Abstract
Background
High sodium intake is an important risk factor for hypertension and cardiovascular disease. However, the association between gut microbiota composition and metabolomic profiles with dietary sodium intake and blood pressure (BP) is not well-understood. The metabolome, microbiome, and dietary salt intervention (MetaSalt) study aimed to investigate microbial and metabolomic profiles related to dietary sodium intake and BP regulation.
Methods
This family-based intervention study was conducted in four communities across three provinces in rural northern China in 2019. Probands with untreated prehypertension or stage-1 hypertension were identified through community-based BP screening, and family members including siblings, offspring, spouses, and parents were subsequently included. All participants participated in a 3-day baseline examination with usual diet consumption, followed by a 10-day low-salt diet (3 g/d of salt or 51.3 mmol/d of sodium) and a 10-day high-salt diet (18 g/d of salt or 307.8 mmol/d of sodium). Differences in mean BP levels were compared according to the intervention phases using a paired Student's t-test.
Results
A total of 528 participants were included in this study, with a mean age of 48.1 years, 36.7% of whom were male, 76.8% had a middle school (69.7%) or higher (7.1%) diploma, 23.4% had a history of smoking, and 24.4% were current drinkers. The mean arterial pressure at baseline was 97.2 ± 10.5 mm Hg for all participants, and significantly decreased during the low-salt intervention (93.8 ± 9.3, P < 0.0001) and subsequently increased during the high-salt intervention (96.4 ± 10.0, P < 0.0001).
Conclusions
Our dietary salt intervention study has successfully recruited participants and will facilitate to evaluate the effects of gut microbiota and metabolites on BP regulation in response to sodium burden, which will provide important evidence for investigating the underlying mechanisms in the development of hypertension and subsequent cardiovascular diseases.
Trial registration
The study was registered in the Chinese Clinical Trial Registry database (ChiCTR1900025171).
Keywords: Dietary sodium, Metabolome, Microbiota, Blood pressure, Hypertension
Introduction
Hypertension is a major risk factor for cardiovascular diseases and the leading cause of death and disability, accounted for 19.2% of all deaths and 9.3% of all disability-adjusted life years worldwide in 2019.1, 2, 3 The positive and significant relationship between dietary salt (sodium) intake and blood pressure (BP) has been demonstrated by a series of prospective studies, which suggest that dietary salt interventions could be an effective and low-cost measure to control hypertension.4,5 However, changes in BP in response to sodium intake vary among individuals, known as salt sensitivity, but knowledge of the underlying physiological mechanisms are not completely understood.6
Previously, we conducted the Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study, which identified several susceptibility genes that might play important roles in determining individual BP response to dietary sodium intake, and provided substantial evidence to support a genetic basis for the variation in BP response to salt.6,7 Findings from the GenSalt study suggest that genetic variants in the endothelial system, sympathetic nervous system, and ion and water channels could influence sodium sensitivity of BP.8, 9, 10, 11, 12 Moreover, recent advances have revealed that dietary sodium intake is associated with changes in gut microbiota and circulating or urinary metabolites, and these changes might further influence BP through different pathways.13,14 For example, previous research suggests that excess dietary salt is associated with increases in several intestinal microbes that cause microbiome-induced inflammation, resulting in higher BP in humans and mice.15 Several metabolites that could be affected by the interaction of intestinal microbiota and dietary sodium intake, such as triglycerides, linoleate, lipopolysaccharide, short-chain fatty acids, dihomo-linolenate, bile acids, trimethylamine-N-oxide, sphingomyelins, and uremic toxins, have been associated with BP regulation and cardiovascular health.16, 17, 18, 19 Fumarase, an enzyme in the tricarboxylic acid cycle, was also found to be significantly reduced in a Dahl salt-sensitive rat, a widely used animal model of human salt-sensitive hypertension, suggesting that abnormalities in cellular intermediary metabolism might affect the salt sensitivity of BP.20 To our knowledge, previous findings on the association of dietary sodium intake with microbial and metabolomic profiling were mainly based on animal experiments or observational studies.16,21, 22, 23 Only a few intervention trials have been performed to verify their causal relationship in humans, and none of these trials were conducted in the Chinese population.24,25
Accordingly, the metabolome, microbiome, and dietary salt intervention (MetaSalt) study was designed to identify the key pathways in the gut microbiota and plasma metabolites that may contribute to BP regulation and subsequent cardiovascular diseases. Herein, we present the study protocol as well as the general characteristics of the participants and preliminary findings regarding the levels of mean arterial pressure (MAP), systolic BP (SBP), and diastolic BP (DBP) levels in different intervention phases.
Methods
Ethical approval
The protocol of this study was developed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Fuwai Hospital, and written informed consent was obtained from all participants.
Study design
The MetaSalt study is a family-based, multicenter dietary salt intervention trial conducted in rural areas of northern China. The study was registered in the Chinese Clinical Trial Registry database on August 15, 2019 (registration number: ChiCTR1900025171).
Study sites and population
The MetaSalt study was carried out in four rural field centers in Hebei (Xinle City and Nangong City), Shanxi (Yu County), and Henan (Changge County) provinces in rural northern China. The study sites were selected in terms of their similar geographical, environmental, cultural, and habitual diets. The study participants were recruited from families with a high risk of developing hypertension because of the association with sensitivity to dietary sodium intake.6,26
Participants
The recruitment of participants for the MetaSalt study was conducted in 2019. All participants in the MetaSalt study were rural residents aged 18–60 years living in one of the four study sites. According to the inclusion and exclusion criteria, the potential probands were first identified using both a short questionnaire and community-based BP screening in the selected villages. Family members (parents, spouses, siblings, and offspring) were selected based on the same criteria. To confirm the eligibility of each participant, we carried out the second round of BP screening among the identified participants on the following day. Finally, the confirmed participants were invited to sign an informed consent before they were officially enrolled. According to a sample size calculation conducted before recruitment, a sample size of 456 would be necessary to achieve 90% power to detect a SBP difference of 2.0 mmHg at a significance level (alpha) of 10−5. Therefore, we planned to enroll 500 participants to allow for some participant dropouts.
The inclusion criteria for the participants were: (1) adults aged 18–60 years who provided informed consent; (2) probands: SBP 130–159 mmHg and/or DBP 85–99 mmHg (mean value of three measurements); (3) family members: SBP <160 mmHg and DBP <100 mmHg (mean value of three measurements); (4) no antihypertensive drugs or drugs affecting BP taken within one month before the screening visit; (5) negative urine protein test; (6) normal blood glucose level (fasting blood glucose < 7.0 mmol/L and postprandial blood glucose < 11.1 mmol/L).
The exclusion criteria were: (1) unwilling to sign the informed consent; (2) pregnant; (3) adhering to a low-sodium diet; (4) drinking alcohol almost every day; (5) use of antibiotics or antihypertensive drugs within one month before the screening visit; (6) defecation interval time ≥ 3 d; (7) stage-2 or more serious hypertension patients (SBP ≥ 160 mmHg and/or DBP ≥ 100 mmHg); (8) history of cardiovascular disease, including coronary heart disease, heart failure, stroke, peripheral arterial disease, etc.; (9) history of peptic ulcer disease or liver disease that required treatment during the past 2 years; (10) kidney disease (positive urine protein test) or diabetes (fasting blood glucose ≥ 7.0 mmol/L and postprandial blood glucose ≥ 11.1 mmol/L) or use of insulin or oral hypoglycemic drugs.
Intervention
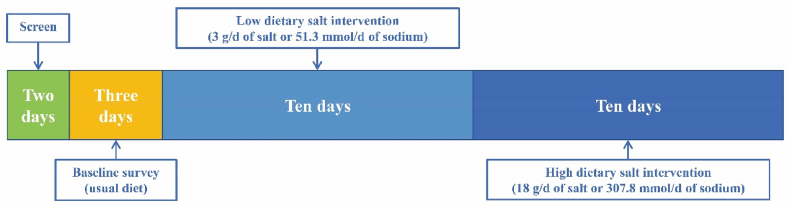
The MetaSalt study was conducted for 25 consecutive days, including two days of screening, three days of baseline observation, ten days of low dietary sodium intervention, and another ten days of high dietary sodium intervention among the participants. A flowchart of the intervention strategy is presented in Fig. 1. During the baseline observation, all participants kept their usual diet, and the intervention was started on the fourth day. First, all participants ate a low-salt diet (3 g/d of salt or 51.3 mmol/d of sodium) from days 4–13, followed by a high-salt diet (18 g/d of salt or 307.8 mmol/d of sodium) from days 14–23. During the intervention period, all participants ate three meals per day (i.e., breakfast, lunch, and dinner) cooked by the study staff at specified canteens. The sodium intake of each participant was strictly controlled through pre-packaged salt that was added to the foods by the study staff. In addition, daily recipes were determined by professional dietitians. Dietary salt intake was equal for all subjects, and their total dietary energy intake was estimated using a food frequency questionnaire.
Fig. 1.
Flowchart of the study.
Data collection and measurements
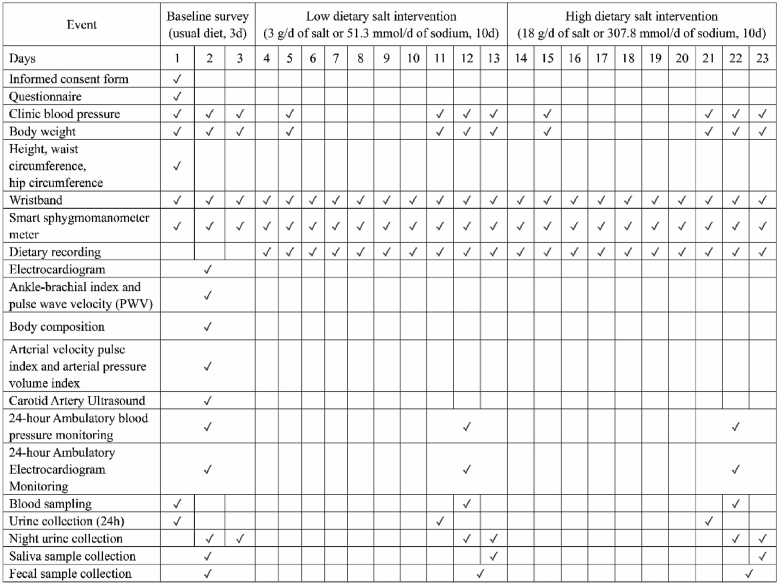
Data collection at each stage of the study is summarized in Fig. 2. During the baseline survey, all participants were interviewed face-to-face by trained investigators to complete a questionnaire regarding their sociodemographic characteristics (such as sex, age, ethnicity, family pedigree, marital status, education, occupation, and family income), medical history, and lifestyle factors (such as physical activity and dietary behaviors). Smoking history was defined as having smoked at least 100 cigarettes in the past, and drinking was defined as having drunk more than 12 times in the past 12 months.
Fig. 2.
Schedule of the MetaSalt study.
BP was measured three times at each clinical visit by trained and certified staff, in accordance with the professional BP monitor instructions (Omron® HBP-1300, Japan). For at least 60 minutes before the measurements, the participants were instructed to refrain from smoking, exercising, eating, taking medicine, or drinking caffeine-containing beverages such as tea, coffee, and colas. Immediately before the measurement, they were instructed to sit in a warm quiet room for five minutes of rest. BP was also self-measured at least once in the morning (after morning urine and before breakfast), once before lunch, and once at night (before sleep) each day during the study period using a smart sphygmomanometer meter (Lifesense® i5, China) that relayed the data automatically to the study staff. Furthermore, 24-hour ambulatory BP monitoring was conducted using a KANG® monitor (KC-2300A, China), a device certified according to the standards of the Association for the Advancement of Medical Instrumentation and the British Hypertension Society.
Various parameters were measured using simple non-invasive devices (Fig. 2). Resting electrocardiogram (ECG) was determined using a 12-lead electrocardiograph (FUKUDA®, Japan), while a 12-lead Holter ECG recorder (Jinco® MIC-12H–3S or Biomedical Instruments® TeleAECG BI9900, China) was used for the 24-hour ECG. The ankle-brachial index and pulse wave velocity were determined using the Omron® BP-203RPEIII (Japan). A portable cardiovascular measuring instrument (PASESA® Japan) was used to measure arterial velocity pulse index and arterial pressure volume index; body composition and body fat percentage were determined using a professional body analyzer (Tanita®, TBF-418B, USA), and the carotid artery ultrasound tests were performed using a portable ultrasound system (Philips®, CX30, the Netherlands).
We also measured the participants’ body weight, height, waist circumference, and hip circumference in the first day of the baseline survey. The participants were required to wear a smart wristband (Lifesense Mambo HR2, China) to collect data on their movement, sleep, and heart rate during the entire study period.
Sample collection and preparation
Fasting whole blood was sampled in both ethylene diamine tetraacetic acid and non-additive blood collection tubes, and the serum and plasma were separately stored at −80 °C until assayed. At baseline and during each intervention phase, one 24 h and two overnight (8 h) urine samples were collected for each participant and stored at −20 °C until assayed. At each stage, we used a saliva microbiome DNA collection kit (Genotek OMR-501, USA) to collect the saliva samples and a gut microbiome DNA collection kit (Genotek OMR-200, USA) to collect the fecal samples, which were stored at −80 °C until assayed. Metabolomic profiling will be analyzed using serum and urine samples, and the compositions of oral and gut microbiota will be determined using saliva and fecal samples and reported in forthcoming manuscripts.
Quality control
A series of quality control measures were taken for the successful implementation of the MetaSalt study. Study staff were trained according to a training program protocol, which included conducting and interpreting the face-to-face interviews. The study staff also had to be well acquainted with the questionnaires, measurements, sample collection, methodologies, and the operation of the instruments, using standard criteria for each participant during the investigation. All observers, who were blinded to the intervention, attended special training and needed to pass an examination on the standard techniques for BP measurement prior to the study. After each interview, all data from the questionnaires were entered into the MetaSalt study database and subjected to a check for completeness, followed by another round of review at the coordinating center in Beijing, and discrepancies or illogical data were sent back to the study sites for corrections. To improve compliance with the study protocol, the study staff organized and held meetings with the local populations to introduce the aims and process of the MetaSalt study, and carried out adequate propaganda through leaflets, handbooks, and local broadcasts before the baseline survey. During the entire study period, all participants were required to have breakfast, lunch, and dinner at specified canteens under the supervision of the investigators, and their food consumption at each meal was recorded. Additionally, an independent coordination center was set up to monitor and review the entire field investigation, and advise the steering committee on modifications to the trial should it become necessary.
Data analysis
The characteristics of participants are described as N (%) for categorical variables and mean ± standard deviation (SD) for continuous variables. Changes in the levels of MAP, SBP, and DBP between baseline and the low-salt intervention and between the low- and high-salt interventions were calculated, and the differences were compared using the paired Student's t-test. Statistical analyses were conducted using R version 3.6.3 (Austria), and a two-tailed P-value < 0.05 was considered statistically significant.
Results
A total of 528 participants from four sites were recruited, including 359 probands and 169 family members. Among the participants, 512 completed the low-salt intervention (dropout rate: 3.03%), and 503 finished the entire study (dropout rate: 4.73%) with complete questionnaire and anthropometric data, as well as all samples (blood, saliva, excrement, and urine).
Table 1 shows the baseline characteristics of the study participants. The mean age of all participants was 48.1 ± 9.3 years, with 48.9 ± 8.6 and 46.4 ± 10.3 years for the probands and their family members, respectively. About 36.7% of the participants were male, 76.8% had a middle school (69.7%) or higher (7.1%) diploma, 23.4% had a history of smoking, and 24.4% were current drinkers. The mean BMI was 26.5 ± 3.6 kg/m2 and the mean waist circumference was 88.7 ± 9.6 cm. The mean heart rate was 75.6 ± 8.9 beats per minute.
Table 1.
Baseline characteristics of the participants.
| Characteristics | All (n = 528) | Probands (n = 359) | Family members (n = 169) |
|---|---|---|---|
| Age, years | 48.1 ± 9.3 | 48.9 ± 8.6 | 46.4 ± 10.3 |
| Male | 194 (36.7) | 136 (37.9) | 58 (34.3) |
| Education | |||
| Primary or lower | 122 (23.3) | 78 (21.9) | 44 (26.2) |
| Middle school | 365 (69.7) | 248 (69.7) | 117 (69.6) |
| Higher | 37 (7.1) | 30 (8.4) | 7 (4.2) |
| History of smoking | 123 (23.4) | 86 (24.1) | 37 (22.0) |
| Drinking | 128 (24.4) | 98 (27.5) | 30 (18.0) |
| Body mass index, kg/m2 | 26.5 ± 3.6 | 27.0 ± 3.6 | 25.5 ± 3.4 |
| Waist circumference, cm | 88.7 ± 9.6 | 90.0 ± 9.8 | 85.7 ± 8.6 |
| Heart rate, beats per minute | 75.6 ± 8.9 | 75.7 ± 8.9 | 75.2 ± 9.1 |
Data were presented as n(%) or mean ± standard deviation.
The mean SBP, DBP, and MAP levels of the participants at baseline and during the low-salt and high-salt interventions are shown in Table 2. The measurements were lower during the low-salt intervention compared with baseline and increased during the high-salt intervention in comparison to the low-salt intervention. Specifically, the mean SBP, DBP, and MAP levels were 129.5 ± 13.7, 81.0 ± 9.8, and 97.2 ± 10.5 mmHg, respectively, at baseline; 124.5 ± 12.0, 78.4 ± 8.8, and 93.8 ± 9.3 mmHg, respectively, during the low-salt intervention; and 129.2 ± 13.6, 80.1 ± 9.2, and 96.4 ± 10.0 mmHg during the high-salt intervention, respectively. Compared to the baseline period, the mean SBP, DBP, and MAP of all participants during the low-salt intervention were significantly lower by −5.0 ± 8.0, −2.5 ± 4.8 and −3.3 ± 5.5 mmHg, respectively (all P < 0.0001), with increases of 4.8 ± 8.1 mmHg (P < 0.0001), 1.8 ± 4.6 mmHg (P = 0.003) and 2.8 ± 5.3 mmHg (P < 0.0001) from the low-salt intervention to the high-salt intervention.
Table 2.
Mean systolic and diastolic blood pressure and mean arterial pressure.
| BP (mmHg) | Baseline (n = 528) | Low dietary salt intervention (n = 512) |
High dietary salt intervention (n = 503) |
||||
|---|---|---|---|---|---|---|---|
| Blood pressure | Change | P-value | Blood pressure | Change | P-value | ||
| SBP | 129.5 ± 13.7 | 124.5 ± 12.0 | −5.0 ± 8.0 | <0.0001 | 129.2 ± 13.6 | 4.8 ± 8.1 | <0.0001 |
| DBP | 81.0 ± 9.8 | 78.4 ± 8.8 | −2.5 ± 4.8 | <0.0001 | 80.1 ± 9.2 | 1.8 ± 4.6 | 0.003 |
| MAP | 97.2 ± 10.5 | 93.8 ± 9.3 | −3.3 ± 5.5 | <0.0001 | 96.4 ± 10.0 | 2.8 ± 5.3 | <0.0001 |
Data were presented as mean ± standard deviation.
The change and P-value indicate the difference in BP data in comparison with the prior stages.
BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure.
Discussion
The MetaSalt study aimed to identify the key pathways in the gut microbiota and plasma metabolites that contribute to BP regulation and subsequent cardiovascular diseases. Our preliminary results from 528 participants who completed both low- and high-salt interventions show that the SBP, DBP, and MAP levels of the participants were significantly lower during the low-salt intervention compared to the baseline measures and were higher during the high-salt intervention compared to the low-salt phase. In a subsequent study, the blood and fecal samples will be analyzed to report the association of plasma metabolites and gut microbiota with sodium burden as indicators of the key pathways in the gut microbiota and plasma metabolites that contribute to BP regulation.
Previous findings indicate that gut microbiota and metabolomic profiles might play important roles in the association between dietary sodium intake and BP, which might further translate into substantial impacts on cardiovascular health.27,28 For example, recent evidence indicates that high-salt diets could damage intestinal anatomy and promote tissue inflammation, while the gut microbiota may mediate the inflammatory responses that contribute to the pathogenesis of hypertension.29 Moreover, there is strong evidence that changes in the circulating metabolome and intestinal microbiota are closely associated with cardiovascular health status, and microbial and metabolomic profiling can be used to identify the crucial mechanisms responsible for the long-term outcomes.17,18,30 Previous findings also suggested that high sodium intake may promote BP elevation by reducing arachidonic acid and B. fragilis levels in the intestine, which may further increase the intestinal-derived corticosterone level in the serum and intestine.31 However, evidence from population studies on the relationships between dietary sodium intake, metabolomic profiling, and gut microbiota with BP are quite limited. The MetaSalt study, with an intervention design at the population level, may provide significant evidence regarding the mechanisms of BP sensitivity by assessing microbial and metabolomic profiles after a dietary salt intervention.
In contrast to the GenSalt study, which focused on identifying genes related to BP responses to dietary sodium and potassium intake,7 the MetaSalt study aimed to study the impacts of low and high dietary sodium intake on microbial and metabolomic profiling and changes in SBP, DBP, and MAP. The two studies adopted a similar protocol of using a family-based design, but the MetaSalt study had a longer intervention duration of ten days for each phase.7,32 As expected, we observed similar patterns in BP changes during the low-salt and high-salt interventions in both studies.32
The MetaSalt study has several strengths. First, the intervention design is a distinct advantage for establishing a causal relationship between the intervening measures and the outcome of interest. Second, the dietary sodium intake of each meal was strictly controlled by pre-packaged salt, and all the participants were required to take each of their meals at fixed canteens, with their food consumption recorded during the entire study period, providing an accurate measurement of dietary sodium intake. Another merit of the MetaSalt study includes the use of several smart devices, such as the sphygmomanometer meters and wearable wristbands. In addition, although a few participants quit the study during the intervention, we still maintained a high retention rate due to the family-based recruitment of the participants.
In conclusion, the MetaSalt study was conducted to address an important public health question regarding the associations between dietary sodium intake, BP, and microbial and metabolomic profiles. The findings from this study are intended to provide a scientific evidence base for the physiological influence of gut microbiota and metabolites on BP regulation, and therefore may help prioritize strategies for the prevention of cardiovascular diseases.
Acknowledgement
The authors deeply appreciate the invaluable support from Dongmei Xing and Qing Wang (Nangong New Greatwall Hospital, Nangong, Hebei), Xu Ji (Xinle Traditional Chinese Medicine Hospital, Xinle, Hebei), Xingqiang Bai (Changshou Community Health Service Center, Xinle, Hebei), Dongsheng Hu (College of Public Health, Zhengzhou University, Zhengzhou, Henan), Ming Zhang (Shenzhen University Health Science Center, Shenzhen, Guangdong), Dongshuang Guo and Shengying Liang (People's Hospital of Yu County, Yangquan, Shanxi) during our field investigation in the four study sites.
Funding
This study was supported by grants from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-2-003, 2017-I2M-1-004).
Conflicts of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Dongfeng Gu, Email: gudongfeng@cashq.ac.cn.
Xiangfeng Lu, Email: xiangfenglu@sina.com.
References
- 1.Murray C.J.L., Aravkin A.Y., Zheng P., et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar M.H., Liu P., Roth G.A., et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. J Am Med Assoc. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 3.Mallah M.A., Liu M., Liu Y., et al. Association of handgrip strength with the prevalence of hypertension in a Chinese Han population. Chronic Dis Transl Med. 2019;5:113–121. doi: 10.1016/j.cdtm.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strazzullo P., D'Elia L., Kandala N.-B., Cappuccio F.P. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guideline W.H.O. World Health Organization; Geneva: 2012. Sodium Intake for Adults and Children. [PubMed] [Google Scholar]
- 6.Elijovich F., Weinberger M.H., Anderson C.A.M., et al. Salt sensitivity of blood pressure. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 7.The GenSalt Collaborative Research G. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–646. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defagó M.D., Gu D., Hixson J.E., et al. Common genetic variants in the endothelial system predict blood pressure response to sodium intake: the GenSalt study. Am J Hypertens. 2013;26:643–656. doi: 10.1093/ajh/hps099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X., Hu Z., Chen J., et al. Associations between genetic variants of NADPH oxidase-related genes and blood pressure responses to dietary sodium intervention: the GenSalt study. Am J Hypertens. 2017;30:427–434. doi: 10.1093/ajh/hpw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Frame A.A., Williams J.S., Wainford R.D. GNAI2 polymorphic variance associates with salt sensitivity of blood pressure in the Genetic Epidemiology Network of Salt Sensitivity study. Physiol Genom. 2018;50:724–725. doi: 10.1152/physiolgenomics.00141.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu X., Gu D., He J., et al. Resequencing epithelial sodium channel genes identifies rare variants associated with blood pressure salt-sensitivity: the GenSalt study. Am J Hypertens. 2017;31:205–211. doi: 10.1093/ajh/hpx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray E.C., Chen J., Kelly T.N., et al. Human epithelial Na+ channel missense variants identified in the GenSalt study alter channel activity. Am J Physiol Ren Physiol. 2016;311:F908–F914. doi: 10.1152/ajprenal.00426.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elijovich F., Laffer C.L., Sahinoz M., Pitzer A., Ferguson J.F., Kirabo A. The gut microbiome, inflammation, and salt-sensitive hypertension. Curr Hypertens Rep. 2020;22:79. doi: 10.1007/s11906-020-01091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y., Song H., Pan X., et al. Urinary metabolites associated with blood pressure on a low- or high-sodium diet. Theranostics. 2018;8:1468–1480. doi: 10.7150/thno.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson J.F., Aden L.A., Barbaro N.R., et al. High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folz J., Oh Y.T., Blaženović I., Richey J., Fiehn O., Youn J.H. Interaction of gut microbiota and high-sodium, low-potassium diet in altering plasma triglyceride profiles revealed by lipidomics analysis. Mol Nutr Food Res. 2019;63:1900752. doi: 10.1002/mnfr.201900752. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein & Cell. 2018;9:416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Wang H., Howard Annie G., et al. Gut microbiota and host plasma metabolites in association with blood pressure in Chinese adults. Hypertension. 2021;120:16154. doi: 10.1161/HYPERTENSIONAHA.120.16154. 0: HYPERTENSIONAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S., Liu J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med. 2017;3:89–94. doi: 10.1016/j.cdtm.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang M. Hypertension as a mitochondrial and metabolic disease. Kidney Int. 2011;80:15–16. doi: 10.1038/ki.2011.84. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty S., Galla S., Cheng X., et al. Salt-Responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25:677–689. doi: 10.1016/j.celrep.2018.09.058. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres S.J., Grimes C., Nowson C.A., et al. Urinary sodium is positively associated with urinary free cortisol and total cortisol metabolites in a cross-sectional sample of Australian schoolchildren aged 5–12 years and their mothers. Br J Nutr. 2019;121:164–171. doi: 10.1017/S0007114518003148. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Wang H., Howard A.G., et al. Associations of sodium and potassium consumption with the gut microbiota and host metabolites in a population-based study in Chinese adults. Am J Clin Nutr. 2020;112:1599–1612. doi: 10.1093/ajcn/nqaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derkach A., Sampson J., Joseph J., Playdon M.C., Stolzenberg-Solomon R.Z. Effects of dietary sodium on metabolites: the dietary approaches to stop hypertension (DASH)-Sodium feeding study. Am J Clin Nutr. 2017;106:1131–1141. doi: 10.3945/ajcn.116.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Lichtenstein A.H., Wong K.E., Appel L.J., Coresh J., Rebholz C.M. Urine metabolites associated with the dietary approaches to stop hypertension (DASH) diet: results from the DASH-sodium trial. Mol Nutr Food Res. 2020:2000695. doi: 10.1002/mnfr.202000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balafa O., Kalaitzidis R.G. Salt sensitivity and hypertension. J Hum Hypertens. 2020 doi: 10.1038/s41371-020-00407-1. [DOI] [PubMed] [Google Scholar]
- 27.Wilck N., Matus M.G., Kearney S.M., et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jablonski K.L., Klawitter J., Chonchol M., Bassett C.J., Racine M.L., Seals D.R. Effect of dietary sodium restriction on human urinary metabolomic profiles. Clin J Am Soc Nephrol. 2015;10:1227. doi: 10.2215/CJN.11531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smiljanec K., Lennon S.L. Sodium, hypertension, and the gut: does the gut microbiota go salty? Am J Physiol Heart Circ Physiol. 2019;317:H1173–H1182. doi: 10.1152/ajpheart.00312.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayor M., Shah Ravi V., Miller Patricia E., et al. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation. 2020;142:1905–1924. doi: 10.1161/CIRCULATIONAHA.120.050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan X., Jin J., Su X., et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126:839–853. doi: 10.1161/CIRCRESAHA.119.316394. [DOI] [PubMed] [Google Scholar]
- 32.He J., Gu D., Chen J., et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/HJH.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]