Abstract
Live and deep imaging play a significant role in the physiological and biological study of organisms. Two-photon excitation microscopy (2PEM), also known as multiphoton excitation microscopy, is a fluorescent imaging technique that allows deep imaging of living tissues. Two-photon lasers use near-infrared (NIR) pulse lasers that are less invasive and permit deep tissue penetration. In this review, recent advances in two-photon imaging and their applications in plant studies are discussed. Compared to confocal microscopy, NIR 2PEM exhibits reduced plant-specific autofluorescence, thereby achieving greater depth and high-resolution imaging in plant tissues. Fluorescent proteins with long emission wavelengths, such as orange–red fluorescent proteins, are particularly suitable for two-photon live imaging in plants. Furthermore, deep- and high-resolution imaging was achieved using plant-specific clearing methods. In addition to imaging, optical cell manipulations can be performed using femtosecond pulsed lasers at the single cell or organelle level. Optical surgery and manipulation can reveal cellular communication during development. Advances in in vivo imaging using 2PEM will greatly benefit biological studies in plant sciences.
Keywords: Deep imaging, Fluorophore, Laser ablation, Live imaging, Plant clearing, Two-photon microscopy
Introduction
Over the past few decades, fluorescence imaging has gained increased importance, particularly for biological imaging. The British scientist, George G. Stokes, first described the term ‘fluorescence’ in 1852 when he observed the autofluorescence of mineral fluorspar (Ceredig 2020). Since the early 1900s, when the fluorescence microscope was developed, various fluorophores have been used to visualize specific tissues and cells. The discovery of green fluorescent protein (GFP) from Aequorea victoria in 1962 truly revolutionized molecular biological studies, including fluorescence microscopy studies (Shimomura et al. 1962, Prasher et al. 1992, Heim et al. 1994). GFP is one of the most widely used and studied fluorescent markers in molecular biology. GFP and other related fluorescent proteins can be genetically encoded in biological samples for visualization of cell and protein dynamics (Shaner et al. 2007). As a result, studies of gene and cell function have advanced significantly.
Live-cell imaging with various fluorescent proteins allows us to study the dynamics of biological events in vivo. Three-dimensional (3D) high-resolution imaging has also become necessary to visualize protein localization and cell dynamics in thick specimens, such as neural networks in the mouse brain (Kobat et al. 2011, Kawakami et al. 2013). Recent advances in imaging techniques based on various fluorescent probes have revealed dynamic events at the subcellular level. Confocal laser scanning microscopy (CLSM) is a fluorescent imaging technique used to increase the optical resolution and contrast of a micrograph by rejecting out-of-focus fluorescent light by using a spatial pinhole (Hovis and Heuer 2010). Although CLSM is a powerful tool for biological imaging in the field of plant sciences, there are certain limitations. A visible-light laser produces excitation regions, thereby generating heat and light dispersion around the focus plane, leading to photodamage and photobleaching of living tissues (Magidson and Khodjakov 2013). In addition, confocal lasers are less suitable for deep tissue imaging because of strong light scattering and plant-specific autofluorescence can mask the fluorescent signals of the probe (Hutzler et al. 1998).
Two-photon absorption was originally predicted by Göppert-Mayer (1931) and was first observed experimentally by Kaiser and Garrett (1961). Two-photon excitation microscopy (2PEM), also known as multiphoton microscopy, was developed approximately 30 years ago (Denk et al. 1990) and is a fluorescence imaging technique that allows for the visualization of cells at depths unachievable by confocal microscopy (Denk et al. 1995, Helmchen and Denk 2005). When fluorophores are illuminated with a suitable light source, electrons are transferred to an excited state by the absorbed light. After that, when the electrons return to the ground state, energy is released as a photon, which causes fluorescence emission (Fig. 1A). Through this process, when one photon excites a fluorophore, a linear single-photon absorption process occurs. In a two-photon absorption, two photons of approximately twice the wavelength are simultaneously absorbed by a fluorophore. Two-photon absorption is rare in nature; thus, in 2PEM, an extremely high photon density is achieved by a femtosecond pulsed laser. Very high light intensities provided by lasers cause a phenomenon called a nonlinear optical phenomenon, a response that is nonlinearly dependent on the intensity of the applied optical field (Boyd 2020). Multiphoton absorption is spatially confined to the perifocal region under nonlinear contrast mechanisms of photon density. Thus, photodamage was reduced, except in the vicinity of the focal spot, and tissue viability increased (Fig. 1A). Additionally, 2PEM typically excites samples using near-infrared (NIR) wavelengths, which penetrate biological structures to a great extent. Due to these characteristics, NIR light does not only penetrate deeper tissue but is also less endogenously absorbed by most tissues and therefore is less phototoxic. Therefore, 2PEM has become an indispensable tool for live-tissue studies and has been widely used in biological studies, such as in neuroscience at the depths of several hundred micrometers (Yamaguchi et al. 2020), in pathology of living animals (Svoboda and Yasuda 2006) and in plant studies (Feijó and Moreno 2004, Cheung et al. 2010, Kimata et al. 2019). In addition to live-cell imaging, deep two-photon imaging of fixed samples based on new clearing methods has also been recently developed (Kurihara et al. 2015, 2021, Hasegawa et al. 2016). Furthermore, laser disruption by two-photon lasers is useful for studying developmental biology. This review highlights the advances in two-photon imaging and the manipulation of cells of interest in various plant tissues.
Fig. 1.
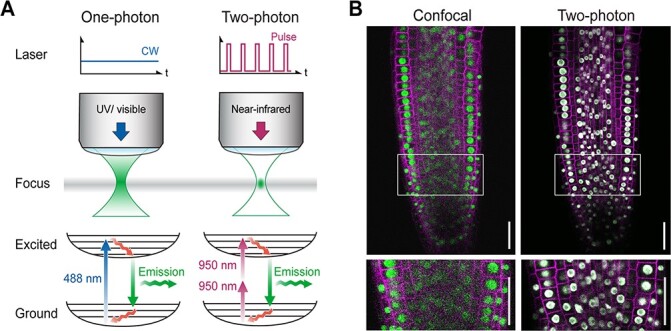
Differences between single- and two-photon microscopies. (A) Schematic representation of single- and two-photon microscopies. Representative excitation wavelengths are indicated. Visible light from continuous wave (CW) laser is used in a single-photon microscopy, whereas femtosecond NIR pulsed laser is used in a two-photon microscopy. In single-photon microscopy, an entire cone of fluorescence light (green) is generated, whereas in two-photon microscopy, fluorescence light is generated at the vicinity of the focal spot by absorbing two photons. This enables clear imaging without noise above and below the focal plane, as there are almost no excitations away from the focal point. (B) Comparison of imaging penetration between single- (confocal microscopy) and two-photon imaging of the root tip of Arabidopsis thaliana expressing RPS5Apro::H2B-sGFP and RRPS5Apro::tdTomato-LTI6b. The nuclei and cell membranes are labeled with fluorescent proteins sGFP (green) and tdTomato (magenta), respectively. This transgenic line has been previously described (Susaki et al. 2020). The sections 54 μm deep from the root surface were excited at 488 and 561 nm for confocal imaging, or 980 nm for two-photon imaging. The magnified images are shown as green rectangles. The microscope is A1R MP (Nikon), and the detailed imaging system has been described in a previous study (Mizuta et al. 2015). Scale bars, 50 μm.
Excitation Wavelength and Fluorescent Probes for Two-Photon Live-Imaging
In general, light absorption, scattering and tissue autofluorescence affect the resolution of deep biological imaging. For in vivo live imaging, a less-invasive method is also required. Plant deep tissue imaging has certain barriers, such as strong plant-specific autofluorescence (Müller et al. 2013) and interference due to optically dense tissues with multiple light-scattering structures (Tamada et al. 2014). Plant chloroplasts, which contain pigments such as chlorophyll, are the main source of optical disturbances in plant cells. The Ti:sapphire lasers used for NIR excitation are usually tunable from 690 to 1,050 nm, reducing autofluorescence and diminishing phototoxicity to living cells (Weissleder and Ntziachristos 2003). Usually, wavelengths of 900–950 nm are used for the excitation of GFPs, but plant tissue compounds such as chlorophyll are also strongly excited, thus masking the target fluorescence (Mizuta et al. 2015). Longer wavelengths, such as 980–1,000 nm NIR femtosecond lasers, clearly visualize GFP with reduced autofluorescence (Svoboda and Yasuda 2006, Mizuta et al. 2015). Fig. 1A shows the optical section of the Arabidopsis root tip, whose cell membranes and nuclei are labeled with green (sGFP) and orange (tdTomato) fluorescent proteins. Compared to the confocal image, the number and shape of cells and nuclei were much more clearly visible, especially at the center of the tissue, under the 2PEM with a 990 nm excitation wavelength. Thus, even with the same NIR light, longer-wavelength excitation over 980 nm is more suitable for deep imaging with reduced autofluorescence in plants.
In biological samples, there are specific regions in the NIR spectral range where the level of absorption and scattering is minimal, termed as the three optical windows (Golovynskyi et al. 2018). The first optical window is 700–1,000 nm (NIR-I), which is used for two-photon imaging by Ti:sapphire lasers of live tissues; however, the tissue penetration depth is still limited. The second and third optical windows are between 1,000 and1,350 nm (NIR-II) and 1,550 and 1,870 nm (NIR-III), respectively, are useful for achieving deeper tissue imaging, but the lack of biocompatible fluorescent probes and sensitivity of cameras have prevented these ranges for in vivo imaging (Smith et al. 2009). Owing to the recent advances in multiphoton microscopy, two-, three- and four-photon excitation used at these long wavelengths, especially multiphoton excitation at the NIR-III, has enabled the deepest imaging in vivo (Guesmi et al. 2018, Liu et al. 2019). In addition to these optical systems, various fluorophores, still under development, can undergo multiphoton excitation and emission in these optical windows (Onishi et al. 2020). Recently, numerous fluorophores for the second optical window have been reported, such as small-molecule dyes (Antaris et al. 2016), quantum dots (QDs) (Zhang et al. 2012) and single-walled carbon nanotubes (Welsher et al. 2009). Even in plant tissues, the emission of fluorophores with longer wavelengths than orange, such as TagRFP and tdTomato, is optimal for in vivo live imaging with long-wavelength excitation by 2PEM (Drobizhev et al. 2009, Mizuta et al. 2015), whereas there are still few a applications of NIR-II optical window and the newly developed fluorophores (Zubkovs et al. 2018). Next-generation NIR fluorescent proteins (Matlashov et al. 2020) and various multiphoton fluorescent probes, such as sense-specific ions or membrane potential, have also been developed (Ouzounov et al. 2017, Ricard et al. 2018). The development of such probes and microscopic applications will become essential for in vivo live imaging and analysis located deep within multicellular organisms such as plants.
Simultaneous Multicolor Imaging by 2PEM
Owing to the development of several fluorescent probes, it is possible to label each target with probes that emit different wavelengths. This enables the simultaneous analysis of labeled targets within the same tissue or cell. Generally, it is necessary to excite at different wavelengths to observe two or more targets labeled with different fluorescent probes separately. Two-photon lasers, usually with femtosecond Ti:sapphire sources, have tunable excitation wavelengths as mentioned above; however, the change in wavelength requires a few seconds to a several tens of seconds. Therefore, the simultaneous analysis of different fluorescent probes is an issue. In the NIR two-photon excitation, the excitation spectra of some fluorescent probes are blue-shifted or broadened compared to twice the single-photon absorption wavelengths (Blab et al. 2001, Drobizhev et al. 2011). For example, the commonly used dyes, cascade blue, fluorescein and rhodamine have absorption maxima in ultraviolet (360 nm), blue (480 nm) and green (540 nm) wavelengths, respectively, under single-photon excitation, which are difficult to excite simultaneously by single-wavelength excitation. However, under two-photon excitation, all three dyes can be excited by 800 nm because of the blueshift of the two-photon spectra with respect to the one-photon spectra of fluorescein and rhodamine (Xu and Webb 1996, Yamanaka et al. 2015, Ricard et al. 2018). This two-photon excitation achieves simultaneous multicolor imaging, including in plant samples (Mizuta et al. 2015). Fig. 2A shows simultaneous multicolor live imaging of Arabidopsis pollen tubes. Pollen expressing five different fluorescent proteins, mTFP1, sGFP, Venus, TagRFP and mRFP, were mixed and pollinated onto the stigma of the pistil. After pollination, the style was cut and placed on the agarose medium and pollen tubes that emerged from the cut end of the style were excited by single-wavelength two-photon excitation at 980 nm. An image was acquired in sequential bandwidths of 6.0 nm spanning the wavelength range of 463.9–649.2 nm to generate a lambda stack containing 32 images (Mizuta et al. 2015). This reduces the time required to capture the images separately, resulting in less live tissue photodamage and photobleaching. However, it should be noted that these features can also be disadvantageous if each fluorescent probe is required to be exited individually. Recently, some improved fluorescent proteins with a large Stokes shift have been used for multiple fluorescent excitations in 2PEM (Kogure et al. 2008, Pan et al. 2017, Chen et al. 2020). The Stokes shift is the difference between the band maxima of the fluorescence absorption and emission spectra. A large Stokes shift results in excellent background reduction, as light scattering can be easily filtered out (Guan et al. 2015).
Fig. 2.
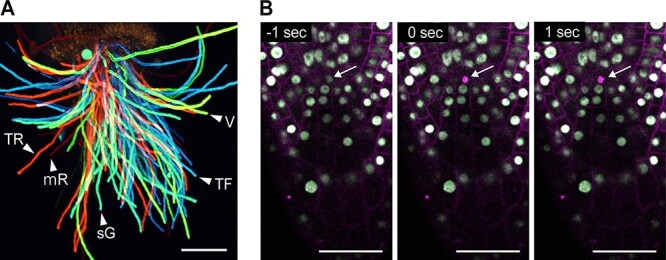
Simultaneous multicolor imaging and laser ablation by two-photon excitation. (A) Simultaneous multicolor two-photon imaging of Arabidopsis thaliana pollen tubes. Pollen tubes emerging from the end of cut style were observed 5 h after pollination. Each pollen tube expresses one of the following five fluorescent proteins—mTFP1 (TF), sGFP (sG), Venus (V), TagRFP (TR) or mRFP (mR). Emitted fluorescence signals were detected using a 32-channel PMT array detector ranging from 463.9 to 649.2 nm at 6.0 nm intervals. Maximum-intensity projections of Z-stack images at 0–126 μm depth were captured using 22 z-planes with 6 μm intervals after excitation at 980 nm. Spectrum analysis and adding color were also processed by NIS-Elements (Nikon). The microscope is A1R MP (Nikon). Imaging system and detail of marker lines have been described in a previous study (Mizuta et al. 2015). Scale bars, 100 μm. (B) Laser ablation of a root stem cell as a single cell level on the Arabidopsis thaliana root tips. Two-photon imaging of the root tip of Arabidopsis thaliana expressing RPS5Apro::H2B-sGFP and RRPS5Apro::tdTomato-LTI6b is shown. Excitation and irradiation wavelength used was 980 nm. Time indicates the elapsed time from the start of laser irradiation. Two-photon laser was used to irradiate a root stem cell at the single cell level (arrow) for 0.5 s. Scale bars, (A) 100 μm and (B) 50 μm.
The wavelength mixing of two synchronized pulses at different excitation wavelengths also enables simultaneous multicolor two-photon imaging (Mahou et al. 2012, Stringari et al. 2017). The combination of the two laser sources with different excitation wavelengths generates another wavelength excitation, which is used as an additional virtual excitation wavelength for two-photon excitation. For example, when pulses are synchronized, 850 and 1,100 nm excitation wavelengths generate another excitation wavelength at 959 nm, which enables simultaneous multicolor imaging of Brainbow-labeled mouse cortical samples (Mahou et al. 2012). Using this method, simultaneous multicolor two-photon imaging can also be used for a sample observed by a non-optimal single wavelength at a low excitation efficiency. Since their application to plants is still limited, future application development is expected.
Imaging Deep Inside the Opaque Plant Bodies by Clearing
In addition to live-cell imaging, high-resolution 3D deep imaging of fixed tissues is important for understanding morphological structures and gene function. Some clearing methods, such as Scale (Hama et al. 2011), SeeDB (Ke et al. 2013), CUBIC (Susaki et al. 2014) and CLARITY (Chung et al. 2013), have been developed primarily for whole-mount imaging of animal tissues. These clearing methods turn fixed samples, including whole-mount mouse brains; they become transparent after several days without quenching fluorescent dyes and proteins. Similar plant-specific clearing methods, such as ClearSee (Gooh et al. 2015) and TOMEI (Hasegawa et al. 2016), have also been reported. ClearSee is a sugar-based reagent consisting urea and detergent, whereas TOMEI is composed of 2,2′-thiodiethanol and propyl gallate. In both regents, whole-plant imaging with the maintenance of fluorescent proteins can be performed by adjusting the refractive index mismatch. This allows for the acquisition of high-resolution 3D images 200–350 mm deep by CLSM without sectioning (Kurihara et al. 2015, Hasegawa et al. 2016). This also enables for the analysis of cell morphological characteristics and protein localization in plant tissues. Even in the cleared leaves and roots, the 2PEM images showed sharp-shaped signals and a higher contrast than those from CLSM (Kurihara et al. 2015). Selecting proper fluorescent proteins is important for imaging, even when clearing samples. As mentioned above, orange fluorescent proteins are suitable for two-photon live imaging (Drobizhev et al. 2009, Mizuta et al. 2015). However, of the orange–red fluorescent proteins, such as TagRFP are not suitable for imaging in ClearSeeAlpha-treated samples because its fluorescence intensity specifically decreases (Kurihara et al. 2021). In recent years, ClearSee solution with fluorescent proteins as well as a combination of fluorescent dyes and immunostaining has been developed (Nagaki et al. 2017, Tofanelli et al. 2019). Depending on whether the samples are live or fixed, choosing both suitable fluorescent probes and excitation wavelengths, while taking autofluorescence into consideration is important for clear visualization of plant tissues.
Precise Optical Manipulation by Two-Photon Laser
In addition to less-invasive and deep-penetrated imaging, the femtosecond pulse laser is also used for laser ablation (also known as disruption) and spatial cell manipulation at the single-cell level (Tirlapur and König 2002, Ellis-Davies 2019). Two-photon absorption occurs in the sub-femtoliter range, which achieves precise optical manipulation by changing the laser irradiation site using an NIR pulsed laser. It is used for destroying an entire cell, specific organelles or to release caged compounds for the analysis of cell responses without impairing cell activity. In animals, a two-photon laser enables focal optical surgery of single neural cells, including those in living mouse brains, with high spatial precision (Hill et al. 2017, Yamaguchi et al. 2020). In plants, two-photon disruption/ablation of targets has been used to study multicellular tissues. In Arabidopsis roots, single-cell wounding by two-photon laser ablation was performed to analyze wound responses and signal communication to neighboring tissues (Marhavý et al. 2019). To analyze intercellular communications during Arabidopsis embryogenesis, single-cell laser disruption of the apical cell was performed (Gooh et al. 2015). The shoot apical meristem was wounded by an NIR laser to investigate auxin-dependent gene expression during leaf development (Caggiano et al. 2017). By adjusting the intensity and irradiation range of the pulsed laser, single-cell ablation is possible, such as in the stem cells of Arabidopsis roots (Fig. 2B). As shown in Fig. 2B, a stem cell collapsed upon laser irradiation and showed autofluorescence derived from cell damage. At the single-cell level, only a single organelle, such as an actin filament or a mitochondrion in tobacco BY-2 cells, can be disrupted (Hasegawa et al. 2014).
A two-photon laser was also used for fluorescence bleaching in cells. Fluorescence recovery after photobleaching is used to study the mobility and metabolism of fluorescent molecules, such as cell membrane proteins in Arabidopsis, tobacco leaves and protoplasts (Martinière et al. 2012). With better light-inducibility and selective ablation, inactivation of a target protein with high spatiotemporal resolution has also been achieved inside living cells by chromophore-assisted light inactivation (CALI) (Tour et al. 2003). CALI uses a photosensitizer, such as KillerRed, which efficiently generates short-lived reactive oxygen species that damage proteins in the immediate vicinity of the chromophore (Bulina et al. 2006). CALI is useful for spatiotemporal knockdown or loss-of-function of target molecules in plant cells, as in the study of chromosome morphogenesis (Matsunaga et al. 2012).
In addition to the above methods, various optical manipulations and analytical methods have been combined with pulsed multiphoton lasers. Fluorescent lifetime imaging (FLIM) is an imaging technique based on the differences in the excited-state decay rate of a fluorescent probe. In plants, not only confocal-, but multiphoton-based FLIM analysis has been used to quantify metabolism in living tissues, such as metal ion uptake in roots and photosynthesis in leaves (Babourina and Rengel 2009, Iermak et al. 2016). Caged compounds have been widely used by neuroscientists to study cellular signaling (Amatrudo et al. 2015). Various exogenous photoactivatable probes, such as ATP, calcium, gamma aminobutyric acid and glutamate, have been developed (Bort et al. 2013). The NIR laser is suitable for generating photolysis (also referred to as uncaging) of such caged probes because two-photon absorption only occurs in a spatially confined region. Its usefulness has also been demonstrated in plants, such as in the study of plant hormones and signal transduction in metabolism (Herbivo et al. 2013, Hayashi et al. 2015). These optical manipulations with innovative fluorescent molecules may be useful for visualizing spatiotemporal dynamics in living tissues using 2PEM. Further research on plants based on these imaging methods will provide new insights into plant developmental biology.
Perspectives
It has been 90 years since two-photon absorption was originally predicted by Maria Goeppert-Mayer. Since the development of the 2PEM 30 years ago, the field of optics for microscopy, such as lasers, detectors, lenses, hardware and software, has greatly progressed. A wide variety of fluorescent probes and observation methods have also been developed. Previous studies demonstrated that QDs have a large two-photon absorption, which is 100–1,000 times higher than that of organic dyes (Resch-Genger et al. 2008). Although it is necessary to verify toxicity, these fluorescent probes may be able to achieve more high-resolution deep imaging on overcoming autofluorescence. In recent years, multiphoton imaging with two or more photons, such as three-photon imaging, has also become commonly used (Liu et al. 2020). In the future, excitation light will be commonly used at longer wavelengths (1,100 or 1,700 nm), which could improve the resolution of tissues with irregular scattering. However, the primary limitation of 2PEM is that a specialized multiphoton laser is extremely expensive and delicate. Therefore, 2PEM has not yet been routinely used in plant fields. Further development of optic technologies, including multiphoton lasers, will be more widely used in any field of plant research. A Galvano scanner and a resonant scanner are mainly used for scanning, but the advancement of adaptive optics will facilitate the improvement of scanning speed and area. In addition to two-photon imaging, various combinations with other microscopies will be tried, such as two-photon spinning disk confocal microscopy (Otomo et al. 2015), two-photon light sheet microscopy (Mahou et al. 2012), and two-photon super-resolution microscopy (Winter et al. 2014). These technical advances will increase the applicability of multiphoton imaging in plant cell biology and will allow us to observe various aspects of plants that we have never seen yet.
Acknowledgements
I sincerely thank Y. Hamamura for her helpful suggestions and discussions. I thank D. Kurihara, S. Nagahara and T. Shinagawa for their materials.
Data Availability
Source data for Figs. 1 and 2 are provided in the paper.
Funding
Japan Society for the Promotion of Science [Grant-in-Aid for Transformative Research Areas (20H05778, 20H05779) and Grant-in-Aid for Scientific Research on Innovative Areas (JP16H06464, JP16K21727)] and the Foundation of Kinoshita Memorial Enterprise (2020 Grant-in-Aid for Academic Research).
Disclosures
The author has no conflicts of interest to declare.
References
- Amatrudo J.M., Olson J.P., Agarwal H.K. and Ellis-Davies G.C.R. (2015) Caged compounds for multichromic optical interrogation of neural systems. Eur. J. Neurosci. 41: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antaris A.L., Chen H., Cheng K., Sun Y., Hong G., Qu C., et al. (2016) A small-molecule dye for NIR-II imaging. Nat. Mater. 15: 235–242. [DOI] [PubMed] [Google Scholar]
- Babourina O. and Rengel Z. (2009) Uptake of aluminium into Arabidopsis root cells measured by fluorescent lifetime imaging. Ann. Bot. 104: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blab G.A., Lommerse P.H.M., Cognet L., Harms G.S. and Schmidt T. (2001) Two-photon excitation action cross-sections of the autofluorescent proteins. Chem. Phys. Lett. 350: 71–77. [Google Scholar]
- Bort G., Gallavardin T., Ogden D. and Dalko P.I. (2013) From one-photon to two-photon probes: ‘caged’ compounds, actuators, and photoswitches. Angew. Chem. Int. Ed. Engl. 52: 4526–4537. [DOI] [PubMed] [Google Scholar]
- Boyd R.W. (2020) Chapter 1–the nonlinear optical susceptibility. InNonlinear Optics, 4th edn. Edited by Boyd, R.W. pp. 1–64. Academic Press, San Diego. [Google Scholar]
- Bulina M.E., Lukyanov K.A., Britanova O.V., Onichtchouk D., Lukyanov S. and Chudakov D.M. (2006) Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat. Protoc. 1: 947–953. [DOI] [PubMed] [Google Scholar]
- Caggiano M.P., Yu X., Bhatia N., Larsson A., Ram H., Ohno C.K., et al. (2017) Cell type boundaries organize plant development. eLife 6: e27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R. (2020) George Gabriel Stokes as a biologist. Philos. Trans. R. Soc. A 378: 20200105. [DOI] [PubMed] [Google Scholar]
- Chen G., Xu J., Zhan Z., Gao X., Jiao M., Ren M., et al. (2020) A bright two-photon fluorescence probe with large stokes shift for deep tissue imaging of H2S during metabolism. Dyes Pigm. 172: 107850. [Google Scholar]
- Cheung A.Y., Boavida L.C., Aggarwal M., Wu H.M. and Feijo J.A. (2010) The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J. Exp. Bot. 61: 1907–1915. [DOI] [PubMed] [Google Scholar]
- Chung K., Wallace J., Kim S.-Y., Kalyanasundaram S., Andalman A.S., Davidson T.J., et al. (2013) Structural and molecular interrogation of intact biological systems. Nature 497: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W., Holt J.R., Shepherd G.M. and Corey D.P. (1995) Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15: 1311–1321. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. and Webb W. (1990) Two-photon laser scanning fluorescence microscopy. Science 248: 73–76. [DOI] [PubMed] [Google Scholar]
- Drobizhev M., Makarov N.S., Tillo S.E., Hughes T.E. and Rebane A. (2011) Two-photon absorption properties of fluorescent proteins. Nat. Methods 8: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobizhev M., Tillo S., Makarov N.S., Hughes T.E. and Rebane A. (2009) Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B 113: 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G.C.R. (2019) Two-photon uncaging of glutamate. Front. Synaptic. Neurosci. 10: 48.doi: 10.3389/fnsyn.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó J.A. and Moreno N. (2004) Imaging plant cells by two-photon excitation. Protoplasma 223: 1–32. [DOI] [PubMed] [Google Scholar]
- Golovynskyi S., Golovynska I., Stepanova L.I., Datsenko O.I., Liu L., Qu J., et al. (2018) Optical windows for head tissues in near-infrared and short-wave infrared regions: approaching transcranial light applications. J. Biophotonics 11: e201800141. [DOI] [PubMed] [Google Scholar]
- Gooh K., Ueda M., Aruga K., Park J., Arata H., Higashiyama T., et al. (2015) Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev. Cell 34: 242–251. [DOI] [PubMed] [Google Scholar]
- Göppert-Mayer M. (1931) Über elementarakte mit zwei quantensprüngen. Ann. Phys. 401: 273–294. [Google Scholar]
- Guan Y., Meurer M., Raghavan S., Rebane A., Lindquist J.R., Santos S., et al. (2015) Live-cell multiphoton fluorescence correlation spectroscopy with an improved large Stokes shift fluorescent protein. Mol. Biol. Cell 26: 2054–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesmi K., Abdeladim L., Tozer S., Mahou P., Kumamoto T., Jurkus K., et al. (2018) Dual-color deep-tissue three-photon microscopy with a multiband infrared laser. Light: Sci. Appl. 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., et al. (2011) Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14: 1481–1488. [DOI] [PubMed] [Google Scholar]
- Hasegawa J., Higaki T., Hamamura Y., Kurihara D., Kutsuna N., Higashiyama T., et al. (2014) Increase in invaginated vacuolar membrane structure caused by plant cell expansion by genotoxic stress induced by DNA double-strand breaks. Cytologia 79: 467–474. [Google Scholar]
- Hasegawa J., Sakamoto Y., Nakagami S., Aida M., Sawa S. and Matsunaga S. (2016) Three-dimensional imaging of plant organs using a simple and rapid transparency technique. Plant Cell Physiol. 57: 462–472. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Kusaka N., Yamasaki S., Zhao Y. and Nozaki H. (2015) Development of 4-methoxy-7-nitroindolinyl (MNI)-caged auxins which are extremely stable in planta. Bioorg. Med. Chem. Lett. 25: 4464–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R., Prasher D.C. and Tsien R.Y. (1994) Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 91: 12501–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F. and Denk W. (2005) Deep tissue two-photon microscopy. Nat. Methods 2: 932–940. [DOI] [PubMed] [Google Scholar]
- Herbivo C., Omran Z., Revol J., Javot H. and Specht A. (2013) Synthesis and characterization of cell-permeable caged phosphates that can be photolyzed by visible light or 800 nm two-photon photolysis. Chembiochem 14: 2277–2283. [DOI] [PubMed] [Google Scholar]
- Hill R.A., Damisah E.C., Chen F., Kwan A.C. and Grutzendler J. (2017) Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat. Commun. 8: 15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis D.B. and Heuer A.H. (2010) The use of laser scanning confocal microscopy (LSCM) in materials science. J. Microsc. 240: 173–180. [DOI] [PubMed] [Google Scholar]
- Hutzler P., Fischbach R., Heller W., Jungblut T.P., Reuber S., Schmitz R., et al. (1998) Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 49: 953–965. [Google Scholar]
- Iermak I., Vink J., Bader A.N., Wientjes E. and van Amerongen H. (2016) Visualizing heterogeneity of photosynthetic properties of plant leaves with two-photon fluorescence lifetime imaging microscopy. Biochim. Biophys. Acta 1857: 1473–1478. [DOI] [PubMed] [Google Scholar]
- Kaiser W. and Garrett C.G.B. (1961) Two-photon excitation in CaF2: Eu2+. Phys. Rev. Lett. 7: 229–231. [Google Scholar]
- Kawakami R., Sawada K., Sato A., Hibi T., Kozawa Y., Sato S., et al. (2013) Visualizing hippocampal neurons with in vivo two-photon microscopy using a 1030 nm picosecond pulse laser. Sci. Rep. 3: 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M.-T., Fujimoto S. and Imai T. (2013) SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 16: 1154–1161. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Kato T., Higaki T., Kurihara D., Yamada T., Segami S., et al. (2019) Polar vacuolar distribution is essential for accurate asymmetric division of Arabidopsis zygotes. Proc. Natl. Acad. Sci. USA 116: 2338–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobat D., Horton N. and Xu C. (2011) In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J. Biomed. Opt. 16: 106014. [DOI] [PubMed] [Google Scholar]
- Kogure T., Kawano H., Abe Y. and Miyawaki A. (2008) Fluorescence imaging using a fluorescent protein with a large Stokes shift. Methods 45: 223–226. [DOI] [PubMed] [Google Scholar]
- Kurihara D., Mizuta Y., Nagahara S. and Higashiyama T. (2021) ClearSeeAlpha: advanced optical clearing for whole-plant imaging. Plant Cell Physiol. 62: 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara D., Mizuta Y., Sato Y. and Higashiyama T. (2015) ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142: 4168–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.J., Roy A., Simons A.A., Farinella D.M. and Kara P. (2020) Three-photon imaging of synthetic dyes in deep layers of the neocortex. Sci. Rep. 10: 16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Deng X., Tong S., He C., Cheng H., Zhuang Z., et al. (2019) In vivo deep-brain structural and hemodynamic multiphoton microscopy enabled by quantum dots. Nano. Lett. 19: 5260–5265. [DOI] [PubMed] [Google Scholar]
- Magidson V., Khodjakov A. (2013) Chapter 23 - circumventing photodamage in live-cell microscopy. InMethods Cell Biology. Edited by Sluder, G. and Wolf, D.E. pp. 545–560. Academic Press, San Diego. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahou P., Zimmerley M., Loulier K., Matho K., Labroille G., Morin X., et al. (2012) Multicolor two-photon tissue imaging by wavelength mixing. Nat. Methods 9: 815–818. [DOI] [PubMed] [Google Scholar]
- Marhavý P., Kurenda A., Siddique S., Dénervaud Tendon V., Zhou F., Holbein J., et al. (2019) Single-cell damage elicits regional, nematode-restricting ethylene responses in roots. EMBO J. 38: e100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A., Lavagi I., Nageswaran G., Rolfe D.J., Maneta-Peyret L., Luu D.-T., et al. (2012) Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. USA 109: 12805–12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashov M.E., Shcherbakova D.M., Alvelid J., Baloban M., Pennacchietti F., Shemetov A.A., et al. (2020) A set of monomeric near-infrared fluorescent proteins for multicolor imaging across scales. Nat. Commun. 11: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S., Takata H., Morimoto A., Hayashihara K., Higashi T., Akatsuchi K., et al. (2012) RBMX: a regulator for maintenance and centromeric protection of sister chromatid cohesion. Cell Rep. 1: 299–308. [DOI] [PubMed] [Google Scholar]
- Mizuta Y., Kurihara D. and Higashiyama T. (2015) Two-photon imaging with longer wavelength excitation in intact Arabidopsis tissues. Protoplasma 252: 1231–1240. [DOI] [PubMed] [Google Scholar]
- Müller S., Galliardt H., Schneider J., Barisas B. and Seidel T. (2013) Quantification of Förster resonance energy transfer by monitoring sensitized emission in living plant cells. Front. Plant Sci. 4: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Yamaji N. and Murata M. (2017) ePro-ClearSee: a simple immunohistochemical method that does not require sectioning of plant samples. Sci. Rep. 7: 42203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Suzuki Y., Ano H. and Kawamata J. (2020) Water-soluble red-fluorescent dyes for two-photon deep-tissue imaging. Bull. Chem. Soc. Jpn. 93: 1226–1233. [Google Scholar]
- Otomo K., Hibi T., Murata T., Watanabe H., Kawakami R., Nakayama H., et al. (2015) Multi-point scanning two-photon excitation microscopy by utilizing a high-peak-power 1042-nm laser. Anal. Sci. 31: 307–313. [DOI] [PubMed] [Google Scholar]
- Ouzounov D.G., Wang T., Wang M., Feng D.D., Horton N.G., Cruz-Hernández J.C., et al. (2017) In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 14: 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Luo F., Liu X., Liu W., Chen W., Liu F., et al. (2017) A novel two-photon fluorescent probe with a long Stokes shift and a high signal-to-background ratio for human NAD(P)H: quinone oxidoreductase 1 (hNQO1) detection and imaging in living cells and tissues. Analyst 142: 2624–2630. [DOI] [PubMed] [Google Scholar]
- Prasher D.C., Eckenrode V.K., Ward W.W., Prendergast F.G. and Cormier M.J. (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111: 229–233. [DOI] [PubMed] [Google Scholar]
- Resch-Genger U., Grabolle M., Cavaliere-Jaricot S., Nitschke R. and Nann T. (2008) Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 5: 763–775. [DOI] [PubMed] [Google Scholar]
- Ricard C., Arroyo E.D., He C.X., Portera-Cailliau C., Lepousez G., Canepari M., et al. (2018) Two-photon probes for in vivo multicolor microscopy of the structure and signals of brain cells. Brain Struct. Funct. 223: 3011–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N.C., Patterson G.H. and Davidson M.W. (2007) Advances in fluorescent protein technology. J. Cell Sci. 120: 4247–4260. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F.H. and Saiga Y. (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59: 223–239. [DOI] [PubMed] [Google Scholar]
- Smith A.M., Mancini M.C. and Nie S. (2009) Second window for in vivo imaging. Nat. Nanotechnol. 4: 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringari C., Abdeladim L., Malkinson G., Mahou P., Solinas X., Lamarre I., et al. (2017) Multicolor two-photon imaging of endogenous fluorophores in living tissues by wavelength mixing. Sci. Rep. 7: 3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki D., Suzuki T., Maruyama D., Ueda M., Higashiyama T. and Kurihara D. (2020) Dynamics of the cell fate specifications during female gametophyte development in Arabidopsis. PLoS biology 19: e3001123.doi: 10.1101/2020.04.07.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki E.A., Tainaka K., Perrin D., Kishino F., Tawara T., Watanabe T.M., et al. (2014) Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157: 726–739. [DOI] [PubMed] [Google Scholar]
- Svoboda K. and Yasuda R. (2006) Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50: 823–839. [DOI] [PubMed] [Google Scholar]
- Tamada Y., Murata T., Hattori M., Oya S., Hayano Y., Kamei Y., et al. (2014) Optical property analyses of plant cells for adaptive optics microscopy. Int. J. Optomechatronics 8: 89–99. [Google Scholar]
- Tirlapur U.K. and König K. (2002) Femtosecond near-infrared laser pulses as a versatile non-invasive tool for intra-tissue nanoprocessing in plants without compromising viability. Plant J. 31: 365–374. [DOI] [PubMed] [Google Scholar]
- Tofanelli R., Vijayan A., Scholz S. and Schneitz K. (2019) Protocol for rapid clearing and staining of fixed Arabidopsis ovules for improved imaging by confocal laser scanning microscopy. Plant Methods 15: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour O., Meijer R.M., Zacharias D.A., Adams S.R. and Tsien R.Y. (2003) Genetically targeted chromophore-assisted light inactivation. Nat. Biotechnol. 21: 1505–1508. [DOI] [PubMed] [Google Scholar]
- Weissleder R. and Ntziachristos V. (2003) Shedding light onto live molecular targets. Nat. Med. 9: 123–128. [DOI] [PubMed] [Google Scholar]
- Welsher K., Liu Z., Sherlock S.P., Robinson J.T., Chen Z., Daranciang D., et al. (2009) A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 4: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter P.W., York A.G., Nogare D.D., Ingaramo M., Christensen R., Chitnis A., et al. (2014) Two-photon instant structured illumination microscopy improves the depth penetration of super-resolution imaging in thick scattering samples. Optica 1: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C. and Webb W.W. (1996) Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13: 481–491. [Google Scholar]
- Yamaguchi K., Kitamura R., Kawakami R., Otomo K. and Nemoto T. (2020) In vivo two-photon microscopic observation and ablation in deeper brain regions realized by modifications of excitation beam diameter and immersion liquid. PLoSOne 15: e0237230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M., Saito K., Smith N., Arai Y., Uegaki K., Yonemaru Y., et al. (2015) Visible-wavelength two-photon excitation microscopy for fluorescent protein imaging. J. Biomed. Opt. 20: 101202. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hong G., Zhang Y., Chen G., Li F., Dai H., et al. (2012) Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 6: 3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkovs V., Antonucci A., Schuergers N., Lambert B., Latini A., Ceccarelli R., et al. (2018) Spinning-disc confocal microscopy in the second near-infrared window (NIR-II). Sci. Rep. 8: 13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data for Figs. 1 and 2 are provided in the paper.


