Abstract
Background
The objective of this study was to evaluate the impact of the time interval between planning imaging and stereotactic radiosurgery (SRS) delivery on tumor volumes and spatial anatomic displacements of brain metastases (BM).
Methods
Consecutive patients diagnosed with BM treated with SRS over a 3-year period were evaluated. Only patients who underwent an institutionally standardized diagnostic MRI (MRI-1) and a treatment planning MRI (MRI-2) were included. The impact of histology, inter-scan time interval, lesion location, tumor volume, and diameter were evaluated on final lesion diameter, volume, anatomic displacement, and ultimate need for change in management (ie, expanding margins, rescanning).
Results
101 patients (531 lesions) with a median inter-scan time interval of 8 days (range: 1-42 days) met the inclusion criteria. The median percentage increase in BM diameter and volume were 9.5% (IQR: 2.25%-24.0%) and 20% (IQR: 0.7%-66.7%). Overall, 147 lesions (27.7%) in 57 patients (56.4%) required a change in management. There was a statistically significant relationship between initial tumor diameter (cm) and change in management (OR: 2.69, 95% CI: 1.93-3.75; P < .001). Each day between MRI-1 and MRI-2 was associated with a change in management with an OR of 1.05 (95% CI: 1.03-1.07; P < .001).
Conclusions
Changes in tumor diameter, volume, and spatial position occur as a function of time. Planning imaging for SRS is recommended to occur in close temporal proximity to treatment; for those with delays, a larger setup margin may need to be used to ensure tumor coverage and account for positional changes.
Keywords: brain metastasis, MRI timing, radiosurgery, SRS, tumor dynamics
Brain metastases (BM) occur in approximately 30% of all cancer patients and are increasing in incidence as patients are living longer from their systemic disease, greater emphasis is placed on screening and surveillance for asymptomatic patients, and sensitivity of magnetic resonance imaging (MRI) continues to improve.1 Stereotactic radiosurgery (SRS) is increasingly being adopted as the primary treatment option over whole-brain radiotherapy (WBRT), but SRS is technologically more complex to plan and deliver. In the traditional sense, SRS is delivered in a single high-dose fraction, and the complications from such an approach are highly correlated with the volume of normal brain irradiated.2,3 Because of this, additional planning margins need to be either minimized or eliminated for treatment. To confidently achieve this, sophisticated MRI scans and high precision in treatment planning and delivery are required. This high-quality rigor often also adds time to the process of scheduling and performing SRS.3 Institutional protocols, departmental workflows, patient social circumstances, and insurance prior authorization may further compound to lengthen the time interval between the treatment planning MRI and SRS delivery. During this time period, tumor displacements and volumetric changes of the BM can occur and influence the overall quality of SRS delivery.
To date, there are limited data examining the effect of time from diagnosis to treatment on SRS planning for BM. In a recent study of 34 patients (59 lesions), Salkeld et al demonstrated that half of the patients required a change in planning margins with longer intervals between imaging studies exhibiting a larger effect.4 Moreover, information regarding lesion volume changes from MRI scans acquired at different time points during the treatment planning process as a function of primary tumor type, initial lesion size, tumor spatial location, or effects of systemic therapies remains limited. Because of this, this study seeks to characterize the influence of these variables on tumor volume dynamics in patients treated with SRS.
Methods
Patient Selection
Following Institutional Review Board (IRB) approval, we conducted a retrospective review of consecutive patients with BM treated with SRS from July 2017 to June 2020. All patients who underwent 2 brain MRIs with identical imaging parameters (three-dimensional [3D] magnetization-prepared rapid gradient-echo [MPRAGE] sequences with identical magnet strength and slice thickness/in-plane resolution) obtained within 42 days of each other prior to treatment were included in this study. Lesions present on both the initial diagnostic MRI (MRI-1) and the treatment planning MRI (MRI-2) were reviewed. Patients were excluded from the review if they did not meet the above criteria or if: (1) either imaging was obtained at an outside facility; (2) either image was distorted due to patient motion preventing analytical assessment; (3) the lesion underwent previous resection; (4) no MRI scanner information was available; or (5) if the lesion of interest underwent staged radiotherapy treatment (and therefore subject to tumor change in between treatments).
Data Gathering
Relevant patient data including patient’s sex, age, tumor histology and associated mutations, corticosteroid use, and systemic therapy around SRS were collected. In addition, imaging information, including the dates of the MRIs of interest (MRI-1 and MRI-2) were recorded. Radiotherapy information, including the number of lesions, location of the centroid of the lesion in the right to left (R-L), superior to inferior (S-I), and anterior to posterior (A-P) planes, maximum lesion dimension (cm), and lesion volume (cc) were recorded.
Contouring Procedures
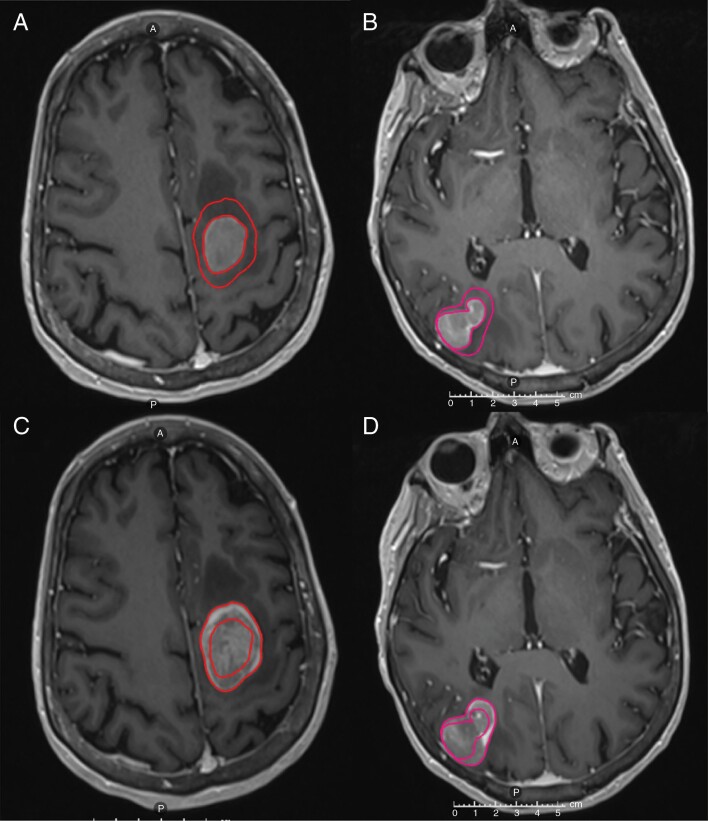
All contouring was performed on RayStation v.5.3.027 (RaySearch Laboratories, Stockholm, Sweden). The MRI-2 images were coregistered to the MRI-1 images using a standardized departmental protocol via the rigid fusion algorithm of RayStation with region of interest (ROI) limited to the brain. A secondary, independent radiation oncology physicist reviewed the fusion registration and verified the fidelity of the coregistration by validating anatomy landmarks. The gross tumor volumes (GTVs) were contoured as the contrast-enhanced lesions on the MRI-1 and MRI-2 T1, post-contrast MPRAGE sequences (Figure 1). The GTVs were contoured by the same radiation oncologist on the MRI-1 and the MRI-2 images. All contours were reviewed by one other observer to ensure target volume delineation fidelity without initial comparison between the imaging studies.
Figure 1.
Axial T1-weighted post-contrast images of a patient with BRAF-mutated metastatic melanoma with brain metastases in the left frontal (A) and right parietal lobes (B) visualized on the diagnostic MRI (MRI-1). Comparison with the treatment planning MRI (MRI-2) performed at SRS 8 days later demonstrated significant interval growth with comparative contours displayed (C, D). Abbreviations: MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery.
Tumor Dynamics Assessment
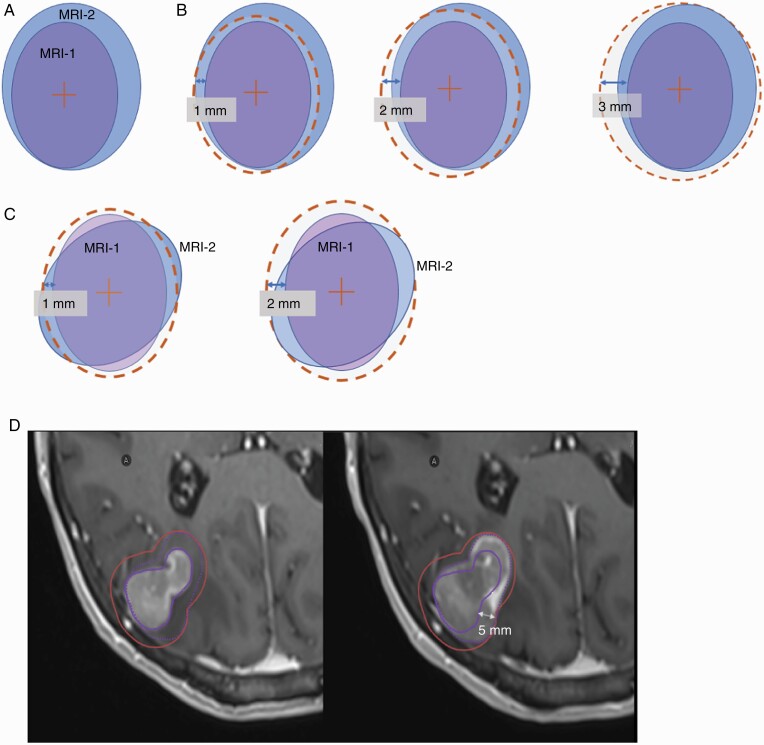
GTV volume, maximum tumor diameter, and geometric location of tumor centroid in A-P, S-I, and R-L axes were recorded for all lesions on both the MRI-1 and MRI-2 image sets. The absolute and percentage differences in volume and maximum diameter between GTVs on MRI-1 and MRI-2 were also calculated. A systematic approach was used to evaluate the margin needed to cover the complete tumor visualized on MRI-2 (in comparison with MRI-1) by creating uniform expansions of the GTV in 1-mm increments. This was applied to all targets identified on MRI-1 to account for changes in size, position, and location. Illustrations of this methodology and case examples are provided in Figures 2 and 3.
Figure 2.
Schematic 2D illustration of the systematic approach to evaluating tumor dynamics used in this study. An example GTV volume on MRI-1 is shown in purple and in blue to represent the GTV on MRI-2 (A). In the first scenario, there is a tumor volume enlargement observed on comparison on MRI-2 to MRI-1. Consequently, a uniform expansion with 1-, 2-, and 3-mm margin is implemented to ensure target volume coverage. In this example, a 3 mm covered the GTV on MRI-2 (B). In a second scenario, the tumor has not changed in size between the MRIs but has changed in shape, also resulting in undercoverage of the target volume. An expansion margin of 2 mm is needed to ensure target volume coverage (C). Actual case example of a treatment planning MRI for a right parietal brain metastasis treated with SRS. Comparison of initial tumor size required a 5-mm margin to adequately cover the disease extent at the time of treatment (D). Abbreviations: GTV, gross tumor volume; MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery.
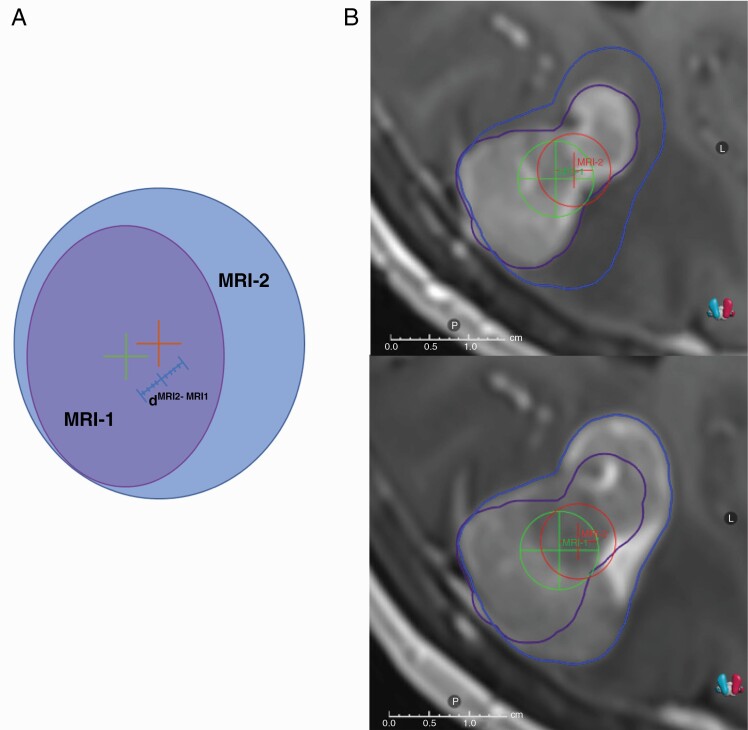
Figure 3.
Schematic 2D view of the distances between the centroids of the target volumes visualized on MRI-1 and MRI-2 (A). Tumor centroid of the GTV on MRI-1 (dMRI-1) is shown with a green plus sign and the tumor centroid of the GTV on MRI-2 is shown with orange plus sign. The maximum distance between the tumor centroids (dMRI-1 to dMRI-2 distance) is shown with a blue line (A). Actual view of tumor displacement on an example MRI-1 and MRI-2. The patient had a tumor centroid displacement of 2.3 mm in R-L direction, 1 mm in S-I direction, and 1.1 mm in P-A direction. Therefore, the overall dMRI-1 to dMRI-2 distance was 2.74 mm (B). Abbreviations: GTV, gross tumor volume; MRI, magnetic resonance imaging.
Statistical Analysis
Descriptive statistics were computed. The impact of histology, lesion spatial location, initial volume, and initial diameter size was evaluated on final lesion size, volume, and spatial position and correlated with the number of days between MRI-1 and MRI-2. For continuous variables, the median and interquartile range (IQR) were presented; sample sizes and percentages were presented for categorical variables. The Kruskal-Wallis rank-sum test was used for comparisons. The primary endpoint of interest in this analysis was a change in treatment management, defined as the need for replanning, an expansion of >1 mm was needed to cover the extent of the disease. To assess factors related to the outcome of change in management, a logistic regression model was fit to the data. The covariates were ECOG (Eastern Cooperative Oncology Group) performance status, systemic therapy, corticosteroid usage, initial tumor diameter, histology, and time interval between MRIs. Wald’s test was used to determine the statistical significance. The level of significance was set to P < .05. Statistical analysis was performed using SPSS, version 27 (SPSS Inc, Chicago, IL, USA).
Results
Patient and Lesion Characteristics
From July 2017 to June 2020, 531 BM in 101 consecutive patients met inclusion criteria for this study (Supplementary Table 1). The median age was 60.5 years (range: 20-89 years) and 45.5% were male. The most common primary tumor was lung (51.5%) and then followed by breast cancer (24.8%). Fifty-six patients (55.4%) were on corticosteroids at the time of treatment and 15 (14.9%) received concurrent systemic therapy at the time of SRS. The three most common locations of BM were the frontal lobe (181 lesions, 34.1%), temporal lobe (96, 18.0%), and cerebellum (91, 17.1%). Overall, 147 (27.7%) of 531 lesions met the criteria for change in management. Table 1 demonstrates the characteristics of lesions for all patients and the lesions that met the need for change in management based on MRI-2.
Table 1.
Lesion-Based Characteristics and Margin Assessment
| All Lesions | Number and Percentage of Lesions Requiring >1-mm Margin to Cover Disease Extent at Time of Treatment | |||
|---|---|---|---|---|
| N | % | N | % | |
| Location of the lesion | ||||
| Frontal lobe | 181 | 34.1 | 59 | 40.1 |
| Temporal lobe | 96 | 18.0 | 26 | 17.7 |
| Cerebellum | 91 | 17.1 | 20 | 13.6 |
| Occipital lobe | 76 | 14.3 | 23 | 15.6 |
| Parietal lobe | 66 | 12.4 | 16 | 10.9 |
| Brainstem | 21 | 4.1 | 3 | 2.0 |
| Concurrent systemic therapy | ||||
| Yes | 95 | 17.9 | 16 | 10.9 |
| No | 436 | 82.1 | 131 | 89.1 |
| Corticosteroids | ||||
| Yes | 243 | 45.7 | 83 | 56.5 |
| No | 288 | 54.3 | 64 | 43.5 |
| Histology of primary lesion | ||||
| Lung cancer | 256 | 48.2 | 73 | 49.7 |
| Malign melanoma | 26 | 4.9 | 11 | 7.5 |
| Breast cancer | 181 | 34.1 | 44 | 29.9 |
| Colorectal cancer | 23 | 4.3 | 0 | 0.0 |
| Other | 45 | 8.5 | 19 | 12.9 |
| Total | 531 | 100 | 147 | 100 |
Tumor Volume, Diameter Change, and Tumor Spatial Displacement
Tumor volume and diameter change
The median MRI-1 tumor diameter and volume were 0.50 cm (IQR: 0.33-0.89 cm) and 0.06 cm3 (IQR: 0.02-0.31), respectively. In comparison, the median MRI-2 tumor diameter and volume were 0.57 cm (IQR: 0.4-0.96 cm) and 0.076 cm3 (IQR: 0.03-0.38), respectively. Thus, the median percentage increase in lesion diameter and volume were 9.5% (IQR: 2.25%-24.0%) and 20% (IQR: 0.7%-66.7%). Overall, there was a statistically significant increase in median tumor volume between MRI-1 and MRI-2 (P = .01). Statistically significant differences in tumor volume were also observed across tumor histology (P = .01); melanoma BM had the largest tumor volume changes across the interval.
Tumor spatial displacement
Directional displacements for the R-L axis were observed in 280 lesions (median: 0.2 mm, IQR: 0.1-0.4 mm), S-I displacements in 304 lesions (median: 0.3 mm, IQR: 0.1-0.6 mm), and P-A displacements in 294 lesions (median: 0.2 mm, IQR: 0.1-0.5 mm). Directional tumor displacements of the centroids were observed in 355 lesions (median: 0.49 mm, IQR: 0.24-0.86 mm) (Supplementary Figure 1). Centroid displacement of more than 1 mm (meeting criteria for change in management) was observed in 66 (12.4%) lesions. Directional displacement analysis revealed displacements in the R-L direction in 16 (3%) lesions, S-I direction in 27 (5%) lesions, and P-A direction in 23 (4.3%) lesions. Overall, 355 (67%) lesions had any displacement in at least 1 direction between MRI-1 and MRI-2, and 205 (38%) lesions had displacements in all 3 directions.
Tumor displacements also differed across various histologies with largest displacements in renal cell carcinoma metastases (P < .001). The magnitude of displacement was also significantly associated with tumor location (P = .03) with the largest displacements observed in parietal lobe lesions. There were no differences observed on tumor displacements with concurrent systemic therapy or corticosteroids (P = .1 and P = .1, respectively).
Tumor margin
Expansions were added to the MRI-1 to cover the new tumor volume visualized on MRI-2 and analyzed to determine the increased margin needed to cover the additional BM extent. Overall, 147 lesions (27.7%) in 57 patients (56.4%) required the addition of >1-mm margin to cover the tumor volume delineated on MRI-2—meeting the study defined criteria for a change in management. Descriptive statistics for these lesions and patients are shown in Table 1. Of the lesions requiring margin additions to cover the changes, the number of lesions covered with a 1-mm uniform expansion were 384 (72.3%), while 482 (90.8%) were covered with a 2-mm uniform expansion, A 3-mm expansion covered 510 (96.1%) lesions while the remaining 21 lesions (3.9%) required >3-mm expansion (Table 2).
Table 2.
Evaluation of the Margins Needed to the Target Volume Based on the Diagnostic MRI (MRI-1) Needed to Cover the Extent of Disease on the Treatment Planning MRI (MRI-2)
| Uniform Margin Expansion | N | % |
|---|---|---|
| 1 mm | 384 | 72.3 |
| 2 mm | 482 | 90.8 |
| 3 mm | 510 | 96.1 |
| >3 mm | 531 | 100 |
Based on logistic regression, systemic therapy was statistically significantly associated with a change in management (odds ratio [OR] = 0.47, 95% CI: 0.24-0.88, P = .02) and having systemic therapy decreased the OR by 54%. Initial tumor diameter was statistically significantly associated with a change in management (OR = 2.69, 95% CI: 1.93-3.75, P < .001) and an increase of 1 mm in diameter increasing the OR by 9%. Corticosteroid usage, tumor histology, and ECOG performance status were not statistically significantly associated with a change in management (P ≥ .05) (Table 3).
Table 3.
Results of Logistic Regression With Change in Management as the Outcome Variable
| Estimate | Std. Error | z Value | P Value | |
|---|---|---|---|---|
| Intercept | −1.71 | 0.42 | −4.04 | <.001 |
| ECOG performance scale | −0.30 | 0.17 | −1.78 | .08 |
| Concurrent systemic therapy | −0.77 | 0.33 | −2.35 | .02 |
| Corticosteroid | 0.15 | 0.24 | 0.61 | .54 |
| Initial tumor diameter | 0.99 | 0.17 | 5.97 | <.001 |
| Histology: breast cancer | Reference | |||
| Histology: colorectal cancer | −17.34 | 768.78 | −0.02 | .98 |
| Histology: malign melanoma | 0.19 | 0.50 | 0.37 | .71 |
| Histology: lung cancer | −0.35 | 0.29 | −1.21 | .22 |
| Histology: other | −0.65 | 0.54 | −1.21 | .23 |
| Histology: renal cell carcinoma | 1.25 | 0.65 | 1.93 | .05 |
| Histology: soft tissue sarcoma | 0.68 | 0.86 | 0.78 | .43 |
| Time between the MRIs | 0.05 | 0.01 | 3.83 | <.001 |
Abbreviations: ECOG: Eastern Cooperative Oncology Group, MRI, magnetic resonance imaging.
Timing Results
The median time between MRI-1 and MRI-2 was 8 days (range: 1-42 days). The time was 28 days or fewer for 503 (94.7%) lesions, 14 days or fewer less for 391 (73.6%) lesions, and 7 days or fewer for 234 (44.0%) lesions (Supplementary Figure 2). For those with the shortest inter-scan time interval (7 days or fewer), 48 (20.5%) lesions were >1 mm outside the initial target volume. The median margin needed to encompass the entire extent of disease on MRI-2 compared to MRI-1 for this subset of patients was 0.77 mm (IQR: 0.41-1.0 mm) for all lesions and 1.57 mm (IQR: 1.37-2.28 mm) for the lesions that needed >1-mm margin. For patients with more than 7 days between MRI-1 and MRI-2, 99 (33.3%) lesions were >1 mm outside the initial target volume. When the inter-scan time interval exceeded 7 days, the median margin needed to encompass the extent of disease was 0.97 mm (IQR: 0.54-1.39 mm) for all lesions and 1.85 mm (IQR: 1.38-2.41 mm) for the lesions that needed >1-mm margin. The median tumor growth was 14.6% (IQR: 1.21%-50%) for the patients with a scan time interval of 7 days or fewer, compared to 33.3% (IQR: 0%-81.35%) for scan time interval of >7 days (Supplementary Table 2). With an inter-scan time interval of 14 days or fewer, 94 (24.0%) lesions would require a change in management. For patients with more than 14 days between MRI-1 and MRI-2, 53 (37.9%) lesions would require a change in management.
Based on logistic regression, an increase in interval (days) between MRI-1 and MRI-2 was statistically significantly associated with a change in management (OR = 1.05, 95% CI: 1.03-1.07; P < .001) (Table 3). Each additional day between MRI-1 and MRI-2 was associated with increased odds of tumor margin expansion beyond 1 mm of 5%.
Discussion
SRS is delivered via stereotactic guidance with submillimeter accuracy.4 Although frame-based SRS technologies have traditionally used same-day treatment planning MRIs for target volume delineation, the recent increase of non–frame-based approaches and availability of pre-frame MRI workflows have resulted in a nonstandardized approach regarding the timing of MRI used for treatment planning. In our study, we evaluated tumor centroid displacement, tumor volume and diameter change, and evaluation of uniform margin expansion needed for tumor coverage with varying intervals between the diagnostic and treatment planning MRI. To our knowledge, our study is the largest analysis to date that investigates each of these variables independently using dedicated MRI sequences specifically designed to evaluate changes that occur during SRS.
We demonstrated that measurable volume, lesion diameter, and geometric location of centroid changes occur in BM between MRIs and this time interval negatively impacts the accuracy of SRS delivery. In fact, in this study, the median tumor volume growth was 20% in a median of 8 days. Overall, more than half of the patients and almost a third of lesions required a change in management due to a >1-mm difference between MRIs. In other words, almost 3 out of 10 BM need an alteration in their management in just 1 week. Moreover, an increase in the days between diagnostic and planning MRI images was statistically significantly associated with the need for a change in management or new MRI for treatment planning. Strikingly, each day between these 2 MRIs was associated with an increased tumor margin expansion beyond 1 mm of 5%. Taken together, these findings support the current practice of a treatment planning MRI performed as close to the treatment as possible to ensure no tumor displacements or volume changes.
There are limited data in the literature to guide clinicians as to how closely to monitor changes in diameter and volume of BM during planning and determine the optimal window from treatment planning scan to SRS delivery. SRS is a complex treatment that requires interdepartmental coordination for accurate and safe administration. There are several variables that must be accounted for in the interval from planning to treatment. Socioeconomic variables, such as insurance approval, coordination for transportation for a patient to treatment, treatment time availability, and weekends and national holidays may lengthen the time interval between treatment planning imaging and treatment delivery. Typically ranges 5-10 days between treatment planning imaging and delivery vary in the published literature.5–7 Each of these should be considered and accounted for when scheduling and planning SRS cases.
Three key studies have evaluated the time from diagnosis MRI to treatment planning MRI for BM patients, each reporting an increase in tumor size between the studies. Although these provide important insights into differences in lesion volume across MRI timings, the current analysis builds on this prior work to provide additional comprehensive evaluations in a large cohort of patients on the effect of lesion volume, size, histology, and effect of systemic agents on final lesion size, volume, and position. For example, Garcia et al reported a median relative change of a 1.44-fold increase in volume between diagnostic to treatment planning MRI for 165 patients treated to 411 lesions. In this study, the mean time interval between the scans was about 3 weeks, which is considerably longer than scan to treatment interval for most institutions and potentially represents diagnosis to treatment planning interval or referral from satellite center to main campus, rather than the treatment planning MRI to treatment.8 Seymour et al have also explored patient outcomes for SRS for BM based on studying this time interval and have demonstrated that local control was lower in metastases with a planning MRI performed ≥14 days before treatment; however, potential tumor volume or displacement changes during this interval was suggested but not evaluated as a rationale for a marginal miss. Nevertheless, this study has since led to the recommendation that the delay from MRI to SRS should be fewer than 14 days.9 In a recent analysis with the shortest inter-scan time, Salkeld et al demonstrated that a change in management was required in 46% of lesions with a 7-day inter-scan time interval, increasing to 62% of lesions at the intervals longer than 7 days. These changes were most commonly a result of change in the tumor volume. In their analysis, the overall mean change was 0.15 cm3 for BM in ~1 week.4 In our study, the median tumor volume change was 0.016 cm3 in a similar time period and 20.5% of lesions needed a change in management in 7 days and 33.3% of lesions needed remanagement longer than 7 days. The differences in results are hypothesized to be due to differences in median initial tumor volume between the studies as the initial tumor volume was approximately 40 times larger in Salkeld et al’s study compared to our analysis (2.13 cm3 vs 0.06 cm3). Differences could also be attributed to sample size differences between the 2 studies as they evaluated 44 BM compared to 531 in our study. Our results demonstrate that the chance of requiring a margin larger than 1 mm increased at a rate of 5% per day; keeping this risk under 10% requires a 48-hour maximum interval from treatment planning MRI to treatment if no additional planning margin is used at the time of treatment.
In the delivery of SRS, many institutions require positioning of the skull in stereotactic frames or utilize mask-based approaches with cone-beam computed tomography (CBCT) image guidance using skull-focused registration. The variation of tumor centroid location on the day of SRS is often unknown and may be reliant upon an MRI study obtained days to weeks earlier. During this time, tumor shifts can occur even if there is no tumor volume change resulting in inaccurate target coverage. In our study, we evaluated the degree of tumor centroid location displacement as a function of time between 2 MRIs obtained days to weeks apart. In our study, displacement of the tumor centroid was observed in 66.8% of lesions and in 12.4% of cases, the magnitude exceeded 1 mm. Hessen et al evaluated tumor position shifts related to peritumoral volume edema changes over time and found that the median values of the tumor shifts obtained from the diagnostic and planning MRIs were 1.3 mm. The differences between median tumor centroid displacement between our study and the one reported by Hessen et al (0.49 mm vs 1.3 mm, respectively), can be explained by the differences in sample size (531 metastases vs 42 metastases, respectively) and inter-scan time interval (8 days vs 22 days, respectively) between the 2 imaging studies. Interestingly, in their study, in addition to tumor growth, the amount of edema influenced the position of the tumor and thereby the accuracy of dose delivery.10 According to our results, there were no differences observed in terms of tumor displacement with corticosteroids; in part, this reflects the fact that the majority of lesions in our study were rather small and most likely associated with less edema.
The treatment planning process and treatment delivery differ across a variety of SRS platforms. For frameless SRS, 1- to 3-mm setup margin is often used, incorporating the uncertainties of the MRI, registration errors, lesion delineation, and patient setup variability and tumor position variability whereas framed systems usually do not involve a setup margin.11–14 In this study, we observed that a 1-mm uniform expansion was enough to cover the tumor volume, shape, and positional displacement for approximately 72% of the lesions. To cover more than 95% of tumors, a 3-mm uniform margin would be needed at the time of treatment. This added margin significantly increases the irradiated volume. For example, for a 1-cm brain metastasis, a GTV expansion of 3 mm for treatment increases the treated volume by 220%. This margin increase has been demonstrated to be associated with increased risk of treatment-related toxicity. In fact, Kirkpatrick et al demonstrated an increased risk of radiation necrosis in patients treated with a 3-mm setup margin expansion compared to 1-mm expansion (12.5% vs 2.5%) (2). Clearly, time delays between treatment planning MRIs to SRS delivery are associated with inferior targeting, and increasing margins to account for these delays only risks increasing toxicity.
Growth of BM is highly variable among cancer types. The changes in tumor volume, diameter, and centroid displacement induced by timing of SRS may not be of clinical significance for slow-growing histologies but may be more important for rapidly growing histologies or for patients receiving concurrent systemic therapy and/or corticosteroids. Although melanoma BM had the largest volume of changes and renal cell cancer metastases had the greatest tumor displacement across the imaging intervals in our study, we did not observe any difference between the histologies by need for a change in management according to the logistic regression model. Bronnimann et al evaluated 23 melanoma and 31 non–small-cell lung cancer (NSCLC) BM and assessed the kinetics of tumor growth between diagnostic imaging and planning MRI secondary to melanoma and NSCLC specifically.15 In their study, median GTV was 0.5 cm3 for melanoma and 0.4 cm3 for NSCLC at the diagnostic scan, and there was 1- and 0.4-cm3 tumor volume increase in median 24 days and 29 days for melanoma and NSCLC lesions, respectively. Additional studies might be necessary to identify key characteristics of complex tumors with hemorrhagic or cystic lesions. In our study, systemic therapy was statistically significantly associated with change in patient management, and having any systemic therapy during this time interval decreased the need for a change in management by 54%. Although lesion response for those treated with systemic therapies does not occur immediately, this may be hypothesized as a mechanism of preventing rapid tumor growth in subsets of BM. We also observed that just under half of the patients were on corticosteroids in our study but there was no association between corticosteroid use and tumor volume change or position. In clinical practice, we observe the most significant effect of corticosteroids on edema within 24-72 hours after administration, and the greatest change is in edema volume, which is less of an issue with smaller lesions.16
Our study has several limitations including the fact that this is a single institution retrospective study. A second limitation is related to the imaging parameters evaluated in this study, including MRI quality, potential distortion during registration, or registration errors of 2 MRIs.17,18 In clinical practice, it might be difficult to accurately and sufficiently coregister the 2 image sets, and there will always be some degree of uncertainty. The accepted target registration error is 2-3 mm according to the most recent AAPM report, which is above the threshold for the differences observed in this study.19 Additionally, for most MRIs, the mean deviations of localization uncertainty range between 0.7 ± 0.2 mm and 1.4 ± 0.5 mm, depending on the MRI device and the imaging sequence.20 To account for this, in this study, we used an automated rigid fusion algorithm followed by manual matching to decrease uncertainties, and we accepted 1-mm cutoff margin for a change in management. A third limitation to this study is the generalizability of the results in position and volume displacement with time to all BM histologies. Lung cancer and breast cancer accounted for 82.3% of the total lesion volume. However, certain tumor types, such as renal cell carcinoma, melanoma, thyroid cancer, and small-cell lung cancer, can be associated with intra-tumoral hemorrhage, which can result in unpredictable and substantial changes in tumor volume and spatial position. Given the small numbers of such patients represented in our series, no significant histology-specific conclusions could be made from the data. Yet, it is important to note that 11 of 26 (42.3%) melanoma patients and 8 of 13 (61.5%) renal cell cancer patients required a change in their management. For these patients, we recommend the shortest window possible between imaging and treatment (24-48 hours) until future studies incorporating hemorrhagic brain metastasis are analyzed. Another limitation to this study is that we analyzed the size, volumetric, and positional changes of each lesion on an individual lesion basis and did not account for potential intra-patient associations between these variables.
The precision of MRI in detecting the size, volume, and position of metastases changes with the thickness of MRI slices, field strength, and the timing and technique of image acquisition.21 One of the strengths of this study is that we only included patients who had both the diagnostic and planning MRIs, and the imaging utilized identical slice thickness and field strength. Another key strength was a systematical review of size and location, to better understand the interplay among these. We observed that some lesions had no tumor volume change but due to the change in their geometrical shape and centroid displacement, an additional margin would have been necessitated to cover the lesion. To avoid this pitfall of margin expansion, we perform a treatment planning MRI no more than 48 hours prior to SRS, with a majority obtained in less than 24 hours. Our data suggest that this shorter window provides a more accurate assessment of tumor diameter, volume, and centroid location. One incidental benefit of performing the planning MRI immediately prior to treatment is to uncover potentially new lesions that were not detectable on the initial MRI; this was the case for 134 of our excluded lesions (a subject of a forthcoming analysis).
In summary, this is the largest study to investigate the impact of time between MRIs, concurrent systemic therapy, and corticosteroids and the change in tumor volume, tumor diameter, tumor centroid displacement, and associated margin needed for coverage of tumor growth. It is widely believed that minimizing the interval between treatment planning imaging and SRS is desirable. We recommend that the interval between imaging and treatment be as short as reasonably possible, ideally within 24-48 hours as to avoid replanning or adverse radiation effects to neighboring regions due to margin expansions.
Supplementary Material
Acknowledgments
The preliminary results of this study were submitted to the 63rd ASTRO Annual Meeting and accepted for poster presentation.
Conflict of interest statement. T.K.: None. R.T.: None. A.W.: None. M.C.T.: Honoraria from ViewRay Inc, Research funding from Blue Earth Diagnostics. J.D.V.: None. H.A.: Honoraria from Novocure Inc. M.D.H.: Honorarium from Accuray Inc. Proton Collaborative Group Executive Committee Institutional Representative and Voting Member, Miami Cancer Institute (unpaid). Grant Funding: Live Like Bella Pediatric Cancer Research Initiative, Florida Department of Health Grant 8LA04. D.J.J.W.: None. S.D.: None. M.W.M.: Consultant Deinde Medical and Stryker Medical. M.S.A.: Receipt of grants/research supports: AstraZeneca, AbbVie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck. Stock shareholder: Doctible, MimiVax. Receipt of honoraria or consultation fees: Elsevier, Wiley, AbbVie, VBI Vaccines, Bayer, Karyopharm, Tocagen, Forma Therapeutics. M.P.M.: Consulting for Zap, Mevion, Karyopharm, Tocagen, AstraZeneca. Board of Directors: Oncoceutics. A.N.G.: Honoraria from ViewRay Inc, Elekta AB, IBA S.A.; ownership of uPlan Oncology. R.K.: Honoraria from Elsevier, Elekta AB, Accuray Inc, Novocure Inc, and ViewRay Inc. Research funding from Medtronic Inc, Blue Earth Diagnostics, Novocure Inc, AstraZeneca, Exelixis, and ViewRay Inc. Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Authorship statement. A.W. and T.K. are responsible for statistical analysis.
Funding
None.
References
- 1. Suh JH, Kotecha R, Chao ST, et al. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–108. [DOI] [PubMed] [Google Scholar]
- 4. Salkeld AL, Hau EKC, Nahar N, et al. Changes in brain metastasis during radiosurgical planning. Int J Radiat Oncol Biol Phys. 2018;102(4):727–733. [DOI] [PubMed] [Google Scholar]
- 5. Stross WC, Malouff TD, Trifiletti DM, et al. MRI-based radiosurgical planning: implications in imaging timing. Ann Transl Med. 2019;7(Suppl 6):S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bodensohn R, Kaempfel AL, Fleischmann DF, et al. Simultaneous stereotactic radiosurgery of multiple brain metastases using single-isocenter dynamic conformal arc therapy: a prospective monocentric registry trial. Strahlenther Onkol. 2021;197(7):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minniti G, Scaringi C, Lanzetta G, et al. Comparative effectiveness of multi-fraction stereotactic radiosurgery for surgically resected or intact large brain metastases from non-small-cell lung cancer (NSCLC). Lung Cancer. 2019;132:119–125. [DOI] [PubMed] [Google Scholar]
- 8. Garcia MA, Anwar M, Yu Y, et al. Brain metastasis growth on preradiosurgical magnetic resonance imaging. Pract Radiat Oncol. 2018;8(6):e369–e376. [DOI] [PubMed] [Google Scholar]
- 9. Seymour ZA, Fogh SE, Westcott SK, et al. Interval from imaging to treatment delivery in the radiation surgery age: how long is too long? Int J Radiat Oncol Biol Phys. 2015;93(1):126–132. [DOI] [PubMed] [Google Scholar]
- 10. Hessen ED, van Buuren LD, Nijkamp JA, et al. Significant tumor shift in patients treated with stereotactic radiosurgery for brain metastasis. Clin Transl Radiat Oncol. 2017;2:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartgerink D, Swinnen A, Roberge D, et al. LINAC based stereotactic radiosurgery for multiple brain metastases: guidance for clinical implementation. Acta Oncol. 2019;58(9):1275–1282. [DOI] [PubMed] [Google Scholar]
- 12. Kocher M, Wittig A, Piroth MD, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190(6):521–532. [DOI] [PubMed] [Google Scholar]
- 13. Palmer JD, Trifiletti DM, Gondi V, et al. Multidisciplinary patient-centered management of brain metastases and future directions. Neurooncol Adv. 2020; 2(1):vdaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McClelland S 3rd, Watson GA. Impact of MRI timing on accuracy of stereotactic radiosurgical planning: visualizing the forest from the trees. Int J Radiat Oncol Biol Phys. 2019;103(4):1012–1013. [DOI] [PubMed] [Google Scholar]
- 15. Bronnimann C, Huchet A, Benech-Faure J, et al. Interval between planning and frameless stereotactic radiosurgery for brain metastases: are our margins still accurate? Neurooncol Pract. 2020;7(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. West J, Fitzpatrick JM, Wang MY, et al. Comparison and evaluation of retrospective intermodality brain image registration techniques. J Comput Assist Tomogr. 1997;21(4):554–566. [DOI] [PubMed] [Google Scholar]
- 18. Pappas EP, Alshanqity M, Moutsatsos A, et al. MRI-related geometric distortions in stereotactic radiotherapy treatment planning: evaluation and dosimetric impact. Technol Cancer Res Treat. 2017;16(6):1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brock KK, Mutic S, McNutt TR, et al. Use of image registration and fusion algorithms and techniques in radiotherapy: report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys. 2017;44(7):e43–e76. [DOI] [PubMed] [Google Scholar]
- 20. Karger CP, Hipp P, Henze M, et al. Stereotactic imaging for radiotherapy: accuracy of CT, MRI, PET and SPECT. Phys Med Biol. 2003;48(2):211–221. [DOI] [PubMed] [Google Scholar]
- 21. Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol. 2018;149:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.