublished documents (on 18 study reports), met the eligibility for inclusion. About half of the published literature and 83% of the unpublished literature were from the UK. Most of the studies were on interventions in patients with lung cancer. Rapid referral pathways and technology for supporting and streamlining the cancer diagnosis process were the most studied interventions. Interventions were mostly complex and organisation-specific. Common themes among the studies that concluded intervention was effective were multidisciplinary collaboration and the use of a nurse navigator.
Conclusions
Multidisciplinary cooperation and involvement of a nurse navigator may be unique features to consider when designing, delivering and evaluating interventions focused on improving accurate and timely cancer diagnosis among symptomatic individuals. Future research should examine the effectiveness of the interventions identified through this review.
Keywords: oncology, preventive medicine, primary care, public health
Strengths and limitations of this study.
A knowledge synthesis librarian developed the search strategy for this review and this was peer-reviewed by an independent knowledge synthesis librarian using the Peer Review of Electronic Search Strategies checklist.
The literature search was limited to evidence from the last 4 years and only evidence from English-language publications and organisational websites.
This review did not summarise the effectiveness of interventions across cancer patient types and regions.
We adhered to known guidelines and standards in the conduct and reporting of the review.
In line with the Joanna Briggs Institute’s guidance for the conduct of scoping reviews, we did not attempt to evaluate the quality of the included studies or provide an assessment of the quality of the evidence.
Introduction
Cancer is the second leading cause of death globally, with about one in six deaths attributable to the disease.1 It was estimated in 2020 that over 19 million new cases and about 10 million deaths were attributable to cancer globally.2 This rate is estimated to be over 28 million new cases by 2040.2 High Human Development Index countries such as Canada will likely experience the greatest increase in incidence in absolute cancer burden, with an estimated over 4 million new cases more in 2040 compared with 2020.2 This is mostly due to the growth and ageing of the population and increasing prevalence of cancer risk factors.2 Estimates from Canada alone suggest that every day 617 people in Canada will be diagnosed with cancer, with about 228 also dying from the disease.3
Although cancer can occur at any age, the risk of the disease increases with age.4 Globally, cancer incidence rates vary, mostly because of differences in risk factors and early detection practices. Likewise, cancer death rates vary, partly because of differences in availability and effectiveness of cancer control strategies, such as early diagnosis and access to timely and effective treatment.2 With timely diagnosis and treatment initiation, significant improvements can be made in the lives of patients with cancer. Moreover, many cancers have higher curative and survival rates if diagnosed early. This means that the cancer burden could be reduced substantially through early detection and management of patients who present with symptoms.5
When not diagnosed following early symptomatic presentation, cancer diagnosis often occurs at more advanced stages of the disease, when treatment may be less effective and cancer prognosis will be poor. Early cancer diagnosis of symptomatic individuals entails carefully planned, well-integrated, culturally safe and equitable clinical evaluation and diagnostic services.5 These services should be designed to reduce delays in and barriers to diagnosis to allow detection at earlier stages of the disease and commence treatment in a timely manner.
Various service-focused interventions to improve early cancer diagnosis of symptomatic individuals have been implemented in various jurisdictions with varying levels of success. Knowledge of the available interventions, strategies used to implement them, and how successful they might have been is necessary to inform the development, implementation and evaluation of effective early cancer diagnosis initiatives.
Methods
This report is a summary of the study commissioned by the Canadian Partnership Against Cancer (the Partnership). The Partnership contributed to specifying the study objectives and questions, and in summarising the evidence.
We undertook a scoping review following the Joanna Briggs Institute’s (JBI’s) guidance for the conduct of scoping reviews.6 This framework includes defining and aligning the objective(s) and question(s) for the review, developing and aligning the inclusion criteria with the review objective(s) and question(s), and describing the planned approach to evidence searching. It also includes selecting, extracting and charting of evidence; summarising the evidence in relation to the objectives and questions; and consultation of information scientists, librarians and/or experts throughout the process. Online supplemental appendix 1 is the work plan approved by the Partnership for the scoping review.
bmjopen-2021-055488supp001.pdf (178.6KB, pdf)
We summarised the current evidence regarding interventions focused on improving accurate and timely cancer diagnosis among symptomatic individuals, including practice guidelines, care pathways or targets for wait times, streamlined or rapid diagnostic services, multidisciplinary teams, and patient navigation strategies. We also summarised innovative interventions (eg, those with a technological component) and approaches to seamless (minimally disruptive) care of symptomatic individuals and identified performance metrics that can be used to measure improvements in the prediagnosis phase. Additionally, we summarised the key points of the patient trajectory from initial symptom presentation to cancer diagnosis.
We report our findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist.7
Search strategy
A knowledge synthesis librarian (NA) designed a search strategy for MEDLINE (Ovid). This search strategy was peer-reviewed independently by another knowledge synthesis librarian using the Peer Review of Electronic Search Strategies (PRESS) checklist.8 The revised search strategy was then adapted for Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) and PsycINFO (Ovid) bibliographic databases. The search strategy for each of the databases is presented in online supplemental appendices 2–4. In addition to searching bibliographic databases, we searched websites of relevant organisations and professional bodies (online supplemental appendix 5) and hand-searched reference lists of potentially relevant publications.
Study selection criteria and data extraction
We sought to summarise practice guidelines, care pathways and initiatives such as benchmarks/targets for wait times, streamlined or rapid diagnostic services, multidisciplinary teams and patient navigation strategies that have been found to enhance accurate and timely cancer diagnosis in symptomatic individuals. We also sought to summarise the leading interventions to seamless care in the cancer prediagnosis phase, performance metrics that can be used to measure the suspicion to diagnosis phase and how these metrics have been used. Further, we sought for specific considerations for underserviced populations in studies, including considerations for Indigenous, rural and remote populations.
Published (peer-reviewed) and unpublished (grey literature) articles in the English language from January 2017 to January 2021 were included. The decision to include articles from 2017 was because the Partnership had previously summarised prior evidence, not included in this current report.9 Study participants were individuals of any age presenting in any clinical settings with symptoms. Interventions included practice guidelines, care pathways or other initiatives focused on achieving predefined benchmarks or targets for wait times, streamlined or rapid diagnostic services, multidisciplinary teams and patient navigation strategies. Outcomes included accuracy and timeliness of cancer diagnosis.
All retrieved citations from the literature search were imported and managed in EndNote (V.X9). One reviewer (GNO or OLTL or VKR or LC) screened each citation for eligibility. Two reviewers (GNO, OLTL, VKR, and LC in pairs) independently screened the full texts of relevant citations and reviewed the reference list of the included full-text articles for potentially relevant citations. Disagreements between the reviewers were resolved through discussion or involvement of a third reviewer (AMA-S). The number of screened citations and both the number and reason for exclusion of full-text articles were documented. One reviewer (GNO or OLTL or VKR or LC) performed data extraction and charting, and another reviewer (GNO or OLTL or VKR or LC) independently checked the extracted and charted data for errors. Disagreements between the reviewers were resolved through discussion or involvement of a third reviewer (AMA-S).
Data synthesis and analysis
Characteristics of the included published articles are presented in a tabular form and descriptive analysis is reported graphically and descriptively. Characteristics of the included unpublished articles are reported descriptively only. Relevant findings from the review of both published and unpublished articles are summarised separately and descriptively, by review question, focusing on the interventions related to each question. Interventions are grouped as centralised or coordinated diagnostic service; interventions to enhance diagnostic services; multidisciplinary team; patient navigation; rapid referral pathway; remote or rural populations-focused; standardised care pathway; support for primary care providers (PCP); target or benchmark; and technology to support the diagnostic process. These interventions are defined in online supplemental appendix 6. We determined the effectiveness of an intervention based on study findings and conclusions reported by the primary study’s authors with respect to intervention effect. As such, effective interventions were those interventions that were found to have had a statistically significant positive effect on an author-determined outcome for effectiveness evaluation. It is important to note that the authors of this scoping review did not assess risk of bias nor rate the quality of evidence and thus definitive conclusions on effectiveness cannot be drawn.
Patient and public involvement
There was no active engagement of patients and/or members of the public.
Results
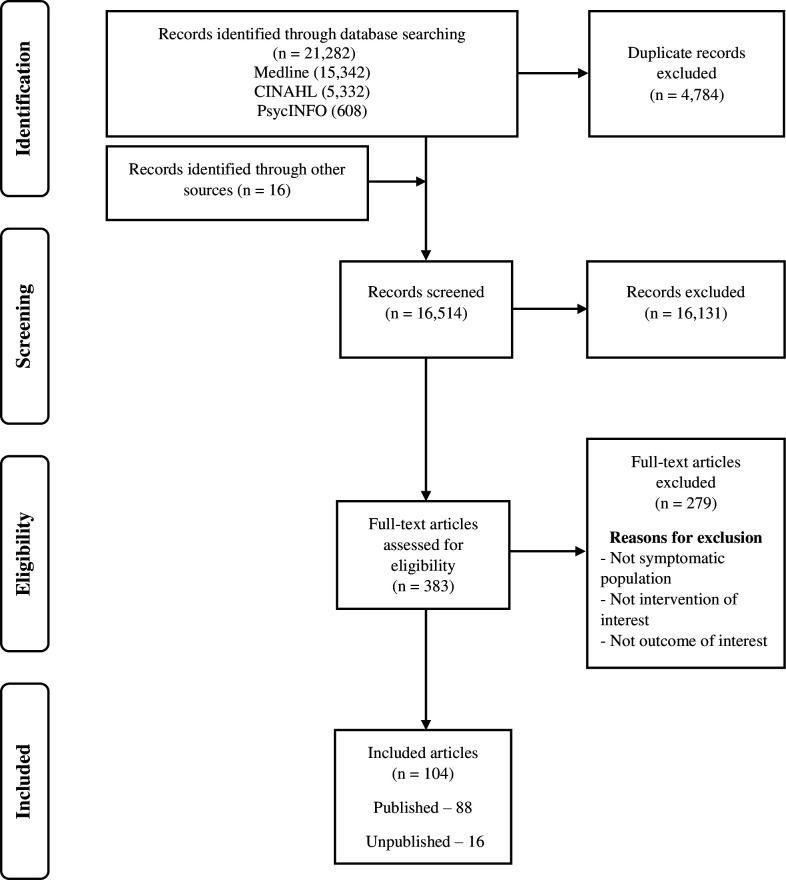
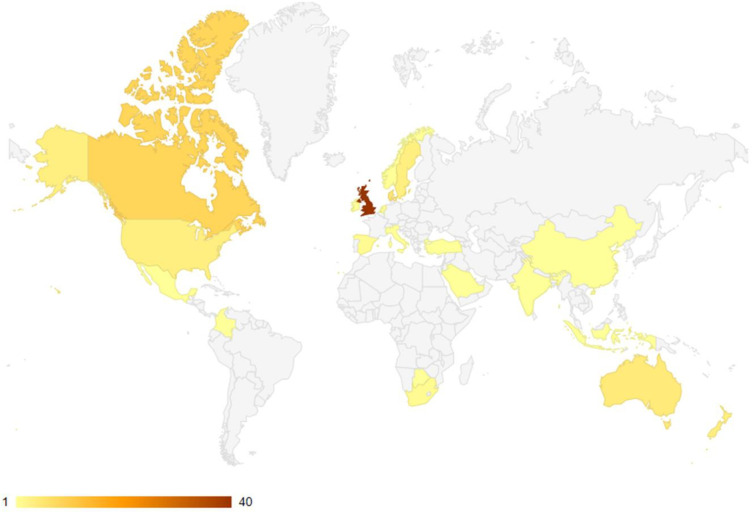
Out of a total of 21 298 retrieved citations, 88 unique published articles10–97 and 16 unique unpublished (grey literature representing 18 different reports)98–113 met the inclusion criteria. The article selection process is detailed below (figure 1). Fifty-seven of the published articles were from Europe, 14 articles from North America, 9 articles from Oceania, 3 articles each from Africa and Asia and 1 article each from the Middle East and South America. Almost half of these articles (n=40) were from the UK alone. A geographic map of published articles is shown in figure 2.
Figure 1.
Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
Figure 2.
Geographical mapping of the included published articles.
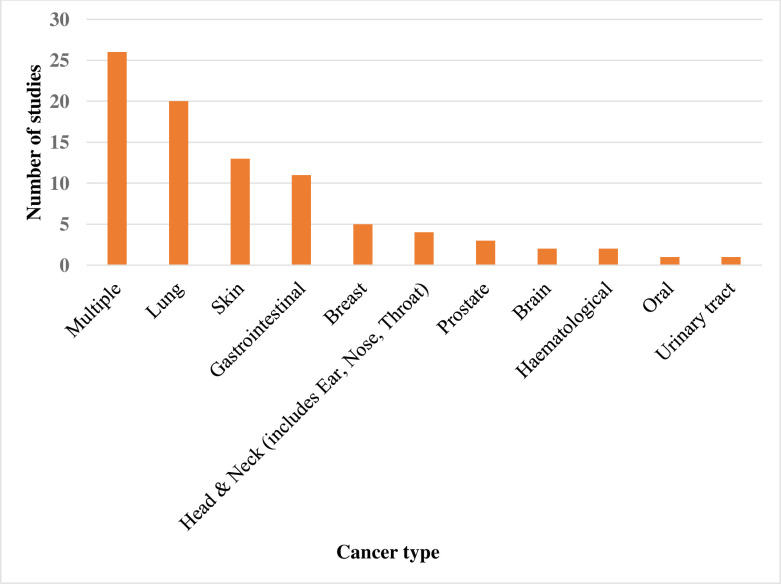
Of the 18 unpublished reports (16 articles), 83% were from the UK, 11% from Canada and 6% from the USA. Forty per cent (n=35) of the published articles were for case–control studies, 29% (n=26) for cross-sectional studies, 22% (n=19) for before-and-after studies, 7% (n=6) for randomised controlled studies and 1% (n=1) each for guideline development and mixed methods studies. In terms of the unpublished articles, 89% (n=16) were before-and-after studies and the rest (n=2) were cross-sectional studies. Figure 3 shows the distribution of the cancer types reported by the published articles; approximately 30% (n=26) reported on multiple cancer types, while the rest reported on specific cancer types, of which lung cancer was the most frequent (about 23% of the publications (n=20)). Of the unpublished articles, half reported on lung cancer, 28% on multiple cancer types, 11% on breast cancer and 5.5% each on brain and gastrointestinal cancers.
Figure 3.
Summary of cancer types reported by the included published articles.
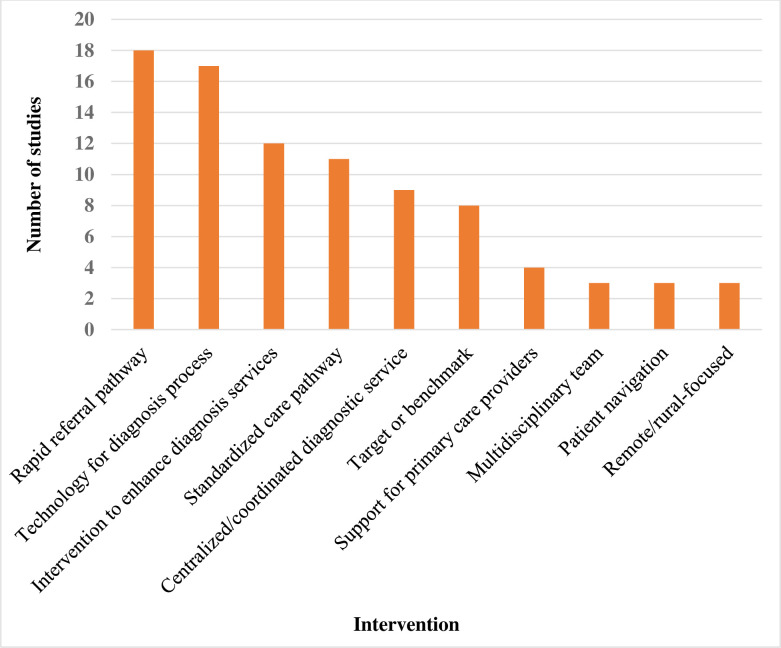
Figure 4 shows the distribution of intervention types across the published articles. Nearly 20% of the published articles were on rapid referral pathway interventions while less than 1% each were on multidisciplinary team, patient navigation and remote/rural-focused interventions. Of the unpublished articles, half reported on rapid referral pathway interventions, 11% each reported on standardised care pathway, target/ benchmark for wait times and technology to support the diagnosis process, and 5.5% each reported on centralised or coordinated diagnostic service and interventions to enhance diagnostic services. Most of the published articles (94%; n=83) reported a performance metric used to measure an improvement in the suspicion to diagnosis phase of cancer.
Figure 4.
Summary of intervention types reported by the included published articles.
Eighty-three per cent (n=73) of the articles reported either a practice guideline, care pathway or an initiative such as benchmark/target for wait times, streamlined or rapid diagnostic service, multidisciplinary team development and a patient navigation strategy to enhance accurate and timely cancer diagnosis. Thirty-one per cent (n=27) of the articles reported (not explicitly) on a key point of care as patients navigate the health system, from initial suspicion to diagnosis of cancer. Twenty-nine per cent (n=25) of the articles reported on a leading innovative intervention or approach to seamless care in the precancer diagnosis phase, while 4.5% (n=4) of the articles reported on some form of consideration for underserved populations. Some of the articles reported on two or more of the above. Details of relevant characteristics of the published articles are presented in table 1 (those reporting effective interventions) and online supplemental appendix 7 (those reporting ineffective interventions) and online supplemental appendix 8 (those focused on remote/and rural populations).
Table 1.
Summary of the characteristics of the included published articles that reported data on effective interventions
| Intervention | Article | Study country (region) | Study type (study years) | Cancer type (population) (sample size) |
Assessment metric | Results |
| Centralised or coordinated diagnostic service | Christensen and Huniche20 | Denmark (Odense) |
Cross-sectional (2016–2017) | Lung (adult) (20) |
Patients’ perspective, experiences and expectations | Although patients experienced anxiety with the fast-track diagnostic pathway, they still wanted to move through with diagnosis as quickly as possible (effective) |
| Common et al23 | Canada (Newfoundland) | Case–control (2015–2016) | Lung (adult) (133) |
Time from first abnormal image to biopsy | There was a statistically significant decline in wait times for patients from 61.5 to 36.0 days (p<0.0001) (effective) | |
| Evison et al32 | UK (Manchester) |
Before-and-after (2016–2019) | Lung (adult) (1035) |
Mean time from referral to CT | The median time from referral to CT was 3 days. Overall 56% and 90% of patients had completed a CT and consultation within 3 and 7 days of referral, respectively (0% and 24% prior to implementation) (effective) | |
| Ezer et al33 | Canada (Montreal) |
Case–control (2010–2011) | Lung (adult) (327 (195 RIC; 132 non-RIC)) |
Time from first contact with physician to diagnosis | Time from first contact to pathological diagnosis was shorter (median (M) 26 days; IQR 14–42 days) vs control patients (M 40 days; IQR 16–68 days) (effective) | |
| Jiang et al44 | Canada (Ontario) |
Case–control (2011) | Breast (adult) (4381) |
Time to diagnosis | The Canadian timeliness targets (time from patients’ first referral or test to the cancer diagnosis) were achieved more often than for usual care (71.7% vs 58.1%, respectively), with associated 10-day (95% CI 7.8 to 11.9) reduction in the median diagnostic interval (effective) | |
| McKevitt et al54 | Canada (British Columbia) |
Case–control (2009) | Breast (NR) (373) |
Diagnostic wait time | Patients had a decreased time to surgical consultation (33 vs 86 days, p<0.0001) for both malignant (36 vs 59 days, p=0.0007) and benign diagnoses (31 vs 95 days, p<0.0001) (effective) | |
| McKevitt et al55 | Canada (Vancouver) | Case–control (2012) | Breast (NR) (176 (40 RABC; 136 TS)) |
Time from presentation to surgical consultation | Time from presentation to surgeon evaluation was shorter in the RABC group for patients with breast symptoms (81 vs 35 days, p<0.0001) (effective) | |
| Moodley et al56 | South Africa (Western Cape province) | Cross-sectional (2015–2016) | Breast (adult) (201) |
Time between first healthcare provider visit and date of diagnosis | The median time between the first healthcare visit and a breast cancer diagnosis was 28 days (IQR 13–58 days). Women whose initial reaction was denial of the breast symptom had a significantly shorter diagnostic interval (11 days vs 29 days, p=0.010) (effective) | |
| Williams et al93 | New Zealand (Northland district) | Before-and-after (2015–2016) | Lung (adult) (212 (70 in phase 1, 46 in phase 2 and 71 in phase 3)) |
Time from GP referral to first specialist appointment | Time from GP referral to first specialist appointment improved significantly (p=0.005) (effective) | |
| Interventions to enhance diagnostic services | Chapman et al17 | UK (Nottingham) |
Cross-sectional (2017–2018) | Gastrointestinal (adult) (1934) |
Colorectal cancer (CRC) detection rate after a FIT | The symptomatic pathway incorporating FIT was feasible and appeared more clinically effective than pathways based on age and symptoms alone, with FIT results identifying patients with a significantly higher risk of CRC (effective) |
| Cotton et al24 | Canada (Ontario) |
Before-and-after (2017–2018) | Lung (NR) (NR) |
Referral to diagnosis | Monthly patient volumes increased by 65%, and wait time improved by 60% (effective) | |
| Laudicella et al52 | UK (England) |
Case–control (2006–2009) | Multiple (adult) (372 353) |
Survival of patients | Rerouting patients from emergency presentation to new referral resulted in better patient survival in all cancer cohorts (effective) | |
| Nixon et al64 | Canada (Ontario) |
Case–control (2015–2017) | Haematological (adult) (126) |
Time from initial consultation to diagnosis of lymphoma | Median time to lymphoma diagnosis was 16 days for patients assessed in the nurse practitioner–led lymphoma rapid diagnosis clinic and 28 days for historical controls (p<0.001) (effective) | |
| Sardi et al75 | Colombia (Cali) |
Before-and-after (2012–2016) | Multiple (NR) (114) |
Time from initial consultation to biopsy | The average time from initial consult to biopsy decreased from 65 to 20 days and from biopsy to diagnosis from 33 to 4 days (effective) | |
| Setyowibowo et al77 | Indonesia (Bandung West Java) | RCT (2017) |
Breast (adult) (107) |
Time between first visit to the hospital and a definitive diagnosis | The intervention reduced the time to definitive diagnosis: mean difference=−13.26, 95% CI −24.51 to −2.00, p=0.02) (effective) | |
| Skevington et al78 | UK (Manchester) |
RCT (2015–2016) |
Multiple (adult) (107) |
Quality of life | Psychological quality of life increased (effective) | |
| Stenman et al80 | Sweden (Kristianstad) | Cross-sectional (2015) | Multiple (adult) (290) |
Total diagnostic interval | Shorter diagnostic interval (time from referral decision in primary care to diagnosis). The median primary care interval was 21 days, and the median diagnostic interval was 11 days (effective) | |
| Tafuri et al83 | USA (NR) |
Case–control (2016–2018) | Prostate (adult) (370) |
Time from multiparametric MRI (mpMRI) to biopsy | One-stop patients experienced shorter time from mpMRI to biopsy (0 vs 7 days; p<0.01) (effective) | |
| Williams et al94 | Botswana (Gaborone) | Before-and-after (2015–2017) | Skin (adult) (218) |
Diagnostic histology turnaround times | Median turnaround in the post dermatology quality improvement interval was 11 days (IQR, 12–23 days) compared with 32 days in the pre-dermatology quality improvement interval (IQR, 24–56 days; p<0.001) (effective) | |
| Multidisciplinary team | Phillips et al68 | USA (NR) |
Case–control (2014–2016) | Lung (NR) (218) |
Time to diagnosis | Compared with controls, patients with lung cancer in the Lung Cancer Strategist Programme cohort had an expedited time from suspicious finding to diagnosis (34 vs 44 days, p=0.027) (effective) |
| Patient navigation | Chavarri-Guerra et al18 | Mexico (Mexico City) |
Before-and-after (2016–2017) | Multiple (adult) (70) |
Feasibility | 91% of patients successfully obtained appointments at cancer centres in <3 months (effective) |
| Drudge-Coates et al28 | UK (London) |
Before-and-after (2012–2015) | Prostate (adult) (60) |
Waiting times from the GP referral to initial clinic assessment | Compared with the previous physician-led service, waiting times for patient appointment fell by 52% over a 3-year study period (effective) | |
| Whitley et al92 | USA (Boston, Denver, San Antonio, and Tampa) |
Case–control (2007–2011) | Multiple (adult) (6349) |
Delays in diagnostic resolution based on Charlson Comorbidity Index score | Patient navigation reduced delays in diagnostic resolution, with the greatest benefits seen for those with a Charlson Comorbidity Index score ≥2 (effective) | |
| Rapid referral pathway | Antel et al13 | South Africa (Cape Town) |
Before-and-after (2017–2019) | Haematological (adult) (130) |
Diagnostic interval | Compared with a historical cohort, the diagnostic interval (time from first health visit to diagnostic biopsy) for patients with lymphoma was significantly shorter, 13.5 vs 48 days (p=0.002) (effective) |
| Arhi et al14 | UK (National) |
Case–control (2000–2013) | Gastrointestinal (adult) (7130) |
HRs of death | Patients referred between 2 weeks to 3 months, and after 3 months with red-flag symptoms demonstrated a significantly worse prognosis than patients who were referred within 2 weeks (effective) | |
| Chng et al19 | UK (Newcastle-upon-Tyne) |
Case–control (2015–2019) | Brain (adult) (101) |
Tumour detection rate | With guideline adherence, the brain tumour detection rate was threefold higher (36.0% vs 11.5%, p=0.02) (effective) | |
| Creak et al25 | UK (Brighton; Sussex) |
Cross-sectional (2015–2018) | Multiple (adult) (258) |
Time to diagnosis | Direct GP referrals were feasible and manageable within a tertiary clinic and resulted in high rates of cancer diagnoses and early contact with an oncologist and nurse specialist, cutting short the ‘limbo’ time of high anxiety before diagnosis (effective) | |
| Hennessy et al36 | Ireland (Dublin) |
Case–control (2012–2018) | Lung (NR) (864) |
Time to diagnosis | Time to diagnosis was longer in those who had attended a post Rapid Access Lung Cancer Clinic CT (34.5 vs 21 days) (effective) |
|
| Jones et al45 | UK (East Midlands) |
Case–control (2013–2015) | Gastrointestinal (NR) (1401 (340 STTP, 495 traditional pathway, 566 control trusts)) |
Time from referral to diagnosis | The pathway saved a mean of 7 days from referral to treatment (with a 95% CI of 3 to 11 days, p<0.008) and a mean of 16 days from referral to diagnosis, when compared with a traditional pathway (effective) | |
| Joyce et al46 | UK (National) |
Cross-sectional (2017–2018) | Multiple (mixed age) (NR) |
Proportion with emergency diagnosis of cancer | A lower proportion of emergency diagnosis of cancer was found with higher 2 weeks wait referral conversion rate (effective) | |
| Pearson et al67 | UK (National) |
Case–control (2014) | Multiple (mixed age) (12 873) |
Primary care interval | Compared with patients with a specific alarm symptom, patients with non-specific but concerning symptoms had higher odds of having longer primary care intervals (adjusted OR: 1.24 (1.11 to 1.36)) (effective) | |
| Round et al72 | UK (National) |
Case–control (2011–2017) | Multiple (mixed age) (1 469 103) |
Risk of death | Cancer patients from the highest referring practices had a lower hazard of death (HR=0.96; 95% CI 0.95 to 0.97) (effective) | |
| Sandager et al74 | Denmark (National) | Cross-sectional (2010) | Multiple (adult) (2256) |
Patient experience | Overall, pathway referred patients were 21% more likely than non‐pathway referred patients to report a positive experience (PR=1.21 (95% CI 1.11 to 1.30)) (effective) | |
| Thanapal et al86 | UK (London) |
Before-and-after (2012–2018) | Gastrointestinal (adult) (1648) |
Time to diagnosis | Patients on the pathway took 25 days to obtain results as compared with 40 days in the standard pathway (effective) | |
| Vijayakumar et al90 | UK (Buckinghamshire) | Cross-sectional (2018) | Lung (NR) (111) |
Patient satisfaction | High satisfaction with the service, with scores above 93% in all parameters (effective) | |
| Standardised care pathway | Alonso-Abreu et al12 | Spain (Tenerife) |
Case–control (2008–2010) | Gastrointestinal (adult) (257) |
Survival rates | Survival rates at 12 and 60 months after treatment were significantly higher in the early colonoscopy group compared with the standard schedule colonoscopy group (p<0.001) (effective) |
| Dahl et al26 | Denmark (Countrywide) | Before-and-after (2004–2010) | Multiple (adult) (3292) |
Patient satisfaction for waiting time from referral to consultation at a hospital | Implementation of pathway was associated with a reduced level of patient-reported dissatisfaction with long waiting time from the time of referral to the first consultation at the hospital (effective) | |
| Laerum et al49 | Norway (Kristiansand) | Before-and-after (2007–2016) | Lung (adult) (780) |
Referral interval | The median referral interval among all patients was reduced by 2 days from baseline to the next time period when the local diagnostic algorithm was streamlined (effective) | |
| Mullin et al59 | Canada (Ontario) |
Before-and-after (2018–2019) | Lung (NR) (833) |
Time from referral to diagnosis | Time from referral to positron emission tomography decreased (from 38.5 to 15.7 days), time from referral to brain imaging decreased (from 33.4 to 13.1 days) and time from referral to diagnosis decreased (from 38.0 to 22.7 days), all demonstrating special-cause variation (effective) | |
| Nilbert et al63 | Sweden (Skane County) |
Case–control (2015–2016) | Urinary tract (adult) (1871) |
Time from sign/symptom to diagnosis | The standardised care pathway shortened the diagnostic delay to a median of 25 days compared with 35 days for regular referral (p=0.01) (effective) | |
| Rankin et al71 | Australia (New South Wales) |
Cross-sectional (2014) | Lung (adult) (19) |
Patient concerns urgency, advocacy and referral | Patients and general practitioners expressed similar themes across the diagnostic and pretreatment intervals (effective) | |
| Target or benchmark for wait times | Jeyakumar et al42 | Australia (Victoria) | Case–control (2018) | Lung (adult) (46) |
Mean time from initial CT to tissue diagnosis | The Standard Care group met the target for treatment commencement in 33.3% of cases whereas the Rapid Access Clinic group achieved this in 77% (effective) |
| Jiang et al43 | China (Shanghai) |
Case–control (2011–2015) | Lung (NR) (4000) |
Time from initial respiratory consultation to treatment decision | Takes a median 4 workdays (range 3–6) for a new patient from initial respiratory consultation to treatment decision, whereas in many countries, 14 workdays are considered a reasonable timeline (effective) | |
| Sagar et al73 | UK (Milton, Somerset) |
Before-and-after (2019–2020) | Gastrointestinal (mixed age) (1255) |
28-day target attainment | Attainment of the 28-day diagnosis target for all suspected colorectal cancer referrals improved following the establishment of a new pathway (88% vs 82%, p<0.0001) (effective) | |
| Stevenson-Hornby et al81 | UK (Wigan) |
Before-and-after (2017) | Gastrointestinal (NR) (NR) |
Percentage diagnosed | 55% of all referrals were found to have hepatobiliary-pancreatic cancer after pathway trial compared with 19% before (effective) | |
| Zhu et al96 | Sweden (Orebro) | RCT (2015–2018) |
Prostate (adult) (204) |
Self-reported symptoms of stress | Significant changes in depression symptoms and self-rated sleep quality suggested a benefit of the fast-track workup intervention (effective) | |
| Piano et al69 * | UK (Guildford, Bradford) |
Cross-sectional (NR) | Multiple (adult)29 |
Patient attitudes within the context of their recent referral experiences | Most patients had experienced swift referral. It was difficult for patients to understand how the new standard could affect on the time that it takes to progress through the system. Responsibility for meeting the standard was also a concern as patients did not see their own behaviours as a form of involvement (NA) | |
| Technology to support diagnosis process | Cazzaniga et al16 | Italy (Bergamo) |
Case–control (2017) | Skin (adult) (232) |
Diagnostic accuracy | The diagnostic accuracy of the online assessment compared with direct clinical examination was significant (effective) |
| Cock et al22 | UK (NR) |
Guideline development (2014–2016) | Gastrointestinal (adult) (NR) |
Patient satisfaction | Audits were being conducted to assess and compare patient satisfaction with face-to-face vs telephone assessments, although intervention was well-received (effective) | |
| Eastham et al29 | UK (Leeds) |
Before-and-after (2015–2016) | Multiple (adult) (NR) |
Form completion rates and time spent processing forms | Form completion rates improved from a mean of 44% of forms at baseline (n=210) to 99% postintervention n=236). Time spent processing forms also decreased from a mean of 96 s to 35 s postintroduction of the new system (effective) | |
| Hirst et al37 | UK (London) |
Cross-sectional (2016) | Multiple (adult) (NR) |
GP perspectives on txt-netting | Text messages were perceived to be an acceptable potential strategy for safety netting patients with low-risk cancer symptoms (effective) | |
| Hunt et al38 | UK (England) |
Case–control (2018) | Skin (adult) (150 (75 consecutive TD referrals paired with 75 standard ‘Face to Face’ controls)) |
Time from referral to first appointment and diagnostic rates | There was a 23% absolute and 37% relative increase in diagnostic completion rates in the mobile van compared with the central hospital facility (p=0.0001) (effective) | |
| Moor et al57 | UK (Newcastle-upon-Tyne; Birmingham) |
Case–control (2007–2010) | Head and neck (mixed age) (4715) |
Diagnostic accuracy | Machine learning algorithms accurately and effectively classify patients referred with suspected head and neck cancer symptoms (effective) | |
| Moreno-Ramirez et al58 | Spain (Southern region) |
Case–control (2004–2015) | Skin (NR) (2009) |
Waiting times for referral | Waiting times for referral for teledermatology network vs conventional letter referral system 12.31 (8.22 to 16.40) vs 88.62 (38.42 to 138.82) (effective) | |
| Nicholson et al62 | UK (London) |
Cross-sectional (2018–2019) | Skin (NR) (60) |
Patient satisfaction | Over 80% (49) would recommend the service, and the majority felt confident with the teledermatology model. Overall, patients would be happy to complete electronic questionnaires and receive results electronically, with younger patients being more amenable to this (effective) | |
| Orchard et al65 | UK (Bristol) |
Before-and-after (2014–2017) | Gastrointestinal (mixed age) (11 357) |
Time from referral to diagnosis | Time from referral to diagnosis reduced from 39 to 21 days and led to a dramatic improvement in patients starting treatment within 62 days (effective) | |
| Snoswell et al79 | New Zealand (Countrywide) | Not clear (2012) |
Skin (adult) (300) |
Time to clinical resolution | Mean time to clinical resolution was 9 days (range, 1–50 days) with teledermoscopy referral compared with 35 days (range, 0–138 days) with usual care alone (difference, 26 days; 95% credible interval 13 to 38 days) (effective) | |
| Sunderland et al82 | New Zealand (Auckland) | Case–control (2016) | Skin (NR) (809) |
Efficacy of diagnostic tool | A positive predictive value (PPV) of 38.1% and number needed to excise (NNE) of 2.6, with less than 10% of referrals triaged for teledermatoscopy confirmed as melanoma (24/264) (effective) | |
| Uthoff et al87 | India (Bangalore, Dimapur) |
Case–control (NR) | Oral (adult) (99) |
Diagnostic accuracy | Sensitivities, specificities, positive predictive values and negative predictive values ranged from 81.25% to 94.94% (effective) | |
| Vestergaard et al89 | Denmark (Southern Denmark) | Case–control (2018) | Skin (adult) (519) |
Percentage of lesions not requiring further in-person assessment | On evaluation by teledermoscopy, 31.5% of lesions did not need further in-person assessment (effective) |
*Effective but not applicable.
CT, computed tomography; FIT, faecal immunochemical testing; GP, general practitioner; NR, not reported; RABC, rapid access breast clinic; RCT, randomised controlled trial; RIC, rapid investigation clinic; STTP, straight to test pathway; TD, teledermatology; TS, traditional system.
Initiatives to enhance accurate and timely cancer diagnosis
This review identified various initiatives to enhance accurate and timely cancer diagnosis. These were often designed, developed and implemented often with the involvement of PCP (physicians and nurses), but not patients. These initiatives are grouped into related interventions and the evidence regarding each intervention is discussed below.
Centralised or coordinated diagnostic services
Nine published articles on centralised or coordinated diagnostic services for adult lung cancer (n=5) and breast cancer (n=4) patients were identified.20 23 32 33 44 54–56 93 Five were from Canada,23 33 44 54 55 and there was one each from Denmark,20 New Zealand,93 South Africa56 and the UK.32 The focus and metrics for assessment of the effectiveness of these diagnostic services varied, but all were found to be effective. These include the rapid access to pulmonary investigation and diagnosis programme in Wythenshawe Hospital, Manchester, UK with expedited (next working day) CT and reporting in suspected lung cancer cases,32 and the Thoracic Triage Panel in a tertiary care centre in St. John’s, Newfoundland, Canada, a multidisciplinary centralised referral programme, whose key components include a nurse navigator who coordinates patient care and act as the contact person for patients and clinicians involved in the programme, weekly multidisciplinary (thoracic specialists) meetings and regular communications with the primary care provider.23 The diagnostic services also include the rapid investigation clinic in a tertiary health centre in Montreal, Canada established to coordinate and accelerate the workup of patients with suspected lung cancer,33 the improved respiratory fast track clinic in Northland district of New Zealand that comprises reserved slots for CT for those referred with a suspicion of lung cancer, bronchoscopy slots and CT-guided biopsy,93 and the Danish lung cancer package at the Center for Lung Cancer, Odense University Hospital, Odense, Denmark, a fast-track diagnostic pathway in the hospital setting.20 Further, there was the rapid access breast clinic in British Columbia, Canada that provides close collaboration between clinicians and radiologists, facilitated by clinical pathways and nurse navigation,54 55 the diagnostic assessment units in Ontario, Canada, focusing on diagnosis at a dedicated breast assessment unit,44 and the breast clinic at a tertiary hospital in Western Cape Province of South Africa, an open-access one-stop diagnostic breast clinic where women may present with a letter from a primary level provider (nurse practitioner or doctor) and receive the same day clinical and cytological evaluation with referral to the combined breast clinic if the breast cytology is positive for malignancy.56
In addition to the above, one unpublished article was identified.113 This was for the Breast ACCESS Project in Ohio, USA, which scheduled patients for a surgical consult within 2 days and a biopsy within 5 days after the surgical consult, with the aim of reducing wait times between abnormal diagnostic mammogram findings to biopsy from 26 to 7 days (7-day ACCESS goal).
Interventions to enhance diagnostic services
Twelve published articles on interventions to enhance diagnostic services were identified.10 17 24 52 53 64 75 77 78 80 83 94 These articles were focused on varied cancer types; four on multiple cancers, two on lung cancer, two on skin cancer and one each on breast, gastrointestinal, haematological and prostate cancers. Four articles were from the UK,17 52 53 78 two articles each from Canada24 64 and Sweden,10 80 and one article each from Botswana,94 Columbia,75 Indonesia77 and the USA.83 The focus and metrics for assessment of the effectiveness of the interventions varied across the publications, and while most were effective, one intervention for lung cancer and one intervention for skin cancer in the UK53 and Sweden,10 respectively, were ineffective. The effective interventions were reducing diagnosis through emergency presentation by improving general practice referral in England, UK,52 the guided personal quality of life (QoL) feedback intervention during the Cancer Research UK’s North West regional summer roadshow in Manchester, UK, aimed at offering guided feedback about personal QoL to adults with potential cancer symptoms, living in deprived communities to promote help seeking in primary care among the communities,78 the mandatory primary care access to faecal immunochemical testing in Nottingham, UK, integrated with the 2-week wait pathway, aimed at improving gastrointestinal cancer diagnosis rather than relying on age and symptoms alone,17 the Stronach Regional Cancer Centre lung diagnostic assessment programme at Southlake Regional Health Centre, Ontario, Canada, aimed at using learnings from a Lean improvement event to provide coordinated, expedited care for all patients undergoing a possible lung cancer diagnosis and to achieve/improve on the provincial wait time target from consultation to diagnosis for patients with lung cancer,24 the nurse practitioner-led lymphoma rapid diagnosis clinic in a tertiary care cancer centre (Princess Margaret Cancer Centre, part of University Health Network) in Ontario, Canada, aimed at reducing wait times for a definitive diagnosis of lymphoma,64 the expedited one-stop prostate cancer diagnosis using advanced imaging and biopsy techniques in a health institution (name not reported) in the USA, aimed at expediting prostate cancer diagnosis.83 There was also the Swedish Diagnostic Center at the Central Hospital of Kristianstad, Sweden, introduced as a separate outpatient unit within the Department of Internal Medicine to expedite diagnostics,80 the Partners for Cancer Care and Prevention action plan in Cali, Columbia, aimed at improving access to a coordinated programme of screening and early diagnosis of breast and cervical cancers in three healthcare centres that serve subsidised populations,75 the dermatology-led quality improvement initiatives in Gaborone, Botswana, aimed at improving multispecialty care coordination,94 and the culturally sensitive, narrative self-help intervention named PERANTARA (PEngantar peRAwataN kesehaTAn payudaRA (translated as introduction to breast health treatment)) across four hospitals in Bandung, West Java, Indonesia, aimed at reducing time to diagnosis in women with breast cancer symptoms.77 In addition to the above, one unpublished article on the Accelerate, Coordinate, Evaluate programme in the UK was identified.100 This programme was an early cancer diagnosis initiative and focused on testing innovations that either identify individuals at high risk of cancer earlier or streamline diagnostic pathways.
The ineffective interventions were the standardised care diagnostic pathway at the Department of Clinical Pathology, Akademiska University Hospital in Uppsala, Sweden (introduced by the Swedish health authorities to eliminate unwanted delay in the diagnostics of melanoma)10 and the 4-week national lung cancer symptom awareness campaign in Wales, UK, aimed at increasing urgent suspected cancer referrals and clinical outcomes.53
Multidisciplinary team
Three multidisciplinary team lung cancer approaches were identified from published articles: from the USA68 85 and Australia.50 The focus and metrics for assessment of the effectiveness of the approaches varied across the publications. One approach from the USA was found to be effective,68 whereas the others were found to be ineffective. The effective approach was the lung cancer strategist programme, a thoracic surgeon-guided, multidisciplinary (disciplines not reported) care programme in hospitals in Massachusetts, USA, aimed at improving timeliness of lung cancer diagnosis and treatment.68 The ineffective approaches were the pre-diagnosis multidisciplinary tumour board (physicians from radiology, medical and radiation oncology, and pulmonary medicine) discussions in a clinic in Cleveland, USA aimed at improving the timeliness of diagnostic evaluation in lung cancer,85 and the Victorian lung cancer service redesign project in Victoria, Australia, which involved multidisciplinary (patients, governance, administration, clinicians and health information services) evaluation aimed at quality improvement collaborative on timeliness and management in lung cancer.50 In addition, nine unpublished articles from the UK were identified.99 101–103 106 108 109 112 These included four articles regarding a ‘straight to CT access’ pathway, on community pharmacy direct referral to lung cancer pathway, rapid colorectal diagnostic pathway, and optometrist direct referral to neuroscience pathway. All but the chest X-ray pathway109 were found to be effective.
Standardised care pathways
Eleven published articles on standardised care pathways were identified.11 12 26 35 39 41 49 59 63 70 71 These articles were focused on varied cancer types (four each for multiple cancers, and one each for ear-nose-throat, urinary tract and gastrointestinal cancers). Three articles were from Denmark,26 39 41 two from the UK35 70 and one each from Canada,59 Norway,49 Sweden,63 Spain12 and Saudi Arabia.11 The publications were on adult patient populations with one also involving paediatric patients. The focus and metrics for assessment of the effectiveness of the pathways varied across the publications. The main effective pathways were the national diagnostic cancer pathway in Norway, with recommended maximum limits for time spent in the diagnostic process as well as mandatory reporting of the actual time intervals for all patients with suspected lung cancer,49 and the standardised triage process in the Southeastern Ontario, Canada, which entailed a two times-weekly nurse–physician triage, preordered staging tests and scheduling according to urgency, redirection and recommendations for inappropriate referrals, and new small nodule clinic.59 Other main effective pathways were the standardised diagnostic pathway for suspected urothelial cancer initiated by primary healthcare providers and specialists in Skane County, Sweden and comprises CT urography, urinary cytology and cystoscopy,63 the early colonoscopy track (within 30 days from referral) in a tertiary referral hospital in Tenerife, Spain,12 and the fast-track cancer care pathway in Denmark (national), with maximum acceptable time thresholds from referral to diagnosis and treatment.39 In addition, two unpublished articles from Canada111 and the UK98 focusing on breast and lung cancers, respectively, were identified. These were the Alberta Health Services Diagnostic Assessment Pathway and the Somerset Integrated Lung Cancer Pathway. While the Canadian pathway was found to be effective, the pathway from the UK was not effective.
Support for PCP
There were four publications on support for PCP, all from the UK.27 31 48 97 Two were focused on multiple cancer types, and one each focused on gastrointestinal and brain cancers. The publications were on adult patient populations with one being also involving paediatric patients. The focus and metrics for assessment of the effectiveness of the support packages (all educational and informational) varied across the publications. None of the support packages was found to be effective, with the identified common theme being a lack of awareness of referral guidelines and associated knowledge by general practitioners (GPs). These ineffective support packages were the use of the Kernick and NICE guidelines as evidence-based support to assist primary care physicians in identifying patients most at risk of having a brain tumour, but also on the fastest route to achieve diagnosis (eg, direct access imaging vs urgent secondary care referral) in Scotland, the UK,97 the use of the national cancer waiting times monitoring dataset for system performance assessment by primary care physicians in England, the UK,27 and the use of safety netting by primary care physicians in Oxfordshire, UK to ensure that patients are monitored until their symptoms or signs are explained, and to guard against delays in diagnosis.31
Target or benchmark for wait times
There were eight published articles related to targets or benchmarks for wait times.15 42 43 69 73 81 88 96 Three of these articles were from the UK,69 73 81 two articles from Australia42 88 and one article each from China,43 Sweden96 and New Zealand.15 These publications were focused on varied cancer types (two each for multiple, lung and gastrointestinal cancers, and one each for prostate and skin cancers), and were on adult patient populations, with one publication involving paediatric patients. The focus and metrics for assessment of the effectiveness of the target or benchmarks varied across the publications, and all but two targets/benchmarks15 88 were found to be effective. The effective targets or benchmarks were the 28-day faster diagnosis standard in the National Health Service England, UK, defined as the time within which the patient is informed whether they do or do not have cancer,73 the fast-track diagnostic workup for men with suspected prostate cancer at the Urology Department at Orebro University Hospital in Sweden, which entailed targeting the shortest possible waiting-time for a diagnostic workup process,96 and the optimal timeframes for referral and diagnosis of lung lesion at Latrobe Regional Hospital in Victoria, Australia established by the National Cancer Expert Reference Group as part of the optimal care pathway for people with lung cancer.42 The ineffective targets or benchmarks were the New Zealand Ministry of Health’s ‘faster cancer treatment’ standards of service provision for melanoma patients, with a target of histopathological diagnosis of melanoma reported within five working days in 80% of cases, and all cases reported in 10 working days.15 In addition, two unpublished articles from Canada105 and the UK107 focusing on multiple cancers were identified, and these were the ‘2-week wait’ benchmark in the UK (already discussed under rapid referral pathways) and the Canadian Breast Cancer Screening Network targets for diagnostic intervals: ≥90% of abnormal screens to be resolved within 5 weeks if no biopsy is required and ≥90% within 7 weeks if a tissue biopsy is required.
Innovative interventions to enhanced care in cancer pre-diagnosis phase
This review identified 17 published articles related to technological interventions for enhanced care in the prediagnosis phase of cancer.16 21 22 29 37 38 51 57 58 62 65 66 79 82 87 89 91 Ten of these articles were from the UK,22 29 37 38 51 57 62 65 66 91 two articles were from New Zealand79 82 and one article each was from Denmark,89 Netherlands,21 Italy,16 India87 and Spain.58 These publications focused on varied cancer types in adult patient populations, with two also involving paediatric patients. The interventions had little patient input in their design, development or implementation. The focus and metrics for assessment of the effectiveness of the interventions varied across the publications. The main identified interventions were the use of teledermatology in skin cancer diagnosis. This involved the taking of images, including dermoscopy by GPs and sending them for evaluation to specialised dermatologists.38 62 79 89 The process is embedded in an e-referral system developed in Auckland, New Zealand for suspected skin malignancy,82 and included teledermatology images triaged as confirmed, likely or suspected melanoma, the use of a web-based referral tool for head and neck cancers at two different hospitals in Birmingham, West Midlands, and Wexham, Berkshire, UK.51 There was also the use of the Digitally Assembled Referral Toolkit for 2-week referral, accessible via a cloud-based template, which contained new referral forms native to GP clinical systems in the UK.29 Additionally, there was the use of an electronic straight-to-test pathway at a large tertiary referral hospital in England, UK to remove hospital-based triage from suspected colorectal cancer pathways; this allows GPs to book tests supported by a decision aid based on the NICE guidance, thus, eliminating the need for a standard referral form or triage process.65 Further, there was the use of electronic clinical decision support for melanoma in four general practices in the Southeast of England, UK, which involved the use of an electronic-based 7-point checklist to assess pigmented lesions,66 the use of machine learning algorithms in Newcastle, UK to classify patients referred on the 2-week wait pathway for suspected head and neck cancer into different diagnostic groups, although very broad ones: cancer and non-cancer,57 the use of nurse-led assessments to evaluate certain groups of patients suspected to have bowel cancer in England, the UK,22 and the use of varied smartphone-based skin and oral self-monitoring and screening applications, in England, UK91 and in the India,87 respectively. In addition, two unpublished articles from the UK were identified.106 110 These were for a cancer decision support tool (computer-based programmes integrated into a GP’s usual patient management system) in Gateshead, London, and a clinical web portal (CWP) electronic system in Manchester, England, with the fundamental part of the CWP being that local clinicians had to take personal responsibility for data input.
Performance metrics to measure improvements in suspicion to diagnosis phase
Varied performance metrics were identified by this review. The main metrics are summarised according to intervention type (online supplemental appendix 9). While performance metrics appear to be mainly intervention-dependent, time from presentation in primary care to diagnosis and from referral from primary care to specialist consultation, appear to be the most consistent metrics used for evaluation. Performance metrics to measure patients’ experience mainly centred on patients’ satisfaction and QoL.
Specific considerations for underserved populations
Four published articles focused on issues related specifically to underserved populations, with all focused on remote/rural populations.18 30 60 88 These publications were from the UK,60 Australia30 88 and Mexico.18 A fifth publication only used the patients’ area of residence as part of their model.95 All of the publications were on multiple cancer types and adult populations, although one included a paediatric population. The specific considerations for underserved populations and the evidence regarding them included a publication from Scotland, the UK, a national audit of cancer diagnosis in Scottish and English general practices, exploring and comparing patient characteristics, diagnostic intervals and routes to diagnosis,60 the publication from New South Wales, Australia on a study that examined geographic variations in time intervals leading up to treatment for head and neck cancer, with assessment of differences based on remoteness of residence (regional/remote or metropolitan) at two tertiary referral centres,88 a publication from Mexico City, Mexico on evaluation of a patient navigation programme to reduce referral time to cancer centres for underserved patients with a suspicion or diagnosis of cancer at a public general hospital,18 and a publication from Western Australia, a cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural patients with cancer with the aim of measuring the effect of community-based symptom awareness and general practice-based educational interventions on the time to diagnosis in rural patients presenting with breast, prostate, colorectal or lung cancer.30
Discussion
This scoping review of 88 published and 16 unpublished documents from January 2017 to January 2021 summarises the evidence on current interventions focused on improving accurate and timely cancer diagnosis among symptomatic individuals. The identified articles were from varied study designs including case–control (most common), cross-sectional, before-and-after, and mixed methods studies, and randomised controlled trials. There was little evidence to suggest that patients were involved in the design, development or implementation of interventions to enhanced care in cancer prediagnosis phase.
The evidence suggests that interventions focused on improving accurate and timely cancer diagnosis among symptomatic individuals are active topics of research. The UK appears to be championing this area of research, contributing about half of all identified published literature and 83% of the identified unpublished literature. Of the specific cancer patient types, patients with lung cancer appear to be the most researched, ranking highest among the patient populations of published and unpublished literature. Of the studied interventions, rapid referral pathways and technology for supporting and streamlining the diagnosis process were the two most reported interventions. Overall, varied national and regional centralised or coordinated diagnostic services, interventions to enhance diagnostic services, multidisciplinary team approaches, patient navigation approaches, rapid referral pathways, standardised care pathways, support for PCP, target or benchmarks, technologies to support diagnosis process, and insights regarding variations between remote/rural and urban populations have been reported although there were no articles that focused specifically on Indigenous populations. Many of these intervention types could be adapted to suit different health systems and jurisdictions around the world.
The interventions mostly comprised multiple interventions/changes to the healthcare pathway. As such, the interventions examined varied widely across the studies. This was true even when applied to the same cancer patient populations and in the same jurisdictions/countries, including those where an intervention was part of the standard care pathway. As such, it is difficult, perhaps impossible, to identify one main approach alone that drives an intervention. Methodological approaches also varied significantly with regard to outcome assessment. A common theme among the effective centralised or coordinated diagnostic services, interventions to enhance diagnostic services, patient navigation approaches and standardised care pathways is multidisciplinary collaboration and the involvement of a nurse navigator.
The findings from this scoping review compare considerably with those of the previously summarised evidence (prior to the ongoing COVID-19 pandemic) not included in this review.9 However, while the previous evidence summary identified similar leading interventions to enhance seamless and coordinated cancer care in symptomatic individuals, intervention effectiveness was not summarised to enable comparison with the findings from this current review. As a result, assessment of the potential impact of the COVID-19 pandemic on intervention effectiveness was not possible; despite reports of decline and delays in cancer diagnosis of symptomatic individuals even in jurisdictions that use interventions that have been found to be effective from this review.114 115 A survey by the Canadian Cancer Survivor Network showed that 54% of those surveyed (with about 75% of prediagnosis and recently diagnosed patients among them) have had their cancer care appointments cancelled, postponed or rescheduled because of COVID-19.116 Further, a modelling study in England, by Maringe and colleagues concluded that substantial increases should be expected in the number of avoidable cancer deaths as a result of diagnostic delays due to the COVID-19 pandemic.117 The conclusions of the available evidence reviews suggest that cancer screening programmes and diagnoses in symptomatic individuals, have been clearly interrupted since the onset of the COVID-19 pandemic, with delayed diagnosis and marked increases in the numbers of avoidable cancer deaths.118 119
It was difficult to determine a specific intervention or a stand-alone approach to an intervention from this scoping review. It was also difficult to assess the true effectiveness of many of the interventions, especially considering the differing composite nature of the interventions, the fact that the evidence is mostly from observational studies, and the range of outcome measures used to measure effectiveness. While many of the interventions could be adapted to suit different health systems and jurisdictions, emphasis should be on the context and the strengths and limitations of the individual health system, and a clear evidence-based performance metric for appropriate evaluation of effectiveness of an intervention ought to be determined a priori. Diagnosing cancer faster and more accurately at an earlier stage is a key priority of the 2019–2029 Canadian Strategy for Cancer Control.120 Over the next 5 years, the Canadian Partnership Against Cancer will leverage findings from this scoping review, as one of several inputs, and partner with Canadian jurisdictions to continue to test innovative models of care that expedite cancer diagnosis, especially for Indigenous and underserved populations.
Limitations and merits
There are some limitations to this study. The literature search was developed by a knowledge synthesis librarian and peer-reviewed by an independent knowledge synthesis librarian using the PRESS checklist. We searched appropriate databases and websites for literature, and adhered to known guidelines and standards in the conduct and reporting of the review. Even so, the literature search was limited to evidence from the last 4 years and only evidence from English-language publications and organisational websites. As such, potentially eligible articles could have been missed.
The eligibility criteria for inclusion were not limited to only comparative studies. This meant that the focus of some of the included studies was not specifically on the assessment of effectiveness of an intervention and therefore, effectiveness may have been underreported for some interventions. Moreover, an intervention’s effectiveness assessment was based solely on author-determined outcome, which may or may not have been an appropriate outcome for assessing effectiveness of certain interventions. As such, an intervention that appeared effective in a study may be ineffective in another study depending on the assessed outcome, with no clear reason for such a discrepancy. Furthermore, this review did not assess effectiveness of interventions across cancer patient types and jurisdictions/regions. This would have allowed assessment of any differences in intervention effectiveness by patient type and study jurisdiction. Finally, and in line with the JBI’s guidance for the conduct of scoping reviews, we did not attempt to provide an assessment of the quality of the evidence and, as such, the risk of bias in randomised controlled trials and quality assessment of observational studies, including assessment for important potential biases such as selection, case ascertainment and measurement biases, and potential confounders in studies were not considered in this review; hence, the findings on effectiveness are not conclusive of the performance of the interventions.
Conclusions
The evidence suggests that interventions focused on improving accurate and timely cancer diagnosis among symptomatic individuals are active topics of research, particularly in lung cancer patient populations, and that the UK is championing this area of research. While the themes of the studied interventions are similar, the interventions differ in many ways within the same intervention group. Multidisciplinary cooperation and involvement of a nurse navigator appeared to be unique features of many of the effective interventions. Canadian and other jurisdictions can leverage these lessons learnt to develop and implement strategies adapted to local health system needs to improve the cancer prediagnosis phase. Future research should examine the effectiveness of the interventions identified through this review.
Supplementary Material
Footnotes
Contributors: Conceptualisation (AP, JS, WZ, ACT); methodology (GNO, AP, JS, WZ, ACT and AMA-S); data acquisition (GNO, OLTL, VKR, LC, NA and AMA-S); formal analysis (GNO); draft manuscript (GNO and AMA-S); final manuscript revisions (GNO, OLTL, VKR, LC, NA, AP, JS, SRK, RL, WZ, ACT and AMA-S); guarantor (GNO and AMA-S). The corresponding author (the manuscript’s guarantor) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded in part by the Canadian Partnership Against Cancer (the Partnership) and the Canadian Institutes of Health Research (CIHR) under the Strategy for Patient Oriented-Research (SPOR) initiative, through the SPOR Evidence Alliance. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.The Canadian Cancer Society . Cancer statistics at a glance, 2021. Available: https://bit.ly/2OjjxOO
- 4.Smetana K, Lacina L, Szabo P, et al. Ageing as an important risk factor for cancer. Anticancer Res 2016;36:5009–17. 10.21873/anticanres.11069 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Fact sheets: cancer, 2018. Available: https://bit.ly/3c0xaL4
- 6.Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020;18:2119–26. 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- 7.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 8.McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 9.Canadian Partnership Against Cancer . Leading practices to create a seamless patient experience for the pre-diagnosis phase of care: an environmental scan. Toronto, Canada: Canadian Partnership Against Cancer, 2018. [Google Scholar]
- 10.Agnarsdóttir M, Päären H, Vassilaki I. The impact of standardized care pathway on reporting time for invasive melanoma - results from one pathology department in Sweden. Ups J Med Sci 2019;124:260–4. 10.1080/03009734.2019.1675102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almuammar A. Primary health care factors associated with late presentation of cancer in Saudi Arabia. J Radiother Pract 2020;19:71–5. 10.1017/S1460396919000232 [DOI] [Google Scholar]
- 12.Alonso-Abreu I, Alarcón-Fernández O, Gimeno-García AZ, et al. Early colonoscopy improves the outcome of patients with symptomatic colorectal cancer. Dis Colon Rectum 2017;60:837–44. 10.1097/DCR.0000000000000863 [DOI] [PubMed] [Google Scholar]
- 13.Antel K, Louw VJ, Maartens G, et al. Diagnosing lymphoma in the shadow of an epidemic: lessons learned from the diagnostic challenges posed by the dual tuberculosis and HIV epidemics. Leuk Lymphoma 2020;61:1–5. 10.1080/10428194.2020.1815016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arhi CS, Burns EM, Bottle A, et al. Delays in referral from primary care worsen survival for patients with colorectal cancer: a retrospective cohort study. Br J Gen Pract 2020;70:e463–71. 10.3399/bjgp20X710441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J 2017;130:54–61. [PubMed] [Google Scholar]
- 16.Cazzaniga S, Castelli E, Di Landro A, et al. Mobile teledermatology for melanoma detection: assessment of the validity in the framework of a population-based skin cancer awareness campaign in northern Italy. J Am Acad Dermatol 2019;81:257–60. 10.1016/j.jaad.2019.02.036 [DOI] [PubMed] [Google Scholar]
- 17.Chapman C, Thomas C, Morling J, et al. Early clinical outcomes of a rapid colorectal cancer diagnosis pathway using faecal immunochemical testing in Nottingham. Colorectal Dis 2020;22:679–88. 10.1111/codi.14944 [DOI] [PubMed] [Google Scholar]
- 18.Chavarri-Guerra Y, Soto-Perez-de-Celis E, Ramos-López W, et al. Patient navigation to enhance access to care for underserved patients with a suspicion or diagnosis of cancer. Oncologist 2019;24:1195–200. 10.1634/theoncologist.2018-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.M C, Ic C, S S, Chng M, Coulter IC, Surash S. Impact of the updated NICE referral pathway for patients with suspected brain cancer on a neuroscience service. Br J Neurosurg 2020:1–5. 10.1080/02688697.2020.1823317 [DOI] [PubMed] [Google Scholar]
- 20.Christensen HM, Huniche L. Patient perspectives and experience on the diagnostic pathway of lung cancer: a qualitative study. SAGE Open Med 2020;8:2050312120918996. 10.1177/2050312120918996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung Y, van der Sande AAJ, de Roos KP, et al. Poor agreement between the automated risk assessment of a smartphone application for skin cancer detection and the rating by dermatologists. J Eur Acad Dermatol Venereol 2020;34:274–8. 10.1111/jdv.15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cock K, Evans D, Collins R. Nurse-Led service development for suspected bowel cancer. Cancer Nursing Practice 2017;16:28–31. 10.7748/cnp.2017.e1371 [DOI] [Google Scholar]
- 23.Common JL, Mariathas HH, Parsons K, et al. Reducing wait time for lung cancer diagnosis and treatment: impact of a multidisciplinary, centralized referral program. Can Assoc Radiol J 2018;69:322–7. 10.1016/j.carj.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 24.Cotton C, Mahut C, Blyth J, et al. Using lean to improve wait time performance in diagnostic assessment for lung cancer. Healthc Q 2020;22:59–63. 10.12927/hcq.2020.26082 [DOI] [PubMed] [Google Scholar]
- 25.Creak A. Prospective cohort of referrals to a cancer of unknown primary clinic, including direct access from primary care. Clin Oncol 2020;32:e87–92. 10.1016/j.clon.2019.09.059 [DOI] [PubMed] [Google Scholar]
- 26.Dahl TL, Vedsted P, Jensen H. The effect of standardised cancer pathways on Danish cancer patients' dissatisfaction with waiting time. Dan Med J 2017;64. [PubMed] [Google Scholar]
- 27.Di Girolamo C, Walters S, Gildea C, et al. Can we assess cancer waiting time targets with cancer survival? a population-based study of individually linked data from the National cancer waiting times monitoring dataset in England, 2009-2013. PLoS One 2018;13:e0201288. 10.1371/journal.pone.0201288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drudge-Coates L, Khati V, Ballesteros R, et al. A nurse practitioner model for the assessment of suspected prostate cancer referrals is safe, cost and time efficient. Ecancermedicalscience 2019;13:994. 10.3332/ecancer.2019.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastham R, Duffy S, Foster C, et al. Dart: a new look at the 2-week wait suspected cancer referral process. Br J Gen Pract 2017;67:560. 10.3399/bjgp17X693713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emery JD, Gray V, Walter FM, et al. The improving rural cancer outcomes trial: a cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural cancer patients in Western Australia. Br J Cancer 2017;117:1459–69. 10.1038/bjc.2017.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans J, Ziebland S, MacArtney JI, et al. Gps' understanding and practice of safety netting for potential cancer presentations: a qualitative study in primary care. Br J Gen Pract 2018;68:e505–11. 10.3399/bjgp18X696233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evison M, Hewitt K, Lyons J, et al. Implementation and outcomes of the rapid programme: addressing the front end of the lung cancer pathway in Manchester. Clin Med 2020;20:401–5. 10.7861/clinmed.2019-0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezer N, Navasakulpong A, Schwartzman K, et al. Impact of rapid investigation clinic on timeliness of lung cancer diagnosis and treatment. BMC Pulm Med 2017;17:178. 10.1186/s12890-017-0504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallon M, Adil MT, Ahmed K, et al. Impact of 'two-week wait' referral pathway on the diagnosis, treatment and survival in upper and lower gastrointestinal cancers. Postgrad Med J 2019;95:470–5. 10.1136/postgradmedj-2019-136507 [DOI] [PubMed] [Google Scholar]
- 35.Gardner D, Nixon IJ. An analysis of waiting times in patients with thyroid cancer. Surg 2020;18:e51–4. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy M, Ryan D, Clarke S, et al. Optimal timing of CT scanning in the rapid access lung cancer clinic. Ir Med J 2020;113:121. [PubMed] [Google Scholar]
- 37.Hirst Y, Lim AWW,. Acceptability of text messages for safety netting patients with low-risk cancer symptoms: a qualitative study. Br J Gen Pract 2018;68:e333–41. 10.3399/bjgp18X695741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt WTN, Ali L, Marder H, et al. A service evaluation between 2-week wait (2WW) skin cancer referrals via teledermatology and the standard face-to-face pathway at a teaching hospital. Clin Exp Dermatol 2020;45:473–6. 10.1111/ced.14137 [DOI] [PubMed] [Google Scholar]
- 39.Iachina M, Jakobsen E, Fallesen AK, et al. Transfer between hospitals as a predictor of delay in diagnosis and treatment of patients with Non-Small Cell Lung Cancer - a register based cohort-study. BMC Health Serv Res 2017;17:267. 10.1186/s12913-017-2230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson L, Atkin K, Sheridan R, et al. Non-attendance at urgent referral appointments for suspected cancer: a qualitative study to gain understanding from patients and GPs. Br J Gen Pract 2019;69:e850–9. 10.3399/bjgp19X706625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen H, Tørring ML, Vedsted P. Prognostic consequences of implementing cancer patient pathways in Denmark: a comparative cohort study of symptomatic cancer patients in primary care. BMC Cancer 2017;17:627. 10.1186/s12885-017-3623-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeyakumar HS, Wright A. Improving regional lung cancer optimal care pathway compliance through a rapid-access respiratory clinic. Intern Med J 2020;50:805–10. 10.1111/imj.14465 [DOI] [PubMed] [Google Scholar]
- 43.Jiang T, Ren S, Li X, et al. The changing diagnostic pathway for lung cancer patients in Shanghai, China. Eur J Cancer 2017;84:168–72. 10.1016/j.ejca.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 44.Jiang L, Gilbert J, Langley H, et al. Is being diagnosed at a dedicated breast assessment unit associated with a reduction in the time to diagnosis for symptomatic breast cancer patients? Eur J Cancer Care 2018;27:e12864. 10.1111/ecc.12864 [DOI] [PubMed] [Google Scholar]
- 45.Jones JA, Catton J, Howard G, et al. Impact of straight to test pathways on time to diagnosis in oesophageal and gastric cancer. BMJ Open Qual 2018;7:e000328. 10.1136/bmjoq-2018-000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyce K, Zermanos T, Badrinath P. Factors associated with variation in emergency diagnoses of cancer at general practice level in England. J Public Health 2020:fdaa142. 10.1093/pubmed/fdaa142 [DOI] [PubMed] [Google Scholar]
- 47.Kassirian S, Dzioba A, Hamel S, et al. Delay in diagnosis of patients with head-and-neck cancer in Canada: impact of patient and provider delay. Curr Oncol 2020;27:e467–77. 10.3747/co.27.6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kidney E, Greenfield S, Berkman L, et al. Cancer suspicion in general practice, urgent referral, and time to diagnosis: a population-based GP survey nested within a feasibility study using information technology to flag-up patients with symptoms of colorectal cancer. BJGP Open 2017;1:bjgpopen17X101109. 10.3399/bjgpopen17X101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laerum D, Brustugun OT, Gallefoss F, et al. Reduced delays in diagnostic pathways for non-small cell lung cancer after local and national initiatives. Cancer Treat Res Commun 2020;23:100168. 10.1016/j.ctarc.2020.100168 [DOI] [PubMed] [Google Scholar]
- 50.Geraldine L, Peter B, Heather D, Largey G, Briggs P, Davies H, et al. The Victorian lung cancer service redesign project: impacts of a quality improvement collaborative on timeliness and management in lung cancer. Intern Med J 2020:08:08. 10.1111/imj.15043 [DOI] [PubMed] [Google Scholar]
- 51.Lau K, Wilkinson J, Moorthy R. A web-based prediction score for head and neck cancer referrals. Clin Otolaryngol 2018;43:15:15. 10.1111/coa.13098 [DOI] [PubMed] [Google Scholar]
- 52.Laudicella M, Walsh B, Burns E, et al. What is the impact of rerouting a cancer diagnosis from emergency presentation to GP referral on resource use and survival? Evidence from a population-based study. BMC Cancer 2018;18:394. 10.1186/s12885-018-4274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCutchan G, Smits S, Ironmonger L, et al. Evaluation of a national lung cancer symptom awareness campaign in Wales. Br J Cancer 2020;122:491–7. 10.1038/s41416-019-0676-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKevitt EC, Dingee CK, Leung S-P, et al. Reduced time to breast cancer diagnosis with coordination of radiological and clinical care. Cureus 2017;9:e1919. 10.7759/cureus.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKevitt E, Dingee C, Warburton R, et al. Patient navigation reduces time to care for patients with breast symptoms and abnormal screening mammograms. Am J Surg 2018;215:805–11. 10.1016/j.amjsurg.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 56.Moodley J, Cairncross L, Naiker T, et al. From symptom discovery to treatment - women's pathways to breast cancer care: a cross-sectional study. BMC Cancer 2018;18:312. 10.1186/s12885-018-4219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moor JW, Paleri V, Edwards J. Patient classification of two-week wait referrals for suspected head and neck cancer: a machine learning approach. J Laryngol Otol 2019;133:875–8. 10.1017/S0022215119001634 [DOI] [PubMed] [Google Scholar]
- 58.Moreno-Ramírez D, Raya-Maldonado J, Morales-Conde M, et al. Increasing frequency of seborrheic keratosis diagnoses as a favorable consequence of Teledermatology-Based skin cancer screening: a cross-sectional study of 34,553 patients. Am J Clin Dermatol 2017;18:681–5. 10.1007/s40257-017-0283-z [DOI] [PubMed] [Google Scholar]
- 59.Mullin MLL, Tran A, Golemiec B, et al. Improving timeliness of lung cancer diagnosis and staging investigations through implementation of standardized triage pathways. JCO Oncol Pract 2020;16:e1202–8. 10.1200/JOP.19.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murchie P, Adam R, McNair E, et al. Cancer diagnosis in Scottish primary care: results from the National cancer diagnosis audit. Eur J Cancer Care 2020;29:e13234. 10.1111/ecc.13234 [DOI] [PubMed] [Google Scholar]
- 61.Neal RD, Barham A, Bongard E, et al. Immediate chest X-ray for patients at risk of lung cancer presenting in primary care: randomised controlled feasibility trial. Br J Cancer 2017;116:293–302. 10.1038/bjc.2016.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholson P, Macedo C, Fuller C, et al. Patient satisfaction with a new skin cancer teledermatology service. Clin Exp Dermatol 2020;45:691–8. 10.1111/ced.14191 [DOI] [PubMed] [Google Scholar]
- 63.Nilbert M, Bläckberg M, Ceberg J, et al. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scand J Urol 2018;52:237–43. 10.1080/21681805.2018.1498124 [DOI] [PubMed] [Google Scholar]
- 64.Nixon S, Bezverbnaya K, Maganti M, et al. Evaluation of lymphadenopathy and suspected lymphoma in a lymphoma rapid diagnosis clinic. JCO Oncol Pract 2020;16:e29–36. 10.1200/JOP.19.00202 [DOI] [PubMed] [Google Scholar]
- 65.Orchard P, Arvind N, Wint A, et al. Removing hospital-based triage from suspected colorectal cancer pathways: the impact and learning from a primary care-led electronic straight-to-test pathway. BMJ Qual Saf 2021;30:11:11. 10.1136/bmjqs-2019-009975 [DOI] [PubMed] [Google Scholar]
- 66.Pannebakker MM, Mills K, Johnson M, et al. Understanding implementation and usefulness of electronic clinical decision support (eCDS) for melanoma in English primary care: a qualitative investigation. BJGP Open 2019;3:bjgpopen18X101635. 10.3399/bjgpopen18X101635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearson C, Poirier V, Fitzgerald K, et al. Cross-Sectional study using primary care and cancer registration data to investigate patients with cancer presenting with non-specific symptoms. BMJ Open 2020;10:e033008. 10.1136/bmjopen-2019-033008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips WW, Hofferberth SC, Armitage J, et al. PD01.19 lung cancer Strategist program: a novel care delivery model to improve timeliness of diagnosis and treatment. Journal of Thoracic Oncology 2019;14:S1140. 10.1016/j.jtho.2019.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piano M, Black G, Amelung D, et al. Exploring public attitudes towards the new faster diagnosis standard for cancer: a focus group study with the UK public. Br J Gen Pract 2019;69:e413–21. 10.3399/bjgp19X702677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price S, Spencer A, Zhang X, et al. Trends in time to cancer diagnosis around the period of changing national guidance on referral of symptomatic patients: a serial cross-sectional study using UK electronic healthcare records from 2006-17. Cancer Epidemiol 2020;69:101805. 10.1016/j.canep.2020.101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rankin NM, York S, Stone E, et al. Pathways to lung cancer diagnosis: a qualitative study of patients and general practitioners about diagnostic and pretreatment intervals. Ann Am Thorac Soc 2017;14:742–53. 10.1513/AnnalsATS.201610-817OC [DOI] [PubMed] [Google Scholar]
- 72.Round T, Gildea C, Ashworth M, et al. Association between use of urgent suspected cancer referral and mortality and stage at diagnosis: a 5-year national cohort study. Br J Gen Pract 2020;70:e389–98. 10.3399/bjgp20X709433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sagar A, Mai DVC, Divya GS, et al. A colorectal straight-to-test cancer pathway with general-practitioner-guided triage improves attainment of the 28-day diagnosis target and increases outpatient clinic capacity. Colorectal Dis 2021;23:19:19. 10.1111/codi.15410 [DOI] [PubMed] [Google Scholar]
- 74.Sandager M, Jensen H, Lipczak H, et al. Cancer patients' experiences with urgent referrals to cancer patient pathways. Eur J Cancer Care 2019;28:e12927. 10.1111/ecc.12927 [DOI] [PubMed] [Google Scholar]
- 75.Sardi A, Orozco-Urdaneta M, Velez-Mejia C, et al. Overcoming barriers in the implementation of programs for breast and cervical cancers in Cali, Colombia: a pilot model. J Glob Oncol 2019;5:1–9. 10.1200/JGO.19.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott SE, Oakley R, Møller H, et al. Tracking cancer occurrence in the 5 years after referral for suspected head and neck cancer. Oral Oncol 2020;109:104955. 10.1016/j.oraloncology.2020.104955 [DOI] [PubMed] [Google Scholar]
- 77.Setyowibowo H, Hunfeld JAM, Iskandarsyah A, et al. A self-help intervention for reducing time to diagnosis in Indonesian women with breast cancer symptoms. Psychooncology 2020;29:696–702. 10.1002/pon.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skevington SM, Long H, Gartland N. Does quality of life feedback promote seeking help for undiagnosed cancer? Qual Life Res 2020;29:1609–19. 10.1007/s11136-020-02431-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snoswell CL, Caffery LJ, Whitty JA, et al. Cost-Effectiveness of skin cancer referral and consultation using Teledermoscopy in Australia. JAMA Dermatol 2018;154:694–700. 10.1001/jamadermatol.2018.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stenman E, Palmér K, Rydén S, et al. Diagnostic spectrum and time intervals in Sweden's first diagnostic center for patients with nonspecific symptoms of cancer. Acta Oncol 2019;58:296–305. 10.1080/0284186X.2018.1537506 [DOI] [PubMed] [Google Scholar]
- 81.Stevenson-Hornby V. A rapid-access diagnostic pathway in suspected pancreatic cancer. Nursing Times 2018;114:13–11. [Google Scholar]
- 82.Sunderland M, Teague R, Gale K, et al. E-referrals and teledermatoscopy grading for melanoma: a successful model of care. Australas J Dermatol 2020;61:147–51. 10.1111/ajd.13230 [DOI] [PubMed] [Google Scholar]
- 83.Tafuri A, Ashrafi AN, Palmer S, et al. One-Stop MRI and MRI/transrectal ultrasound fusion-guided biopsy: an expedited pathway for prostate cancer diagnosis. World J Urol 2020;38:949–56. 10.1007/s00345-019-02835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talwar C, McClune A, Kelly D, et al. Two-Week rule: suspected head and neck cancer referrals from a general medical practice perspective. Br J Oral Maxillofac Surg 2020;58:981–5. 10.1016/j.bjoms.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 85.Thalanayar Muthukrishnan P, Ratnam M, Nguyen M-T, et al. Pre-Diagnosis multidisciplinary tumor board and time to staging in lung cancer: the case Western MetroHealth experience. Cureus 2020;12:e6595. 10.7759/cureus.6595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thanapal MR, Thin N, Alagaratnam S, et al. Straight-to-test colonoscopy: has it improved the detection of colorectal cancer? A 7- year review. Surgeon 2021;19:26:26. 10.1016/j.surge.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 87.Uthoff RD, Song B, Sunny S, et al. Point-Of-Care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS One 2018;13:e0207493. 10.1371/journal.pone.0207493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venchiarutti RL, Clark JR, Palme CE, et al. Influence of remoteness of residence on timeliness of diagnosis and treatment of oral cavity and oropharynx cancer: a retrospective cohort study. J Med Imaging Radiat Oncol 2020;64:261–70. 10.1111/1754-9485.12990 [DOI] [PubMed] [Google Scholar]
- 89.Vestergaard T, Prasad SC, Schuster A, et al. Introducing teledermoscopy of possible skin cancers in general practice in southern Denmark. Fam Pract 2020;37:513–8. 10.1093/fampra/cmaa041 [DOI] [PubMed] [Google Scholar]
- 90.Vijayakumar B, Shahidi M, Thakkar R, et al. A novel 2-week wait lung cancer pathway starting with a telephone consultation, with patient satisfaction survey results. Future Healthc J 2020;7:s15. 10.7861/fhj.7.1.s15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walter FM, Pannebakker MM, Barclay ME, et al. Effect of a skin self-monitoring smartphone application on time to physician consultation among patients with possible melanoma: a phase 2 randomized clinical trial. JAMA Netw Open 2020;3:e200001. 10.1001/jamanetworkopen.2020.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitley EM, Raich PC, Dudley DJ, et al. Relation of comorbidities and patient navigation with the time to diagnostic resolution after abnormal cancer screening. Cancer 2017;123:312–8. 10.1002/cncr.30316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams S, Davies P, Johnson B, et al. A fast track clinic improves diagnosis and treatment times for those investigated for lung cancer in Northland district health board. N Z Med J 2018;131:29–37. [PubMed] [Google Scholar]
- 94.Williams VL, Narasimhamurthy M, Rodriguez O, et al. Dermatology-Driven quality improvement interventions to decrease diagnostic delays for Kaposi sarcoma in Botswana. J Glob Oncol 2019;5:1–7. 10.1200/JGO.19.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fİ Y, Çimen F, Ş A. Factors affecting delay in diagnosis and treatment of lung cancer. Journal of Surgery & Medicine 2020;4:720–4. [Google Scholar]
- 96.Zhu J, Chen R, Davidsson S, et al. Psychological and physiological impacts of a fast-track diagnostic workup for men with suspected prostate cancer: preliminary report from a randomized clinical trial. Cancer Commun 2020;40:239–42. 10.1002/cac2.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zienius K, Chak-Lam I, Park J, et al. Direct access CT for suspicion of brain tumour: an analysis of referral pathways in a population-based patient group. BMC Fam Pract 2019;20:118. 10.1186/s12875-019-1003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ACE Programme . Somerset integrated lung cancer pathway. phases one and two final report final report. In: Group SCC, ed. UK: NHS, 2017: 1–12. [Google Scholar]
- 99.NHS England, Cancer Research UK and Macmillan Cancer Support . Improving diagnostic pathways for patients with suspected colorectal cancer. In: Accelerate CEP, ed. UK: NHS England, Cancer Research UK and Macmillan Cancer Support, 2017: 39. [Google Scholar]
- 100.NHS England . Improving diagnostic pathways for patients with vague symptoms. In: Accelerate CEP, ed. UK. UK: NHS England, 2017: 49. [Google Scholar]
- 101.NHS England, Cancer Research UK and Macmillan Cancer Support . Pharmacy training for early diagnosis of cancer. In: accelerate CEP, ed. UK: NHS England, Cancer Research UK and Macmillan Cancer Support, 2017: 27. [Google Scholar]
- 102.NHS England . Streamlining the lung diagnostic pathway (A87). In: trust CCCGwS, Sussex Healthcare NHS, eds. UK: NHS England, 2017: 14. [Google Scholar]
- 103.NHS England . Streamlining the lung diagnostic pathway (A14). In: Trust H, Mid Sussex CCGwB, Sussex University H, eds. UK: NHS England, 2017: 27. [Google Scholar]
- 104.NHS England, Cancer Research UK and Macmillan Cancer Support . Multidisciplinary Diagnostic Centre (MDC) based pathways for patients with non-specific but concerning symptoms. Interim report. In: Accelerate CEP, ed. UK: NHS England, Cancer Research UK and Macmillan Cancer Support, 2018. [Google Scholar]
- 105.New Brunswick Cancer Network . Cancer system performance 2019. New Brunswick, Canada: Government of New Brunswick, 2020: 36. [Google Scholar]
- 106.Gill B. Improving diagnostic pathways for patients with suspected lung cancer. In: Accelerate CEP, ed. UK: NHS England, Cancer Research UK and Macmillan Cancer Support, 2017. [Google Scholar]
- 107.Hamilton M, Hodgson O, Dai D. Waiting times for suspected and diagnosed cancer patients. 2017-18 annual report. In: NHS, ed. UK: NHS England, 2018: 21. [Google Scholar]
- 108.Robinson S, Fuller E. South Tees optical referral project (STORP). A project summary. In: Accelerate CEP, ed. UK: NHS England, 2017: 14. [Google Scholar]
- 109.Robinson S, Fuller E. A lung health service – Doncaster pharmacy direct referral for chest X-ray. A project summary. In: Accelerate CEP, ed. UK: NHS England, Cancer Research UK and Macmillan Cancer Support, 2017: 12. [Google Scholar]
- 110.Robinson S, Poirier V, Watson S. Using cancer decision support tools to support the early diagnosis of cancer. In: Accelerate CEP, ed. UK: NHS, 2017: 39. [Google Scholar]
- 111.Services AH . Cancer SCN quarterly update. July to September 2018. Canada: Alberta Health Services, 2018. [Google Scholar]
- 112.Stacey C, D S, M E, K S, A T . Implementing new models and standards for earlier and faster diagnosis of cancer. Paper presented at: Health and Care Innovation Expo2018; Manchester, UK 2018. [Google Scholar]
- 113.Inzetta SL, Musarra LL. Breast care access project. Oncology Issues 2018;33:34–45. 10.1080/10463356.2018.1427990 [DOI] [Google Scholar]
- 114.Patt D LG. M D, et al. impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020;4:1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacob L, Loosen SH, Kalder M, et al. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers 2021;13:408–11. 10.3390/cancers13030408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen-See S. Disruption of cancer care in Canada during COVID-19. Lancet Oncol 2020;21:e374. 10.1016/S1470-2045(20)30397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34. 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alkatout I, Biebl M, Momenimovahed Z, et al. Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol 2021;11:675038. 10.3389/fonc.2021.675038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riera R, Bagattini Ângela Maria, Pacheco RL, et al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol 2021;7:311–23. 10.1200/GO.20.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Canadian Partnership Against Cancer . Canadian strategy for cancer control (2019 to 2029): doing together what cannot be done alone. Toronto, Canada: Canadian Partnership Against Cancer 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055488supp001.pdf (178.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.