Abstract
Production of organophosphate esters (OPEs), which represent a major flame-retardant class present in consumer goods, has increased over the past 2 decades. Experimental studies suggest that OPEs may be associated with thyroid hormone disruption, but few human studies have examined this association. We quantified OPE metabolites in the urine of 298 pregnant women from Cincinnati, Ohio, in the Health Outcomes and Measures of the Environment Study (enrolled 2003–2006) at 3 time points (16 and 26 weeks’ gestation, and at delivery), and thyroid hormones in 16-week maternal and newborn cord sera. Urinary bis(1,3-dichloro-2-propyl)-phosphate concentrations were generally associated with decreased triiodothyronine and thyroxine levels and increased thyroid-stimulating hormone levels in maternal and newborn thyroid hormones in quartile dose–response analyses and multiple informant models. There was weaker evidence for thyroid hormone alterations in association with diphenyl-phosphate and di-n-butyl-phosphate. Bis-2-chloroethyl-phosphate was not associated with alterations in thyroid hormones in any analyses. We did not observe any evidence of effect modification by infant sex. These results suggest that gestational exposure to some OPEs may influence maternal and neonatal thyroid function, although replication in other cohorts is needed.
Keywords: cohort studies, flame retardants, pregnancy, thyroid hormones
Abbreviations
- BCEP
bis-2-chloroethyl phosphate
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- CI
confidence interval
- DNBP
di-n-butyl phosphate
- DPHP
diphenyl phosphate
- ln
natural log
- OPE
organophosphate ester
- T3
triiodothyronine
- T4
thyroxine
- TSH
thyroid-stimulating hormone
Thyroid hormones are critical for brain development during fetal and early life. Thyroid hormones facilitate programmed neuron proliferation, migration, and myelination during fetal development and early childhood, and the fetus is either fully or partially dependent on maternal thyroxine (T4) throughout gestation (1). The thyroid gland releases T4, which circulates in blood bound to thyroxin-binding globulin. It is measured in its unbound fraction (free T4) and in total quantities (total T4). Once free T4 reaches target tissues, local enzymes convert it into its biologically active form, triiodothyronine (T3) (2). Thyroid-stimulating hormone (TSH) stimulates the thyroid to produce more T4 when levels drop, and it is regulated directly and indirectly through levels of T4 and T3 (3).
Children whose mothers have untreated hypothyroidism during pregnancy, diagnosed by elevated TSH levels, are more likely to experience intellectual disabilities, diminished reflexes, hearing loss, aphasia, and cerebral palsy (4). Even women who have thyroid hormone levels in the low to normal range are more likely to have children with poorer intellectual development (5). Research by Korevaar et al. (6) also indicates there is an inverted U-shaped association between maternal thyroid hormone levels and child IQ and volumetric brain measures, even among euthyroid women, indicating that tight maternal thyroid hormone regulation during gestation is essential for optimal brain development.
Environmental chemicals, including phthalates, bisphenol A, and polychlorinated biphenyls, have been associated with thyroid hormone disruption in humans (7, 8). Several international clinical and health policy organizations have issued statements regarding the threat that endocrine-disrupting chemicals pose to public health, especially when exposure occurs during a sensitive developmental period such as gestation (7). Organophosphate esters (OPEs) are a class of flame-retardant and plasticizing chemicals that are suspected of affecting thyroid hormone homeostasis, but their potential for endocrine disruption remains to be studied in humans.
The use of OPEs has grown in the last 2 decades since the phase out of polybrominated diphenyl ethers as flame-retardant chemicals, due to evidence of those chemicals’ adverse health effects (9, 10). OPEs can be found in a variety of consumer products, such as furniture, electronics, food packaging, and textiles. OPEs are not physically bound to products, so they readily leach out and contaminate home and office environments as well as foods (11–13). OPEs are quickly metabolized in the body and excreted into the urine, where the chemical metabolites are readily detectable (14, 15). Studies in zebrafish (16), chickens (17), and rodents (18) show that OPEs can affect thyroid hormones, and studies of human exposure to OPEs and thyroid hormones in pregnant women and developing fetuses are warranted.
The objectives of this study were to examine whether exposure to gestational OPE metabolites was associated with alterations in maternal thyroid hormone measured during pregnancy and neonatal thyroid hormone concentrations measured at birth in cord serum.
METHODS
Study participants
A total of 468 pregnant women were enrolled in the Health Outcomes and Measures of the Environment Study between March 2003 and February 2006 in Cincinnati, Ohio. Women were eligible for participation if they were 1) 18 years old or older, 2) at 16 ± 3 weeks’ gestation, and 3) living in a home built before 1978. We excluded women who were taking medication for thyroid disorders or seizures; HIV positive; had a diagnosis of bipolar disorder, schizophrenia, diabetes, or cancer that required radiation or chemotherapy; not fluent in English; or planning to move outside of the Greater Cincinnati area. More detailed enrollment criteria are described elsewhere (19).
For this study, we included women who delivered live, singleton infants, gave at least 1 urine sample during pregnancy or at delivery, and who had at least 1 thyroid hormone measurement during pregnancy or in their newborn (n = 298). All women gave informed consent for themselves and their children, and the Centers for Disease Control and Prevention deferred to the Institutional Review Board at Cincinnati Children’s Hospital Medical Center, which approved the study protocol.
Quantification of urinary OPE metabolites
Pregnant women provided urine samples in polypropylene specimen cups at approximately 16 and 26 weeks’ gestation and within 48 hours of delivery. Samples were aliquoted and frozen at −20°C until analysis for the presence of 4 OPE metabolites: bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), the metabolite of tris(1,3-dichloro-2-propyl) phosphate; bis-2-chloroethyl phosphate (BCEP), the metabolite of tris(2-chloroethyl) phosphate; diphenyl phosphate (DPHP), the metabolite of triphenyl phosphate; and di-n-butyl phosphate (DNBP), the metabolite of tri-n-butyl phosphate. Metabolite concentrations were measured at the Centers for Disease Control and Prevention’s National Center for Environmental Health Laboratory. Details of the analysis, including quality control procedures, are described elsewhere (20). In brief, after enzymatic hydrolysis of the metabolites’ conjugates in 200 μL of urine, the target metabolites underwent automated, off-line, solid-phase extraction; separation via reversed-phase high-performance liquid chromatography; and detection by isotope dilution-electrospray ionization tandem mass spectrometry (21, 22). The limit of detection was 0.10 μg/L for all metabolites, and we replaced concentrations below the limit of detection with values calculated as follows: limit of detection divided by the square root of 2 (23). The percentages of samples with nondetectable concentrations were 11.33%–16.33% for BCEP, 4.27%–10.47% for BDCIPP, 17.00%–40.13% for DNBP, and 0.64%–2.62% for DPHP, depending on the exposure time point (Web Table 1) (available at https://doi.org/10.1093/aje/kwab086).
Thyroid hormone measurement
We collected venous blood from women at approximately 16 weeks of pregnancy and from newborn umbilical cords at birth. Serum was separated and stored at −80°C until analysis. Maternal and cord sera were analyzed at the Department of Laboratory Medicine at the University of Washington for TSH (measured in μIU/mL), free T3 (measured in pg/mL), free T4 (measured in ng/dL), total T3 (measured in ng/dL), and total T4 (measured in μg/dL) using an Access2 automated clinical immunoassay analyzer (Beckman Coulter Inc., Fullerton, California). Two levels of quality-control materials (Bio-Rad Liquicheck or Bio-Rad Immunoassay Plus; Bio-Rad Laboratories, Hercules, California) were analyzed with each assay every day (n = 22). All results were double-checked for errors by a second technologist (8).
Statistical analysis
Urinary OPE metabolite concentrations were divided by creatine concentrations to standardize for urine dilution. OPEs and TSH were log-normally distributed, so we natural-log (ln) transformed these variables.
We tested correlations among urinary OPE metabolites and among thyroid hormones at each time point. Additionally, we tested the intraclass correlations among urinary OPE metabolite measurement time points. To assess for nonlinearity in the associations between urinary OPE metabolites and thyroid hormones, we used generalized additive models. We observed some nonlinear associations and decided to use quartiles of exposure for dose–response analysis (Web Figure 1). We examined associations between concurrent OPE metabolites and thyroid hormone concentrations in maternal and cord sera, using linear regression to estimate β values and 95% confidence intervals for each of the OPEs modeled in quartiles. We also assessed linear trend by taking the median value of each quartile as a continuous variable in linear regression models (24). Subsequently, we analyzed prospective associations between quartiles of each OPE metabolite during pregnancy (at 16 and 26 weeks) and cord serum thyroid hormones.
To explore the potential associations between multiple urinary OPE metabolites and thyroid hormone concentrations, we created an arithmetic sum of urinary OPE metabolites that includes creatinine-standardized and ln-transformed BCEP, BDCIPP, DPHP, and DNBP.
Finally, we used a multiple-informant model to test for differences in the associations of OPE metabolite concentrations across the 3 time points (16 weeks, 26 weeks, and delivery) with thyroid hormones in cord serum (25). As described by Sánchez et al. (25), multiple-informant models can be used in the context of environmental exposures to test whether exposure during specific time windows has the same association with the outcome of interest. It has the advantage of formally testing for differences in association between OPE exposure windows. Additionally, multiple-informant models are not subject to limitations of collinearity among time points of exposure measurement.
We adjusted all models for maternal age at delivery, education, and race (White vs. non-White), year of delivery, and infant sex based on a directed acyclic graph. We performed a sensitivity analysis testing for the impact of maternal iodine status in which we fit linear regression models for maternal urinary OPEs and thyroid hormones with either a full set of covariates or a full set of covariates plus maternal iodine concentrations, and we computed analysis of variance tables for comparable models. Finally, we tested for sex-by-exposure interaction by including a sex × exposure product term in regression models. All analyses were performed using R, version 3.6.1 (26), including packages geepack (27) and mgcv (28).
RESULTS
Participant characteristics
This study included 298 women and their infants who provided at least 1 of 3 urine specimens during pregnancy or at delivery and at least 1 of 2 venous blood samples during pregnancy (maternal blood, n = 226) or at delivery (infant cord blood, n = 294). The majority of women (66.8%) were White, 80.2% had more than a high school education, their average age at delivery was 30.1 years, and 54.7% of infants were female (Table 1). Geometric means of creatinine-standardized urinary OPE metabolite concentrations (in μg/g creatinine) at 16 weeks, 26 weeks, and delivery were: 0.59, 0.67 and 0.72 for BCEP; 0.75, 0.74, and 0.81 for BDCIPP; 1.74, 1.73, and 2.10 for DPHP; and 0.25, 0.26, and 0.21 for DNBP (Web Table 1). Maternal thyroid hormone levels measured at 16 weeks’ gestation were normally distributed (Table 2) and consistent with reference values for pregnant women (29). None of the infants in the study were diagnosed with congenital hypothyroidism. Pearson correlation coefficients among urinary OPE metabolites and thyroid hormones are presented in Web Tables 2 and 3, respectively. Intraclass correlations among creatinine-standardized and ln-transformed urinary OPE metabolites at 16 weeks, 26 weeks, and birth were low (BCEP: 0.30, BDCIPP: 0.34, DPHP: 0.14, and DNBP: 0.19).
Table 1.
Demographic Characteristics of Participants Included in Analyses (n = 298), Health Outcomes and Measures of the Environment Study, 2003–2006a
| Characteristic | No | % |
|---|---|---|
| Maternal race | ||
| White | 199 | 66.8 |
| Non-White | 99 | 33.2 |
| Maternal education | ||
| High school or less | 59 | 19.8 |
| Some college | 72 | 24.2 |
| Bachelor’s degree | 95 | 31.9 |
| Graduate school | 66 | 22.1 |
| Maternal age at delivery, yearsb | 30.1 (18.7–45.0) | |
| Year of birth | ||
| 2003 | 30 | 10.1 |
| 2004 | 121 | 40.6 |
| 2005 | 106 | 35.6 |
| 2006 | 40 | 13.4 |
| Infant sex | ||
| Female | 163 | 54.7 |
| Male | 135 | 45.3 |
a Participants have ≥1 thyroid hormone measurement and ≥1 urinary organophosphate ester metabolite measurement.
b Values are expressed as mean (range).
Table 2.
Thyroid Hormone Concentrations in Mothers During Pregnancy and Neonates (n = 298), Health Outcomes and Measures of the Environment Study, 2003–2006
| Hormone | 16-Week Maternal Serum Level, mean (IQR) | Infant Cord Serum Level, mean (IQR) |
|---|---|---|
| Free T3, pg/mL | 3.20 (3.00–3.40) | 1.66 (1.44–1.80) |
| Free T4, ng/dL | 0.69 (0.60–0.73) | 0.99 (0.90–1.10) |
| Total T3, ng/dL | 160 (142.00–175.00) | 51.40 (40.00–57.30) |
| Total T4, μg/dL | 10.40 (9.00–11.5) | 9.61 (8.60–10.80) |
| TSH, μIU/mLa | 1.21 (0.89–1.98) | 7.11 (5.10–9.83) |
Abbreviations: IQR, interquartile range; T3, triiodothyronine; T4, thyroxine.
a Values are geometric means.
Associations between urinary OPE metabolites and thyroid hormones
Maternal thyroid hormone analysis.
At 16 weeks’ gestation, we observed a weakly negative linear trend between BDCIPP and maternal free T4 across quartiles of urinary BDCIPP, but other OPEs were not associated with maternal thyroid hormones at this time point (Table 3). An analysis of the arithmetic sum of all 4 urinary OPE metabolites and maternal thyroid hormones at 16 weeks’ gestation did not reveal any associations (Web Table 4).
Table 3.
Associations Between Creatinine-Standardized and ln-Transformed Urinary Organophosphate Ester Metabolites at 16 Weeks and Maternal Thyroid Hormone Levels at 16 Weeks (n = 298), Health Outcomes and Measures of the Environment Study, 2003–2006a
| Parameter | FT3, pg/mL | FT4, ng/dL | TT3, ng/dL | TT4, μg/dL | Log TSH, μIU/mL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| BCEP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | −0.05 | −0.18, 0.07 | −0.01 | −0.05, 0.03 | −8.02 | −18.20, 2.15 | −0.52 | −1.32, 0.28 | 0.98 | 0.71, 1.36 |
| 3 | 0.04 | −0.08, 0.16 | 0.01 | −0.03, 0.05 | −1.67 | −11.85, 8.52 | −0.42 | −1.22, 0.38 | 0.96 | 0.69, 1.33 |
| 4 | −0.03 | −0.15, 0.10 | 0.00 | −0.05, 0.05 | −4.69 | −14.68, 5.31 | −0.45 | −1.24, 0.33 | 1.20 | 0.89, 1.65 |
| P for trend | 0.82 | 0.83 | 0.65 | 0.46 | 0.18 | |||||
| BDCIPP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | 0.15 | 0.03, 0.27 | 0.02 | −0.02, 0.06 | 8.43 | −1.68, 18.54 | 0.25 | −0.55, 1.04 | 1.01 | 0.73, 1.40 |
| 3 | 0.06 | −0.06, 0.19 | 0.02 | −0.03, 0.06 | 8.73 | −1.59, 19.05 | 0.27 | −0.55, 1.08 | 1.10 | 0.79, 1.54 |
| 4 | 0.06 | −0.06, 0.19 | −0.03 | −0.07, 0.01 | 5.52 | −4.68, 15.72 | 0.07 | −0.96, 0.72 | 1.12 | 0.81, 1.56 |
| P for trend | 0.93 | 0.04 | 0.70 | 0.91 | 0.48 | |||||
| DPHP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | 0.05 | −0.08, 0.17 | −0.01 | −0.05, 0.03 | −0.38 | −10.83, 10.08 | 0.25 | −0.56, 1.07 | 1.22 | 0.88, 1.70 |
| 3 | 0.02 | −0.11, 0.14 | 0.01 | −0.03, 0.05 | −0.74 | −11.01, 9.54 | 0.40 | −0.40, 1.20 | 0.95 | 0.68, 1.32 |
| 4 | 0.03 | −0.10, 0.15 | −0.01 | −0.06, 0.03 | −0.79 | −10.93, 9.35 | 0.15 | −0.64, 0.94 | 0.92 | 0.66, 1.26 |
| P for trend | 0.89 | 0.56 | 0.89 | 0.92 | 0.27 | |||||
| DNBP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | 0.04 | −0.08, 0.17 | −0.02 | −0.06, 0.02 | 0.70 | −9.70, 11.10 | −0.44 | −1.25, 0.37 | 0.87 | 0.62, 1.21 |
| 3 | 0.03 | −0.09, 0.16 | 0.01 | −0.04, 0.05 | −0.13 | −10.78, 11.10 | −0.64 | −1.48, 0.18 | 1.10 | 0.78, 1.54 |
| 4 | −0.06 | −0.21, 0.09 | 0.01 | −0.06, 0.04 | −0.50 | −11.23, 10.23 | −0.32 | −1.15, 0.52 | 1.01 | 0.72, 1.42 |
| P for trend | 0.25 | 0.48 | 0.87 | 0.57 | 0.62 | |||||
Abbreviations: BCEP, bis-2-chloroethyl phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DNBP, di-n-butyl phosphate; DPHP, diphenyl phosphate; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
a Models were adjusted for mother’s age at delivery, mother’s education, race, year of birth, and infant sex.
Umbilical cord thyroid hormone analysis.
At delivery, we found that increasing maternal urinary BDCIPP and DPHP levels were associated with decreased concentrations of free and total T3 thyroid hormones and increased concentrations of TSH in cord serum, when examined by quartiles of exposure (Table 4). Maternal urinary OPEs had weak or null associations with cord free and total T4 levels at delivery. We also created longitudinal linear regression models of quartiles of maternal concentrations of OPE metabolites at either 16 or 26 weeks’ gestation and infant cord serum thyroid hormone levels. Web Table 5 indicates that maternal urinary BDCIPP concentrations at 16 weeks’ gestation are associated with decreased free and total T4 in cord serum, and we observed a positive linear trend between 16-week maternal urinary DNBP and cord TSH levels (P for trend = 0.01). Maternal urinary BDCIPP concentrations at 26 weeks’ gestation were associated with decreased free T3 and total T4 concentrations in infant cord serum; maternal urinary DNBP concentrations were associated with decreased free and total T3 concentrations in cord serum (Web Table 6). In an analysis of potential joint effects of multiple OPE metabolites at birth, we observed associations between the arithmetic sum of urinary OPE metabolites and a 0.04 pg/mL decrease in free T3 (95% CI: −0.08, 0.01), a 2.35 ng/dL decrease in total T3 (95% CI: −4.96, 0.26), and an 8% increase in TSH (95% CI: −1.0%, 16%) (Web Table 4).
Table 4.
Associations Between Creatinine-Standardized and ln-Transformed Urinary Organophosphate Ester Metabolites at Birth by Quartile and Cord Blood Thyroid Hormones (n = 298), Health Outcomes and Measures of the Environment Study, 2003–2006a
| Parameter | FT3, pg/mL | FT4, ng/dL | TT3, ng/dL | TT4, μg/dL | Log TSH, μIU/mL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| BCEP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | −0.05 | −0.17, 0.06 | −0.05 | −0.10, 0.01 | 0.73 | −6.38, 7.83 | −0.05 | −0.75, 0.65 | 0.83 | 0.68, 1.02 |
| 3 | −0.05 | −0.16, 0.07 | −0.02 | −0.07, 0.04 | −2.54 | −9.63, 4.55 | −0.50 | −1.20, 0.21 | 0.98 | 0.80, 1.21 |
| 4 | −0.07 | −0.18, 0.05 | 0.03 | −0.03, 0.09 | −3.09 | −10.23, 4.05 | 0.19 | −0.51, 0.90 | 1.05 | 0.85, 1.30 |
| P for trend | 0.39 | 0.05 | 0.31 | 0.41 | 0.19 | |||||
| BDCIPP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | 0.04 | −0.07, 0.15 | 0.01 | −0.04, 0.07 | 3.64 | −3.40, 10.68 | −0.11 | −0.82, 0.60 | 1.26 | 1.02, 1.55 |
| 3 | −0.07 | −0.19, 0.04 | 0.02 | −0.03, 0.08 | −3.68 | −10.74, 3.38 | −0.25 | −0.96, 0.46 | 0.99 | 0.81, 1.22 |
| 4 | −0.11 | −0.22, 0.00 | 0.03 | −0.03, 0.09 | −5.18 | −12.09, 1.72 | −0.28 | −0.97, 0.41 | 1.24 | 1.01, 1.52 |
| P for trend | 0.02 | 0.35 | 0.04 | 0.48 | 0.14 | |||||
| DPHP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | 0.01 | −0.10, 0.13 | −0.01 | −0.06, 0.05 | −1.36 | −8.30, 5.58 | 0.07 | −0.63, 0.77 | 1.06 | 0.86, 1.30 |
| 3 | −0.12 | −0.24, −0.01 | 0.02 | −0.04, 0.08 | −8.19 | −15.29, −1.09 | −0.35 | −1.06, 0.37 | 1.23 | 1.00, 1.52 |
| 4 | −0.07 | −0.19, 0.04 | 0.01 | −0.05, 0.06 | −4.75 | −11.75, 2.25 | −0.42 | −1.12, 0.28 | 1.25 | 1.01, 1.54 |
| P for trend | 0.17 | 0.75 | 0.22 | 0.16 | 0.04 | |||||
| DNBP, quartile | ||||||||||
| 1 | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 1.00 | Referent |
| 2 | −0.04 | −0.15, 0.08 | 0.04 | −0.02, 0.09 | −2.55 | −9.72, 4.62 | −0.38 | −1.09, 0.34 | 0.98 | 0.80, 1.22 |
| 3 | −0.01 | −0.12, 0.11 | 0.05 | −0.00, 0.11 | −1.48 | −8.57, 5.62 | 0.23 | −0.47, 0.93 | 1.05 | 0.85, 1.29 |
| 4 | 0.00 | −0.12, 0.12 | 0.01 | −0.05, 0.07 | −1.75 | −9.01, 5.59 | −0.33 | −1.06, 0.41 | 1.17 | 0.94, 1.45 |
| P for trend | 0.76 | 0.91 | 0.82 | 0.56 | 0.09 | |||||
Abbreviations: BCEP, bis-2-chloroethyl phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DNBP, di-n-butyl phosphate; DPHP, diphenyl phosphate; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
a Models were adjusted for mother’s age at delivery, mother’s education, race, year of birth, and infant sex.
Analysis of all exposure time points.
Using multiple informant models (Figure 1, Web Table 7), because the interaction terms for time point by OPE were significant (using a P < 0.2 level of significance) and not homogeneous, we retained the interaction term to provide window-specific estimates rather than dropping it and reporting estimates of pooled exposure. A ln-unit increase in BDCIPP concentration at 26 weeks and at delivery was associated with a 0.04 pg/mL decrease in infant cord free T3 concentration (26-week 95% CI: −0.08, 0.003; delivery 95% CI: −0.08, −0.01), and at delivery was associated with a 2.29 ng/dL decrease in total T3 concentration (95% CI: −4.15, −0.43). At both 16 and 26 weeks’ gestation, maternal urinary BDCIPP level was associated with a 0.24 μg/dL decrease in infant cord total T4 concentrations (16-week 95% CI: −0.45, −0.03; 26-week 95% CI: −0.41, −0.06). We also observed a 5.8% increase in infant TSH concentration in association with a 1 ln-unit increase in maternal urinary BDCIPP level at 26 weeks and at delivery (26-week 95% CI: −1.0%, 12.2%; delivery 95% CI: −1.0%, 11.3%).
Figure 1.
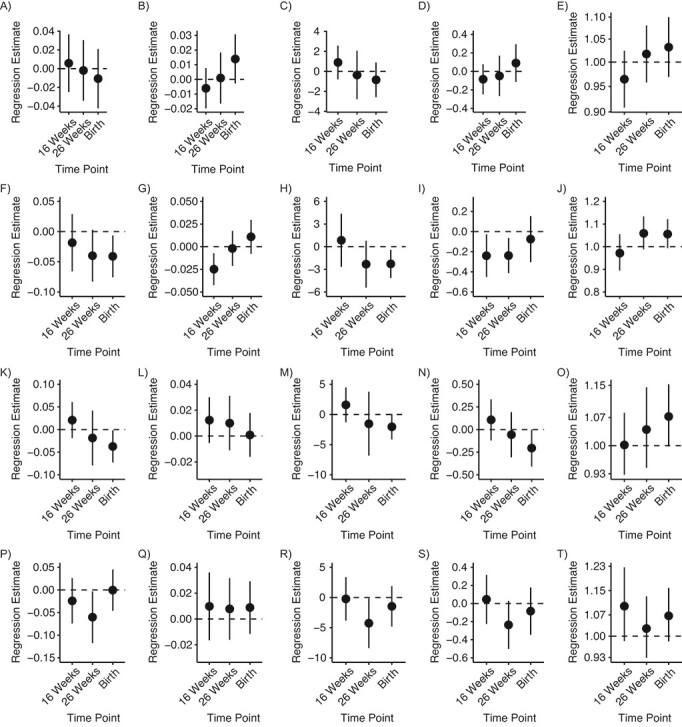
Line plot of multiple informant model results with 3 time points of creatinine standardized and ln-transformed, maternal urinary organophosphate metabolite measurements in association with newborn cord blood thyroid hormone concentrations in the Health Outcomes and Measures of the Environment (HOME) study, 2003–2006. Models were adjusted for mother’s age at delivery, mother’s education, race, year of birth, and infant sex. Levels of A) bis-2-chloroethyl phosphate (BCEP) and free triiodothyronine (T3; pg/mL); B) BCEP and free thyroxine (T4; ng/dL); C) BCEP and total T3 (ng/dL); D) BCEP and total T4 (μg/dL); E) BCEP and log of thyroid-stimulating hormone (TSH; μIU/mL); F) bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) and free T3 (pg/mL); G) BDCIPP and free T4 (ng/dL); H) BDCIPP and total T3 (ng/dL); I) BDCIPP and total T4 (μg/dL); J) BDCIPP and log-TSH (μIU/mL); K) diphenyl phosphate (DPHP) and free T3 (pg/mL); L) DPHP and free T4 (ng/dL); M) DPHP and total T3 (ng/dL); N) DPHP and total T4 (μg/dL); O) DPHP and log-TSH (μIU/mL); P) DNBP and free T3 (pg/mL); Q) DNBP and free T4 (ng/dL); R) DNBP and total T3 (ng/dL); S) DNBP and total T4 (μg/dL); and T) DNBP and log-TSH (μIU/mL).
Increasing concentrations of maternal urinary DPHP at delivery were associated with a 0.04 pg/mL decrease in infant cord free T3 concentration (95% CI: −0.07, −0.00), a 0.21 μg/dL decrease in total T4 concentration (95% CI: −0.41, 0.00), and a 6.8% increase in TSH concentration (95% CI: 0.0%, 14.0%). Maternal urinary DNBP concentrations at 26 weeks were associated with decreased infant cord free T3, total T3, and total T4 concentrations, and 16-week and delivery concentrations were associated with increased infant cord log-TSH concentration. BCEP was not associated with cord serum thyroid hormones in multiple informant models.
Sensitivity analyses.
In a sensitivity analysis of maternal iodine status, we did not observe significant differences between models that included iodine and those without, so we excluded iodine from our models to preserve degrees of freedom (Web Table 8). We also tested for effect modification by infant sex in multiple informant models, but we did not observe significant chemical-by-sex interactions at any time point (using a P < 0.2 level of significance) (Web Table 9).
DISCUSSION
In this study of women and their infants with typical exposures to OPEs measured between 2003 and 2006 (20, 30), we found maternal urinary concentrations of OPE metabolites were associated with alterations in thyroid hormone concentrations in both maternal and neonatal sera. In multiple informant models, increasing maternal urinary BDCIPP, DPHP, and DNBP concentrations were associated with decreased infant free T3, free T4, total T3, total T4, and increased log-TSH concentrations. The arithmetic sum of urinary OPE metabolites was associated with decreased free T3, decreased total T3, and increased TSH concentrations at birth, and appears to be driven by BDCIPP and DPHP.
We observed some evidence that maternal urinary DPHP and DNBP concentrations were linearly associated with increased maternal TSH concentration. Although there was only weak evidence of alterations in the other maternal thyroid hormones, Andersen et al. (31) have discussed that abnormal TSH values are indicative of abnormal T3 and T4 levels, even when those values are within reference ranges, due to the logarithmic response in TSH to deviations in the other hormones.
During early brain development, the fetus is entirely dependent on maternal thyroid hormones during the first trimester. The fetus begins to produce a higher proportion of thyroid hormones throughout the second and third trimesters, becoming successively less dependent on maternal contributions (1). Additionally, levels of fetal T3 and T4 are associated with maternal T4, but not T3; small changes in maternal T4 concentration cause large changes in fetal thyroid hormones, due to differences in the binding of carrier proteins between adults and fetuses (32). Therefore, our observation of maternal urinary concentrations of OPE metabolites being associated with decreased fetal T4 concentration may reflect both direct effects from fetal exposure to OPEs that crossed the placenta and indirect effects of decreased maternal T4 concentration, although reliance on maternal T4 in the third trimester is relatively small compared with earlier in gestation (33). Although T3 is the biologically active thyroid hormone, T3 levels are directly influenced by circulating levels of T4, so altered levels of T4 in either maternal or cord serum are indicative of altered biological activity at the cellular level.
Findings from multiple informant models reinforce our conclusions from regression models using quartiles of exposure. Of the 4 OPE metabolites examined, BDCIPP was the most consistently associated with alterations in thyroid hormone concentrations. There is weak evidence for the associations between DPHP and DNBP and thyroid hormone alterations. The associations between BDCIPP and DPHP and infant cord free T3 concentration that we observed in multiple informant models (−0.04 pg/mL) are equivalent to a 2.4% decrease from the cohort mean free T3 concentration for every log-unit increase in OPE metabolite, and the −0.06 pg/mL change in free T3 concentration associated with maternal urinary DNBP concentration at 26 weeks is equivalent to a 3.6% decrease for every log-unit increase in DNBP concentration. Only maternal urinary BDCIPP level at 16 weeks was associated with infant free T4 concentration (−0.03 ng/dL), and the change was equivalent to a 3% decrease from the mean for every log-unit increase in BDCIPP concentration. For every log-unit increase in maternal urinary DNBP level at 26 weeks, we observed a 4.28 ng/dL decrease in infant cord total T3 concentration, which is equivalent to an 8.3% decrease from the mean. We also observed associations between maternal urinary BDCIPP, DPHP, and DNBP concentrations and decreasing level of total T4. The observed 0.24 μg/dL decrease is equivalent to a 2.5% decrease from the mean. Literature has shown in both human and animal studies that small (25%–30%) changes in maternal T4 concentration during critical developmental windows cause structural and functional neurological deficits in offspring (34–36). Furthermore, thyroid hormones are more tightly regulated in individuals than in populations, so small variances in population averages would correspond to relatively large changes in an individual’s serum hormone levels (31). Miller et al. (37) concluded that thyroid disruption in population-based studies should be taken as evidence of adverse effects among individuals.
Although the mechanisms by which OPEs disrupt thyroid hormones have not been fully elucidated, experimental studies offer some insights. Wang et al. (16) showed that treating zebrafish with tris(1,3-dichloro-2-propyl) phosphate, the parent compound of BDCIPP, caused decreased transcription of genes related to the hypothalamic–pituitary-thyroid axis. Hill et al. (38) explored the potential for OPEs to disturb thyroid hormone transport and found that increased exposure to these chemicals enhanced the binding of T4 to its transporter protein in vitro. Increased activation of thyroid hormone nuclear receptors and enhanced T4 binding to transporter proteins could cause decreased pressure on the negative feedback loop that regulates thyroid hormone homeostasis, consistent with our finding of lower T4 levels among participants with higher concentrations of urinary OPE metabolites. Interestingly, several experimental studies showed that increased OPE exposure caused levels of thyroid hormones to increase (39–41), and some reported only sex-specific associations on thyroid hormone levels (42). One other study, in Dalian, China, also recently investigated these associations in pregnant women and newborns (43). The researchers observed associations between maternal urinary DNBP levels during pregnancy and increased TSH concentrations in newborns and also between cross-sectional maternal urinary DPHP levels during pregnancy and maternal TSH levels. Although our findings also support an association between urinary OPE metabolites and increased maternal and newborn TSH concentrations, we did not see a cross-sectional association between maternal DPHP and TSH levels during pregnancy, and we only observed an association between DNBP concentrations and newborn TSH concentrations at 16 weeks, not at 26 weeks. Yao et al. (43) also reported effect modification by sex, which we did not observe. The differences in findings between the 2 cohorts could be due to a variety of factors. The concentrations of most urinary OPE metabolites were much lower in the Chinese women than in our participants, and Yao et al. measured OPE metabolites and thyroid hormones across a wide range of time during pregnancy (8–37 weeks’ gestation), which fails to account for differences in maternal and fetal physiology across pregnancy.
Limitations and strengths
Tight regulation of maternal thyroid hormone levels is critical for fetal development in the first trimester of gestation. However, we only had measurements of maternal urinary OPE metabolites and serum hormones at 16 weeks. We cannot rule out the possibility of residual confounding in this study even though we controlled for multiple potential factors. Another limitation of this study is the relatively high percentage of measurements below the limit of detection for DNBP (17%–43%). Because of methodological limitations, we were unable to use a multiple imputation approach for the quartile-based regression (44).
Although we observed some evidence of a linear response in thyroid hormone alterations in association with OPE metabolites, we were likely underpowered to fully assess the shape of the dose–response curve. Future studies should use very large cohorts to detect true dose–response patterns when effect sizes are relatively small on a population scale, such as in this case. We also recommend that studies focus on early gestation, if possible, when exploring the associations between environmental chemicals and maternal thyroid hormone levels. Replication of our results at multiple time points during gestation and in maternal and cord blood is a major strength of the study and lends weight to our findings, as does the biological plausibility of the pattern of decreased T3 and T4 levels paired with increased TSH level.
CONCLUSIONS
We observed relatively consistent evidence of second- and third-trimester maternal BDCIPP concentration associated with decreased T3 and T4 levels and increased TSH level in infants, weak evidence of thyroid hormone alterations in association with maternal DPHP and DNBP concentrations, and no evidence of thyroid hormone alterations in association with BCEP. There was no evidence of sex-specific effect measure modification. Replication in other birth cohorts is of public health interest because of the ubiquitous nature of OPEs and the critical role of thyroid hormones in human development.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental and Public Health Sciences, University of Cincinnati, Cincinnati, Ohio, United States (Zana Percy, Changchun Xie, Kim M. Cecil, Kim N. Dietrich, Kimberly Yolton); School of Public Health, University of Nevada, Las Vegas, Las Vegas, Nevada, United States (Ann M. Vuong); Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, United States (Yingying Xu, Kim M. Cecil, Kimberly Yolton); National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia, United States (Maria Ospina, Antonia M. Calafat); Department of Laboratory Medicine, University of Washington, Seattle, Washington, United States (Andy Hoofnagle), Faculty of Health Sciences, Simon Fraser University, Burnaby, British Columbia, Canada (Bruce P. Lanphear); Department of Epidemiology, Brown University, Providence, Rhode Island, United States (Joseph M. Braun); Department of Radiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States (Kim M. Cecil); and Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States (Aimin Chen).
This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS grants P01 ES11261, R01 ES014575, R01 ES020349, R01 ES027224, R01 ES028277, P30 ES006096), the US Environmental Protection Agency (EPA grant P01 R829389), and the University of Cincinnati Medical Scientist Training Program (grant 2T32GM063483-1). We also thank the L.B. Research and Education Foundation for their support of this work.
We thank Nayana Jayatilaka, Paula Restrepo, Zack Davis, and Meghan Vidal, of the Centers for Disease Control and Prevention, for providing the OPE metabolites measurements.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC.
J.M.B. was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures and received an honorarium for serving on an advisory board to Quest Diagnostics. J.M.B. served as an expert witness in litigation related to perfluorooctanoic acid contamination in drinking water in New Hampshire. Any funds he received from this arrangement were or are paid to Brown University and cannot be used for his direct benefit (e.g., salary/fringe, travel). The other authors report no conflicts.
REFERENCES
- 1. Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20(6):784–794. [DOI] [PubMed] [Google Scholar]
- 2. Moog NK, Entringer S, Heim C, et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper DS, Ladenson PW. Chapter 7. The thyroid gland. In: Gardner DG, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 9th ed. New York, NY: The McGraw-Hill Companies; 2011. [Google Scholar]
- 4. de Escobar GM, Obregón MJ, del Rey F. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18(2):225–248. [DOI] [PubMed] [Google Scholar]
- 5. Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24(1):150–157. [DOI] [PubMed] [Google Scholar]
- 6. Korevaar TIM, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):35–43. [DOI] [PubMed] [Google Scholar]
- 7. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vuong AM, Webster GM, Romano ME, et al. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: the HOME study, Cincinnati, USA. Environ Health Perspect. 2015;123(10):1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Environmental Protection Agency . Polybrominated diphenylethers (PBDEs) significant new use rules (SNUR). https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenylethers-pbdes-significant-new-use. Accessed June 30, 2020.
- 10. Wei GL, Li DQ, Zhuo MN, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut. 2015;196:29–46. [DOI] [PubMed] [Google Scholar]
- 11. Han L, Sapozhnikova Y, Nuñez A. Analysis and occurrence of organophosphate esters in meats and fish consumed in the United States. J Agric Food Chem. 2019;67(46):12652–12662. [DOI] [PubMed] [Google Scholar]
- 12. He C, Wang X, Tang S, et al. Concentrations of organophosphate esters and their specific metabolites in food in Southeast Queensland, Australia: is dietary exposure an important pathway of organophosphate esters and their metabolites? Environ Sci Technol. 2018;52(21):12765–12773. [DOI] [PubMed] [Google Scholar]
- 13. van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–1153. [DOI] [PubMed] [Google Scholar]
- 14. Nomeir AA, Kato S, Matthews HB. The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (fyrol FR-2) in the rat. Toxicol Appl Pharmacol. 1981;57(3):401–413. [DOI] [PubMed] [Google Scholar]
- 15. Cequier E, Marcé RM, Becher G, et al. A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography-high resolution mass spectrometry. Anal Chim Acta. 2014;845:98–104. [DOI] [PubMed] [Google Scholar]
- 16. Wang Q, Liang K, Liu J, et al. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol. 2013;126:207–213. [DOI] [PubMed] [Google Scholar]
- 17. Farhat A, Crump D, Chiu S, et al. In ovo effects of two organophosphate flame retardants—TCPP and TDCPP—on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134(1):92–102. [DOI] [PubMed] [Google Scholar]
- 18. Springer C, Dere E, Hall SJ, et al. Rodent thyroid, liver, and fetal testis toxicity of the monoester metabolite of bis-(2-ethylhexyl) tetrabromophthalate (TBPH), a novel brominated flame retardant present in indoor dust. Environ Health Perspect. 2012;120(12):1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braun JM, Kalloo G, Chen A, et al. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) Study. Int J Epidemiol. 2017;46(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Percy Z, Vuong AM, Ospina M, et al. Organophosphate esters in a cohort of pregnant women : variability and predictors of exposure. Environ Res. 2020;184:109255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayatilaka NK, Restrepo P, Williams L, et al. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction- high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(5):1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jayatilaka NK, Restrepo P, Davis Z, et al. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere. 2019;235:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 24. Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. [DOI] [PubMed] [Google Scholar]
- 25. Sánchez BN, Hu H, Litman HJ, et al. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; R Foundation for Statistical Computing; 2017. [Google Scholar]
- 27. Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1–11. [Google Scholar]
- 28. Wood S. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B. 2011;73(1):3–36. [Google Scholar]
- 29. Zhang D, Cai K, Wang G, et al. Trimester-specific reference ranges for thyroid hormones in pregnant women. Medicine (Baltimore). 2019;98(4):e14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Percy Z, La MJ, Xu Y, et al. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the HOME study during the PBDE phase-out. Chemosphere. 2020;239:124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersen S, Pedersen KM, Bruun NH, et al. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87(3):1068–1072. [DOI] [PubMed] [Google Scholar]
- 32. Contempré B, Jauniaux E, Calvo R, et al. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993;77(6):1719–1722. [DOI] [PubMed] [Google Scholar]
- 33. Ding J, Xu Z, Huang W, et al. Organophosphate ester flame retardants and plasticizers in human placenta in eastern China. Sci Total Environ. 2016;554-555:211–217. [DOI] [PubMed] [Google Scholar]
- 34. Gilbert ME, Sui L. Developmental exposure to perchlorate alters synaptic transmission in hippocampus of the adult rat. Environ Health Perspect. 2008;116(6):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ausó E, Lavado-Autric R, Cuevas E, et al. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037–4047. [DOI] [PubMed] [Google Scholar]
- 36. Haddow JE, Palomaki GE, Williams J. Thyroid-stimulating-hormone concentrations and risk of hypothyroidism. Lancet. 2002;360(9350):2081–2082. [DOI] [PubMed] [Google Scholar]
- 37. Miller MD, Crofton KM, Rice DC, et al. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117(7):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hill KL, Hamers T, Kamstra JH, et al. Organophosphate triesters and selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol Lett. 2018;285:87–93. [DOI] [PubMed] [Google Scholar]
- 39. Liu X, Jung D, Jo A, et al. Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environ Toxicol Chem. 2016;35(9):2288–2296. [DOI] [PubMed] [Google Scholar]
- 40. Kim S, Jung J, Lee I, et al. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol. 2015;160:188–196. [DOI] [PubMed] [Google Scholar]
- 41. Fernie KJ, Palace V, Peters LE, et al. Investigating endocrine and physiological parameters of captive American kestrels exposed by diet to selected organophosphate flame retardants. Environ Sci Technol. 2015;49(12):7448–7455. [DOI] [PubMed] [Google Scholar]
- 42. Xu T, Wang Q, Shi Q, et al. Bioconcentration, metabolism and alterations of thyroid hormones of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in zebrafish. Environ Toxicol Pharmacol. 2015;40(2):581–586. [DOI] [PubMed] [Google Scholar]
- 43. Yao Y, Li M, Pan L, et al. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy : thyroid endocrine disruption and mediation role of oxidative stress. Environ Int. 2021;146:106215. [DOI] [PubMed] [Google Scholar]
- 44. Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


