Abstract
Background
The development of brain metastases (BM) is one of the most feared complications of cancer due to the substantial neurocognitive morbidity and a grim prognosis. In the past decade, targeted therapies and checkpoint inhibitors have demonstrated promising intracranial response rates for tumors of multiple histologies. As overall survival for these patients improves, there is a growing need to identify issues surrounding patient survivorship and to standardize physician practice patterns for these patients. To date, there has not been an adequate study to specifically explore these questions of survivorship and practice standardization for patients with advanced cancer and BM.
Methods
Here, we present results from a cross-sectional survey in which we analyze responses from 237 patients, 209 caregivers, and 239 physicians to identify areas of improvement in the clinical care of BM.
Results
In comparing physician and patient/caregiver responses, we found a disparity in the perceived discussion of topics pertaining to important aspects of BM clinical care. We identified variability in practice patterns for this patient population between private practice and academic physicians. Many physicians continue to have patients with BM excluded from clinical trials. Finally, we obtained patient/physician recommendations on high-yield areas for federal funding to improve patient quality of life.
Conclusion
By identifying potential areas of unmet need, we anticipate this wealth of actionable information will translate into tangible benefits for both patients and caregivers. Future studies are needed to validate our findings.
Keywords: brain metastases, patient advocacy, patient survivorship
Brain metastases (BM) are the most common central nervous system (CNS) malignancy and portend a grim prognosis. Current estimates suggest that approximately 50 000-150 000 patients are diagnosed annually in the United States alone, and historically, median survival has been on the order of a few months after diagnosis.1–5 As progression of intracranial disease is the cause of death in up to 50% of patients with BM,2 treatments for BM are an emerging unmet need in modern oncology. To this end, recent clinical trials evaluating checkpoint inhibitors6,7 and targeted therapies8–12 have demonstrated promising intracranial activity. However, as prognoses for these patients improve, there has been increased awareness of issues surrounding patient survivorship and the variability of practice4 across oncologists for this challenging patient population. These questions have major ramifications for adequately treating patient symptoms and improving quality of life (QoL).
Historically, clinical outcomes, such as overall survival, progression-free survival, and treatment-related toxicity, have been used to evaluate therapeutic efficacy in oncology. These metrics, however, do not capture the holistic effect of treatment on a patient’s everyday life. For example, patient symptoms and psychosocial stressors are difficult to quantify and may be under-recognized in clinical practice, resulting in greater morbidity and decreased QoL for the patient.13–16 In recent years, patient-related outcomes (PROs), defined as a report of the status of a patient’s health that comes directly from the patient without interpretation by a health care professional,17 have emerged as a popular tool to longitudinally track facets of a patient’s well-being. Consequently, PROs are being incorporated in standard clinical workflows and have accelerated a paradigm shift in oncology towards patient-centered care.18–20
To date, relatively few PRO studies have been performed for brain tumor patients. Brain tumors, compared to systemic cancers, present unique challenges due to long-term neurocognitive sequelae.21 From the patients’ perspective, brain tumors are catastrophic events, as they usually present after an unexpected event (eg, seizure, hemiparesis) and are accompanied by persistent limitations in neurologic function due to subsequent supportive and tumor-directed therapies.22–24 Further compounding this issue is the difficulty from the physician perspective in integrating recent advances in a rapidly evolving field without a clear standard of care. This conundrum may result in variability in physician practices and messages relayed to patients/caregivers that can evoke stress and confusion. Current PRO studies in neuro-oncology have predominantly surveyed glioma patients, and have revealed important insights about an inadequate understanding of the disease process from patients,25,26 and unmet financial and psychosocial needs for caregivers.21,27–30 However, to our knowledge, there has not been a dedicated study evaluating these endpoints or physician practice patterns, specific to the BM population. Hence, using a cross-sectional survey of patients, caregivers, and physicians, we sought to collect information on unmet patient/caregiver needs, physician practice regimens for BM, and patient-caregiver-physician recommendations on ways to improve patient care and increase federal resources for BM. We anticipate this information will improve patient-caregiver-clinician communication, standardize treatment recommendations in a rapidly evolving field, and facilitate the development of new therapeutics.
Methods
Participants
The subjects of the survey were patients, caregivers, and physicians. All patients carried a diagnosis of BM, with histologically confirmed disease from any metastatic solid tumor. A caregiver was defined as an adult individual (eg, family members, nursing staff), who was not a clinician, that provided support (eg, medical, financial, emotional, physical) to a patient with a confirmed diagnosis of BM. Physicians provided direct clinical care to patients carrying a diagnosis of BM and included neuro-oncologists, medical oncologists, neurosurgeons, and radiation oncologists. Patient, caregiver, and physician survey answers were not matched on the patient or physician level. All participants were required to be able to read and respond to questions in English.
Study Measures
The study was conceptualized, developed, and sponsored by the American Brain Tumor Association (ABTA), a nonprofit organization dedicated to brain tumor patient services and research. Surveys were administered by Penn, Schoen, and Berland (PSB) to PSB survey panels. Commercial panel providers, such as PSB, continuously solicit cancer patients, caregivers, and physicians from a diverse background, and are frequently used by pharmaceutical companies due to their broad reach in recruiting participants. Additionally, surveys were also provided to patients, caregivers, and physicians on lists provided by partner organizations (Kidney Cancer Association, LUNGevity Foundation, Melanoma Research Foundation, and Society for Neuro-Oncology). Eligible participants were contacted via email with a link to an online questionnaire. There were no reminder emails sent, and there was no additional re-contact with survey responders’ post-survey completion.
A predetermined goal of approximately 200 survey responders for each survey population (ie, patient, caregiver, and physician) was targeted. As stated above, we employed a recruiting strategy using lists provided by partner organizations and commercial survey panels to maximize the diversity of our cohort. However, this strategy results in a non-probabilistic sample, as not everyone in the total population of metastatic brain tumor patients and caregivers would theoretically be able to take the survey as potential respondents voluntarily joined survey or partner organization panels. For non-probabilistic samples such as these, a margin of error equivalent is often used. However, this calculation is dependent on knowing the approximate size of the metastatic brain tumor population, which to our knowledge, does not exist in current literature. Therefore, a conservative estimate of total population of adults as the base was used. This yielded a sampling margin of error equivalent of ±6.9% at the 95% confidence level.
Patient-caregiver-physician surveys contained questions about demographics, BM symptoms, discussion of BM diagnosis by the clinician, psychosocial concerns from the patient, available treatment options for BM, advocacy resources specific to patients with BM, BM-specific clinical trials, and the level of familiarity and expectation of the ABTA and other brain tumor patient advocacy organizations. Physicians were also asked about their level of experience in treating general oncology patients, and whether they worked in an academic or private setting.
Statistical Analyses
Descriptive statistics were used to summarize survey results from each survey population, and within the physician category by workplace type (eg, private, academic, and other). For patients, 3 summary tables were generated, stratifying patients by sex, age at diagnosis of BM, and histology of primary cancer. Chi-square tests were used to test for differences in sex, age, and primary cancer type, using P < .05 as a significance threshold. For caregivers, 3 summary tables were generated, stratifying caregivers by sex, age at patients’ diagnosis of BM, and histology of primary cancer for the patient of whom he/she took care of. Chi-square tests were used to test for differences in sex, age, and histology of primary cancer for the patient, using P < .05 as a significance threshold. For physicians, 4 summary tables were generated, stratifying physicians by sex, age, number of cancer patients managed per month, and workplace type (private, academic, and other). Chi-square tests were used to test for differences by workplace type, using P < .05 as a significance threshold. Questions that overlapped between groups were also analyzed to assess differences between respective respondent groups using chi-square tests. All analyses of this study were approved by the Piedmont Health and Case Western Reserve University Institutional Review Boards and performed using R (v3.6.2) and its libraries.
Results
Participants
We identified 45 133 patients, 1582 caregivers, and 2019 physicians, and contacted them via email. From August 13 to September 16, 2018, 1841 patients agreed to participate in the survey and 237 of these patients were identified as eligible for our study based on the screening questions. A total of 209 caregivers completed our survey. From June 16 to 25, 2019, 239 physicians completed our survey. Additional survey responders after these time frames were not included in our analysis, given financial constraints and our pre-specified goal of approximately 200 survey responders for each population. 200 survey responses from each cohort were obtained through PSB panels. The remainder of responses was obtained through social media postings by the ABTA or partner organizations.
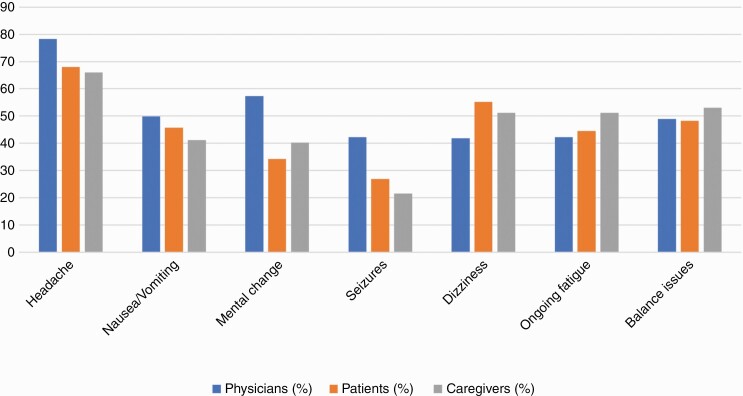
The majority of patients and caregivers were Caucasian, young or middle-aged adults (Table 1), and had at least a college education (Supplementary Table 1). The most common symptom that patients endorsed were headaches, dizziness, and balance issues (Figure 1). Caregivers provided physical, emotional, and financial support to patients, which frequently had emotional (87.6%) and mental (70.8%) impacts on caregiver well-being (Supplementary Table 2). A significant portion of caregivers reported feeling sad (48.8%), depressed (32.5%), and overwhelmed (40.7%). Social, psychiatric, and physical coping means were used by caregivers to assist with these emotional burdens. Surveyed physicians were predominantly male (80%) and Caucasian (58%; Table 1), and worked in a private practice setting (Table 2). Most (78.2%) were medical oncologists with 10+ years of experience.
Table 1.
Physician, Patient, and Caregiver Characteristics
| Characteristics Summary | Physicians | Patients | Caregivers | P Value for Differences by Groupa |
|---|---|---|---|---|
| N (Sample size) | 239 | 234 | 209 | |
| Sex (%) | <.001 | |||
| Male | 191 (79.9) | 105 (44.9) | 96 (45.9) | |
| Female | 48 (20.1) | 129 (55.1) | 113 (54.1) | |
| Age at time of survey (%) | <.001 | |||
| Under 35 | 28 (11.7) | 84 (35.9) | 70 (33.4) | |
| 35-44 | 88 (36.8) | 78 (33.3) | 67 (32.1) | |
| 45-54 | 69 (28.9) | 37 (15.8) | 30 (14.4) | |
| 55-64 | 45 (18.8) | 25 (10.7) | 32 (15.3) | |
| 65+ | 9 (3.8) | 10 (4.2) | 10 (4.7) | |
| Region (%) | .331 | |||
| Northeast | 62 (25.9) | 57 (24.4) | 42 (20.1) | |
| Midwest | 54 (22.6) | 40 (17.1) | 53 (25.4) | |
| South | 81 (33.9) | 90 (38.5) | 71 (34.0) | |
| West | 42 (17.6) | 47 (20.1) | 43 (20.6) | |
| Race (%)—multiple | ||||
| White/Caucasian | 139 (58.2) | 194 (82.9) | 174 (83.3) | <.001 |
| Black/African American/Caribbean-American | 10 (4.2) | 23 (9.8) | 23 (11.0) | .017 |
| Asian | 69 (28.9) | 11 (4.7) | 7 (3.3) | <.001 |
| American Indian or Alaska Native | 6 (2.5) | 1 (0.4) | 3 (1.4) | .169 |
| Native Hawaiian or Pacific Islander | 4 (1.7) | 2 (0.9) | 1 (0.5) | .434 |
| The time when the patient was first diagnosed with metastatic brain tumors (%) | <.001 | |||
| 0-3 mo ago | — | 27 (11.5) | 6 (2.9) | |
| 3-6 mo ago | — | 61 (26.1) | 21 (10.0) | |
| 7 mo to <12 mo ago | — | 59 (25.2) | 41 (19.6) | |
| 1-3 yr ago | — | 65 (27.8) | 77 (36.8) | |
| 4-5 yr ago | — | 13 (5.6) | 36 (17.2) | |
| 6-10 yr ago | — | 7 (3.0) | 13 (6.2) | |
| 11 or more years ago | — | 1 (0.4) | 14 (6.7) | |
| Don’t know | — | 1 (0.4) | 1 (0.5) |
aGenerated by a chi-square test.
bThe first current primary cancer type of patients under the care of caregivers.
Figure 1.
Most common symptoms endorsed by patients diagnosed with brain metastases.
Table 2.
Summary of Physician Characteristics by Workplace
| Physician Characteristics | Privatea | Academic | Other | P Value for Differences by Workplaceb | P Value for Differences Between Private and Academicb |
|---|---|---|---|---|---|
| N (Sample size) | 108 | 68 | 63 | ||
| Age of physicians at the time of survey (%) | .021 | .035 | |||
| 25-34 | 6 (5.6) | 10 (14.7) | 12 (19.0) | ||
| 35-44 | 36 (33.3) | 26 (38.2) | 26 (41.3) | ||
| 45-54 | 35 (32.4) | 23 (33.8) | 11 (17.5) | ||
| 55-64 | 24 (22.2) | 9 (13.2) | 12 (19.0) | ||
| 65+ | 7 (6.5) | 0 (0.0) | 2 (3.2) | ||
| Sex of physicians (%) | .088 | .06 | |||
| Male | 93 (86.1) | 50 (73.5) | 48 (76.2) | ||
| Female | 15 (13.9) | 18 (26.5) | 15 (23.8) | ||
| Your role (%) | .001 | <.001 | |||
| Practicing physician | 107 (99.1) | 56 (82.4) | 54 (85.7) | ||
| Fellow | 1 (0.9) | 11 (16.2) | 7 (11.1) | ||
| Resident | 0 (0.0) | 1 (1.5) | 2 (3.2) | ||
| Your field(s) (%)—multiple | |||||
| Neurologist | 1 (0.9) | 3 (4.4) | 0 (0.0) | .103 | .321 |
| Neuro oncologist | 7 (6.5) | 19 (27.9) | 7 (11.1) | <.001 | <.001 |
| Medical oncologist or hematologist | 96 (88.9) | 47 (69.1) | 44 (69.8) | .001 | .002 |
| Neurosurgeon | 4 (3.7) | 7 (10.3) | 1 (1.6) | .052 | .15 |
| Pediatric oncologist | 2 (1.9) | 2 (2.9) | 5 (7.9) | .12 | >.999 |
| Radiation oncologist | 4 (3.7) | 6 (8.8) | 9 (14.3) | .045 | .274 |
| Your expertise/sub-specialization in one or more disease sites (%)—multiple | |||||
| Head/neck | 54 (50.0) | 22 (32.4) | 27 (42.9) | .071 | .032 |
| Breast | 63 (58.3) | 32 (47.1) | 31 (49.2) | .279 | .192 |
| Thoracic | 56 (51.9) | 21 (30.9) | 34 (54.0) | .009 | .01 |
| GI | 56 (51.9) | 23 (33.8) | 28 (44.4) | .064 | .029 |
| GU | 53 (49.1) | 19 (27.9) | 25 (39.7) | .021 | .009 |
| Gyn | 51 (47.2) | 13 (19.1) | 17 (27.0) | <.001 | <.001 |
| Sarcoma | 49 (45.4) | 18 (26.5) | 22 (34.9) | .037 | .019 |
| Lymphoma | 56 (51.9) | 25 (36.8) | 34 (54.0) | .083 | .072 |
| Pediatrics | 27 (25.0) | 10 (14.7) | 12 (19.0) | .244 | .149 |
| Melanoma | 58 (53.7) | 25 (36.8) | 28 (44.4) | .084 | .042 |
| I do not have any expertise/sub-specialization | 31 (28.7) | 9 (13.2) | 8 (12.7) | .01 | .028 |
| How long you have worked in oncology since the end of your training (%) | .002 | <.001 | |||
| <1 yr | 0 (0.0) | 4 (5.9) | 5 (7.9) | ||
| 1-3 yr | 3 (2.8) | 13 (19.1) | 7 (11.1) | ||
| 4-5 yr | 8 (7.4) | 6 (8.8) | 5 (7.9) | ||
| 6-10 yr | 21 (19.4) | 14 (20.6) | 16 (25.4) | ||
| 11-15 yr | 36 (33.3) | 12 (17.6) | 10 (15.9) | ||
| 16-20 yr | 9 (8.3) | 8 (11.8) | 8 (12.7) | ||
| 21 yr or more | 31 (28.7) | 11 (16.2) | 12 (19.0) | ||
| The number of cancer patients you personally manage within your practice each month (%) | .05 | .043 | |||
| <20 | 1 (0.9) | 2 (2.9) | 3 (4.8) | ||
| 20-50 | 9 (8.3) | 13 (19.1) | 6 (9.5) | ||
| 51-80 | 11 (10.2) | 13 (19.1) | 8 (12.7) | ||
| 81-100 | 14 (13.0) | 9 (13.2) | 15 (23.8) | ||
| 101-150 | 15 (13.9) | 10 (14.7) | 8 (12.7) | ||
| 151-200 | 23 (21.3) | 11 (16.2) | 13 (20.6) | ||
| 201+ | 35 (32.4) | 10 (14.7) | 10 (15.9) | ||
| Region of physicians (%) | .013 | .003 | |||
| Northeast | 17 (15.7) | 27 (39.7) | 18 (28.6) | ||
| Midwest | 27 (25.0) | 11 (16.2) | 16 (25.4) | ||
| South | 42 (38.9) | 23 (33.8) | 16 (25.4) | ||
| West | 22 (20.4) | 7 (10.3) | 13 (20.6) | ||
| The majority of your time spent (%) | .043 | .039 | |||
| Clinical work | 108 (100.0) | 64 (94.1) | 62 (98.4) | ||
| Research | 0 (0.0) | 3 (4.4) | 0 (0.0) | ||
| Administration | 0 (0.0) | 0 (0.0) | 1 (1.6) | ||
| Other | 0 (0.0) | 1 (1.5) | 0 (0.0) |
aPhysician workplace was coded as academic, private (which included “private practice as a solo partner,” “private practice with multiple practitioners,” and “private clinical research”) and other.
Participant-Caregiver-Physician Concerns About Clinical Care of Brain Metastases
In our survey, we found discrepancies in the perceived discussion of the risk and implications of developing BM, from the patient/caregiver and physician perspective (Table 3). While patient and caregiver responses were not linked, the provided responses were largely similar. These discussions generally first occurred after the initial diagnosis of a metastatic solid tumor. Given the stress associated with this diagnosis, it can be difficult for the physician, within the constraints of a clinic visit, to present all necessary information to the patient, and even more challenging for the patient/caregiver to process a great deal of potentially life-altering information. Many topics, such as a general overview of BM, worrisome symptoms, treatment options, and patient advocacy resources, were felt to have been discussed more frequently from the perspective of physicians than from that of patients or caregivers. Consistent with this, a higher percentage of patients/caregivers, compared to physicians, indicated a desire for increased discussion on these issues. All parties felt that more detailed discussion regarding the prognostic and therapeutic implications of BM was desired in the visits following a diagnosis of metastatic cancer. The most common discussion points that patients/caregivers wanted more information on included: survival rates of BM, treatment options, and patient advocacy support. 91.5% of patients felt that the information provided by the physician about treatments targeting the BM were either “very helpful” or “somewhat helpful” (Supplementary Table 3).
Table 3.
Topics of Discussion Between Patient/Caregiver and Physician Regarding the Risk of Developing Brain Metastases, and Topic of Information on Brain Metastases Each Group Wished They Would Like to See More of
| Topic of Discussion Between Patient/Caregiver and Physician | Physicians | Patients | Caregivers | P Value for Differences by Groupa |
|---|---|---|---|---|
| Overview of brain metastases | 161 (67.4) | 105 (44.9) | 111 (53.1) | <.001 |
| Symptoms to be aware of | 191 (79.9) | 112 (47.9) | 123 (58.9) | <.001 |
| Plan/schedule for testing | 139 (58.2) | 107 (45.7) | 78 (37.3) | <.001 |
| Where to go for information/support | 66 (27.6) | 99 (42.3) | 78 (37.3) | .003 |
| Survival rates | 101 (42.3) | 102 (43.6) | 85 (40.7) | .825 |
| Caregiver support | 67 (28.0) | 91 (38.9) | 67 (32.1) | .04 |
| Treatment options | 157 (65.7) | 118 (50.4) | 107 (51.2) | .001 |
| Topic of Information You Would Like to Know More About | Physicians | Patients | Caregivers | P Value for Differences by Groupa |
| Overview of brain metastases | 60 (25.1) | 97 (41.5) | 92 (44.0) | <.001 |
| Symptoms to be aware of | 61 (25.5) | 102 (43.6) | 93 (44.5) | <.001 |
| Plan/schedule for testing | 57 (23.8) | 87 (37.2) | 56 (26.8) | .004 |
| Where to go for information/support | 68 (28.5) | 101 (43.2) | 94 (45.0) | <.001 |
| Survival rates | 92 (38.5) | 122 (52.1) | 98 (46.9) | .01 |
| Caregiver support | 80 (33.5) | 87 (37.2) | 89 (42.6) | .138 |
| Treatment options | 105 (43.9) | 109 (46.6) | 96 (45.9) | .834 |
a P values are generated by a chi-square test.
Given the substantial neurologic morbidity and grim prognosis, a diagnosis of a BM can be a life-altering event for patients that can evoke many questions and concerns. In our survey, the most common questions that were asked by patients/caregivers to the physician after a diagnosis of BM included: worrisome symptoms, treatment options/success, and impact on QoL (Supplementary Table 4). After the diagnosis of a BM, physicians commonly referred patients to patient support groups, published research, and online educational resources for more information (Supplementary Table 5). About 80%-90% of patients felt that the information provided by the physician for social or financial support was helpful (Supplementary Table 3).
Next, we queried physicians on their greatest concerns for patients with a diagnosis of BM (Supplementary Table 6). The most common concerns included: neurologic symptoms, treatment options/success, and impact of BM on patient QoL. Significantly more private practice-affiliated physicians, compared to academic physicians, were concerned about their patients’ neurologic symptoms (50.0% vs 30.9%; P = .019). Academic physicians were more likely to be worried about the current state of published research for BM and patient eligibility for clinical trials.
Treatment Options
Patients in our cohort received care from oncology/neuro-oncology (43.2/38.5%), radiation oncology (32.5%), neurosurgery (28.6%), and palliative care (17.5%; Supplementary Table 3). Physicians, patients, and caregivers indicated that the most popular recommended treatment options, following the diagnosis of a BM, were stereotactic radiosurgery (SRS), whole-brain radiation therapy (WBRT), and chemotherapy (Table 4). Participation in a clinical trial was among the least recommended options (23.0% of physicians and 17.9% of patients). Private practice physicians, compared to academic physicians, were significantly more likely to recommend WBRT (61.1 vs 39.7%, P = .009). About 88.5% of patients reported satisfaction with the choice of BM-targeted treatment (Supplementary Table 3).
Table 4.
Treatment Options Recommended by Physicians Following BM Diagnosis, Stratified by Group and Physician Workplace
| Recommended Treatment Option (%) | Physiciansa | Patients | Caregivers | P Value for Differences by Groupb |
|---|---|---|---|---|
| Surgery | 53 (22.2) | 60 (25.6) | 35 (16.7) | .075 |
| Stereotactic radiation | 126 (52.7) | 56 (23.9) | 35 (16.7) | <.001 |
| Whole brain radiation | 123 (51.5) | 63 (26.9) | 63 (30.1) | <.001 |
| Chemotherapy | 125 (52.3) | 78 (33.3) | 96 (45.9) | <.001 |
| Homeopathic treatment | 12 (5.0) | 20 (8.5) | 12 (5.7) | .261 |
| Participation in a clinical trial | 55 (23.0) | 42 (17.9) | 23 (11.0) | .004 |
| Observation | 18 (7.5) | 33 (14.1) | 33 (15.8) | .017 |
| Recommended Treatment Option (Physicians only) (%) | Privatec | Academic | Other | P Value for Differences Between Private and Academicb |
| Surgery | 30 (27.8) | 10 (14.7) | 12 (19.0) | .067 |
| Stereotactic radiation | 56 (51.9) | 38 (55.9) | 33 (52.4) | .714 |
| Whole brain radiation | 66 (61.1) | 27 (39.7) | 30 (47.6) | .009 |
| Chemotherapy | 56 (51.9) | 33 (48.5) | 36 (57.1) | .784 |
| Homeopathic treatment | 9 (8.3) | 2 (2.9) | 1 (1.6) | .263 |
| Participation in a clinical trial | 24 (22.2) | 17 (25.0) | 13 (20.6) | .809 |
| Observation | 10 (9.3) | 4 (5.9) | 4 (6.3) | .603 |
aThe counts of treatment options recommended by the physician to his/her patient(s) are calculated based on the average of the recommended options across combinations of primary cancer types and brain metastases types.
b P values are generated by a chi-square test.
cPhysician workplace was coded as academic, private (which included “private practice as a solo partner,” “private practice with multiple practitioners,” and “private clinical research”) and other.
Physicians stated that patient QoL, intracranial and extracranial disease burden, the presence of neurologic symptoms, and the number of viable systemic options were the most important factors in deciding on BM-directed treatments (Supplementary Table 7). Academic physicians were more likely to consider clinical research and treatment toxicity in their decision-making process (Supplementary Table 8). The most preferred resources for physicians in the treatment of BM patients were National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) published guidelines.
Finally, more than half of all surveyed physicians indicated that more clinical trials for BM patients were needed (Supplementary Figure 1). A large barrier to effective treatments for BM is the relative paucity of clinical trials specifically for patients diagnosed with BM, due to perceived poor prognosis. The majority of physicians (59.1% private, 71.9% academic) stated that one or more patients in their care were denied participation in clinical trials, specifically due to the presence of BM (Supplementary Table 9). The most desired trial designs were those evaluating novel systemic therapies, followed by those using novel radiation approaches to avoid WBRT (Supplementary Table 10).
Federal Government Advocacy
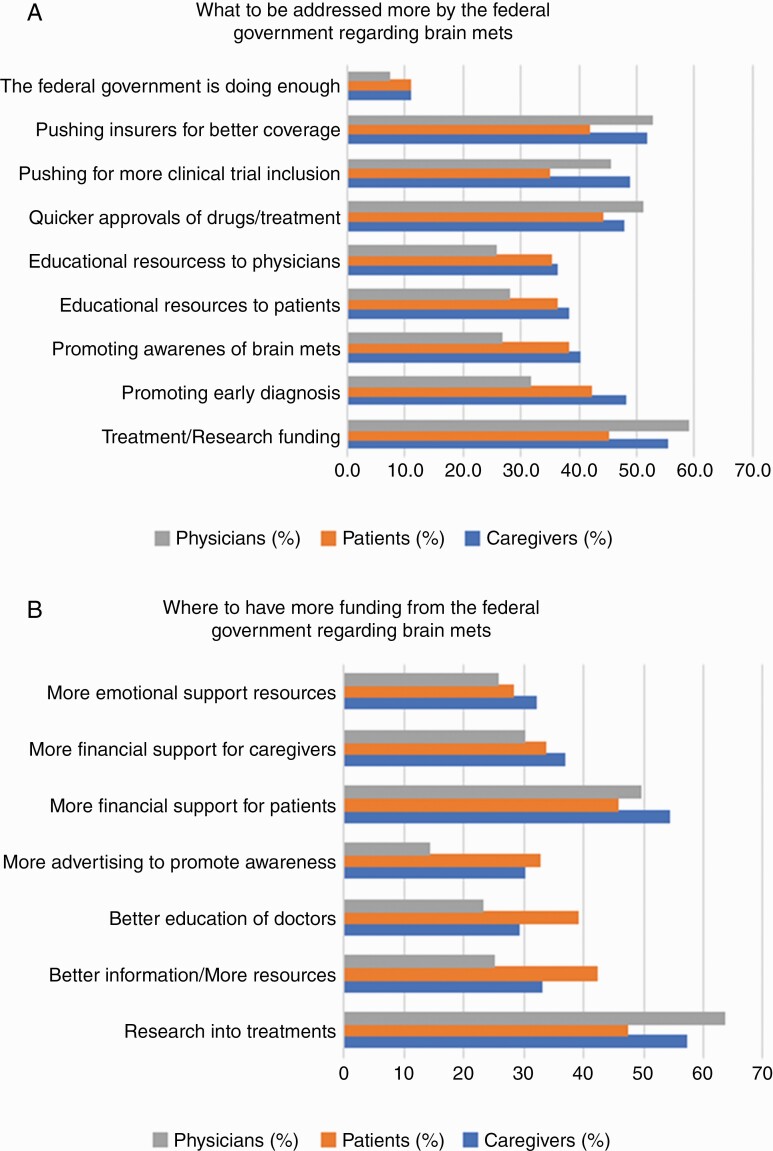
Very few survey responders (7.5% of physicians, 11.0% of patients) felt that the federal government was doing enough for patients with advanced cancer and BM (Figure 2). The consensus among physicians, patients, and caregivers was that the highest yield area for federal assistance is increased treatment/research funding for BM, followed by quicker FDA approvals of BM treatments. Other desired areas of improvement included more clinical trial availability and patient advocacy resources. When physician responses were stratified based on workplace, academic physicians were more likely to advocate for increased treatment/research funding and clinical trial availability for patients with BM (Supplementary Figures 1 and 2). Increased federal government funding for BM treatments and research remained the most popular area of advocacy among all physicians.
Figure 2.
High-yield areas of federal intervention for patients with brain metastases.
Discussion
Our findings represent one of the first patient/caregiver-centered studies designed specifically to evaluate the complex and unique needs of patients diagnosed with BM. We then integrated these insights with input from clinicians on practice patterns and high-yield areas of improvement. A central finding of our study was a disparity in the perceived discussion of topics pertaining to BM, from physicians and patients/caregivers. These topics included especially important issues, such as prognosis and treatment intent. Our findings are consistent with existing data; for example, more than half of patients with advanced cancer have an overly optimistic perception of their prognosis.31,32 Furthermore, our data suggest that a significant portion of patients diagnosed with BM may be making treatment decisions without fully understanding treatment ramifications and expected outcomes.
Patients, caregivers, and physicians reported QoL as a paramount concern and the most influential factor dictating selection of treatment. Therefore, interventions directed at improving prognostic awareness, with a focus on QoL, are needed. More effective patient-clinician communication and additional patient-centric resources would enable patients to make more informed decisions about their treatment and likely have downstream benefits in psychological well-being. In our study, only 17.5% of patients saw a palliative care physician during their treatment course. As palliative or best supportive care is an emerging treatment option in consensus guidelines,33 we recommend consideration of palliative care referrals for this patient population. QoL efforts should also be directed towards caregiver well-being, as a significant number of caregivers endorsed deleterious psychosocial effects from caregiver burden. Caregivers who suffer emotional distress stemming from their loved one’s illness have worse physical and psychological health, which may translate into worse outcomes for the patient.34–37
Another novel aspect of our study is our assessment of physician practice patterns and recommendations for the field of BM. Due to the recent emergence of CNS-penetrant targeted therapies8,10–12,38–40 and immune checkpoint inhibitors6,7 for BM, some oncologists now consider up-front systemic therapy in asymptomatic BM’s in order to delay surgery or radiation until BM progression and to minimize surgical morbidity41 or radiation-induced neurotoxicity. Given rapidly evolving treatment paradigms for BM, we hypothesized that there would be variability in treatment recommendations across physicians. In our survey, private practice physicians, compared to academic physicians, were significantly more likely to recommend WBRT as a treatment for BM. Additionally, private practice physicians were more likely to be concerned about treating neurologic symptoms. As physicians stated that their most preferred educational resources for management of BM were NCCN and ASCO guidelines, we recommend continued correspondence to our oncology colleagues using these resources. Furthermore, our study adds to the growing body of evidence illustrating the paucity in clinical trials specific for the BM population. As patients continue to be denied participation in a clinical trial due to the presence of BM, we urge our colleagues to prioritize planning of trials evaluating intracranial efficacy of novel systemic therapies or radiation approaches, with flexible inclusion criteria for patients with BM.
Finally, very few survey responders felt the federal government is currently doing enough for patients diagnosed with BM. The area of highest need was unanimous among patients, caregivers, and physicians: more research funding for BM treatments. Therefore, we recommend increased federal resources to better understand BM pathophysiology and design more effective treatments. Additionally, we note that supportive care of patients/caregivers is a frequently overlooked and unmet need, which can result in deleterious effects on QoL and emotional well-being.34–37 Funding for patient advocacy efforts, focusing on psychological well-being through one-on-one counseling and social/emotional resources,42 is needed.
Our study has several important limitations. First, given the lack of an established reporting system that facilitates quantitation of the metastatic brain tumor population, we were not able to perform a statistically rigorous calculation for sample size. The majority of our patient/caregiver cohort were young or middle-aged adults and primarily Caucasian. These factors may limit the generalizability of our findings. Additionally, more than 95% of our patient/caregiver cohort endorsed symptomatic BM. Therefore, our findings may only reflect the experiences of patients with symptomatic BM, rather than those with asymptomatic intracranial disease. Future studies are needed to capture the needs of patients with relatively small and asymptomatic BM, which are increasing in incidence41 due to guidelines43 that have resulted in increased screening for BM in cancer patients. Next, the vast majority of physician responders were medical oncologists. There may be important concerns from other physician specialists that were not captured. Similarly, our study did not gauge the experience of patients and caregivers on the impact of interdisciplinary care coordination.
Another limitation is the fact that patient-caregiver-physician survey responses were not matched, thereby interpretation of results cannot be extrapolated to reflect experiences of patient/caregiver, patient/physician, or caregiver/physician pairs. While the patient and caregiver responses were not linked, the provided responses within these cohorts were largely similar, which gives some credence to the collected data on general symptoms and unmet needs for the BM population. Additionally, the majority of patients, caregivers, and physicians that were contacted did not respond to our survey, which may be a source of participation bias. While the response rate for caregivers (13%) and physicians (10%) are in line with historical norms for surveys in which there is no reward, other than altruism, for participating,44 we note the low response rate from the patient cohort (4%) and acknowledge that our population may be skewed towards patients of high functional status. Many patients carrying a diagnosis of BM also have concomitant extracranial disease, which may inhibit survey engagement due to limitations in functional status. Finally, our study did not differentiate between patients with different histologies and treatments received (eg, steroids, immunotherapy), as we tried to capture broadly applicable aspects of BM clinical care as a first step. To address these issues, we will plan future longitudinal studies by surveying the patient population of a specific physician at different time points. These efforts would capture patients of poor functional status, assess the needs of patients with asymptomatic BM, link patient/caregiver/physician responses, and measure the impact of various interventions (eg, steroids, memantine after WBRT, anticonvulsants) during the patient’s treatment course.
In summary, we performed a large cross-sectional survey in which we compared responses from physicians, caregivers, and patients to identify areas of improvement in the clinical care of BM. Our study is one of the first studies tailored specifically for these patients, a unique population due to their neurocognitive sequelae and limited prognosis. We collected actionable information on patient/caregiver psychosocial needs, variability in physician practice patterns, and recommendations on high-yield areas for federal funding to improve the clinical care of BM. Our conclusions are tempered by the low response rate from patients and the lack of patient/caregiver/physician-matched data and will require prospective validation in future studies. Nonetheless, our hope is that these findings are a first step towards planning larger studies that identify survivorship issues for a specific subset within the BM population (eg, histology-specific, neurologically asymptomatic patients), evaluate the longitudinal impact of specific interventions on patient QoL, and obtain input from other physician specialists. Results from these studies may inspire future quality improvement measures to improve specific facets of the care of patients with BM. These efforts will be instrumental towards improving outcomes for a dismal disease.
Supplementary Material
Acknowledgments
First and foremost, we thank the patients, caregivers, families, and physicians for contributing to our research efforts. Additionally, we thank our partner organizations in Kidney Cancer Association, LUNGevity Foundation, Melanoma Research Foundation, and Society for Neuro-Oncology, for their assistance in the development of survey questions, distribution of the survey to their constituents, and input on our manuscript. We also thank our partner organizations, Living Beyond Breast Cancer and Fight Colorectal Cancer, for their feedback on our manuscript.
Conflict of interest statement. M.P.M. reports advisory board honoraria from Zap, Mevion, Karyopharm, Tocagen, AstraZeneca; and Board of Directors options from Oncoceutics. P.K.B. has consulted for Angiochem, Genentech-Roche, Lilly, Tesaro, ElevateBio, Pfizer, SK Life Sciences, and Dantari, received grant/research support to MGH from Merck, BMS and Lilly and honoraria from Merck, Genentech-Roche, Pfizer, and Lilly.
Funding
This study was funded by Accuray, Agios, Bristol Myers Squibb, Brainlab, Celgene, Genentech, and Novocure through grants to the American Brain Tumor Association. A.E.K. is supported by an American Brain Tumor Association Basic Research Fellowship In Honor of Paul Fabbri (BRF1900017). P.K.B. is supported by the NIH (5R01CA244975, 5R01CA227156, and 5R21CA220253), Damon Runyon Cancer Research Foundation, Breast Cancer Research Foundation, and the MGH Research Scholars Program.
References
- 1. Brastianos HC, Cahill DP, Brastianos PK. Systemic therapy of brain metastases. Curr Neurol Neurosci Rep. 2015;15:518. [DOI] [PubMed] [Google Scholar]
- 2. Brastianos PK, Curry WT, Oh KS. Clinical discussion and review of the management of brain metastases. JNCCN J Natl Compr Cancer Netw. 2013;11:1153–1164. [DOI] [PubMed] [Google Scholar]
- 3. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. [DOI] [PubMed] [Google Scholar]
- 4. Chow E, Davis L, Panzarella T, et al. Accuracy of survival prediction by palliative radiation oncologists. Int J Radiat Oncol Biol Phys. 2005;61:870–873. [DOI] [PubMed] [Google Scholar]
- 5. Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol. 2018;149:27–42. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: data from a randomized Phase III trial (Aura3). J Clin Oncol 2018;36:2702–2709. [DOI] [PubMed] [Google Scholar]
- 10. Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. [DOI] [PubMed] [Google Scholar]
- 12. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 13. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanson-Fisher R, Girgis A, Boyes A, et al. The unmet supportive care needs of patients with cancer. Cancer. 2000;88:225-236. [DOI] [PubMed] [Google Scholar]
- 15. Laugsand EA, Sprangers MAG, Bjordal K, et al. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes. 2010;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graves KD. Cancer care for the whole patient: meeting psychosocial health needs. Written by the Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting Board on Health Care Services. Institute of Medicine. The National Academies Press. Psychooncology. 2009;18:305–306. [Google Scholar]
- 17. Strong LE. The past, present, and future of patient-reported outcomes in oncology. Am Soc Clin Oncol Educ B. 2015;35:e616–e620. [DOI] [PubMed] [Google Scholar]
- 18. Howell D, Molloy S, Wilkinson K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26:1846–1858. [DOI] [PubMed] [Google Scholar]
- 19. Tevis SE, James TA, Kuerer HM, et al. Patient-reported outcomes for breast cancer. Ann Surg Oncol. 2018;25:2839–2845. [DOI] [PubMed] [Google Scholar]
- 20. LeBlanc TW, Abernethy AP. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14:763–772. [DOI] [PubMed] [Google Scholar]
- 21. Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro Oncol. 2008;10:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fox S, Lantz C. The brain tumor experience and quality of life: a qualitative study. J Neurosci Nurs. 1998;30:245–252. [DOI] [PubMed] [Google Scholar]
- 23. Leavitt MB, Lamb SA, Voss BS. Brain tumor support group: content themes and mechanisms of support. Oncol Nurs Forum. 1996;23:1247–1256. [PubMed] [Google Scholar]
- 24. Keir ST, Guill AB, Carter KE, et al. Differential levels of stress in caregivers of brain tumor patients - observations from a pilot study. Support Care Cancer. 2006;14:1258–1261. [DOI] [PubMed] [Google Scholar]
- 25. Deborah AF, Katharine MQ, Kevin P, et al. Psychological distress and prognostic understanding in patients with malignant gliomas and their caregivers. Neuro Oncol. 2017;19:vi170–vi170. [Google Scholar]
- 26. Forst DA, Quain K, Landay SL, et al. Perceptions of prognosis and goal of treatment in patients with malignant gliomas and their caregivers. Neurooncol Pract. 2020;7:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boele FW, Given CW, Given BA, et al. Family caregivers’ level of mastery predicts survival of patients with glioblastoma: a preliminary report. Cancer. 2017;123:832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Long A, Halkett GKB, Lobb EA, et al. Carers of patients with high-grade glioma report high levels of distress, unmet needs, and psychological morbidity during patient chemoradiotherapy. Neurooncol Pract. 2016;3:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flechl B, Ackerl M, Sax C, et al. The caregivers’ perspective on the end-of-life phase of glioblastoma patients. J Neurooncol. 2013;112:403–411. [DOI] [PubMed] [Google Scholar]
- 30. Onken J, Goerling U, Heinrich M, et al. Patient reported outcome (PRO) among high-grade glioma patients receiving TTFields treatment: a two center observational study. Front Neurol. 2019;10:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120:278–285. [DOI] [PubMed] [Google Scholar]
- 32. Chen CH, Kuo SC, Tang ST. Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: systematic review and meta-regression analysis. Palliat Med. 2017. 2017;31:406–418. [DOI] [PubMed] [Google Scholar]
- 33. Silvestre J, Gosse T, Read P, et al. Genesis of quality measurements to improve the care delivered to patients with brain metastases. JCO Oncol Pract. 2020;17:e397–e405. [DOI] [PubMed] [Google Scholar]
- 34. Nijboer C, Tempelaar R, Sanderman R, et al. Cancer and caregiving: the impact on the caregiver’s health. Psychooncology. 1998;7:3–13. [DOI] [PubMed] [Google Scholar]
- 35. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. J Am Med Assoc. 1999;282:2215–2219. [DOI] [PubMed] [Google Scholar]
- 36. Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: across the trajectory of the illness. Cancer. 2008;112:2556–2568. [DOI] [PubMed] [Google Scholar]
- 37. Shaffer KM, Kim Y, Carver CS, Cannady RS. Depressive symptoms predict cancer caregivers’ physical health decline. Cancer. 2017;123:4277–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34:2858–2865. [DOI] [PubMed] [Google Scholar]
- 39. Costa DB, Shaw AT, Ou SHI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 41. Loh D, Hogg F, Edwards P, et al. Two-year experience of multi-disciplinary team (MDT) outcomes for brain metastases in a tertiary neuro-oncology centre. Br J Neurosurg. 2018;32:53–60. [DOI] [PubMed] [Google Scholar]
- 42. Papadakos J, Agarwal A, Charow R, et al. Informational needs of brain metastases patients and their caregivers. Neurooncol Pract. 2019;6:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gradishar WJ, Anderson BO, Balassanian R, et al. Clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw. 2018;16:310–320. [DOI] [PubMed] [Google Scholar]
- 44. Cohen AJ, Washington S, Butler C, et al. Altruistic donation to improve survey responses: a global randomized trial. BMC Res Notes. 2019;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.