Abstract
The relationship of body mass index (BMI) with lung function and COPD has been previously described in several high-income settings. However, few studies have examined this relationship in resource-limited settings where being underweight is more common. We evaluated the association between BMI and lung function outcomes across 14 diverse low- and middle-income countries. We included data from 12,396 participants aged 35–95 years and used multivariable regressions to assess the relationship between BMI with either COPD and lung function while adjusting for known risk factors. An inflection point was observed at a BMI of 19.8 kg/m2. Participants with BMI<19.8 kg/m2 were 2.28 times (95% CI 1.83–2.86) more likely to have COPD and had a 0.21 (0.13–0.30) lower FEV1 and 0.34 (0.27–0.41) lower FEV1/FVC z-score compared to those with BMI≥19.8 kg/m2. The association with lung function remained even after excluding participants with COPD. Individuals with lower BMI were more likely to have COPD and had lower lung function compared to those in higher BMI. The association with lung function remained positive even after excluding participants with COPD, suggesting that being underweight may also play a role in having worse lung function.
INTRODUCTION
COPD is a chronic respiratory disease marked by fixed, non-reversible airflow obstruction and is the third leading cause of death globally [1–3]. The vast majority of morbidity and mortality related to COPD occurs in low- and middle-income countries (LMICs) where the burden of disease is expected to rise over the next decade.3 COPD is a largely heterogeneous disease with different phenotypic expression and outcomes. While the phenotypes and risk factors for COPD have been well described in high-income settings, less is known about risk factors for COPD and phenotypes in LMICs where other more prevalent exposures, such as biomass fuel smoke, that can predispose individuals to COPD [4].
Body mass index (BMI) is associated with COPD, FEV1 decline and mortality in high-income countries (HIC) [1,5,6]. BMI is an important component in prognostic tools for COPD-related mortality such as the Body mass index, airflow Obstruction, Dyspnea and Exercise capacity (BODE) or Age, Dyspnea and airflow Obstruction (ADO) indexes in high income settings [1,7]. Previous studies in HICs have demonstrated the risk of COPD is associated with BMI, and COPD severity is higher in lower BMI categories [8]. In contrast, the relationship between BMI and COPD in LMIC settings, where being underweight is more common, is not as well defined [4]. Individuals in LMICs have unique nutrition-related risk factors, which predispose to poor lung health over the course of the lifespan. Maternal malnutrition and micronutrient deficiencies in childhood have been associated with lower levels of lung function in and higher rates of COPD in adulthood [9].
In this study, we sought to describe the relationship between BMI and lung function outcomes among fourteen low- and middle-income settings in Argentina, Bangladesh, Chile, Peru, Uganda and Uruguay. Our sample encompassed sites with a diversity of geographies, ethnicities, variations in altitude, and degrees of urbanization.
METHODS
Study setting
This study utilized pooled data from multiple population-based studies spanning six countries and thirteen cities in Latin America, Africa and Asia [10–13]. The Pulmonary Risk in South America (PRISA) study was conducted by the Institute for Clinical Effectiveness and Health Policy, the CRONICAS (Spanish for chronic) study was conducted by the CRONICAS Center of Excellence for Chronic Diseases at Universidad Peruana Cayetano Heredia, the Bangladesh study was conducted by the Centre for Control of Chronic Diseases (CCD) at icddrb, the Lung Function in Nakaseke and Kampala (LiNK) study was conducted by the Johns Hopkins University and Makerere University, and the FRESH AIR study was conducted by the University of Groningen and Makerere University [10–13]. Further details about the study populations can be found in previous publications and in Table 1 [10–15]. All countries included were low- to middle-income countries at the date of data collection (PRISA: Argentina, Chile, Uruguay; CRONICAS: Peru; CCD study: Bangladesh; LiNK: Uganda; FRESH AIR: Uganda). Specifically, data from Argentina, Chile and Uruguay were collected in 2011 when these countries were considered middle-income by World Bank classification.
Table 1:
Sociodemographic characteristics stratified by site.
| Bariloche | Canelones | Dhaka | Kampala | Lima | Marcos Paz | Masindi | Matlab | Nakaseke | Rural Puno | Temuco | Tumbes | Urban Puno | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of people | 1099 | 851 | 1672 | 596 | 997 | 1236 | 414 | 1824 | 721 | 500 | 1038 | 945 | 503 |
| BMI (kg/m2), mean (SD) | 29.0 (5.8) | 29.6 (6.2) | 24.6 (4.9) | 26.0 (5.3) | 28.4 (4.4) | 30.1 (5.8) | 22.9 (4.3) | 20.6 (3.6) | 23.8 (4.5) | 25.2 (3.7) | 29.1 (4.9) | 28.3 (4.7) | 27.9 (4.4) |
| BMI ≤ 19.8 kg/m2, % (n) | 2.5 (27) | 2.7 (23) | 16.4 (274) | 10.2 (61) | 0.9 (9) | 1.4 (17) | 21.3 (88) | 47.6 (868) | 14.3 (103) | 4.8 (24) | 1.2 (12) | 1.9 (18) | 1.8 (9) |
| BMI categories (kg/m2), % (n) | |||||||||||||
| 0–18.5 | 0.9 (10) | 1.1 (9) | 10.3 (173) | 3.4 (20) | 0.1 (1) | 0.5 (6) | 9.4 (39) | 30.5 (556) | 8.2 (59) | 1.6 (8) | 0.5 (5) | 0.4 (4) | 0.6 (3) |
| 18.5–25 | 25.0 (275) | 20.0 (170) | 44.7 (748) | 46.5 (277) | 22.0 (219) | 17.9 (221) | 67.1 (278) | 58.3 (1063) | 61.0 (440) | 50.4 (252) | 16.7 (173) | 23.7 (224) | 23.3 (117) |
| 25–30 | 36.2 (398) | 36.0 (306) | 32.7 (546) | 28.9 (172) | 45.4 (453) | 35.1 (434) | 17.4 (72) | 9.6 (176) | 21.1 (152) | 37.2 (186) | 46.2 (480) | 44.3 (419) | 49.7 (250) |
| 30+ | 37.9 (416) | 43.0 (366) | 12.3 (205) | 21.3 (127) | 32.5 (324) | 46.5 (575) | 6.0 (25) | 1.6 (29) | 9.7 (70) | 10.8 (54) | 36.6 (380) | 31.5 (298) | 26.4 (133) |
| Age in years, mean (SD) | 57.6 (7.8) | 59.6 (8.5) | 51.8 (9.3) | 44.2 (8.9) | 54.9 (11.8) | 58.8 (8.2) | 49.7 (12.5) | 55.1 (10.6) | 49.1 (11.2) | 55.5 (12.5) | 59.2 (8.5) | 55.5 (13.1) | 55.2 (12.1) |
| Height in centimeters, mean (SD) | 161.2 (9.8) | 162.2 (9.3) | 155.5 (9.0) | 162.1 (8.7) | 154.8 (8.5) | 161.7 (9.4) | 161.3 (9.3) | 153.9 (8.3) | 159.9 (8.0) | 155.4 (8.0) | 160.3 (9.1) | 158.4 (8.7) | 156.9 (9.0) |
| Number of males, % (n) | 61.2 (673) | 61.7 (525) | 45.5 (761) | 48.7 (290) | 49.2 (491) | 59.9 (740) | 50.0 (207) | 46.0 (839) | 45.9 (331) | 47.0 (235) | 54.7 (568) | 50.2 (474) | 49.5 (249) |
| Education ≥ secondary, % (n) | 49.9 (548) | 49.8 (424) | 44.1 (738) | 20.8 (124) | 45.7 (456) | 34.3 (424) | 16.9 (70) | 18.8 (342) | 7.1 (51) | 33.4 (167) | 70.7 (734) | 36.0 (340) | 65.2 (328) |
| Biomass as primary source of fuel, % (n) | 23.1 (254) | 5.1 (43) | 3.6 (61) | 93.5 (557) | 2.4 (24) | 0.5 (6) | 92.8 (384) | 98.1 (1789) | 99.6 (718) | 95.4 (477) | 22.4 (233) | 16.7 (158) | 2.0 (10) |
| Daily smokers, % (n) | 23.9 (263) | 21.7 (185) | 6.5 (108) | 8.7 (52) | 3.2 (32) | 22.7 (281) | 36.2 (150) | 9.0 (164) | 6.9 (50) | 0.2 (1) | 14.9 (155) | 5.6 (53) | 2.2 (11) |
| Pack-years smoked, mean (SD) | 23.0 (18.3) | 35.2 (30.0) | 20.6 (18.0) | 8.7 ( 9.4) | 2.7 ( 9.0) | 31.4 (22.1) | 6.7 (13.1) | 19.8 (15.9) | 6.5 ( 7.7) | 1.3 ( 4.3) | 13.0 (14.2) | 5.3 (12.9) | 2.7 ( 7.2) |
Study Design
Both the PRISA and CRONICAS studies used age- and sex-stratified random sampling while the Bangladesh study used simple random sampling of available census data at each site. The LiNK study used a sampling technique outlined by the WHO Expandable Programme on Immunization, while the FRESH AIR study used a multi-level sampling approach [10–14,16,17]. Adults between the ages of 35 to 95 years were included for analysis due to restrictions of reference equations for adults beyond 95 years [18]. All studies obtained informed consent and required confidentiality training for field workers [10–14].
Spirometry
Spirometry was conducted using American Thoracic Society and European Respiratory Society guidelines [19]. PRISA, CRONICAS, LiNK, and the Bangladesh utilized Easy-On PC (ndd), while the FRESH AIR study used Pneumotrac (Vitalograph) spirometers [10–13]. Post-bronchodilator readings were taken on all individuals in PRISA and CRONICAS studies, while FRESH AIR and LiNK conducted spirometry on those who screened positive for obstruction (FEV1/FVC ≤ 0.7 in FRESH AIR and the Bangladesh studies, and FEV1/FVC ≤ lower limit of normal in LiNK).
Definitions
We used the Global Lung Function Initiative 2012 (GLI2012) mixed ethnic reference population to calculate z-scores for lung function [18]. We refer to forced expiratory volume at 1 second (FEV1) or forced vital capacity (FVC) as forced expiratory volumes (FEVs). For this analysis, COPD was defined as having a post-bronchodilator FEV1/FVC 1.64 SDs below median (i.e., z-score < −1.64). BMI was defined as weight/height2 (kg/m2). Underweight, normal, overweight, and obese individuals were classified as having a BMI between 0–18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2, respectively [20]. COPD severity was stratified into mild, moderate, severe, and very severe using percent predicted FEV1 values based on GOLD criteria [2]. Participants were also considered to have symptomatic COPD if they had wheeze, cough, or phlegm currently or in the last 12 months. Daily smoking was defined as having one or more cigarette/day, biomass use was defined as using biomass fuel as the primary source for cooking, and education was defined as having completed secondary education or higher.
Biostatistical methods
The main objectives of this study were to analyze associations between BMI and lung function outcomes, including COPD as defined by post-bronchodilator spirometry and pre-bronchodilator FEVs. We limited our analysis to pre-bronchodilator FEVs because two of the study sites (Bangladesh and Uganda) only administered a bronchodilator to individuals who demonstrated obstruction on screening [12,13]. We also conducted secondary analyses to assess the association between BMI and other COPD outcomes, namely severity and the presence of concomitant respiratory symptoms, and pre-bronchodilator FEVs.
We used alternating logistic regression (ALR) models to model the odds of COPD and linear mixed-effects (LME) models to model lung function as function of BMI. All models adjusted for age, sex, daily smoking, self-reported biomass fuel use, education level, and history of tuberculosis [21,22]. We chose ALR and LME models because they allowed us to address the potential of having non-independence due to intra-site correlation [21,22]. In our study, ALR was used with pairwise odds ratios between sites, while LME was used with random intercept and slopes by site. We used natural cubic splines of BMI to investigate the potential non-linear relationships with either COPD or lung function [23]. We also investigated potential effect modification by sex, age (≥55 or <55 years), self-reported biomass fuel smoke exposure, self-reported daily cigarette smoking, and having secondary education.
Regression tree analysis and plotting area under the receiver operating curve (AUC) estimates from multivariable logistic regressions for varying BMI cutoffs were used to help choose a BMI cutoff that most accurately represents the underlying associations [24]. Similarly, we plotted normalized mean squared error (nMSE) estimates for varying FEV1/FVC cutoffs to assess consistency with logistic regression models [25]. For secondary analyses, we used multivariable random effects ordinal logistic regressions to examine the association between BMI and COPD severity (none, mild, moderate or severe/very severe COPD) or symptomatic COPD (none, asymptomatic COPD and symptomatic COPD) adjusted for confounders.
Sensitivity analysis was performed by using the GLI2012 Caucasian reference values, performing leave-one-out analysis by site, analyzing BMI broken down by WHO categories with normal weight as reference, excluding COPD positive individuals from pre-bronchodilator FEVs regression models to avoid reverse causality, limiting analyses to people without severe or very-severe COPD, and using logistic mixed effects models to check for robustness of our model selection when analyzing odds of COPD. Analyses were performed in R version 3.4.4 (www.r-project.org) using the lme4, alr, randomForest, partykit, ggplot2, and gmodels packages [26].
RESULTS
Population Characteristics
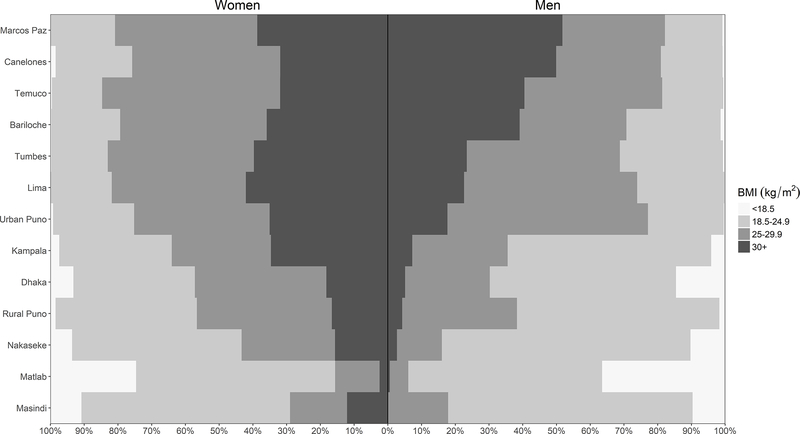
A total of 13,023 participants were included in this study, however, 12,396 met eligibility criteria and were included for analysis. Data was collected across five countries spanning Latin America, Southeast Asia, and Sub-Saharan Africa. People in this study had an average age of 54.9 years (range of mean age across settings 44.2–59.6 years), were 48.5% women (range of proportions across settings 38.3%−54.5%), and 38.3% completed secondary education or higher (range of proportions across settings 7.1%−71.0%). Among our study sample, 7.2% of participants were underweight, 36.0% were normal weight, 32.6% were overweight, and 24.2% were obese; however, there was also heterogeneity across sites and sex (Figure 1). Average BMI was 26.4 kg/m2, ranging from 20.6 kg/m2 in Matlab to 30.1 kg/m2 in Marcos Paz. There was no difference in prevalence of COPD (p=0.97) between those who are included or excluded from our analyses; however, the in-sample population were younger on average (46.0 years vs. 54.9 years, p<0.001), had fewer men (48.5% vs. 54.9%; p=0.002), and had lower BMI on average (25.3 kg/m2 vs. 26.4 kg/m2, p<0.001).
Figure 1: Sex-, site- and category-stratified prevelances of Body Mass Index (BMI).
The prevalence of BMI was stratified by sex (women on the left, men on the right) and WHO classification for underweight, normal, overweight, and obese individuals (BMI between 0–18.5, 18.5–25, 25–30, and over 30 kg/m2, respectively) across the 13 LMIC sites. Sites were ordered according to the overall prevalence of Obesity in Men from highest (top) to lowest (bottom).
Body Mass Index and COPD Outcomes
The overall prevalence of COPD was 8.8%, ranging from 1.7% in Kampala, Uganda to 15.5% in Masindi, Uganda. Among the 1,091 COPD positive participants, 394 (36.1%) were mild, 524 (48.0%) were moderate, 143 (13.1%) were severe, and 30 (2.7%) were very severe. Prevalence of COPD was highest among those with BMI <18.5 kg/m2 and moved progressively lower as BMI increased (Figure E1). It was also apparent that among the lowest BMI category in Figure E1, men had a higher prevalence of COPD than women (31.3% vs 14.8%, respectively, p<0.001).
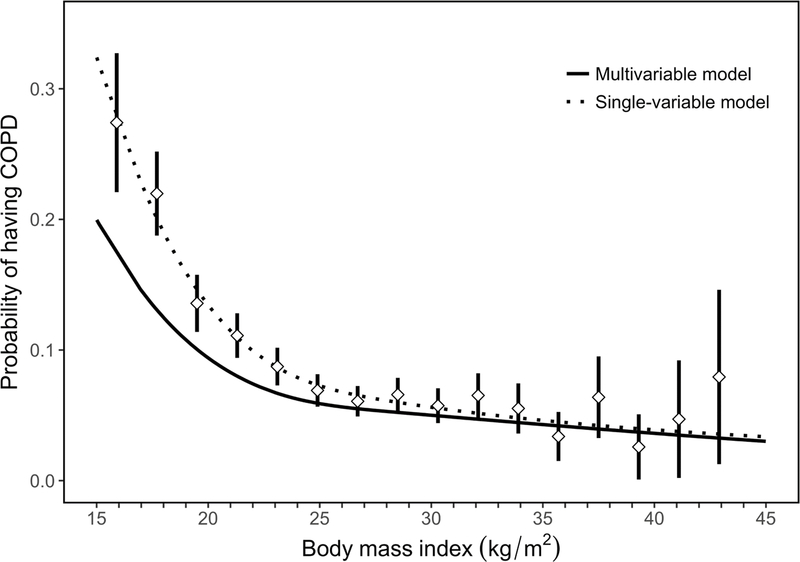
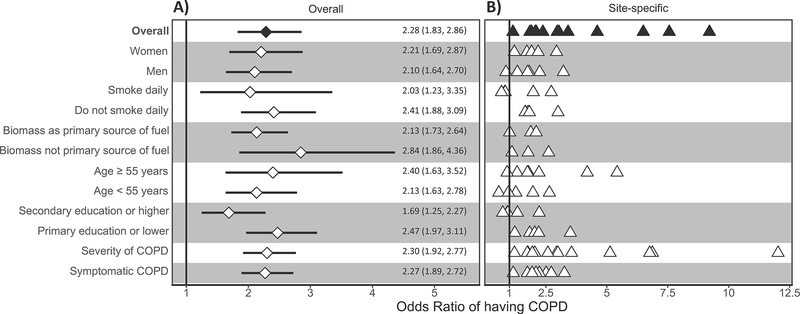
We observed an inflection point for BMI at 19.8 kg/m2, such that the probability of having COPD increased at a faster pace at lower BMI outcomes prior to this threshold in both single variable and multivariable logistic regression analysis (Figure 2). We found complimentary results when using multivariable logistic regression and the AUC as a measure for the area under the Receiver Operating Characteristic curve, with highest predictive accuracy occurring around the 19.5–20.0 kg/m2 (Figure E2). In multivariable regression analysis, participants with BMI <19.8 kg/m2 were 2.28 times (95% CI 1.83–2.86) more likely to have COPD compared to everyone with BMI ≥19.8 kg/m2. We plotted interaction effects between BMI and potential effect modifiers on the odds ratio of having COPD (Figure 3) and found that the association between having a BMI <19.8 kg/m and COPD was greater among those with primary education or less (OR=2.47, 1.97–3.11) vs. secondary education or higher (OR=1.69, 1.25–2.27) when compared to those with BMI ≥19.8 kg/m2. Additionally, participants with BMI <19.8 kg/m2 had higher odds of having more severe COPD (adjusted OR=2.30, 95% CI 1.92–2.77) or having more symptoms (adjusted OR=2.27, 1.89–2.72) when compared to those with BMI ≥19.8 kg/m2.
Figure 2: Body mass index vs. the probability of having COPD in our sample.
We plotted probabilities from a multivariable adjusted model (solid line), single-variable non-adjusted model (broken line), and binned probabilities with corresponding 95% confidence intervals. The multivariable model was adjusted for age, sex, daily cigarette smoking, level of education completed, and post-treatment pulmonary tuberculosis.
Figure 3: Associations between low body mass index (BMI < 19.8 kg/m2) and COPD outcomes obtained from multivariable regression models, and interaction effects with sex, smoking status, biomass use, age, and educational attainment.
Panel A shows estimates using data from all sites, while panel B shows site-specific estimates. In panel A, odds ratios and the corresponding 95% confidence intervals are represented by diamonds and lines, respectively. We also tabulated numerical values for the odds ratios and the corresponding 95% confidence intervals. In panel B, site-specific odds ratios are presented by triangles. In the overall model, we evaluated the association between having a BMI < 19.8 kg/m2 and COPD prevalence adjusted for age, sex, biomass use, daily cigarette smoking, post-treatment pulmonary tuberculosis, and secondary education. We then evaluated for interaction effects between having a BMI < 19.8 kg/m2 and either sex, smoking status, biomass use, age, or educational attainment on COPD outcomes. Models stratified by sex were adjusted for age, daily cigarette smoking, biomass, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by smoking status were adjusted for age, sex, biomass, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by biomass use were adjusted for sex, daily cigarette smoking, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by age were adjusted for sex, daily cigarette smoking, biomass, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by educational attainment were adjusted for age, sex, daily cigarette smoking, biomass, and post-treatment pulmonary tuberculosis. Models with severity and symptom status of COPD as outcomes were adjusted for age, sex, daily cigarette smoking, biomass, post-treatment pulmonary tuberculosis, and secondary education.
In sensitivity analyses, we found that using the GLI2012 Caucasian reference population did not affect the direction of reported exposure-outcome associations (Table E1 and Table E2). Using the WHO BMI categories with normal weight as reference revealed consistent results, with underweight participants having increased odds of COPD and overweight or obese participants having decreased odds of COPD (Table E3). Leave-one-site-out analysis revealed that no single site had particularly heavily influence on association between having a BMI < 19.8 kg/m2 and COPD compared with other sites (Table E4). Moreover, when running models for each site we found that having BMI <19.8 kg/m2 was consistently associated with increased odds of COPD when compared to those with BMI ≥19.8 kg/m2 (Figure 3 and Table E5).
Body Mass Index and Lung Function
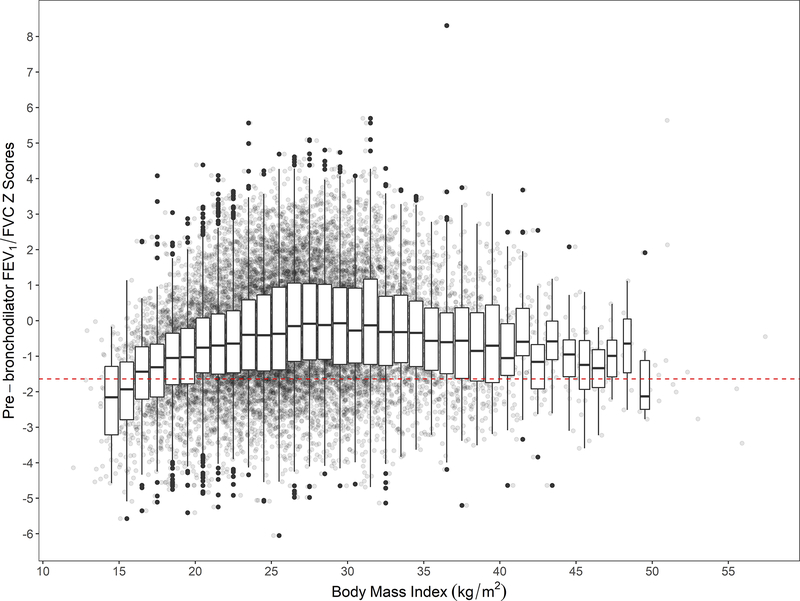
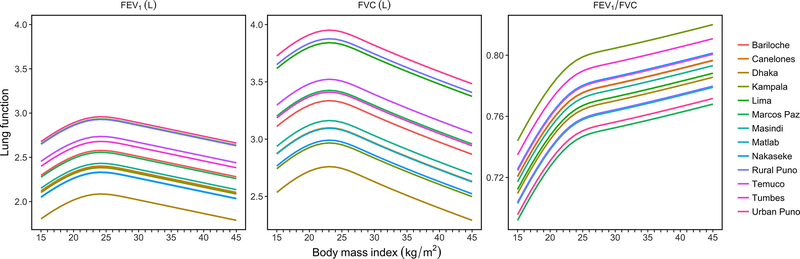
We plotted BMI (kg/m2) vs. pre-bronchodilator FEV1/FVC z-scores with individual boxplots per each unit BMI (Figure 4), and found that participants with lower BMI also have lower FEV1/FVC z-scores. This finding was consistent in multivariable analysis (Figure 5 and Figure E3). On average, people with more extreme values of BMI, i.e. underweight or obese, had lower pre-bronchodilator FEV1 and FVC (Figure 5). When using multivariable linear regression and the plotting the nMSE estimates at varying BMI cutoffs (Figure E4), we observed the highest predictive accuracy around 19.5–20.0 kg/m2. This range shifted to around 22.0–22.5 kg/m2 when COPD positive participant were removed from analysis (Figure E4). In multivariable adjusted analysis, we found that participants with BMI <19.8 kg/m2 had 0.21 (95% CI 0.13–0.30) lower FEV1 z-scores and 0.34 (95% CI 0.27–0.41) lower FEV1/FVC z-scores compared to those with BMI ≥19.8 kg/m2. We observed no difference in pre-bronchodilator z-scores for FVC (−0.04, 95% CI −0.13 to 0.04). These effects remained significant but were slightly lower in magnitude when participants with COPD positive excluded from analyses. Specifically, we found that participants with BMI <19.8 kg/m2 had 0.09 (95% CI 0.01–0.18) lower FEV1 z-scores and 0.08 (95% CI 0.02–0.14) lower FEV1/FVC z-scores compared to those with BMI ≥19.8 kg/m2, with no observable difference in FVC z-scores (−0.07; 95% CI −0.16 to 0.02).
Figure 4: Body mass index vs. pre-bronchodilator FEV1/FVC z-scores.
Crude lung function values were plotted behind boxplots. Each boxplot encompasses values within one-unit of BMI (i.e., 1 kg/m2). We included a broken red line at a pre-bronchodilator FEV1/FVC z-score of −1.64 to help visualize which values are below the lower limit of normal (bottom 5th percentile).
Figure 5: Body mass index vs. fitted values for FEV1, FVC, and FEV1/FVC, stratified by site.
All models were adjusted for age, sex, daily cigarette smoking, level of education completed, and post-treatment pulmonary tuberculosis. We used linear mixed-effects models with random intercepts by site.
DISCUSSION
In this multi-country population-based study, there was a dynamic relationship between BMI and lung function. Although low BMI may be the result of cachexia secondary to COPD as demonstrated in high-income settings, we conducted sensitivity analysis by excluding individuals with COPD and found a similar relationship between lung function and low BMI across the sites [27]. We examine the relationship between BMI and lung function continuously, demonstrating an inflection point at which this change occurs. A similar inflection and parabolic curve occurs for lung function as measured by FEV1 and FVC suggesting that both under- and over-nutrition have deleterious effects of lung function. These models were adjusted for environmental, lifestyle, and socioeconomic variables, suggesting that this non-linear association of BMI is present beyond a priori risk factors. While tobacco continues to be a major risk factor for COPD and lung function decline in high income settings, having low BMI (i.e., underweight or <19.8 kg/m2) may be a potential risk factor for both poor lung function as well as COPD in LMIC settings.
The prevalence of obesity in our LMIC settings is comparable to that of high-income settings. We found that 56.8% of our sample was overweight or obese with a BMI ≥25 kg/m2 (ranging from 11.2% in Matlab to 82.9% in Temuco) compared to 70.7% in the US, reflecting the nutritional transition occurring in many LMICs [28]. The Latin American Project for the Investigation of Obstructive Lung Disease (PLATINO) investigators found higher rates of obesity among the South American population studied than the present analysis, possibly reflecting the urban settings where PLATINO was conducted [29]. The percentage of underweight individuals is higher in the sampled LMIC settings. 7.2% of individuals in our sample were underweight with a BMI <18.5 kg/m2 compared to a rate of 1.7% in the US. We found similar rates of underweight individuals in the overall population when compared to other studies in LMICs [29,30]. Although the PLATINO investigators demonstrated an association between low BMI and COPD, BMI was analyzed as a categorical variable [29]. This study utilizes BMI as a continuous variable, gaining valuable information (inflection at specific BMI) that could potentially influence medical interventions by targeting individuals among more specific BMI ranges.
Few studies in high-income settings and none in low-income settings have analyzed BMI and lung function as continuous values at a population-level. The CARDIA study examined lung function longitudinally over a 10-year period and found FEV1 to decrease with increasing weight across BMI categories [31]. Although lung function in high-income settings appears to worsen with increasing BMI, the opposite is true of COPD-related mortality, probably a result of cachexia from advanced disease. The Copenhagen City Heart Study showed that among COPD positive individuals, relative risk of COPD-related mortality was greatest among those in the lowest BMI category (<20.0 kg/m2) [8]. Relative risk seemed to plateau after individuals reached the next BMI category (20.0–24.9 kg/m2), with small to no change in relative risk with increasing BMI after this point [8]. The results of the Copenhagen City Heart Study informed the development of the BODE index which included a cut-off of ≤21.0 kg/m2 for increased risk of death among those with COPD [27]. Our results demonstrated a similar inflection at 19.8 kg/m2.
Nutritional status and socioeconomic status have been proposed as important risk factors alongside household and environmental air pollution in LMICs [32]. While a lower BMI may be protective for declined lung function in high-income populations, we found the opposite to be true among populations in low- and middle-income countries [31]. Compared to HICs, lower BMI in LMICs is more likely to represent poorer overall nutritional status and micronutrient deficiencies. Nutrition across the lifespan has been linked to FEV1 [9]. Antenatal and childhood micronutrient supplementation with Vitamin A, D and E have all been studied in relationship to subsequent childhood lung function, albeit with mixed results [33,34]. While Vitamin A is thought to play a role in fetal lung development, Vitamin E and C are thought to provide antioxidant functions which decrease inflammatory state leading to lung function decline [35]. Cross-sectional studies have demonstrated lower levels of lung function among individuals with reduced Vitamin C and E intake [35].
In high-income populations, obesity has been associated with faster lung function decline when compared with underweight individuals [31]. Higher BMI has been known to reduce both FEV1 and FVC due to decreasing lung wall compliance, though elevated BMI has mixed effects with the FEV1/FVC ratio [31]. As people grow older, decreased lung elasticity results in lower FEV1/FVC ratio. However, among those with elevated BMI, the FEV1/FVC ratio may be higher due to greater effects of obesity on FVC than FEV1. Additionally, obesity has been linked to a range of cytokines including increased levels of TNF-alpha, IL-6 and decreased levels of adiponectin which result in a proinflammatory state [31,36,37]. Similarly, lower levels of cytokines and baseline inflammation among those with lower BMI in high income settings has been postulated as protective for future lung function decline [31].
There were several limitations in our study. First, we were limited in our adjustment of SES, a known independent risk factor for COPD and lung function which is also associated with BMI [14]. Second, the Global Lung Function Initiative mixed rthnic lung reference population used for diagnosis of COPD may not accurately represent all individuals in our study. Because of this, we performed sensitivity analysis using GLI Caucasian reference population which showed results consistent with that of the GLI mixed ethnic population. Third, understanding a causal relationship between BMI and COPD is limited by the cross-sectional nature of our sample. Also, severe COPD is known to result in poor nutritional status and lower BMI. Due to this, we performed sensitivity analysis by removing individuals with COPD, again showing results consistent with our main analysis. Fourth, we do not have information about early life exposures in our study participants which may affect lung function trajectories. Early life exposures — such as in utero and childhood exposures including micronutrient deficiencies, air pollution, cigarette smoking and childhood pneumonia — play a critical role in lung function development and may be as important as exposures experienced during adulthood in the development of COPD.
Our study also had potential strengths. While the association between COPD and BMI has been previously described, we provide new insights using statistical methods that capture a non-linear relationship between BMI and either the odds of COPD or lung function. Furthermore, this study utilized a large and diverse sample with properly harmonized variables, allowing for the adjustment of priori risk factors for COPD (e.g. smoking and using biomass fuels). Also, we used the most current, more accurate definition for COPD diagnosis (FEV1/FVC ≤ lower limit of normal) instead of the fixed-cutoff method [38].
CONCLUSIONS
The results of this large, multi-country analysis further characterizes the association between body mass index and lung function outcomes. Underweight individuals had lower lung function compared to those in higher BMI ranges, even when those with COPD were excluded. While COPD is well known to be a systemic disease that leads to cachexia, our data also suggests that low body mass index is independently associated with lower lung function. Specifically, undernutrition as measured by a low body mass index may be associated with worse lung function outcomes in adults living in low- and middle-income country settings.
Supplementary Material
ACKNOWLEDGEMENTS
This study was sponsored and funded by the National Heart, Lung, and Blood Institute (NHLBI), a division of the National Institute of Health in the United States, under contract no HHSN268200900033C and HHSN26820900032C. WC is additionally supported under UM1HL134590. TS was supported by a National Research Service Award through the National Institute of Environmental Health Sciences of the National Institutes of Health (1F32ES028577). AR was supported by the National Institutes of Health Office of the Director, Fogarty International Centre and National Heart, Blood, and Lung Institute through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
DECLARATION OF INTEREST STATEMENT
Dr. Jones reports grants, personal fees and non-financial support from Astra Zeneca, personal fees from Boehringher Ingelheim, personal fees from Chiesi, personal fees and non-financial support from GSK, personal fees from Novartis, personal fees from Pfizer, personal fees from Nutricia, outside the submitted work. All other authors report no conflicts of interest.
REFERENCES
- 1.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2003; 187:347–365. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis 2015; 19: 10–20. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Aymerich J, Gómez FP, Benet M, et al. ; PAC-COPD Study Group. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 2011; 66: 430–437. [DOI] [PubMed] [Google Scholar]
- 6.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 2002; 121: 370–376. [DOI] [PubMed] [Google Scholar]
- 7.Puhan MA, Hansel NN, Sobradillo P, et al. ; International COPD Cohorts Collaboration Working Group. Large-scale international validation of the ADO index in subjects with COPD: an individual subject data analysis of 10 cohorts. BMJ Open 2012; 2:e002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:1856–1861. [DOI] [PubMed] [Google Scholar]
- 9.Checkley W, Pollard SL, Siddharthan T, et al. Managing threats to respiratory health in urban slums. Lancet Respir Med 2016; 4:852–854. [DOI] [PubMed] [Google Scholar]
- 10.Jaganath D, Miranda JJ, Gilman RH, et al. ; CRONICAS Cohort Study Group. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res 2015; 16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinstein AL, Irazola VE, Bazzano LA, et al. Detection and follow-up of chronic obstructive pulmonary disease (COPD) and risk factors in the Southern Cone of Latin America: the pulmonary risk in South America (PRISA) study. BMC Pulm Med 2011; 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health 2015; 3:e44–e51. [DOI] [PubMed] [Google Scholar]
- 13.Alam DS, Chowdhury MA, Siddiquee AT, et al. Prevalence and Determinants of Chronic Obstructive Pulmonary Disease (COPD) in Bangladesh. COPD 2015; 12:658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigsby M, Siddharthan T, Chowdhury MA, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis 2016; 11:2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddharthan T, Grigsby MR, Goodman D, et al. Association between Household Air Pollution Exposure and Chronic Obstructive Pulmonary Disease Outcomes in 13 Low- and Middle-Income Country Settings. Am J Respir Crit Care Med 2018; 197:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostoen K, Chalabi Z. Optimization of household survey sampling without sample frames. Int J Epidemiol 2006; 35:751–755. [DOI] [PubMed] [Google Scholar]
- 17.Chao LW, Szrek H, Peltzer K, et al. A Comparison of EPI Sampling, Probability Sampling, and Compact Segment Sampling Methods for Micro and Small Enterprises. J Dev Econ 2012; 98:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, et al. ; ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26:319–338. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva; 1995. [PubMed] [Google Scholar]
- 21.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika 1993; 80:517–526. [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38:963–974. [PubMed] [Google Scholar]
- 23.Hastie TJ. Generalized additive models. In: Chambers JM, Hastie TJ, eds. Statistical Models in S. Bota Raton: CRC Press; 1982: 249–304. [Google Scholar]
- 24.Breiman L Classification and regression trees. Bota Raton: Chapman & Hall/CRC Press; 1984. [Google Scholar]
- 25.Grigsby MR, Di J, Leroux A, et al. Novel metrics for growth model selection. Emerg Themes Epidemiol 2018; 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 27.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Eng J Med 2004; 350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. 2017. [PubMed] [Google Scholar]
- 29.Montes de Oca M, Tálamo C, Perez-Padilla R, et al. ; PLATINO Team. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med 2008; 102:642–650. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Zhou M, Smith M, et al. Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol 2010; 39:1027–1036. [DOI] [PubMed] [Google Scholar]
- 31.Thyagarajan B, Jacobs DR Jr, Apostol GG, et al. Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res 2008; 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Checkley W, Ghannem H, Irazola V, et al. ; GRAND South Network, UnitedHealth Group/National Heart, Lung, and Blood Institute Centers of Excellence. Management of NCD in low-and middle-income countries. Glob Heart 2014; 9:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devakumar D, Stocks J, Ayres JG, et al. Effects of antenatal multiple micronutrient supplementation on lung function in mid-childhood: follow-up of a double-blind randomised controlled trial in Nepal. Eur Respir J 2015; 45:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Checkley W, West KP Jr, Wise RA, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med 2010; 362:1784–1794. [DOI] [PubMed] [Google Scholar]
- 35.Gilliland FD, Berhane KT, Li Y-F, et al. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol 158:576–584. [DOI] [PubMed] [Google Scholar]
- 36.Cancello R, Tounian A, Poitou C, et al K. Adiposity signals, genetic and body weight regulation in humans. Diabetes Metab 2003; 30:215–227. [DOI] [PubMed] [Google Scholar]
- 37.Steffes MW, Gross MD, Schreiner PJ, et al. Serum adiponectin in young adults—interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol 2004; 14:492–498. [DOI] [PubMed] [Google Scholar]
- 38.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008; 63:1046–1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.